Review of Recent Nuclear Magnetic Resonance Studies of Ion Transport in Polymer Electrolytes
Abstract
1. Introduction
2. PEO and Ceramic Composite Electrolytes
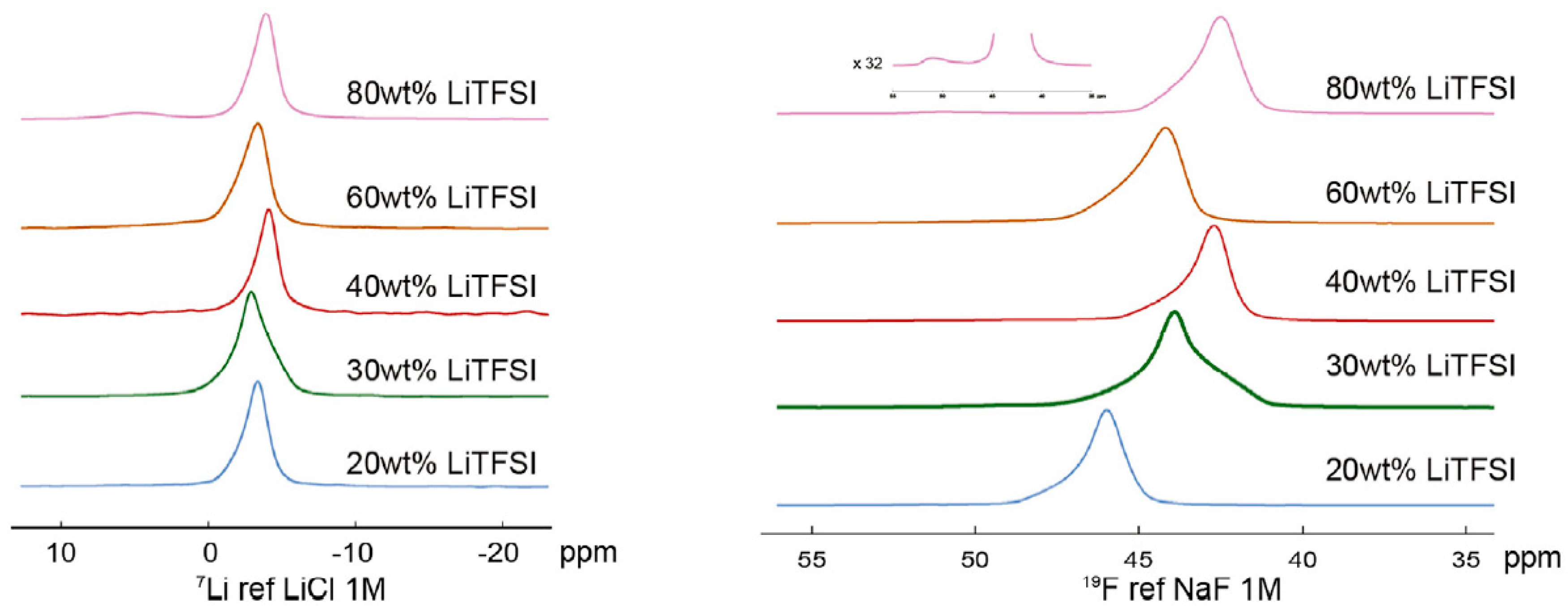
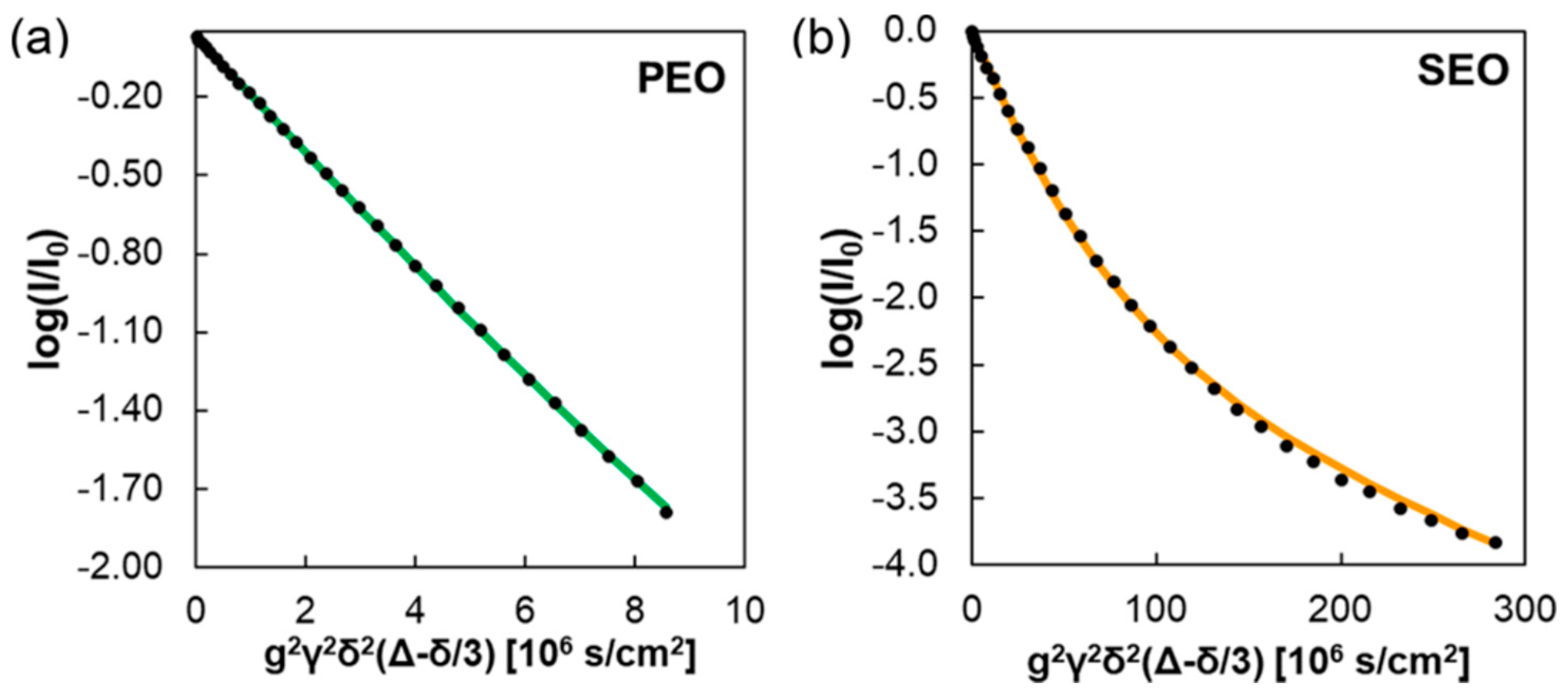
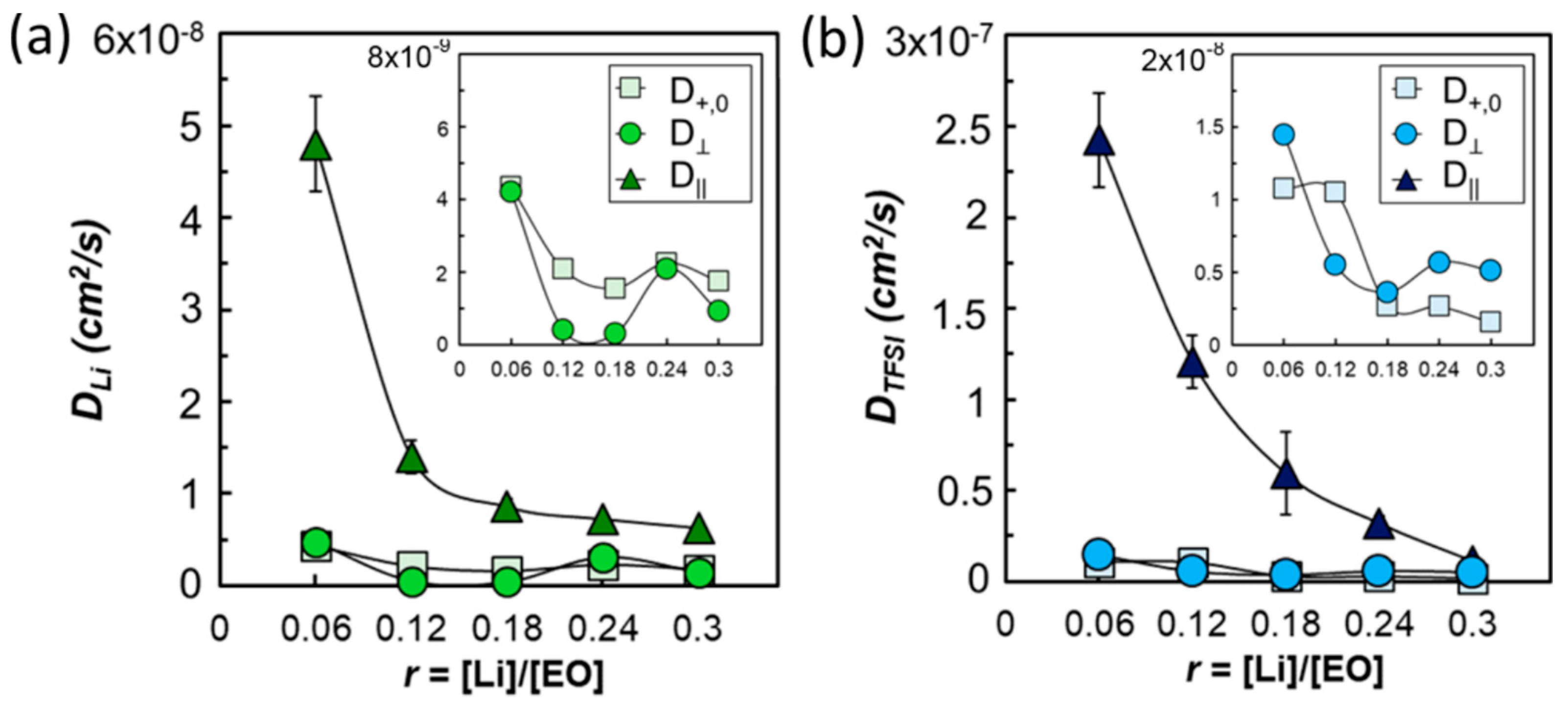
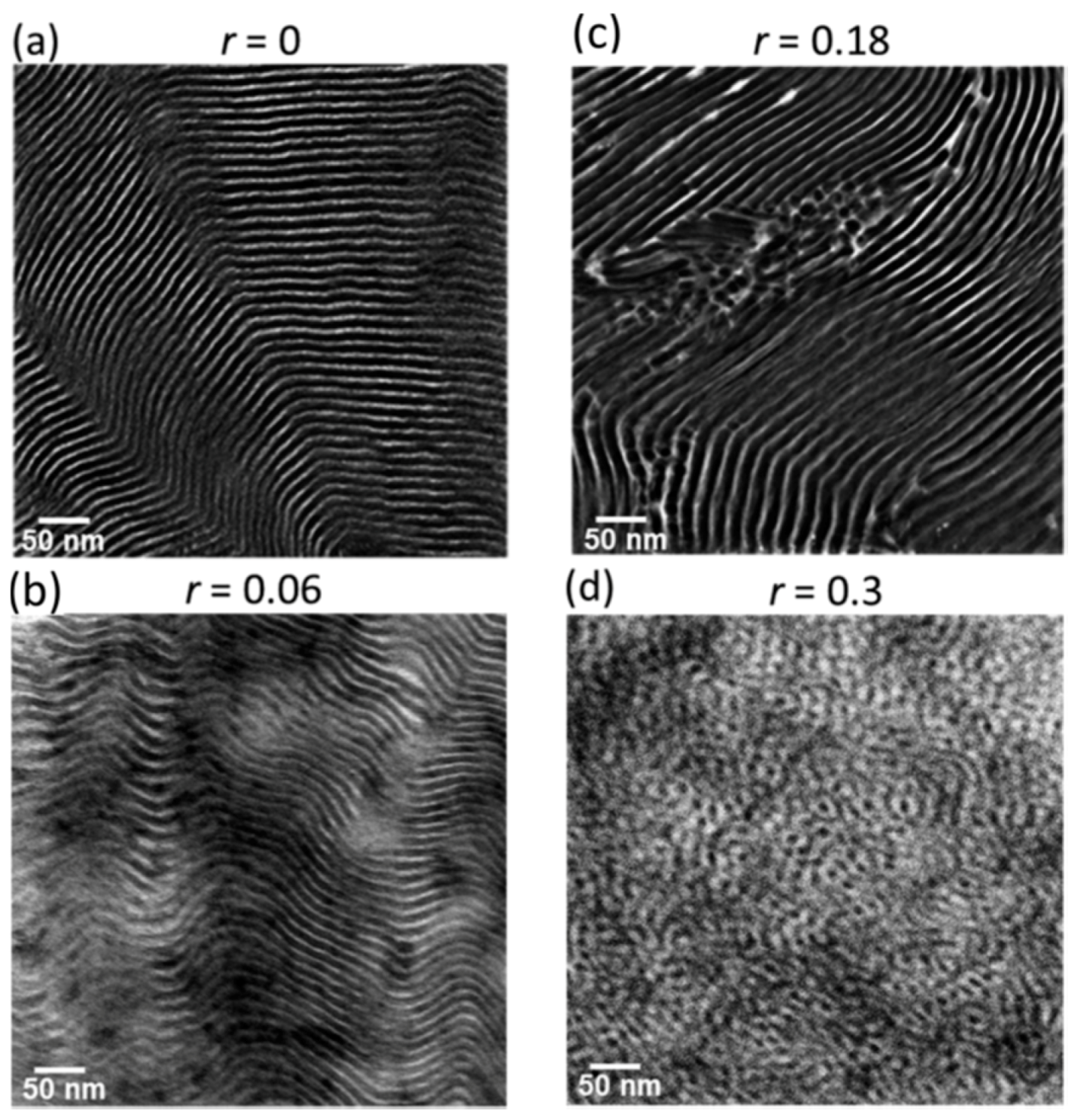
3. Copolymers, Block Copolymers, and Polymer Blends
4. Crystalline Polymer Electrolytes
5. Sodium-Conducting Electrolytes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, E.J.R.; Vincent, C.A. Polymer Electrolyte Reviews; Elsevier Applied Science: London, UK, 1989. [Google Scholar]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.; Johansson, S.; Zick, K.; Schmedt auf der Günne, J.; Dehnen, S.; Roling, B. Li10SnP2S12: An Affordable Lithium Superionic Conductor. J. Am. Chem. Soc. 2013, 135, 15694–15697. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Grundish, N.; Murchison, A.; Goodenough, J. Alternative strategy for a safe rechargeable battery. Energy Environ. Sci. 2017, 10, 331–336. [Google Scholar] [CrossRef]
- Yu, X.; Bates, J.; Jellison, G.; Hart, F. A Stable Thin—Film Lithium Electrolyte: Lithium Phosphorus Oxynitride. J. Electrochem. Soc. 1997, 144, 524–532. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Armand, M.B.; Chabagno, J.M.; Duclot, M.J. Fast Ion Transport in Solids. In Proceedings of the International Conference on Fast Ion Transport in Solids, Electrodes and Electrolytes., Lake Geneva, WI, USA, 21–25 May 1979; p. 131. [Google Scholar]
- Berthier, C.; Gorecki, W.; Minier, M.; Armand, M.B.; Chabagno, J.M.; Rigaud, P. Microscopic investigation of ionic conductivity in alkali metal salts-poly(ethylene oxide) adducts. Solid State Ionics 1983, 11, 91–95. [Google Scholar] [CrossRef]
- Adamić, K.J.; Greenbaum, S.G.; Wintersgill, M.C.; Fontanella, J.J. Ionic conductivity in solid, crosslinked dimethylsiloxane-ethylene oxide copolymer networks containing sodium. J. Appl. Phys. 1986, 60, 1342–1345. [Google Scholar] [CrossRef]
- Chung, S.H.; Wang, Y.; Persi, L.; Croce, F.; Greenbaum, S.G.; Scrosati, B.; Plichta, E. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides. J. Power Sources 2001, 97–98, 644–648. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.B.; Bruce, P.G.; Forsyth, M.; Scrosati, B.; Wieczorek, W. Polymer Electrolytes. In Energy Materials; Bruce, D.W., Dermot, O.H., Wlton, R.I., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kovarsky, R.; Golodnitsky, D.; Peled, E.; Khatun, S.; Stallworth, P.E.; Greenbaum, S.; Greenbaum, A. Conductivity enhancement induced by casting of polymer electrolytes under a magnetic field. Electrochimica Acta 2011, 57, 27–35. [Google Scholar] [CrossRef]
- Wetjen, M.; Kim, G.-T.; Joost, M.; Winter, M.; Passerini, S. Temperature dependence of electrochemical properties of cross-linked poly(ethylene oxide)–lithium bis(trifluoromethanesulfonyl)imide–N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide solid polymer electrolytes for lithium batteries. Electrochimica Acta 2013, 87, 779–787. [Google Scholar] [CrossRef]
- Maranski, K.; Andreev, Y.G.; Bruce, P.G. Synthesis of Poly(ethylene oxide) Approaching Monodispersity. Angew. Chem. Int. Ed. 2014, 53, 6411–6413. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, F.; Jin, L.; O’Dell, L.A.; Forsyth, M. Insight into Local Structure and Molecular Dynamics in Organic Solid-State Ionic Conductors. ChemPhysChem 2014, 15, 3720–3724. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, F.; Belieres, J.P.; Kuwata, N.; Pradel, A.; Ribes, M.; Angell, C.A. Highly decoupled ionic and protonic solid electrolyte systems, in relation to other relaxing systems and their energy landscapes. J. Non-Cryst. Solids 2006, 352, 5147–5155. [Google Scholar] [CrossRef]
- Wang, Y.; Agapov, A.L.; Fan, F.; Hong, K.; Yu, X.; Mays, J.; Sokolov, A.P. Decoupling of Ionic Transport from Segmental Relaxation in Polymer Electrolytes. Phys. Rev. Lett. 2012, 108, 088303. [Google Scholar] [CrossRef] [PubMed]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Hovington, P.; Lagacé, M.; Guerfi, A.; Bouchard, P.; Mauger, A.; Julien, C.M.; Armand, M.; Zaghib, K. New Lithium Metal Polymer Solid State Battery for an Ultrahigh Energy: Nano C-LiFePO4 versus Nano Li1.2V3O8. Nano Lett. 2015, 15, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Lago, N.; Garcia-Calvo, O.; Lopez del Amo, J.M.; Rojo, T.; Armand, M. All-Solid-State Lithium-Ion Batteries with Grafted Ceramic Nanoparticles Dispersed in Solid Polymer Electrolytes. ChemSusChem 2015, 8, 3039–3043. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.-P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Javier, A.E.; Patel, S.N.; Hallinan, D.T.; Srinivasan, V.; Balsara, N.P. Simultaneous Electronic and Ionic Conduction in a Block Copolymer: Application in Lithium Battery Electrodes. Angew. Chem. Int. Ed. 2011, 50, 9848–9851. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, J.; Hosono, E.; Yoshio, M.; Ichikawa, T.; Matsumoto, T.; Ohno, H.; Zhou, H.; Kato, T. Liquid Crystals: Liquid-Crystalline Electrolytes for Lithium-Ion Batteries: Ordered Assemblies of a Mesogen-Containing Carbonate and a Lithium Salt (Adv. Funct. Mater. 8/2015). Adv. Funct. Mater. 2015, 25, 1205. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Seki, S.; Mita, Y.; Ohno, Y.; Miyashiro, H.; Charest, P.; Guerfi, A.; Zaghib, K. High reversible capacities of graphite and SiO/graphite with solvent-free solid polymer electrolyte for lithium-ion batteries. J. Power Sources 2008, 185, 542–548. [Google Scholar] [CrossRef]
- Seki, S.; Kobayashi, Y.; Miyashiro, H.; Mita, Y.; Iwahori, T. Fabrication of High-Voltage, High-Capacity All-Solid-State Lithium Polymer Secondary Batteries by Application of the Polymer Electrolyte/Inorganic Electrolyte Composite Concept. Chem. Mater. 2005, 17, 2041–2045. [Google Scholar] [CrossRef]
- He, R.; Echeverri, M.; Ward, D.; Zhu, Y.; Kyu, T. Highly conductive solvent-free polymer electrolyte membrane for lithium-ion batteries: Effect of prepolymer molecular weight. J. Membr. Sci. 2016, 498, 208–217. [Google Scholar] [CrossRef]
- Porcarelli, L.; Gerbaldi, C.; Bella, F.; Nair, J.R. Super Soft All-Ethylene Oxide Polymer Electrolyte for Safe All-Solid Lithium Batteries. Sci. Rep. 2016, 6, 19892. [Google Scholar] [CrossRef] [PubMed]
- Porcarelli, L.; Aboudzadeh, M.A.; Rubatat, L.; Nair, J.R.; Shaplov, A.S.; Gerbaldi, C.; Mecerreyes, D. Single-ion triblock copolymer electrolytes based on poly(ethylene oxide) and methacrylic sulfonamide blocks for lithium metal batteries. J. Power Sources 2017, 364, 191–199. [Google Scholar] [CrossRef]
- He, W.; Cui, Z.; Liu, X.; Cui, Y.; Chai, J.; Zhou, X.; Liu, Z.; Cui, G. Carbonate-linked poly(ethylene oxide) polymer electrolytes towards high performance solid state lithium batteries. Electrochim. Acta 2017, 225, 151–159. [Google Scholar] [CrossRef]
- Wong, S.; Vaia, R.A.; Giannelis, E.P.; Zax, D.B. Dynamics in a poly(ethylene oxide)-based nanocomposite polymer electrolyte probed by solid state NMR. Solid State Ionics 1996, 86–88, 547–557. [Google Scholar] [CrossRef]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption; World Scientific Publishing Co. Pte. Ltd.: Singapore, 1990; pp. 411–444. [Google Scholar]
- Stejskal, E.O.; Tanner, J.E. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Böhmer, R.; Jeffrey, K.R.; Vogel, M. Solid-state Li NMR with applications to the translational dynamics in ion conductors. Prog. Nucl. Magn. Reson. Spectrosc. 2007, 50, 87–174. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Y.; Greenbaum, S.G.; Bajue, S.A.; Golodnitsky, D.; Ardel, G.; Strauss, E.; Peled, E. Electrical, thermal and NMR investigation of composite solid electrolytes based on PEO, LiI and high surface area inorganic oxides. Electrochim. Acta 1998, 43, 1557–1561. [Google Scholar] [CrossRef]
- Croce, F.; Bonino, F.; Panero, S.; Scrosati, B. Properties of mixed polymer and crystalline ionic conductors. Philos. Mag. B 1989, 59, 161–168. [Google Scholar] [CrossRef]
- Capuano, F.; Croce, F.; Scrosati, B. Composite Polymer Electrolytes. J. Electrochem. Soc. 1991, 138, 1918–1922. [Google Scholar] [CrossRef]
- Wieczorek, W.; Florjanczyk, Z.; Stevens, J.R. Composite polyether based solid electrolytes. Electrochim. Acta 1995, 40, 2251–2258. [Google Scholar] [CrossRef]
- Przyluski, J.; Siekierski, M.; Wieczorek, W. Effective medium theory in studies of conductivity of composite polymeric electrolytes. Electrochim. Acta 1995, 40, 2101–2108. [Google Scholar] [CrossRef]
- Golodnitsky, D.; Strauss, E.; Peled, E.; Greenbaum, S. Review—On Order and Disorder in Polymer Electrolytes. J. Electrochem. Soc. 2015, 162, A2551–A2566. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Sun, J.; Hsu, P.-C.; Li, Y.; Lee, H.-W.; Cui, Y. Ionic Conductivity Enhancement of Polymer Electrolytes with Ceramic Nanowire Fillers. Nano Lett. 2015, 15, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lee, S.W.; Lin, D.; Shi, F.; Wang, S.; Sendek, A.D.; Cui, Y. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat. Energy 2017, 2, 17035. [Google Scholar] [CrossRef]
- Weston, J.E.; Steele, B.C.H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ionics 1982, 7, 75–79. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.-P.; Fan, L.-Z.; Nan, C.-W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Aetukuri, N.B.; Kitajima, S.; Jung, E.; Thompson, L.E.; Virwani, K.; Reich, M.-L.; Kunze, M.; Schneider, M.; Schmidbauer, W.; Wilcke, W.W.; et al. Flexible Ion-Conducting Composite Membranes for Lithium Batteries. Adv. Energy Mater. 2015, 5, 1500265. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, C.; Peng, G.; Chen, X.; Yao, X.; Bai, Y.; Wu, F.; Chen, S.; Xu, X. A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries. J. Power Sources 2016, 301, 47–53. [Google Scholar] [CrossRef]
- Tu, Z.; Kambe, Y.; Lu, Y.; Archer, L.A. Nanoporous Polymer-Ceramic Composite Electrolytes for Lithium Metal Batteries. Adv. Energy Mater. 2013. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, Y.-Y. New Insights into the Compositional Dependence of Li-Ion Transport in Polymer–Ceramic Composite Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 4113–4120. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tang, M.; Hu, Y.-Y. Lithium Ion Pathway within Li7La3Zr2O12-Polyethylene Oxide Composite Electrolytes. Angew. Chem. Int. Ed. 2016, 55, 12538–12542. [Google Scholar] [CrossRef] [PubMed]
- Villaluenga, I.; Bogle, X.; Greenbaum, S.; Gil de Muro, I.; Rojo, T.; Armand, M. Cation only conduction in new polymer–SiO2 nanohybrids: Na+ electrolytes. J. Mater. Chem. A 2013, 1, 8348–8352. [Google Scholar] [CrossRef]
- Croce, F.; Sacchetti, S.; Scrosati, B. Advanced, lithium batteries based on high-performance composite polymer electrolytes. J. Power Sources 2006, 162, 685–689. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.A.; Cai, W.; Rohan, R.; Xu, G.; Sun, Y.; Cheng, H. Design and synthesis of a single ion conducting block copolymer electrolyte with multifunctionality for lithium ion batteries. RSC Adv. 2014, 4, 43857–43864. [Google Scholar] [CrossRef]
- Chen, B.; Xu, Q.; Huang, Z.; Zhao, Y.; Chen, S.; Xu, X. One-pot preparation of new copolymer electrolytes with tunable network structure for all-solid-state lithium battery. J. Power Sources 2016, 331, 322–331. [Google Scholar] [CrossRef]
- Boden, N.; Leng, S.A.; Ward, I.M. Ionic conductivity and diffusivity in polyethylene oxode/electrolyte solutions as models for polymer electrolytes. Solid State Ionics 1991, 45, 261–270. [Google Scholar] [CrossRef]
- Yuan, R.; Teran, A.A.; Gurevitch, I.; Mullin, S.A.; Wanakule, N.S.; Balsara, N.P. Ionic Conductivity of Low Molecular Weight Block Copolymer Electrolytes. Macromolecules 2013, 46, 914–921. [Google Scholar] [CrossRef]
- Niitani, T.; Amaike, M.; Nakano, H.; Dokko, K.; Kanamura, K. Star-Shaped Polymer Electrolyte with Microphase Separation Structure for All-Solid-State Lithium Batteries. J. Electrochem. Soc. 2009, 156, A577–A583. [Google Scholar] [CrossRef]
- Alloin, F.; Sanchez, J.Y.; Armand, M. Triblock copolymers and networks incorporating oligo (oxyethylene) chains. Solid State Ionics 1993, 60, 3–9. [Google Scholar] [CrossRef]
- Daigle, J.-C.; Arnold, A.; Vijh, A.; Zaghib, K. Solid-State NMR Study of New Copolymers as Solid Polymer Electrolytes. Magnetochemistry 2018, 4, 13. [Google Scholar] [CrossRef]
- Lin, C.-L.; Kao, H.-M.; Wu, R.-R.; Kuo, P.-L. Multinuclear Solid-State NMR, DSC, and Conductivity Studies of Solid Polymer Electrolytes Based on Polyurethane/Poly(dimethylsiloxane) Segmented Copolymers. Macromolecules 2002, 35, 3083–3096. [Google Scholar] [CrossRef]
- Meabe, L.; Huynh, T.V.; Lago, N.; Sardon, H.; Li, C.; O’Dell, L.A.; Armand, M.; Forsyth, M.; Mecerreyes, D. Poly(ethylene oxide carbonates) solid polymer electrolytes for lithium batteries. Electrochim. Acta 2018, 264, 367–375. [Google Scholar] [CrossRef]
- Morioka, T.; Nakano, K.; Tominaga, Y. Ion-Conductive Properties of a Polymer Electrolyte Based on Ethylene Carbonate/Ethylene Oxide Random Copolymer. Macromol. Rapid Commun. 2017, 38, 1600652. [Google Scholar] [CrossRef] [PubMed]
- Mindemark, J.; Sun, B.; Törmä, E.; Brandell, D. High-performance solid polymer electrolytes for lithium batteries operational at ambient temperature. J. Power Sources 2015, 298, 166–170. [Google Scholar] [CrossRef]
- Timachova, K.; Villaluenga, I.; Cirrincione, L.; Gobet, M.; Bhattacharya, R.; Jiang, X.; Newman, J.; Madsen, L.A.; Greenbaum, S.G.; Balsara, N.P. Anisotropic Ion Diffusion and Electrochemically Driven Transport in Nanostructured Block Copolymer Electrolytes. J. Phys. Chem. B 2018, 122, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Vashishta, P.; Mundy, J.N. Fast Ion Transport in Solids: Electrodes and Electrolytes; Elsevier North Holland, Inc.: Amsterdam, NY, USA, 1979. [Google Scholar]
- Papke, B.L.; Dupon, R.; Ratner, M.A.; Shriver, D.F. Ion-pairing in polyether solid electrolytes and its influence on ion transport. Solid State Ionics 1981, 5, 685–688. [Google Scholar] [CrossRef]
- Chintapalli, M.; Le, T.N.P.; Venkatesan, N.R.; Mackay, N.G.; Rojas, A.A.; Thelen, J.L.; Chen, X.C.; Devaux, D.; Balsara, N.P. Structure and Ionic Conductivity of Polystyrene-block-poly(ethylene oxide) Electrolytes in the High Salt Concentration Limit. Macromolecules 2016, 49, 1770–1780. [Google Scholar] [CrossRef]
- Singh, M.; Odusanya, O.; Wilmes, G.M.; Eitouni, H.B.; Gomez, E.D.; Patel, A.J.; Chen, V.L.; Park, M.J.; Fragouli, P.; Iatrou, H.; et al. Effect of Molecular Weight on the Mechanical and Electrical Properties of Block Copolymer Electrolytes. Macromolecules 2007, 40, 4578–4585. [Google Scholar] [CrossRef]
- Liu, T.-M.; Saikia, D.; Ho, S.-Y.; Chen, M.-C.; Kao, H.-M. High ion-conducting solid polymer electrolytes based on blending hybrids derived from monoamine and diamine polyethers for lithium solid-state batteries. RSC Adv. 2017, 7, 20373–20383. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Costa, C.M.; Lanceros-Méndez, S. Polymer composites and blends for battery separators: State of the art, challenges and future trends. J. Power Sources 2015, 281, 378–398. [Google Scholar] [CrossRef]
- Nguyen, C.A.; Xiong, S.; Ma, J.; Lu, X.; Lee, P.S. High ionic conductivity P(VDF-TrFE)/PEO blended polymer electrolytes for solid electrochromic devices. Phys. Chem. Chem. Phys. 2011, 13, 13319–13326. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.A.M.; Ahmad, A.; Talib, I.A.; Rahman, M.Y.A. Morphology, chemical interaction, and conductivity of a PEO-ENR50 based on solid polymer electrolyte. Ionics 2010, 16, 161–170. [Google Scholar] [CrossRef]
- Borodin, O.; Suo, L.; Gobet, M.; Ren, X.; Wang, F.; Faraone, A.; Peng, J.; Olguin, M.; Schroeder, M.; Ding, M.S.; et al. Liquid Structure with Nano-Heterogeneity Promotes Cationic Transport in Concentrated Electrolytes. ACS Nano 2017, 11, 10462–10471. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.H.; Bianchi, R.F.; Dahmouche, K.; Judeinstein, P.; Faria, R.M.; Bonagamba, T.J. Solid-State NMR, Ionic Conductivity, and Thermal Studies of Lithium-doped Siloxane−Poly(propylene glycol) Organic−Inorganic Nanocomposites. Chem. Mater. 2001, 13, 3685–3692. [Google Scholar] [CrossRef]
- Gadjourova, Z.; Andreev, Y.G.; Tunstall, D.P.; Bruce, P.G. Ionic conductivity in crystalline polymer electrolytes. Nature 2001, 412, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.M.; Lilley, S.J.; Staunton, E.; Andreev, Y.G.; Bruce, P.G. Increasing the conductivity of crystalline polymer electrolytes. Nature 2005, 433, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Staunton, E.; Andreev, Y.G.; Bruce, P.G. Raising the Conductivity of Crystalline Polymer Electrolytes by Aliovalent Doping. J. Am. Chem. Soc. 2005, 127, 18305–18308. [Google Scholar] [CrossRef] [PubMed]
- Staunton, E.; Andreev, Y.G.; Bruce, P.G. Factors influencing the conductivity of crystalline polymer electrolytes. Faraday Discuss. 2007, 134, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Golodnitsky, D.; Livshits, E.; Peled, E. Highly conductive oriented PEO-based polymer electrolytes. Macromol. Symp. 2003, 203, 27–46. [Google Scholar] [CrossRef]
- Golodnitsky, D.; Livshits, E.; Ulus, A.; Barkay, Z.; Lapides, I.; Peled, E.; Chung, S.H.; Greenbaum, S. Fast Ion Transport Phenomena in Oriented Semicrystalline LiI−P(EO)n-Based Polymer Electrolytes. J. Phys. Chem. A 2001, 105, 10098–10106. [Google Scholar] [CrossRef]
- Yan, X.; Peng, B.; Hu, B.; Chen, Q. PEO-urea-LiTFSI ternary complex as solid polymer electrolytes. Polymer 2016, 99, 44–48. [Google Scholar] [CrossRef]
- Liu, Y.; Antaya, H.; Pellerin, C. Characterization of the stable and metastable poly(ethylene oxide)–urea complexes in electrospun fibers. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1903–1913. [Google Scholar] [CrossRef]
- Chenite, A.; Brisse, F. Structure and conformation of poly(ethylene oxide), PEO, in the trigonal form of the PEO-urea complex at 173 K. Macromolecules 1991, 24, 2221–2225. [Google Scholar] [CrossRef]
- Liu, Y.; Pellerin, C. Highly Oriented Electrospun Fibers of Self-Assembled Inclusion Complexes of Poly(ethylene oxide) and Urea. Macromolecules 2006, 39, 8886–8888. [Google Scholar] [CrossRef]
- Liang, H.; Li, H.; Wang, Z.; Wu, F.; Chen, L.; Huang, X. New Binary Room-Temperature Molten Salt Electrolyte Based on Urea and LiTFSI. J. Phys. Chem. B 2001, 105, 9966–9969. [Google Scholar] [CrossRef]
- Fu, X.-B.; Yang, G.; Wu, J.-Z.; Wang, J.-C.; Chen, Q.; Yao, Y.-F. Fast Lithium-Ion Transportation in Crystalline Polymer Electrolytes. ChemPhysChem 2018, 19, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Chen, L.; He, X.; Chen, Y. Sequential effect and enhanced conductivity of star-shaped diblock liquid-crystalline copolymers for solid electrolytes. J. Power Sources 2014, 247, 786–793. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Pringle, J.M.; Sun, J.; Annat, G.; Neil, W.; Izgorodina, E.I. Ionic Liquids in Electrochemical Devices and Processes: Managing Interfacial Electrochemistry. Acc. Chem. Res. 2007, 40, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Huang, J.; Forsyth, M. Lithium-doped plastic crystal electrolytes exhibiting fast ion conduction for secondary batteries. Nature 1999, 402, 792–794. [Google Scholar] [CrossRef]
- Chen, F.; Pringle, J.M.; Forsyth, M. Insights into the Transport of Alkali Metal Ions Doped into a Plastic Crystal Electrolyte. Chem. Mater. 2015, 27, 2666–2672. [Google Scholar] [CrossRef]
- Wanakule, N.S.; Panday, A.; Mullin, S.A.; Gann, E.; Hexemer, A.; Balsara, N.P. Ionic Conductivity of Block Copolymer Electrolytes in the Vicinity of Order−Disorder and Order−Order Transitions. Macromolecules 2009, 42, 5642–5651. [Google Scholar] [CrossRef]
- Choi, I.; Ahn, H.; Park, M.J. Enhanced Performance in Lithium–Polymer Batteries Using Surface-Functionalized Si Nanoparticle Anodes and Self-Assembled Block Copolymer Electrolytes. Macromolecules 2011, 44, 7327–7334. [Google Scholar] [CrossRef]
- Sun, J.; Liao, X.; Minor, A.M.; Balsara, N.P.; Zuckermann, R.N. Morphology-Conductivity Relationship in Crystalline and Amorphous Sequence-Defined Peptoid Block Copolymer Electrolytes. J. Am. Chem. Soc. 2014, 136, 14990–14997. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Chen, L.; He, X.; Chen, Y. Free Mesogen Assisted Assembly of the Star-shaped Liquid-crystalline Copolymer/Polyethylene Oxide Solid Electrolytes for Lithium Ion Batteries. Electrochim. Acta 2014, 118, 33–40. [Google Scholar] [CrossRef]
- Lin, D.; Liu, W.; Liu, Y.; Lee, H.R.; Hsu, P.-C.; Liu, K.; Cui, Y. High Ionic Conductivity of Composite Solid Polymer Electrolyte via In Situ Synthesis of Monodispersed SiO2 Nanospheres in Poly(ethylene oxide). Nano Lett. 2016, 16, 459–465. [Google Scholar] [CrossRef] [PubMed]
- MacGlashan, G.S.; Andreev, Y.G.; Bruce, P.G. Structure of the polymer electrolyte poly(ethylene oxide)6:LiAsF6. Nature 1999, 398, 792–794. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Wei, D.-X.; Xu, M.; Yao, Y.-F.; Chen, Q. Transferring Lithium Ions in Nanochannels: A PEO/Li+ Solid Polymer Electrolyte Design. Angew. Chem. 2014, 126, 3705–3709. [Google Scholar] [CrossRef]
- Fu, X.-B.; Yang, L.-Y.; Ma, J.-Q.; Yang, G.; Yao, Y.-F.; Chen, Q. Revealing structure and dynamics in host–guest supramolecular crystalline polymer electrolytes by solid-state NMR: Applications to β-CD-polyether/Li+ crystal. Polymer 2016, 105, 310–317. [Google Scholar] [CrossRef]
- Okumura, H.; Kawaguchi, Y.; Harada, A. Preparation and Characterization of Inclusion Complexes of Poly(dimethylsiloxane)s with Cyclodextrins. Macromolecules 2001, 34, 6338–6343. [Google Scholar] [CrossRef]
- Li, J.; Ni, X.; Zhou, Z.; Leong, K.W. Preparation and Characterization of Polypseudorotaxanes Based on Block-Selected Inclusion Complexation between Poly(propylene oxide)-Poly(ethylene oxide)-Poly(propylene oxide) Triblock Copolymers and α-Cyclodextrin. J. Am. Chem. Soc. 2003, 125, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, X.; Yan, D.; Chen, Y.; Chen, Q.; Yao, Y. Controlling Polymer Architecture through Host–Guest Interactions. Angew. Chem. Int. Ed. 2006, 45, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gamble, S.; Ainsworth, D.; Slawin, A.M.Z.; Andreev, Y.G.; Bruce, P.G. Alkali metal crystalline polymer electrolytes. Nat. Mater. 2009, 8, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.R.; Romanenko, K.; MacFarlane, D.R.; Forsyth, M.; O’Dell, L.A. Sodium ion dynamics in a sulfonate based ionomer system studied by 23Na solid-state nuclear magnetic resonance and impedance spectroscopy. Electrochim. Acta 2015, 175, 62–67. [Google Scholar] [CrossRef]
- Tiyapiboonchaiya, C.; Pringle, J.M.; MacFarlane, D.R.; Forsyth, M.; Sun, J. Polyelectrolyte-in-Ionic-Liquid Electrolytes. Macromol. Chem. Phys. 2003, 204, 2147–2154. [Google Scholar] [CrossRef]
- Ünal, H.I.; Yilmaz, H. Electrorheological properties of poly(lithium-2-acrylamido-2-methyl propane sulfonic acid) suspensions. J. Appl. Polym. Sci. 2002, 86, 1106–1112. [Google Scholar] [CrossRef]
- Noor, S.A.M.; Sun, J.; MacFarlane, D.R.; Armand, M.; Gunzelmann, D.; Forsyth, M. Decoupled ion conduction in poly(2-acrylamido-2-methyl-1-propane-sulfonic acid) homopolymers. J. Mater. Chem. A 2014, 2, 17934–17943. [Google Scholar] [CrossRef]
- Wang, W.; Tudryn, G.J.; Colby, R.H.; Winey, K.I. Thermally Driven Ionic Aggregation in Poly(ethylene oxide)-Based Sulfonate Ionomers. J. Am. Chem. Soc. 2011, 133, 10826–10831. [Google Scholar] [CrossRef] [PubMed]
- Tudryn, G.J.; Liu, W.; Wang, S.-W.; Colby, R.H. Counterion Dynamics in Polyester−Sulfonate Ionomers with Ionic Liquid Counterions. Macromolecules 2011, 44, 3572–3582. [Google Scholar] [CrossRef]
- Mandai, T.; Nozawa, R.; Tsuzuki, S.; Yoshida, K.; Ueno, K.; Dokko, K.; Watanabe, M. Phase Diagrams and Solvate Structures of Binary Mixtures of Glymes and Na Salts. J. Phys. Chem. B 2013, 117, 15072–15085. [Google Scholar] [CrossRef] [PubMed]
- Freitag, K.M.; Walke, P.; Nilges, T.; Kirchhain, H.; Spranger, R.J.; van Wüllen, L. Electrospun-sodiumtetrafluoroborate-polyethylene oxide membranes for solvent-free sodium ion transport in solid state sodium ion batteries. J. Power Sources 2018, 378, 610–617. [Google Scholar] [CrossRef]
- Freitag, K.M.; Kirchhain, H.; Wüllen, L.V.; Nilges, T. Enhancement of Li Ion Conductivity by Electrospun Polymer Fibers and Direct Fabrication of Solvent-Free Separator Membranes for Li Ion Batteries. Inorg. Chem. 2017, 56, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
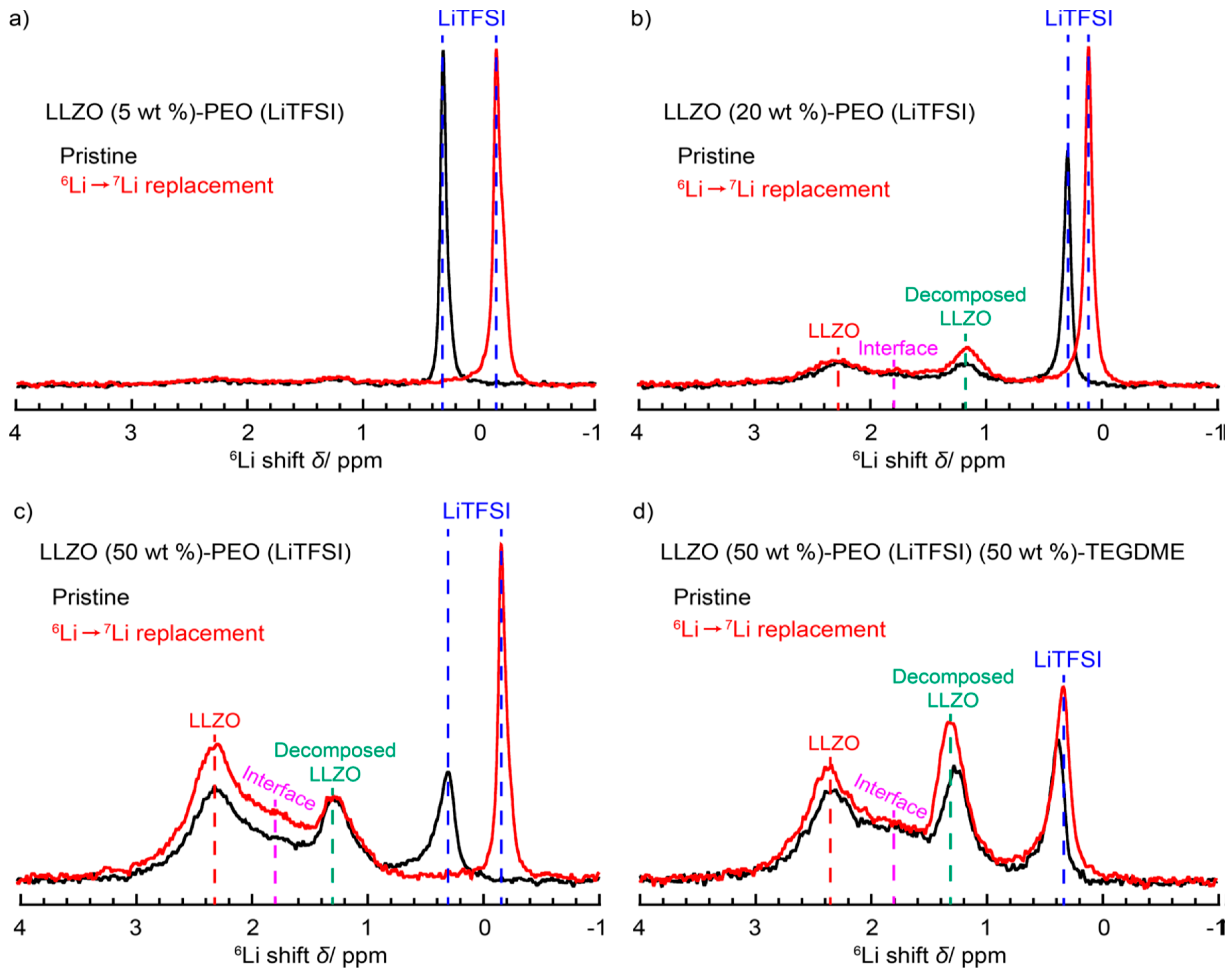
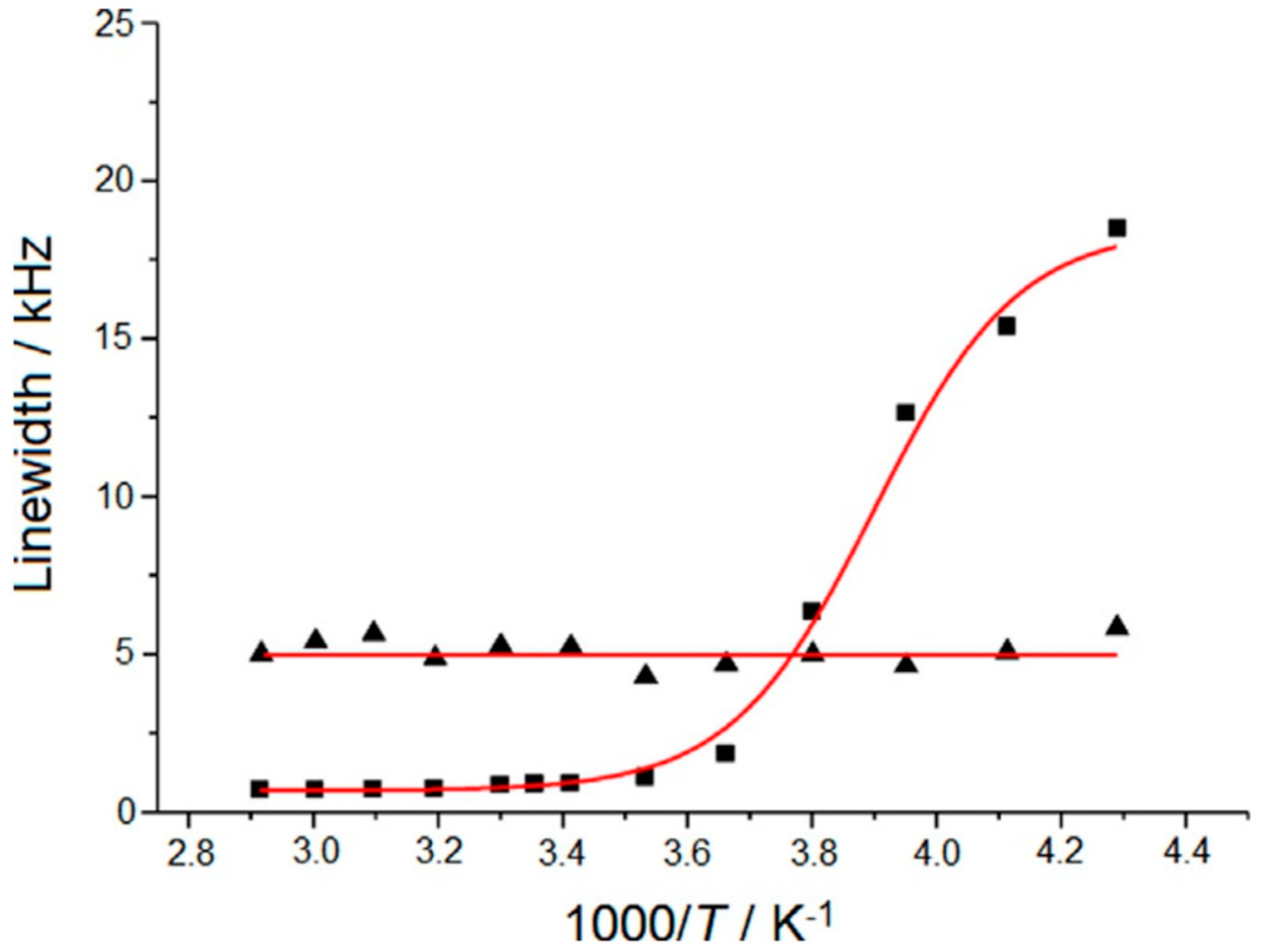
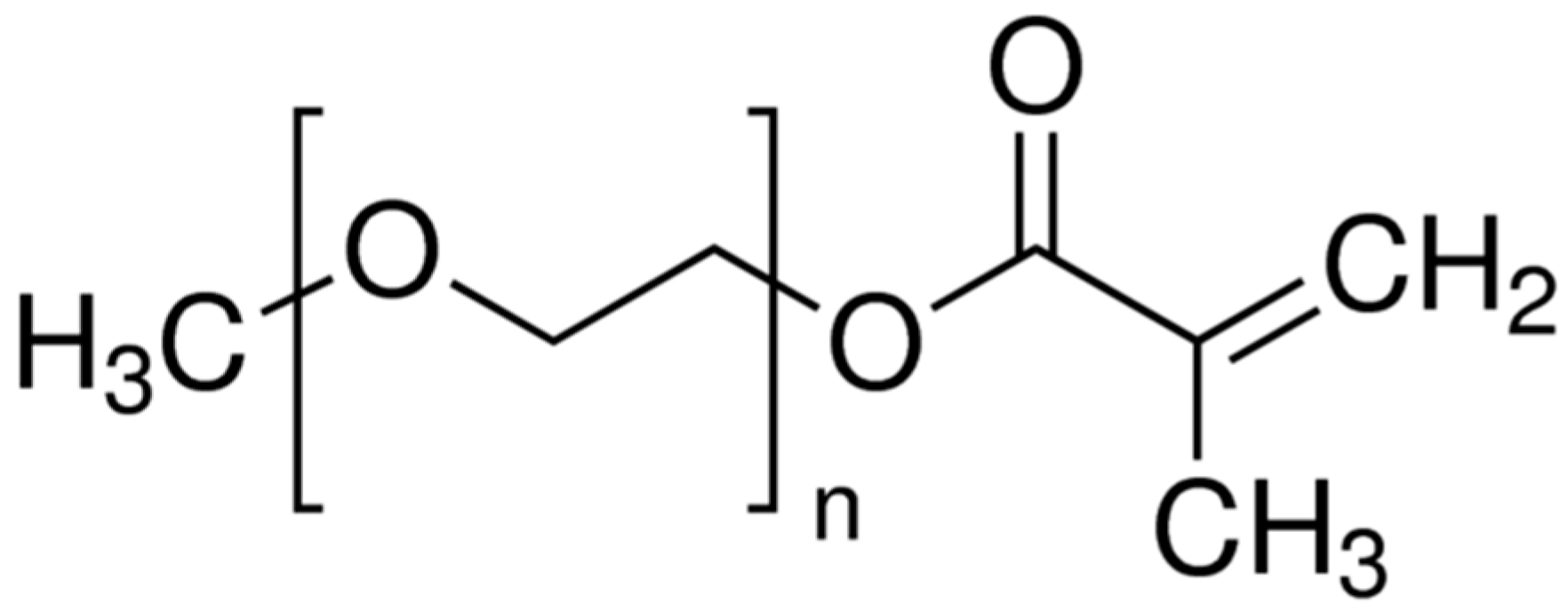
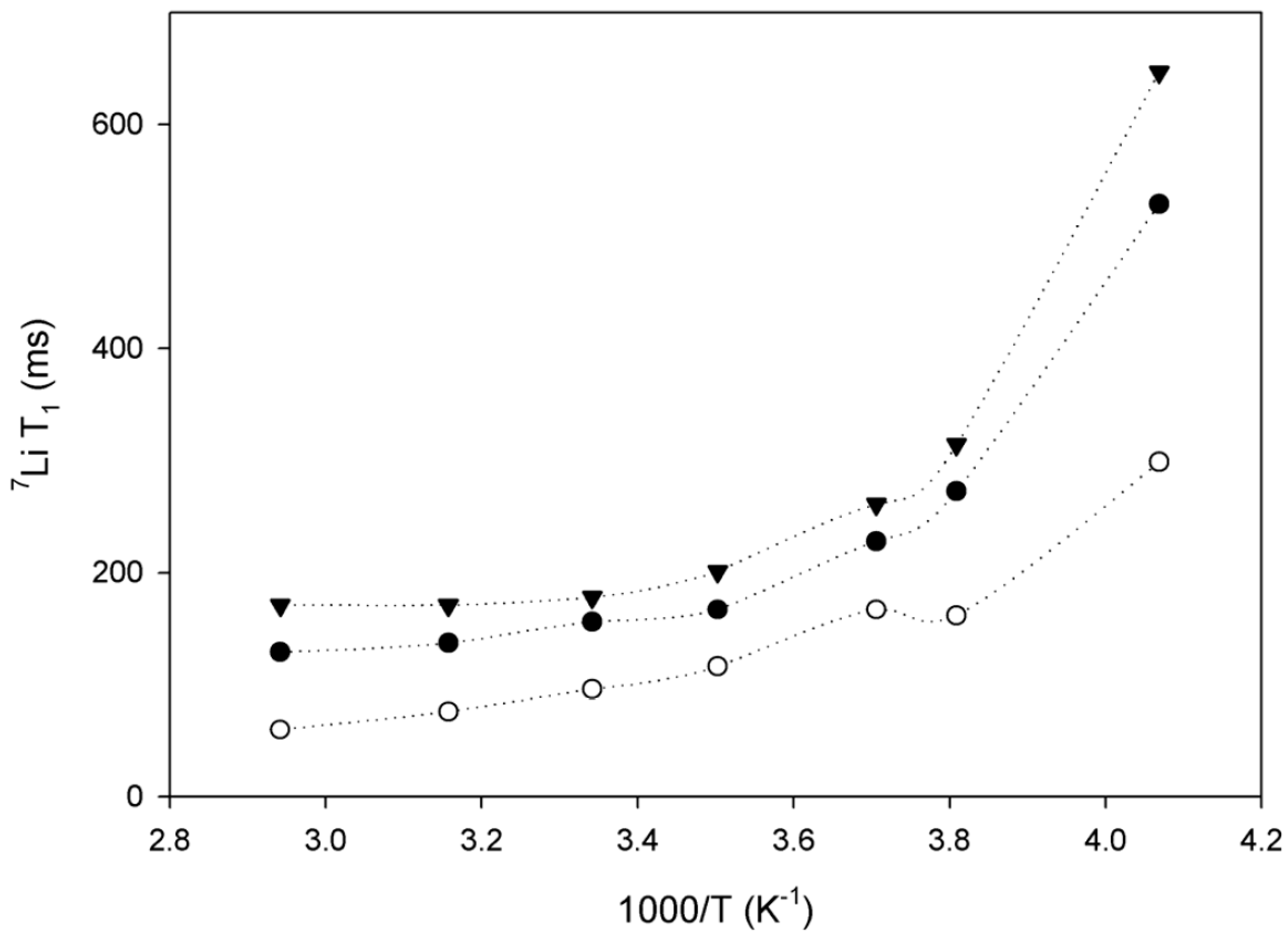
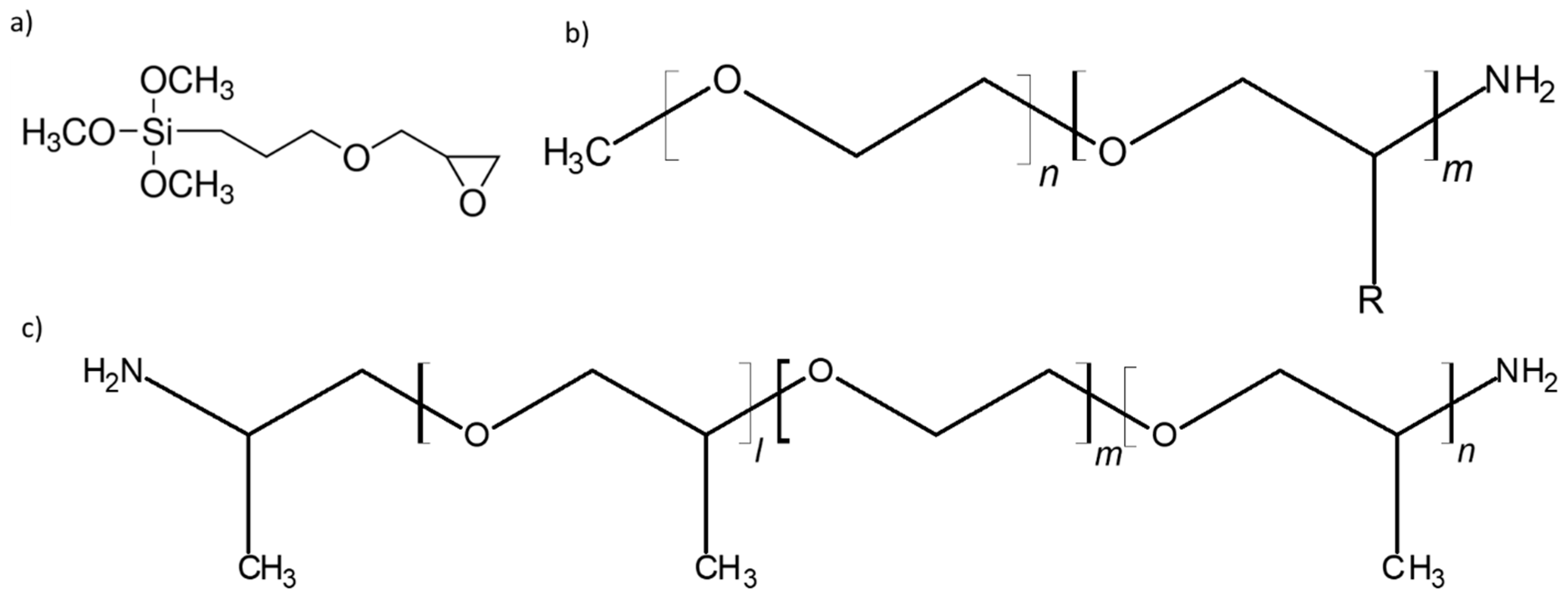
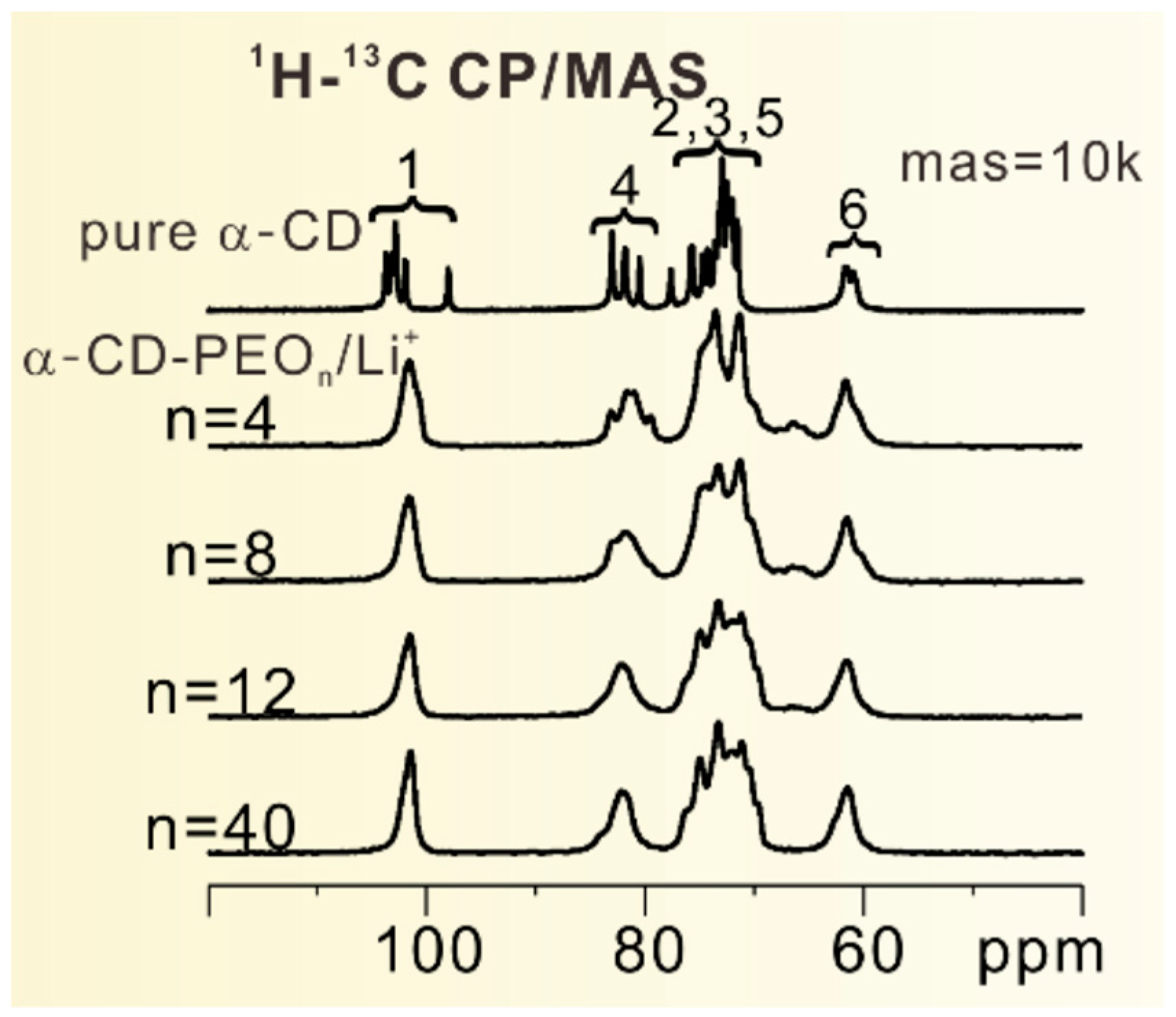
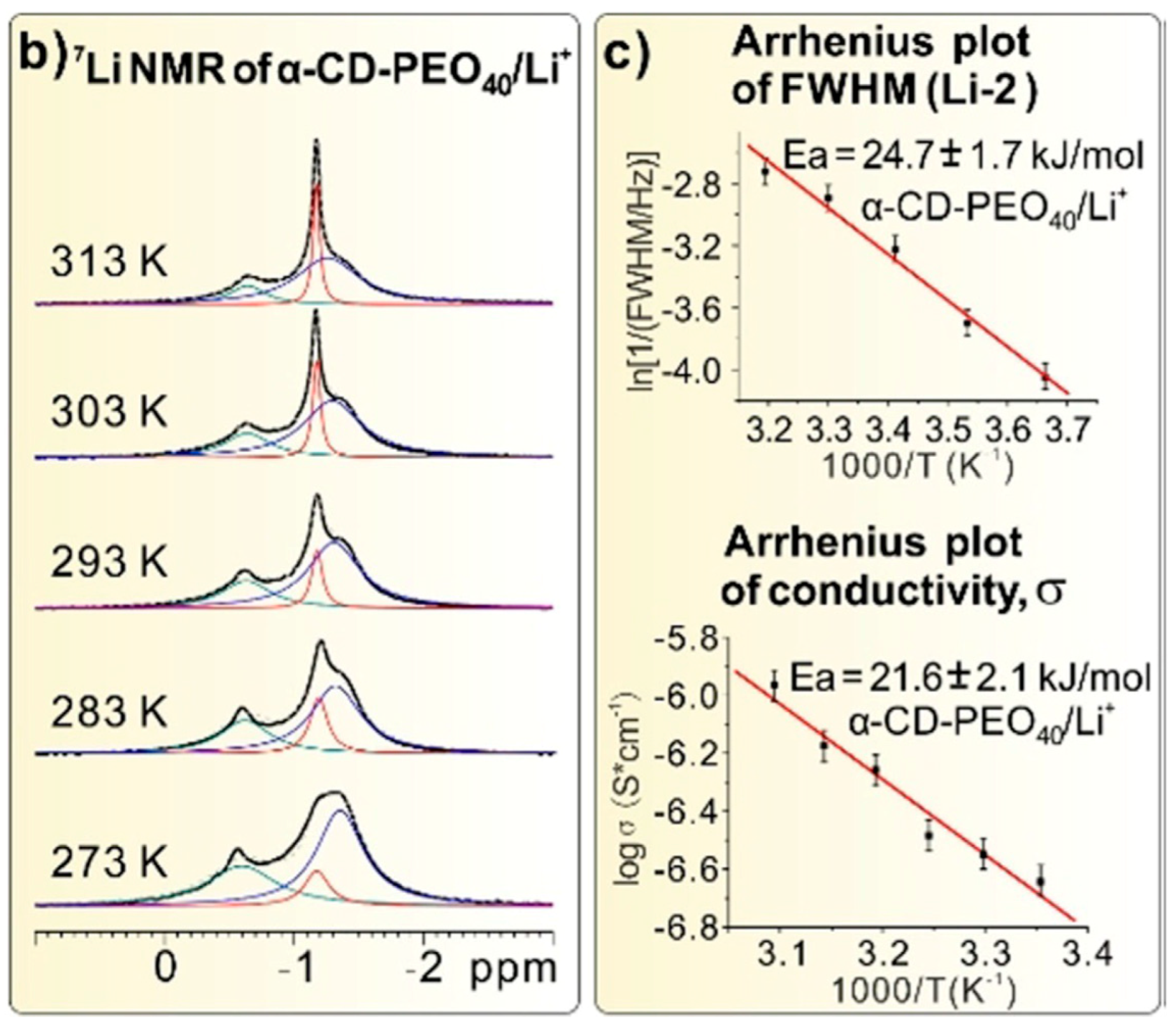

| Dry Polymer Electrolyte Type | Room Temperature Conductivity Scm−1 | Cathode Type Used | Cathode Loading Used (wt. % Active) | Areal Cathode Capacity | Test Fixture Format | Testing Temperature Used | # of Full/Deep Cycles Demonstrated |
|---|---|---|---|---|---|---|---|
| PEO/nanocomposite [21] | ~10−5 or lower | LiFePO4 (<3.5 V) | 60% | ~1 mAh/cm2 | Coin cell | 100 °C | 100 |
| Polyether/LiFTSI [22] | ~8 × 10−5 | LiFePO4 (<3.5 V) | 54% | Undisclosed | Coin cell | 80 °C | 1300 |
| PEO/nano particle composite [23] | ~5 × 10−5 | LiFePO4 (<3.5 V) | 63% | Undisclosed | Coin cell | 70 °C | 130 |
| Single-ion BAB triblock copolymer [24] | Lower than 10−6 | LiFePO4 (<3.5 V) | 60% | 8 mAh/cm2 | Coin cell | 80 °C | ~100 |
| Block Co-polymer (P3HT-PEO) [25] | ~10−5 or lower | LiFePO4 (<3.5 V) | 50% | Undisclosed | Coin cell | 90 °C | 10’s |
| Ordered Liquid Crystalline (meogen/Li salt) [26] | ~10−6 Scm−1 | LiFePO4 (<3.5 V) | 65% | Undisclosed | Coin cell | 60 °C | 30 |
| PEO/MEEGE [27] | ~ lower than 10−5 | LiFePO4 (<3.5 V) | 83% | Undisclosed | Pouch Cell | 60 °C | 250 |
| P(EO/MEEGE/AGE) [28] | lower than 10−5 | Nano-coated LiCoO2 | 82% | ~1 mAh/cm2 | Coin cell | 60 °C | 25 (not fully stable at cathode potentials) |
| PEM [29] | <10−3 | LiFePO4 | 80% | 0.8–1.5 mg/cm2 | Coin cell | ambient | 50 cycles (80% capacity after) |
| Interlinked solid polymer electrolyte [30] | ~10−4 | LiFePO4 (2.5–4 V) | Undisclosed | ~0.1 mAh/cm2 | Coin cell | 20 °C | 50 |
| Single ion triblock copolymer [31] | <10−7 | LiFePO4 | 60% | Undisclosed | Undisclosed | 70 °C | 300 (77% capacity retention) |
| Carbonate-linked PEO electrolyte [32] | <10−5 | LiFePO4 | 80% | 1.3–1.8 mAh/cm2 | Coin | 25 °C | 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munoz, S.; Greenbaum, S. Review of Recent Nuclear Magnetic Resonance Studies of Ion Transport in Polymer Electrolytes. Membranes 2018, 8, 120. https://doi.org/10.3390/membranes8040120
Munoz S, Greenbaum S. Review of Recent Nuclear Magnetic Resonance Studies of Ion Transport in Polymer Electrolytes. Membranes. 2018; 8(4):120. https://doi.org/10.3390/membranes8040120
Chicago/Turabian StyleMunoz, Stephen, and Steven Greenbaum. 2018. "Review of Recent Nuclear Magnetic Resonance Studies of Ion Transport in Polymer Electrolytes" Membranes 8, no. 4: 120. https://doi.org/10.3390/membranes8040120
APA StyleMunoz, S., & Greenbaum, S. (2018). Review of Recent Nuclear Magnetic Resonance Studies of Ion Transport in Polymer Electrolytes. Membranes, 8(4), 120. https://doi.org/10.3390/membranes8040120






