An Efficient Polymer Inclusion Membrane-Based Device for Cd Monitoring in Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Polymer Inclusion Membranes Preparation and Stability Test
2.3. Instrumentation
2.4. Transport and Preconcentration Experiments
3. Results
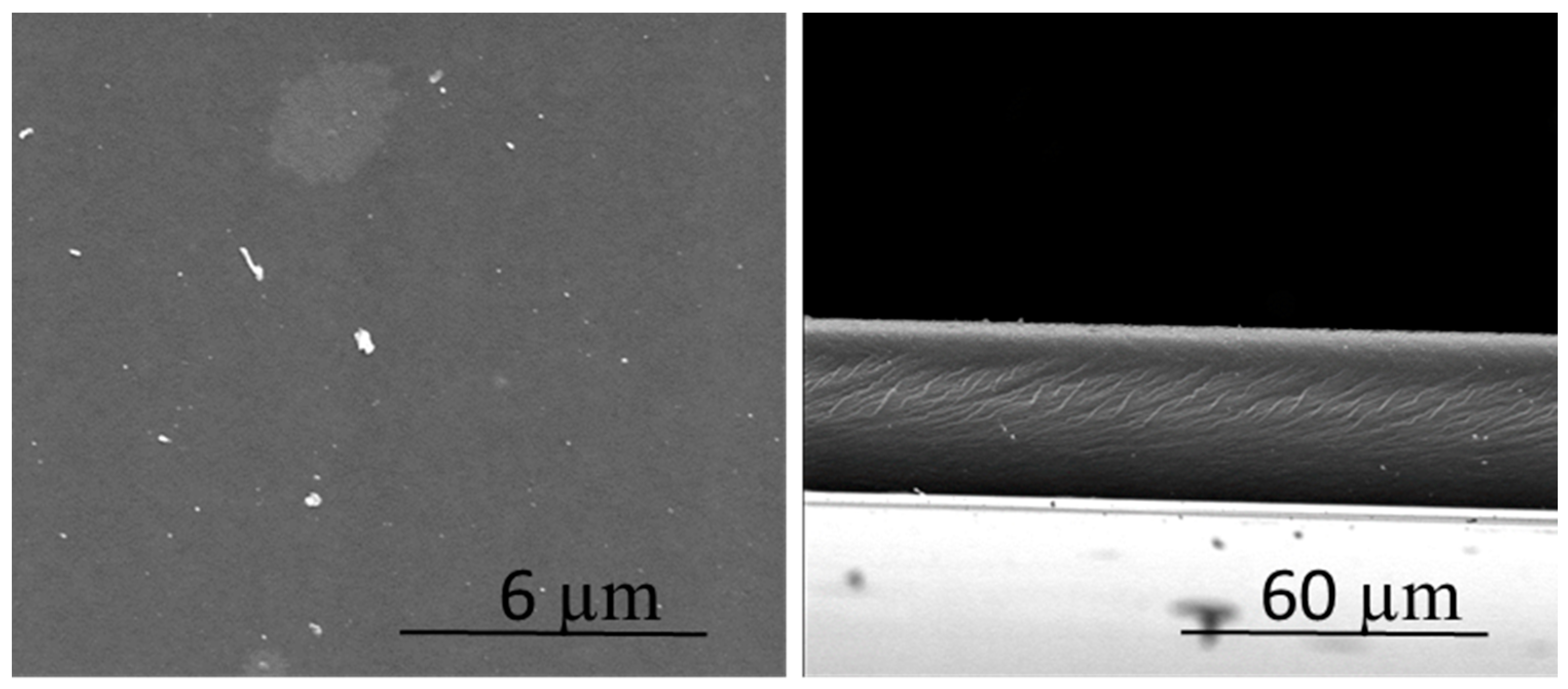
3.1. Characteristics of the Polymer Inclusion Membranes (PIMs)
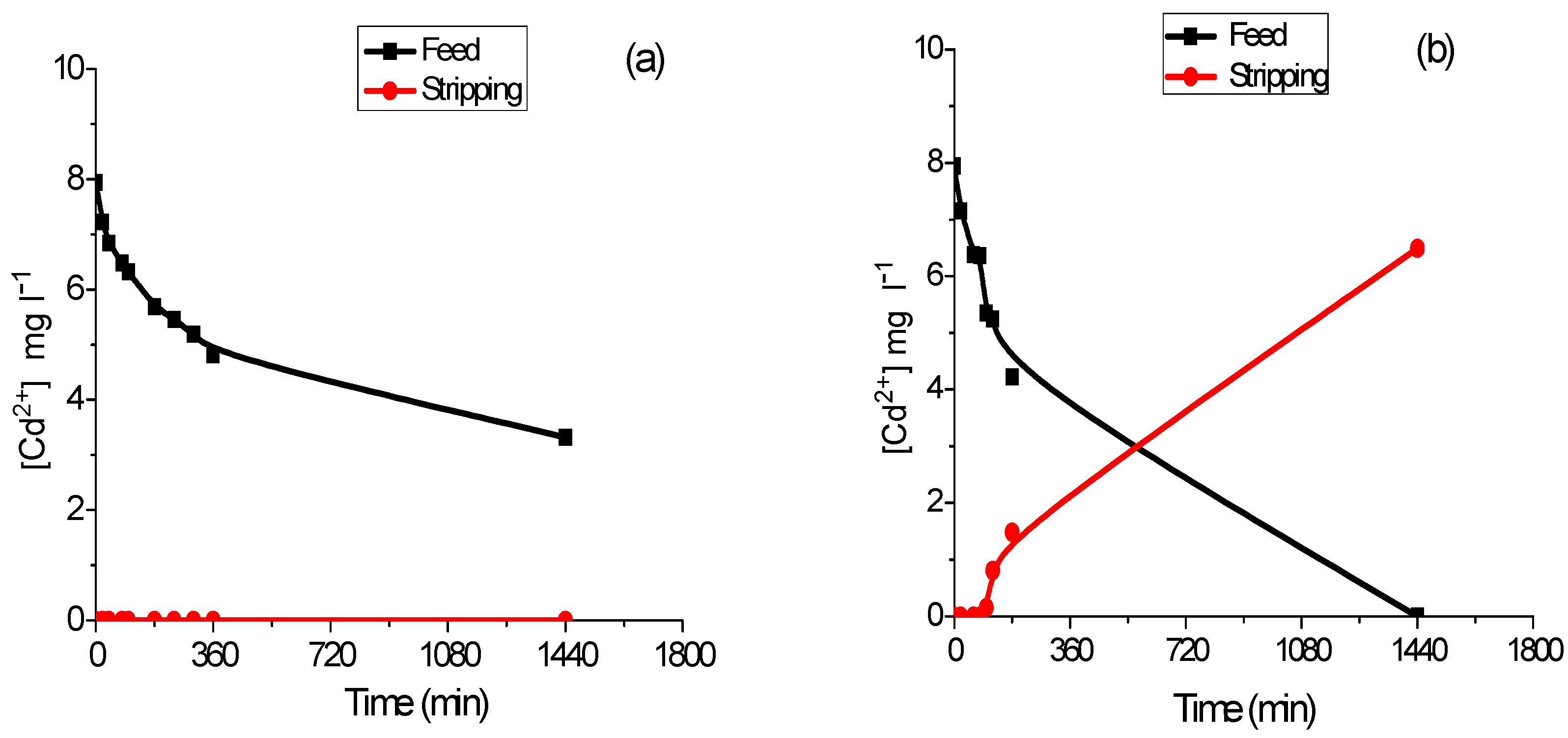
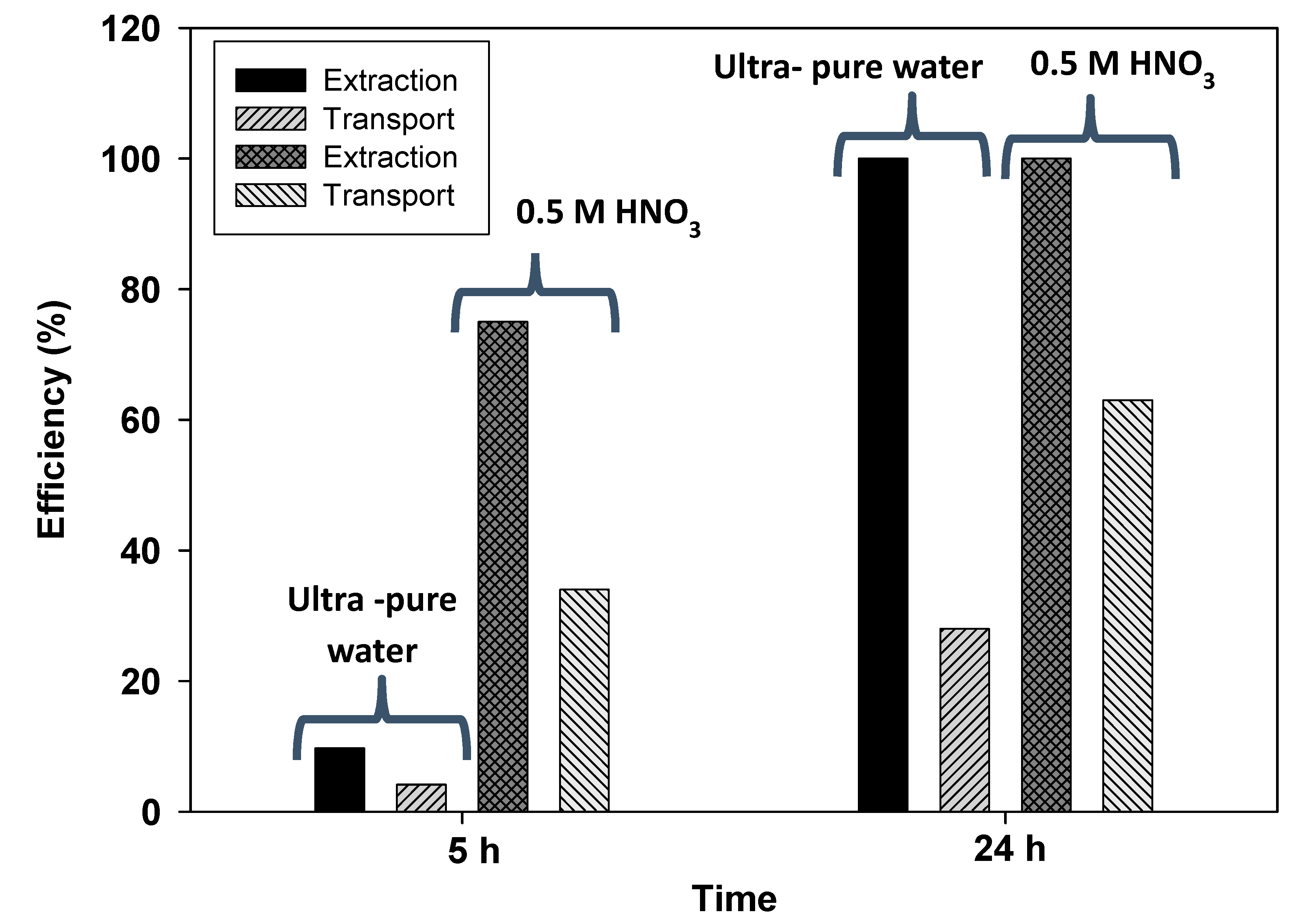
3.2. Transport Experiments
3.3. Cadmium Preconcentration Using the PIM-Device
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Angeletti, R.; Binato, G.; Guidotti, M.; Morelli, S.; Pastorelli, A.A.; Sagratella, E.; Ciardullo, S.; Stacchini, P. Cadmium bioaccumulation in mediterranean spider crab (Maya squinado): Human consumption and health implications for exposure in italian population. Chemosphere 2014, 100, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Herce-Sesa, B.; López-López, J.A.; Moreno, C. Ionic liquid solvent bar micro-extraction of CdCln(n−2)-species for ultra-trace Cd determination in seawater. Chemosphere 2018, 193, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Pont, N.; Salvadó, V.; Fontàs, C. Applicability of a supported liquid membrane in the enrichment and determination of cadmium from complex aqueous samples. Membranes 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Mahandra, H.; Gupta, B. Solvent extraction studies on cadmium and zinc using Cyphos IL 102 and recovery of zinc from zinc-plating mud. Hydrometallurgy 2017, 172, 11–18. [Google Scholar] [CrossRef]
- Pospiech, B. Studies on extraction and permeation of cadmium(II) using Cyphos IL 104 as selective extractant and ion carrier. Hydrometallurgy 2015, 154, 88–94. [Google Scholar] [CrossRef]
- Arias, A.; Saucedo, I.; Navarro, R.; Gallardo, V.; Martinez, M.; Guibal, E. Cadmium(II) recovery from hydrochloric acid solutions using Amberlite XAD-7 impregnated with a tetraalkyl phosphonium ionic liquid. React. Funct. Polym. 2011, 71, 1059–1070. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis—A review. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fontàs, C.; Vera, R.; Batalla, A.; Kolev, S.D.; Anticó, E. A novel low-cost detection method for screening of arsenic in groundwater. Environ. Sci. Pollut. Res. 2014, 21, 11682–11688. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodríguez, A.; Fontàs, C.; Matamoros, V.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Development of a polymer inclusion membrane-based passive sampler for monitoring of sulfamethoxazole in natural waters. Minimizing the effect of the flow pattern of the aquatic system. Microchem. J. 2016, 124, 175–180. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Chan, C.; Pettigrove, V.J.; Cattrall, R.W.; Kolev, S.D. Development of a passive sampler for Zinc(II) in urban pond waters using a polymer inclusion membrane. Environ. Pollut. 2014, 193, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.G.S.; Silva, A.M.L.; Coleman, R.A.; Pettigrove, V.J.; Cattrall, R.W.; Kolev, S.D. Development of a passive sampler based on a polymer inclusion membrane for total ammonia monitoring in freshwaters. Anal. Bioanal. Chem. 2016, 408, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodríguez, A.; Matamoros, V.; Kolev, S.D.; Fontàs, C. Development of a polymer inclusion membrane (PIM) for the preconcentration of antibiotics in environmental water samples. J. Membr. Sci. 2015, 492, 32–39. [Google Scholar] [CrossRef]
- Kagaya, S.; Ryokan, Y.; Cattrall, R.W.; Kolev, S.D. Stability studies of poly(vinyl chloride)-based polymer inclusion membranes containing Aliquat 336 as a carrier. Sep. Purif. Technol. 2012, 101, 69–75. [Google Scholar] [CrossRef]
- Fontàs, C.; Salvadó, V.; Hidalgo, M. Selective enrichment of palladium from spent automotive catalysts by using a liquid membrane system. J. Membr. Sci. 2003, 223, 39–48. [Google Scholar] [CrossRef]
- Vera, R.; Fontàs, C.; Galceran, J.; Serra, O.; Anticó, E. Polymer inclusion membrane to access Zn speciation: Comparison with root uptake. Sci. Total Environ. 2018, 622, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Fontàs, C.; Tayeb, R.; Dhahbi, M.; Gaudichet, E.; Thominette, F.; Roy, P.; Steenkeste, K.; Fontaine-Aupart, M.-P.; Tingry, S.; Tronel-Peyroz, E.; et al. Polymer inclusion membranes: The concept of fixed sites membrane revised. J. Membr. Sci. 2007, 290, 62–72. [Google Scholar] [CrossRef]
- Argiropoulos, G.; Cattrall, R. The study of a membrane for extracting gold (III) from hydrochloric acid solutions. J. Membr. Sci. 1998, 138, 279–285. [Google Scholar] [CrossRef]
- Pont, N.; Salvadó, V.; Fontàs, C. Selective transport and removal of Cd from chloride solutions by polymer inclusion membranes. J. Membr. Sci. 2008, 318, 340–345. [Google Scholar] [CrossRef]
- Vera, R.; Gelde, L.; Anticó, E.; Martínez de Yuso, M.V.; Benavente, J.; Fontàs, C. Tuning physicochemical, electrochemical and transport characteristics of polymer inclusion membrane by varying the counter-anion of the ionic liquid Aliquat 336. J. Membr. Sci. 2017, 529, 87–94. [Google Scholar] [CrossRef]
| PIM | Polymer (CTA) (%) | Carrier (THTDPCl) (%) | Plasticizer | TE (%) | ||
|---|---|---|---|---|---|---|
| Content (%) | Viscosity (cP) | Dielectric Constant (ε) | ||||
| 1 | 70 | 30 | 0 | - | - | 0 |
| 2 | 50 | 30 | NPOE (20) | 12.8 | 23.1 | 82.2 |
| 3 | 30 | FPNPOE (20) | 13 | 50 | 71.8 | |
| 4 | 30 | DBS (20) | 9.5 | 4.5 | 84.1 | |
| Sample | Cd Stripping Phase (µg L−1) | TE (%) | Recovery (%) ± SD |
|---|---|---|---|
| 0.5 M NaCl + 10 µg L−1 Cd | 238.33 | 119 | 108 ± 15 |
| 194.30 | 98 | ||
| Seawater + 10 µg L−1 Cd | 202.20 | 101 | 99 ± 4 |
| 191.43 | 96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Khaldoun, I.; Mitiche, L.; Sahmoune, A.; Fontàs, C. An Efficient Polymer Inclusion Membrane-Based Device for Cd Monitoring in Seawater. Membranes 2018, 8, 61. https://doi.org/10.3390/membranes8030061
Ait Khaldoun I, Mitiche L, Sahmoune A, Fontàs C. An Efficient Polymer Inclusion Membrane-Based Device for Cd Monitoring in Seawater. Membranes. 2018; 8(3):61. https://doi.org/10.3390/membranes8030061
Chicago/Turabian StyleAit Khaldoun, Ibrahim, Lynda Mitiche, Amar Sahmoune, and Clàudia Fontàs. 2018. "An Efficient Polymer Inclusion Membrane-Based Device for Cd Monitoring in Seawater" Membranes 8, no. 3: 61. https://doi.org/10.3390/membranes8030061
APA StyleAit Khaldoun, I., Mitiche, L., Sahmoune, A., & Fontàs, C. (2018). An Efficient Polymer Inclusion Membrane-Based Device for Cd Monitoring in Seawater. Membranes, 8(3), 61. https://doi.org/10.3390/membranes8030061






