Preparation and Characterization of TiO2/g-C3N4/PVDF Composite Membrane with Enhanced Physical Properties
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of Carbon Nitride (g-C3N4)
2.3. Preparation of Membrane
2.4. Contact Angle Measurement (Sessile-Drop Method)
2.5. Porosity Measurement
2.6. Characterization
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shu, C.; Song, B.; Wei, X.; Liu, Y.; Tan, Q.; Chong, S.; Chen, Y.; Yang, X.-D.; Yang, W.-H.; Liu, Y. Mesoporous 3D nitrogen-doped yolk-shelled carbon spheres for direct methanol fuel cells with polymer fiber membranes. Carbon 2018, 129, 613–620. [Google Scholar] [CrossRef]
- Colò, F.; Bella, F.; Nair, J.R.; Gerbaldi, C. Light-cured polymer electrolytes for safe, low-cost and sustainable sodium-ion batteries. J. Power Sources 2017, 365, 293–302. [Google Scholar] [CrossRef]
- Bella, F.; Pugliese, D.; Zolin, L.; Gerbaldi, C. Paper-based quasi-solid dye-sensitized solar cells. Electrochim. Acta 2017, 237, 87–93. [Google Scholar] [CrossRef]
- Hu, E.N.; Lin, C.X.; Liu, F.H.; Wang, X.Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Poly(arylene ether nitrile) anion exchange membranes with dense flexible ionic side chain for fuel cells. J. Membr. Sci. 2018, 550, 254–265. [Google Scholar] [CrossRef]
- Nair, J.R.; Bella, F.; Angulakshmi, N.; Stephan, A.M.; Gerbaldi, C. Nanocellulose-laden composite polymer electrolytes for high performing lithium–sulphur batteries. Energy Storage Mater. 2016, 3, 69–76. [Google Scholar] [CrossRef]
- Bella, F.; Ozzello, E.D.; Bianco, S.; Bongiovanni, R. Photo-polymerization of acrylic/methacrylic gel–polymer electrolyte membranes for dye-sensitized solar cells. Chem. Eng. J. 2013, 225, 873–879. [Google Scholar] [CrossRef]
- Jha, R.; Dulikravich, G.S.; Chakraborti, N.; Fan, M.; Schwartz, J.; Koch, C.C.; Colaco, M.J.; Poloni, C.; Egorov, I.N. Algorithms for design optimization of chemistry of hard magnetic alloys using experimental data. J. Alloys Compd. 2016, 682, 454–467. [Google Scholar] [CrossRef]
- Fan, M.; Liu, Y.; Jha, R.; Dulikravich, G.S.; Schwartz, J.; Koch, C.C. On the Formation and Evolution of Cu-Ni-Rich Bridges of Alnico Alloys with Thermomagnetic Treatment. IEEE Trans. Magn. 2016, 52, 1–10. [Google Scholar] [CrossRef]
- Wang, R.; Yang, C.; Fan, M.; Wu, M.; Wang, C.; Yu, X.; Zhu, J.; Zhang, J.; Li, G.; Huang, Q.; et al. Phase relationship of the TbO1.81–Mn3O4–Fe2O3 system synthesized at 1200 °C. J. Alloys Compd. 2013, 554, 385–394. [Google Scholar] [CrossRef]
- Fan, M.; Liu, Y.; Jha, R.; Dulikravich, G.S.; Schwartz, J.; Koch, C.C. On the evolution of Cu-Ni-rich bridges of Alnico alloys with tempering. J. Magn. Magn. Mater. 2016, 420, 296–302. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.; Li, K. Preparation of porous PVDF hollow fibre membrane via a phase inversion method using lithium perchlorate (LiClO4) as an additive. J. Membr. Sci. 2005, 258, 16–22. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Xu, Z.-L.; Shen, H.-M.; Yang, H. Preparation and characterization of PVDF–SiO2 composite hollow fiber UF membrane by sol–gel method. J. Membr. Sci. 2009, 337, 257–265. [Google Scholar] [CrossRef]
- Cha, S.; Kim, S.M.; Kim, H.; Ku, J.; Sohn, J.I.; Park, Y.J.; Song, B.G.; Jung, M.H.; Lee, E.K.; Choi, B.L.; et al. Porous PVDF as Effective Sonic Wave Driven Nanogenerators. Nano Lett. 2011, 11, 5142–5147. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Chou, M.H. Polymorphism, piezoelectricity and sound absorption of electrospun PVDF membranes with and without carbon nanotubes. Compos. Sci. Technol. 2016, 127, 127–133. [Google Scholar] [CrossRef]
- Zaddach, A.J.; Niu, C.; Oni, A.A.; Fan, M.; LeBeau, J.M.; Irving, D.L.; Koch, C.C. Structure and magnetic properties of a multi-principal element Ni–Fe–Cr–Co–Zn–Mn alloy. Intermetallics 2016, 68, 107–112. [Google Scholar] [CrossRef]
- Zhang, G.; Fan, M. ChemInform Abstract: Synthesis and Magnetic Properties of Double B Mixed Perovskite Series La0.75K0.25Mn1−xFexO3. ChemInform 2011, 42. [Google Scholar] [CrossRef]
- Sahoo, B.N.; Balasubramanian, K. Facile synthesis of nano cauliflower and nano broccoli like hierarchical superhydrophobic composite coating using PVDF/carbon soot particles via gelation technique. J. Colloid Interface Sci. 2014, 436, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.-M.; Wang, H.-Y.; Zhang, Y.-H.; Qi, J.-Q. Morphology and Dielectric Property of Homogenous BaTiO3/PVDF Nanocomposites Prepared via the Natural Adsorption Action of Nanosized BaTiO3. Macromol. Rapid Commun. 2005, 26, 1185–1189. [Google Scholar] [CrossRef]
- Li, M.; Shi, J.; Chen, C.; Li, N.; Xu, Z.; Li, J.; Lv, H.; Qian, X.; Jiao, X. Optimized permeation and antifouling of PVDF hybrid ultrafiltration membranes: Synergistic effect of dispersion and migration for fluorinated graphene oxide. J. Nanopart. Res. 2017, 19, 114. [Google Scholar] [CrossRef]
- Liu, J.; Tian, C.; Xiong, J.; Wang, L. Polypyrrole blending modification for PVDF conductive membrane preparing and fouling mitigation. J. Colloid Interface Sci. 2017, 494, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Feng, S.; Li, J.; Chen, J.; Li, F.; Lin, H.; Yu, G. Surface modification of polyvinylidene fluoride (PVDF) membrane via radiation grafting: Novel mechanisms underlying the interesting enhanced membrane performance. Sci. Rep. UK 2017, 7, 2721. [Google Scholar] [CrossRef] [PubMed]
- Khayet, M.; Villaluenga, J.; Valentin, J.L.; Lopez-Manchado, M.; Mengual, J.I.; Seoane, B. Filled Poly(2,6-dimethyl-1,4-phenylene Oxide) Dense Membranes by Silica and Silane Modified Silica Nanoparticles: Characterization and Application in Pervaporation. Polymer 2005, 46, 9881–9891. [Google Scholar] [CrossRef]
- Liu, Z.; Pang, L.; Li, Q.; Zhang, S.; Li, J.; Tong, H.; Xu, Z.; Yi, C. Hydrophilic porous polyimide/β-cyclodextrin composite membranes with enhanced gas separation performance and low dielectric constant. High Perform. Polym. 2017. [Google Scholar] [CrossRef]
- Jia, H.; Wu, Z.; Liu, N. Effect of nano-ZnO with different particle size on the performance of PVDF composite membrane. Plast. Rubber Compos. 2017, 46, 1–7. [Google Scholar] [CrossRef]
- Albo, J.; Santos, E.; Neves, L.A.; Simeonov, S.P.; Afonso, C.A.M.; Crespo, J.G.; Irabien, A. Separation performance of CO2 through Supported Magnetic Ionic Liquid Membranes (SMILMs). Sep. Purif. Technol. 2012, 97, 26–33. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface Modifications for Antifouling Membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Manawi, Y.; Kochkodan, V.; Mahmoudi, E.; Johnson, D.J.; Mohammad, A.W.; Atieh, M.A. Characterization and Separation Performance of a Novel Polyethersulfone Membrane Blended with Acacia Gum. Sci. Rep. UK 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Kim, Y.; Matsuura, T.; Arafat, H.A. Development of antifouling thin-film-composite membranes for seawater desalination. J. Membr. Sci. 2011, 367, 110–118. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Tran, T.; Ramanan, S.; Lin, H. Membranes with Surface-Enhanced Antifouling Properties for Water Purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, J.; Shi, X.; Ren, Z. Effect of TiO2 nanoparticle size on the performance of PVDF membrane. Appl. Surf. Sci. 2006, 253, 2003–2010. [Google Scholar] [CrossRef]
- Wang, X.; Xinxin Zhao, C.; Xu, G.; Chen, Z.-K.; Zhu, F. Degradation mechanisms in organic solar cells: Localized moisture encroachment and cathode reaction. Sol. Energy Mater. Sol. C 2012, 104, 1–6. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, C.X.; Zhi, M.M.; Wang, K.; Deng, L.; Xu, G. Alkaline direct oxidation glucose fuel cell system using silver/nickel foams as electrodes. Electrochim. Acta 2012, 66, 133–138. [Google Scholar] [CrossRef]
- Deng, L.L.; Zhao, C.X.; Ma, Y.; Chen, S.S.; Xu, G. Low cost acetone sensors with selectivity over water vapor based on screen printed TiO2 nanoparticles. Anal. Methods 2013, 5, 3709–3713. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, N.; Gao, F.; Chen, H.; Jiang, F. All-solid-state Z-scheme 3,4-dihydroxybenzaldehyde-functionalized Ga2O3/graphitic carbon nitride photocatalyst with aromatic rings as electron mediators for visible-light photocatalytic nitrogen fixation. Appl. Catal. B Environ. 2017, 218, 600–610. [Google Scholar] [CrossRef]
- Samanta, S.; Khilari, S.; Pradhan, D.; Srivastava, R. An Efficient, Visible Light Driven, Selective Oxidation of Aromatic Alcohols and Amines with O2 Using BiVO4/g-C3N4 Nanocomposite: A Systematic and Comprehensive Study toward the Development of a Photocatalytic Process. ACS Sustain. Chem. Eng. 2017, 5, 2562–2577. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Zhao, C.X.; Wang, X.; Zeng, W.; Chen, Z.K.; Ong, B.S.; Wang, K.; Deng, L.; Xu, G. Organic photovoltaic power conversion efficiency improved by AC electric field alignment during fabrication. Appl. Phys. Lett. 2011, 99. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hang, Z.S.; Lu, X.M.; Ying, S.J. Preparation and pyrolysis performance of g-C3N4/PVDF composite membrane. Mod. Chem. Ind. 2013, 33, 77–81. [Google Scholar]
- Yan, H.; Zhao, C.; Wang, K.; Deng, L.; Ma, M.; Xu, G. Negative dielectric constant manifested by static electricity. Appl. Phys. Lett. 2013, 102, 062904. [Google Scholar] [CrossRef]
- Zhao, C.X.; Mao, A.Y.; Xu, G. Junction capacitance and donor-acceptor interface of organic photovoltaics. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Thomas, E.; Parvathy, C.; Balachandran, N.; Bhuvaneswari, S.; Vijayalakshmi, K.P.; George, B.K. PVDF-ionic liquid modified clay nanocomposites: Phase changes and shish-kebab structure. Polymer 2017, 115, 70–76. [Google Scholar] [CrossRef]
- Adams, F.V.; Nxumalo, E.N.; Krause, R.W.M.; Hoek, E.M.V.; Mamba, B.B. Preparation and characterization of polysulfone/β-cyclodextrin polyurethane composite nanofiltration membranes. J. Membr. Sci. 2012, 405–406, 291–299. [Google Scholar] [CrossRef]
- Yan, J.; Rodrigues, M.-T.F.; Song, Z.; Li, H.; Xu, H.; Liu, H.; Wu, J.; Xu, Y.; Song, Y.; Liu, Y.; et al. Reversible Formation of g-C3N4 3D Hydrogels through Ionic Liquid Activation: Gelation Behavior and Room-Temperature Gas-Sensing Properties. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural Characterization of Thin-Film Polyamide Reverse Osmosis Membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Ogawa, H.; Kanaya, T.; Nishida, K.; Matsuba, G. Phase separation and dewetting in polystyrene/poly(vinyl methyl ether) blend thin films in a wide thickness range. Polymer 2008, 49, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.-H.; Zhu, L.-P.; Li, X.-L.; Xu, Y.-Y.; Zhu, B.-K. Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. J. Membr. Sci. 2010, 364, 194–202. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of membrane support layer hydrophobicity on water flux in osmotically driven membrane processes. J. Membr. Sci. 2008, 318, 458–466. [Google Scholar] [CrossRef]
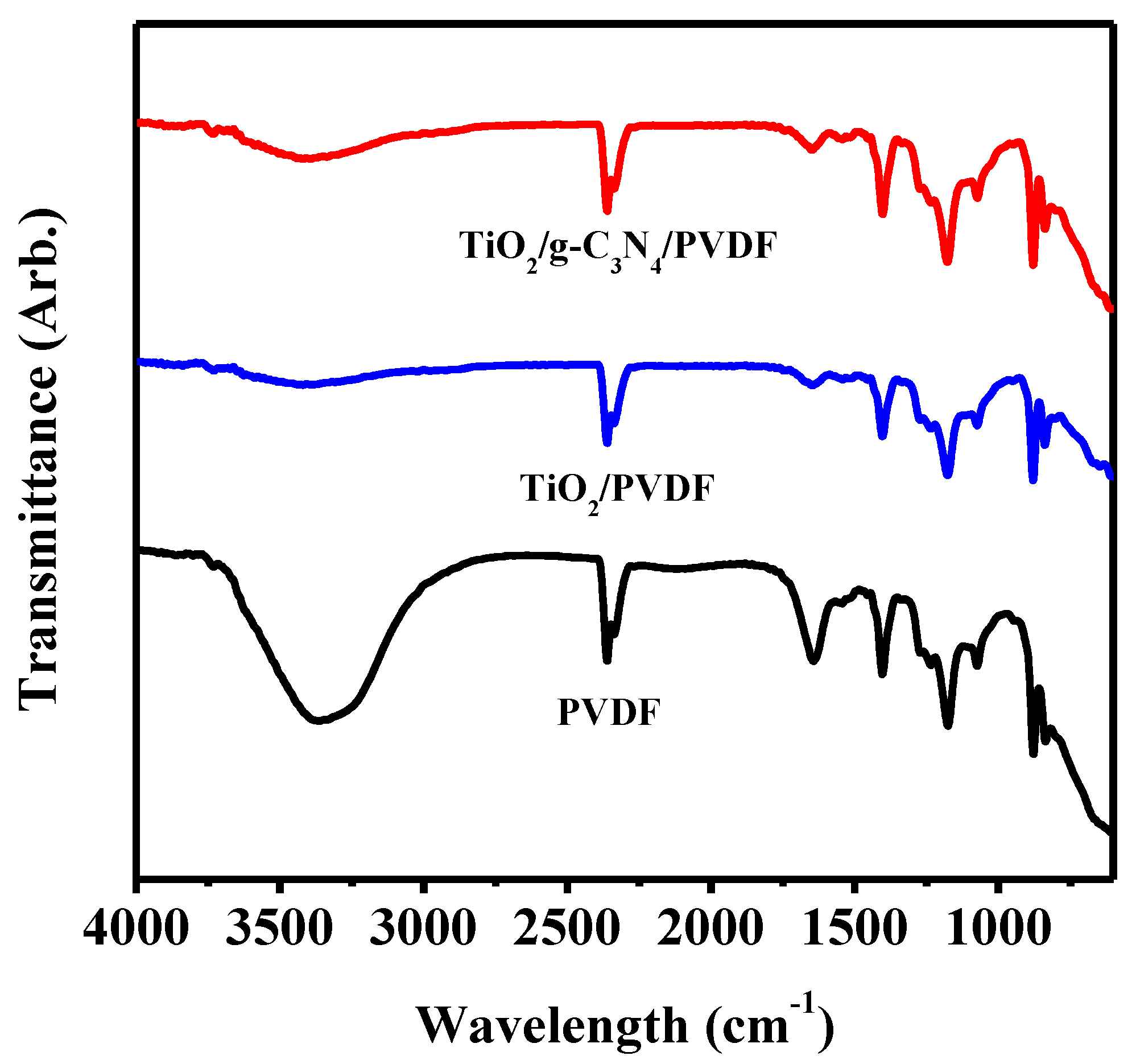
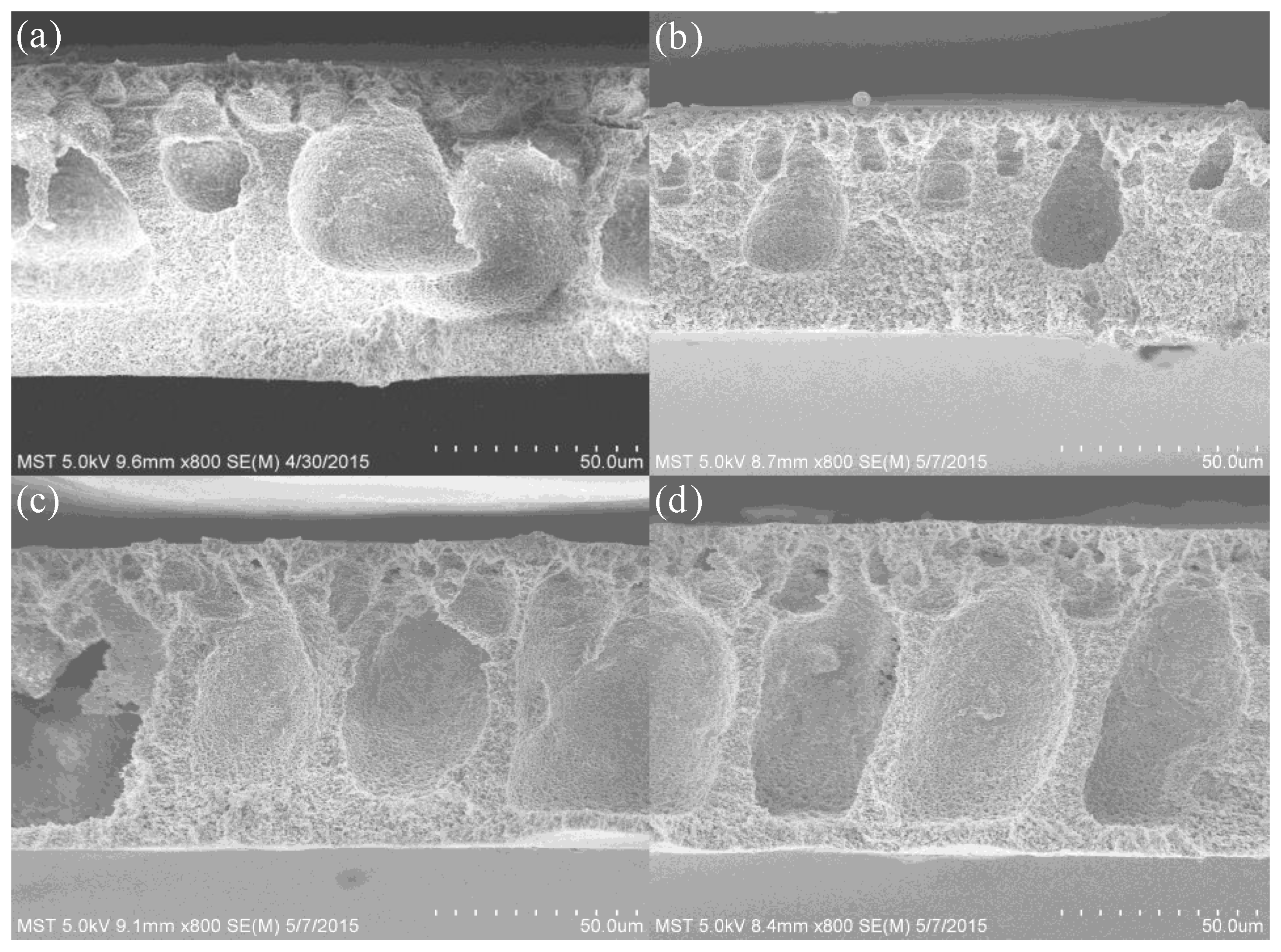
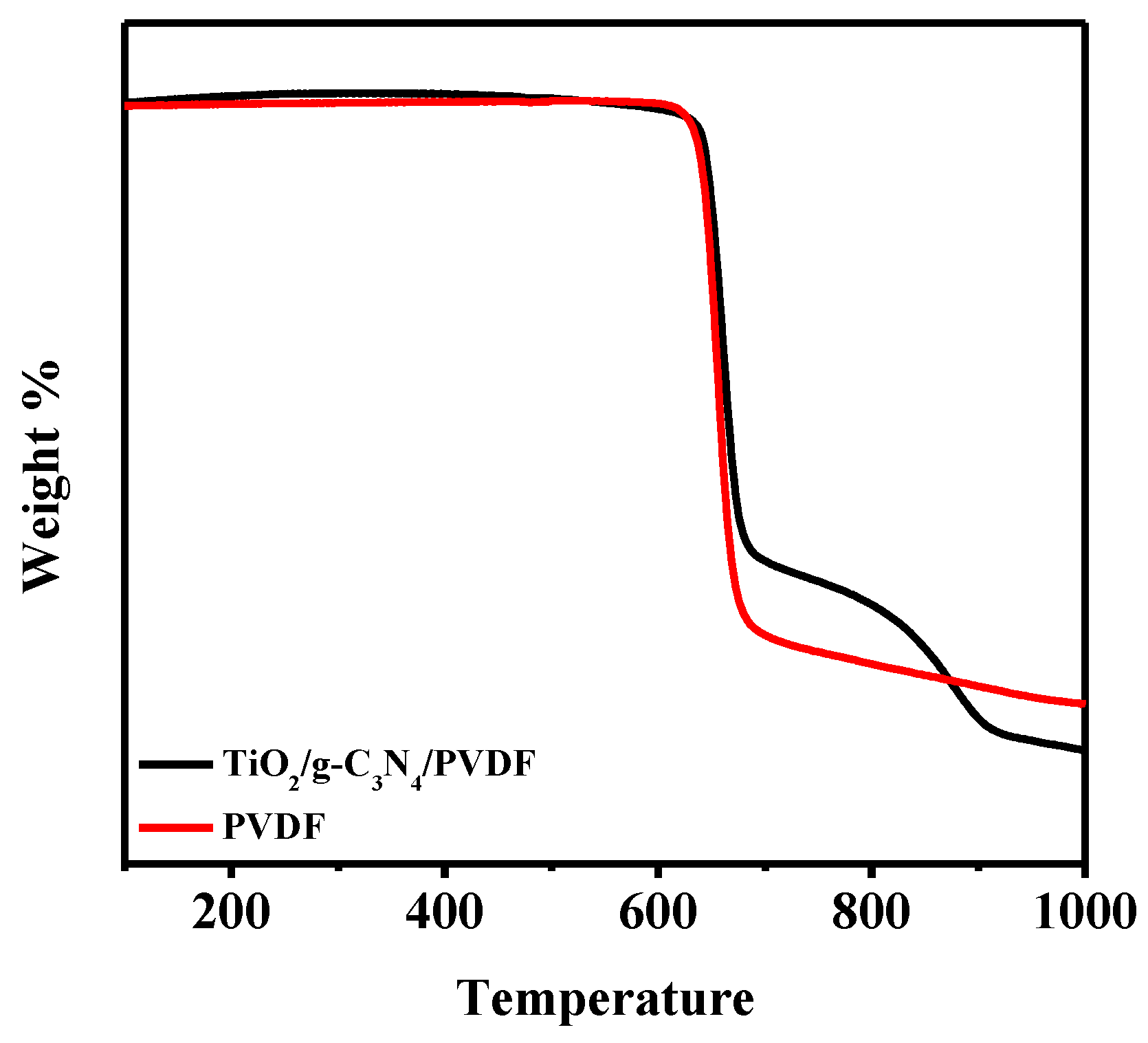
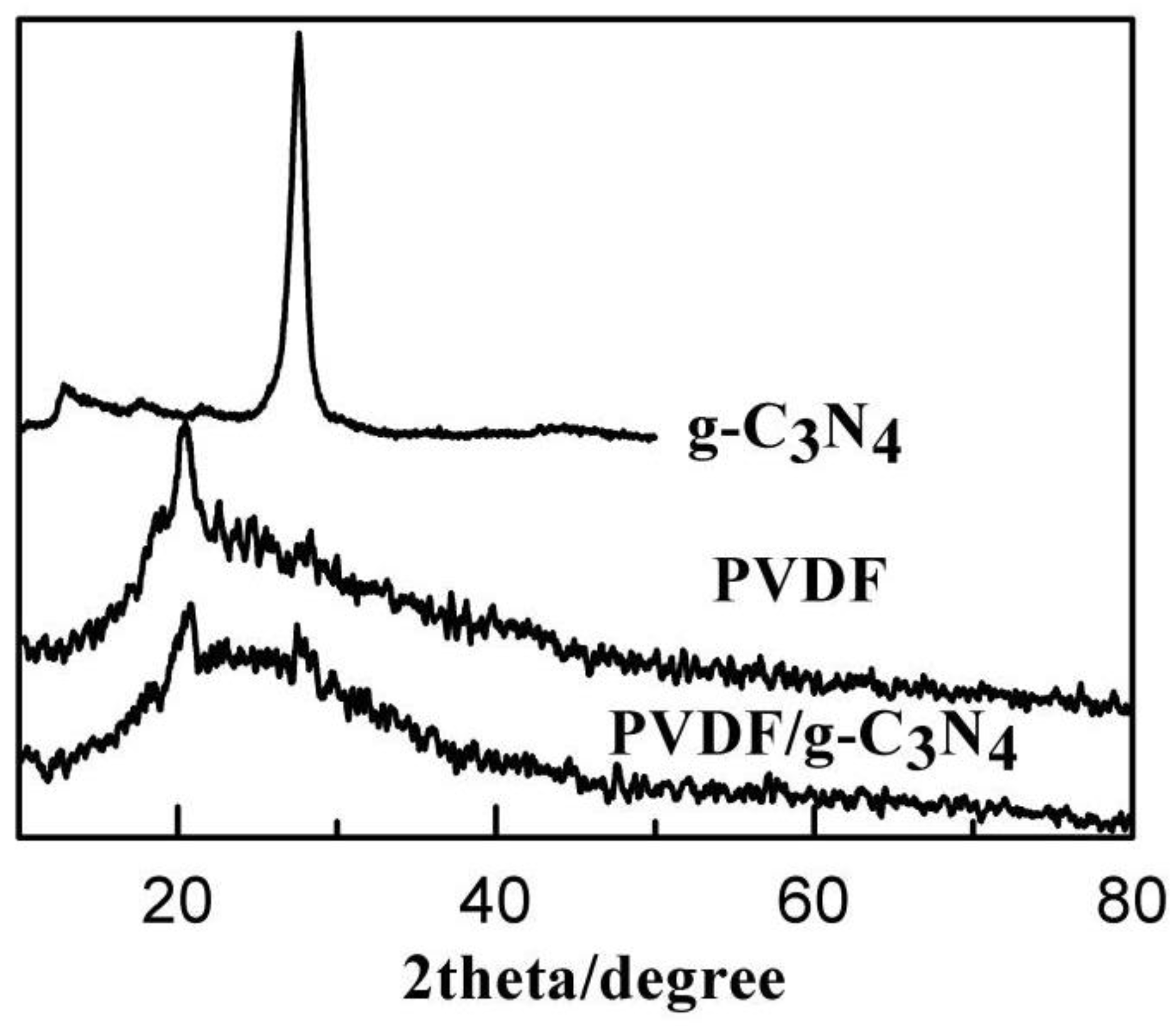
| Membrane | Composition (wt %) | UTS (MPa) | Contact Angle | Porosity | Water Content |
|---|---|---|---|---|---|
| PVDF | - | 0.27 | 75.98 | 50 | 90.3 |
| TiO2/PVDF | - | 0.29 | 56.22 | 71 | 111.3 |
| g-C3N4/PVDF | - | 0.29 | 58.64 | 71 | 127.7 |
| TiO2/g-C3N4/PVDF | 0.75:0.25 | 0.33 | 62.57 | 67 | 143.2 |
| TiO2/g-C3N4/PVDF | 0.5:0.5 | 0.29 | 70.89 | 60 | 124.5 |
| Parametric Membrane | Composition (wt %) | Contact Angle | Water Content | ||||
|---|---|---|---|---|---|---|---|
| Initial State | 30 s | 60 s | Container Quality | 3 min Later Quality | JW L/(m2·h) | ||
| PVDF | - | 82.34 | 72.62 | 75.98 | 1.9164 | 7.7294 | 90.3 |
| TiO2/PVDF | - | 73.27 | 68.06 | 56.22 | 4.0196 | 10.6646 | 111.3 |
| g-C3N4/PVDF | - | 75.53 | 65.94 | 58.64 | 3.9521 | 11.5824 | 127.7 |
| TiO2/g-C3N4/PVDF | 0.75:0.25 | 79.29 | 70.93 | 62.57 | 3.8871 | 12.4376 | 143.2 |
| TiO2/g-C3N4/PVDF | 0.5:0.5 | 77.53 | 79.42 | 70.89 | 2.7236 | 9.8236 | 124.5 |
| Parametric Membrane | Composition (wt %) | Porosity (%) | ||
|---|---|---|---|---|
| mwet (g) | mdry (g) | ε | ||
| PVDF | - | 0.06 | 0.03 | 50% |
| TiO2/PVDF | - | 0.07 | 0.02 | 71% |
| g-C3N4/PVDF | - | 0.07 | 0.02 | 71% |
| TiO2/g-C3N4/PVDF | 0.75:0.25 | 0.06 | 0.02 | 67% |
| TiO2/g-C3N4/PVDF | 0.5:0.5 | 0.05 | 0.02 | 60% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Gong, R.; Qian, X. Preparation and Characterization of TiO2/g-C3N4/PVDF Composite Membrane with Enhanced Physical Properties. Membranes 2018, 8, 14. https://doi.org/10.3390/membranes8010014
Wang H, Gong R, Qian X. Preparation and Characterization of TiO2/g-C3N4/PVDF Composite Membrane with Enhanced Physical Properties. Membranes. 2018; 8(1):14. https://doi.org/10.3390/membranes8010014
Chicago/Turabian StyleWang, Huiya, Ran Gong, and Xinliang Qian. 2018. "Preparation and Characterization of TiO2/g-C3N4/PVDF Composite Membrane with Enhanced Physical Properties" Membranes 8, no. 1: 14. https://doi.org/10.3390/membranes8010014
APA StyleWang, H., Gong, R., & Qian, X. (2018). Preparation and Characterization of TiO2/g-C3N4/PVDF Composite Membrane with Enhanced Physical Properties. Membranes, 8(1), 14. https://doi.org/10.3390/membranes8010014




