Thickness Effect on CO2/N2 Separation in Double Layer Pebax-1657®/PDMS Membranes
Abstract
1. Introduction
2. Materials and Methods
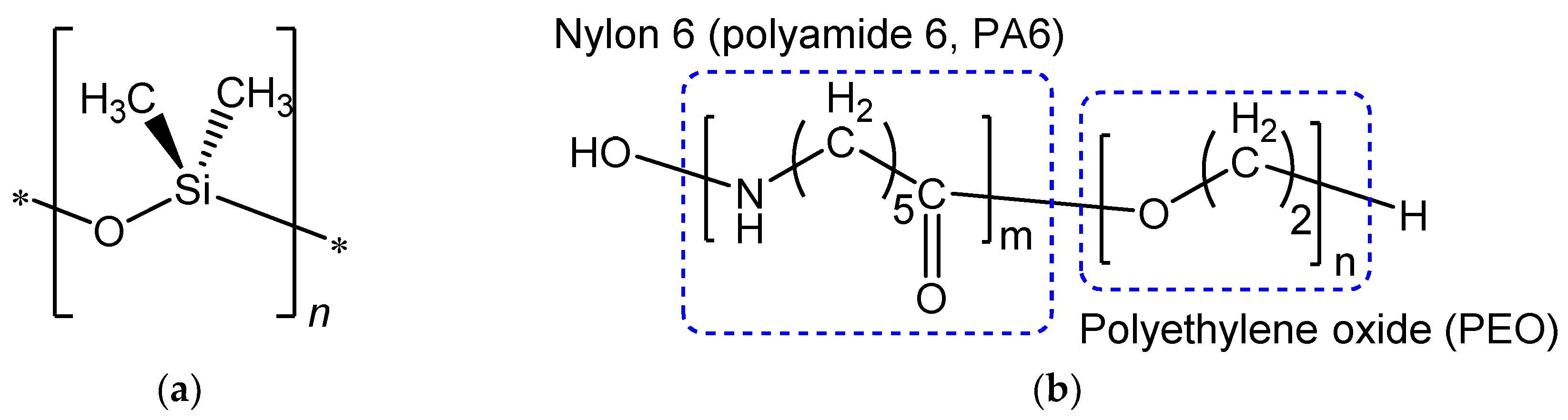
2.1. Materials
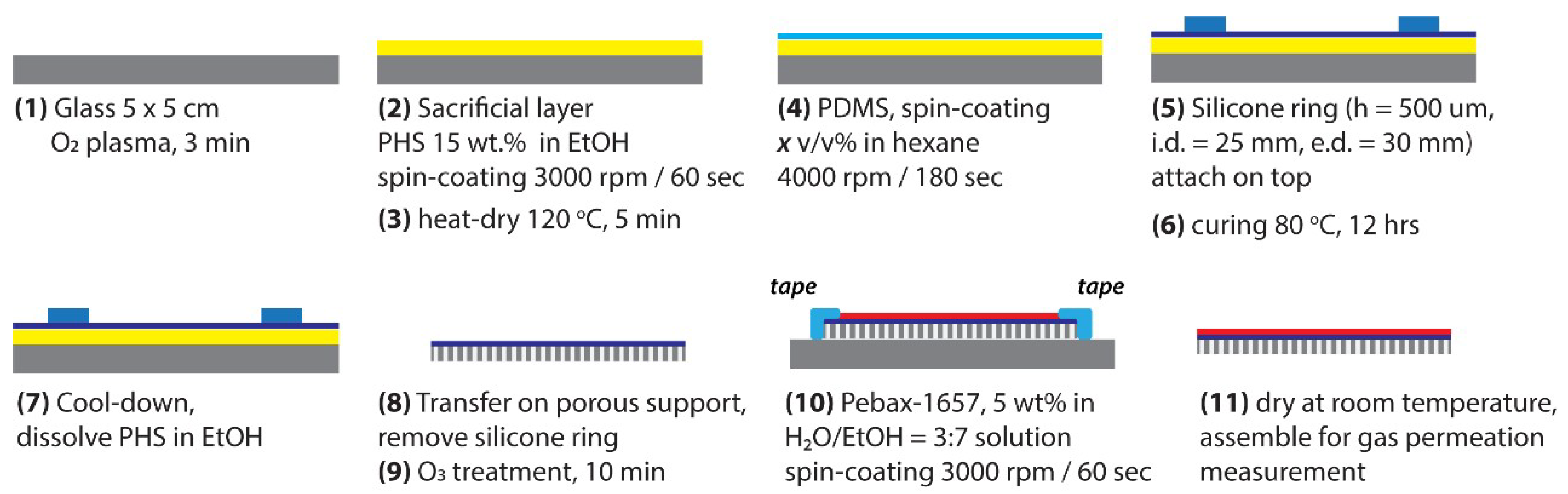
2.2. Membrane Fabrication
2.3. Gas Permeation Measurement
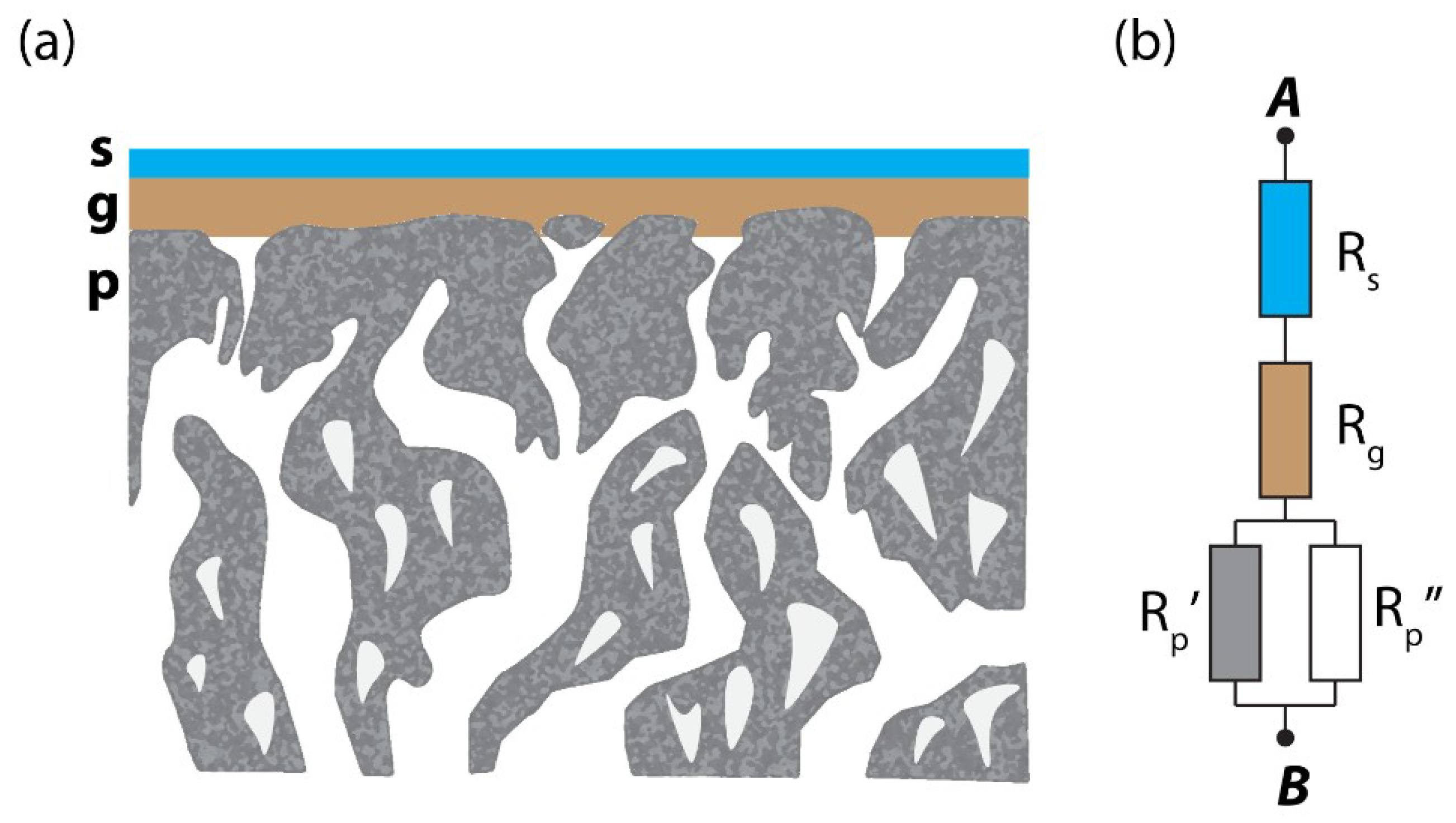
2.4. Resistance Model for Gas Permeation Description on Three-Layer System
3. Results
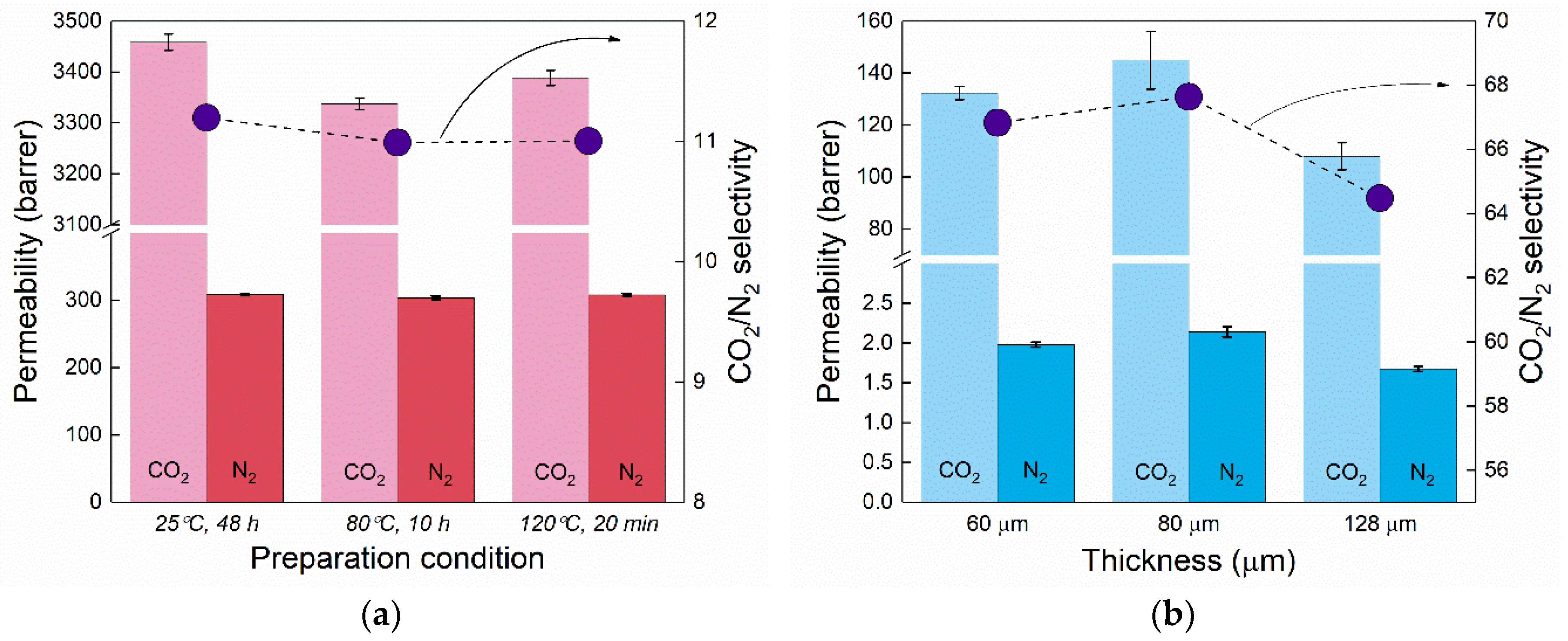
3.1. Confirmation of the Bulk Permeability Values of PDMS and Pebax-1657
3.2. Resistance Model Predictions for Double Layer Pebax-1657/PDMS Membranes
3.3. Cross Section Morphology of the Thin-Film Composite Membranes
3.4. Comparison of the Experimental Results with Resistance in Series Model Predictions
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hägg, M.B. Membranes for environmentally friendly energy processes. Membranes (Basel) 2012, 2, 706–726. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membrane Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membrane Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Selyanchyn, R.; Fujikawa, S. Membrane thinning for efficient CO2 capture. Sci. Technol. Adv. Mater. 2017, 18, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.R.; Smith, Z.P.; Lin, H.; Freeman, B.D.; Paul, D.R. Gas permeation in thin films of “high free-volume” glassy perfluoropolymers: Part I. Physical aging. Polymer (United Kingdom) 2014, 55, 5788–5800. [Google Scholar] [CrossRef]
- Yave, W.; Huth, H.; Car, A.; Schick, C. Peculiarity of a CO2-philic block copolymer confined in thin films with constrained thickness: “a super membrane for CO2-capture”. Energy Environ. Sci. 2011, 4, 4656–4661. [Google Scholar] [CrossRef]
- Selyanchyn, R.; Staykov, A.; Fujikawa, S. Incorporation of CO2 philic moieties into a TiO2 nanomembrane for preferential CO2 separation. RSC Adv. 2016, 6, 88664–88667. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Deng, L. Recent advances in multi-layer composite polymeric membranes for CO2 separation: A review. Green Energy Environ. 2016, 1, 102–128. [Google Scholar] [CrossRef]
- Fam, W.; Mansouri, J.; Li, H.; Hou, J.; Chen, V. Gelled Graphene Oxide-Ionic Liquid Composite Membranes with Enriched Ionic Liquid Surfaces for Improved CO2 Separation. ACS Appl. Mater. Interfaces 2018, 10, 7389–7400. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Zulkifli, M.Y.; Li, H.; Zhang, Y.; Liang, W.; D’Alessandro, D.M.; Chen, V. Surface functionalized UiO-66/Pebax-based ultrathin composite hollow fiber gas separation membranes. J. Mater. Chem. A 2018, 6, 918–931. [Google Scholar] [CrossRef]
- Kim, J.; Fu, Q.; Scofield, J.M.P.; Kentish, S.E.; Qiao, G.G. Ultra-thin film composite mixed matrix membranes incorporating iron(III)–dopamine nanoparticles for CO2 separation. Nanoscale 2016, 8, 8312–8323. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Fu, Q.; Xie, K.; Scofield, J.M.P.; Kentish, S.E.; Qiao, G.G. CO2 separation using surface-functionalized SiO2 nanoparticles incorporated ultra-thin film composite mixed matrix membranes for post-combustion carbon capture. J. Membrane Sci. 2016, 515, 54–62. [Google Scholar] [CrossRef]
- Chen, H.Z.; Thong, Z.; Li, P.; Chung, T.S. High performance composite hollow fiber membranes for CO2/H2 and CO2/N2 separation. Int. J. Hydrogen Energy 2014, 39, 5043–5053. [Google Scholar] [CrossRef]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas sorption and characterization of poly(ether-b-amide) segmented block copolymers. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2463–2475. [Google Scholar] [CrossRef]
- Henis, J.M.S.; Tripodi, M.K. Composite hollow fiber membranes for gas separation: The resistance model approach. J. Membrane Sci. 1981, 8, 233–246. [Google Scholar] [CrossRef]
- Mersha, A.; Selyanchyn, R.; Fujikawa, S. Preparation of large, ultra-flexible and free-standing nanomembranes of metal oxide–polymer composite and their gas permeation properties. Clean Energy 2017, 1, 80–89. [Google Scholar] [CrossRef]
- Fujikawa, S.; Ariyoshi, M.; Shigyo, E.; Fukakusa, C.; Selyanchyn, R.; Kunitake, T. Preferential CO2 separation over nitrogen by a free-standing and nanometer-thick membrane. Energy Procedia 2017, 114, 608–612. [Google Scholar] [CrossRef]
- Bayer, T.; Selyanchyn, R.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. Spray-painted graphene oxide membrane fuel cells. J. Membrane Sci. 2017, 541, 347–357. [Google Scholar] [CrossRef]
- Berean, K.; Ou, J.Z.; Nour, M.; Latham, K.; McSweeney, C.; Paull, D.; Halim, A.; Kentish, S.; Doherty, C.M.; Hill, A.J.; et al. The effect of crosslinking temperature on the permeability of PDMS membranes: Evidence of extraordinary CO2 and CH4 gas permeation. Sep. Purif. Technol. 2014, 122, 96–104. [Google Scholar] [CrossRef]
- Allen, S.M.; Fujii, M.; Stannett, V.; Hopfenberg, H.B.; Williams, J.L. The barrier properties of polyacrylonitrile. J. Membrane Sci. 1977, 2, 153–163. [Google Scholar] [CrossRef]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas sorption, diffusion, and permeation in poly (dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- Houston, K.S.; Weinkauf, D.H.; Stewart, F.F. Gas transport characteristics of plasma treated poly(dimethylsiloxane) and polyphosphazene membrane materials. J. Membrane Sci. 2002, 205, 103–112. [Google Scholar] [CrossRef]
- De Bo, I.; Van Langenhove, H.; Pruuost, P.; De Neve, J.; Pieters, J.; Vankelecom, I.F.J.; Dick, E. Investigation of the permeability and selectivity of gases and volatile organic compounds for polydimethylsiloxane membranes. J. Membrane Sci. 2003, 215, 303–319. [Google Scholar] [CrossRef]
- Surya Murali, R.; Sridhar, S.; Sankarshana, T.; Ravikumar, Y.V.L. Gas permeation behavior of Pebax-1657 nanocomposite membrane incorporated with multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2010, 49, 6530–6538. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.S. Molecular-level mixed matrix membranes comprising Pebax® and POSS for hydrogen purification via preferential CO2 removal. Int. J. Hydrogen Energy 2010, 35, 10560–10568. [Google Scholar] [CrossRef]
- Bernardo, P.; Jansen, J.C.; Bazzarelli, F.; Tasselli, F.; Fuoco, A.; Friess, K.; Izák, P.; Jarmarová, V.; Kačírková, M.; Clarizia, G. Gas transport properties of Pebax®/room temperature ionic liquid gel membranes. Sep. Purif. Technol. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Neumann, S.; Bolmer, S.; Khan, M.M.; Abetz, V. PEBAX® with PEG functionalized POSS as nanocomposite membranes for CO2 separation. J. Membrane Sci. 2013, 437, 286–297. [Google Scholar] [CrossRef]
- Yu, B.; Cong, H.; Li, Z.; Tang, J.; Zhao, X.S. Pebax-1657 nanocomposite membranes incorporated with nanoparticles/ colloids/carbon nanotubes for CO2/N2 and CO2/H2 separation. J. Appl. Polym. Sci. 2013, 130, 2867–2876. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Li, X.; Deng, M. Gas separation properties of poly(amide-6-b-ethylene oxide)/amino modified multi-walled carbon nanotubes mixed matrix membranes. J. Membrane Sci. 2014, 467, 41–47. [Google Scholar] [CrossRef]
- Ramon, G.Z.; Wong, M.C.Y.; Hoek, E.M. V Transport through composite membrane, Part 1: Is there an optimal support membrane? J. Membrane Sci. 2012, 415–416, 298–305. [Google Scholar] [CrossRef]
- Fu, Y.J.; Qui, H.Z.; Liao, K.S.; Lue, S.J.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Effect of UV-Ozone treatment on poly(dimethylsiloxane) membranes: Surface characterization and gas separation performance. Langmuir 2010, 26, 4392–4399. [Google Scholar] [CrossRef] [PubMed]
- Wijmans, J.G.; Hao, P. Influence of the porous support on diffusion in composite membranes. J. Membrane Sci. 2015, 494, 78–85. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Physical aging of thin glassy polymer films monitored by gas permeability. Polymer (Guildf) 2004, 45, 8377–8393. [Google Scholar] [CrossRef]
- Müller, N.; Handge, U.A.; Abetz, V. Physical aging and lifetime prediction of polymer membranes for gas separation processes. J. Membrane Sci. 2016, 516, 33–46. [Google Scholar] [CrossRef]
- Firpo, G.; Angeli, E.; Repetto, L.; Valbusa, U. Permeability thickness dependence of polydimethylsiloxane (PDMS) membranes. J. Membrane Sci. 2015, 481, 1–8. [Google Scholar] [CrossRef]
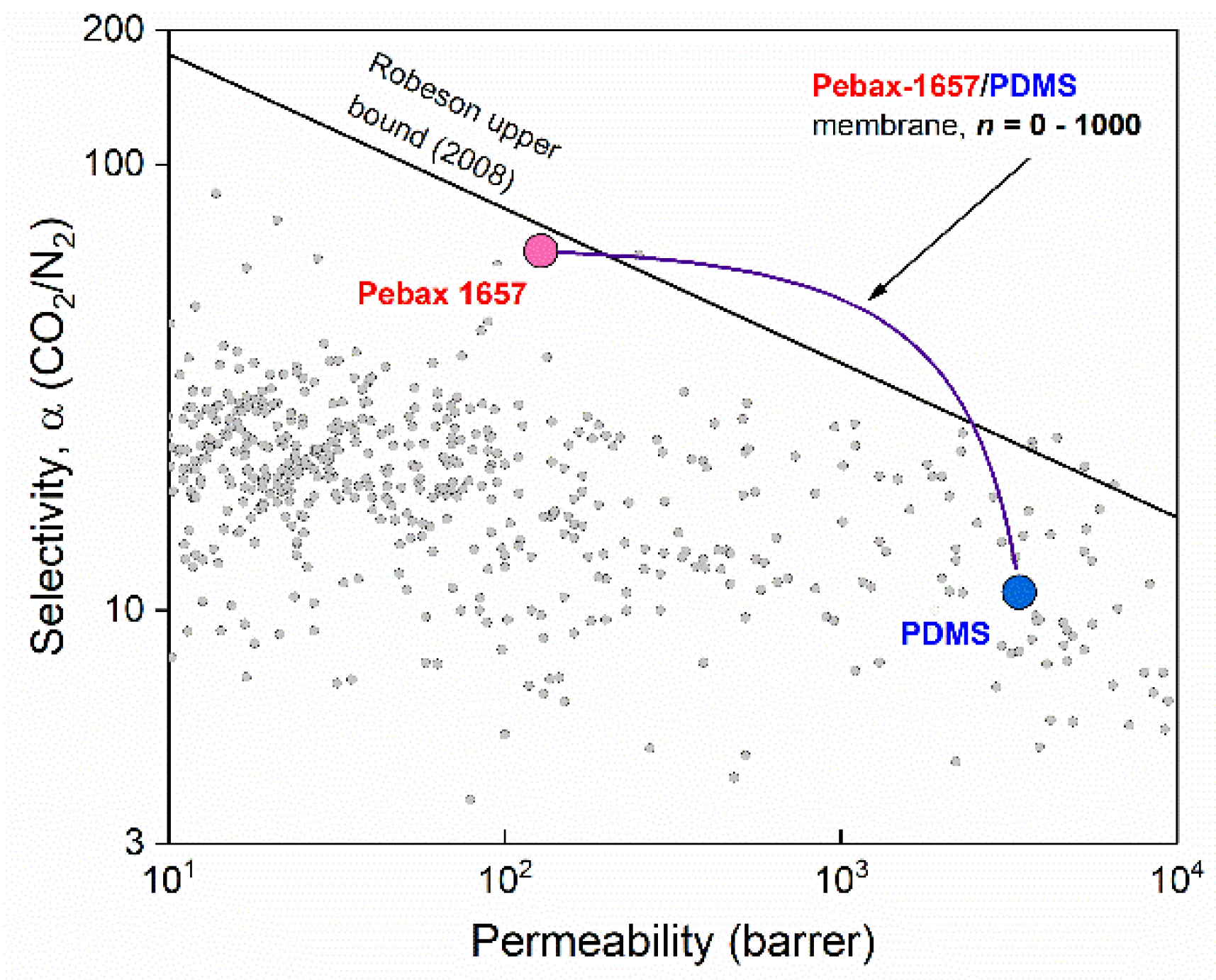
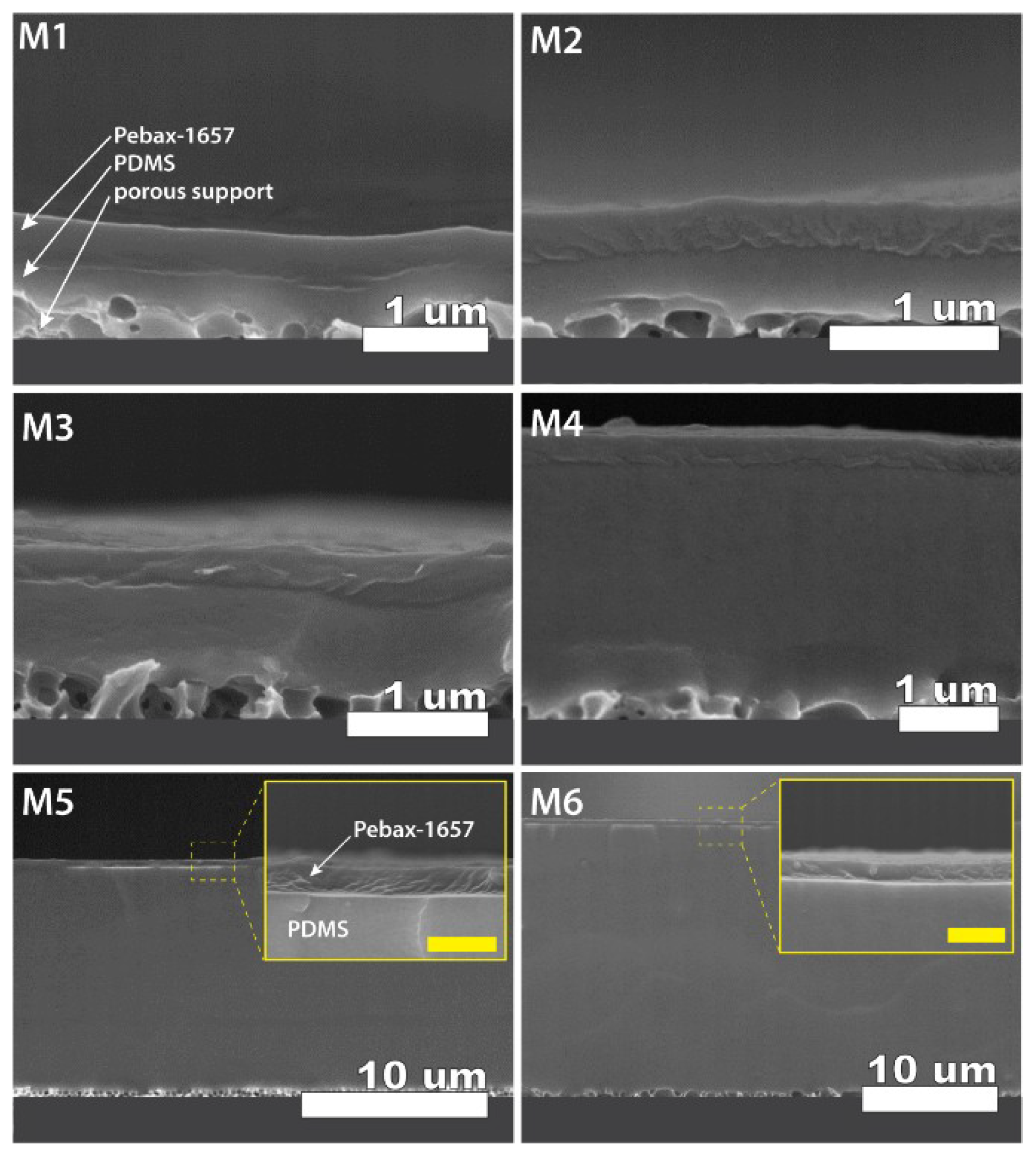
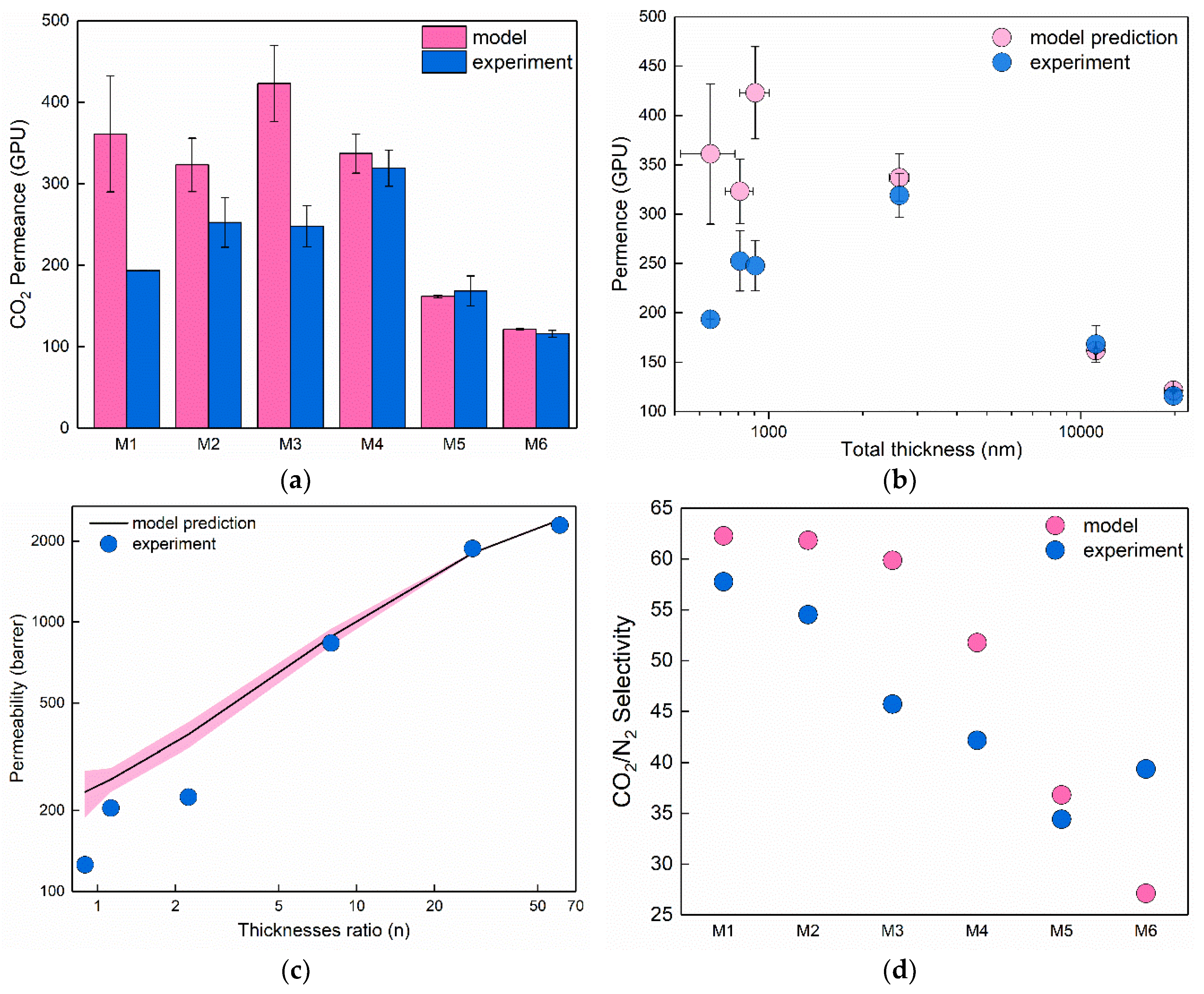
| Material | CO2 Permeability, Barrer | N2 Permeability, Barrer | Ideal Selectivity | Reference |
|---|---|---|---|---|
| PDMS | 3395 ± 55 | 307 ± 3 | 11 | This work |
| 3100 a | 290 | 10.7 | [23] | |
| 3632 a | 330 c | 11 | [24] | |
| 3800 ± 70 b | 400 ± 10 b | 9.5 | [21] | |
| Pebax-1657 | 128 ± 17 | 2.0 ± 0.4 | 64 | This work |
| 55.8 | 1.4 | 40.2 | [25] | |
| 80 | 1.1 | 70 | [29] | |
| 133 | 2.6 c | 52 | [30] |
| Membrane # | Thickness of Pebax-1657 Layer, nm (ls ± Δls) 1 | Thickness of PDMS Layer, nm (lg ± Δlg) 1 | Ratio of Thicknesses, n = lg/ls | Model-Predicted CO2 Permeability, Barrer | Model-Predicted CO2 Permeance, GPU 2 | Model-Predicted Selectivity |
|---|---|---|---|---|---|---|
| M1 | 343 ± 58 | 307 ± 70 | 0.90 | 235 | 361 ± 71 | 62.3 |
| M2 | 380 ± 29 | 428 ± 53 | 1.13 | 261 | 323 ± 33 | 61.8 |
| M3 | 279 ± 55 | 626 ± 45 | 2.24 | 383 | 432 ± 47 | 59.9 |
| M4 | 292 ± 23 | 2327 ± 164 | 7.97 | 883 | 337 ±24 | 51.8 |
| M5 | 385 ± 15 | 10,775 ± 90 | 27.99 | 1805 | 162 ± 2 | 36.8 |
| M6 | 320 ± 15 | 19,469 ± 165 | 60.84 | 2403 | 121 ± 1 | 27.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selyanchyn, R.; Ariyoshi, M.; Fujikawa, S. Thickness Effect on CO2/N2 Separation in Double Layer Pebax-1657®/PDMS Membranes. Membranes 2018, 8, 121. https://doi.org/10.3390/membranes8040121
Selyanchyn R, Ariyoshi M, Fujikawa S. Thickness Effect on CO2/N2 Separation in Double Layer Pebax-1657®/PDMS Membranes. Membranes. 2018; 8(4):121. https://doi.org/10.3390/membranes8040121
Chicago/Turabian StyleSelyanchyn, Roman, Miho Ariyoshi, and Shigenori Fujikawa. 2018. "Thickness Effect on CO2/N2 Separation in Double Layer Pebax-1657®/PDMS Membranes" Membranes 8, no. 4: 121. https://doi.org/10.3390/membranes8040121
APA StyleSelyanchyn, R., Ariyoshi, M., & Fujikawa, S. (2018). Thickness Effect on CO2/N2 Separation in Double Layer Pebax-1657®/PDMS Membranes. Membranes, 8(4), 121. https://doi.org/10.3390/membranes8040121






