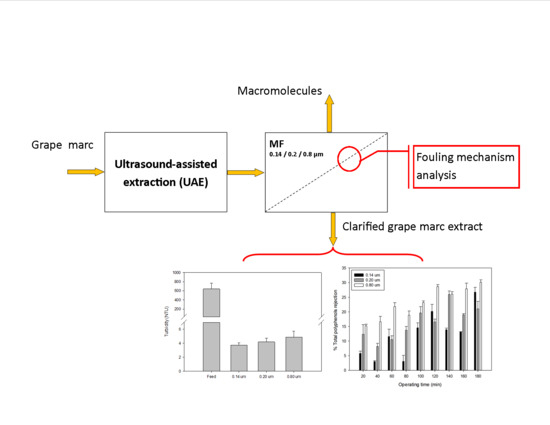
Impact of Membrane Pore Size on the Clarification Performance of Grape Marc Extract by Microfiltration
Abstract
1. Introduction
2. Materials and Methods
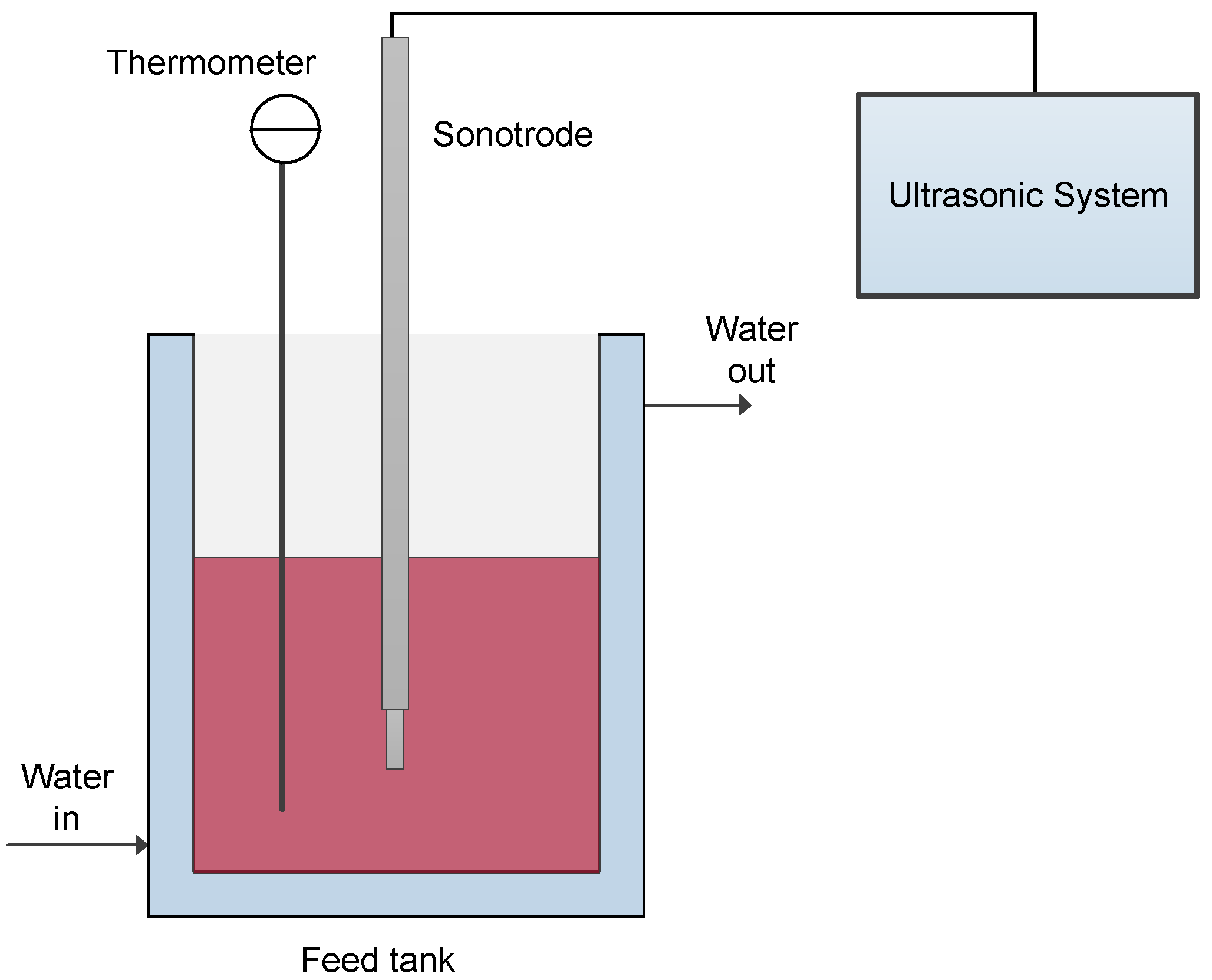
2.1. Grape Marc Extract
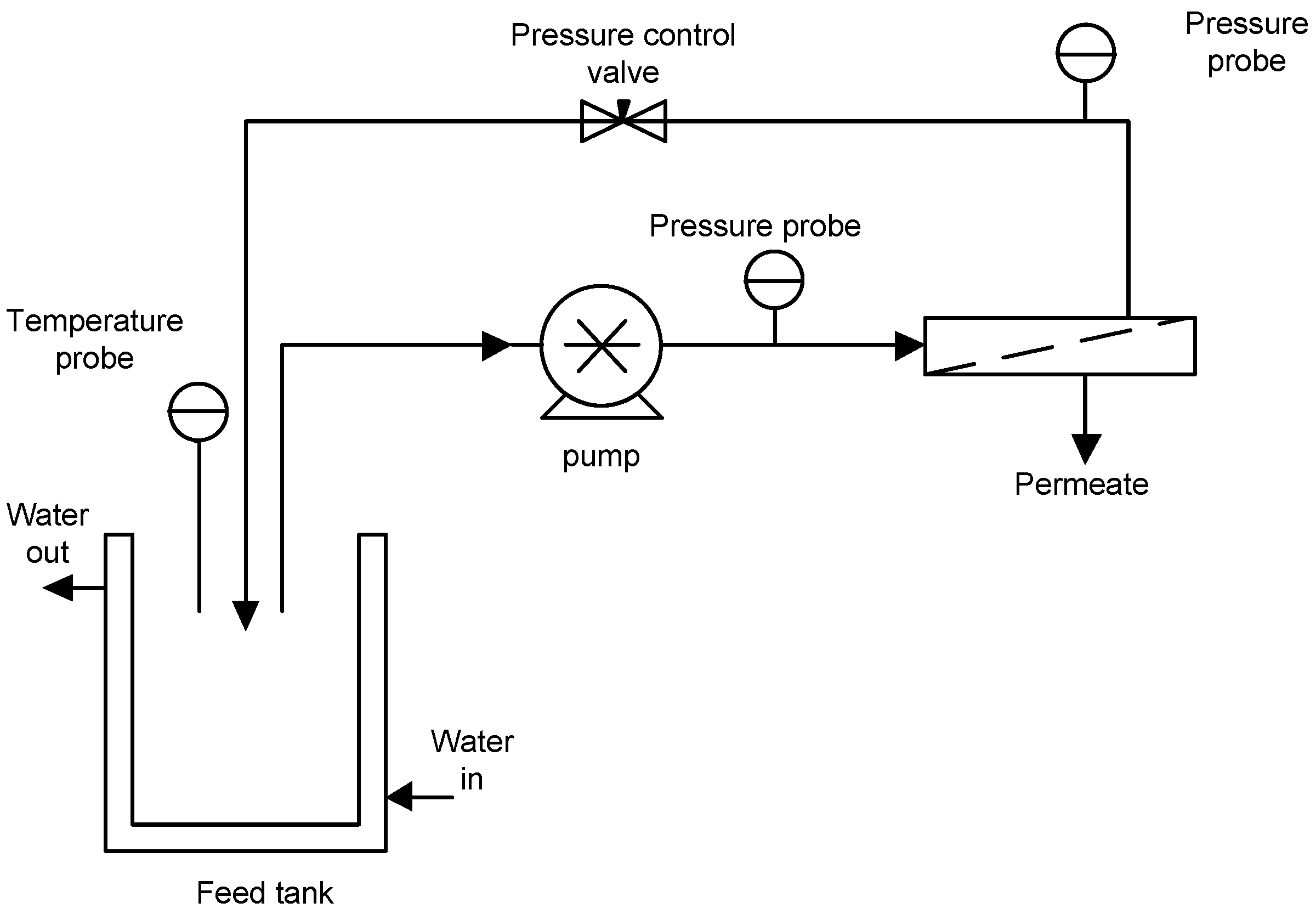
2.2. MF Set-Up and Procedure
2.3. Membrane Fouling Analysis
2.3.1. Hermia’s Model Modified for Cross-Flow Filtration
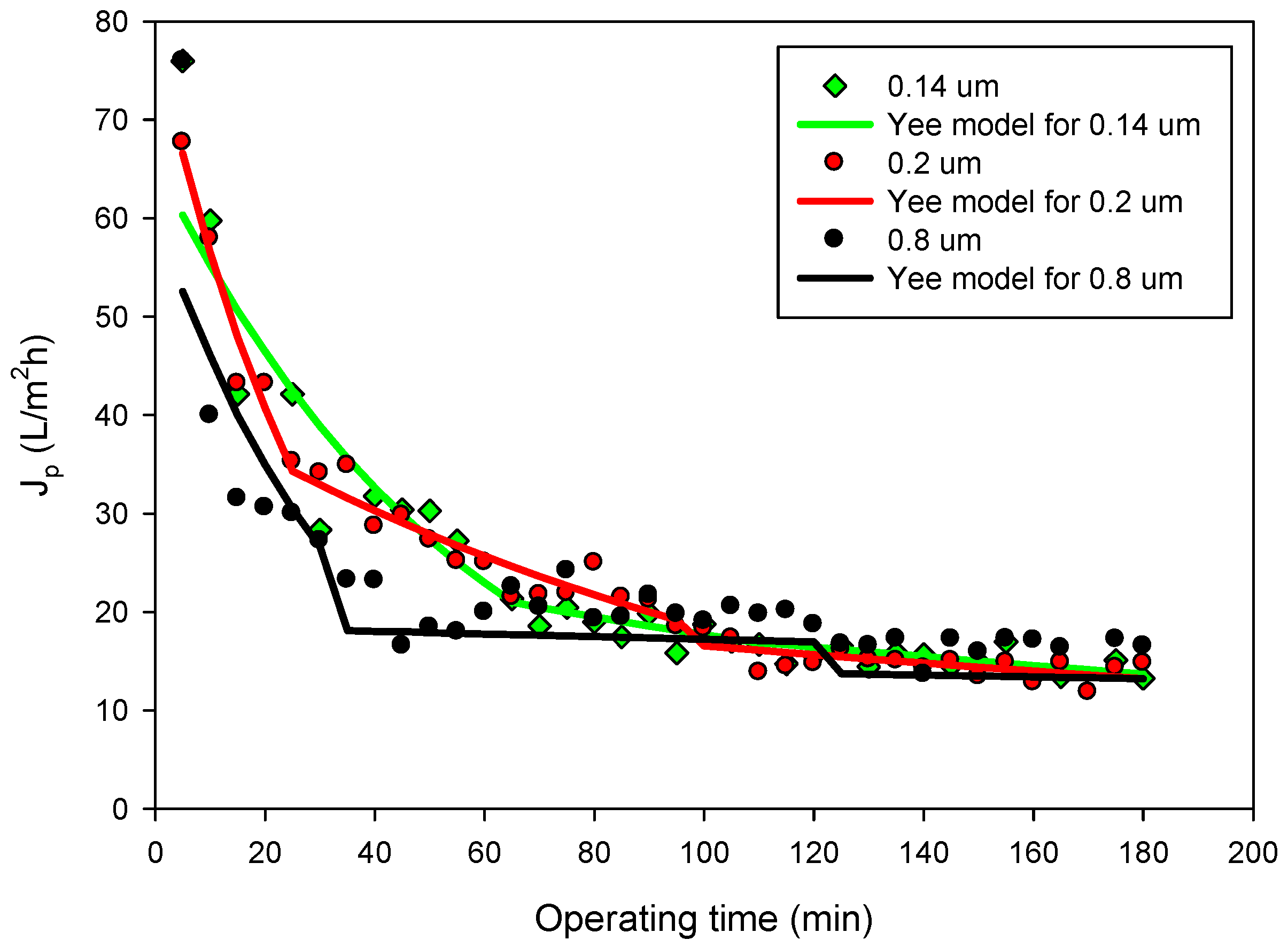
2.3.2. Unified Model of Flux Decline
2.4. Analytical Measurements
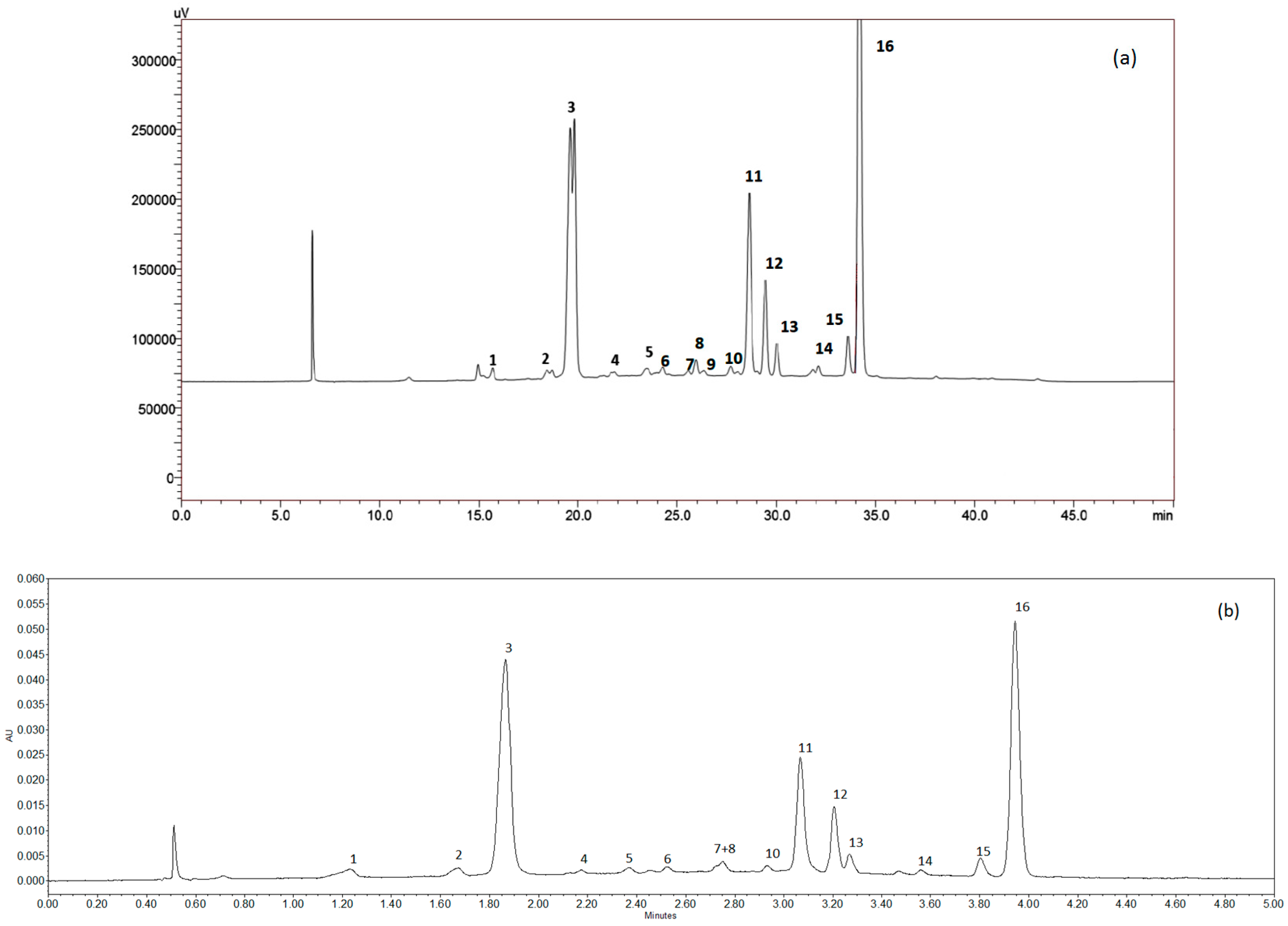
2.4.1. Anthocyanins Analysis
2.4.2. Total Phenolic Compound Analysis
2.4.3. Soluble and Suspended Solids Measurements
2.5. Data Analysis
3. Results and Discussion
3.1. UAE Extract Characterization
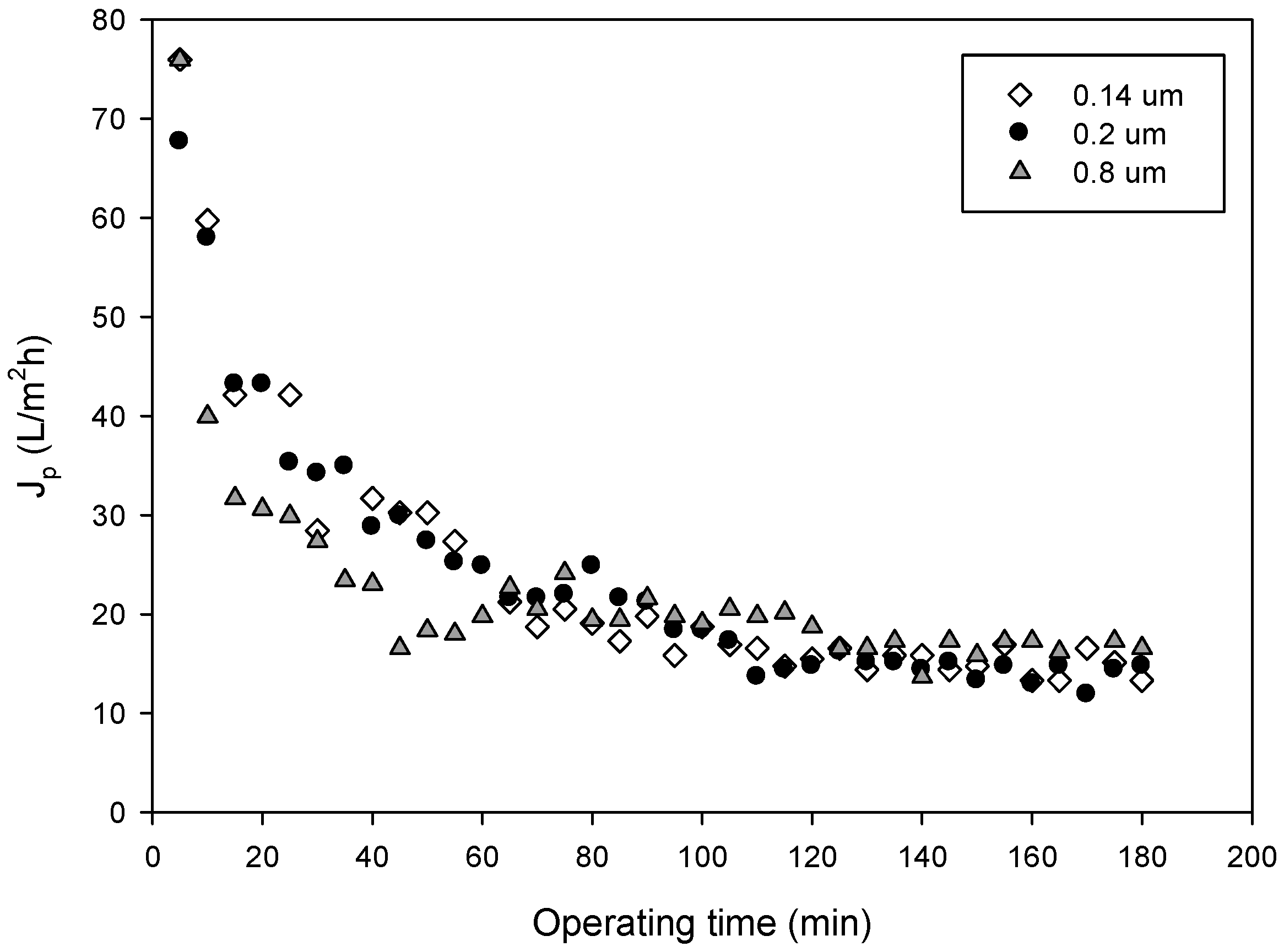
3.2. Permeate Flux and Membrane Fouling Analysis
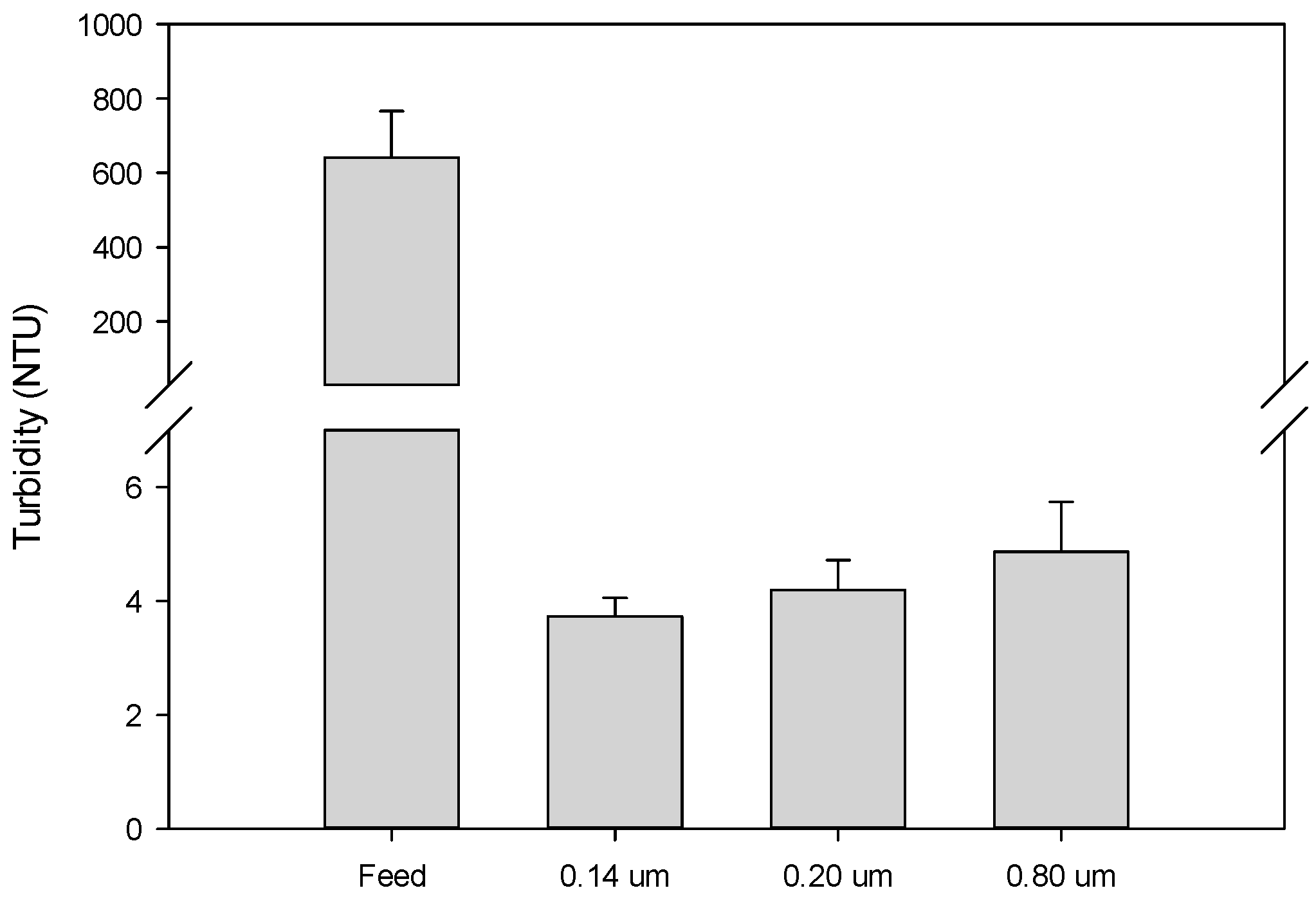
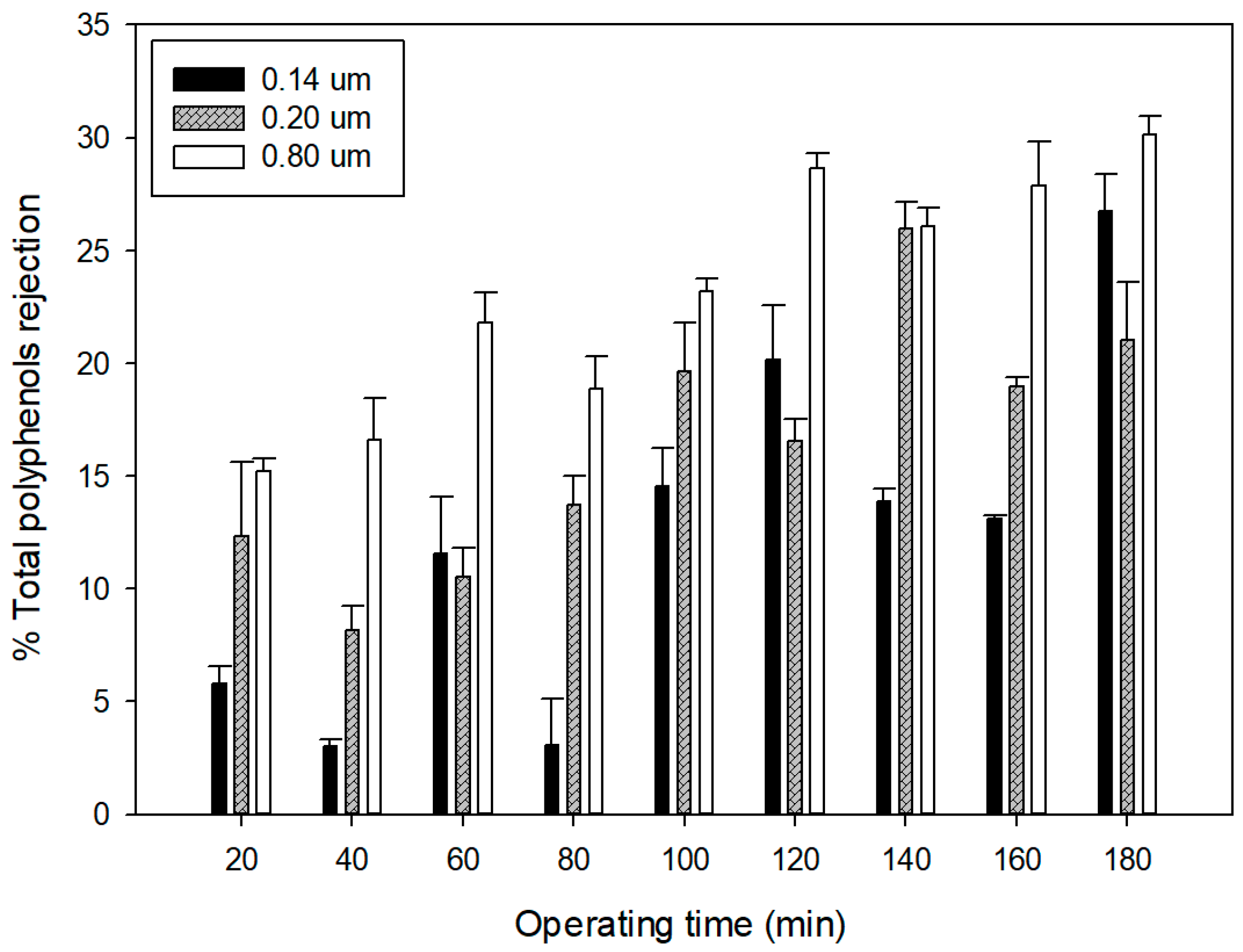
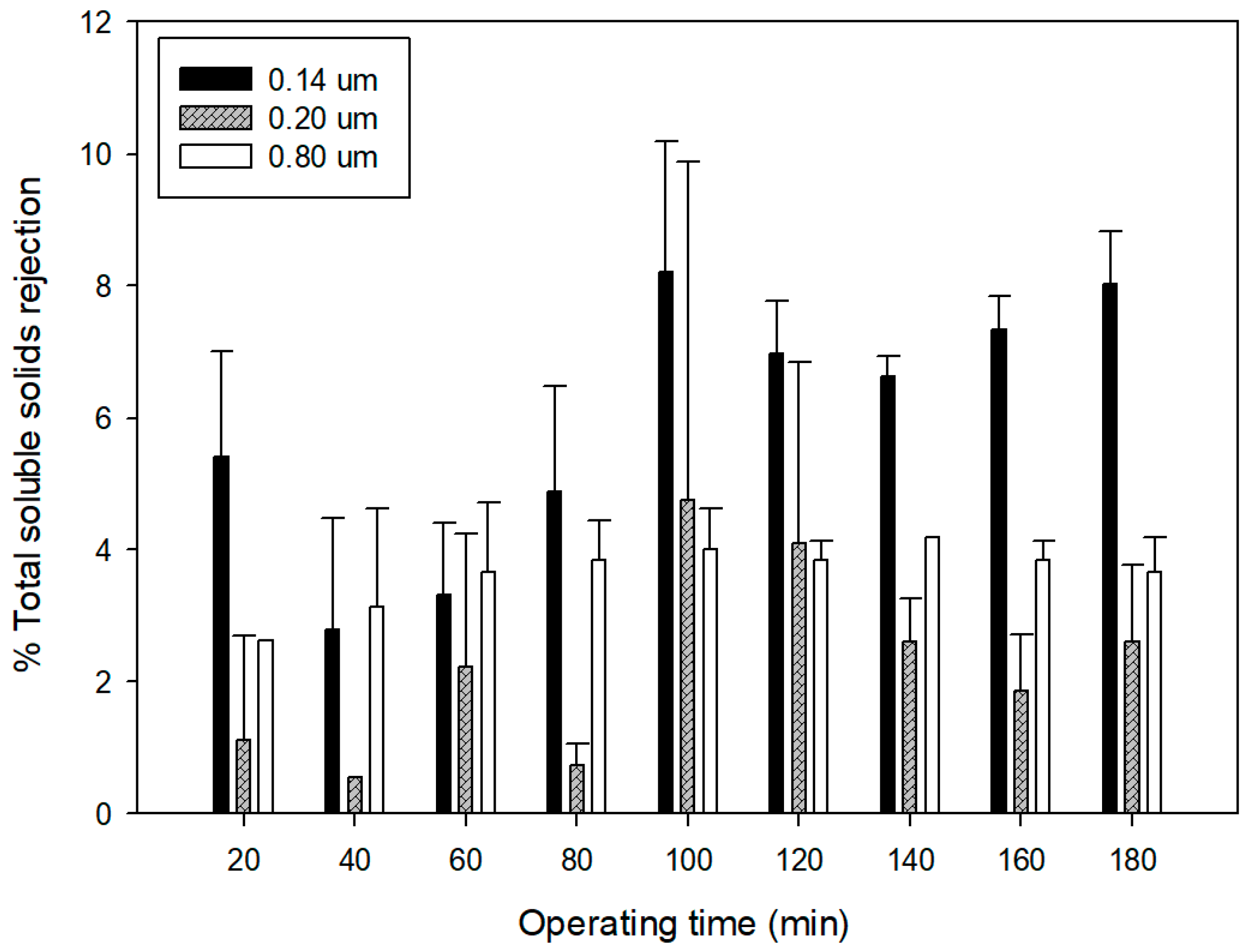
3.3. Quality of Microfiltered Grape Marc Extract
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andersen, M.S. An introductory note on the environmental economics of the circular economy. Sustain. Sci. 2007, 2, 133–140. [Google Scholar] [CrossRef]
- Rodrigues, F.; Palmeira-de-Oliveira, A.; das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B. Medicago spp. extracts as promising ingredients for skin care products. Ind. Crops Prod. 2013, 49, 634–644. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed]
- International Organisation of Vine and Wine. 2019 Statistical Report on World Vitiviniculture; International Organisation of Vine and Wine: Paris, France, 2019; 23p. [Google Scholar]
- Corbin, K.R.; Hsieh, Y.S.Y.; Betts, N.S.; Byrt, C.S.; Henderson, M.; Stork, J.; DeBolt, S.; Fincher, G.B.; Burton, R.A. Grape marc as a source of carbohydrates for bioethanol: Chemical composition, pre-treatment and saccharification. Bioresour. Technol. 2015, 193, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, C.X.; Cáceres, R.E.; Hein, D.; Molina, M.G.; Pia, J.M. Biogas production from grape pomace: Thermodynamic model of the process and dynamic model of the power generation system. Int. J. Hydrog. Energy 2012, 37, 10111–10117. [Google Scholar] [CrossRef]
- Rivera-Dominguez, M.; Wlodarchak, N.; Kushad, M.; Yahia, E.M. Identification and quantification of phenolic compounds in grapes. Acta Hortic. 2010, 877, 1233–1240. [Google Scholar] [CrossRef]
- Frum, A.; Georgescu, C.; Gligor, F.; Lengyel, E.; Stegarus, D.; Dobrea, C.; Tita, O. Identification and quantification of phenolic compounds from red grape pomace. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 45–52. [Google Scholar]
- Jara-Palacios, J.M.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Antioxidant potential of white grape pomaces: Phenolic composition and antioxidant capacity measured by spectrophotometric and cyclic voltammetry methods. Food Res. Int. 2014, 66, 150–157. [Google Scholar] [CrossRef]
- Ajami, M.; Pazoki-Toroudi, H.; Amani, H.; Nabavi, S.F.; Braidy, N.; Vacca, R.A.; Atanasov, A.G.; Mocan, A.; Nabavi, S.M. Therapeutic role of sirtuins in neurodegenerative disease and their modulation by polyphenols. Neurosci. Biobehav. Rev. 2017, 73, 39–47. [Google Scholar] [CrossRef]
- Rasines-Perea, Z.; Teissedre, P.L. Grape Polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.I.; Mishra, N. Anti-oxidant effect of quercetin on type 2 diabetic erythrocytes. J. Food Biochem. 2009, 33, 404–415. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phyther. Res. 2016, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Ladas, D.; Mavromatis, A. Potential uses and applications of treated wine waste: A review. Int. J. Food Sci. Technol. 2006, 41, 475–487. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Bleve, M.; Ciurlia, L.; Erroi, E.; Lionetto, G.; Longo, L.; Rescio, L.; Schettino, T.; Vasapollo, G. An innovative method for the purification of anthocyanins from grape skin extracts by using liquid and sub-critical carbon dioxide. Sep. Purif. Technol. 2008, 64, 192–197. [Google Scholar] [CrossRef]
- Brianceau, S.; Turk, M.; Vitrac, X.; Vorobiev, E. Combined densification and pulsed electric field treatment for selective polyphenols recovery from fermented grape pomace. Innov. Food Sci. Emerg. Technol. 2015, 29, 2–8. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; de la Ossa, E.J.M.; Roldán, A.; De Ory, I.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Domingues, R.C.C.; Ramos, A.A.; Cardoso, V.L.; Reis, M.H.M. Microfiltration of passion fruit juice using hollow fibre membranes and evaluation of fouling mechanisms. J. Food Eng. 2014, 121, 73–79. [Google Scholar] [CrossRef]
- He, Y.; Ji, Z.; Li, S. Effective clarification of apple juice using membrane filtration without enzyme and pasteurization pretreatment. Sep. Purif. Technol. 2007, 57, 366–373. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saxena, V.K.; Dutta, S. Fruit juice processing using membrane technology: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 136–153. [Google Scholar] [CrossRef]
- Mirsaeedghazi, H.; Emam-Djomeh, Z.; Mousavi, S.M.; Aroujalian, A.; Navidbakhsh, M. Clarification of pomegranate juice by microfiltration with PVDF membranes. Desalination 2010, 264, 243–248. [Google Scholar] [CrossRef]
- de Barros, S.T.D.; Andrade, C.M.G.; Mendes, E.S.; Peres, L. Study of fouling mechanism in pineapple juice clarification by ultrafiltration. J. Memb. Sci. 2003, 215, 213–224. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Stokłosa, D. Effect of different sizes of ceramic membranes in the process of microfiltration on physicochemical parameters of chokeberry juice. Eur. Food Res. Technol. 2019, 245, 1263–1275. [Google Scholar] [CrossRef]
- Jin, L.; Ong, S.L.; Ng, H.Y. Comparison of fouling characteristics in different pore-sized submerged ceramic membrane bioreactors. Water Res. 2010, 44, 5907–5918. [Google Scholar] [CrossRef] [PubMed]
- Nittami, T.; Hitomi, T.; Matsumoto, K.; Nakamura, K.; Ikeda, T.; Setoguchi, Y.; Motoori, M. Comparison of polytetrafluoroethylene flat-sheet membranes with different pore sizes in application to submerged membrane bioreactor. Membranes 2012, 2, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Lau, E.; Huang, S.; Moraru, C.I. The effect of apple cider characteristics and membrane pore size on membrane fouling. LWT Food Sci. Technol. 2015, 64, 974–979. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical flux concept for microfiltration fouling. J. Memb. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Yee, K.W.K.; Wiley, D.E.; Bao, J. A unified model of the time dependence of flux decline for the long-term ultrafiltration of whey. J. Memb. Sci. 2009, 332, 69–80. [Google Scholar] [CrossRef]
- Hermia, J. Constant Pressure Blocking Filtration Laws-Application to Power-Law Non-Newtonian Fluids. Trans. Inst. Chem. Eng. 1982, 60, 183–187. [Google Scholar]
- Vincent Vela, M.C.; Blanco, S.Á.; García, J.L.; Rodríguez, E.B. Analysis of membrane pore blocking models applied to the ultrafiltration of PEG. Sep. Purif. Technol. 2008, 62, 489–498. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr.; Rossi, J.A., Jr. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Huaman-Castilla, N.L.; Mariotti-Celis, M.S.; Perez-Correa, J.R. Polyphenols of Carménère Grapes. Mini. Rev. Org. Chem. 2017, 14, 176–186. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Prodanov, M.; Vacas, V.; Sánchez, A.M.; Alonso, G.L.; Elorza, Á.; Lucendo, C.; Hernández, T.; Estrella, I. Industrial clarification of anthocyanin-rich grape pomace extracts by crossflow membrane filtration. Int. J. Food Sci. Technol. 2013, 48, 1426–1435. [Google Scholar] [CrossRef]
- Qin, G.; Lü, X.; Wei, W.; Li, J.; Cui, R.; Hu, S. Microfiltration of kiwifruit juice and fouling mechanism using fly-ash-based ceramic membranes. Food Bioprod. Process. 2015, 96, 278–284. [Google Scholar] [CrossRef]
- Hwang, K.J.; Liao, C.Y.; Tung, K.L. Effect of membrane pore size on the particle fouling in membrane filtration. Desalination 2008, 234, 16–23. [Google Scholar] [CrossRef]
- Razi, B.; Aroujalian, A.; Fathizadeh, M. Modeling of fouling layer deposition in cross-flow microfiltration during tomato juice clarification. Food Bioprod. Process. 2012, 90, 841–848. [Google Scholar] [CrossRef]
| Function | n Value | Model | Equation |
|---|---|---|---|
| Complete pore blocking | n = 2 | (4) | |
| Standard pore blocking | n = 1.5 | (5) | |
| Intermediate pore blocking | n = 1 | (6) | |
| Cake formation | n = 0 | (7) |
| Peak | Compound | tR (min) | λ (nm) | [M-H]+ | Product Ions |
|---|---|---|---|---|---|
| 1 | Petunidin-3-glucoside | 16.11 | 280, 523 | 479 | 317, 302, 274 |
| 2 | Peonidin-3-glucoside | 18.62 | 280, 327, 520 | 463 | 301, 286 |
| 3 | Malvidin-3-O-glucoside ** | 20.15 | 280, 350, 528 | 493 | 331, 315, 287 |
| 4 | Quercetin-3-glucuronide *** | 21.94 | 250, 353 | 479 | 303 |
| 5 | Quercetin 3-glucoside *** | 22.75 | 250, 351 | 465 | 303 |
| 6 | Petunidin 3-(acetyl)-glucoside | 24.55 | 280, 530 | 521 | 317, 302 |
| 7 | Petunidin 3-(caffeoyl)-glucoside | 25.83 | 280, 530 | 641 | 317, 302, 274 |
| 8 | Delphinidin 3-rutinoside | 26.20 | 280, 320, 529 | 611 | 303 |
| 9 | n.i. * | 26.63 | 276, 500 | 559 | 355, 339, 311 |
| 10 | Peonidin 3-(acetyl)-glucoside | 27.70 | 280, 312,520 | 505 | 301, 286 |
| 11 | Malvidin 3-(acetyl)-glucoside | 28.90 | 276, 529 | 535 | 331, 315, 287 |
| 12 | Malvidin 3-(caffeoyl)-glucoside | 29.71 | 281, 327, 530 | 655 | 331, 315, 287 |
| 13 | Petunidin 3-(coumaroyl)-glucoside | 30.30 | 281, 532 | 625 | 317, 302 |
| 14 | Malvidin-3-rutinoside | 32.40 | 280, 530 | 639 | 331, 315, 287 |
| 15 | Peonidin 3-(coumaroyl)-glucoside | 33.85 | 283, 310, 523 | 609 | 301, 286 |
| 16 | Malvidin 3-(coumaroyl)-glucoside | 34.40 | 282, 532 | 639 | 331, 315, 287 |
| Fouling Model | Membrane Pore Size | ||||
|---|---|---|---|---|---|
| 0.14 µm | 0.2 µm | 0.8 µm | |||
| Hermia’s models adapted for cross-flow microfiltration [34] | Complete pore blocking | Kc | 32.535 | 26.001 | 95.9345 |
| R2 | 95.2028 | 96.3153 | 91.6064 | ||
| RMSE | 0.0049 | 0.0017 | 0.0038 | ||
| S-W test (p-value) | 7.83 × 10−10 | 0.2746 | 4.3 × 10−6 | ||
| K-S test (p-value) | 0.000657 | 0.6340 | 0.0846100 | ||
| Standard pore blocking | Ki | 0.656384 | 0.73382 | 0,67193 | |
| R2 | 67.13958 | 55.6330 | 89.655 | ||
| RMSE | 0.0042 | 0.0013 | 0.0028 | ||
| S-W test (p-value) | 1.43 × 10−3 | 0.0157 | 2.89 × 10−9 | ||
| K-S test (p-value) | 0.022328 | 0.4564 | 0.006085 | ||
| Intermediate pore blocking | Ks | 36.9425 | 47.5505 | 129.562 | |
| R2 | 95.2028 | 97.8559 | 90.5891 | ||
| RMSE | 0.0035 | 0.0008 | 0.0023 | ||
| S-W test (p-value) | 9.49 × 10−10 | 0.0003 | 5.95 × 10−9 | ||
| K-S test (p-value) | 0.000516 | 0.2808 | 0.037512 | ||
| Cake formation | Kg | 5,800,344 | 8,642,697 | 11,030,594 | |
| R2 | 88.53165 | 97.5383 | 82.30814 | ||
| RMSE | 0.0031 | 0.0013 | 0.0015 | ||
| S-W test (p-value) | 2.89 × 10−8 | 3.76 × 10−6 | 3.74 × 10−11 | ||
| K-S test (p-value) | 0.004099 | 0.33078 | 0.0104003 | ||
| Unified model for flux decline Yee et al. [35] | K1 | 0.000292 | 0.00054 | 4.55 × 10−4 | |
| b1 | −10.9087 | −10.734 | −10,9964 | ||
| K2 | 0.0000811 | 0.00013 | 1.291 × 10−5 | ||
| b2 | −11.7368 | −11.3531 | −12.1731 | ||
| K3 | 5.05 × 10−5 | 4.7 × 10−5 | 1.115× 10−5 | ||
| b3 | −11.9331 | −12.0056 | −12.3943 | ||
| R2 | 90.594 | 97.35 | 80.576 | ||
| RMSE | 3.3 × 10−3 | 0.5 × 10−3 | 1.6 × 10−2 | ||
| S-W test (p-value) | 5.86 × 10−10 | 0.61 | 1.13 × 10−6 | ||
| K-S test (p-value) | 9.41 × 10−5 | 0.7652 | 0.04760 | ||
| Compound | Sample | Membrane Pore Size | ||
|---|---|---|---|---|
| 0.14 µm | 0.2 µm | 0.8 µm | ||
| Malvidin-3-O-glucoside (mg/L) | Feed | 43.77 ± 0.58 | 43.29 ± 2.1 | 40.62 ± 1.8 |
| Permeate * | 42.95 ± 0.71 | 42.72 ± 1.26 | 39.44 ± 0.22 | |
| Malvidin 3-(acetyl)-glucoside (mg/L) ** | Feed | 18.81 ± 4.8 | 19.13 ± 1.3 | 17.49 ± 2.6 |
| Permeate * | 18.01 ± 0.39 | 18.08 ± 0.30 | 16.76 ± 0.12 | |
| Malvidin 3-(coumaroyl)-glucoside ** | Feed | 79.46 ± 4.7 | 79.72 ± 5.1 | 76.10 ± 3.2 |
| Permeate * | 75.71 ± 2.18 | 74.91 ± 1.60 | 68.65 ± 12.85 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, F.; Pérez, K.; Quezada, C.; Herrera, C.; Cassano, A.; Ruby-Figueroa, R. Impact of Membrane Pore Size on the Clarification Performance of Grape Marc Extract by Microfiltration. Membranes 2019, 9, 146. https://doi.org/10.3390/membranes9110146
Mora F, Pérez K, Quezada C, Herrera C, Cassano A, Ruby-Figueroa R. Impact of Membrane Pore Size on the Clarification Performance of Grape Marc Extract by Microfiltration. Membranes. 2019; 9(11):146. https://doi.org/10.3390/membranes9110146
Chicago/Turabian StyleMora, Francisca, Karla Pérez, Carolina Quezada, Carla Herrera, Alfredo Cassano, and René Ruby-Figueroa. 2019. "Impact of Membrane Pore Size on the Clarification Performance of Grape Marc Extract by Microfiltration" Membranes 9, no. 11: 146. https://doi.org/10.3390/membranes9110146
APA StyleMora, F., Pérez, K., Quezada, C., Herrera, C., Cassano, A., & Ruby-Figueroa, R. (2019). Impact of Membrane Pore Size on the Clarification Performance of Grape Marc Extract by Microfiltration. Membranes, 9(11), 146. https://doi.org/10.3390/membranes9110146