Current and Emerging Techniques for High-Pressure Membrane Integrity Testing
Abstract
1. Introduction
1.1. Log Reduction Value
1.2. The Target—Poliovirus
1.3. The “Ideal” Integrity Test
2. Direct Integrity Monitoring
2.1. Vacuum Decay Testing
2.2. Pressure Decay Testing
3. Indirect Integrity Monitoring
3.1. Naturally Occurring Substances
3.1.1. Particle and Turbidity Monitoring
3.1.2. Total Organic Carbon
3.1.3. Sulphate Monitoring
3.1.4. Electrical Conductivity
3.1.5. Periodic Testing
3.2. Challenge Testing
3.2.1. Dyes and Tracer Chemicals
3.2.2. Spiked Integrity Monitoring
3.2.3. Pulse Integrity Testing
3.2.4. Microbial Surrogates
3.2.5. Non-Microbial Nanoparticle Surrogates
4. Integrated and Multi-Parameter Monitoring Systems
4.1. TRASAR® Testing
4.2. Small Sensor Cell Membrane Testing
4.3. Binary Gas Integrity Testing
4.4. ZAPS LiquID Station
5. Emerging Techniques
5.1. Pathogen Detection Systems
5.1.1. BioSentry Device
5.1.2. Real-Time Polymerase Chain Reaction Monitoring
5.1.3. Evanescent Wave Fiber Optic Sensors
5.1.4. Surface Generated Acoustic Wave Biosensors
5.1.5. RAPTOR Fiber Optic Biosensors
5.1.6. Block II Chemical Biological Mass Spectrometer
5.1.7. Miniaturized Portable Biosensors
5.1.8. Microarray Biosensors
5.1.9. Surface Plasmon Resonance Biosensors
5.1.10. Quantum Dot Based DNA Nanosensors
5.1.11. Laser Scanning Cytometry
5.1.12. Microfluidic Biochip Systems
5.2. Other Detection Systems
5.2.1. NanoSight Particle Tracking
5.2.2. Online Chemical Oxygen Demand
5.2.3. Whispering Gallery Microlasers
5.2.4. Fluorescence Emission Excitation Spectroscopy
5.2.5. Quantum Dots
6. Assessing the Current and Emerging Techniques
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bartels, C.R.; Wilf, M.; Andes, K.; Iong, J. Design considerations for wastewater treatment by reverse osmosis. Water Sci. Technol. 2005, 51, 473–482. [Google Scholar] [CrossRef] [PubMed]
- DeCarolis, J.; Adham, S.; Kumar, M.; Pearce, B.; Wasserman, L. Integrity and Performance Evaluation of New Generation Desalting Membranes During Municipal Wastewater Reclamation. In Proceedings of the Water Environment Federation (WEFTEC 2005), Washington, DC, USA, 29 October–2 November 2005; pp. 3518–3529. [Google Scholar]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, H.F.; Gale, J.D.; Hughes, Z.E.; Stewart, M.B.; Orbell, J.D.; Gray, S.R. Molecular scale modeling of membrane water treatment processes. In Functional Nanostructured Materials and Membranes for Water Treatment; Duke, M., Zhao, D., Semiat, R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; Volume 10, pp. 249–299. [Google Scholar]
- Paul, D.R. Reformulation of the solution-diffusion theory of reverse osmosis. J. Membr. Sci. 2004, 241, 371–386. [Google Scholar] [CrossRef]
- Adham, S.; Gagliardo, P.; Smith, D.; Ross, D.; Gramith, K.; Trussell, R. Monitoring the integrity of reverse osmosis membranes. Desalination 1998, 119, 143–150. [Google Scholar] [CrossRef]
- Crozes, G.F.; Sethi, S.; Mi, B.; Curl, J.; Mariñas, B. Improving membrane integrity monitoring indirect methods to reduce plant downtime and increase microbial removal credit. Desalination 2002, 149, 493–497. [Google Scholar] [CrossRef]
- Kitis, M.; Lozier, J.C.; Kim, J.H.; Mi, B.; Marinas, B.J. Microbial removal and integrity monitoring of RO and NF membranes. J. Am. Water Works Assoc. 2003, 95, 105–119. [Google Scholar] [CrossRef]
- Lozier, J.; Kitis, M.; Colvin, C.; Kim, J.-H.; Mi, B.; Marinas, B. Microbial Removal and Integrity Monitoring of High-Pressure Membranes; IWA Publishing: London, UK, 2003; p. 220. [Google Scholar]
- Surawanvijit, S.; Thompson, J.; Rahardianto, A.; Frenkel, V.; Cohen, Y. Pulsed marker method for real-time detection of reverse osmosis membrane integrity loss. Desalination 2015, 370, 25–32. [Google Scholar] [CrossRef]
- Huang, X.; Min, J.H.; Lu, W.; Jaktar, K.; Yu, C.; Jiang, S.C. Evaluation of methods for reverse osmosis membrane integrity monitoring for wastewater reuse. J. Water Proc. Eng. 2015, 7, 161–168. [Google Scholar] [CrossRef]
- Min, J.H.; Yu, C.; Jiang, S. New Techniques for Real-Time Monitoring of Membrane Integrity for Virus Removal Using Submicron Particle Characterization Methods. Water Reuse Foundation Report WRF-09-06a-1. 2013, p. 92. Available online: https://watereuse.org/watereuse-research/09-06a-new-techniques-for-real-time-monitoring-of-membrane-integrity-for-virus-removal-using-submicron-particle-characterization-methods/ (accessed on 7 August 2018).
- Zhang, J.; Cran, M.; Northcott, K.; Packer, M.; Duke, M.; Milne, N.; Scales, P.; Knight, A.; Gray, S.R. Assessment of pressure decay test for RO protozoa removal validation in remote operations. Desalination 2016, 386, 19–24. [Google Scholar] [CrossRef]
- Victorian Government Department of Health. Guidelines for validating treatment processes for pathogen reduction. In Supporting Class A Recycled Water Schemes in Victoria; Department of Health, Australia: Canberra, Australia, 2013; p. 96. [Google Scholar]
- Kumar, M.; Adham, S.; DeCarolis, J. Reverse osmosis integrity monitoring. Desalination 2007, 214, 138–149. [Google Scholar] [CrossRef]
- Casani, S.; Hansen, T.B.; Christensen, J.; Knøchel, S. Comparison of methods for assessing reverse osmosis membrane treatment of shrimp process water. J. Food Prot. 2005, 68, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Gitis, V.; Adin, A.; Nasser, A.; Gun, J.; Lev, O. Fluorescent dye labeled bacteriophages—A new tracer for the investigation of viral transport in porous media: 1. Introduction and characterization. Water Res. 2002, 36, 4227–4234. [Google Scholar] [CrossRef]
- DeCarolis, J.; Adham, S.; Kumar, M.; Hirani, Z. Reverse Osmosis Membrane Integrity; MWH Global Inc.: Denver, CO, USA, 2006; p. 117. [Google Scholar]
- Antony, A.; Blackbeard, J.; Leslie, G. Removal efficiency and integrity monitoring techniques for virus removal by membrane processes. Crit. Rev. Environ. Sci. Technol. 2012, 42, 891–933. [Google Scholar] [CrossRef]
- Pype, M.-L.; Lawrence, M.G.; Keller, J.; Gernjak, W. Reverse osmosis integrity monitoring in water reuse: The challenge to verify virus removal—A review. Water Res. 2016, 98, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Lidén, A.; Lavonen, E.; Persson, K.M.; Larson, M. Integrity breaches in a hollow fiber nanofilter—Effects on natural organic matter and virus-like particle removal. Water Res. 2016, 105, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A. Drinking water: Pathogen removal from water—Technologies and techniques. Filtr. Separ. 2008, 45, 14–16. [Google Scholar] [CrossRef]
- Hill, D. Basic Microbiology for Drinking Water Personnel; American Water Works Association: Denver, CO, USA, 2006. [Google Scholar]
- Adu, F.D. The virology of the polio virus. Ann. Ibadan Postgrad. Med. 2005, 3, 13–19. [Google Scholar] [CrossRef][Green Version]
- American Water Works Association. Manual of Water Supply Practices (M48): Waterborne Pathogens; AWWA: Denver, CO, USA, 1999. [Google Scholar]
- U.S. Environmental Protection Agency. Membrane Filtration Guidance Manual; EPA 815-R-06-009; U.S. Environmental Protection Agency: Washington, DC, USA, 2005; p. 332.
- ASTM D 3923-08. Standard Practices for Detecting Leaks in Reverse Osmosis and Nanofiltration Devices. 2014. Available online: http://www.astm.org/Standards/D3923.htm (accessed on 7 August 2018).
- ASTM D 6908-06. Standard Practice for Integrity Testing of Membrane Filtration Systems. 2017. Available online: http://www.astm.org/Standards/D6908.htm (accessed on 7 August 2018).
- Kruithof, J.C.; Kamp, P.C.; Folmer, H.C.; Nederlof, M.M.; van Hoof, S.C.J.M. Development of a membrane integrity monitoring strategy for the UF/RO Heemskerk drinking water treatment plant. Water Sci. Technol. Water Suppl. 2001, 1, 261–271. [Google Scholar] [CrossRef]
- Eriksson, P.; Mueller, P. Vacuum Decay test of membrane systems with spiral wound elements. In Proceedings of the AWWA Membrane Technology Conference, Atlanta, GA, USA, 2–5 March 2003. [Google Scholar]
- Pearce, B.; DeCarolis, J.; Wetterau, G. Evaluation of performance monitoring methods for advanced water purification processes: City of San Diego. In Proceedings of the 16th Annual Wate Reuse & Desalination Research Conference, San Diego, CA, USA, 4–5 June 2012. [Google Scholar]
- Jons, S.D. Method of Testing the Integrity of Spiral Wound Modules. U.S. Patent 2011/0091094A1, 2011. Available online: https://patents.google.com/patent/US20110091094A1 (accessed on 7 August 2018).
- Wilbert, M.C.; Linton, K. Methods for Monitoring the Integrity of Reverse Osmosis and Nanofiltration Membrane Systems; US Department of Interior: Denver, CO, USA, 2000; p. 239.
- Zhang, J.; Knight, A.; Duke, M.; Northcott, K.; Packer, M.; Scales, P.J.; Gray, S.R. A new integrated potable reuse process for a small remote community in Antarctica. Proc. Saf. Environ. Protect. 2016, 104, 196–208. [Google Scholar] [CrossRef]
- Johnson, J.; Busch, M. Engineering aspects of reverse osmosis module design. Desal. Water Treat. 2010, 15, 236–248. [Google Scholar] [CrossRef]
- Banerjee, A.; Lambertson, M.; Lozier, J.; Colvin, C. Monitoring membrane integrity using high sensitivity laser turbidimetry. Water Sci. Technol. Water Suppl. 2001, 1, 273–276. [Google Scholar] [CrossRef]
- Naismith, J. Membrane integrity—Direct turbidity measurement of filtrate from mf membrane modules at an operating potable water treatment plant. Desalination 2005, 179, 25–30. [Google Scholar] [CrossRef]
- Seah, H. Newater: Regulatory Framework in Singapore. In Proceedings of the International Conference on Water Reuse for Drinking Purposes, Umhlanga, KwaZulu-Natal, South Africa, 9–11 October 2012. [Google Scholar]
- Pype, M.-L.; Patureau, D.; Wery, N.; Poussade, Y.; Gernjak, W. Monitoring reverse osmosis performance: Conductivity versus fluorescence excitation–emission matrix (EEM). J. Membr. Sci. 2013, 428, 205–211. [Google Scholar] [CrossRef]
- Hydranautics. Procedure for Pressure Vessel Probing, Technical Service Bulletin TSB114.03. Available online: http://www.membranes.com/docs/tsb/tsb114.pdf (accessed on 19 November 2012).
- Montgomery Watson. Water Re-Purification Project: Final Report on Membrane Prequalification Pilot Study; MWH Global Inc.: Denver, CO, USA, 1997; p. 121. [Google Scholar]
- Binet, S.; Jomard, H.; Lebourg, T.; Guglielmi, Y.; Tric, E.; Bertrand, C.; Mudry, J. Experimental analysis of groundwater flow through a landslide slip surface using natural and artificial water chemical tracers. Hydrol. Process. 2007, 21, 3463–3472. [Google Scholar] [CrossRef]
- Harden, H.S.; Chanton, J.P.; Rose, J.B.; John, D.E.; Hooks, M.E. Comparison of sulfur hexafluoride, fluorescein and rhodamine dyes and the bacteriophage prd-1 in tracing subsurface flow. J. Hydrol. 2003, 277, 100–115. [Google Scholar] [CrossRef]
- Ford, D.E.; Thornton, K.W. Lissamine ff fluorescent dye. J. Environ. Eng. 1983, 109, 952–955. [Google Scholar] [CrossRef]
- Chua, L.H.C.; Robertson, A.P.; Yee, W.K.; Shuy, E.B.; Lo, E.Y.M.; Lim, T.T.; Tan, S.K. Use of fluorescein as a ground water tracer in brackish water aquifers. Ground Water 2007, 45, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Stanbro, W.D.; Pyrch, D.A. Stability of rhodamine WT in saline waters. Water Resour. Res. 1979, 15, 1631–1632. [Google Scholar] [CrossRef]
- Flury, M.; Fluehler, H. Tracer characteristics of Brilliant Blue FCF. Soil Sci. Soc. Am. J. 1995, 59, 22–27. [Google Scholar] [CrossRef]
- Reinken, G.; Kale, S.P.; Fuehr, F. Distribution pattern of organic carbon in soils and its role in preferential movement of brilliant blue in a structured silty soil. Sci. Soils. 1996. Available online: http://hintze-online.com/sos/1996/Articles/Art2/art2.html (accessed on 9 August 2018).
- Smart, P.L.; Laidlaw, I.M.S. An evaluation of some fluorescent dyes for water tracing. Water Resour. Res. 1977, 13, 15–33. [Google Scholar] [CrossRef]
- Sutton, D.J.; Kabala, Z.J.; Francisco, A.; Vasudevan, D. Limitations and potential of commercially available rhodamine WT as a groundwater tracer. Water Resour. Res. 2001, 37, 1641–1656. [Google Scholar] [CrossRef]
- Magal, E.; Weisbrod, N.; Yakirevich, A.; Yechieli, Y. The use of fluorescent dyes as tracers in highly saline groundwater. J. Hydrol. 2008, 358, 124–133. [Google Scholar] [CrossRef]
- YSI Environmental. Water Tracing, in Situ Dye Fluorometry and the YSI 6130 Rhodamine WT Sensor. Available online: http://www.ysi.com (accessed on 3 February 2012).
- Aqualab Scientific. Rhodamine WT. Available online: http://www.aqualab.com.au/products/rhodamine-wt.html (accessed on 12 January 2010).
- Kitis, M.; Lozier, J.C.; Kim, J.H.; Mi, B.; Marinas, B.J. Evaluation of biologic and non-biologic methods for assessing virus removal by and integrity of high pressure membrane systems. Water Sci. Technol. Water Suppl. 2003, 3, 81–92. [Google Scholar] [CrossRef]
- Australian Water Recycling Centre of Excellence. Roadmap for a National Validation Framework; Water Quality Research Australia Limited: Adelaide, Australia, 2011; Available online: http://www.australianwaterrecycling.com.au/coe/images/stories/publications/NatVal_Road_Map_Report_2011.pdf (accessed on 19 July 2012).
- Lozier, J.C.; Zornes, G.L.; Jansen, E. Validation testing of a full-scale RO system for pathogen removal. In Proceedings of the IDA World Congress, Perth, WA, USA, 4–9 September 2011. [Google Scholar]
- Lozier, J.C.; Edwards, B.; Scholes, E. Use and suitability of dye-based pathogen integrity testing of RO systems within advanced water recycling schemes. In Proceedings of the AWA Water Recycling Conference, Brisbane, Australia, 1–4 July 2013; p. 10. [Google Scholar]
- Ostarcevic, E.; Cran, M.; Gray, S. Screening fluorescent chemicals and nanoparticles as potential surrogates for real-time reverse osmosis integrity monitoring. In Proceedings of the AWA Water Recycling Conference, Brisbane, Australia, 1–4 July 2013; p. 8. [Google Scholar]
- Van Hoof, S.C.J.M.; Broens, L.; Nahrstedt, A.; Panglisch, S.; Gimbel, R. Development of a new integrity testing system. Water Sci. Technol. Water Suppl. 2003, 3, 101–108. [Google Scholar] [CrossRef]
- Jons, S.D.; Haynes, T.N.; Nathan, J.S. Sensitive integrity test for RO/NF elements. In Proceedings of the International Water Conference, Orlando, FL, USA, 9–13 October 2005. [Google Scholar]
- Jons, S.D.; Johnson, J.E.; Fialkowski, M.A. Method for Testing Separation Modules. U.S. Patent US 7,698,928 B2, 2010. Available online: https://patents.google.com/patent/US7698928B2 (accessed on 8 August 2018).
- Langlet, J.; Gaboriaud, F.; Gantzer, C. Effects of ph on plaque forming unit counts and aggregation of ms2 bacteriophage. J. Appl. Microbiol. 2007, 103, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Eaton, C.L.; Kim, J.-H.; Colvin, C.K.; Lozier, J.C.; Marinas, B.J. Removal of biological and non-biological viral surrogates by spiral-wound reverse osmosis membrane elements with intact and compromised integrity. Water Res. 2004, 38, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Jacangelo, J.G.; Laine, J.M.; Carns, K.E.; Cummings, E.W.; Mallevialle, J. Low-pressure membrane filtration for removing giardia and microbial indicators. J. Am. Water Works Assoc. 1991, 83, 97–106. [Google Scholar] [CrossRef]
- Wu, B.; Wang, R.; Fane, A.G. The roles of bacteriophages in membrane-based water and wastewater treatment processes: A review. Water Res. 2017, 110, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Pype, M.-L.; Donose, B.C.; Marti, L.; Patureau, D.; Wery, N.; Gernjak, W. Virus removal and integrity in aged RO membranes. Water Res. 2016, 90, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Jacangelo, J.G.; Brown, N.L.P.; Madec, A.; Schwab, K.; Huffman, D.; Amy, G.; Mysore, C.; Leparc, J.; Prescott, A. Micro- and Ultrafiltration Performance Specifications Based on Microbial Removal; IWA Publishing: London, UK, 2006; p. 10. [Google Scholar]
- Adham, S.S.; Trussell, R.S.; Gagliardo, P.F.; Trussell, R.R. Rejection of ms-2 virus by RO membranes. J. Am. Water Works Assoc. 1998, 90, 130–135. [Google Scholar] [CrossRef]
- Colvin, C.K.; Acker, C.L.; Marinas, B.J.; Lozier, J.C. Microbial removal by NF/RO. In Proceedings of the American Water Works Association Annual Conference, Denver, CO, USA, 11–15 June 2000; pp. 1275–1293. [Google Scholar]
- Acker, C.L.; Colvin, C.K.; Marinas, B.J.; Lozier, J.C. Assessing the integrity of reverse osmosis spiral-wound membrane elements with biological and non-biological surrogate indicators. In Membrane Practices for Water Treatment; Duranceau, S.J., Ed.; American Water Works Association: Denver, CO, USA, 2001; Volume 10, pp. 464–484. [Google Scholar]
- California Department of Public Health. California Surface Water Treatment Rule, Alternative Filtration Technology Demonstration Report; California Department of Public Health: Sacramento, CA, USA, 2001; p. 75.
- DeCarolis, J.; Adham, S.; Hirani, Z.; Pearce, W. Integrity evaluation of new generation reverse osmosis membranes during municipal wastewater reclamation. In Proceedings of the Water Quality Technology Conference, Denver, CO, USA, 5–9 November 2006. [Google Scholar]
- Pontius, F.W.; Amy, G.L.; Hernandez, M.T. Fluorescent microspheres as virion surrogates in low-pressure membrane studies. J. Membr. Sci. 2009, 335, 43–50. [Google Scholar] [CrossRef]
- Gitis, V.; Haught, R.C.; Clark, R.M.; Gun, J.; Lev, O. Application of nanoscale probes for the evaluation of the integrity of ultrafiltration membranes. J. Membr. Sci. 2006, 276, 185–192. [Google Scholar] [CrossRef]
- Deluhery, J.; Rajagopalan, N. Use of paramagnetic particles in membrane integrity testing. J. Membr. Sci. 2008, 318, 176–181. [Google Scholar] [CrossRef]
- Guo, H.; Wyart, Y.; Perot, J.; Nauleau, F.; Moulin, P. Application of magnetic nanoparticles for UF membrane integrity monitoring at low-pressure operation. J. Membr. Sci. 2010, 350, 172–179. [Google Scholar] [CrossRef]
- Choi, S.H.; Yang, J.; Suh, C.; Cho, J. Use of fluorescent silica particles for checking the integrity of microfiltration membranes. J. Membr. Sci. 2011, 367, 306–313. [Google Scholar] [CrossRef]
- Lohwacharin, J.; Takizawa, S. Effects of nanoparticles on the ultrafiltration of surface water. J. Membr. Sci. 2009, 326, 354–362. [Google Scholar] [CrossRef]
- O’Malley, E. Fate and toxicity of engineered nanomaterials in treated wastewater. Water J. Aust. Water Assoc. 2015, 42, 66–70. [Google Scholar]
- Koh, I.; Josephson, L. Magnetic nanoparticle sensors. Sensors 2009, 9, 8130–8145. [Google Scholar] [CrossRef] [PubMed]
- Lawler, W.; Antony, A.; Cran, M.; Duke, M.; Leslie, G.; Le-Clech, P. Production and characterisation of UF membranes by chemical conversion of used RO membranes. J. Membr. Sci. 2013, 447, 203–211. [Google Scholar] [CrossRef]
- Zeiher, E.H.K.; Ho, B.; Williams, K.D. A fluorescent technology to ensure RO antiscalant performance. Ultrapure Water 2003, 20, 36–43. [Google Scholar]
- Fane, T. Monitoring membrane processes for improved performance. In Proceedings of the AWA Membranes and Desalination Specialty Conference, Sydney, Australia, 11–13 February 2009. [Google Scholar]
- Giglia, S.; Krishnan, M. High sensitivity binary gas integrity test for membrane filters. J. Membr. Sci. 2008, 323, 60–66. [Google Scholar] [CrossRef]
- Giglia, S.; Krishnan, M. New binary gas integrity test improves membrane quality assurance. BioPharm Int. 2011, 24, 24–28. [Google Scholar]
- ZAPS Technologies Inc. Technical Specification Sheet: The LiquIDTM Station. Available online: http://www.zapstechnologies.com/wp-content/uploads/2012/05/LiquID-Tech-Specifications-July-2013.pdf (accessed on 10 October 2013).
- Optimos Solutions. Solutions/ZAPS Technologies. Available online: http://www.optimos.com.au_zaps-liquid.php (accessed on 10 October 2013).
- ZAPS Technologies Inc. Automated Online Water Qualtiy Monitoring. Available online: http://www.zapstechnologies.com/wp-content/uploads/2013/02/LiquID-2013-Product-Brochure.pdf (accessed on 10 October 2013).
- ZAPS Technologies Inc. Real-Time E. coli Monitoring with the Liquid Station. Available online: http://www.zapstechnologies.com/wp-content/uploads/2012/07/Monitoring-E.coli-in-the-Creek.pdf (accessed on 10 October 2013).
- ZAPS Technologies Inc. Raw Water Monitoring and Event Detection. Available online: http://www.zapstechnologies.com/wp-content/uploads/2012/05/ZAPS-RW-Monitoring-and-Event-Detection.pdf (accessed on 10 October 2013).
- Ivnitski, D.; Abdel-Hamid, I.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Mukundan, H.; Anderson, A.S.; Grace, W.K.; Grace, K.M.; Hartman, N.; Martinez, J.S.; Swanson, B.I. Waveguide-based biosensors for pathogen detection. Sensors 2009, 9, 5783–5809. [Google Scholar] [CrossRef] [PubMed]
- Irudayaraj, J. Pathogen sensors. Sensors 2009, 9, 8610–8612. [Google Scholar] [CrossRef] [PubMed]
- Pejcic, B.; De Marco, R.; Parkinson, G. The role of biosensors in the detection of emerging infectious diseases. Analyst 2006, 131, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Adams, J. Shedding light on continuous water monitoring. J. Am. Water Works Assoc. 2009, 101, 46–48. [Google Scholar] [CrossRef]
- JMar. Contamination Warning and Process Control Device for Waterborne Microorganisms. Available online: http://www.jmar.com/wordpress/?page_id=329 (accessed on 6 September 2013).
- Shirasaki, N.; Matsushita, T.; Matsui, Y.; Kobuke, M.; Ohno, K. Comparison of removal performance of two surrogates for pathogenic waterborne viruses, bacteriophage qβ and MS2, in a coagulation-ceramic microfiltration system. J. Membr. Sci. 2009, 326, 564–571. [Google Scholar] [CrossRef]
- Byrne, B.; Stack, E.; Gilmartin, N.; O’Kennedy, R. Antibody-based sensors: Principles, problems and potential for detection of pathogens and associated toxins. Sensors 2009, 9, 4407–4445. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.V. Detection of microorganisms and toxins with evanescent wave fiber-optic biosensors. Proc. IEEE 2003, 91, 902–907. [Google Scholar] [CrossRef]
- Lee, W.E.; Thompson, H.G. Detection of Newcastle disease virus using an evanescent wave inamno-based biosensor. Can. J. Chem. 1996, 74, 707–712. [Google Scholar] [CrossRef]
- Taitt, C.R.; Anderson, G.P.; Ligler, F.S. Evanescent wave fluorescence biosensors. Biosens. Bioelectron. 2005, 20, 2470–2487. [Google Scholar] [CrossRef] [PubMed]
- Bisoffi, M.; Hjelle, B.; Brown, D.C.; Branch, D.W.; Edwards, T.L.; Brozik, S.M.; Bondu-Hawkins, V.S.; Larson, R.S. Detection of viral bioagents using a shear horizontal surface acoustic wave biosensor. Biosens. Bioelectron. 2008, 23, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Gaso, M.I.; March-Iborra, C.; Montoya-Baides, A.; Arnau-Vives, A. Surface generated acoustic wave biosensors for the detection of pathogens: A review. Sensors 2009, 9, 5740–5769. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.P.; Rowe-Taitt, C.A.; Ligler, F.S. Raptor: A portable, automated biosensor. In Proceedings of the First Joint Conference on Point Detection for Chemical and Biological Defense, Williamsburg, VA, USA, 23–27 October 2000; p. 7. [Google Scholar]
- Nanduri, V.; Kim, G.; Morgan, M.; Ess, D.; Hahm, B.-K.; Kothapalli, A.; Valadez, A.; Geng, T.; Bhunia, A. Antibody immobilization on waveguides using a flow–through system shows improved Listeria monocytogenes detection in an automated fiber optic biosensor: Raptor™. Sensors 2006, 6, 808–822. [Google Scholar] [CrossRef]
- Kramer, M.F.; Lim, D.V. A rapid and automated fiber optic-based biosensor assay for the detection of salmonella in spent irrigation water used in the sprouting of sprout seeds. J. Food Prot. 2004, 67, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, S.D.; Lim, D.V. Rapid ultrafiltration concentration and biosensor detection of Enterococci from large volumes of Florida recreational water. Appl. Environ. Microbiol. 2008, 74, 4792–4798. [Google Scholar] [CrossRef] [PubMed]
- Griest, W.H.; Wise, M.B.; Hart, K.J.; Lammert, S.A.; Hryncewich, A.P.; Sickenberger, D.W. The block ii chemical biological mass spectrometer—Point detection for both chemical and biological warfare agents. In Proceedings of the First Joint Conference on Point Detection for Chemical and Biological Defense, Williamsburg, VA, USA, 23–27 October 2000; p. 10. [Google Scholar]
- Diouani, M.F.; Helali, S.; Hafaid, I.; Hassen, W.M.; Snoussi, M.A.; Ghram, A.; Jaffrezic-Renault, N.; Abdelghani, A. Miniaturized biosensor for avian influenza virus detection. Mater. Sci. Eng. C 2008, 28, 580–583. [Google Scholar] [CrossRef]
- Byungchul, J.; Peiyan, C.; Chevalier, A.; Ellington, A.; Hassibi, A. A CMOS fluorescent-based biosensor microarray. In Proceedings of the Solid-State Circuits Conference, San Francisco, CA, USA, 8–12 February 2009; pp. 436–437. [Google Scholar]
- Weller, M. Optical microarray biosensors. Anal. Bioanal. Chem. 2005, 381, 41–43. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Final Report: Online Water Monitoring Utilizing an Automated Microarray Biosensor Instrument. Available online: http://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.abstractDetail/abstract/8951/report/F (accessed on 6 September 2013).
- Li, Y.; Liu, X.; Lin, Z. Recent developments and applications of surface plasmon resonance biosensors for the detection of mycotoxins in foodstuffs. Food Chem. 2012, 132, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Bedford, E.E.; Spadavecchia, J.; Pradier, C.-M.; Gu, F.X. Surface plasmon resonance biosensors incorporating gold nanoparticles. Macromol. Biosci. 2012, 12, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Oyarzabal, O.A.; Huang, T.-S.; Balasubramanian, S.; Sista, S.; Simonian, A.L. Development of a surface plasmon resonance biosensor for the identification of Campylobacter jejuni. J. Microbiol. Meth. 2007, 69, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, X.; Cui, H. Growth mechanism of flowerlike gold nanostructures: Surface plasmon resonance (SPR) and resonance Rayleigh scattering (RRS) approaches to growth monitoring. J. Phys. Chem. C 2008, 112, 16348–16353. [Google Scholar] [CrossRef]
- Sherry, L.J.; Chang, S.-H.; Schatz, G.C.; Van, D.R.P.; Wiley, B.J.; Xia, Y. Localized surface plasmon resonance spectroscopy of single silver nanocubes. Nano Lett. 2005, 5, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Yeh, H.-C.; Kuroki, M.T.; Wang, T.-H. Single-quantum-dot-based DNA nanosensor. Nature Mater. 2005, 4, 826–831. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Hu, J. Single quantum dot-based nanosensor for multiple DNA detection. Anal. Chem. 2010, 82, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Ladner, D.A.; Lee, B.W.; Clark, M.M. Laser scanning cytometry for enumeration of fluorescent microspheres. J. Am. Water Works Assoc. 2007, 99, 110–117. [Google Scholar] [CrossRef]
- Mairhofer, J.; Roppert, K.; Ertl, P. Microfluidic systems for pathogen sensing: A review. Sensors 2009, 9, 4804–4823. [Google Scholar] [CrossRef] [PubMed]
- Sanvicens, N.; Pastells, C.; Pascual, N.; Marco, M.P. Nanoparticle-based biosensors for detection of pathogenic bacteria. Trends Anal. Chem. 2009, 28, 1243–1252. [Google Scholar] [CrossRef]
- Herrmann, J.; Escher, B.; Jämting, Å.; Neale, P.A. The Fate of Engineered Nanoparticles in Wastewater: Literature Review; Water Quality Research Australia Limited: Adelaide, SA, Australia, 2012; Available online: http://www.waterra.com.au/project-details/135 (accessed on 30 October 2012).
- Carr, B.; Wright, M. Nanoparticle Tracking Analysis. A Review of Applications and Usage 2010—2012; NanoSight Ltd.: Salisbury, UK, 2013; p. 188. [Google Scholar]
- NanoSight. Measuring Concentration with Nanoparticle Tracking Analysis. Available online: http://www.nanosight.com/technology/nta-software/concentration-measurement (accessed on 11 May 2013).
- Defante, A.P.; Vreeland, W.N.; Benkstein, K.D.; Ripple, D.C. Using image attributes to assure accurate particle size and count using nanoparticle tracking analysis. J. Pharmaceut. Sci. 2018, 107, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- AquaDiagnostic. PeCOD™ Laboratory or Field COD Analysis—L100/F100. Available online: http://www.aquadiagnostic.com/downloads/brochures/L100.pdf (accessed on 5 September 2013).
- Vollmer, F.; Arnold, S.; Keng, D. Single virus detection from the reactive shift of a whispering-gallery mode. Proc. Natl. Acad. Sci. USA 2008, 105, 20701–20704. [Google Scholar] [CrossRef] [PubMed]
- Quinten, M. Optical Properties of Nanoparticle Systems; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; p. 488. [Google Scholar]
- He, L.; Ozdemir, S.K.; Zhu, J.; Kim, W.; Yang, L. Detecting single viruses and nanoparticles using whispering gallery microlasers. Nat. Nanotechnol. 2011, 6, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ozdemir, S.K.; Xiao, Y.-F.; Li, L.; He, L.; Chen, D.-R.; Yang, L. On-chip single nanoparticle detection and sizing by mode splitting in an ultrahigh-q microresonator. Nat. Photonics 2010, 4, 46–49. [Google Scholar] [CrossRef]
- Vollmer, F. Optical microresonators: Label-free detection down to single viral pathogens. SPIE News. 2010. [Google Scholar] [CrossRef]
- Johnson, D.W.; Callis, J.B.; Christian, G.D. Rapid scanning fluorescence spectroscopy. Anal. Chem. 1977, 49, 747A–757A. [Google Scholar] [CrossRef]
- Senga, Y.; Minami, S. Excitation-emission matrix scanning spectrofluorometer. Appl. Spectrosc. 1991, 45, 1721–1725. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.; Myrick, M.L. Fluorescence fingerprint of waters: Excitation-emission matrix spectroscopy as a tracking tool. Appl. Spectrosc. 2000, 54, 1539–1542. [Google Scholar] [CrossRef]
- Baker, A. Fluorescence properties of some farm wastes: Implications for water quality monitoring. Water Res. 2002, 36, 189–195. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Carstea, E.M.; Baker, A.; Bieroza, M.; Reynolds, D. Continuous fluorescence excitation–emission matrix monitoring of river organic matter. Water Res. 2010, 44, 5356–5366. [Google Scholar] [CrossRef] [PubMed]
- Hambly, A.; Henderson, R.K.; Baker, A.; Stuetz, R.M.; Khan, S.J. Probabilistic analysis of fluorescence signals for monitoring dual reticulation water recycling schemes. Water Sci. Technol. 2010, 62, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Baker, A. Fluorescence excitation—Emission matrix characterization of some sewage-impacted rivers. Environ. Sci. Technol. 2001, 35, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Baker, A. Fluorescence excitation-emission matrix characterization of river waters impacted by a tissue mill effluent. Environ. Sci. Technol. 2002, 36, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Bieroza, M.Z.; Bridgeman, J.; Baker, A. Fluorescence spectroscopy as a tool for determination of organic matter removal efficiency at water treatment works. Drink. Water Eng. Sci. 2010, 3, 63–70. [Google Scholar] [CrossRef]
- Hambly, A.C.; Henderson, R.K.; Storey, M.V.; Baker, A.; Stuetz, R.M.; Khan, S.J. Fluorescence monitoring at a recycled water treatment plant and associated dual distribution system—Implications forcross-connection detection. Water Res. 2010, 44, 5323–5333. [Google Scholar] [CrossRef] [PubMed]
- Galinha, C.F.; Carvalho, G.; Portugal, C.A.M.; Guglielmi, G.; Reis, M.A.M.; Crespo, J.G. Two-dimensional fluorescence as a fingerprinting tool for monitoring wastewater treatment systems. J. Chem. Technol. Biotechnol. 2011, 86, 985–992. [Google Scholar] [CrossRef]
- Singh, S.; Henderson, R.K.; Baker, A.; Stuetz, R.M.; Khan, S.J. Distinguishing stage 1 and 2 reverse osmosis permeates using fluorescence spectroscopy. Water Sci. Technol. 2009, 60, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Henderson, R.K.; Baker, A.; Stuetz, R.M.; Khan, S.J. Characterisation of reverse osmosis permeates from municipal recycled water systems using fluorescence spectroscopy: Implications for integrity monitoring. J. Membr. Sci. 2012, 421–422, 180–189. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, W. Zno qd@pmaa-co-pdmaema nonviral vector for plasmid DNA delivery and bioimaging. Biomaterials 2010, 31, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-H.; Wu, F.-Y.; Wu, Y.-M.; Zhan, X.-S. Fluorescent method for the determination of sulfide anion with ZnS:Mn quantum dots. J. Fluoresc. 2010, 20, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Knappenberger, K.L.; Wong, D.B.; Romanyuk, Y.E.; Leone, S.R. Excitation wavelength dependence of fluorescence intermittency in cdse/ZnS core/shell quantum dots. Nano Lett. 2007, 7, 3869–3874. [Google Scholar] [CrossRef] [PubMed]
- Ni, X. Photoluminescent, Water-Soluble Mn-Doped ZnS Quantum Dots. Available online: http://nanotechweb.org/cws/article/lab/37256 (accessed on 2 April 2012).
- Borisov, S.M.; Mayr, T.; Karasyov, A.A.; Klimant, I.; Chojnacki, P.; Moser, C.; Nagl, S.; Schaeferling, M.; Stich, M.I.; Kocincova, A.S.; et al. New plastic microparticles and nanoparticles for fluorescent sensing and encoding. In Springer Series on Fluorescence; Springer: Berlin, Germany, 2008; Volume 4, pp. 431–463. [Google Scholar]
- Schneider, R.L.; Balan, L.; Aldeek, F. Synthesis, Characterization and Biological Applications of Water-Soluble ZnO Quantum Dots. In Nanomaterials; Rahaman, M.M., Ed.; InTech: London, UK, 2011; Volume 2, pp. 27–42. [Google Scholar]
- Geszke, M.; Murias, M.; Balan, L.; Medjahdi, G.; Korczynski, J.; Moritz, M.; Lulek, J.; Schneider, R. Folic acid-conjugated core/shell ZnS:Mn/ZnS quantum dots as targeted probes for two photon fluorescence imaging of cancer cells. Acta Biomater. 2011, 7, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Verma, P.; Pandey, A.C. Synthesis of Highly Luminescent Manganese Doped ZnS Nanophosphor. In Proceedings of the ASID ‘06, New Delhi, India, 8–12 October 2006; pp. 259–261. [Google Scholar]
- Hu, S.; Bai, P.; Sun, J.; Cao, S. Fluorescent carbon nanoparticles: Recent achievements and technical challenges. Prog. Chem. 2010, 22, 345–351. [Google Scholar]
- Mochalin, V.N.; Gogotsi, Y. Wet chemistry route to hydrophobic blue fluorescent nanodiamond. J. Am. Chem. Soc. 2009, 131, 4594–4595. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Zboril, R.; Kubala, M.; Stathi, P.; Deligiannakis, Y.; Karakassides, M.A.; Steriotis, T.A.; Stubos, A.K. Fabrication of fluorescent nanodiamond@c core-shell hybrids via mild carbonization of sodium cholate-nanodiamond complexes. J. Mater. Sci. 2011, 46, 7912–7916. [Google Scholar] [CrossRef]
- Wee, T.-L.; Mau, Y.-W.; Fang, C.-Y.; Hsu, H.-L.; Han, C.-C.; Chang, H.-C. Preparation and characterization of green fluorescent nanodiamonds for biological applications. Diamond Relat. Mater. 2009, 18, 567–573. [Google Scholar] [CrossRef]
- Boudou, J.-P.; Tisler, J.; Reuter, R.; Thorel, A.; Curmi, P.A.; Jelezko, F.; Wrachtrup, J. Fluorescent nanodiamonds derived from HPHT with a size of less than 10 nm. Diamond Relat. Mater. 2013, 37, 80–86. [Google Scholar] [CrossRef]
- Havlik, J.; Petrakova, V.; Rehor, I.; Petrak, V.; Gulka, M.; Stursa, J.; Kucka, J.; Ralis, J.; Rendler, T.; Lee, S.-Y.; et al. Boosting nanodiamond fluorescence: Towards development of brighter probes. Nanoscale 2013, 5, 3208–3211. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Zhou, S.; Xu, B.; Chen, P.; Shimotsuma, Y.; Miura, K.; Qiu, J. Simple synthesis of ultra-small nanodiamonds with tunable size and photoluminescence. Carbon 2013, 62, 374–381. [Google Scholar] [CrossRef]
- Liu, J.-H.; Yang, S.-T.; Chen, X.-X.; Wang, H. Fluorescent carbon dots and nanodiamonds for biological imaging: Preparation, application, pharmacokinetics and toxicity. Curr. Drug MeTable 2012, 13, 1046–1056. [Google Scholar] [CrossRef]
- Liang, Q.; Ma, W.; Shi, Y.; Li, Z.; Yang, X. Easy synthesis of highly fluorescent carbon quantum dots from gelatin and their luminescent properties and applications. Carbon 2013, 60, 421–428. [Google Scholar] [CrossRef]
- Boulet, S. Diamonds Could Help Detect Early Stage Cancers, University of Sydney Researchers Say. Available online: http://www.abc.net.au/news/2015-10-10/diamonds-could-help-detect-early-stage-cancers/6844212 (accessed on 12 February 2016).
- Adham, S.S.; Jacangelo, J.G.; Laine, J.M. Low-pressure membranes: Assessing integrity. J. Am. Water Works Assoc. 1995, 87, 62–75. [Google Scholar] [CrossRef]

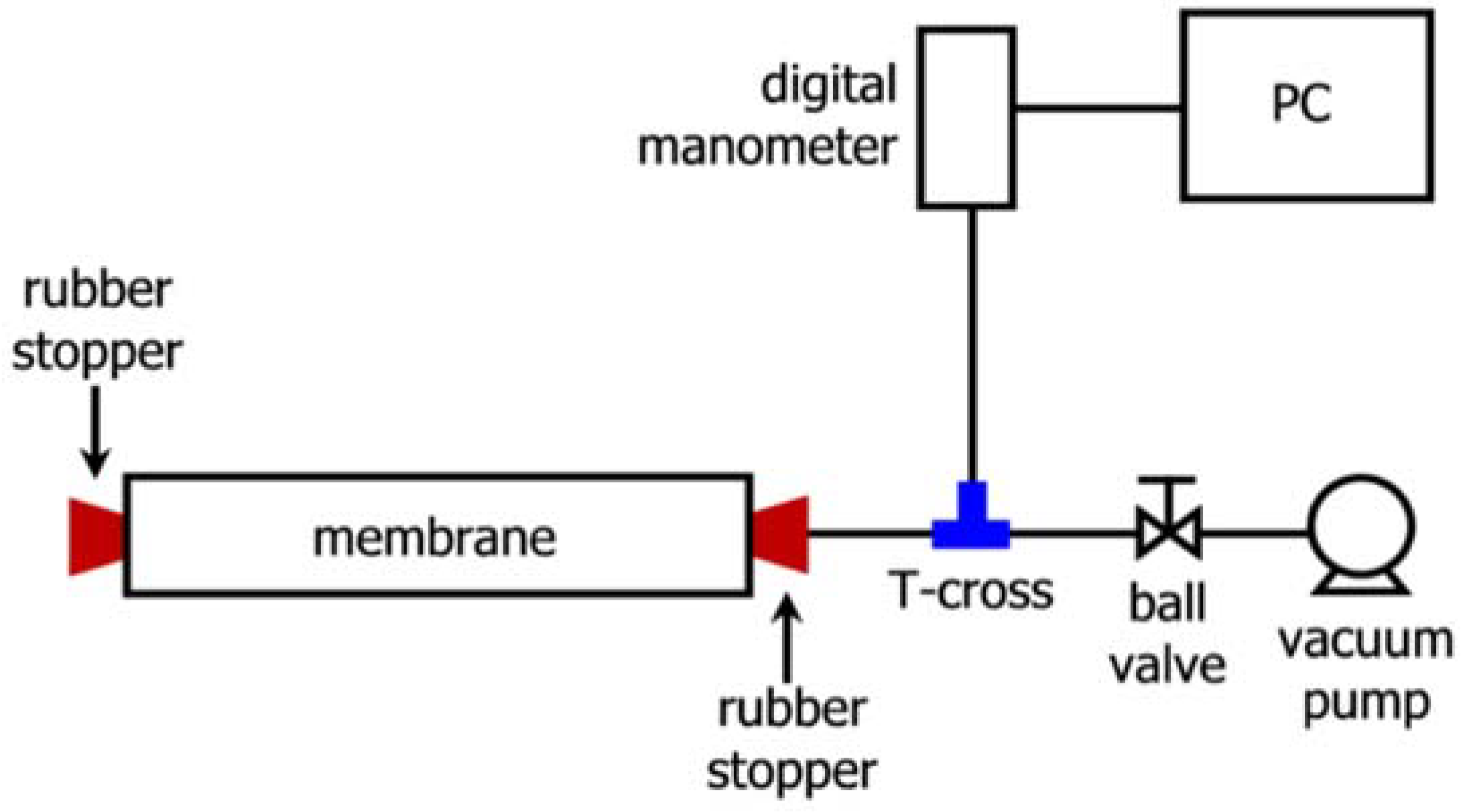
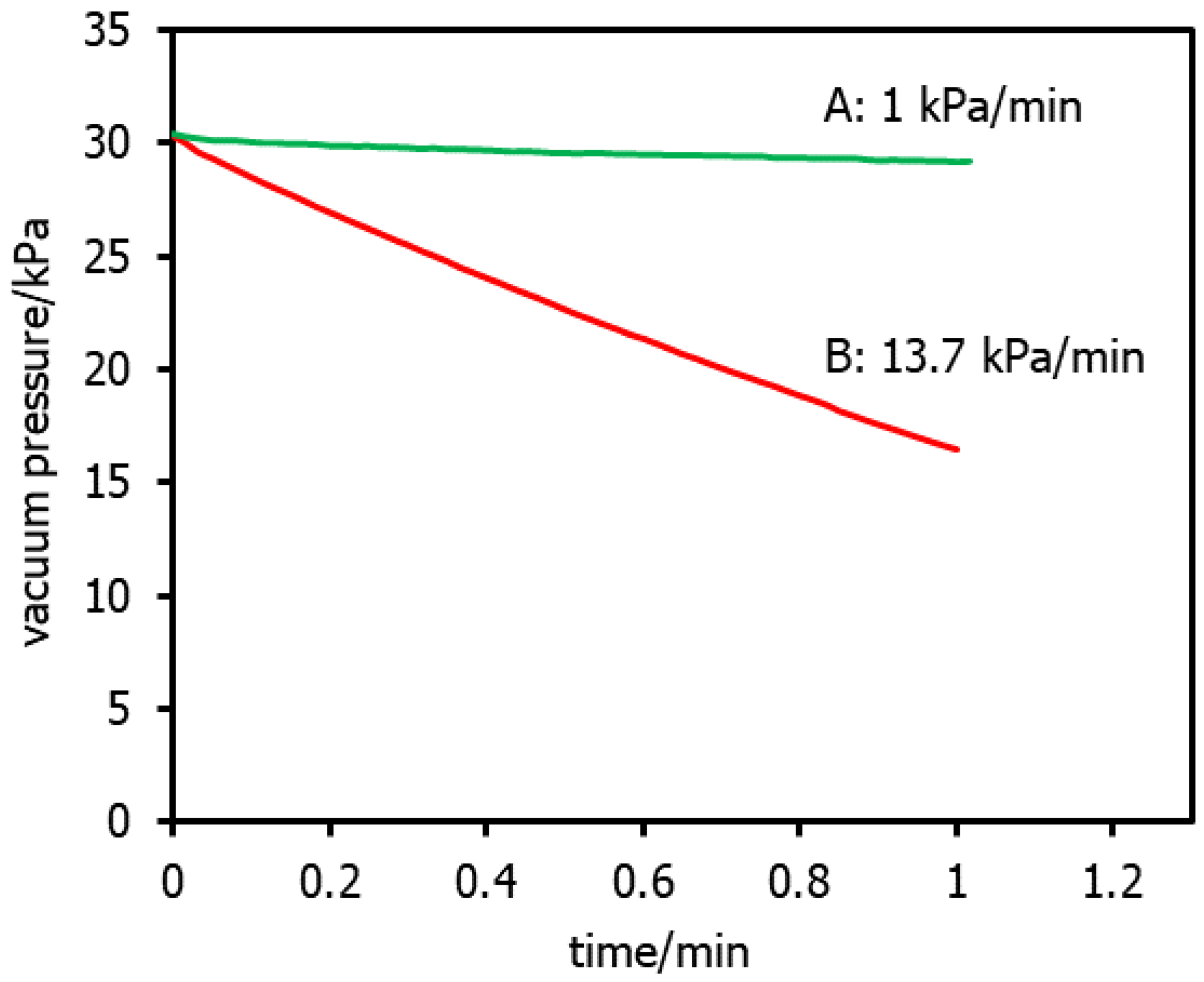
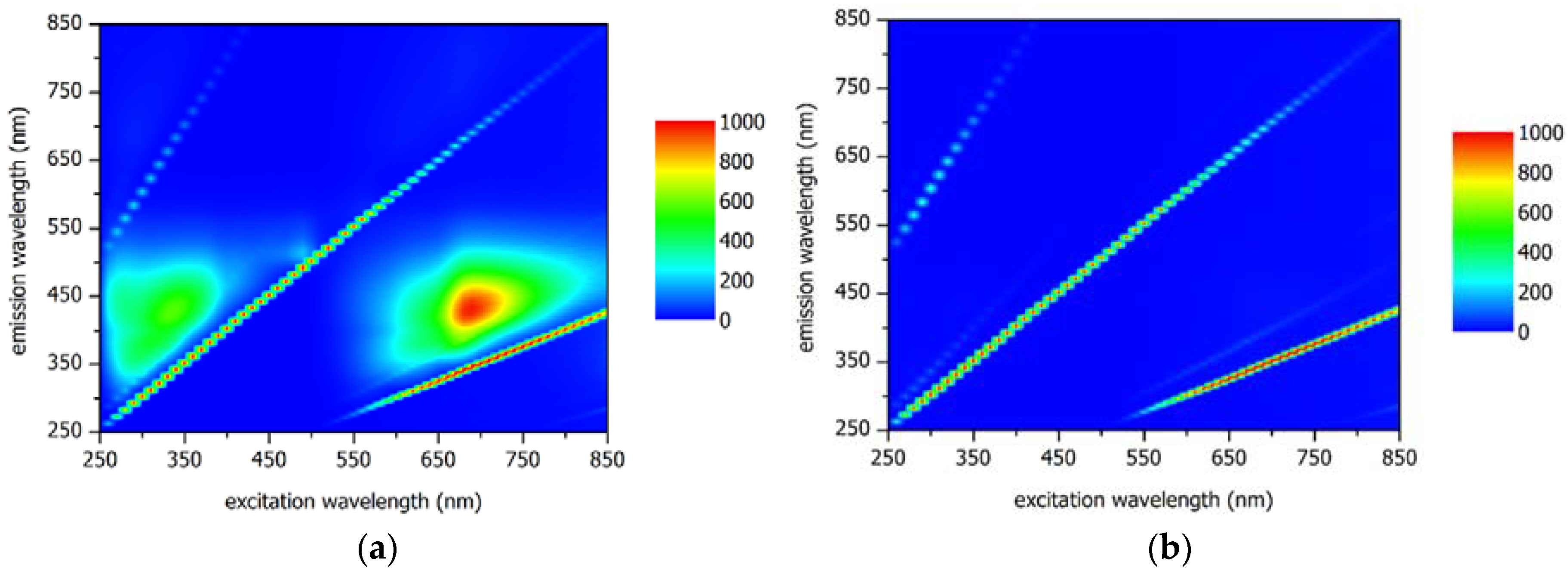
| Criteria | Requirement(s) |
|---|---|
| Technique Considerations | |
| Test type | Test should be online and provide integrity performance (LRV) results in real-time. Detection in permeate from membrane elements, individual pressure vessels or membrane array of complete membrane train. System shutdown unnecessary, normal operations continue during integrity surveillance. |
| Sensitivity | High sensitivity at low challenge species concentration. |
| Selectivity | Challenge species should be representative of the smallest virus rather than chemical compounds and not be subject to changes in detection resulting from variations in environmental or chemical conditions such as NOM, salinity, pH and temperature. |
| Output | Test should deliver minimum LRV of 4 log10 sensitivity. |
| Financial Considerations | |
| Capital cost | In the same order or less of capital cost as existing online real-time systems such as total organic carbon instrumentation. |
| Installation Integration | The ability to be fully integrated into existing systems as well as new systems seamlessly (greenfield and brownfield applications). |
| Operation | Should require minimal training for operators. |
| Running costs | Should not add more than 1–1.5% of the tariff charged to consumers for the provision of treated water. |
| Feed Concentration (mg/L) | Mode * | LRV | Reference |
|---|---|---|---|
| 0.1–1 | C | 3.5–5.3 | [54] |
| 1–2 | C | 3.9 | [8] |
| 1 | C | 2.7–3 | [9] |
| 0.1–1 | C | 2–5 | [57] |
| 0.1 | C | 2.6 | [56] |
| 5–10 | P | >4 | [58] |
| RO Permeate | TDS (mg/L) | COD (ppm) |
|---|---|---|
| 1st pass | 48.2 | 1.57 |
| 2nd pass | 2.4 | 0.27 |
| Monitoring Technique | Membrane Applications | Mode | Description | Scale | Advantages/Limitations | References |
|---|---|---|---|---|---|---|
| Existing Techniques—Direct Monitoring | ||||||
| Vacuum Decay Testing | NF and RO membranes | Offline | Element soaked with RO permeate overnight, drained then capped, vacuum applied; decay monitored over 1 min; fail at >10 kPa/min decay | Post-manufacturing; bench- and pilot-scale | Applies only to individual elements and not to the entire system | [6,9,28,71] |
| Pressure Decay Testing | MF, UF, NF and RO membranes | Offline | One side of the membrane pressurized, pressure loss over time monitored | Bench- and pilot-scale; can be used for entire stage of NF and RO systems | Not practical for full-scale elements due to drainage requirement; pressurizing permeate side can cause damage to NF/RO membrane; not widely used for these systems | [28,33,71,166] |
| Existing Techniques—Indirect Monitoring—Naturally Occurring Substances | ||||||
| Particle Monitoring | MF and UF membranes | Online | Particle concentration measured in feed and permeate | Pilot-scale | Not suitable for NF/RO as particle size is too large; resolution dependent on particle concentration in feed water | [33] |
| Turbidity Monitoring | MF and UF membranes | Online | Similar to particle monitoring, concentration measured in feed and permeate | Full- and pilot-scale | Minimum particle size is 1 µm; low resolution | [36] |
| TOC Monitoring | NF and RO membranes | Online | TOC concentrations measured in feed and permeate | Full-scale; can be used for entire stage of NF and RO systems | Used in several installations but equipment to detect very low levels is expensive | [18,41] |
| Sulphate Monitoring | NF and RO membranes | Offline | Sulphate concentrations measured in feed and permeate | Full-scale; can be used for entire stage of NF and RO systems | Expensive to monitor continuously using ICP | [18] |
| Conductivity Monitoring | NF and RO membranes | Online | Conductivity of feed and permeate monitored | Bench-, pilot-, and full-scale; can be used for entire stage of NF and RO systems | Low resolution; removal limited to 2 log10 for water reuse applications; probing more effective than online monitoring | [41] |
| Periodic Testing | NF and RO elements, trains | Online | Can involve multiple tests including conductivity probing and UV-254 | Full-scale of NF and RO systems | Offers multiple, periodic testing; can locate defects but is complex to implement in full scale applications | [41] |
| Existing Techniques—Indirect Monitoring—Challenge Tests | ||||||
| Dye Testing | NF and RO membranes | Online | Log removal of dye measured by calibrated absorbance or fluorescence at optimum wavelength | Pilot- and full-scale | Can provide up to 4 log10 resolution; fouling can be an issue for some dyes but not RWT | [18,33] |
| Spiked Integrity Monitoring | MF and UF membranes | Online | PAC particles injected in feed side and particle concentration measured in permeate | Full-scale | Applicable only for micron size particles | [59] |
| Pulse Integrity Test | NF and RO | Online | Measures a pulse of highly rejected species (i.e., sulphate) | Pilot scale | Can locate defects if calibrated | [60] |
| Microbial Surrogates (i.e., MS2, E. coli etc.) | MF, UF, NF and RO membranes | Offline | High concentrations of surrogate introduced into feed and concentration measured in permeate | Pilot- and full-scale | Seeding required since MF/UF pretreatment will remove most surrogates; can be expensive | [71] |
| Fluorescent Microspheres | MF and UF membranes | Offline | Microsphere concentration in feed and permeate measured by fluorescence | Pilot- and full-scale | Up to 4 log10 removal reported; expensive due to cost of particles | [18] |
| Existing Techniques—Integrated and Multi-Parameter Monitoring Systems | ||||||
| TRASAR® | NF and RO membranes | Online | Fluorescent molecules injected with antiscalant; fluorescence measured in permeate using trace leak detection | Full-scale; can be used for entire stage of NF and RO systems | Up to 6 log10 removal reported with non-continuous spikes; up to 2 log10 when used with antiscalant | [18] |
| Small Sensor Cell with Collection Membrane | MF and UF membranes | Online | Microsieve sensor membrane placed in permeate side stream; change in TMP of sensor membrane detects breach | Bench- and pilot-scale | Can take >60 min to detect very small breach | [83] |
| Binary Gas Integrity Test | MF and UF membranes | Online | Diffusivity of low permeating gas detected in permeate using mass flowmeters and composition with FTIR | Bench-scale | Complex to implement in larger membrane systems; gas permeability may be an issue as would the cost of inert gases required | [84] |
| ZAPS LiquID Station | General water quality monitoring device; could be applicable for MF, UF, NF and RO | Online | Measures multiple optical parameters simultaneously | Full-scale | Can potentially report high LRVs for TOC and BOD; difficult to quantify system LRV as it uses tryptophan, a common amino acid in many proteins not unique to E. coli and that may limit its sensitivity | [86] |
| Emerging Techniques—Pathogen Detection Systems | ||||||
| BioSentry Device | General water quality monitoring device; could be applicable for MF, UF, NF and RO | Offline | Multi-angle light scattering at 660 nm used to determine particle size, shape and internal structure | Bench-scale | Valid only for particles greater than 0.4 micron | [96] |
| Real-Time Polymerase Chain Reaction | Water quality monitoring specifically for viruses | Offline | Feed and permeate collected and virus detected using centrifugation, filtration and enumeration techniques | Bench-scale | Requires specialized personnel, sample preparation and long time periods for results; expensive | [97] |
| Evanescent Wave Fiber Optic Sensor | Detection of pathogens | Online | Laser derived evanescent wave is excited over sample and fluorescence measured using laser spectrofluorometer | Bench-scale | Long detection time (several h) | [98,99,100] |
| RAPTOR Fiber Optic Biosensor | Detection of pathogens | Online | Monitors complex formation by evanescently exciting surface-bound fluorophores with a diode laser | Bench-scale | Portable; results in less than 10 min | [104] |
| Miniaturized Portable Biosensor | Detection of pathogens | Online | Electrochemical technique (impedance spectroscopy) used to detect virus by immobilization of antibodies onto biofunctionalized gold electrode | Bench-scale | Long detection time (several h) | [109] |
| Microarray Biosensor Instrument | Detection of pathogens | Online | Automated concentration system uses advance array biosensor to detect pathogens in water | Bench-scale | Laboratory-scale systems common | [112] |
| Surface Plasmon Resonance Biosensors | Detection of pathogens | Online | Illumination of a metallic surface by visible or near-infrared radiation from a monochromatic light source via a hemispherical prism; electromagnetic waves are generated and detected | Bench-scale | Not currently available as a commercial technique for field applications | [116] |
| Quantum Dot Based DNA Nanosensors | Detection of pathogens | - | Ultrasensitive nanosensor based on fluorescence resonance for detecting DNA | Bench-scale | Requires specialized personnel; expensive | [119] |
| Laser- Scanning Cytometry | Detection of pathogens | Online | Laser-scanning cytometry used to detect microspheres in feed and permeate samples | Bench-scale | Only applicable for micron-sized particles | [121] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostarcevic, E.R.; Jacangelo, J.; Gray, S.R.; Cran, M.J. Current and Emerging Techniques for High-Pressure Membrane Integrity Testing. Membranes 2018, 8, 60. https://doi.org/10.3390/membranes8030060
Ostarcevic ER, Jacangelo J, Gray SR, Cran MJ. Current and Emerging Techniques for High-Pressure Membrane Integrity Testing. Membranes. 2018; 8(3):60. https://doi.org/10.3390/membranes8030060
Chicago/Turabian StyleOstarcevic, Eddy R., Joseph Jacangelo, Stephen R. Gray, and Marlene J. Cran. 2018. "Current and Emerging Techniques for High-Pressure Membrane Integrity Testing" Membranes 8, no. 3: 60. https://doi.org/10.3390/membranes8030060
APA StyleOstarcevic, E. R., Jacangelo, J., Gray, S. R., & Cran, M. J. (2018). Current and Emerging Techniques for High-Pressure Membrane Integrity Testing. Membranes, 8(3), 60. https://doi.org/10.3390/membranes8030060







