Custom-Made Ion Exchange Membranes at Laboratory Scale for Reverse Electrodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ion Exchange Membranes
2.1.1. Anion Exchange Membrane
2.1.2. Cation Exchange Membrane
2.2. Membrane Characterization
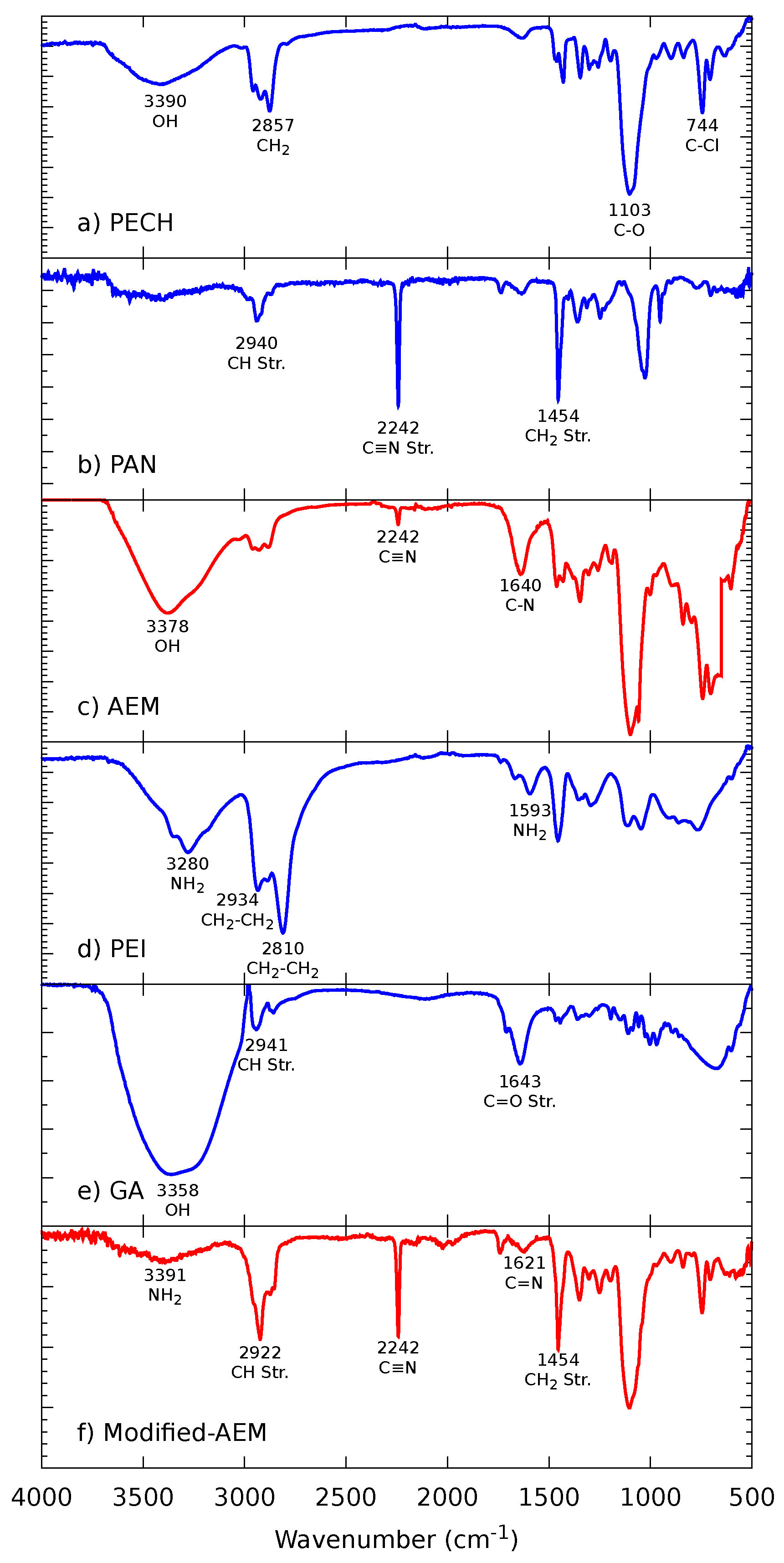
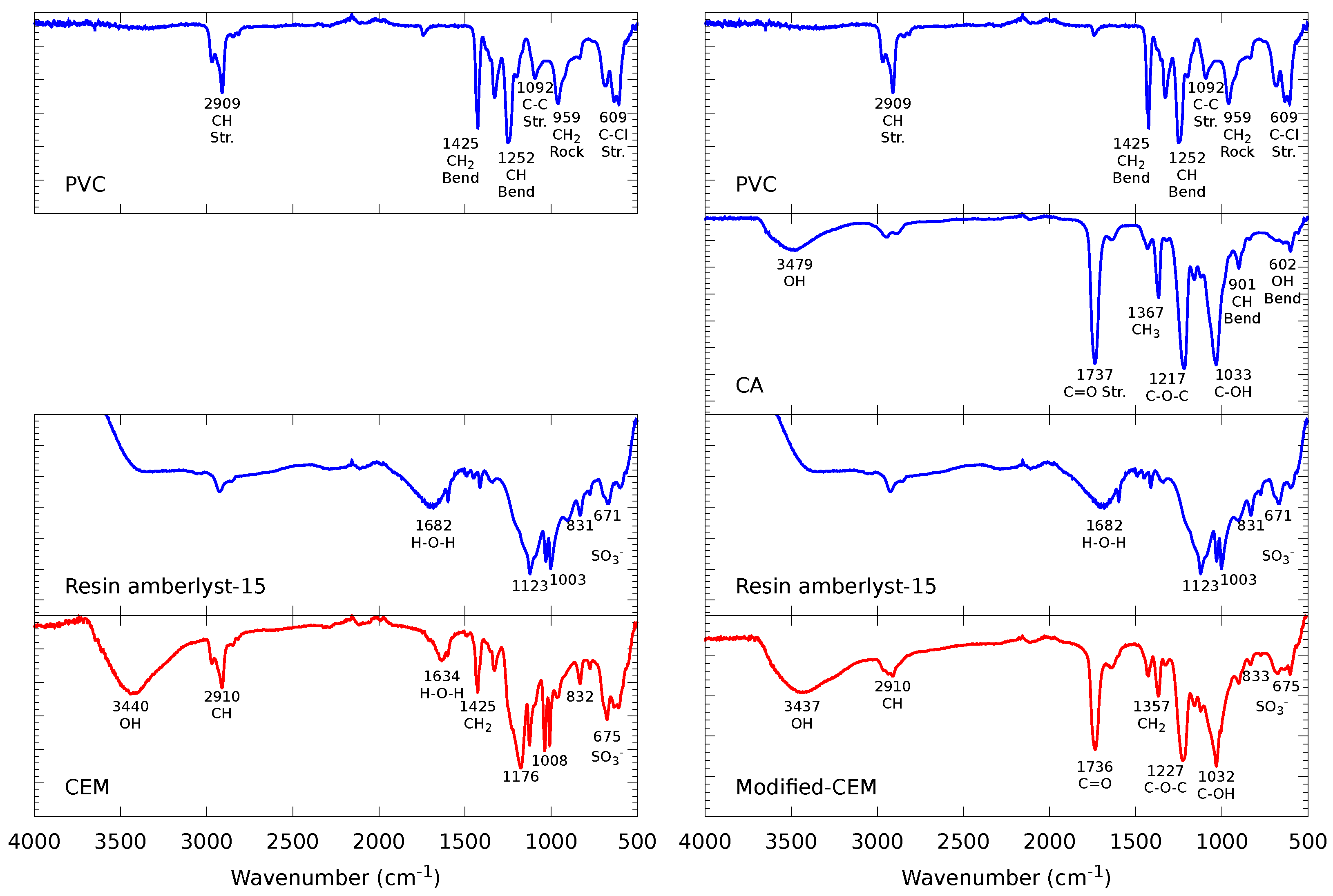
2.2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
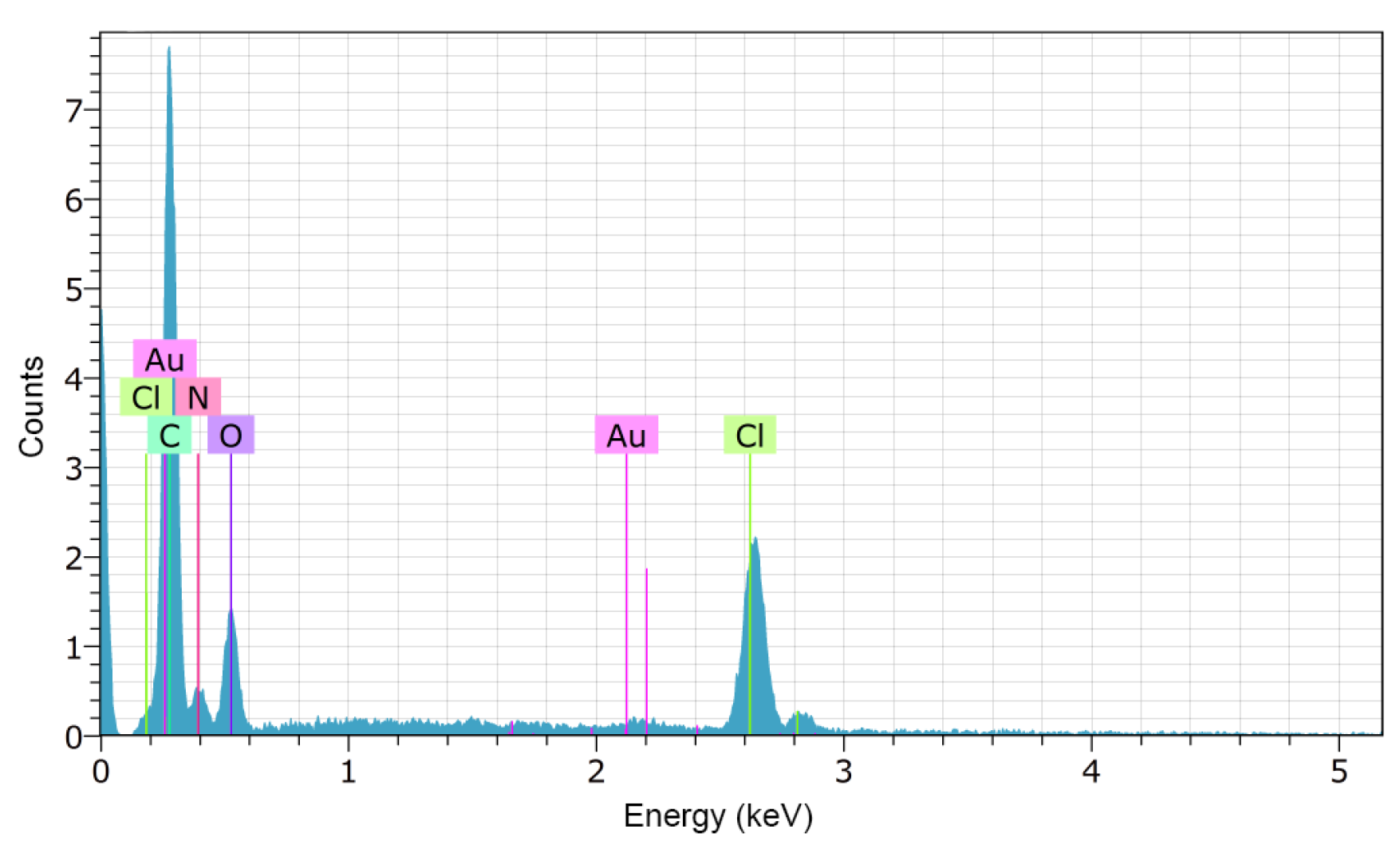
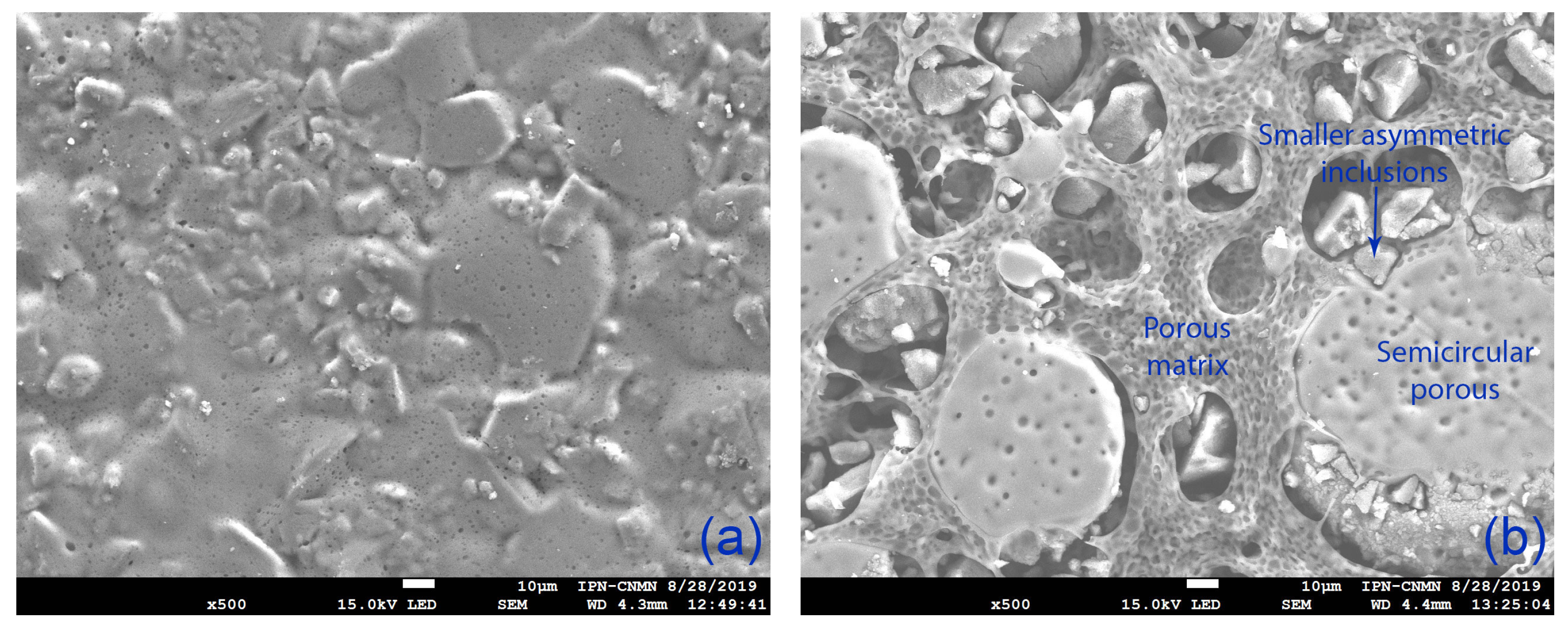
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Ultrapure Water Contact Angle
2.2.4. Swelling Degree
2.2.5. Ion Exchange Capacity
2.2.6. Charge Density
2.2.7. Permselectivity
2.2.8. Electrical Resistance
3. Results and Discussions
3.1. Membranes Characterization
3.1.1. FTIR Spectroscopy
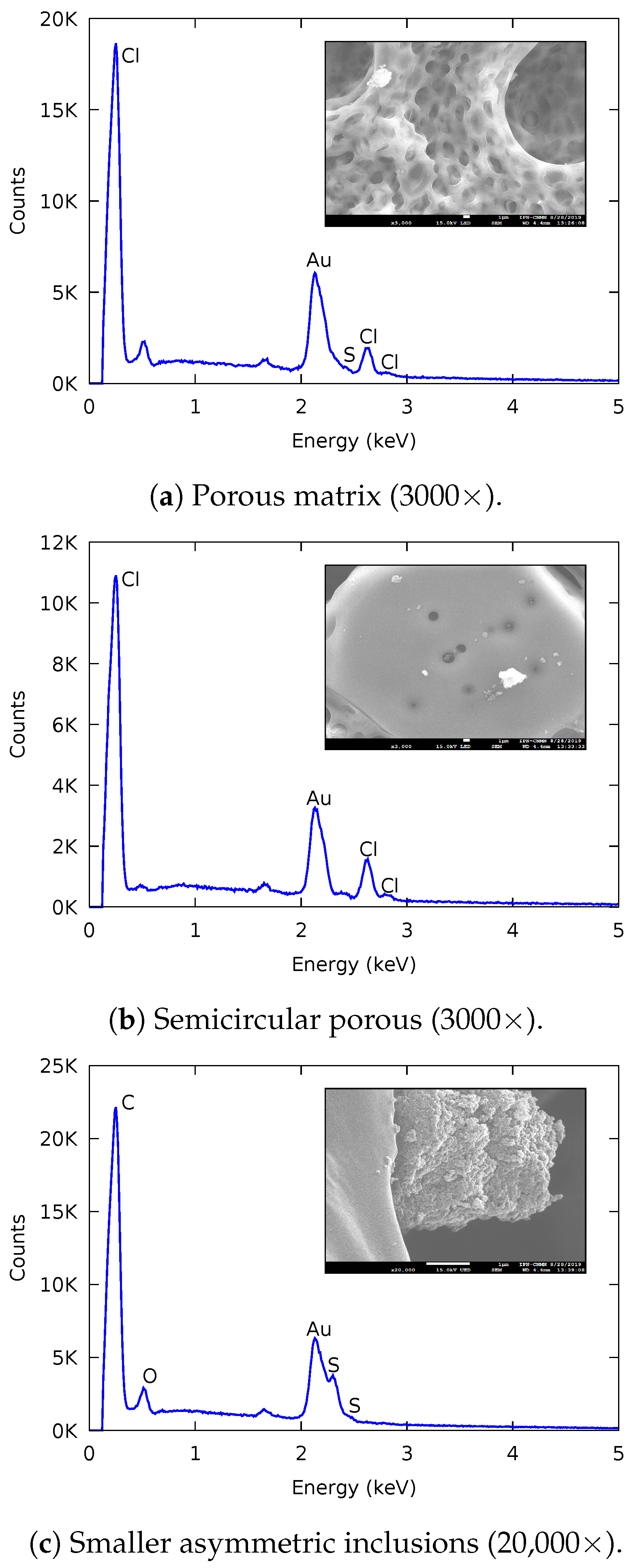
3.1.2. Scanning Electron Microscopy (SEM)
3.1.3. Ultrapure Water Contact Angle and Membrane Thickness
3.1.4. Swelling Degree, Ion Exchange Capacity, Fixed Charge Density, Permselectivity, and Electrical Resistance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- US EIA. Annual Energy Outlook 2019 with Projections to 2050; Technical Report; U.S. Energy Information Administration: Washington, DC, USA, 2019.
- Pattle, R.E. Production of Electric Power by mixing Fresh and Salt Water in the Hydroelectric Piles. Nature 1954, 174, 660. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Crespo, J.G.; Nijmeijer, K.; Curcio, E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Cipollina, A.; Silvestre, M.D.; Giacalone, F.; Micale, G.; Sanseverino, E.R.; Sangiorgio, R.; Tran, Q.; Vaccaro, V.; Zizzo, G. A methodology for assessing the impact of salinity gradient power generation in urban contexts. Sustain. Cities Soc. 2018, 38, 158–173. [Google Scholar] [CrossRef]
- Ortiz-Imedio, R.; Gomez-Coma, L.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Comparative performance of Salinity Gradient Power-Reverse Electrodialysis under different operating conditions. Desalination 2019, 457, 8–21. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Logan, B.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef]
- Post, J.W.; Veerman, J.; Hamelers, H.V.; Euverink, G.J.; Metz, S.J.; Nymeijer, K.; Buisman, C.J. Salinity-gradient power: Evaluation of pressure-retarded osmosis and reverse electrodialysis. J. Membr. Sci. 2007, 288, 218–230. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Tufa, R.A.; Piallat, T.; Hnát, J.; Fontananova, E.; Paidar, M.; Chanda, D.; Curcio, E.; di Profio, G.; Bouzek, K. Salinity gradient power reverse electrodialysis: Cation exchange membrane design based on polypyrrole-chitosan composites for enhanced monovalent selectivity. Chem. Eng. J. 2020, 380, 122461. [Google Scholar] [CrossRef]
- Loeb, S. Method and Apparatus for Generating Power Utilizing Reverse Electrodialysis. U.S. Patent 4171409A, 16 October 1979. [Google Scholar]
- Tedesco, M.; Scalici, C.; Vaccari, D.; Cipollina, A.; Tamburini, A.; Micale, G. Performance of the first reverse electrodialysis pilot plant for power production from saline waters and concentrated brines. J. Membr. Sci. 2016, 500, 33–45. [Google Scholar] [CrossRef]
- Lee, E.S.H.; Xiong, J.Y.; Han, G.; Wan, C.F.; Chong, Q.Y.; Chung, T.S. A pilot study on pressure retarded osmosis operation and effective cleaning strategies. Desalination 2017, 420, 273–282. [Google Scholar] [CrossRef]
- Güler, E.; Elizen, R.; Saakes, M.; Nijmeijer, K. Micro-structured membranes for electricity generation by reverse electrodialysis. J. Membr. Sci. 2014, 458, 136–148. [Google Scholar] [CrossRef]
- Liu, J.; Geise, G.M.; Luo, X.; Hou, H.; Zhang, F.; Feng, Y.; Hickner, M.A.; Logan, B.E. Patterned ion exchange membranes for improved power production in microbial reverse-electrodialysis cells. J. Power Sources 2014, 271, 437–443. [Google Scholar] [CrossRef]
- Güler, E.; van Baak, W.; Saakes, M.; Nijmeijer, K. Monovalent-ion-selective membranes for reverse electrodialysis. J. Membr. Sci. 2014, 455, 254–270. [Google Scholar] [CrossRef]
- Tedesco, M.; Brauns, E.; Cipollina, A.; Micale, G.; Modica, P.; Russo, G.; Helsen, J. Reverse electrodialysis with saline waters and concentrated brines: A laboratory investigation towards technology scale-up. J. Membr. Sci. 2015, 492, 9–20. [Google Scholar] [CrossRef]
- Geise, G.M.; Curtis, A.J.; Hatzell, M.C.; Hickner, M.A.; Logan, B.E. Salt Concentration Differences Alter Membrane Resistance in Reverse Electrodialysis Stacks. Environ. Sci. Technol. Lett. 2014, 1, 36–39. [Google Scholar] [CrossRef]
- Tufa, R.A.; Curcio, E.; van Baak, W.; Veerman, J.; Grasman, S.; Fontananova, E.; Di Profio, G. Potential of brackish water and brine for energy generation by salinity gradient power-reverse electrodialysis (SGP-RE). RSC Adv. 2014, 4, 42617–42623. [Google Scholar] [CrossRef]
- Brauns, E. Salinity gradient power by reverse electrodialysis: Effect of model parameters on electrical power output. Desalination 2009, 237, 378–391. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Power generation using profiled membranes in reverse electrodialysis. J. Membr. Sci. 2011, 385–386, 234–242. [Google Scholar] [CrossRef]
- Długołȩcki, P.; Dąbrowska, J.; Nijmeijer, K.; Wessling, M. Ion conductive spacers for increased power generation in reverse electrodialysis. J. Membr. Sci. 2010, 347, 101–107. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Kunteng, D.; Veerman, J.; Saakes, M.; Nijmeijer, K. Periodic Feedwater Reversal and Air Sparging As Antifouling Strategies in Reverse Electrodialysis. Environ. Sci. Technol. 2014, 48, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; de Hart, N.; Saakes, M.; Nijmeijer, K. CO2 saturated water as two-phase flow for fouling control in reverse electrodialysis. Water Res. 2017, 125, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Güler, E.; Elizen, R.; Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Performance-determining membrane properties in reverse electrodialysis. J. Membr. Sci. 2013, 446, 266–276. [Google Scholar] [CrossRef]
- Długołȩcki, P.; Nymeijer, K.; Metz, S.; Wessling, M. Current status of ion exchange membranes for power generation from salinity gradients. J. Membr. Sci. 2008, 319, 214–222. [Google Scholar] [CrossRef]
- Daniilidis, A.; Herber, R.; Vermaas, D.A. Upscale potential and financial feasibility of a reverse electrodialysis power plant. Appl. Energy 2014, 119, 257–265. [Google Scholar] [CrossRef]
- Tedesco, M.; Hamelers, H.; Biesheuvel, P. Nernst-Planck transport theory for (reverse) electrodialysis: I. Effect of co-ion transport through the membranes. J. Membr. Sci. 2016, 510, 370–381. [Google Scholar] [CrossRef]
- Geise, G.M.; Cassady, H.J.; Paul, D.R.; Logan, B.E.; Hickner, M.A. Specific ion effects on membrane potential and the permselectivity of ion exchange membranes. Phys. Chem. Chem. Phys. 2014, 16, 21673–21681. [Google Scholar] [CrossRef]
- Jones, G.; Sata, T.; Sata, T. Ion Exchange Membranes: Preparation, Characterization, Modification and Application; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Klaysom, C.; Marschall, R.; Moon, S.H.; Ladewig, B.P.; Lu, G.Q.M.; Wang, L. Preparation of porous composite ion-exchange membranes for desalination application. J. Mater. Chem. 2011, 21, 7401–7409. [Google Scholar] [CrossRef]
- Klaysom, C.; Marschall, R.; Wang, L.; Ladewig, B.P.; Lu, G.Q.M. Synthesis of composite ion-exchange membranes and their electrochemical properties for desalination applications. J. Mater. Chem. 2010, 20, 4669–4674. [Google Scholar] [CrossRef]
- Ponce-González, J.; Whelligan, D.K.; Wang, L.; Bance-Soualhi, R.; Wang, Y.; Peng, Y.; Peng, H.; Apperley, D.C.; Sarode, H.N.; Pandey, T.P.; et al. High performance aliphatic-heterocyclic benzyl-quaternary ammonium radiation-grafted anion-exchange membranes. Energy Environ. Sci. 2016, 9, 3724–3735. [Google Scholar] [CrossRef]
- Hnát, J.; Paidar, M.; Schauer, J.; Žitka, J.; Bouzek, K. Polymer anion-selective membranes for electrolytic splitting of water. Part II: Enhancement of ionic conductivity and performance under conditions of alkaline water electrolysis. J. Appl. Electrochem. 2012, 42, 545–554. [Google Scholar] [CrossRef]
- Søndergaard, T.; Cleemann, L.N.; Becker, H.; Aili, D.; Steenberg, T.; Hjuler, H.A.; Seerup, L.; Li, Q.; Jensen, J.O. Long-term durability of HT-PEM fuel cells based on thermally cross-linked polybenzimidazole. J. Power Sources 2017, 342, 570–578. [Google Scholar] [CrossRef]
- Hong, J.G.; Park, T.W.; Dhadake, Y. Property evaluation of custom-made ion exchange membranes for electrochemical performance in reverse electrodialysis application. J. Electroanal. Chem. 2019, 850, 113437. [Google Scholar] [CrossRef]
- Guler, E.; Nijmeijer, K. Reverse Electrodialysis for Salinity Gradient Power Generation: Challenges and Future Perspectives. J. Membr. Sci. Res. 2018, 4, 108–110. [Google Scholar] [CrossRef]
- Guler, E.; Zhang, Y.; Saakes, M.; Nijmeijer, K. Tailor-Made Anion-Exchange Membranes for Salinity Gradient Power Generation Using Reverse Electrodialysis. ChemSusChem 2012, 5, 2262–2270. [Google Scholar] [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis–Effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Membr. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.L.; Jia, Y.X.; Liu, X. An attempt for improving electrodialytic transport properties of a heterogeneous anion exchange membrane. Desalination 2014, 351, 163–170. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Madaeni, S.S.; Asiani, H.; Heidari, A.R. Preparation and Electrochemical Characterization of Monovalent Ion Selective Poly (Vinyl Chloride)-Blend-Poly (Styrene-Co-Butadiene) Heterogeneous Cation Exchange Membrane Coated with Poly (Methyl Methacrylate). Sep. Sci. Technol. 2012, 47, 1443–1454. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Rahzani, B.; Asiani, H.; Khodabakhshi, A.R.; Hamidi, A.R.; Madaeni, S.S.; Moghadassi, A.R.; Seidypoor, A. Surface modification of heterogeneous cation exchange membranes by simultaneous using polymerization of (acrylic acid-co-methyl methacrylate): Membrane characterization in desalination process. Desalination 2014, 345, 13–20. [Google Scholar] [CrossRef]
- Rao, P.S.; Smitha, B.; Sridhar, S.; Krishnaiah, A. Preparation and performance of poly(vinyl alcohol)/polyethyleneimine blend membranes for the dehydration of 1,4-dioxane by pervaporation: Comparison with glutaraldehyde cross-linked membranes. Sep. Purif. Technol. 2006, 48, 244–254. [Google Scholar] [CrossRef]
- Khoiruddin; Ariono, D.; Subagjo; Wenten, I.G. Surface modification of ion-exchange membranes: Methods, characteristics, and performance. J. Appl. Polym. Sci. 2017, 134, 45540. [Google Scholar] [CrossRef]
- Hosseini, S.; Gholami, A.; Madaeni, S.; Moghadassi, A.; Hamidi, A. Fabrication of (polyvinyl chloride/cellulose acetate) electrodialysis heterogeneous cation exchange membrane: Characterization and performance in desalination process. Desalination 2012, 306, 51–59. [Google Scholar] [CrossRef]
- Moghadassi, A.; Rajabi, Z.; Hosseini, S.; Mohammadi, M. Fabrication and modification of cellulose acetate based mixed matrix membrane: Gas separation and physical properties. J. Ind. Eng. Chem. 2014, 20, 1050–1060. [Google Scholar] [CrossRef]
- Diagne, N.W.; Rabiller-Baudry, M.; Paugam, L. On the actual cleanability of polyethersulfone membrane fouled by proteins at critical or limiting flux. J. Membr. Sci. 2013, 425–426, 40–47. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, M.S.; Lee, S.Y.; Choi, Y.W.; Jeong, N.J.; Kim, C.S. High power density of reverse electrodialysis with pore-filling ion exchange membranes and a high-open-area spacer. J. Mater. Chem. A 2015, 3, 16302–16306. [Google Scholar] [CrossRef]
- Nijmeijer, K.; Metz, S. Chapter 5 Salinity Gradient Energy. In Sustainable Water for the Future: Water Recycling versus Desalination; Escobar, I.C., Schäfer, A.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, pp. 95–139. [Google Scholar] [CrossRef]
- Fisher, S.; Kunin, R. Routine Exchange Capacity Determinations of Ion Exchange Resins. Anal. Chem. 1955, 27, 1191–1194. [Google Scholar] [CrossRef]
- Antony, A.; Chilcott, T.; Coster, H.; Leslie, G. In situ structural and functional characterization of reverse osmosis membranes using electrical impedance spectroscopy. J. Membr. Sci. 2013, 425–426, 89–97. [Google Scholar] [CrossRef]
- Anna, K.; Aleksandra, K.; Martyna, J.; Paweł, M. Synthesis of High-Energy Polymer—Glycidyl Azide Polymer (GAP). Probl. Mechatronics. Armament Aviat. Saf. Eng. 2018, 9, 59–72. [Google Scholar] [CrossRef]
- Çetin, M.Z.; Camurlu, P. An amperometric glucose biosensor based on PEDOT nanofibers. RSC Adv. 2018, 8, 19724–19731. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, K.; Chen, B.; Zhou, S.; Li, N.; Liu, C.; Yang, J.; Lin, R.; Zhang, T.; He, W. A polyethylenimine-based diazeniumdiolate nitric oxide donor accelerates wound healing. Biomater. Sci. 2019, 7, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Wu, J.; Liu, D.; Zhao, X.; Lu, W.W. BMP2 gene delivery to bone mesenchymal stem cell by chitosan-g-PEI nonviral vector. Nanoscale Res Lett. 2015, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.S.; Lai, P.L.; Peng, S.; Wu, H.C.; Yu, S.; Tseng, T.Y. Glial cell line-derived neurotrophic factor gene delivery via a polyethylene imine grafted chitosan carrier. Int. J. Nanomed. 2014, 3163–3174. [Google Scholar] [CrossRef][Green Version]
- Patro, T.U.; Wagner, H.D. Influence of graphene oxide incorporation and chemical cross-linking on structure and mechanical properties of layer-by-layer assembled poly(Vinyl alcohol)-Laponite free-standing films. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 2377–2387. [Google Scholar] [CrossRef]
- Tong, W.; Gao, C.; Möhwald, H. Poly(ethyleneimine) microcapsules: Glutaraldehyde-mediated assembly and the influence of molecular weight on their properties. Polym. Adv. Technol. 2008, 19, 817–823. [Google Scholar] [CrossRef]
- Lindén, J.B.; Larsson, M.; Kaur, S.; Skinner, W.M.; Miklavcic, S.J.; Nann, T.; Kempson, I.M.; Nydén, M. Polyethyleneimine for copper absorption II: Kinetics, selectivity and efficiency from seawater. RSC Adv. 2015, 5, 51883–51890. [Google Scholar] [CrossRef]
- Fang, X.; Ma, H.; Xiao, S.; Shen, M.; Guo, R.; Cao, X.; Shi, X. Facile immobilization of gold nanoparticles into electrospun polyethyleneimine/polyvinyl alcohol nanofibers for catalytic applications. J. Mater. Chem. 2011, 21, 4493–4501. [Google Scholar] [CrossRef]
- Dan-asabe, B.; Yaro, S.A.; Yawas, D.S.; Aku, S.Y.; Adesegun Samotu, I.; Abubakar, U.; Obada, D. Mechanical, Spectroscopic and Micro-structural Characterization of Banana Particulate Reinforced PVC Composite as Piping Material. Tribol. Ind. 2016, 38, 255–267. [Google Scholar]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Salem, I.A. Activation of H2O2 by Amberlyst-15 resin supported with copper(II)-complexes towards oxidation of crystal violet. Chemosphere 2001, 44, 1109–1119. [Google Scholar] [CrossRef]
- Hosseini, S.; Alibakhshi, H.; Jashni, E.; Parvizian, F.; Shen, J.; Taheri, M.; Ebrahimi, M.; Rafiei, N. A novel layer-by-layer heterogeneous cation exchange membrane for heavy metal ions removal from water. J. Hazard. Mater. 2020, 381, 120884. [Google Scholar] [CrossRef] [PubMed]
- Sudiarti, T.; Wahyuningrum, D.; Bundjali, B.; Arcana, I.M. Mechanical strength and ionic conductivity of polymer electrolyte membranes prepared from cellulose acetate-lithium perchlorate. IOP Conf. Ser. Mater. Sci. Eng. 2017, 223, 012052. [Google Scholar] [CrossRef]
- Świerczyńska, A.; Bohdziewicz, J.; Kamińska, G.; Wojciechowski, K. Influence of the type of membrane-forming polymer on the membrane fouling. Envir. Prot. Eng. 2016, 42, 197–210. [Google Scholar]
- Hong, J.G.; Zhang, B.; Glabman, S.; Uzal, N.; Dou, X.; Zhang, H.; Wei, X.; Chen, Y. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J. Membr. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Hosseini, S.; Jashni, E.; Habibi, M.; Nemati, M.; der Bruggen, B.V. Evaluating the ion transport characteristics of novel graphene oxide nanoplates entrapped mixed matrix cation exchange membranes in water deionization. J. Membr. Sci. 2017, 541, 641–652. [Google Scholar] [CrossRef]
- Fateh, M.; Pelagia, I.G. Super Water-Repellent Cellulose Acetate Mats. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Moreno, J.; Grasman, S.; van Engelen, R.; Nijmeijer, K. Upscaling Reverse Electrodialysis. Environ. Sci. Technol. 2018, 52, 10856–10863. [Google Scholar] [CrossRef]
- Giorno, L.; Drioli, E.; Strathmann, H. Ion-Exchange Membrane Characterization. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1052–1056. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K. The influence of molecular mass of poly (vinyl chloride) on the structure and transport characteristics of ultrafiltration membranes. J. Membr. Sci. 1991, 61, 131–156. [Google Scholar] [CrossRef]


| Membrane | ± SE | Thickness ± SE |
|---|---|---|
| () | () | |
| AEM | 81 ± 2 | 77 ± 3 |
| Modified-AEM | 58 ± 1 | 154 ± 1 |
| CEM | 99 ± 3 | 109 ± 2 |
| Modified-CEM | 134 ± 1 | 269 ± 2 |
| Membrane | SD ± SE | IEC ± SE | ± SE | S± SE | ER ± SE |
|---|---|---|---|---|---|
| (%) | (meq −1) | (meq −1) | (%) | ( cm) | |
| AEM * | 30.1 ± 1.1 | 1.4 ± 0.1 | 4.5 ± 0.4 | 87.0 ± 0.4 | 1.3 ± 0.3 |
| Modified-AEM * | 25.0 ± 2.1 | 2.5 ± 0.3 | 10.1 ± 1.2 | 94.9 ± 0.2 | 0.9 ± 0.1 |
| AEM † | 49.1 ± 0.21 | 1.68 ± 0.04 | 3.42 ± 0.06 | 87.0 ± 0.01 | 1.32 ± 0.16 |
| AMX ‡ | 16 | 1.25 | 5.4 | 90.7 | 2.35 |
| Fujifilm Type 10 AEM ‡ | 23 | 1.5 | 6.52 | 94.5 | 1.5 |
| Membrane | SD ± SE | IEC ± SE | ± SE | S± SE | ER ± SE |
|---|---|---|---|---|---|
| (%) | (meq −1) | (meq −1) | (%) | ( cm) | |
| CEM * | 4.0 ± 0.1 | 1.3 ± 0.2 | 33.6 ± 4.8 | 90.0 ± 0.3 | 6.77 ± 0.14 |
| Modified-CEM * | 4.5 ± 0.7 | 1.6 ± 0.1 | 36.6 ± 3.0 | 90.9 ± 0.7 | 3.10 ± 0.32 |
| CEM † | 25 | 1.45 | 5.8 | 79 | 23 |
| Modified-CEM † | 43 | 1.53 | 3.5 | 67 | 12 |
| CMX ‡ | 18 | 1.62 | 8.8 | 99 | 2.91 |
| Fujifilm Type 10 CEM ‡ | 21 | 1.67 | 7.95 | 94.7 | 2.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villafaña-López, L.; Reyes-Valadez, D.M.; González-Vargas, O.A.; Suárez-Toriello, V.A.; Jaime-Ferrer, J.S. Custom-Made Ion Exchange Membranes at Laboratory Scale for Reverse Electrodialysis. Membranes 2019, 9, 145. https://doi.org/10.3390/membranes9110145
Villafaña-López L, Reyes-Valadez DM, González-Vargas OA, Suárez-Toriello VA, Jaime-Ferrer JS. Custom-Made Ion Exchange Membranes at Laboratory Scale for Reverse Electrodialysis. Membranes. 2019; 9(11):145. https://doi.org/10.3390/membranes9110145
Chicago/Turabian StyleVillafaña-López, Liliana, Daniel M. Reyes-Valadez, Oscar A. González-Vargas, Victor A. Suárez-Toriello, and Jesús S. Jaime-Ferrer. 2019. "Custom-Made Ion Exchange Membranes at Laboratory Scale for Reverse Electrodialysis" Membranes 9, no. 11: 145. https://doi.org/10.3390/membranes9110145
APA StyleVillafaña-López, L., Reyes-Valadez, D. M., González-Vargas, O. A., Suárez-Toriello, V. A., & Jaime-Ferrer, J. S. (2019). Custom-Made Ion Exchange Membranes at Laboratory Scale for Reverse Electrodialysis. Membranes, 9(11), 145. https://doi.org/10.3390/membranes9110145





