Insignificant Impact of Chemotactic Responses of Pseudomonas aeruginosa on the Bacterial Attachment to Organic Pre-Conditioned RO Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemotaxis Responses of P. aeruginosa PAO1 to the Organic Substances in the Feed Solution
2.2. Swarming Behavior of P. aeruginosa PAO1 on Swarm Plates
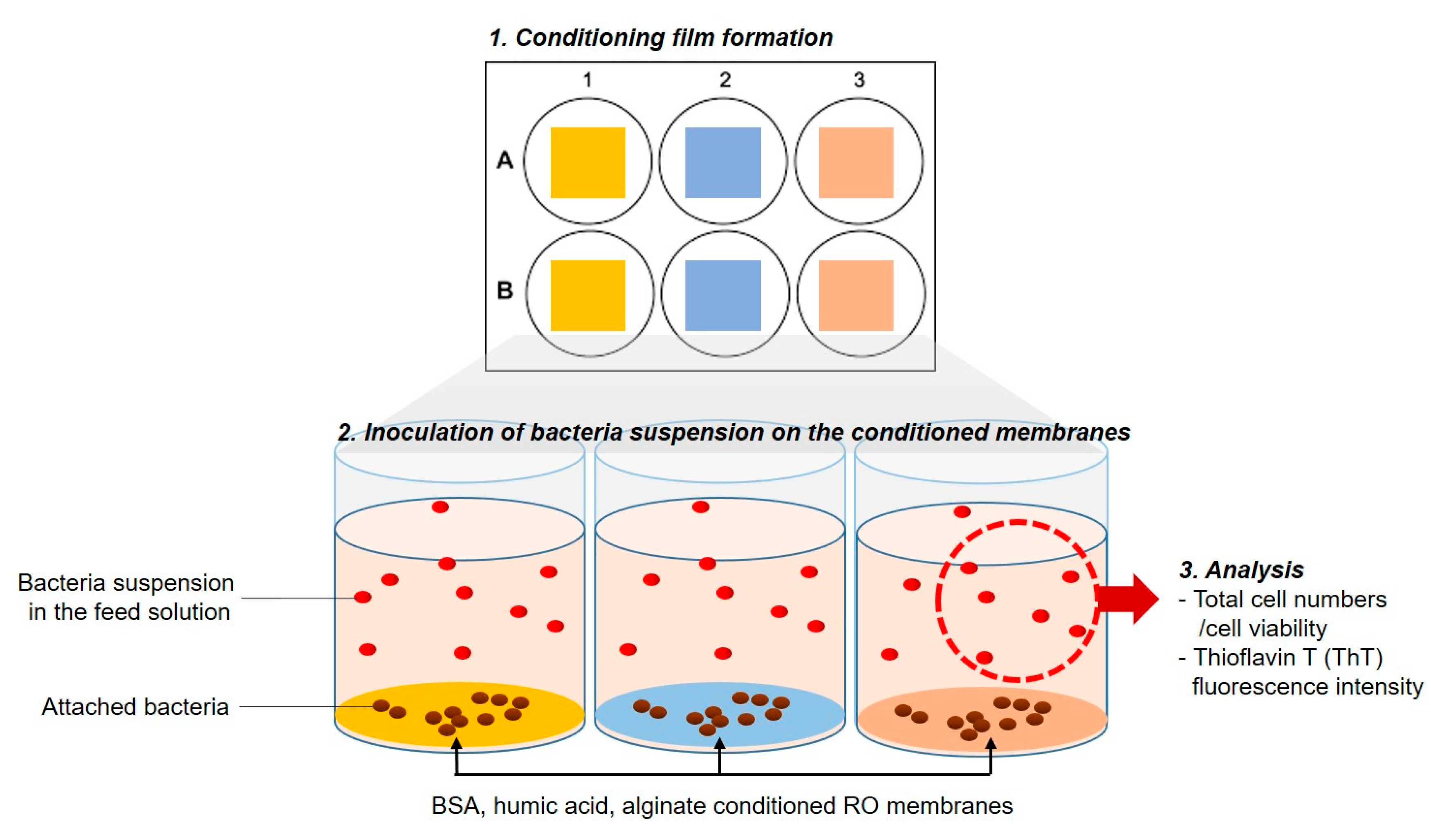
2.3. Impact of Physicochemical Properties of Organic Pre-Conditioned Membranes on Bacterial Attachment
2.4. Impact of Nutrients in the Feed Solution on Bacterial Attachment
2.5. Impact of Microbial Activity in the Feed Solution on Bacterial Attachment
2.6. Statistics Analysis
3. Results and Discussion
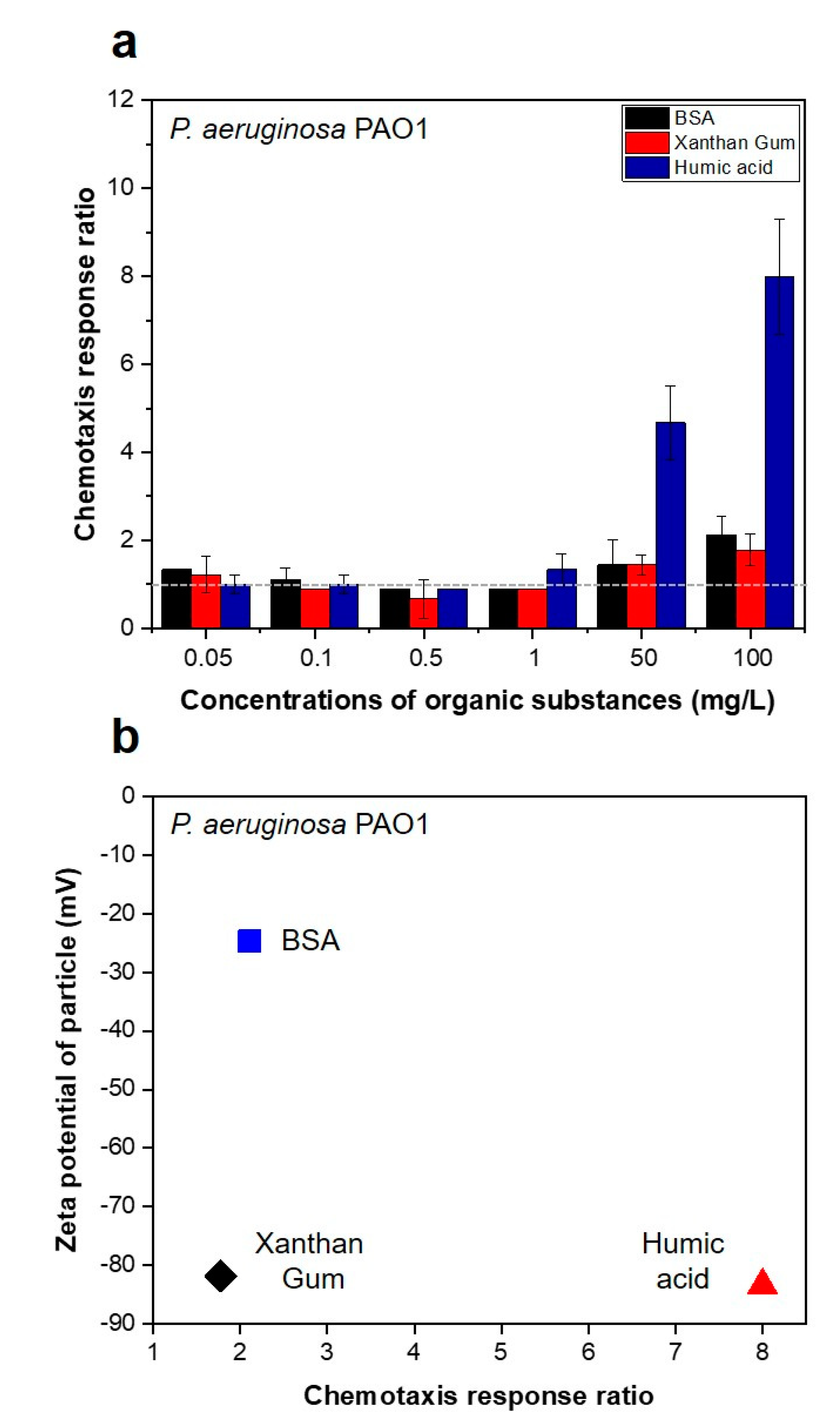
3.1. Strong Chemotactic Responses of P. aeruginosa PAO1 to the HA in the Feed Solution
3.2. Bacterial Movement towards Membranes Pre-Conditioned with SA and BSA Organics
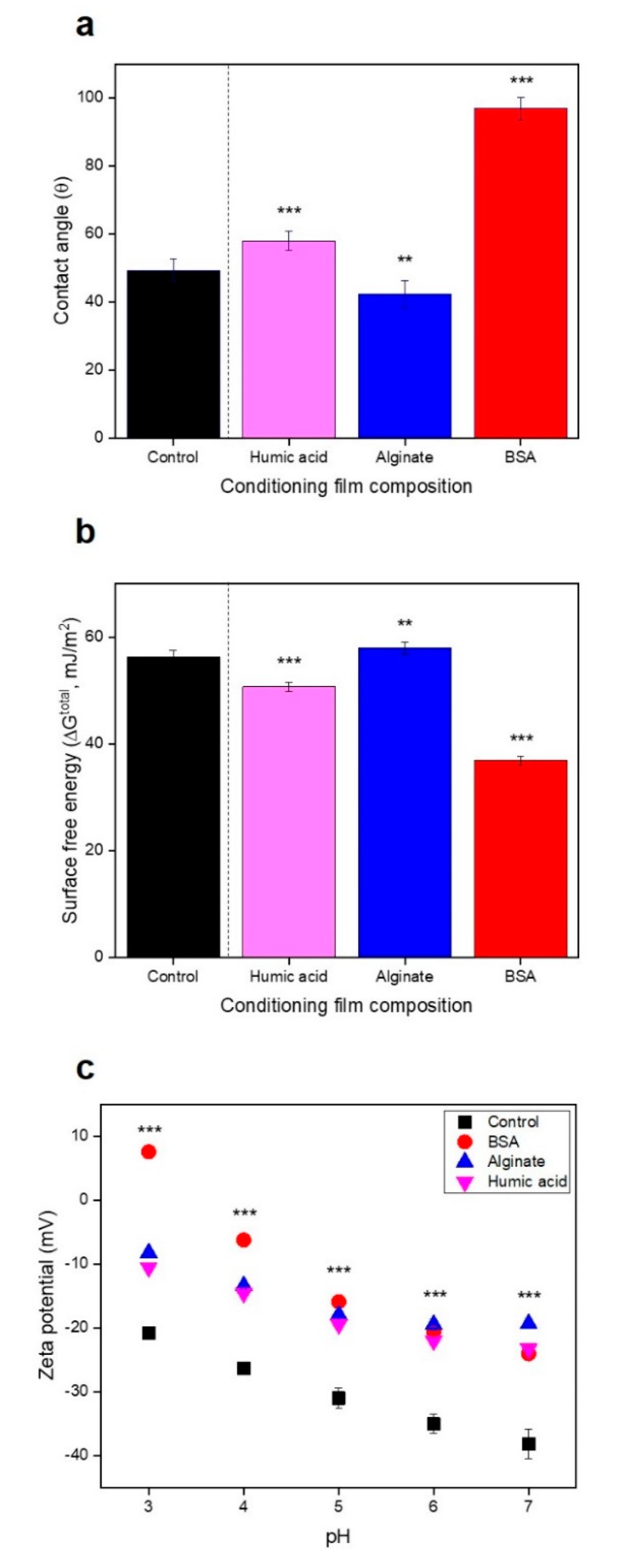
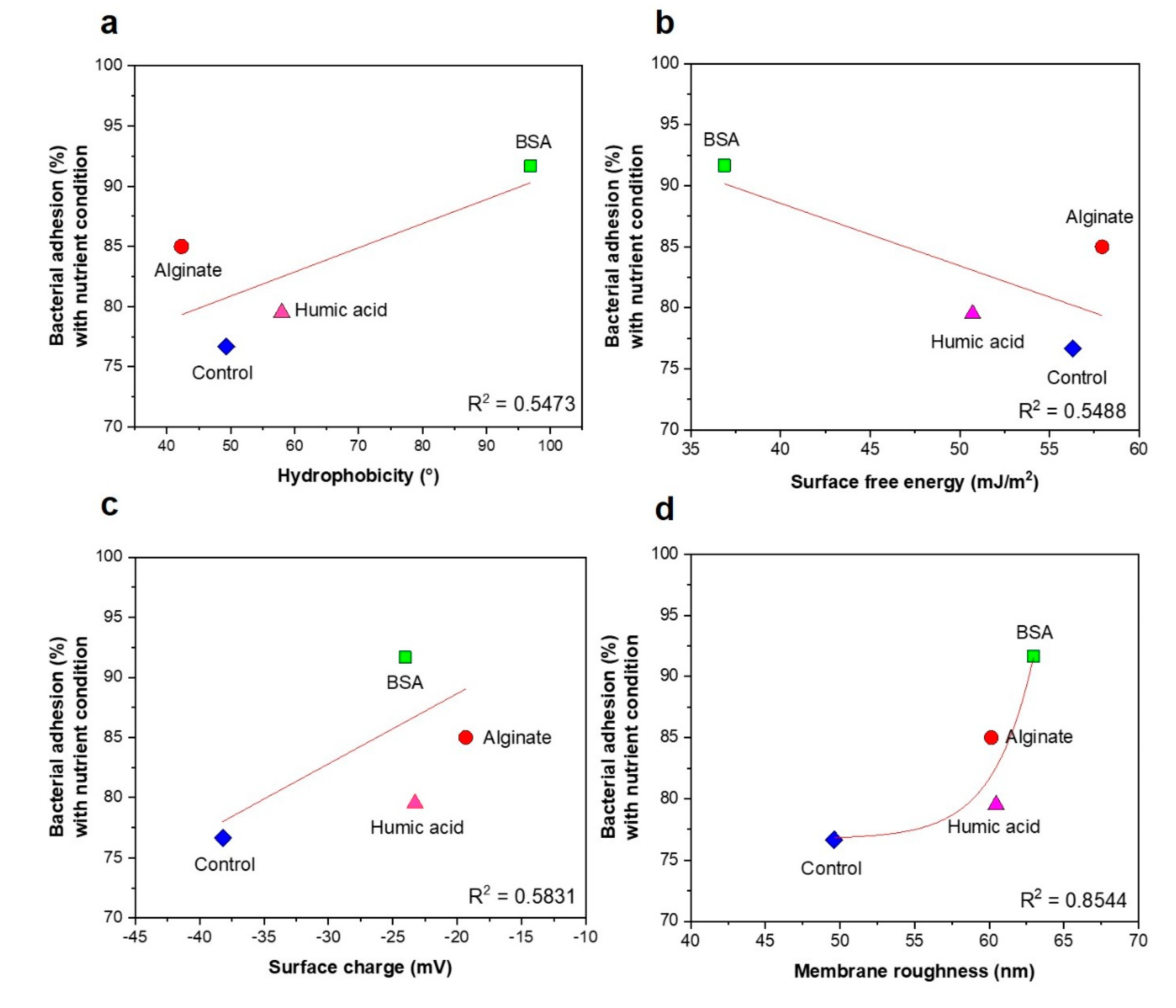
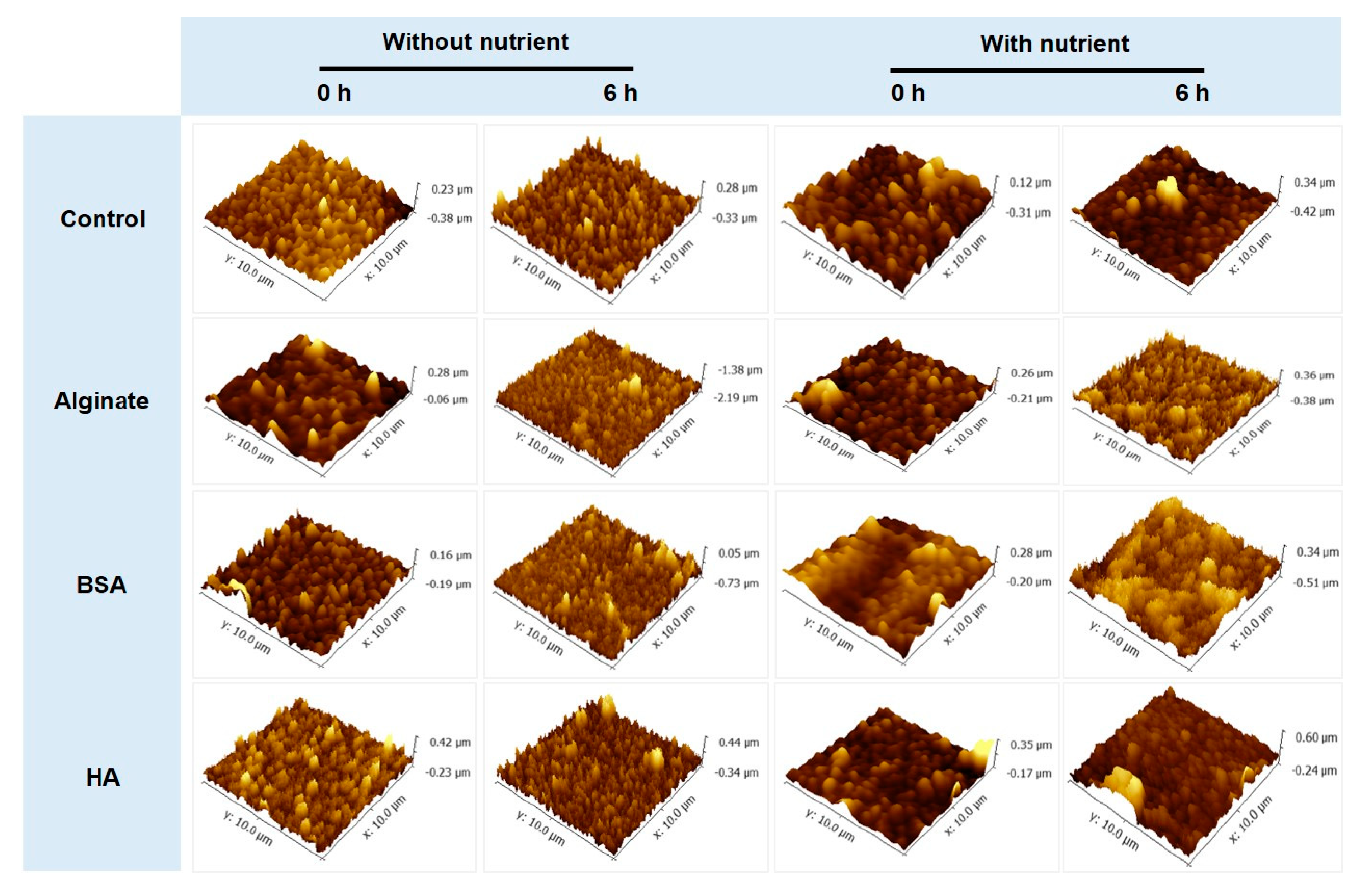
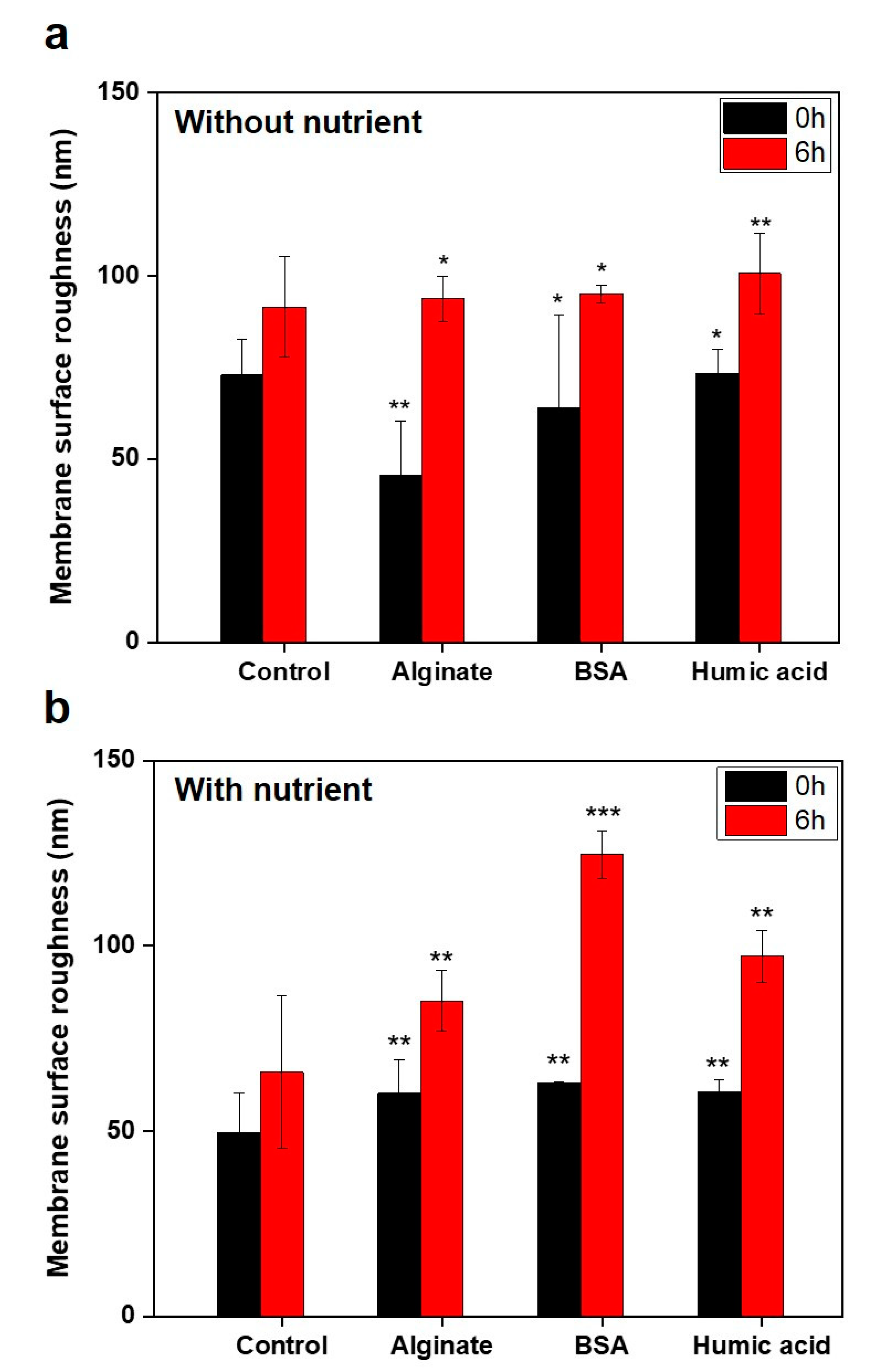
3.3. No Significant Relationships between Bacterial Attachment and Hydrophobicity or Surface Charge, but Exponential Relations with Membrane Roughness of Conditioned Membranes
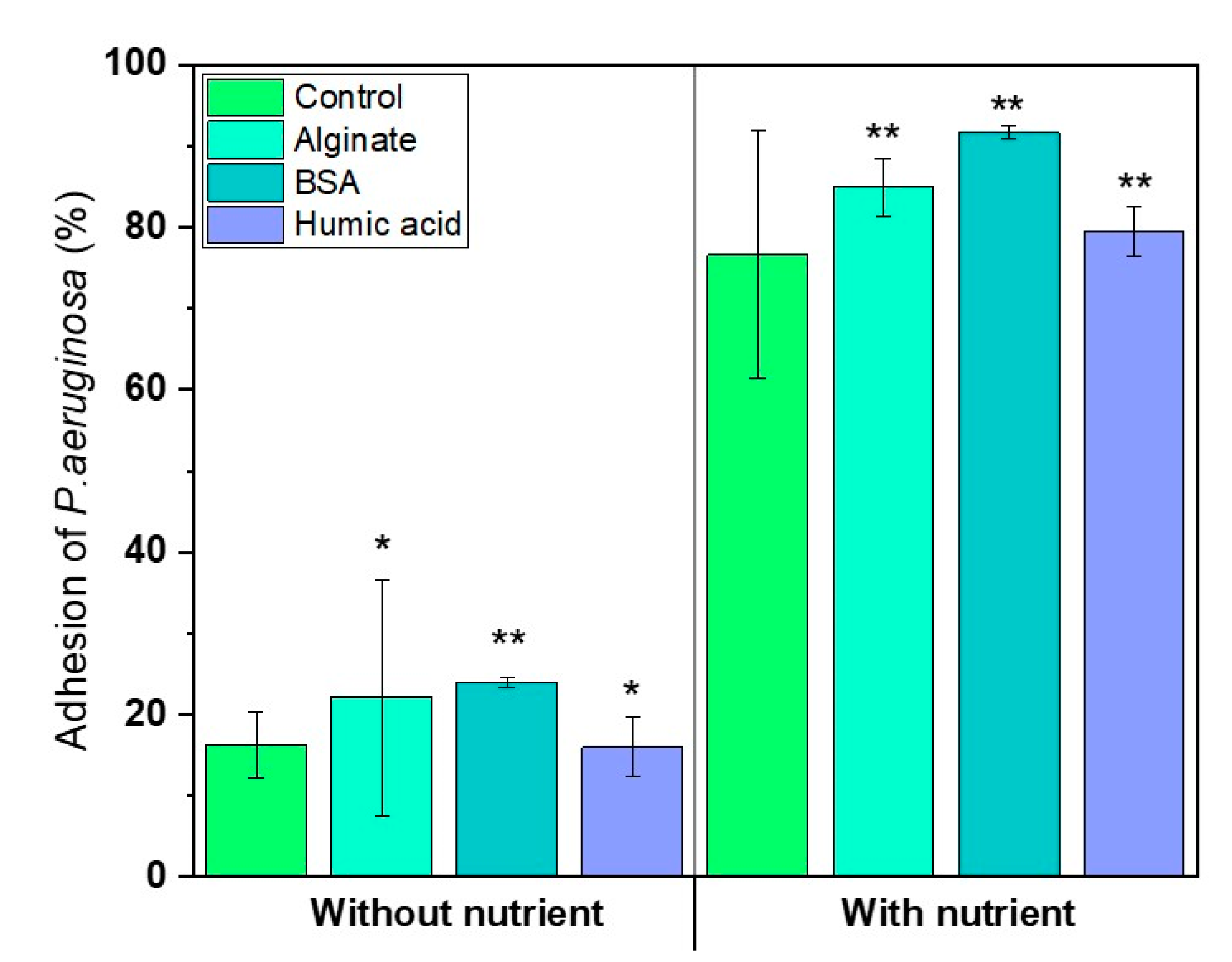
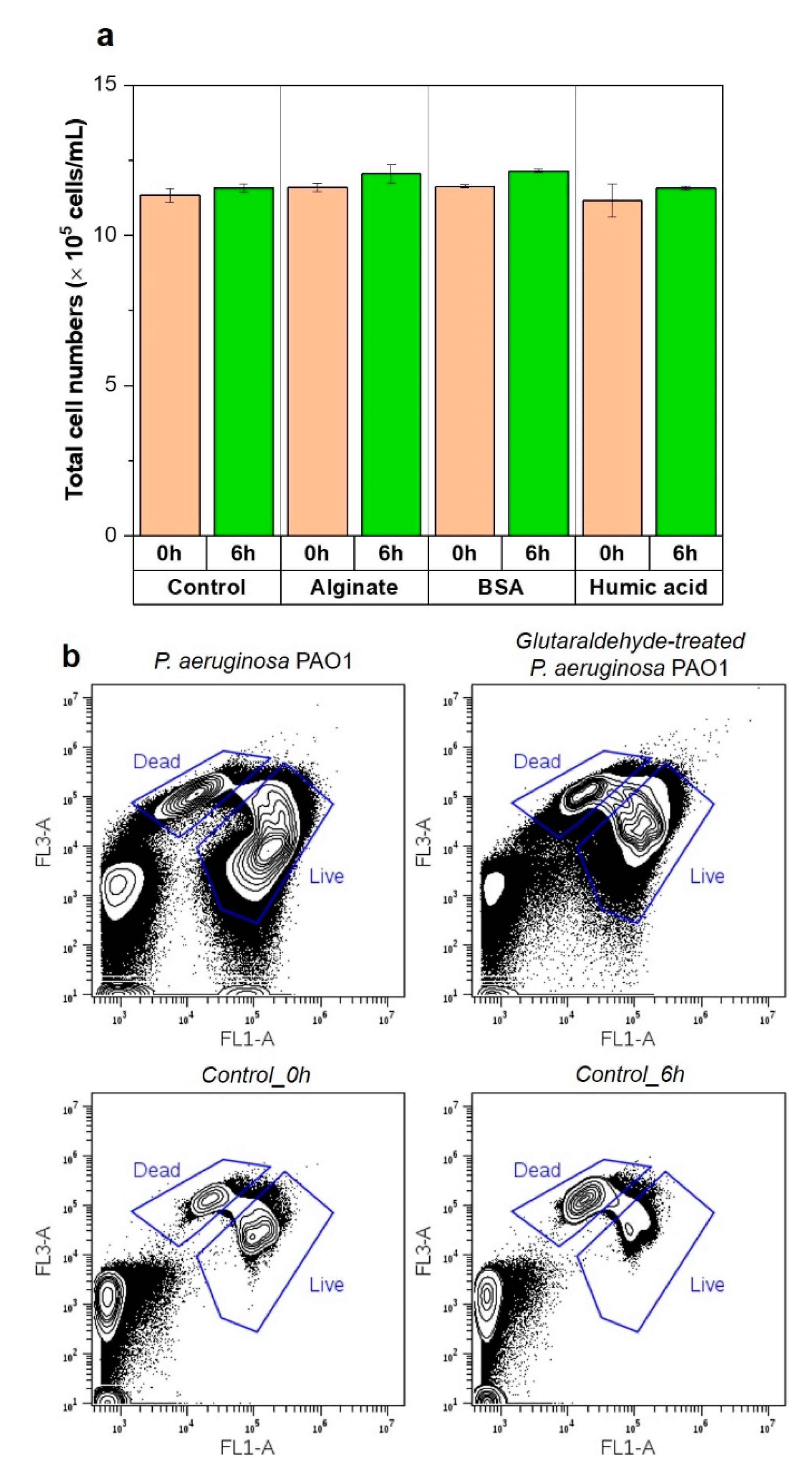
3.4. Nutrients in the Feed Solution Enhances Initial Bacterial Attachment
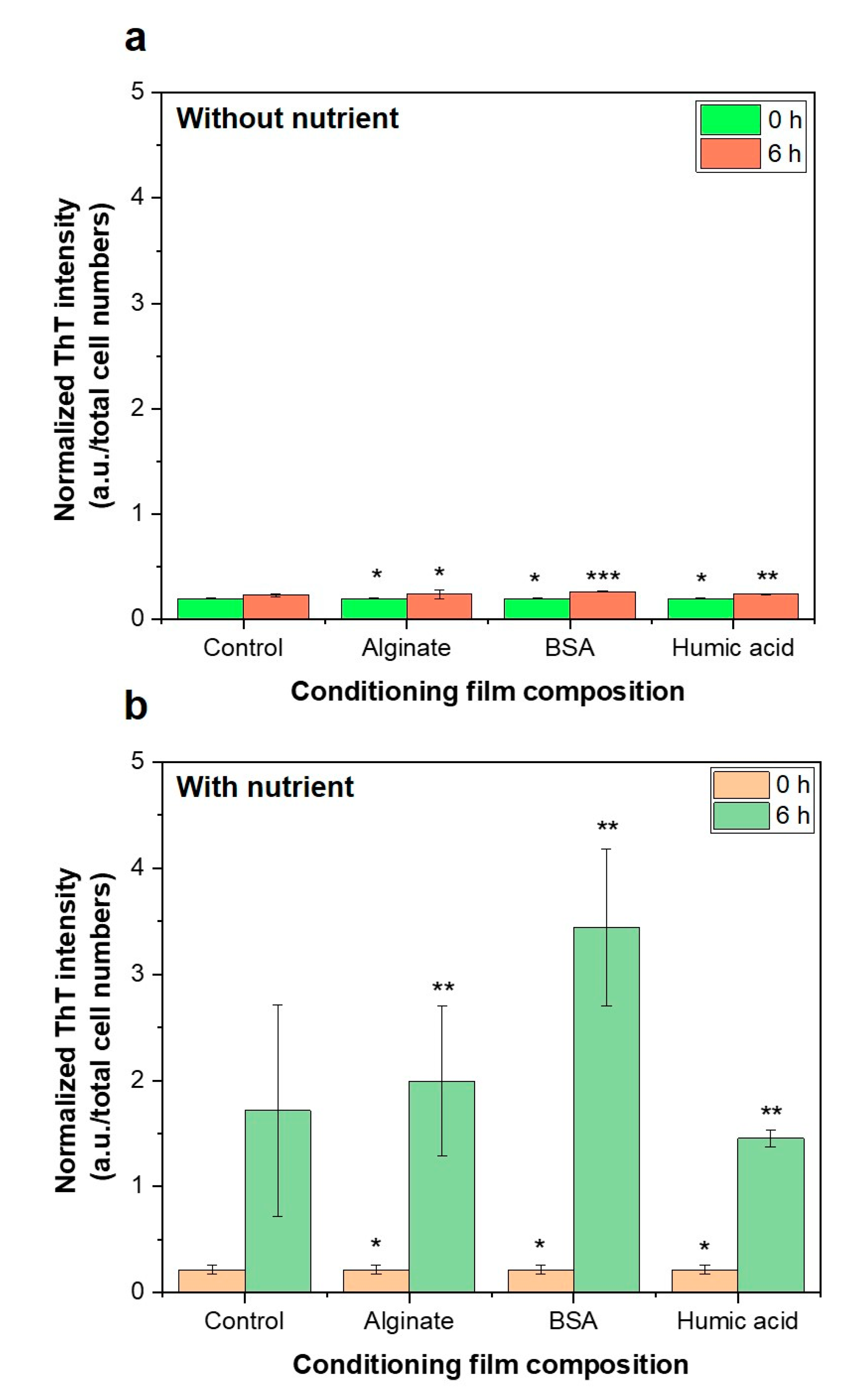
3.5. Importance of Bacterial Metabolic Activity on the Initial Bacterial Attachment
4. Conclusions
- Chemotactic response of P. aeruginosa PAO1 to the organic substances did not influence bacterial attachment on organic pre-conditioned RO membranes.
- Changes of membrane roughness by a conditioning film affected bacterial attachment, whereas hydrophobicity, membrane surface charge and surface free energy did not affect bacterial attachment.
- Presence of nutrients and microbial metabolic activity in the feed solution showed a significant impact on initial bacterial attachment.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.H.; Nava-Ocampo, M.; van Loosdrecht, M.C.M.; Kruithof, J.C.; Vrouwenvelder, J.S. The membrane fouling simulator: Development, application, and early-warning of biofouling in RO treatment. Desalin. Water Treat. 2018, 126, 1–23. [Google Scholar] [CrossRef]
- Matin, A.; Khan, Z.; Zaidi, S.M.J.; Boyce, M.C. Biofouling in reverse osmosis membranes for seawater desalination: Phenomena and prevention. Desalination 2011, 281, 1–16. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Herzberg, M.; Elimelech, M. Physiology and genetic traits of reverse osmosis membrane biofilms: A case study with Pseudomonas aeruginosa. ISME J. 2008, 2, 180–194. [Google Scholar] [CrossRef]
- Hwang, G.; Kang, S.; El-Din, M.G.; Liu, Y. Impact of conditioning films on the initial adhesion of Burkholderia cepacia. Colloids Surf. B Biointerfaces 2012, 91, 181–188. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Subramani, A.; Huang, X.; Hoek, E.M. Direct observation of bacterial deposition onto clean and organic-fouled polyamide membranes. J. Colloid Interface Sci. 2009, 336, 13–20. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, K.; Ren, H.; Ding, L.; Geng, J.; Zhang, Y. Combined effects of organic matter and calcium on biofouling of nanofiltration membranes. J. Membr. Sci. 2015, 486, 177–188. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Ding, S.; Hou, L.-A. Dynamics of biofouling development on the conditioned membrane and its relationship with membrane performance. J. Membr. Sci. 2016, 514, 264–273. [Google Scholar] [CrossRef]
- Heffernan, R.; Habimana, O.; Semiao, A.J.; Cao, H.; Safari, A.; Casey, E. A physical impact of organic fouling layers on bacterial adhesion during nanofiltration. Water Res. 2014, 67, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Garrido, K.D.; Palacios, R.J.S.; Lee, C.; Kang, S. Impact of conditioning film on the initial adhesion of E. coli on polysulfone ultrafiltration membrane. J. Ind. Eng. Chem. 2014, 20, 1438–1443. [Google Scholar] [CrossRef]
- Suwarno, S.R.; Hanada, S.; Chong, T.H.; Goto, S.; Henmi, M.; Fane, A.G. The effect of different surface conditioning layers on bacterial adhesion on reverse osmosis membranes. Desalination 2016, 387, 1–13. [Google Scholar] [CrossRef]
- Lee, H.; Park, C.; Kim, H.; Park, H.; Hong, S. Role of transparent exopolymer particles (TEP) in initial bacterial deposition and biofilm formation on reverse osmosis (RO) membrane. J. Membr. Sci. 2015, 494, 25–31. [Google Scholar] [CrossRef]
- Alshahri, A.H.; Dehwah, A.H.A.; Leiknes, T.; Missimer, T.M. Organic carbon movement through two SWRO facilities from source water to pretreatment to product with relevance to membrane biofouling. Desalination 2017, 407, 52–60. [Google Scholar] [CrossRef]
- Baek, Y.; Yu, J.; Kim, S.-H.; Lee, S.; Yoon, J. Effect of surface properties of reverse osmosis membranes on biofouling occurrence under filtration conditions. J. Membr. Sci. 2011, 382, 91–99. [Google Scholar] [CrossRef]
- Bos, R.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry of initial microbial adhesive interactions- its mechanisms and methods for study. FEMS Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef]
- Dunne, W.M., Jr. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef]
- Long, Z.; Quaife, B.; Salman, H.; Oltvai, Z.N. Cell-cell communication enhances bacterial chemotaxis toward external attractants. Sci. Rep. 2017, 7, 12855. [Google Scholar] [CrossRef]
- Adler, J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. Microbiology 1973, 74, 77–91. [Google Scholar] [CrossRef]
- Ravichandar, J.D.; Bower, A.G.; Julius, A.A.; Collins, C.H. Transcriptional control of motility enables directional movement of Escherichia coli in a signal gradient. Sci. Rep. 2017, 7, 8959. [Google Scholar] [CrossRef]
- Kim, L.H.; Jung, Y.; Yu, H.-W.; Chae, K.-J.; Kim, I.S. Physicochemical interactions between rhamnolipids and Pseudomonas aeruginosa biofilm layers. Environ. Sci. Technol. 2015, 49, 3718–3726. [Google Scholar] [CrossRef] [PubMed]
- Salgın, S.; Salgın, U.; Tuzlalı, N. Determination of correct zeta potential of polyether sulfone membranes using CLC and AGC: Ionic environment effect. Desalin. Water Treat. 2016, 57, 26031–26040. [Google Scholar] [CrossRef]
- Chao, Y.; Zhang, T. Optimization of fixation methods for observation of bacterial cell morphology and surface ultrastructures by atomic force microscopy. Appl. Microbiol. Biotechnol. 2011, 92, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Girgis, H.S.; Liu, Y.; Ryu, W.S.; Tavazoie, S. A comprehensive genetic characterization of bacterial motility. PLoS Genet. 2007, 3, e154. [Google Scholar] [CrossRef] [PubMed]
- Parales, R.E.; Harwood, C.S. Bacterial chemotaxis to pollutants and plant-derived aromatic molecules. Curr. Opin. Microbiol. 2002, 5, 266–273. [Google Scholar] [CrossRef]
- Wei, X.; Bauer, W.D. Starvation-induced changes in motility, chemotaxis, and flagellation of Rhizobium meliloti. Appl. Environ. Microbiol. 1998, 64, 1708–1714. [Google Scholar]
- Pandey, G.; Jain, R.K. Bacterial chemotaxis toward environmental pollutants: Role in bioremediation. Appl. Environ. Microbiol. 2002, 68, 5789–5795. [Google Scholar] [CrossRef]
- Charest, M.-H.; Beauchamp, C.J.; Antoun, H. Effects of the humic substances of de-inking paper sludge on the antagonism between two compost bacteria and Pythium ultimum. FEMS Microbiol. Ecol. 2005, 52, 219–227. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Visser, S.A. Physiological action of humic substances on microbial cells. Soil Bio. Biochem. 1985, 17, 457–462. [Google Scholar] [CrossRef]
- Tikhonov, V.V.; Yakushev, A.V.; Zavgorodnyaya, Y.A.; Byzov, B.A.; Demin, V.V. Effects of humic acids on the growth of bacteria. Eurasian Soil Sci. 2010, 43, 305–313. [Google Scholar] [CrossRef]
- Myint, A.A.; Lee, W.; Mun, S.; Ahn, C.H.; Lee, S.; Yoon, J. Influence of membrane surface properties on the behavior of initial bacterial adhesion and biofilm development onto nanofiltration membranes. Biofouling 2010, 26, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Qi, M.; Gong, X.; Wu, B.; Zhang, G. Landing dynamics of swimming bacteria on a polymeric surface: Effect of surface properties. Langmuir 2017, 33, 3525–3533. [Google Scholar] [CrossRef] [PubMed]
- Habimana, O.; Semião, A.J.C.; Casey, E. The role of cell-surface interactions in bacterial initial adhesion and consequent biofilm formation on nanofiltration/reverse osmosis membranes. J. Membr. Sci. 2014, 454, 82–96. [Google Scholar] [CrossRef]
- Sadr Ghayeni, S.B.; Beatson, P.J.; Schneider, R.P.; Fane, A.G. Adhesion of waste water bacteria to reverse osmosis membranes. J. Membr. Sci. 1998, 138, 29–42. [Google Scholar] [CrossRef]
- Zaky, A.M.; Escobar, I.C.; Gruden, C.L. Application of atomic force microscopy for characterizing membrane biofouling in the micrometer and nanometer scales. Environ. Prog. Sustain. Energy 2013, 32, 449–457. [Google Scholar] [CrossRef]
- Lee, W.; Ahn, C.H.; Hong, S.; Kim, S.; Lee, S.; Baek, Y.; Yoon, J. Evaluation of surface properties of reverse osmosis membranes on the initial biofouling stages under no filtration condition. J. Membr. Sci. 2010, 351, 112–122. [Google Scholar] [CrossRef]
- Semião, A.J.C.; Habimana, O.; Cao, H.; Heffernan, R.; Safari, A.; Casey, E. The importance of laboratory water quality for studying initial bacterial adhesion during NF filtration processes. Water Res. 2013, 47, 2909–2920. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and nanotopography sensitive bacterial attachment mechanisms: A review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Tuson, H.H.; Weibel, D.B. Bacteria-surface interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Taglialegna, A.; Lasa, I.; Valle, J. Amyloid structures as biofilm matrix scaffolds. J. Bacteriol. 2016, 198, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- de Kerchove, A.J.; Elimelech, M. Impact of alginate conditioning film on deposition kinetics of motile and nonmotile Pseudomonas aeruginosa strains. Appl. Environ. Microbiol. 2007, 73, 5227–5234. [Google Scholar] [CrossRef] [PubMed]
- Goulter, R.M.; Gentle, I.R.; Dykes, G.A. Issues in determining factors influencing bacterial attachment: A review using the attachment of Escherichia coli to abiotic surfaces as an example. Lett. Appl. Microbiol. 2009, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fane, A.G.; Coster, H.G.L.; Vigneswaran, S. Direct observation of particle deposition on the membrane surface during crossflow microfiltration. J. Membr. Sci. 1998, 149, 83–97. [Google Scholar] [CrossRef]
- Eshed, L.; Yaron, S.; Dosoretz, C.G. Effect of permeate drag force on the development of a biofouling layer in a pressure-driven membrane separation system. J. Appl. Environ. Microbiol. 2008, 74, 7338–7347. [Google Scholar] [CrossRef]

| Composition of the Conditioning Film | Substratum | Bacteria | Key Findings | Ref. |
|---|---|---|---|---|
| a SA, BSA | NF membrane (DOW Filmtec, USA) | Pseudomonas putida |
| [8] |
| SA, BSA, HA | NF membrane (Synder, USA) | Pseudomonas aeruginosa |
| [9] |
| Synthetic wastewater | RO membrane (DOW Filmtec, USA) | Pseudomonas aeruginosa |
| [10] |
| HA, AA | NF membrane (DOW Filmtec, USA) | Pseudomonas fluorescens |
| [11] |
| BSA, SA, NOM | Ultrafiltration (UF) membrane (GE Osmonics, USA) | Escherichia coli |
| [12] |
| AA, BSA | RO membrane (Toray, Japan) | Pseudomonas aeruginosa PAO1 |
| [13] |
| AA | RO membrane (Hydranuatics, PA) | Pseudomonas aeruginosa PA14 |
| [14] |
| Title | Experimental Scheme | Analysis | Matrix | Results |
|---|---|---|---|---|
| 2.1. Chemotaxis responses of P. aeruginosa PAO1 to the organic substances in the feed solution | 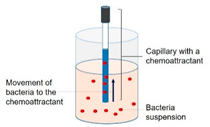 | Capillary test | Liquid solution | Figure 2 |
| 2.2. Swarming behavior of P. aeruginosa PAO1 on swarm plates |  | Swarming motility | Swarm plates | Figure 3 |
| 2.3. Impact of physicochemical properties of organic pre-conditioned membranes on bacterial attachment | 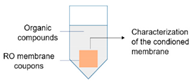 | Characterization of membrane roughness, hydrophobicity, surface charge | RO membrane | Table 3, Figures 4 and 5 |
| 2.4. Impact of nutrients in the feed solution on bacterial attachment |  | Quantification of total cell numbers and bacterial motility in the feed solution | Figures 6–9 | |
| 2.5. Impact of microbial activity in the feed solution on bacterial attachment |  | Figure 10 |
| Composition of the Conditioning Film | Control (in DI Water) | Without Nutrients (in PBS) | With Nutrients (in M9 Medium) | |||
|---|---|---|---|---|---|---|
| Roughness (Sq) (nm) | Skewness (Ssk) | Roughness (Sq) (nm) | Skewness (Ssk) | Roughness (Sq) (nm) | Skewness (Ssk) | |
| Control | 113.3 (±21.2) | −0.4 (±0.3) | 72.9 (±9.7) | 0.9 (±0.6) | 43.9 (±5.4) | 0.9 (±0.1) |
| Humic acid | 153.0 (±53.6) | 1.2 (±0.7) | 73.4 (±6.4) | 1.6 (±0.6) | 60.5 (±3.3) | 1.9 (±1.1) |
| Alginate | 84.6 (±34.9) | 1.1 (±0.4) | 45.5 (±15.0) | 1.4 (±0.8) | 60.1 (±9.2) | 1.7 (±0.4) |
| BSA | 80.3 (±2.1) | 1.0 (±0.2) | 64.0 (±25.2) | 1.2 (±0.2) | 63.0 (±0.4) | 0.4 (±0.3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, L.H.; Vrouwenvelder, J.S. Insignificant Impact of Chemotactic Responses of Pseudomonas aeruginosa on the Bacterial Attachment to Organic Pre-Conditioned RO Membranes. Membranes 2019, 9, 162. https://doi.org/10.3390/membranes9120162
Kim LH, Vrouwenvelder JS. Insignificant Impact of Chemotactic Responses of Pseudomonas aeruginosa on the Bacterial Attachment to Organic Pre-Conditioned RO Membranes. Membranes. 2019; 9(12):162. https://doi.org/10.3390/membranes9120162
Chicago/Turabian StyleKim, Lan Hee, and Johannes S. Vrouwenvelder. 2019. "Insignificant Impact of Chemotactic Responses of Pseudomonas aeruginosa on the Bacterial Attachment to Organic Pre-Conditioned RO Membranes" Membranes 9, no. 12: 162. https://doi.org/10.3390/membranes9120162
APA StyleKim, L. H., & Vrouwenvelder, J. S. (2019). Insignificant Impact of Chemotactic Responses of Pseudomonas aeruginosa on the Bacterial Attachment to Organic Pre-Conditioned RO Membranes. Membranes, 9(12), 162. https://doi.org/10.3390/membranes9120162





