Polysulfone/Polyamide-SiO2 Composite Membrane with High Permeance for Organic Solvent Nanofiltration
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of Mesoporous Silica
2.3. Preparation of PSf Substrates
2.4. Preparation of PSf/PA-SiO2 Nanocomposite Membranes
2.5. Characterization of Membranes
3. Results and Discussion
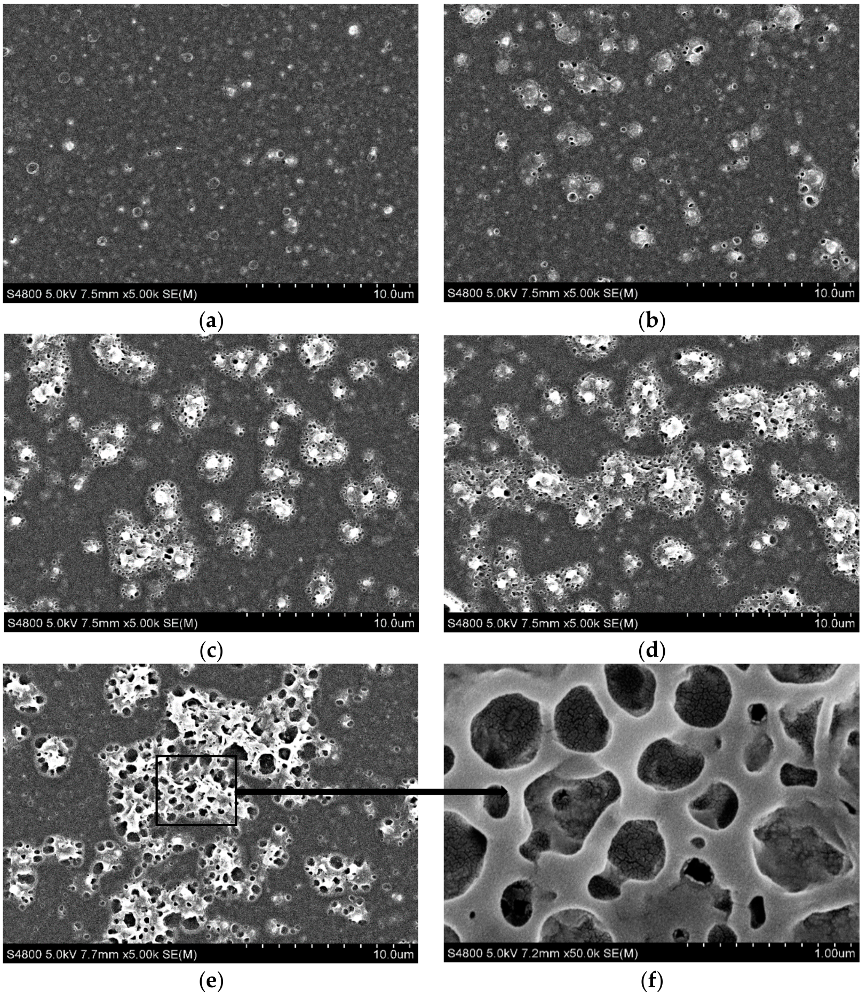
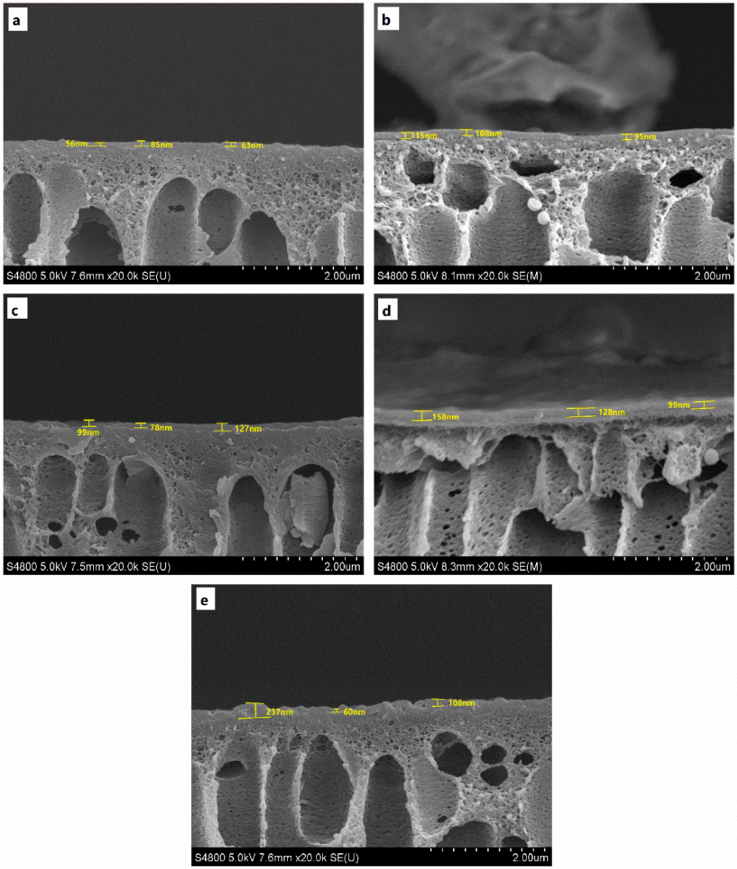
3.1. Membrane Characterization
3.2. Membrane Performance in Organic Solvent
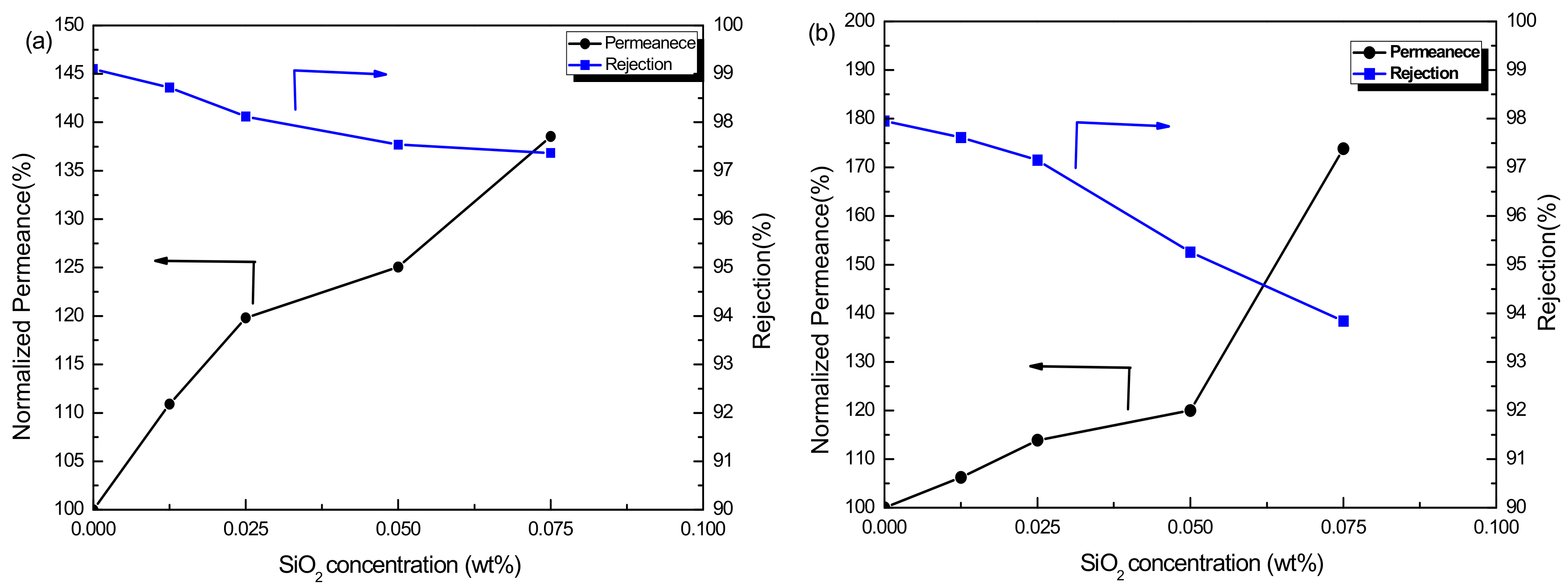
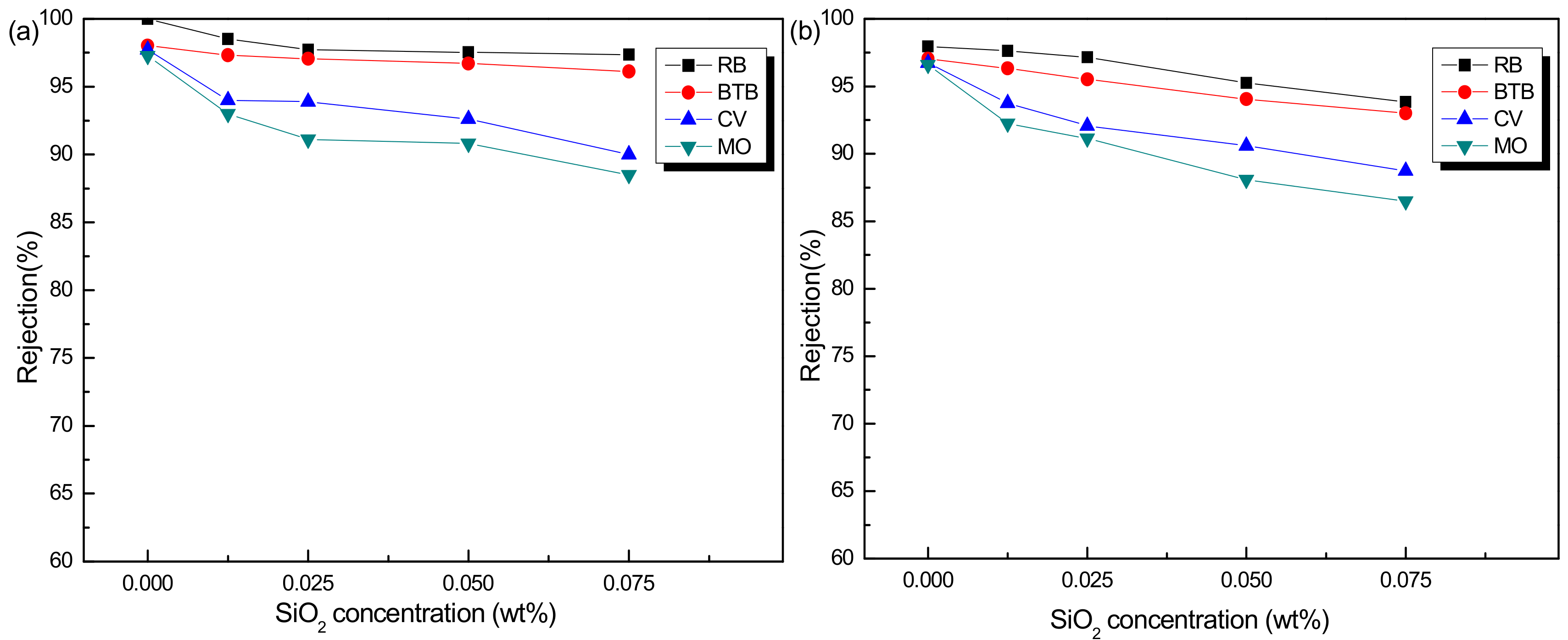
3.2.1. Influence of SiO2 Concentration on Membrane Performance
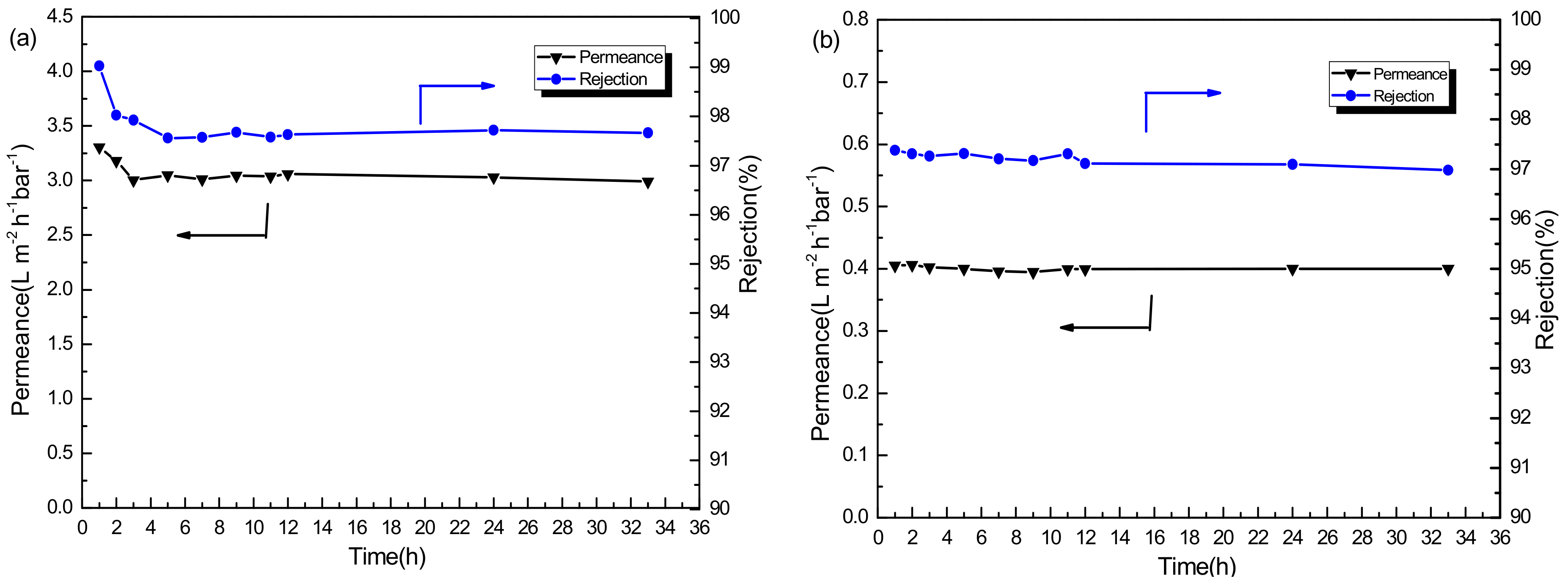
3.2.2. Stability Performance of PSf/PA-SiO2 Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Vandezande, P.; Gevers, L.E.; Vankelecom, I.F. Solvent resistant nanofiltration: Separating on a molecular level. Chem. Soc. Rev. 2008, 37, 365–405. [Google Scholar] [CrossRef] [PubMed]
- Székely, G.; Bandarra, J.; Heggie, W.; Sellergren, B.; Ferreira, F.C. Organic solvent nanofiltration: A platform for removal of genotoxins from active pharmaceutical ingredients. J. Membr. Sci. 2011, 381, 21–33. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Gao, T.T.; Zhang, M.; Li, Y.R.; Dai, L.M.; Qu, L.T.; Shi, G.Q. Reduced graphene oxide membranes for ultrafast organic solvent nanofiltration. Adv. Mater. 2016, 28, 8669–8674. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Tang, Y.P.; Liu, L.F.; Guo, Z.H.; Shao, L. Nanocomposite organic solvent nanofiltration membranes by a highly-efficient mussel-inspired co-deposition strategy. J. Membr. Sci. 2017, 526, 32–42. [Google Scholar] [CrossRef]
- Yang, H.; Wang, N.; Wang, L.; Liu, H.X.; An, Q.F.; Ji, S. Vacuum-assisted assembly of ZIF-8@GO composite membranes on ceramic tube with enhanced organic solvent nanofiltration performance. J. Membr. Sci. 2018, 545, 158–166. [Google Scholar] [CrossRef]
- Zeidler, S.; Puhlfürß, P.; Kätzel, U.; Voigt, I. Preparation and characterization of new low MWCO ceramic nanofiltration membranes for organic solvents. J. Membr. Sci. 2014, 470, 421–430. [Google Scholar] [CrossRef]
- Soroko, I.; Lopes, M.P.; Livingston, A.G. The effect of membrane formation parameters on performance of polyimide membranes for organic solvent nanofiltration (OSN): Part A. effect of polymer/solvent/non-solvent system choice. J. Membr. Sci. 2011, 381, 152–162. [Google Scholar] [CrossRef]
- Soroko, I.; Livingston, A.G. Impact of TiO2, nanoparticles on morphology and performance of crosslinked polyimide organic solvent nanofiltration (OSN) membranes. J. Membr. Sci. 2009, 343, 189–198. [Google Scholar] [CrossRef]
- Valadez-Blanco, R.; Livingston, A.G. Solute molecular transport through polyimide asymmetric organic solvent nanofiltration (OSN) membranes and the effect of membrane-formation parameters on mass transfer. J. Membr. Sci. 2009, 326, 332–342. [Google Scholar] [CrossRef]
- See-Toh, Y.H.; Ferreira, F.C.; Livingston, A.G. The influence of membrane formation on functional performance of organic solvent nanofiltration membranes. Desalination 2007, 199, 242–244. [Google Scholar] [CrossRef]
- Valtcheva, I.B.; Marchetti, P.; Livingston, A.G. Crosslinked polybenzimidazole membranes for organic solvent nanofiltration (OSN): Analysis of crosslinking reaction mechanism and effects of reaction parameters. J. Membr. Sci. 2015, 493, 568–579. [Google Scholar] [CrossRef]
- Namvar-Mahboub, M.; Pakizeh, M. Development of a novel thin film composite membrane by interfacial polymerization on polyetherimide/modified SiO2, support for organic solvent nanofiltration. Sep. Sci. Technol. 2013, 119, 35–45. [Google Scholar] [CrossRef]
- Gevers, L.E.; Vankelecom, I.F.; Jacobs, P.A. Zeolite filled polydimethylsiloxane (PDMS) as an improved membrane for solvent-resistant nanofiltration (SRNF). Chem. Commun. 2005, 19, 2500–2502. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, S.; Kätzel, U.; Kreis, P. Systematic investigation on the influence of solutes on the separation behavior of a PDMS membrane in organic solvent nanofiltration. J. Membr. Sci. 2013, 429, 295–303. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Cai, W.; Wang, T.; Wu, Z.; Li, J. Highly stable PDMS–PTFPMS/PVDF OSN membranes for hexane recovery during vegetable oil production. RSC Adv. 2017, 7, 11381–11388. [Google Scholar] [CrossRef]
- Fritsch, D.; Merten, P.; Heinrich, K.; Lazar, M.; Priske, M. High performance organic solvent nanofiltration membranes: Development and thorough testing of thin film composite membranes made of polymers of intrinsic microporosity (PIMS). J. Membr. Sci. 2012, 401–402, 222–231. [Google Scholar] [CrossRef]
- Li, X.; Vandezande, P.; Vankelecom, I.F.J. Polypyrrole modified solvent resistant nanofiltration membranes. J. Membr. Sci. 2008, 320, 143–150. [Google Scholar] [CrossRef]
- Li, X.; Basko, M.; Prez, F.D.; Vankelecom, I.F.J. Multifunctional membranes for solvent resistant nanofiltration and pervaporation applications based on segmented polymer networks. J. Phys. Chem. B 2008, 112, 16539–16545. [Google Scholar] [CrossRef] [PubMed]
- Ahmadiannamini, P.; Li, X.; Goyens, W.; Joseph, N.; Meesschaert, B.; Vankelecom, I.F.J. Multilayered polyelectrolyte complex based solvent resistant nanofiltration membranes prepared from weak polyacids. J. Membr. Sci. 2012, 394–395, 98–106. [Google Scholar] [CrossRef]
- Machado, D.R.; Hasson, D.; Semiat, R. Effect of solvent properties on permeate flow through nanofiltration membranes. Ph.D. Thesis, Technion-Israel Institute of Technology, Haifa, Israel, May 1998. [Google Scholar]
- Sarango, L.; Paseta, L.; Navarro, M.; Zornoza, B.; Coronas, J. Controlled deposition of MOFs by dip-coating in thin film nanocomposite membranes for organic solvent nanofiltration. J. Ind. Eng. Chem. 2017, 59, 8–16. [Google Scholar] [CrossRef]
- Altun, V.; Remigy, J.C.; Vankelecom, I.F.J. UV-cured polysulfone-based membranes: Effect of co-solvent addition and evaporation process on membrane morphology and srnf performance. J. Membr. Sci. 2017, 524, 729–737. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Majid, M.A.; Ooi, B.S. Functionalized PSf/SiO2, nanocomposite membrane for oil-in-water emulsion separation. Desalination 2011, 268, 266–269. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, K.; Wang, K.; Xie, Z.; Ladewig, B.; Wang, H. Fabrication of polyethersulfone-mesoporous silica nanocomposite ultrafiltration membranes with antifouling properties. J. Membr. Sci. 2012, 423–424, 362–370. [Google Scholar] [CrossRef]
- Shen, L.; Feng, S.; Li, J.; Chen, J.; Li, F.; Lin, H.; Yu, G. Surface modification of polyvinylidene fluoride (PVDF) membrane via radiation grafting: Novel mechanisms underlying the interesting enhanced membrane performance. Sci. Rep. 2017, 7, 2721. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shaibani, M.; Smith, S.J.; Konstas, K.; Hill, M.R.; Wang, H.; Zhang, K.; Xie, Z. Microporous carbon from fullerene impregnated porous aromatic frameworks for improving desalination performance of thin film composite forward osmosis membranes. J. Mater. Chem. A 2018, 6, 11327–11336. [Google Scholar] [CrossRef]
- Ploegmakers, J.; Japip, S.; Nijmeijer, K. Mixed matrix membranes containing MOFs for ethylene/ethane separation Part A: membrane preparation and characterization. J. Membr. Sci. 2013, 428, 445–453. [Google Scholar] [CrossRef]
- Liu, S.; Fang, F.; Wu, J.; Zhang, K. The anti-biofouling properties of thin-film composite nanofiltration membranes grafted with biogenic silver nanoparticles. Desalination 2015, 375, 121–128. [Google Scholar] [CrossRef]
- Wu, X.; Field, R.W.; Wu, J.J.; Zhang, K. Polyvinylpyrrolidone modified graphene oxide as a modifier for thin film composite forward osmosis membranes. J. Membr. Sci. 2017, 540, 251–260. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhang, K. Influence of hydrophilic carbon dots on polyamide thin film nanocomposite reverse osmosis membranes. J. Membr. Sci. 2017, 537, 42–53. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, H.; Wang, J.; Ding, R.; Du, Z.; Liu, J.; Cao, S. Mineralization-inspired preparation of composite membranes with polyethyleneimine–nanoparticle hybrid active layer for solvent resistant nanofiltration. J. Membr. Sci. 2014, 470, 70–79. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Peyravi, M.; Rahimpour, A.; Jahanshahi, M. Thin film composite membranes with modified polysulfone supports for organic solvent nanofiltration. J. Membr. Sci. 2012, 423–424, 225–237. [Google Scholar] [CrossRef]
- Hołda, A.K.; Aernouts, B.; Saeys, W.; Vankelecom, I.F.J. Study of polymer concentration and evaporation time as phase inversion parameters for polysulfone-based SRNF membranes. J. Membr. Sci. 2013, 442, 196–205. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F.J. Integrally skinned PSf-based SRNF-membranes prepared via phase inversion—Part A: Influence of high molecular weight additives. J. Membr. Sci. 2012, 450, 512–521. [Google Scholar] [CrossRef]
- Struzynska-Piron, I.; Loccufier, J.; Vanmaele, L.; Vankelecom, I.; Hermans, S. Cross-linked PSf membranes for SRNF application. Procedia Eng. 2012, 44, 1348–1350. [Google Scholar] [CrossRef]
- Liu, M.; Yu, S.; Qi, M.; Pan, Q.; Gao, C. Impact of manufacture technique on seawater desalination performance of thin-film composite polyamide-urethane reverse osmosis membranes and their spiral wound elements. J. Membr. Sci. 2010, 348, 268–276. [Google Scholar] [CrossRef]
- Solomon, M.F.J.; Bhole, Y.; Livingston, A.G. High flux membranes for organic solvent nanofiltration (OSN)—Interfacial polymerization with solvent activation. J. Membr. Sci. 2012, 423–424, 371–382. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal–organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Peyravi, M.; Jahanshahi, M.; Rahimpour, A.; Javadi, A.; Hajavi, S. Novel thin film nanocomposite membranes incorporated with functionalized TiO2, nanoparticles for organic solvent nanofiltration. Chem. Eng. J. 2014, 241, 155–166. [Google Scholar] [CrossRef]

| Membrane | C | O | S | Cl | N | Si |
|---|---|---|---|---|---|---|
| PSf/PA | 72.21 | 20.41 | 4.37 | 0.46 | 2.55 | - |
| PSf/PA-SiO2 0.075% | 69.18 | 21.30 | 3.36 | 0.51 | 4.77 | 0.88 |
| Dye | RB | BTB | CV | MO |
|---|---|---|---|---|
| MW (Da) | 1017 | 624 | 408 | 327 |
| Membrane Type | Solvent | Permeance (L·m−2·h−1·bar−1) | Marker | Marker MW (g mol−1 ) | Rejection | Reference |
|---|---|---|---|---|---|---|
| PSf/PA-SiO2 0.025% | Methanol | 3.29 | RB | 1017 | 98% | This work |
| BTB | 624 | 97% | ||||
| CV | 408 | 94% | ||||
| MO | 327 | 91% | ||||
| Ethanol | 0.42 | RB | 1017 | 97% | ||
| BTB | 624 | 96% | ||||
| CV | 408 | 92% | ||||
| MO | 327 | 91% | ||||
| PA/PSf | Methanol | 2 | Bromothymol Blue | 624 | >90% | [34] |
| PA/crosslinked P84 polyimide | Methanol | 1.5 | Styrene oligomers | 236 | 98% | [17] |
| PIM–1/polyacrylonitrile | Methanol | 6 | Hexaphenylbenzene | 535 | 73% | [39] |
| Ethanol | 3 | 78% | ||||
| (PIM-1/poly(ethylene imine))/polyacrylonitrile | Methanol | 3.6 | Hexaphenylbenzene | 535 | 91% | |
| Ethanol | 1.4 | 85% | ||||
| (PA/MOFs)/P84 polyimide | Methanol | 3.9 | styrene oligomers | 236 | 96% | [40] |
| TiO 2 nanoparticles + PA/ polyimide | Methanol | 24 | Bromothymol Blue | 624 | >90% | [41] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wu, X.; Zhang, K. Polysulfone/Polyamide-SiO2 Composite Membrane with High Permeance for Organic Solvent Nanofiltration. Membranes 2018, 8, 89. https://doi.org/10.3390/membranes8040089
Liu Q, Wu X, Zhang K. Polysulfone/Polyamide-SiO2 Composite Membrane with High Permeance for Organic Solvent Nanofiltration. Membranes. 2018; 8(4):89. https://doi.org/10.3390/membranes8040089
Chicago/Turabian StyleLiu, Qin, Xing Wu, and Kaisong Zhang. 2018. "Polysulfone/Polyamide-SiO2 Composite Membrane with High Permeance for Organic Solvent Nanofiltration" Membranes 8, no. 4: 89. https://doi.org/10.3390/membranes8040089
APA StyleLiu, Q., Wu, X., & Zhang, K. (2018). Polysulfone/Polyamide-SiO2 Composite Membrane with High Permeance for Organic Solvent Nanofiltration. Membranes, 8(4), 89. https://doi.org/10.3390/membranes8040089




