Performance of Mixed Matrix Membranes Containing Porous Two-Dimensional (2D) and Three-Dimensional (3D) Fillers for CO2 Separation: A Review
Abstract
1. Introduction
2. Metal Organic Frameworks (MOFs)
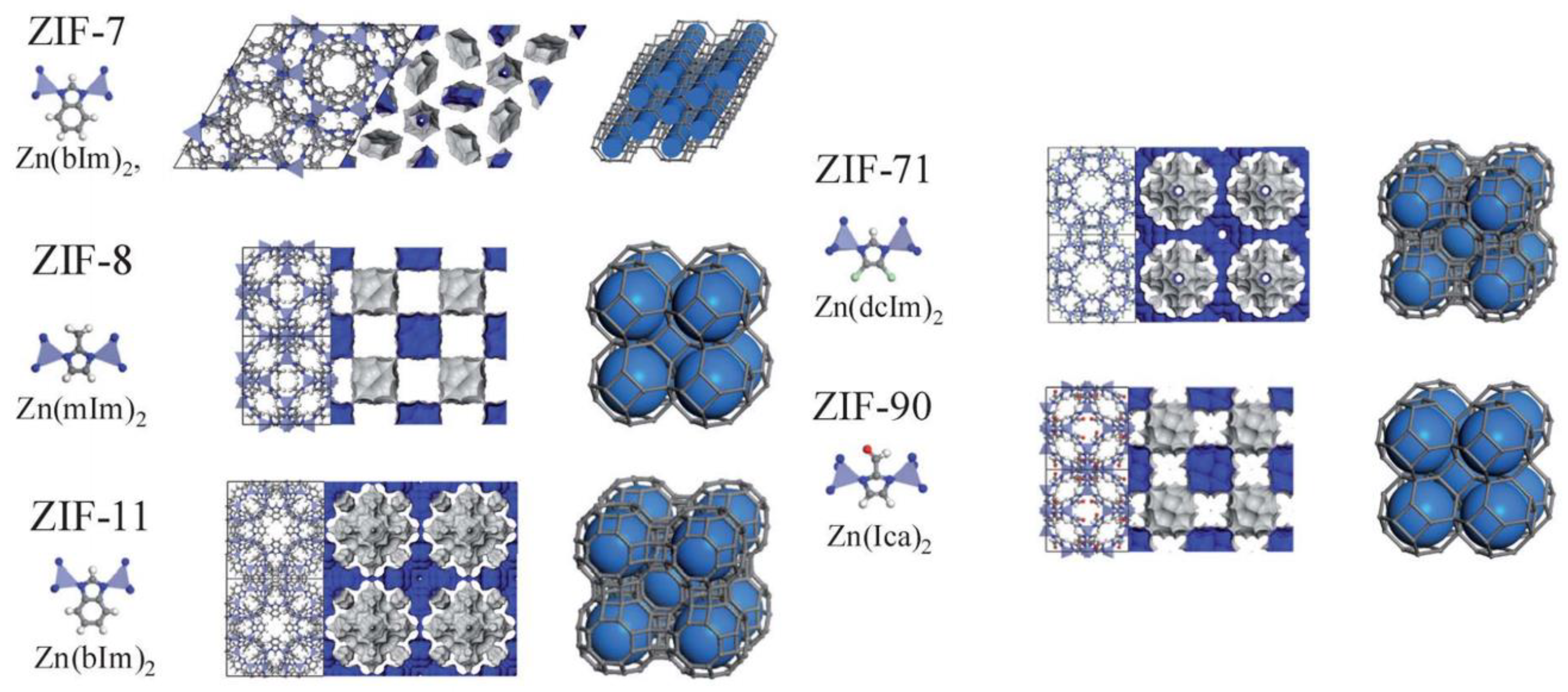
2.1. Zeolitic Imidazolate Frameworks (ZIFs)
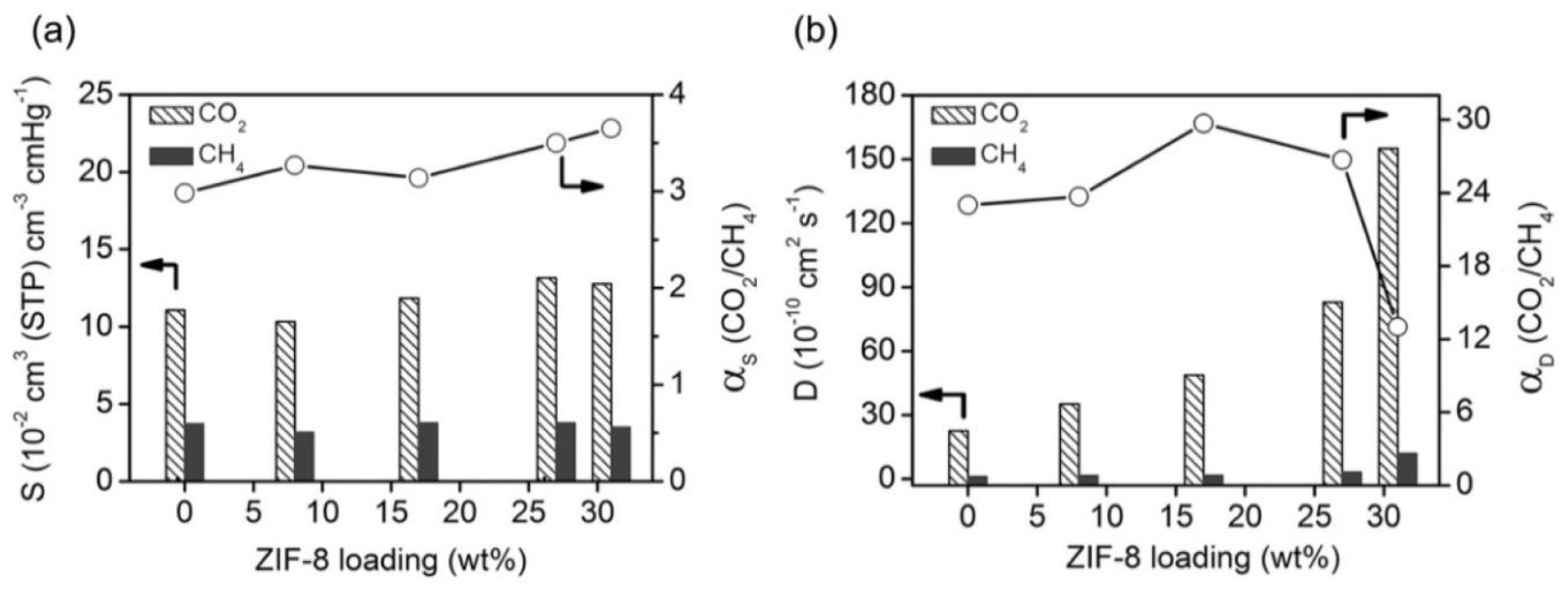
2.1.1. ZIF-8
2.1.2. ZIF-7
2.1.3. ZIF-11, ZIF-71, and ZIF-90
2.2. Zirconium 1,4-Dicarboxybenzene (UiO-66)
2.3. Copper-Based MOFs
2.4. Materials Institute Lavoisier MOFs (MILs)
2.5. Other MOFs
3. Porous Organic Frameworks (POFs)
4. Zeolites
5. Porous Nanosheets
6. Conclusions and Perspective
- to reduce primary particle size of existing MOFs and expedite their incorporation in thin composite polymeric membranes;
- to increase the CO2 affinity and the polymer/particle interactions by novel surface functionalization procedures on the nanoparticle or by post-functionalization after membrane preparation, aiming at improving the CO2 separation performance and simplifying their dispersion in the polymeric phases;
- to tune the structure and morphology of POFs with the aim of enhancing the selectivity of hybrid matrix when used in high free volume polymers;
- to design and fabricate novel 2D MOF frameworks with improved sieving ability that do not sacrifice the gas transport through the selective layer; and,
- to systematically investigate the potential of hybrid membranes in H2 separation, exploiting the exceptional H2 sieving ability of some MOFs.
Funding
Conflicts of Interest
References
- Pera-Titus, M. Porous Inorganic Membranes for CO2 Capture: Present and Prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef] [PubMed]
- Rackley, S.A. Chapter 4—Carbon Capture from Power Generation. In Carbon Capture and Storage; Rackley, S.A., Ed.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 65–93. ISBN 978-1-85617-636-1. [Google Scholar]
- Rackley, S.A. Chapter 5—Carbon Capture from Industrial Processes. In Carbon Capture and Storage; Rackley, S.A., Ed.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 95–102. ISBN 978-1-85617-636-1. [Google Scholar]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernandez, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Dalane, K.; Dai, Z.; Mogseth, G.; Hillestad, M.; Deng, L. Potential applications of membrane separation for subsea natural gas processing: A review. J. Nat. Gas Sci. Eng. 2017, 39, 101–117. [Google Scholar] [CrossRef]
- Ahmadi, M.; Tas, E.; Kilic, A.; Kumbaraci, V.; Talinli, N.; Ahunbay, M.G.; Tantekin-Ersolmaz, S.B. Highly CO2 Selective Microporous Metal-Imidazolate Framework (MMIF) Based Mixed Matrix Membranes. ACS Appl. Mater. Interfaces 2017, 9, 35936–35946. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xie, L.H.; Li, J.R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 Capture and Separations Using MOFs: Computational and Experimental Studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef] [PubMed]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ansaloni, L.; Deng, L. Recent advances in multi-layer composite polymeric membranes for CO2 separation: A review. Green Energy Environ. 2012, 1, 102–128. [Google Scholar] [CrossRef]
- Ansaloni, L.; Deng, L. Advances in polymer-inorganic hybrids as membrane materials. In Recent Developments in Polymer Macro, Micro and Nano Blends: Preparation and Characterisation; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2016; pp. 163–206. ISBN 9780081004272. [Google Scholar]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Memb. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Robeson, L.M.; Smith, Z.P.; Freeman, B.D.; Paul, D.R. Contributions of diffusion and solubility selectivity to the upper bound analysis for glassy gas separation membranes. J. Membr. Sci. 2014, 453, 71–83. [Google Scholar] [CrossRef]
- Park, H.B.; Jung, C.H.; Lee, Y.M.; Hill, A.J.; Pas, S.J.; Mudie, S.T.; Van Wagner, E.; Freeman, B.D.; Cookson, D.J. Polymers with cavities tuned for fast selective transport of small molecules and ions. Science 2007, 318, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas Permeation Properties, Physical Aging, and Its Mitigation in High Free Volume Glassy Polymers. Chem. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Deng, L.; Hägg, M.-B. Role of Facilitated Transport Membranes and Composite Membranes for Efficient CO2 Capture—A Review. ChemBioEng Rev. 2016, 3, 68–85. [Google Scholar] [CrossRef]
- Caro, J. Hierarchy in inorganic membranes. Chem. Soc. Rev. 2016, 45, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; Carson, C.; Ward, J.; Tannenbaum, R.; Koros, W. Metal organic framework mixed matrix membranes for gas separations. Microporous Mesoporous Mater. 2010, 131, 13–20. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Janakiram, S.; Ahmadi, M.; Dai, Z.; Ansaloni, L.; Deng, L. Performance of nanocomposite membranes containing 0D to 2D nanofillers for CO2 separation: A review. Membranes 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Rezakazemi, M.; Ebadi Amooghin, A.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Seoane, B.; Coronas, J.; Gascon, I.; Benavides, M.E.; Karvan, O.; Caro, J.; Kapteijn, F.; Gascon, J. Metal–organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture? Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, N.; Fong Yeong, Y.; Leng Chew, T.; Keong Lau, K.; Mohd Shariff, A. Separation & Purification Reviews Current Development and Challenges of Mixed Matrix Membranes for CO2/CH4 Separation Current Development and Challenges of Mixed Matrix Membranes for CO2/CH4 Separation. Sep. Purif. Rev. 2016, 45, 321–344. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Zhao, S.; Wang, J.; Wang, S. Recent advances on mixed matrix membranes for CO2 separation. Chinese J. Chem. Eng. 2017, 25, 1581–1597. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-Organic Frameworks: Opportunities for Catalysis. Angew. Chemie Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2011, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.; Hwa Lee, J.; Yeong Jung, G.; Kyeong Kim, Y.; Kyung Kim, T.; Jeoung, S.; Kyu Kwak, S.; Moon, D.; Ri Moon, H. Exploration of Gate-Opening and Breathing Phenomena in a Tailored Flexible Metal—Organic Framework. Inorg. Chem 2016, 55, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, S.; Kapteijn, F.; Gascon, J. Photoswitchable metal organic frameworks: turn on the lights and close the windows. CrystEngComm 2016, 18, 4006–4012. [Google Scholar] [CrossRef]
- Alhamami, M.; Doan, H.; Cheng, C.-H. A Review on Breathing Behaviors of Metal-Organic-Frameworks (MOFs) for Gas Adsorption. Materials (Basel) 2014, 7, 3198–3250. [Google Scholar] [CrossRef] [PubMed]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Duren, T. Opening the gate: framework flexibility in ZIF-8 explored by experiments and simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [PubMed]
- Kolokolov, D.I.; Maryasov, A.G.; Ollivier, J.; Freude, D.; Haase, J.; Stepanov, A.G.; Jobic, H. Uncovering the Rotation and Translational Mobility of Benzene Confined in UiO-66 (Zr) Metal–Organic Framework by the 2H NMR–QENS Experimental Toolbox. J. Phys. Chem. C 2017, 121, 2844–2857. [Google Scholar] [CrossRef]
- Damron, J.T.; Ma, J.; Kurz, R.; Saalwächter, K.; Matzger, A.J.; Ramamoorthy, A. The Influence of Chemical Modification on Linker Rotational Dynamics in Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2018, 57, 8678–8681. [Google Scholar] [CrossRef] [PubMed]
- Yoo, G.Y.; Lee, W.R.; Jo, H.; Park, J.; Song, J.H.; Lim, K.S.; Moon, D.; Jung, H.; Lim, J.; Han, S.S.; Jung, Y.; Hong, C.S. Adsorption of Carbon Dioxide on Unsaturated Metal Sites in M2 (dobpdc) Frameworks with Exceptional Structural Stability and Relation between Lewis Acidity and Adsorption Enthalpy. Chem. - A Eur. J. 2016, 22, 7444–7451. [Google Scholar] [CrossRef] [PubMed]
- Poloni, R.; Lee, K.; Berger, R.F.; Smit, B.; Neaton, J.B. Understanding Trends in CO2 Adsorption in Metal−Organic Frameworks with Open-Metal Sites. J. Phys. Chem. Lett. 2014, 5, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.W.; Dubbeldam, D.; Liu, M.S.; Ladewig, B.P.; Hill, A.J.; Hill, M.R. Feasibility of zeolitic imidazolate framework membranes for clean energy applications. Energy Environ. Sci. 2012, 5, 7637–7646. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Jang, M.-S.; Kwon, H.-J.; Ahn, W.-S. Zeolitic imidazolate frameworks: Synthesis, functionalization, and catalytic/adsorption applications. Catal. Surv. Asia 2014, 18, 101–127. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Fairen-Jimenez, D.; Galvelis, R.; Torrisi, A.; Gellan, A.D.; Wharmby, M.T.; Wright, P.A.; Mellot-Draznieks, C.; Düren, T. Flexibility and swing effect on the adsorption of energy-related gases on ZIF-8: combined experimental and simulation study. Dalt. Trans. 2012, 41, 10752–10762. [Google Scholar] [CrossRef] [PubMed]
- Coudert, F.-X. Molecular Mechanism of Swing Effect in Zeolitic Imidazolate Framework ZIF-8: Continuous Deformation upon Adsorption. ChemPhysChem 2017, 18, 2732–2738. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, M.J.C.; Balkus, K.J., Jr.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes. J. Memb. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Song, Q.; Nataraj, S.K.; Roussenova, M.V; Tan, J.C.; Hughes, D.J.; Li, W.; Bourgoin, P.; Alam, M.A.; Cheetham, A.K.; Al-Muhtaseb, S.A.; et al. Zeolitic imidazolate framework (ZIF-8) based polymer nanocomposite membranes for gas separation. Energy Environ. Sci. 2012, 5, 8359–8369. [Google Scholar] [CrossRef]
- Thompson, J.A.; Chapman, K.W.; Koros, W.J.; Jones, C.W.; Nair, S. Sonication-induced Ostwald ripening of ZIF-8 nanoparticles and formation of ZIF-8/polymer composite membranes. Microporous Mesoporous Mater. 2012, 158, 292–299. [Google Scholar] [CrossRef]
- Thompson, J.A.; Vaughn, J.T.; Brunelli, N.A.; Koros, W.J.; Jones, C.W.; Nair, S. Mixed-linker zeolitic imidazolate framework mixed-matrix membranes for aggressive CO2 separation from natural gas. Microporous Mesoporous Mater. 2014, 192, 43–51. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernandez-Barquin, A.; Zornoza, B.; Tellez, C.; Coronas, J.; Irabien, A. Synthesis and characterisation of MOF/ionic liquid/chitosan mixed matrix membranes for CO2/N2 separation. RSC Adv. 2015, 5, 102350–102361. [Google Scholar] [CrossRef]
- Carter, D.; Tezel, F.H.; Kruczek, B.; Kalipcilar, H. Investigation and comparison of mixed matrix membranes composed of polyimide matrimid with ZIF-8, silicalite, and SAPO-34. J. Memb. Sci. 2017, 544, 35–46. [Google Scholar] [CrossRef]
- Guo, A.; Ban, Y.; Yang, K.; Yang, W. Metal-organic framework-based mixed matrix membranes: Synergetic effect of adsorption and diffusion for CO2/CH4 separation. J. Memb. Sci. 2018, 562, 76–84. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Transport properties of mixed matrix membranes encompassing zeolitic imidazolate framework 8 (ZIF-8) nanofiller and 6FDA-durene polymer: Optimization of process variables for the separation of CO2from CH4. J. Clean. Prod. 2017, 149, 80–95. [Google Scholar] [CrossRef]
- Wijenayake, S.N.; Panapitiya, N.P.; Versteeg, S.H.; Nguyen, C.N.; Goel, S.; Balkus, K.J.; Musselman, I.H.; Ferraris, J.P. Surface cross-linking of ZIF-8/polyimide mixed matrix membranes (MMMs) for gas separation. Ind. Eng. Chem. Res. 2013, 52, 6991–7001. [Google Scholar] [CrossRef]
- Zhang, Z.; Xian, S.; Xia, Q.; Wang, H.; Li, Z.; Li, J. Enhancement of CO2 adsorption and CO2/N2 selectivity on ZIF-8 via postsynthetic modification. AIChE J. 2013, 59, 2195–2206. [Google Scholar] [CrossRef]
- Askari, M.; Chung, T.-S. Natural gas purification and olefin/paraffin separation using thermal cross-linkable co-polyimide/ZIF-8 mixed matrix membranes. J. Memb. Sci. 2013, 444, 173–183. [Google Scholar] [CrossRef]
- Nafisi, V.; Hägg, M.-B. Gas separation properties of ZIF-8/6FDA-durene diamine mixed matrix membrane. Sep. Purif. Technol. 2014, 128, 31–38. [Google Scholar] [CrossRef]
- Nafisi, V.; Hägg, M.B. Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2 capture. J. Memb. Sci. 2014, 459, 244–255. [Google Scholar] [CrossRef]
- Sánchez-Laínez, J.; Zornoza, B.; Téllez, C.; Coronas, J. Asymmetric polybenzimidazole membranes with thin selective skin layer containing ZIF-8 for H2/CO2 separation at pre-combustion capture conditions. J. Memb. Sci. 2018, 563, 427–434. [Google Scholar] [CrossRef]
- Dai, Y.; Johnson, J.R.; Karvan, O.; Sholl, D.S.; Koros, W.J. Ultem®/ZIF-8 mixed matrix hollow fiber membranes for CO2/N2 separations. J. Memb. Sci. 2012, 401–402, 76–82. [Google Scholar] [CrossRef]
- Mubashir, M.; Fong, Y.Y.; Leng, C.T.; Keong, L.K. Issues and Current Trends of Hollow-Fiber Mixed-Matrix Membranes for CO2 Separation from N2 and CH4. Chem. Eng. Technol. 2018, 41, 235–252. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, L.; Uliana, A.; Hou, J.; Wang, J.; Zhang, Y.; Li, X.; Yuan, S.; Li, J.; Tian, M.; et al. Elevated Performance of Thin Film Nanocomposite Membranes Enabled by Modified Hydrophilic MOFs for Nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High Flux Thin Film Nanocomposite Membranes Based on Metal–Organic Frameworks for Organic Solvent Nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, B.; Hu, X.; Nair, S.; Chen, Y. Thin film nanocomposite membrane containing zeolitic imidazolate framework-8 via interfacial polymerization for highly permeable nanofiltration. J. Taiwan Inst. Chem. Eng. 2018, 83, 159–167. [Google Scholar] [CrossRef]
- Echaide-Górriz, C.; Navarro, M.; Téllez, C.; Coronas, J. Simultaneous use of MOFs MIL-101(Cr) and ZIF-11 in thin film nanocomposite membranes for organic solvent nanofiltration. Dalt. Trans. 2017, 46, 6244–6252. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Laínez, J.; Paseta, L.; Navarro, M.; Zornoza, B.; Téllez, C.; Coronas, J. Ultrapermeable Thin Film ZIF-8/Polyamide Membrane for H2/CO2 Separation at High Temperature without Using Sweep Gas. Adv. Mater. Interfaces 2018, 1800647. [Google Scholar] [CrossRef]
- Li, Y.; Liang, F.; Bux, H.; Yang, W.; Caro, J. Zeolitic imidazolate framework ZIF-7 based molecular sieve membrane for hydrogen separation. J. Memb. Sci. 2010, 354, 48–54. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Birkett, G.; Zhu, Z.; Rufford, T.E. Gate opening effect of zeolitic imidazolate framework ZIF-7 for adsorption of CH4 and CO2 from N2. J. Mater. Chem. A 2017, 5, 21389–21399. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.; Peinemann, K.-V.; Lai, Z. Carbon dioxide selective mixed matrix composite membrane containing ZIF-7 nano-fillers. J. Memb. Sci. 2013, 425, 235–242. [Google Scholar] [CrossRef]
- Al-maythalony, B.A.; Alloush, A.M.; Faizan, M.; Dafallah, H.; Elgzoly, M.A.A.; Seliman, A.A.A.; Al-ahmed, A.; Yamani, Z.H.; Habib, M.A.M.; Cordova, K.E.; et al. Tuning the Interplay between Selectivity and Permeability of ZIF-7 Mixed Matrix Membranes. ACS Appl. Mater. Interfaces 2017, 9, 33401–33407. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.; Doonan, C.J.; Furukawa, H.; Banerjee, R.; Yaghi, O.M. Crystals as molecules: postsynthesis covalent functionalization of zeolitic imidazolate frameworks. J. Am. Chem. Soc. 2008, 130, 12626–12627. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 58. [Google Scholar] [CrossRef] [PubMed]
- Japip, S.; Xiao, Y.; Chung, T.-S. Particle-Size Effects on Gas Transport Properties of 6FDA-Durene/ZIF-71 Mixed Matrix Membranes. Ind. Eng. Chem. Res. 2016, 55, 9507–9517. [Google Scholar] [CrossRef]
- Ehsani, A.; Pakizeh, M. Synthesis, characterization and gas permeation study of ZIF-11/Pebax® 2533 mixed matrix membranes. J. Taiwan Inst. Chem. Eng. 2016, 66, 414–423. [Google Scholar] [CrossRef]
- Boroglu, M.S.; Yumru, A.B. Gas separation performance of 6FDA-DAM-ZIF-11 mixed-matrix membranes for H2/CH4 and CO2/CH4 separation. Sep. Purif. Technol. 2017, 173, 269–279. [Google Scholar] [CrossRef]
- Hao, L.; Liao, K.-S.; Chung, T.-S. Photo-oxidative PIM-1 based mixed matrix membranes with superior gas separation performance. J. Mater. Chem. A 2015, 3, 17273–17281. [Google Scholar] [CrossRef]
- Bae, T.; Lee, J.S.; Qiu, W.; Koros, W.J.; Jones, C.W.; Nair, S. A High-Performance Gas-Separation Membrane Containing Submicrometer-Sized Metal–Organic Framework Crystals. Angew. Chemie Int. Ed. 2010, 49, 9863–9866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, S.; Weidman, J.R.; Guo, R. Preparation and gas separation performance of mixed-matrix membranes based on triptycene-containing polyimide and zeolite imidazole framework (ZIF-90). Polymer (Guildf) 2017, 131, 209–216. [Google Scholar] [CrossRef]
- DeStefano, M.R.; Islamoglu, T.; Garibay, S.J.; Hupp, J.T.; Farha, O.K. Room-Temperature Synthesis of UiO-66 and Thermal Modulation of Densities of Defect Sites. Chem. Mater. 2017, 29, 1357–1361. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Friebe, S.; Geppert, B.; Steinbach, F.; Caro, J. Metal–Organic Framework UiO-66 Layer: A Highly Oriented Membrane with Good Selectivity and Hydrogen Permeance. ACS Appl. Mater. Interfaces 2017, 9, 12878–12885. [Google Scholar] [CrossRef] [PubMed]
- Kolokolov, D.I.; Stepanov, A.G.; Guillerm, V.; Serre, C.; Frick, B.; Jobic, H. Probing the Dynamics of the Porous Zr Terephthalate UiO-66 Framework Using 2H NMR and Neutron Scattering. J. Phys. Chem. C 2012, 116, 12131–12136. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Li, Q.; Guan, K.; Li, Y.; Jin, W. UiO-66-polyether block amide mixed matrix membranes for CO2 separation. J. Memb. Sci. 2016, 513, 155–165. [Google Scholar] [CrossRef]
- Anjum, M.W.; Vermoortele, F.; Khan, A.L.; Bueken, B.; De Vos, D.E.; Vankelecom, I.F.J. Modulated UiO-66-based mixed-matrix membranes for CO2 separation. ACS Appl. Mater. Interfaces 2015, 7, 25193–25201. [Google Scholar] [CrossRef] [PubMed]
- Venna, S.R.; Lartey, M.; Li, T.; Spore, A.; Kumar, S.; Nulwala, H.B.; Luebke, D.R.; Rosi, N.L.; Albenze, E. Fabrication of MMMs with improved gas separation properties using externally-functionalized MOF particles. J. Mater. Chem. A 2015, 3, 5014–5022. [Google Scholar] [CrossRef]
- Khdhayyer, M.R.; Esposito, E.; Fuoco, A.; Monteleone, M.; Giorno, L.; Jansen, J.C.; Attfield, M.P.; Budd, P.M. Mixed matrix membranes based on UiO-66 MOFs in the polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2017, 173, 304–313. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Zamidi Ahmad, M.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; Fila, V.; Coronas, J. Enhancement of CO2/CH4 separation performances of 6FDA-based co-polyimides mixed matrix membranes embedded with UiO-66 nanoparticles. Sep. Purif. Technol. 2018, 192, 465–474. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; de Vos, W.M.; Benes, N.E.; Konnertz, N.M.; Visser, T.; Semino, R.; et al. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. J. Memb. Sci. 2018, 558, 64–77. [Google Scholar] [CrossRef]
- Yazaydın, A.O.; Benin, A.I.; Faheem, S.A.; Jakubczak, P.; Low, J.J.; Willis, R.R.; Snurr, R.Q. Enhanced CO2 adsorption in metal-organic frameworks via occupation of open-metal sites by coordinated water molecules. Chem. Mater. 2009, 21, 1425–1430. [Google Scholar] [CrossRef]
- Du, M.; Li, L.; Li, M.; Si, R. Adsorption mechanism on metal organic frameworks of Cu-BTC, Fe-BTC and ZIF-8 for CO2 capture investigated by X-ray absorption fine structure. RSC Adv. 2016, 6, 62705–62716. [Google Scholar] [CrossRef]
- Ge, L.; Zhou, W.; Rudolph, V.; Zhu, Z. Mixed matrix membranes incorporated with size-reduced Cu-BTC for improved gas separation. J. Mater. Chem. A 2013, 1, 6350–6358. [Google Scholar] [CrossRef]
- Abedini, R.; Mosayebi, A.; Mokhtari, M. Improved CO2 separation of azide cross-linked PMP mixed matrix membrane embedded by nano-CuBTC metal organic framework. Process Saf. Environ. Prot. 2018, 114, 229–239. [Google Scholar] [CrossRef]
- Tayebeh, K.; Mohammadreza, O.; Serge, K.; Denis, R. Amine-functionalized CuBTC/poly(ether-b-amide-6) (Pebax® MH 1657) mixed matrix membranes for CO2/CH4 separation. Can. J. Chem. Eng. 2017, 95, 2024–2033. [Google Scholar] [CrossRef]
- Perez, E.V; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Metal-organic polyhedra 18 mixed-matrix membranes for gas separation. J. Memb. Sci. 2014, 463, 82–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Musselman, I.H.; Ferraris, J.P.; Balkus, K.J. Gas permeability properties of Matrimid® membranes containing the metal-organic framework Cu–BPY–HFS. J. Memb. Sci. 2008, 313, 170–181. [Google Scholar] [CrossRef]
- Sandrine, B.; Philip, L.L.; Christian, S.; Franck, M.; Thierry, L.; Gérard, F. Different Adsorption Behaviors of Methane and Carbon Dioxide in the Isotypic Nanoporous Metal Terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef]
- Hu, Y.H.; Zhang, L. Hydrogen Storage in Metal-Organic Frameworks. Adv. Mater. 2010, 22, E117–E130. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.K.; Lin, K.-S.; Tu, M.-T. Hydrogen storage capacity enhancement of MIL-53(Cr) by Pd loaded activated carbon doping. J. Taiwan Inst. Chem. Eng. 2016, 63, 463–472. [Google Scholar] [CrossRef]
- Lin, K.-S.; Adhikari, A.K.; Tu, M.-T.; Wang, C.-H.; Chiang, C.-L. Preparation, characterization, and hydrogen storage capacity of MIL-53 metal-organic frameworks. J. Nanosci. Nanotechnol. 2013, 13, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.M.; Assfour, B.; Huot, J.; Dingemans, T.J.; Wagemaker, M.; Ramirez-Cuesta, A.J. Hydrogen in the Metal−Organic Framework Cr MIL-53. J. Phys. Chem. C 2010, 114, 10648–10655. [Google Scholar] [CrossRef]
- Hamon, L.; Llewellyn, P.L.; Devic, T.; Ghoufi, A.; Clet, G.; Guillerm, V.; Pirngruber, G.D.; Maurin, G.; Serre, C.; Driver, G.; et al. Co-adsorption and Separation of CO2−CH4 Mixtures in the Highly Flexible MIL-53(Cr) MOF. J. Am. Chem. Soc. 2009, 131, 17490–17499. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Filinchuk, Y.; Férey, G. How Hydration Drastically Improves Adsorption Selectivity for CO2 over CH4 in the Flexible Chromium Terephthalate MIL-53. Angew. Chemie Int. Ed. 2006, 45, 7751–7754. [Google Scholar] [CrossRef] [PubMed]
- Hamon, L.; Serre, C.; Devic, T.; Loiseau, T.; Millange, F.; Férey, G.; Weireld, G. De Comparative Study of Hydrogen Sulfide Adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) Metal−Organic Frameworks at Room Temperature. J. Am. Chem. Soc. 2009, 131, 8775–8777. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, F.; Omidkhah, M.; Abedini, R. Fabrication and characterization of Matrimid/MIL-53 mixed matrix membrane for CO2/CH4 separation. Chem. Eng. Res. Des. 2014, 92, 2439–2448. [Google Scholar] [CrossRef]
- Hsieh, J.O.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. MIL-53 frameworks in mixed-matrix membranes. Microporous Mesoporous Mater. 2014, 196, 165–174. [Google Scholar] [CrossRef]
- Abedini, R.; Omidkhah, M.; Dorosti, F. Highly permeable poly(4-methyl-1-pentyne)/NH2-MIL 53 (Al) mixed matrix membrane for CO2/CH4 separation. RSC Adv. 2014, 4, 36522–36537. [Google Scholar] [CrossRef]
- Feijani, E.A.; Mahdavi, H.; Tavasoli, A. Poly(vinylidene fluoride) based mixed matrix membranes comprising metal organic frameworks for gas separation applications. Chem. Eng. Res. Des. 2015, 96, 87–102. [Google Scholar] [CrossRef]
- Ahmadi Feijani, E.; Tavasoli, A.; Mahdavi, H. Improving Gas Separation Performance of Poly(vinylidene fluoride) Based Mixed Matrix Membranes Containing Metal–Organic Frameworks by Chemical Modification. Ind. Eng. Chem. Res. 2015, 54, 12124–12134. [Google Scholar] [CrossRef]
- Tien-Binh, N.; Vinh-Thang, H.; Chen, X.Y.; Rodrigue, D.; Kaliaguine, S. Polymer functionalization to enhance interface quality of mixed matrix membranes for high CO2/CH4 gas separation. J. Mater. Chem. A 2015, 3, 15202–15213. [Google Scholar] [CrossRef]
- Zhu, H.; Jie, X.; Wang, L.; Liu, D.; Cao, Y. Polydimethylsiloxane/postmodified MIL-53 composite layer coated on asymmetric hollow fiber membrane for improving gas separation performance. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Hong, D.-Y.; Hwang, Y.K.; Serre, C.; Férey, G.; Chang, J.-S. Porous Chromium Terephthalate MIL-101 with Coordinatively Unsaturated Sites: Surface Functionalization, Encapsulation, Sorption and Catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Naseri, M.; Mousavi, S.F.; Mohammadi, T.; Bakhtiari, O. Synthesis and gas transport performance of MIL-101/Matrimid mixed matrix membranes. J. Ind. Eng. Chem. 2015, 29, 249–256. [Google Scholar] [CrossRef]
- Rajati, H.; Navarchian, A.H.; Tangestaninejad, S. Preparation and characterization of mixed matrix membranes based on Matrimid/PVDF blend and MIL-101(Cr) as filler for CO2/CH4 separation. Chem. Eng. Sci. 2018, 185, 92–104. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Guo, X.; Huang, H.; Zhong, C. Fabrication of mixed-matrix membranes with MOF-derived porous carbon for CO2 separation. AIChE J. 2018. [Google Scholar] [CrossRef]
- Tanh Jeazet, H.B.; Sorribas, S.; Román-Marín, J.M.; Zornoza, B.; Téllez, C.; Coronas, J.; Janiak, C. Increased Selectivity in CO2/CH4 Separation with Mixed-Matrix Membranes of Polysulfone and Mixed-MOFs MIL-101(Cr) and ZIF-8. Eur. J. Inorg. Chem. 2016, 2016, 4363–4367. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; Webley, P.A.; Qiao, G.G. MOF Scaffold for a High-Performance Mixed-Matrix Membrane. Angew. Chemie Int. Ed. 2018. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. High pressure gas separation performance of mixed-matrix polymer membranes containing mesoporous Fe(BTC). J. Memb. Sci. 2014, 459, 33–44. [Google Scholar] [CrossRef]
- Rita, A.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Alves, V.D.; Esteves, I.A.A.C. Separation and Purification Technology CO2/N2 gas separation using Fe(BTC)-based mixed matrix membranes: A view on the adsorptive and filler properties of metal-organic frameworks. Sep. Purif. Technol. 2018, 202, 174–184. [Google Scholar] [CrossRef]
- Dorosti, F.; Alizadehdakhel, A. Fabrication and investigation of PEBAX/Fe-BTC, a high permeable and CO2 selective mixed matrix membrane. Chem. Eng. Res. Des. 2017, 136, 119–128. [Google Scholar] [CrossRef]
- Cadiau, A.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Eddaoudi, M. A metal-organic framework–based splitter for separating propylene from propane. Science 2016, 353, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, K.; Xiang, L.; Dong, X.; Han, Y.; Wang, C.; Sun, L.; Pan, Y. Enhanced CO2/CH4 separation performance of mixed-matrix membranes through dispersion of sorption-selective MOF nanocrystals. J. Membr. Sci. 2018, 563, 360–370. [Google Scholar] [CrossRef]
- Bae, T.-H.; Long, J.R. CO2/N2 separations with mixed-matrix membranes containing Mg2(dobdc) nanocrystals. Energy Environ. Sci. 2013, 6, 3565–3569. [Google Scholar] [CrossRef]
- Smith, Z.P.; Bachman, J.E.; Li, T.; Gludovatz, B.; Kusuma, V.A.; Xu, T.; Hopkinson, D.P.; Ritchie, R.O.; Long, J.R. Increasing M2(dobdc) Loading in Selective Mixed-Matrix Membranes: A Rubber Toughening Approach. Chem. Mater. 2018, 30, 1484–1495. [Google Scholar] [CrossRef]
- Konstas, K.; Taylor, J.W.; Thornton, A.W.; Doherty, C.M.; Lim, W.X.; Bastow, T.J.; Kennedy, D.F.; Wood, C.D.; Cox, B.J.; Hill, J.M. Lithiated porous aromatic frameworks with exceptional gas storage capacity. Angew. Chemie Int. Ed. 2012, 51, 6639–6642. [Google Scholar] [CrossRef] [PubMed]
- Dechnik, J.; Gascon, J.; Doonan, C.; Janiak, C.; Sumby, C.J. Mixed-matrix membranes. Angew. Chemie Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.H.; Trewin, A. Amorphous PAF-1: Guiding the rational design of ultraporous materials. J. Phys. Chem. C 2014, 118, 19712–19722. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. 2009, 121, 9621–9624. [Google Scholar] [CrossRef]
- Li, Y.; Roy, S.; Ben, T.; Xu, S.; Qiu, S. Micropore engineering of carbonized porous aromatic framework (PAF-1) for supercapacitors application. Phys. Chem. Chem. Phys. 2014, 16, 12909–12917. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, J.; Custelcean, R.; Jiang, D. Nitrogen-doped porous aromatic frameworks for enhanced CO2 adsorption. J. Colloid Interface Sci. 2015, 438, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ben, T.; Qiu, S. Porous aromatic frameworks: Synthesis structure and functions. CrystEngComm 2013, 15, 17–26. [Google Scholar] [CrossRef]
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.C.Y.; Sprouster, D.J. Ending aging in super glassy polymer membranes. Angew. Chem. Int. Ed. 2014, 53, 5322–5326. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.V; Bakhtin, D.S.; Kulikov, L.A.; Terenina, M.V.; Golubev, G.S.; Bondarenko, G.N.; Legkov, S.A.; Shandryuk, G.A.; Volkov, V.V; Khotimskiy, V.S. Stabilization of gas transport properties of PTMSP with porous aromatic framework: Effect of annealing. J. Memb. Sci. 2016, 517, 80–90. [Google Scholar] [CrossRef]
- Lau, C.H.; Konstas, K.; Doherty, C.M.; Kanehashi, S.; Ozcelik, B.; Kentish, S.E.; Hill, A.J.; Hill, M.R. Tailoring Physical Aging in Super Glassy Polymers with Functionalized Porous Aromatic Frameworks for CO2 Capture Tailoring Physical Aging in Super Glassy Polymers with Functionalized Porous Aromatic Frameworks for CO2 Capture. Chem. Mater. 2015, 27, 4756–4762. [Google Scholar] [CrossRef]
- Mitra, T.; Bhavsar, R.S.; Adams, D.J.; Budd, P.M.; Cooper, A.I. PIM-1 mixed matrix membranes for gas separations using cost-effective hypercrosslinked nanoparticle fillers. Chem. Commun. 2016, 52, 5581–5584. [Google Scholar] [CrossRef] [PubMed]
- Yaumi, A.L.; Bakar, M.Z.A.; Hameed, B.H. Recent advances in functionalized composite solid materials for carbon dioxide capture. Energy 2017, 124, 461–480. [Google Scholar] [CrossRef]
- Matsukata, M.; Sawamura, K.; Sekine, Y.; Kikuchi, E. Chapter 8—Review on Prospects for Energy Saving in Distillation Process with Microporous Membranes. In Inorganic Polymeric and Composite Membranes; Oyama, S.T., Stagg-Williams, S., Eds.; Elsevier: New York, NY, USA, 2011; Volume 14, pp. 175–193. ISBN 0927-5193. [Google Scholar]
- Rangnekar, N.; Mittal, N.; Elyassi, B.; Caro, J.; Tsapatsis, M. Zeolite membranes—A review and comparison with MOFs. Chem. Soc. Rev. 2015, 44, 7128–7154. [Google Scholar] [CrossRef] [PubMed]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Hosseinzadeh Beiragh, H.; Omidkhah, M.; Abedini, R.; Khosravi, T.; Pakseresht, S. Synthesis and characterization of poly (ether-block-amide) mixed matrix membranes incorporated by nanoporous ZSM-5 particles for CO2/CH4 separation. Asia-Pac. J. Chem. Eng. 2016, 11, 522–532. [Google Scholar] [CrossRef]
- Dorosti, F.; Omidkhah, M.; Abedini, R. Enhanced CO2/CH4 separation properties of asymmetric mixed matrix membrane by incorporating nano-porous ZSM-5 and MIL-53 particles into Matrimid® 5218. J. Nat. Gas Sci. Eng. 2015, 25, 88–102. [Google Scholar] [CrossRef]
- Bryan, N.; Lasseuguette, E.; Van Dalen, M.; Permogorov, N.; Amieiro, A.; Brandani, S.; Ferrari, M.C. Development of mixed matrix mebranes containing zeolites for post-combustion carbon capture. Energy Procedia 2014, 63, 160–166. [Google Scholar] [CrossRef]
- Bezerra, D.P.; da Silva, F.W.M.; de Moura, P.A.S.; Sousa, A.G.S.; Vieira, R.S.; Rodriguez-Castellon, E.; Azevedo, D.C.S. CO2 adsorption in amine-grafted zeolite 13X. Appl. Surf. Sci. 2014, 314, 314–321. [Google Scholar] [CrossRef]
- Surya Murali, R.; Ismail, A.F.; Rahman, M.A.; Sridhar, S. Mixed matrix membranes of Pebax-1657 loaded with 4A zeolite for gaseous separations. Sep. Purif. Technol. 2014, 129, 1–8. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Hua, K.; Deng, M. Poly(amide-6-b-ethylene oxide)/SAPO-34 mixed matrix membrane for CO2 separation. J. Energy Chem. 2014, 23, 227–234. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Falconer, J.L.; Noble, R.D. Hydrogen purification using a SAPO-34 membrane. J. Memb. Sci. 2008, 307, 277–283. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Shahidi, K.; Mohammadi, T. Hydrogen separation and purification using crosslinkable PDMS/zeolite A nanoparticles mixed matrix membranes. Int. J. Hydrog. Energy 2012, 37, 14576–14589. [Google Scholar] [CrossRef]
- Atalay-Oral, Ç.; Tokay, B.; Erdem-Şenatalar, A.; Tantekin-Ersolmaz, B. Ferrierite-poly(vinyl acetate) mixed matrix membranes for gas separation: A comparative study. Microporous Mesoporous Mater. 2018, 259, 17–25. [Google Scholar] [CrossRef]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Palomino, M.; Valencia, S.; Irabien, A. LTA/Poly(1-trimethylsilyl-1-propyne) Mixed-Matrix Membranes for High-Temperature CO2/N2 Separation. Chem. Eng. Technol. 2015, 38, 658–666. [Google Scholar] [CrossRef]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Palomino, M.; Valencia, S.; Irabien, A. Permselectivity improvement in membranes for CO2/N2 separation. Sep. Purif. Technol. 2016, 157, 102–111. [Google Scholar] [CrossRef]
- Gong, H.; Lee, S.S.; Bae, T.-H. Mixed-matrix membranes containing inorganically surface-modified 5A zeolite for enhanced CO2/CH4 separation. Microporous Mesoporous Mater. 2017, 237, 82–89. [Google Scholar] [CrossRef]
- Pakizeh, M.; Hokmabadi, S. Experimental study of the effect of zeolite 4A treated with magnesium hydroxide on the characteristics and gas-permeation properties of polysulfone-based mixed-matrix membranes. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Nasernejad, B. Advances Aminosilane-functionalization of a nanoporous Y-type zeolite for application in a cellulose acetate based mixed matrix membrane for CO2 separation. RSC Adv. 2014, 4, 63966–63976. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Yao, J.; Chen, R.; Low, Z.; He, M.; Liu, J.Z.; Wang, H. Oriented two-dimensional zeolitic imidazolate framework-L membranes and their gas permeation properties. J. Mater. Chem. A 2015, 3, 15715–15722. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K.; et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 2017, 543, 690. [Google Scholar] [CrossRef] [PubMed]
- Varoon, K.; Zhang, X.; Elyassi, B.; Brewer, D.D.; Gettel, M.; Kumar, S.; Lee, J.A.; Maheshwari, S.; Mittal, A.; Sung, C.-Y.; et al. Dispersible Exfoliated Zeolite Nanosheets and Their Application as a Selective Membrane. Science 2011, 334, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jiang, Z.; Cao, K.; Nair, S.; Cheng, X.; Zhao, J.; Gomaa, H.; Wu, H.; Pan, F. Pervaporation performance comparison of hybrid membranes filled with two-dimensional ZIF-L nanosheets and zero-dimensional ZIF-8 nanoparticles. J. Memb. Sci. 2017, 523, 185–196. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.S.; Bucknall, D.G.; Koros, W.J.; Nair, S. Nanoporous layered silicate AMH-3/cellulose acetate nanocomposite membranes for gas separations. J. Memb. Sci. 2013, 441, 129–136. [Google Scholar] [CrossRef]
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Hu, Z.; Tee, L.; Guo, Z.; Zhao, D. Mixed Matrix Membranes (MMMs) Comprising Exfoliated 2D Covalent Organic Frameworks (COFs) for Efficient CO2 Separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X.; Gascon, J. Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2014, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Shete, M.; Kumar, P.; Bachman, J.E.; Ma, X.; Smith, Z.P.; Xu, W.; Mkhoyan, K.A.; Long, J.R.; Tsapatsis, M. On the direct synthesis of Cu(BDC) MOF nanosheets and their performance in mixed matrix membranes. J. Memb. Sci. 2018, 549, 312–320. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Jia, C.; Wang, Y.; Zhai, L.; Wang, Q.; Zhao, D. Ultrathin mixed matrix membranes containing two-dimensional metal-organic framework nanosheets for efficient CO2/CH4 separation. J. Memb. Sci. 2017, 539, 213–223. [Google Scholar] [CrossRef]
- Yang, Y.; Goh, K.; Wang, R.; Bae, T.-H. High-performance nanocomposite membranes realized by efficient molecular sieving with CuBDC nanosheets. Chem. Commun. 2017, 53, 4254–4257. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Peng, Y.; Hu, Z.; Qian, Y.; Chi, C.; Yeo, L.Y.; Tee, L.; Zhao, D. Mixed matrix membranes composed of two-dimensional metal-organic framework nanosheets for pre-combustion CO2 capture: A relationship study of filler morphology versus membrane performance. J. Mater. Chem. A 2015, 3, 20801–20810. [Google Scholar] [CrossRef]
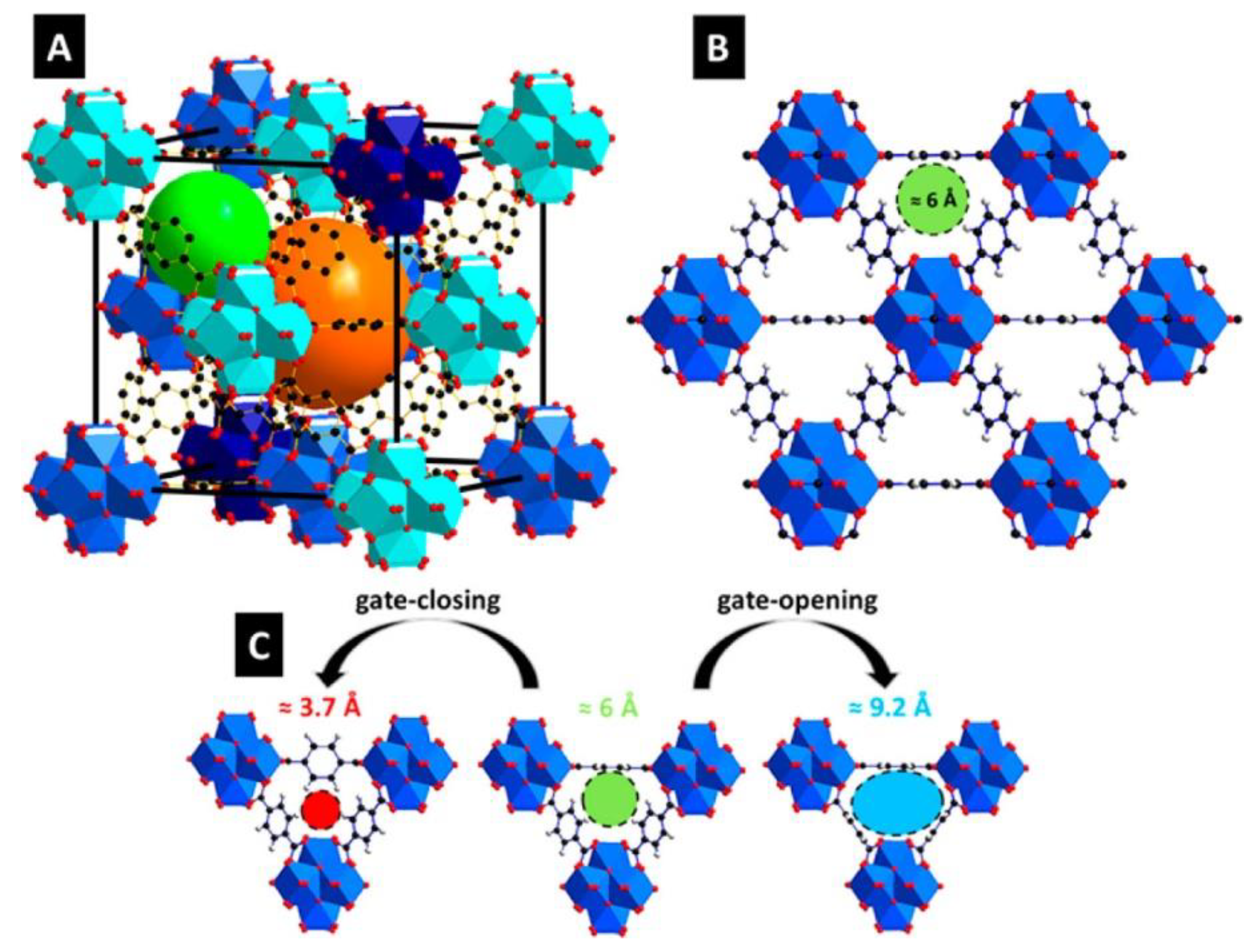
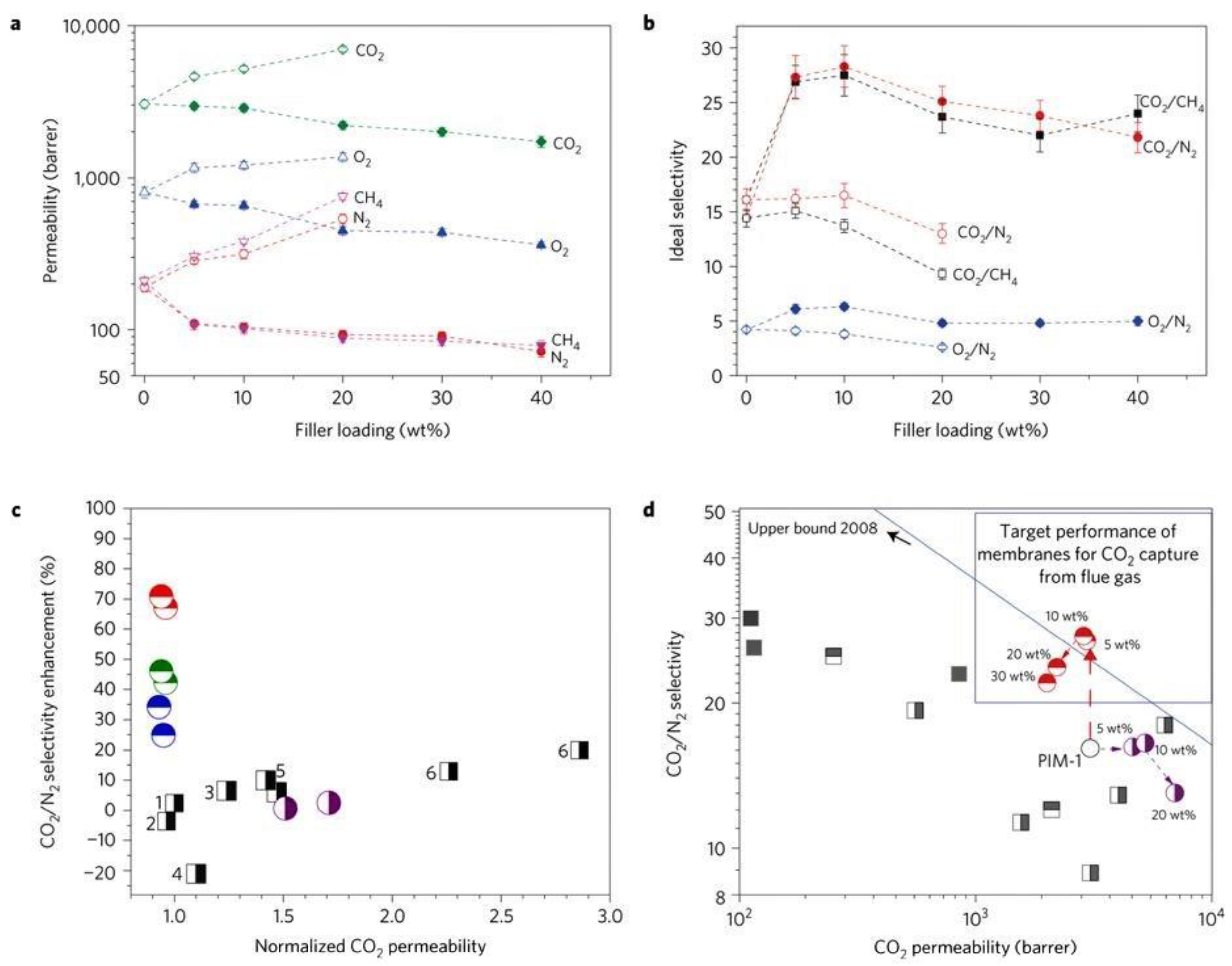
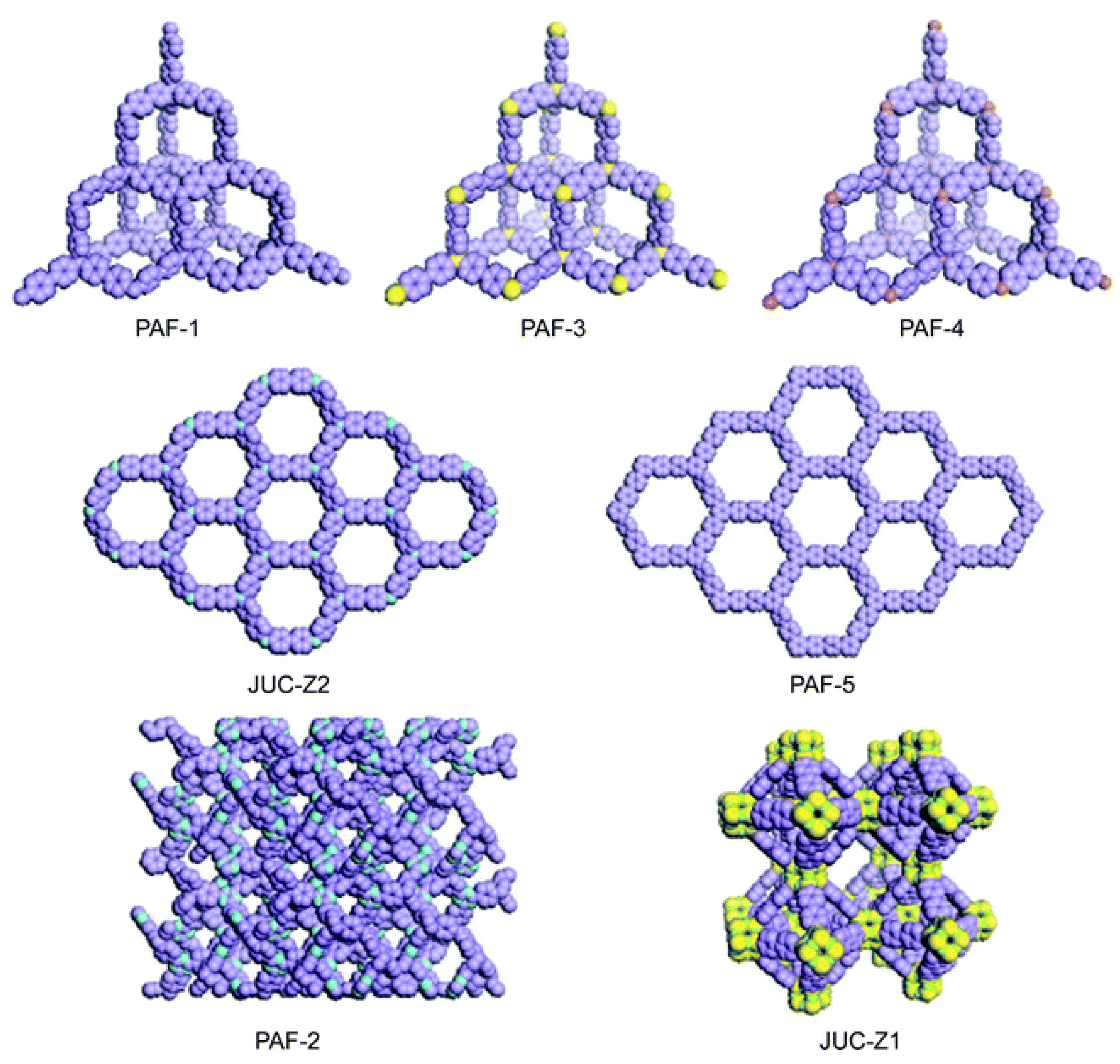
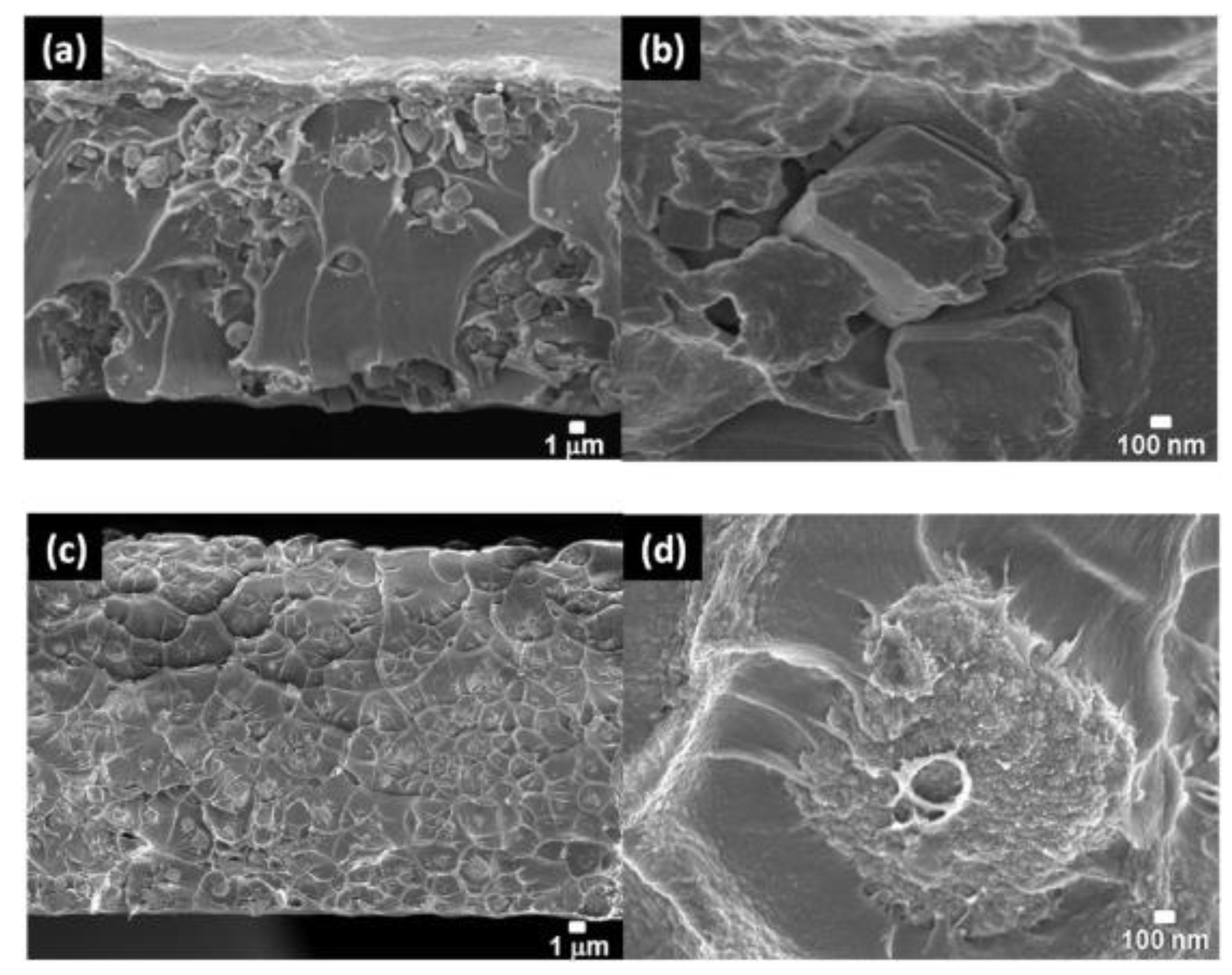
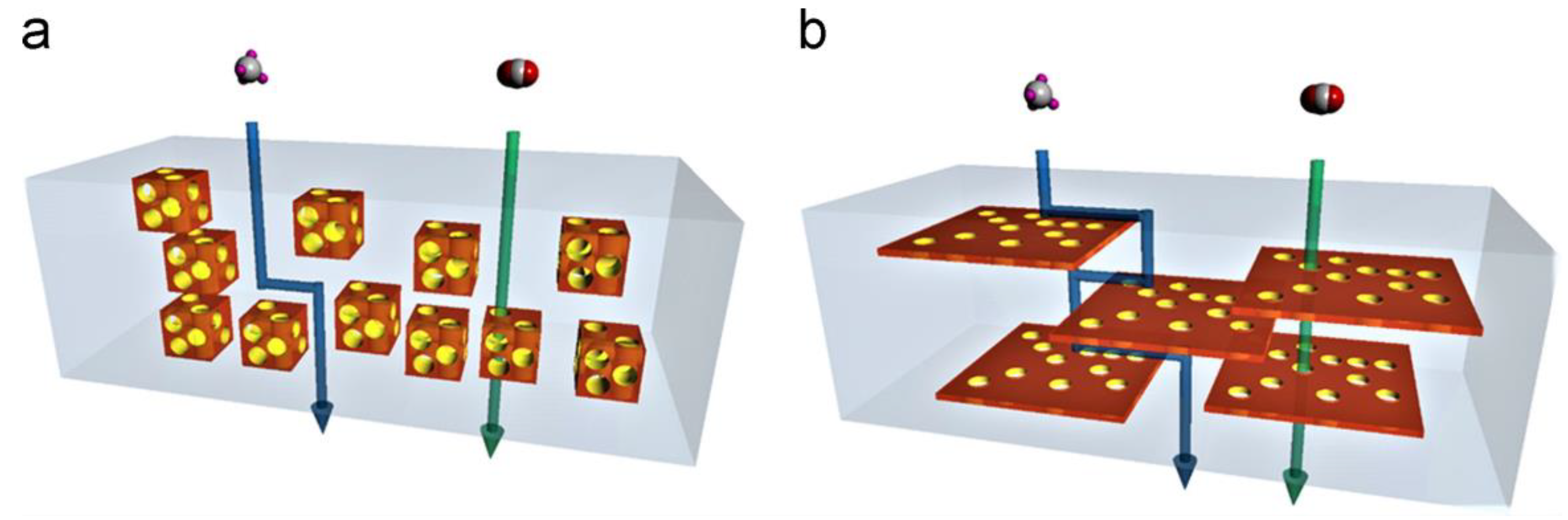
| Filler | Polymer | Loading (wt.%) | PCO2 (Barrer) | α CO2/N2 | α CO2/CH4 | α CO2/H2 | Ref. |
|---|---|---|---|---|---|---|---|
| ZIF-8 | |||||||
| Matrimid 5218 | 0 | 9.5 | 30.7 | 39.8 | 0.34 | [48] | |
| ZIF-8 | 20 | 9.0 | 30.1 | 51.1 | 0.29 | ||
| 50–150 nm | 30 | 14.2 | 24.1 | 38.2 | 0.31 | ||
| 40 | 24.5 | 23.4 | 27.8 | 0.35 | |||
| 50 | 4.7 | 26.2 | 124.9 | 0.35 | |||
| 60 | 8.1 | 18.4 | 80.7 | 0.26 | |||
| Matrimid 9725 | 0 | 0.21 a | 28.0 | [49] | |||
| ZIF-8 250–500 nm | 10 | 0.31 a | 29.5 | ||||
| 20 | 0.42 a | 31.0 | |||||
| 30 | 0.7 a | 31.5 | |||||
| Matrimid 5218 | 0 | 8.1 | 22.4 | 35.2 | [50] | ||
| ZIF-8 | 5 | 10.1 | 21.2 | 39.1 | |||
| 60 nm | 10 | 13.7 | 21.6 | 30.6 | |||
| 20 | 16.6 | 19.0 | 35.8 | ||||
| 30 | 28.7 | 17.1 | 24.9 | ||||
| ZIF-8 | Matrimid | 0 | 10.7 | 33.9 | [51] | ||
| Dir. Son. | 10 | 21.9 | 36.0 | ||||
| 25 | 47.0 | 39.0 | |||||
| Indir. Son. | 10 | 13.2 | 31.0 | ||||
| 25 | 23.2 | 31.9 | |||||
| Matrimid 5218 | 0 | 9.0 | 35.0 | [52] | |||
| ZIF-8 | 15 | 11.3 | 35.0 | ||||
| ZIF-8-ambz | 15 | 10.4 | 36.5 | ||||
| 30 | 10.2 | 38.0 | |||||
| Matrimid 5218 | 0 | 9.5 | 13.6 | 29.8 | 0.31 | [54] | |
| ZIF-8 | 10 | 13.1 | 20.5 | 0.26 | |||
| 95 nm | 10 b | 15.5 | 26.7 | 34.4 | 0.34 | ||
| P84 | 0 | 2.7 c | 54.1 | [55] | |||
| ZIF-8 | 8 | 3.2 c | 63.5 | ||||
| 30 nm | 17 | 6.3 c | 93.6 | ||||
| 27 | 11.0 c | 92.3 | |||||
| 31 | 20.0 c | 45.8 | |||||
| 6FDA-durene | 0 | 468 | 7 | [56] | |||
| ZIF-8 | 5 | 694 | 16.5 | ||||
| 50 nm | 10 | 1427 | 28.7 | ||||
| 15 | 1466 | 11.3 | |||||
| 20 | 1463 | 8.97 | |||||
| 6FDA-durene | 0 | 469 | 13.4 | 15.6 | 0.91 | [57] | |
| ZIF-8 | 33 | 1553 | 11.3 | 11.1 | 0.71 | ||
| 33 d | 23.7 | 11.8 | 16.9 | 0.08 | |||
| 6FDA-durene | 0 | 352 | 16.6 | [59] | |||
| ZIF-8 | T = 200 °C | 20 | 487 | 17.9 | |||
| 80 nm | T = 350 °C | 0 | 432 | 13.8 | |||
| 20 | 857 | 13.1 | |||||
| T = 400 °C | 0 | 541 | 13.1 | ||||
| 20 | 1090 | 13.0 | |||||
| 6FDA-durene | 0 | 1468 | 25.4 | 22.6 | [60] | ||
| ZIF-8 | 3 | 1593 | 25.7 | 21.9 | |||
| 100–200 nm | 5 | 1695 | 22.7 | 20.1 | |||
| 7 | 1774 | 22.1 | 19.4 | ||||
| 10 | 1882 | 20.5 | 19 | ||||
| 15 | 1940 | 18.6 | 18.1 | ||||
| 20 | 2027 | 17.5 | 16.9 | ||||
| 30 | 2186 | 17 | 17.1 | ||||
| PEBAX 2533 | 0 | 351 | 35.1 | 8.3 | [61] | ||
| ZIF-8 | 5 | 305 | 25.4 | 6.8 | |||
| 10 | 427 | 30.5 | 8.5 | ||||
| 15 | 574 | 30.2 | 10.4 | ||||
| 20 | 854 | 28.5 | 9.2 | ||||
| 25 | 1082 | 30.9 | 8.5 | ||||
| 30 | 1176 | 31.8 | 8.7 | ||||
| 35 | 1287 | 32.2 | 9 | ||||
| Ultem 1000 | 0 | 14 e | 30 | [63] | |||
| ZIF-8 | 13 | 26 e | 36 | ||||
| ZIF-7 | |||||||
| PEBAX 1657 | 0 | 72 | 34 | 14 | [72] | ||
| ZIF-7 | 8 | 145 | 68 | 23 | |||
| 40–50 nm | 22 | 111 | 97 | 30 | |||
| 34 | 41 | 105 | 44 | ||||
| PEI | 0 | 82.5 | 3.8 | 4.4 | [73] | ||
| ZIF-7 | 5 | 64.7 | 17 | 12.9 | |||
| PSM-ZIF-7 g | 5 | 246 | 1.3 | 2.3 | |||
| ZIF-11 | |||||||
| PEBAX 2533 | 0 | 232 | 41.3 | 8 | [77] | ||
| ZIF-11 | 10 | 212 | 53 | 9.7 | |||
| 500–5000 nm | 30 | 186 | 47.9 | 11.4 | |||
| 50 | 233 | 46.9 | 11.2 | ||||
| 70 | 402 | 29 | 12.4 | ||||
| 6FDA-DAM | 0 | 21.4 | 32.7 | [78] | |||
| ZIF-11 | 10 | 107 | 31.3 | ||||
| 200–2000 nm | 20 | 273 | 31 | ||||
| 30 | 76.7 | 30.4 | |||||
| ZIF-71 | |||||||
| PIM-1 | 0 | 3265 | 20.1 | 10.2 | [79] | ||
| ZIF-71 | 10 | 4271 | 19.4 | 11.3 | |||
| <1000 nm | 20 | 5942 | 20 | 11.9 | |||
| 30 | 8377 | 18.3 | 11.2 | ||||
| UV-PIM-1 | 0 | 1233 | 29.8 | 34.1 | |||
| UV-ZIF-71 | 10 | 1909 | 29.1 | 35.5 | |||
| <1000 nm | 20 | 2546 | 27.2 | 35.3 | |||
| 30 | 3459 | 26.9 | 35.6 | ||||
| ZIF-71 | 6FDA-Durene | 0 | 805 | 14.7 | 17 | [76] | |
| 30 nm | 20 | 2560 | 13.8 | 14.2 | |||
| 200 nm | 20 | 2744 | 13.2 | 13.9 | |||
| 600 nm | 20 | 1656 | 13.5 | 14.7 | |||
| ZIF-90 | |||||||
| 6FDA-DAM | 0 | 402 | 17.5 | [80] | |||
| ZIF-90 | 15 | 808 | 27.2 | ||||
| 810 nm | Ultem®1000 | 0 | 1.4 | 37.9 | |||
| ZIF-90 | 15 | 2.9 | 38.9 | ||||
| Matrimid | 0 | 7.7 | 34.9 | ||||
| ZIF-90 | 15 | 12.1 | 34.8 | ||||
| 6FDA-DAM h | 0 | 390 | 24 | ||||
| 15 | 720 | 37 | |||||
| 6FDA-TP i | 0 | 20 | 20 | 37 | [81] | ||
| ZIF-90 | 10 | 26 | 24 | 42 | |||
| 60–105 nm | 20 | 29 | 22 | 38 | |||
| 40 | 45 | 20 | 36 | ||||
| 50 | 63 | 20 | 36 | ||||
| Filler | Polymer | Loading (wt.%) | PCO2 (Barrer) | α CO2/N2 | α CO2/CH4 | α CO2/H2 | Ref. |
|---|---|---|---|---|---|---|---|
| PEBAX 1657 | 0 | 51.5 | 42.1 | [86] | |||
| UiO-66 | 5 | 75.0 | 56.0 | ||||
| 60–80 nm | 7.5 | 90.0 | 60.0 | ||||
| 10 | 96.3 | 56.6 | |||||
| 12.5 | 110.5 | 40.0 | |||||
| 15 | 115.0 | 27.0 | |||||
| 20 | 134.0 | 21.0 | |||||
| UiO-66-NH2 60–80 nm | 5 | 71.0 | 68.0 | ||||
| 7.5 | 78.0 | 76.0 | |||||
| 10 | 87.0 | 79.2 | |||||
| 12.5 | 96.0 | 45.0 | |||||
| 15 | 100.0 | 37.5 | |||||
| 20 | 122 | 26 | |||||
| Matrimid 9725 a | 0 | 5.9 | 31.2 | [87] | |||
| UiO-66 | 30 | 15.0 | 35.8 | ||||
| UiO-66-BA | 30 | 17.8 | 42.9 | ||||
| UiO-66-ABA | 30 | 13.6 | 45.1 | ||||
| UiO-66-NH2 | 30 | 17.8 | 37.3 | ||||
| UiO-66-NH2-BA | 30 | 17.4 | 39.3 | ||||
| UiO-66-NH2-ABA | 30 | 38.0 | 47.4 | ||||
| Matrimid 5218 b | 0 | 8.5 | 29 | [88] | |||
| UiO-66 -NH2 | 12 | 18.5 | 33 | ||||
| 200 nm | 23 | 24 | 36 | ||||
| 40 | 28 | 27.5 | |||||
| UiO-66-NH2-PA | 12 | 20.5 | 32.5 | ||||
| 23 | 28 | 36.5 | |||||
| 40 | 31 | 28 | |||||
| UiO-66-NH2-C10 | 23 | 22.5 | 28 | ||||
| UiO-66-NH2-SA | 23 | 20 | 30.5 | ||||
| PIM-1 | 0 | 4770 | 21.8 | 16.7 | 1.76 | [89] | |
| UiO-66 | as cast | 9.1 | 5940 | 23.2 | 16. | 1.93 | |
| 200 nm | 16.6 | 7610 | 20.7 | 14.4 | 1.67 | ||
| 23.1 | 7610 | 20.7 | 14.4 | 1.67 | |||
| 28.6 | 4940 | 13.6 | 11.2 | 0.66 | |||
| UiO-66-(COOH)2 | 9.1 | 4600 | 20.9 | 14.1 | 2.22 | ||
| 200 nm | 16.6 | 5190 | 20.4 | 13.2 | 2.19 | ||
| 23.1 | 5300 | 19.9 | 12.9 | 2.22 | |||
| 28.6 | 6090 | 20.6 | 15.2 | 1.63 | |||
| UiO-66-NH2 | 9.1 | 4810 | 22.2 | 16.5 | 1.62 | ||
| 200 nm | 16.6 | 6340 | 20.9 | 14.9 | 2.03 | ||
| 23.1 | 5070 | 20.1 | 14.7 | 1.58 | |||
| 28.6 | 6310 | 21.5 | 13.3 | 2.10 | |||
| PIM-1 | 0 | 8210 | 21.2 | 15.7 | 1.63 | ||
| UiO-66 | exchanged solvent | 16.6 | 9980 | 21.6 | 17 | 1.23 | |
| 200 nm | 23.1 | 9980 | 21.6 | 17 | 1.23 | ||
| 28.6 | 10,900 | 15.2 | 13.2 | 1.74 | |||
| UiO-66-(COOH)2 | 16.6 | 9720 | 18.9 | 11.7 | 2.28 | ||
| 200 nm | 23.1 | 8770 | 18.1 | 11 | 2.05 | ||
| 28.6 | 9020 | 22.1 | 13.5 | 1.02 | |||
| UiO-66-NH2 | 9.1 | 8740 | 22 | 14.7 | 1.84 | ||
| 200 nm | 16.6 | 10,700 | 21.4 | 13.7 | 1.88 | ||
| 23.1 | 9570 | 23.4 | 13.8 | 1.43 | |||
| 28.6 | 9030 | 19.5 | 13 | 1.70 | |||
| PIM-1 | 0 | 3054 | 16.1 | 14.5 | 1.67 | [90] | |
| UiO-66 | 5 | 4620 | 16.2 | 15.1 | 1.90 | ||
| 100–200 nm | 10 | 5210 | 16.5 | 13.7 | 2.04 | ||
| 20 | 6981 | 13 | 9.3 | 2.60 | |||
| UiO-66-H | 5 | 2765 | 22.9 | 18.2 | 0.88 | ||
| 20–30 nm | 10 | 2631 | 23.5 | 18.8 | 0.88 | ||
| 20 | 2606 | 24.6 | 20.1 | 0.89 | |||
| 30 | 1880 | 18.3 | 16.1 | 1.55 | |||
| 40 | 1023 | 21.4 | 15.8 | 1.67 | |||
| UiO-66-NH2 | 5 | 2952 | 26.9 | 27.3 | 1.11 | ||
| 20–30 nm | 10 | 2869 | 27.5 | 28.3 | 1.09 | ||
| 20 | 2210 | 23.7 | 25.1 | 0.99 | |||
| 30 | 2005 | 22 | 23.8 | 0.99 | |||
| 40 | 1727 | 24 | 21.8 | 0.86 | |||
| UiO-66-Br | 5 | 2890 | 20.1 | 18.1 | 1.49 | ||
| 20–30 nm | 10 | 2846 | 21.6 | 17.1 | 1.25 | ||
| 20 | 2416 | 19.3 | 16.3 | 1.53 | |||
| 30 | 2294 | 19 | 17.1 | 1.57 | |||
| 40 | 1441 | 23.6 | 20.8 | 1.03 | |||
| 6FDA-BisP | 0 | 33.9 | 27.5 | [91] | |||
| UiO-66 | 6 | 56.7 | 33.6 | ||||
| 50–100 nm | 14 | 83.9 | 36.2 | ||||
| 17 | 108 | 41.9 | |||||
| 21 | 155 | 24.6 | |||||
| 6FDA-ODA | 0 | 25.9 | 20.6 | ||||
| UiO-66 | 4 | 30.1 | 38 | ||||
| 50–100 nm | 8 | 37.4 | 51.5 | ||||
| 17 | 43.3 | 57 | |||||
| 23 | 72 | 21.5 | |||||
| 6FDA-DAM | 0 | 997 | 29.2 | ||||
| UiO-66 | 4 | 1283 | 29.6 | ||||
| 50–100 nm | 8 | 1728 | 32 | ||||
| 14 | 1912 | 30.9 | |||||
| 21 | 2358 | 12.7 | |||||
| 6FDA-DAM | 0 | 1010 c | 29.2 | [92] | |||
| UiO-66 | 4 | 1290 c | 29.6 | ||||
| 8 | 1730 c | 32.1 | |||||
| 14 | 1915 c | 31.2 | |||||
| 21 | 2365 c | 12.6 | |||||
| UiO-66-NH2 | 4 | 1295 c | 29.2 | ||||
| 8 | 1300 c | 30.3 | |||||
| 14 | 1345 c | 29.9 | |||||
| 21 | 1585 c | 20.7 | |||||
| UiO-66-NH-COCH3 | 4 | 1081 c | 30.3 | ||||
| 8 | 1171 c | 32.5 | |||||
| 14 | 1266 c | 33.1 | |||||
| 21 | 1417 c | 24.1 |
| Filler | Polymer | Loading (wt.%) | PCO2 (Barrer) | α CO2/N2 | α CO2/CH4 | α CO2/H2 | Ref. |
|---|---|---|---|---|---|---|---|
| Matrimid 9725 | 0 | 0.21 a | 28.0 | [49] | |||
| Cu3(BTC)2 | 10 | 0.3 a | 30.0 | ||||
| 10 μm | 20 | 0.41 a | 31.0 | ||||
| 30 | 0.64 a | 32.5 | |||||
| Cu3(BTC)2 | PPO | 0 | 68.9 | 16.1 | 16.2 | 0.92 | [95] |
| 6 µm | 10 | 87.2 | 23.8 | 28.2 | 0.94 | ||
| PMP | 0 | 76.1 | 20.5 | 15.2 | 7.5 | [96] | |
| Cu3(BTC)2 | 5 | 88.3 | 22.2 | 17.1 | 8.1 | ||
| 100 nm | 10 | 103 | 23.7 | 19.2 | 9.2 | ||
| 15 | 124 | 25.4 | 22.7 | 10.7 | |||
| 20 | 144 | 28.6 | 24.3 | 12.2 | |||
| PEBAX 1657 | 0 | 84.2 | 16.4 | [97] | |||
| Cu3(BTC)2 | 5 | 91.4 | 17.7 | ||||
| 10 | 102.7 | 19 | |||||
| 15 | 128.8 | 20.5 | |||||
| 20 | 167.3 | 19.5 | |||||
| NH2-Cu3(BTC)2 | 5 | 93 | 18.4 | ||||
| 10 | 108.8 | 21 | |||||
| 15 | 135.2 | 23.6 | |||||
| 20 | 170.1 | 26.2 | |||||
| Matrimid 5218 | 0 | 7.3 | 30.5 | 32.8 | 0.43 | [98] | |
| MOP-18 | 23 | 9.4 | 27.6 | 23.2 | 0.53 | ||
| 33 | 14 | 22.9 | 21.8 | 0.63 | |||
| 44 | 15.6 | 26.0 | 16.4 | 0.70 | |||
| MMIF | Matrimid 5218 | 0 | 6.8 | 26.2 | 35.9 | [6] | |
| 50 nm | 10 | 8.1 | 27.3 | 36.9 | |||
| 200 nm | 20 | 8.6 | 27 | 34.6 | |||
| Matrimid 5218 | 0 | 8.0 b | 38.3 | ||||
| 50 nm | 10 | 9.7 b | 81 | ||||
| 200 nm | 20 | 10.1 b | 88 | ||||
| Matrimid 5218 | 0 | 7.1 c | 32.3 | ||||
| 50 nm | 10 | 8.2 c | 38.9 | ||||
| 200 nm | 20 | 11.7 c | 58 | ||||
| CU-BPY-HFS d | Matrimid 5218 | 0 | 7.3 | 33.1 | 34.7 | 0.42 | [99] |
| 200–300 nm | 10 | 7.81 | 32.5 | 31.9 | 0.46 | ||
| 20 | 9.88 | 31.9 | 27.6 | 0.59 | |||
| 30 | 10.36 | 33.4 | 27.4 | 0.51 | |||
| 40 | 15.06 | 30.7 | 25.5 | 0.56 |
| Filler | Polymer | Loading (wt.%) | PCO2 (Barrer) | α CO2/N2 | α CO2/CH4 | α CO2/H2 | Ref. |
|---|---|---|---|---|---|---|---|
| Matrimid 5218 | 0 | 6.2 | 28.2 | [108] | |||
| MIL-53 (Al) | 5 | 6.8 | 29.6 | ||||
| 123–466 nm | 10 | 7.45 | 31 | ||||
| 15 | 12.43 | 51.8 | |||||
| 20 | 14.52 | 15.1 | |||||
| Matrimid 5218 | 0 | 8.4 | 33.6 | 39.4 | 0.33 | [109] | |
| MIL-53-as a | 37.5 | 40 | 95.2 | 90.1 | 0.55 | ||
| MIL-53-ht a | 33.3 | 26.6 | 42.9 | 45.7 | 0.50 | ||
| 50–100 nm | 37.5 | 51 | 28.3 | 47.0 | 0.60 | ||
| PMP | 0 | 98.74 | 8.72 | [110] | |||
| NH2-MIL-53 (Al) | 5 | 107.32 | 11.85 | ||||
| 110 nm | 10 | 118.74 | 12.59 | ||||
| 15 | 139.56 | 15.72 | |||||
| 20 | 164.78 | 18.46 | |||||
| 25 | 203.44 | 20.18 | |||||
| 30 | 226.37 | 20.36 | |||||
| PVDF | 0 | 0.92 | 16.3 | 21.3 | [111] | ||
| MIL-53 | 5 | 1.21 | 16.3 | 21.2 | |||
| 100 nm | 10 | 1.55 | 16.2 | 21.0 | |||
| NH2-MIL-53(Al) | 5 | 1.11 | 17.3 | 23.1 | |||
| 100 nm | 10 | 1.41 | 19.5 | 26.0 | |||
| m-PVDF b | 0 | 1.2 | 27.9 | [112] | |||
| MIL-53 | 5 | 1.75 | 35.8 | ||||
| 100 nm | 10 | 2.45 | 39.6 | ||||
| NH2-MIL-53 | 5 | 1.69 | 37.6 | ||||
| 100 nm | 10 | 2.24 | 43.2 | ||||
| 6FDA–(DAM) | 0 | 316.6 c | 9.76 | [113] | |||
| MIL-53 (Al) | 10 | 331.9 c | 10.19 | ||||
| 190–340 nm | 15 | 354.0 c | 11.46 | ||||
| 6FDA–(DAM)–(HAB) 2:1 | 0 | 115.7 c | 21.65 | ||||
| 10 | 124.2 c | 24.62 | |||||
| 15 | 134.5 c | 26.96 | |||||
| 6FDA–(DAM)–(HAB) 1:1 | 0 | 46.8 c | 34.39 | ||||
| 10 | 55.3 c | 37.15 | |||||
| 15 | 63.0 c | 40.76 | |||||
| 6FDA–(DAM)–(HAB) 1:2 | 0 | 19.6 c | 43.1 | ||||
| 10 | 33.2 c | 47.13 | |||||
| 15 | 42.6 c | 48.83 | |||||
| 6FDA–(DAM) | 0 | 316.2 c | 9.77 | ||||
| NH2-MIL-53 (Al) | 10 | 308.9 | 13.63 | ||||
| 100–200 nm | 15 | 290.7 c | 14.77 | ||||
| 20 | 299.8 c | 8.86 | |||||
| 6FDA–(DAM)–(HAB) 2:1 | 0 | 115.7 c | 21.81 | ||||
| 10 | 112.1 c | 43.63 | |||||
| 15 | 105.7 c | 36.13 | |||||
| 20 | 122.1 c | 29.31 | |||||
| 6FDA–(DAM)–(HAB) 1:1 | 0 | 47.4 c | 34.54 | ||||
| 10 | 43.7 c | 77.72 | |||||
| 15 | 44.6 c | 64.54 | |||||
| 20 | 54.7 c | 35.68 | |||||
| 6FDA–(DAM)–(HAB) 1:2 | 0 | 24.6 c | 53.86 | ||||
| 10 | 20.0 c | 86.81 | |||||
| 15 | 21.9 c | 96.36 | |||||
| 20 | 31.9 c | 55.9 | |||||
| PDMS | 0 | 30 | 23.3 | 27.0 | 0.22 | [114] | |
| P-MIL-53 | 5 | 33.3 | 24.5 | 28.8 | |||
| 500 nm | 10 | 36.0 | 25.8 | 30.5 | 0.24 | ||
| 15 | 40.3 | 28.1 | 32.1 | ||||
| 20 | 42.3 | 27.5 | 28.4 | ||||
| Matrimid 5218 d | 0 | 4.44 | 34 | 35 | [116] | ||
| MIL-101(Cr) | 10 | 6.95 | 52 | 56 | |||
| ~1000 nm | 15 | 5.7 | 44 | 47 | |||
| 20 | 5.85 | 42 | 37 | ||||
| 30 | 7.99 | 47 | 44 | ||||
| Matrimid 5218 f | 0 | 7.33 | 34.9 | [117] | |||
| MIL-101(Cr) | 10 | 12.01 | 52.21 | ||||
| Matrimid/PVDF f | 0 | 9.42 | 42.81 | ||||
| MIL-101(Cr) | 10 | 14.87 | 62 | ||||
| PPO-PEG c,e | 0 | 657 | 18.42 | [118] | |||
| MIL-53(Al)-PC | 5 | 684 | 25.51 | ||||
| 200–250 nm | 10 | 723.6 | 29.23 | ||||
| 15 | 763 | 35.78 | |||||
| 20 | 789 | 40.39 | |||||
| 25 | 1266 | 31.53 | |||||
| MIL-101(Cr)-PC | PPO-PEG c,e | 0 | 657 | 19.26 | |||
| 50–100 nm | 5 | 771 | 22.93 | ||||
| 10 | 874 | 26.61 | |||||
| 15 | 952 | 30.46 | |||||
| 20 | 1056 | 34.66 | |||||
| 25 | 1896 | 29.24 | |||||
| PSF | 0 | 5 | 23 | [119] | |||
| MIL-101 | 8 | 8 | 21 | ||||
| 110–400 nm | 16 | 8.9 | 24 | ||||
| 24 | 18.1 | 28 | |||||
| ZIF-8 | 0 | 5 | 23 | ||||
| 75–100 nm | 8 | 10 | 35 | ||||
| 16 | 14 | 22 | |||||
| 24 | 24 | 24 | |||||
| MIL-101/ZIF-8 | 0 | 4.7 | 23 | ||||
| 8 | 10.6 | 36 | |||||
| 16 | 14.2 | 40 | |||||
| 24 | 24 | 26 | |||||
| 35 | 29.6 | 24 |
| Filler | Polymer | Loading (wt.%) | PCO2 (Barrer) | α CO2/N2 | α CO2/CH4 | α CO2/H2 | Ref. |
|---|---|---|---|---|---|---|---|
| Matrimid 5218 | 0 | 9 | 25 | [121] | |||
| Fe(BTC) | 10 | 9.5 | 27.5 | ||||
| 20 | 10.8 | 28 | |||||
| 30 | 13.1 | 29.5 | |||||
| Matrimid 5218 a | 0 | 14.6 | 4.4 | [122] | |||
| Fe(BTC) | 10 | 84.9 | 43.5 | ||||
| 10–20 um | 20 | 91.2 | 15.4 | ||||
| 30 | 217.9 | 23.1 | |||||
| Pebax 1657 | 0 | 70.67 | 18.4 | [123] | |||
| Fe(BTC) | 5 | 80.79 | 19.3 | ||||
| 10 | 82.32 | 19.4 | |||||
| 15 | 89.63 | 20.8 | |||||
| 20 | 98.32 | 22.2 | |||||
| 25 | 148.44 | 21.9 | |||||
| 30 | 402.69 | 21.5 | |||||
| 40 | 425.5 | 12.3 | |||||
| 0 | 60.35 b | 16.9 | |||||
| 10 | 70.11 b | 17.6 | |||||
| 20 | 85.28 b | 19.3 | |||||
| 30 | 329.7 b | 20.5 | |||||
| 40 | 345.4 b | 13.1 | |||||
| 6FDA Durene | 0 | 759.7 b | 34.7 | [125] | |||
| KAUST-7 | 11 | 895.7 b | 36.2 | ||||
| 80 nm | 22 | 966.9 b | 37.0 | ||||
| 33 | 1038.1 b | 37.6 | |||||
| PDMS | 0 | 3100.0 | 9.5 | [126] | |||
| Mg2(dobdc) | 20 | 2100.0 | 12 | ||||
| 100 nm | XLPEO | 0 | 380.0 | 22 | |||
| 10 | 250.0 | 25 | |||||
| 6FDA-TMPDA | 0 | 650.0 | 14 | ||||
| 10 | 850.0 | 23 |
| Filler | Polymer | Loading (wt.%) | PCO2 (Barrer) | α CO2/N2 | α CO2/CH4 | α CO2/H2 | Ref. |
|---|---|---|---|---|---|---|---|
| PTMSP – 0 d | 0 | 20,000 | 8.7 | [135] | |||
| PAF-1 | 10 | 25,000 | 8.1 | ||||
| PTMSP – 240 d | 0 | 12,400 | 9.8 | ||||
| PAF-1 | 10 | 23,200 | 9.6 | ||||
| PIM-1 – 0 d | 0 | 4000 | 15 | ||||
| PAF-1 | 10 | 15,000 | 12 | ||||
| PIM-1 – 240 d | 0 | 1700 | 19 | ||||
| PAF-1 | 10 | 15,000 | 19 | ||||
| PMP– 0 d | 0 | 6500 | 10.5 | ||||
| PAF-1 | 10 | 11,500 | 9.4 | ||||
| PMP – 240 d | 0 | 3500 | 11 | ||||
| PAF-1 | 10 | 10,500 | 9.4 | ||||
| PTMSP | 0 | 30,000 | 5.6 | [136] | |||
| PAF-11 | 1 | 38,000 | 5.9 | ||||
| 5 | 37,000 | 5.8 | |||||
| 10 | 34,000 | 5.6 | |||||
| 510 hours | 1 | 20,000 | 7 | ||||
| 5 | 19,500 | 6.8 | |||||
| 10 | 23,500 | 6.3 | |||||
| PTMSP | 0 | 30,000 | 5.9 | 2.3 | [137] | ||
| PAF-1 | 10 | 35,500 | 5.7 | 2.3 | |||
| PAF-1-NH2 | 10 | 43,000 | 5.9 | 2.2 | |||
| PAF-1-SO3H | 10 | 32,500 | 5.7 | 2.3 | |||
| PAF-1-C60 | 10 | 33,000 | 5 | 2.1 | |||
| PAF-1-Li6C60 | 10 | 55,000 | 5.4 | 2 | |||
| Aged | 0 | 8000 | 8.8 | 5.3 | |||
| PAF-1 | 10 | 28,000 | 7.4 | 3.1 | |||
| PAF-1-NH2 | 10 | 29,000 | 7.5 | 3.6 | |||
| PAF-1-SO3H | 10 | 23,500 | 6 | 2.6 | |||
| PAF-1-C60 | 10 | 15,000 | 8.3 | 5 | |||
| PAF-1-Li6C60 | 10 | 50,600 | 9 | 3.9 | |||
| PIM-1 - CH2Cl2 | 0 | 2258 | 24 | [138] | |||
| HCP | 5.7 | 4690 | 17.6 | ||||
| 16.67 | 5103 | 13.1 | |||||
| 21.3 | 6331 | 14.1 | |||||
| 150 d | 0 | 1109 | 4.2 | ||||
| HCP | 5.7 | 3616 | 19.7 | ||||
| 21.3 | 5060 | 16 | |||||
| PIM-1 - CHCl3 | 0 | 2660 | 22.3 | ||||
| HCP | 4.6 | 4313 | 19.8 | ||||
| 9.1 | 4700 | 19.3 | |||||
| 16.67 | 10,040 | 17.1 | |||||
| 150 d | 0 | 1225 | 21.5 | ||||
| HCP | 4.6 | 1857 | 22.4 | ||||
| 9.1 | 2043 | 22.2 | |||||
| 16.67 | 4165 | 21.8 |
| Filler | Polymer | Loading (wt.%) | PCO2 (Barrer) | α CO2/N2 | α CO2/CH4 | α CO2/H2 | Ref. |
|---|---|---|---|---|---|---|---|
| PEBAX 1675 | 0 | 120 | 20.3 | [143] | |||
| ZSM-5 | 5 | 230 | 21 | ||||
| 10 | 191 | 32.5 | |||||
| 15 | 170 | 33.9 | |||||
| Matrimid 5218 | 0 | 5.1 a | 14.8 | [144] | |||
| ZSM-5 | 6 | 6.6 a | 15.6 | ||||
| 15 | 11.1 a | 7.2 | |||||
| 24 | 14.5 a | 4.8 | |||||
| 30 | 21 a | 3.6 | |||||
| PEBAX 1675 | 0 | 81.4 | 41 | [145] | |||
| 13X | 10 | 104 | 39 | ||||
| 15 | 114 | 47 | |||||
| PEBAX 1675 | 0 | 55.8 | 39.9 | 18.0 | [147] | ||
| 4A | 5 | 71.4 | 51.0 | 32.5 | |||
| 10 | 97 | 53.9 | 26.2 | ||||
| 20 | 113.7 | 39.2 | 17.5 | ||||
| 30 | 155.8 | 13.0 | 7.9 | ||||
| PEBAX 1675 | 0 | 110 | 54 | 16 | 8.99 | [148] | |
| SAPO-34 | 9 | 100 | 53 | 16.5 | 8.29 | ||
| 23 | 130 | 56 | 21.9 | 6.58 | |||
| 33 | 250 | 55.7 | 16.4 | 8.96 | |||
| 50 | 340 | 55.5 | 16.5 | 8.40 | |||
| PDMS | 0 | 4796 | 3.0 | 4.21 | [150] | ||
| 4A | 10 | 4226 | 2.7 | 1.55 | |||
| 20 | 3691 | 2.6 | 0.61 | ||||
| 30 | 3323 | 2.9 | 0.40 | ||||
| 40 | 2972 | 2.8 | 0.30 | ||||
| 50 | 2886 | 2.9 | 0.27 | ||||
| PVAc | 0 | 2.74 | 28 | 53 | [151] | ||
| Ferrierite | 20 | 3.93 | 61 | 54 | |||
| 40 | 3.93 | 82 | 57 | ||||
| 4A | 20 | 2.55 | 52 | ||||
| 40 | 2.73 | 74 | |||||
| 5A | 20 | 2.77 | 46 | ||||
| 40 | 1.70 | 33 | |||||
| Silicalite-1 | 20 | 3.38 | 42 | ||||
| 40 | 3.52 | 50 | |||||
| PTMSP | 0 | 17430 | 0.9 | [152] | |||
| Zeolite A | 5 | 13029 | 9.7 | ||||
| 20 | 11403 | 76.4 | |||||
| ITQ-29 | 5 | 16501 | 4.4 | ||||
| 20 | 14546 | 1.1 | |||||
| PSF | 0 | 4.9 | 18.5 | [154] | |||
| 4A | 20 | 5 | 12.5 | ||||
| 25 | 6.9 | 7.6 | |||||
| 30 | 7 | 2 | |||||
| 35 | 7.12 | 1.44 | |||||
| treated 4A | 20 | 4.75 | 23.5 | ||||
| 25 | 4.73 | 28 | |||||
| 30 | 4.7 | 31 | |||||
| 35 | 3.7 | 29 | |||||
| Matrimid | 0 | 10.2 | 33.6 | [155] | |||
| 5A | 10 | 26.7 | 31.3 | ||||
| 20 | 31 | 30.8 | |||||
| 5A-Mg(OH)2 | 10 | 19.6 | 35.4 | ||||
| 20 | 22.4 | 36.4 | |||||
| Cellulose Acetate | 0 | 2.2 | 26 | [156] | |||
| Na-Y | 5 | 2.5 | 22.5 | ||||
| 10 | 2.6 | 22 | |||||
| 15 | 3.4 | 21 | |||||
| 20 | 4.95 | 22.5 | |||||
| 25 | 3.5 | 15 | |||||
| Na-Y-NH2 | 5 | 3.2 | 25 | ||||
| 10 | 3.5 | 23 | |||||
| 15 | 3.65 | 22 | |||||
| 20 | 4.1 | 26 | |||||
| 25 | 4.3 | 17 |
| Filler | Polymer | Loading (wt.%) | PCO2 (Barrer) | α CO2/N2 | α CO2/CH4 | α H2/CO2 | Ref. |
|---|---|---|---|---|---|---|---|
| Cellulose Acetate | 0 | 7.55 | 29.61 | [163] | |||
| AMH-3 | 2 | 9.65 | 29.24 | ||||
| 4 | 10.36 | 30.03 | |||||
| 6 | 11.59 | 29.71 | |||||
| Ultem | 0 a | 2.22 | 20.2 | 2.88 | [164] | ||
| NUS-2 | 10 a | 3.75 | 25 | 3.39 | |||
| 20 a | 4.92 | 22.4 | 4.61 | ||||
| 30 a | 8.70 | 12.7 | 1.89 | ||||
| NUS-3 | 10 a | 5.89 | 22.7 | 2.46 | |||
| 20 a | 15 | 28.3 | 2.23 | ||||
| 30 a | 8.11 | 10.7 | 2.45 | ||||
| Matrimid 5218 | 0 | 5.78 | 59.8 | [165] | |||
| ns-CuBDC b | 1.7 | 5.38 | 61.6 | ||||
| 3.7 | 9.91 | 59.5 | |||||
| 4.3 | 4.74 | 63.5 | |||||
| 8.2 | 4.09 | 78.7 | |||||
| b-CuBDC b | 7.9 | 5.21 | 45 | ||||
| nc-CuBDC b | 8.3 | 5.03 | 49.4 | ||||
| Matrimid 5218 | 0 | 7.2 c | 23.7 | [166] | |||
| CuBDC | 4 | 6.4 c | 42.0 | ||||
| 8 | 4.0 c | 48.1 | |||||
| 0 | 15.2 | 25.3 | |||||
| 12 | 6.6 | 40.3 | |||||
| PIM-1 | 0 | 1750 d | 4.4 | [167] | |||
| CuBDC-ns | 2 | 500 d | 10.2 | ||||
| 5 | 490 d | 12.9 | |||||
| 10 | 400 d | 16.0 | |||||
| 15 | 490 d | 11.7 | |||||
| 0 | 161 d | 12.2 | |||||
| 10 | 196 d | 10.8 | |||||
| 10 | 407 d | 15.5 | |||||
| PIM-1 | 0 | 3100 | 17 | [168] | |||
| CuBDC-ns | 2 | 2030 | 24 | ||||
| 4 | 2300 | 22 | |||||
| 6FDA-DAM | 0 | 590 | 30 | ||||
| CuBDC-ns | 2 | 570 | 37 | ||||
| 4 | 430 | 43 | |||||
| PBI | 0 | 3.62 | 9.3 | [169] | |||
| ns-Cu2(ndc)2(dabco) b | 10 | 4.86 | 18.7 | ||||
| 20 | 6.15 | 22.8 | |||||
| 30 | 11.9 | 12.3 | |||||
| 50 | 66.4 | 4.8 | |||||
| bc-Cu2(ndc)2(dabco) b | 20 | 5.18 | 12.6 | ||||
| nc-Cu2(ndc)2(dabco) b | 20 | 5.29 | 17.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, M.; Janakiram, S.; Dai, Z.; Ansaloni, L.; Deng, L. Performance of Mixed Matrix Membranes Containing Porous Two-Dimensional (2D) and Three-Dimensional (3D) Fillers for CO2 Separation: A Review. Membranes 2018, 8, 50. https://doi.org/10.3390/membranes8030050
Ahmadi M, Janakiram S, Dai Z, Ansaloni L, Deng L. Performance of Mixed Matrix Membranes Containing Porous Two-Dimensional (2D) and Three-Dimensional (3D) Fillers for CO2 Separation: A Review. Membranes. 2018; 8(3):50. https://doi.org/10.3390/membranes8030050
Chicago/Turabian StyleAhmadi, Mahdi, Saravanan Janakiram, Zhongde Dai, Luca Ansaloni, and Liyuan Deng. 2018. "Performance of Mixed Matrix Membranes Containing Porous Two-Dimensional (2D) and Three-Dimensional (3D) Fillers for CO2 Separation: A Review" Membranes 8, no. 3: 50. https://doi.org/10.3390/membranes8030050
APA StyleAhmadi, M., Janakiram, S., Dai, Z., Ansaloni, L., & Deng, L. (2018). Performance of Mixed Matrix Membranes Containing Porous Two-Dimensional (2D) and Three-Dimensional (3D) Fillers for CO2 Separation: A Review. Membranes, 8(3), 50. https://doi.org/10.3390/membranes8030050






