Assessment of Blend PVDF Membranes, and the Effect of Polymer Concentration and Blend Composition
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
2.2. Membranes Preparations
2.3. Membrane Characterization
2.4. Membrane Pure Water Permeability
2.4.1. Pressure Driven
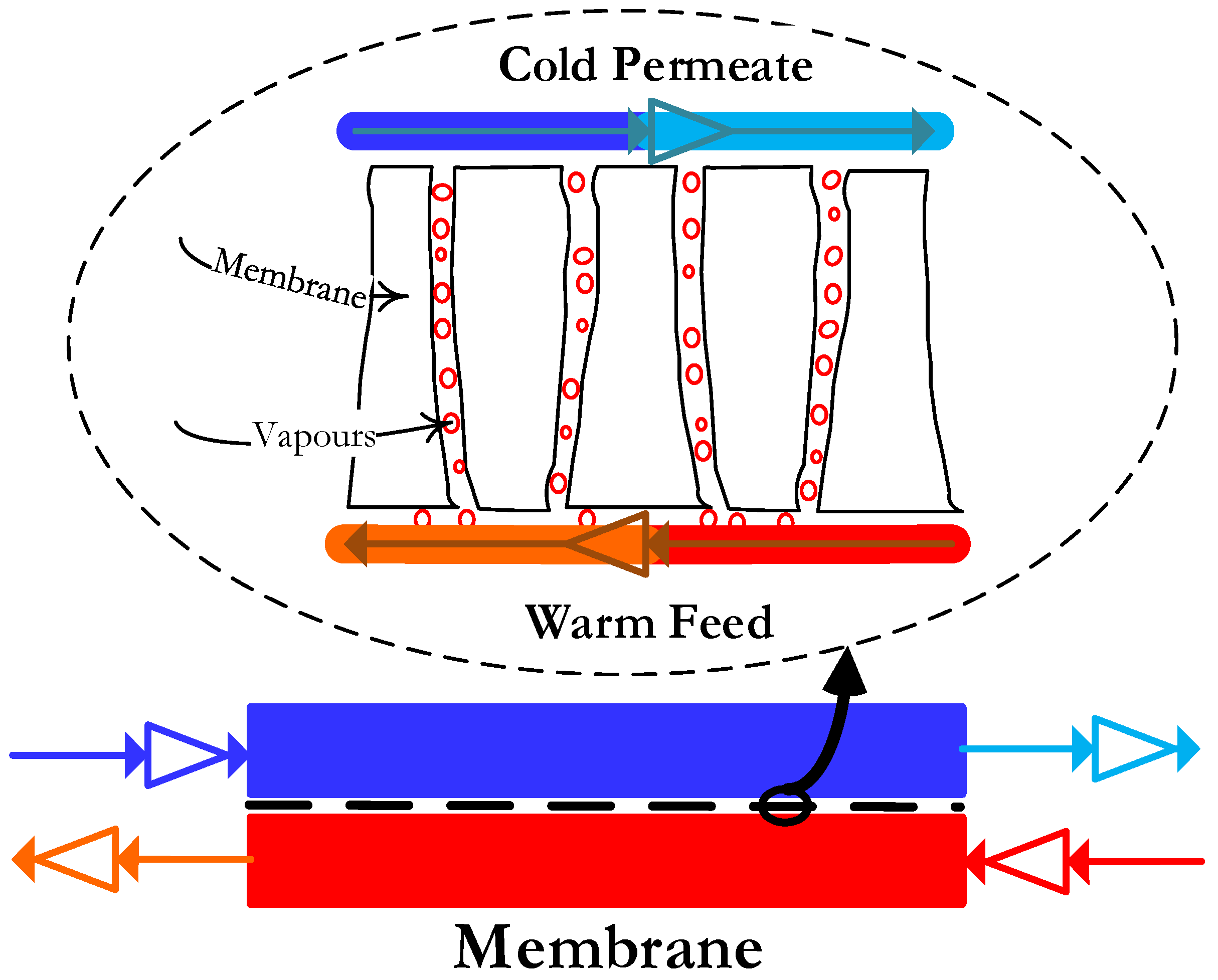
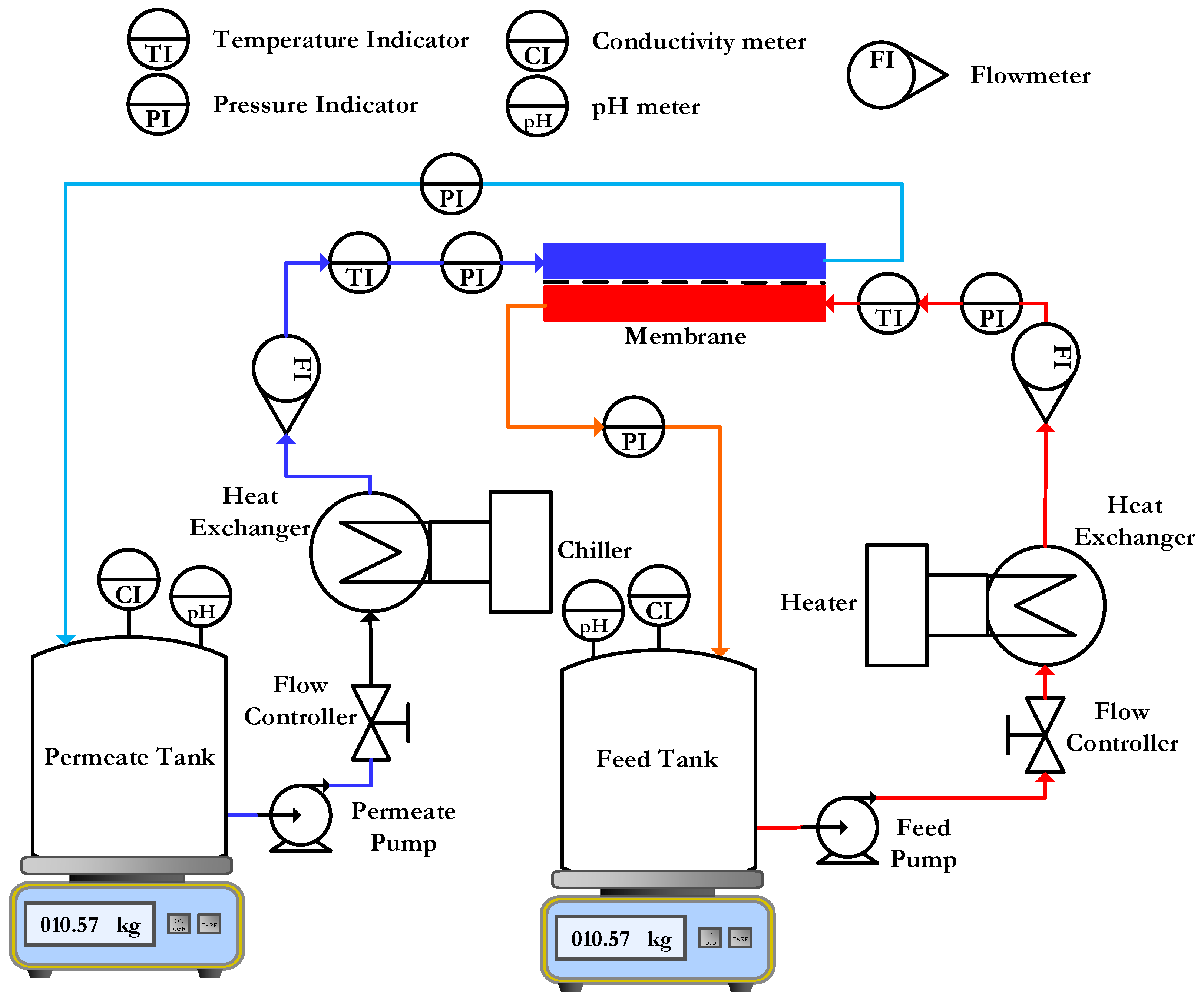
2.4.2. Direct Contact Membrane Distillation
3. Results and Discussion
3.1. Mechanical Properties
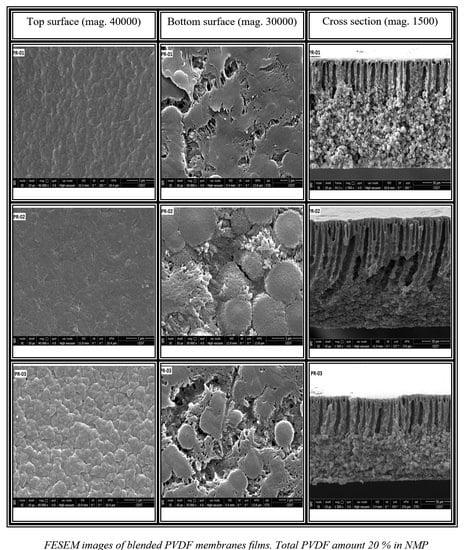
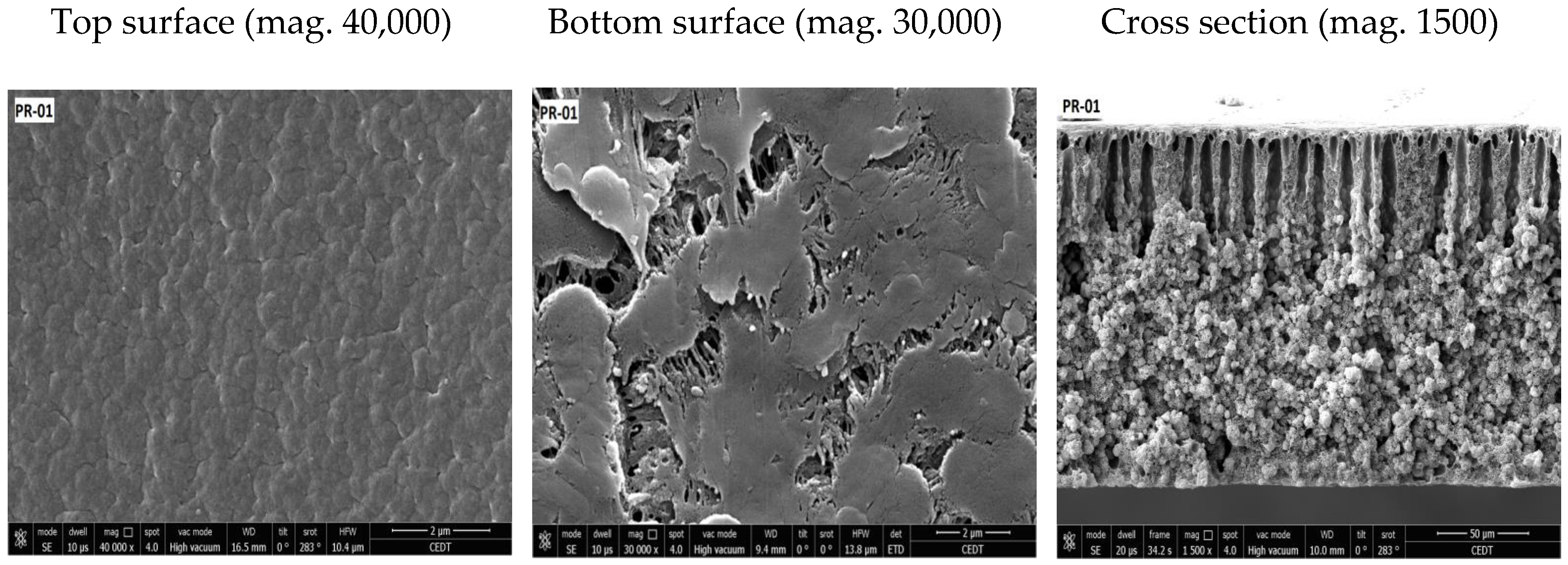
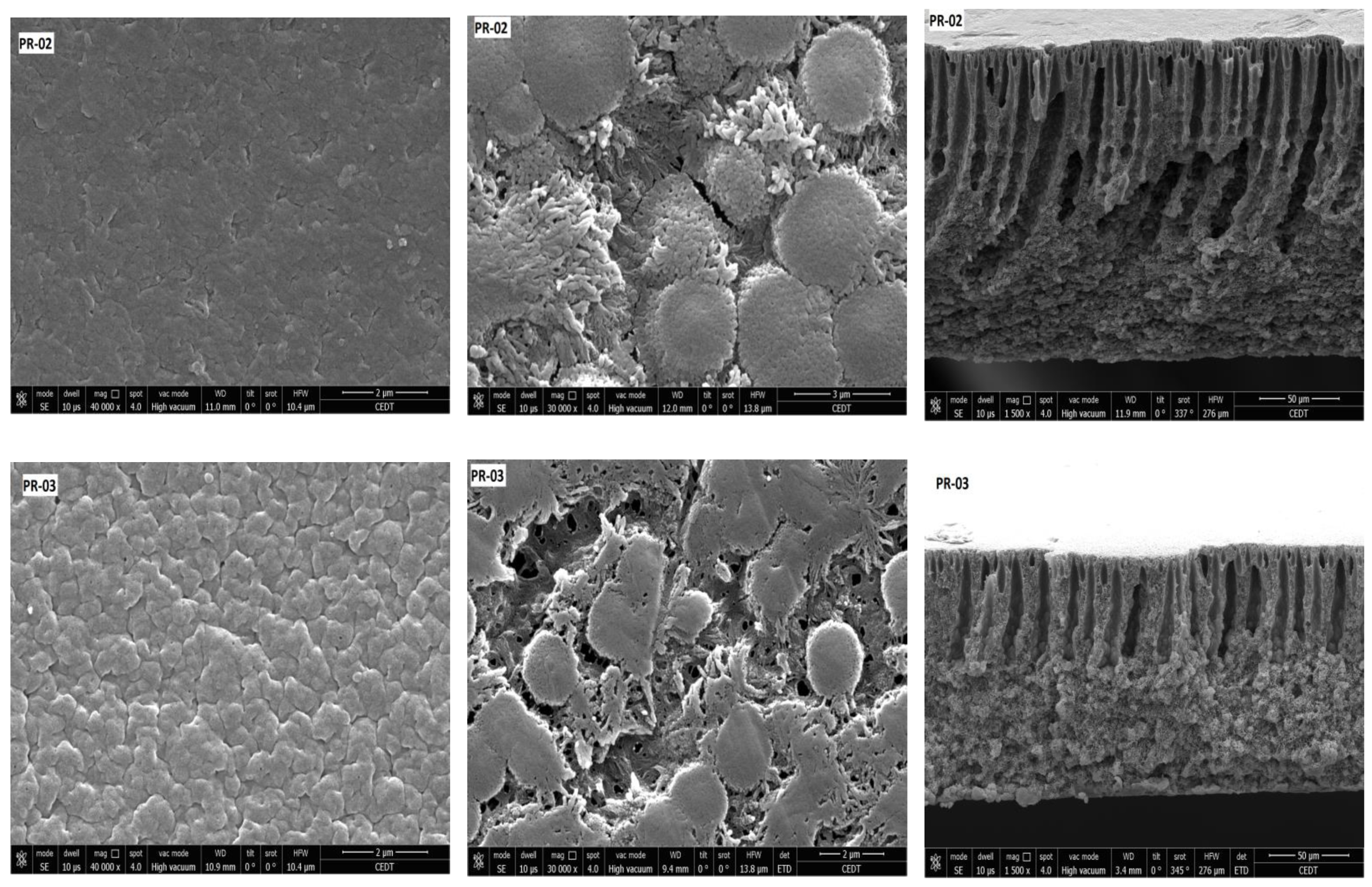
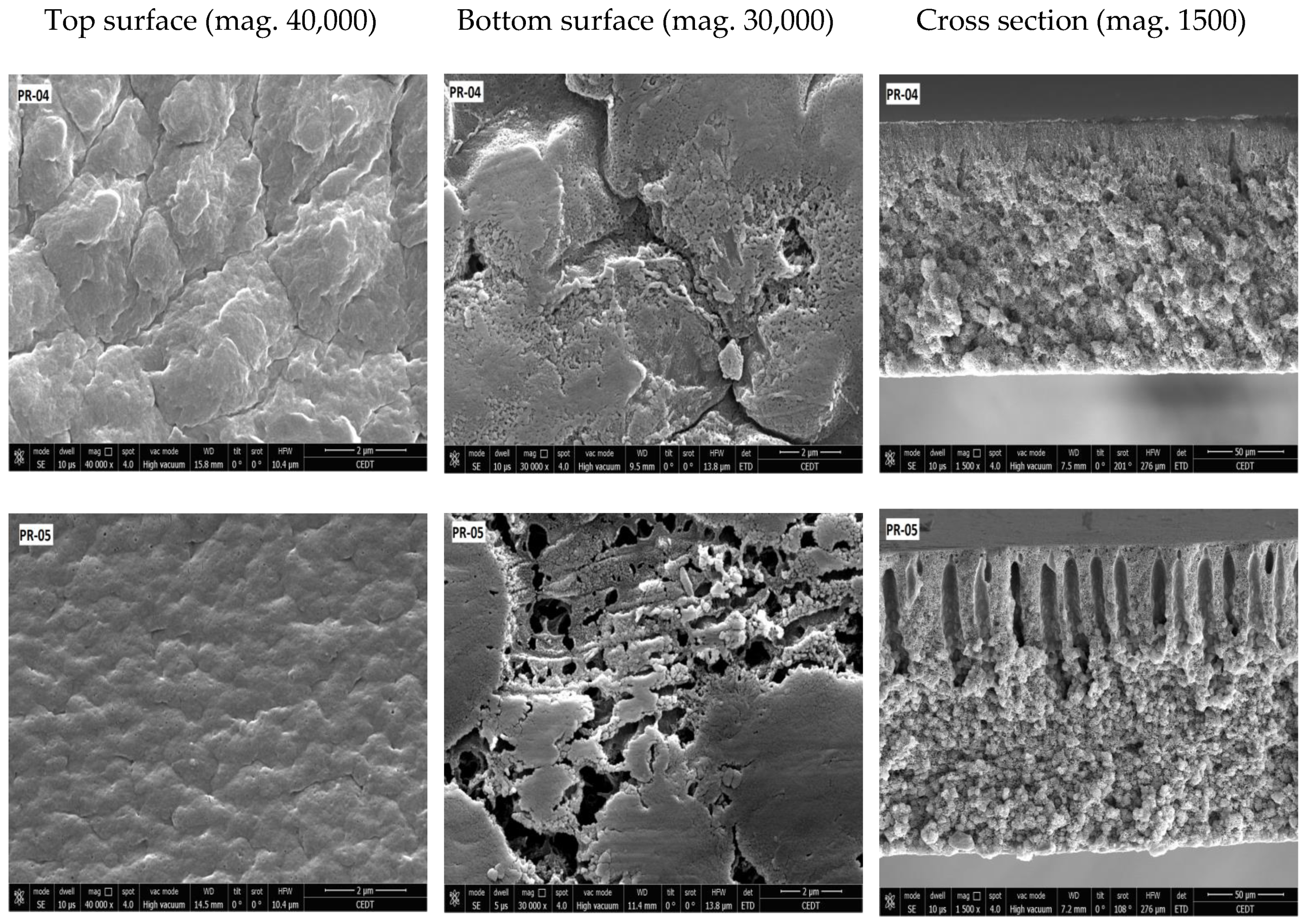
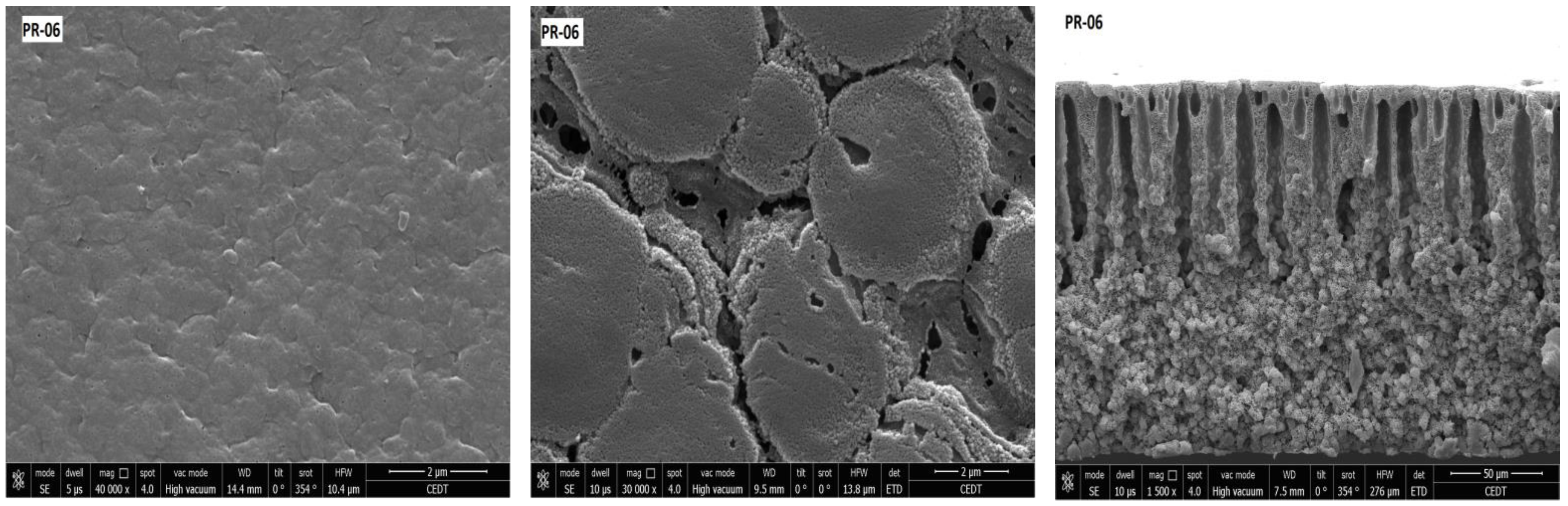
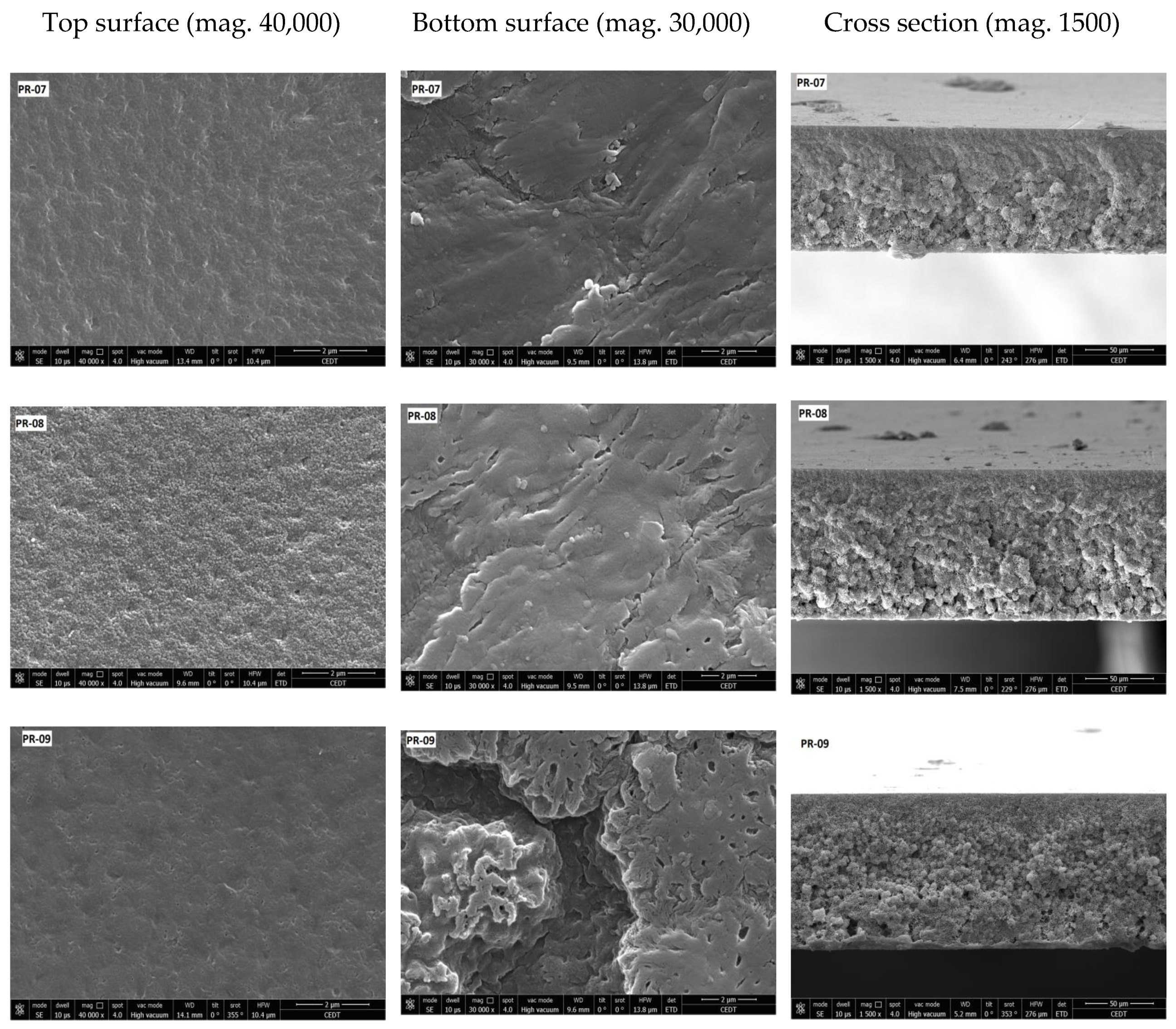
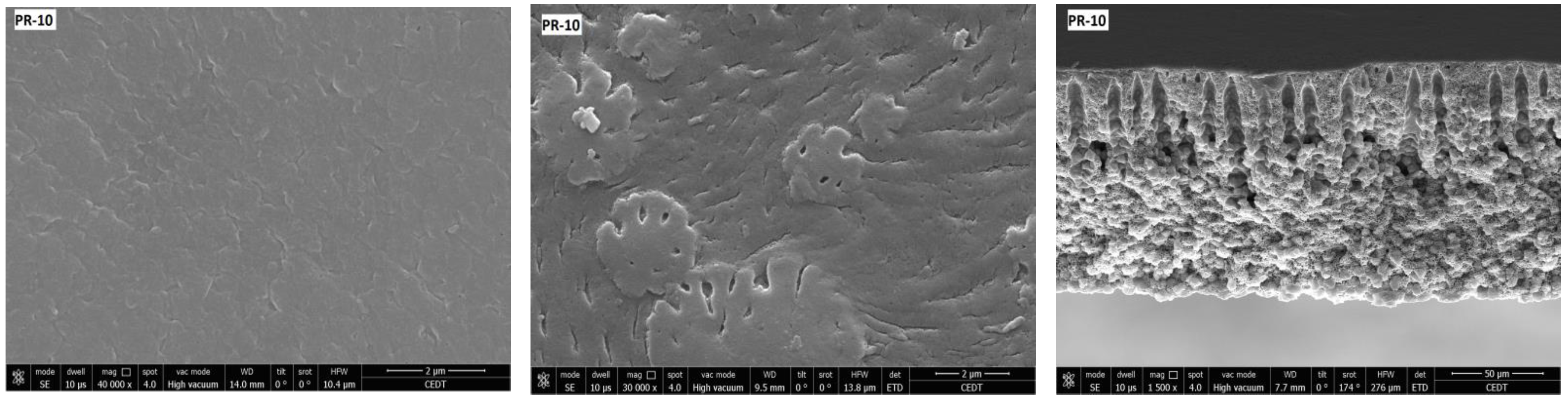
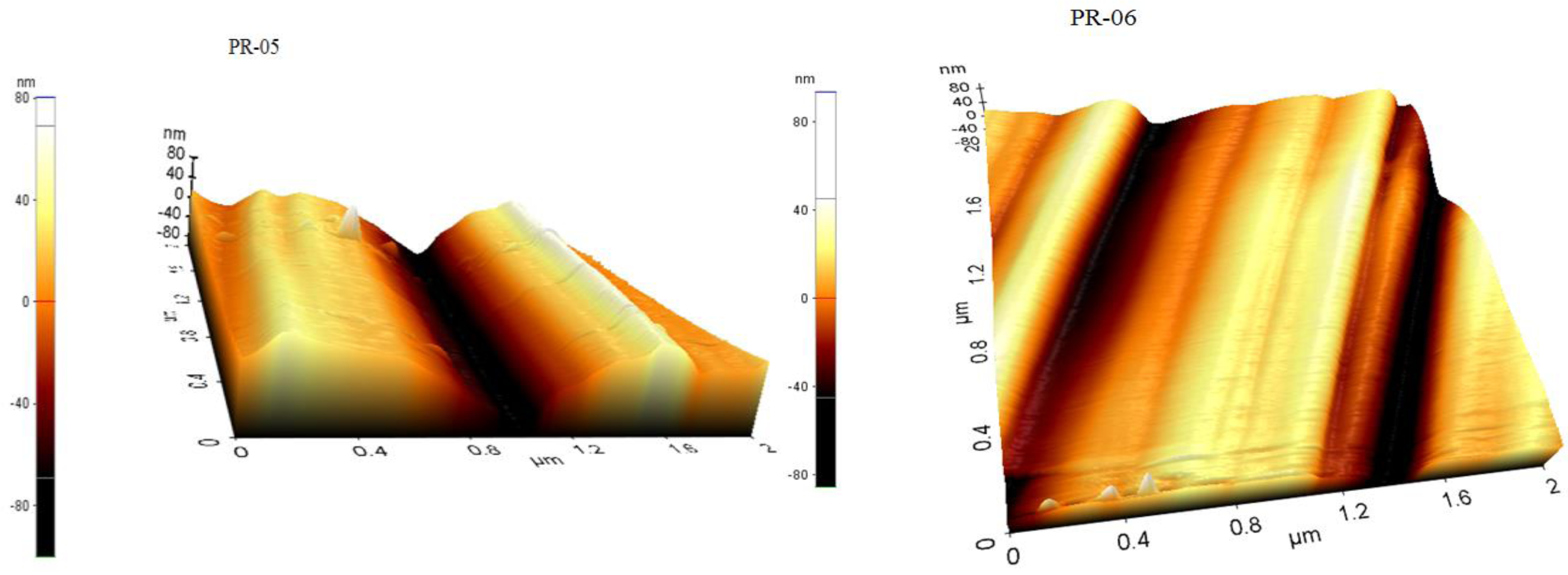
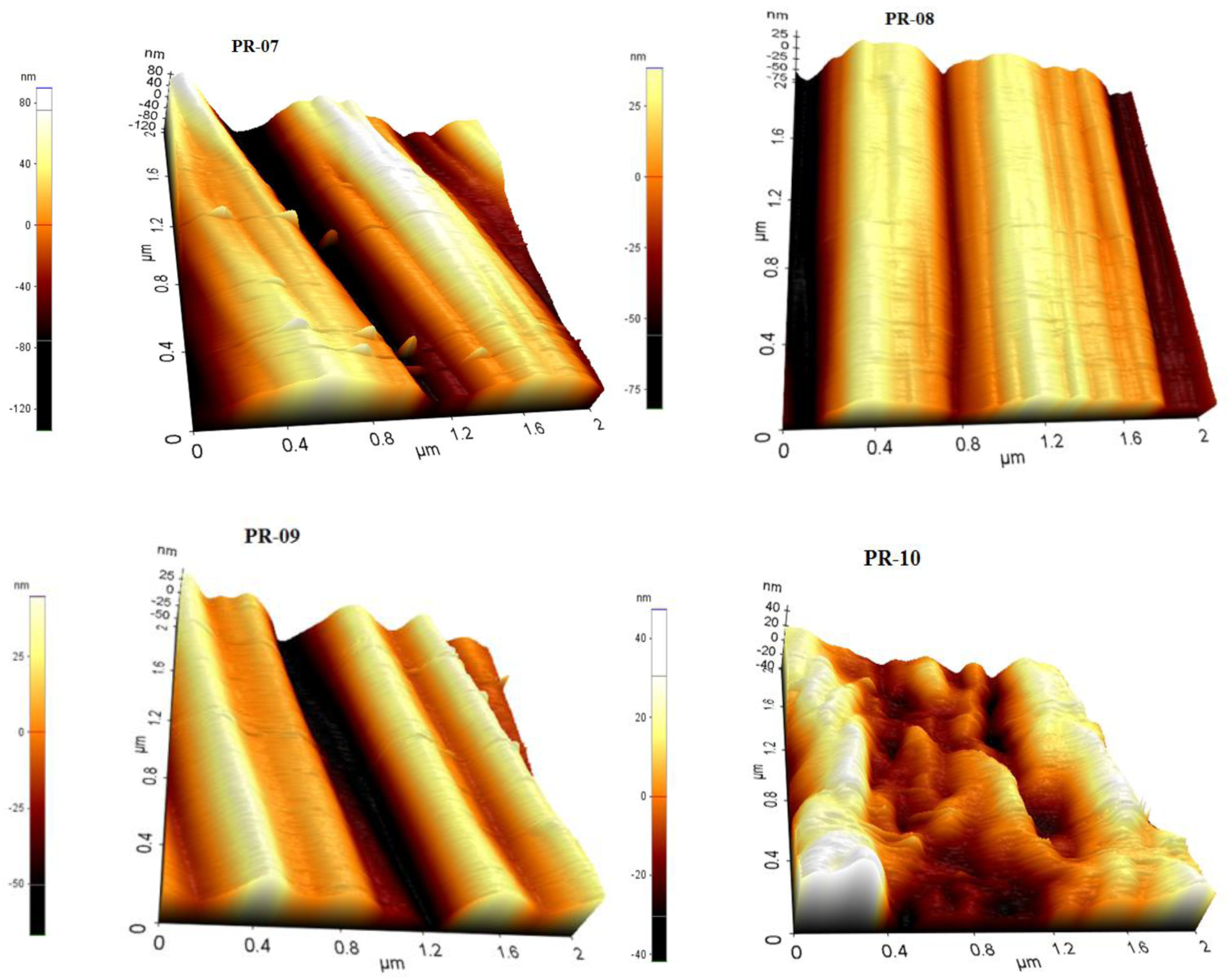
3.2. Membrane Structure
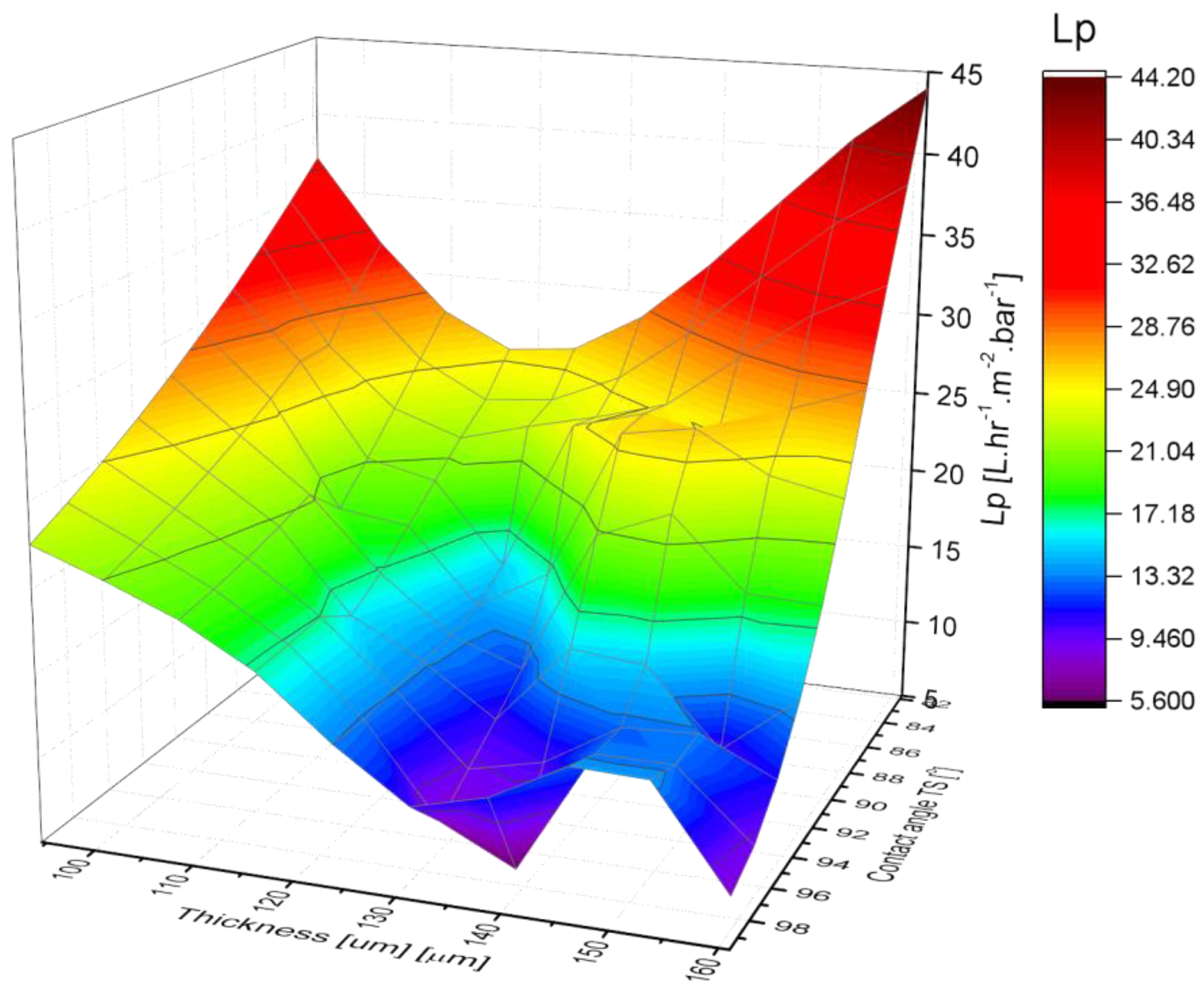
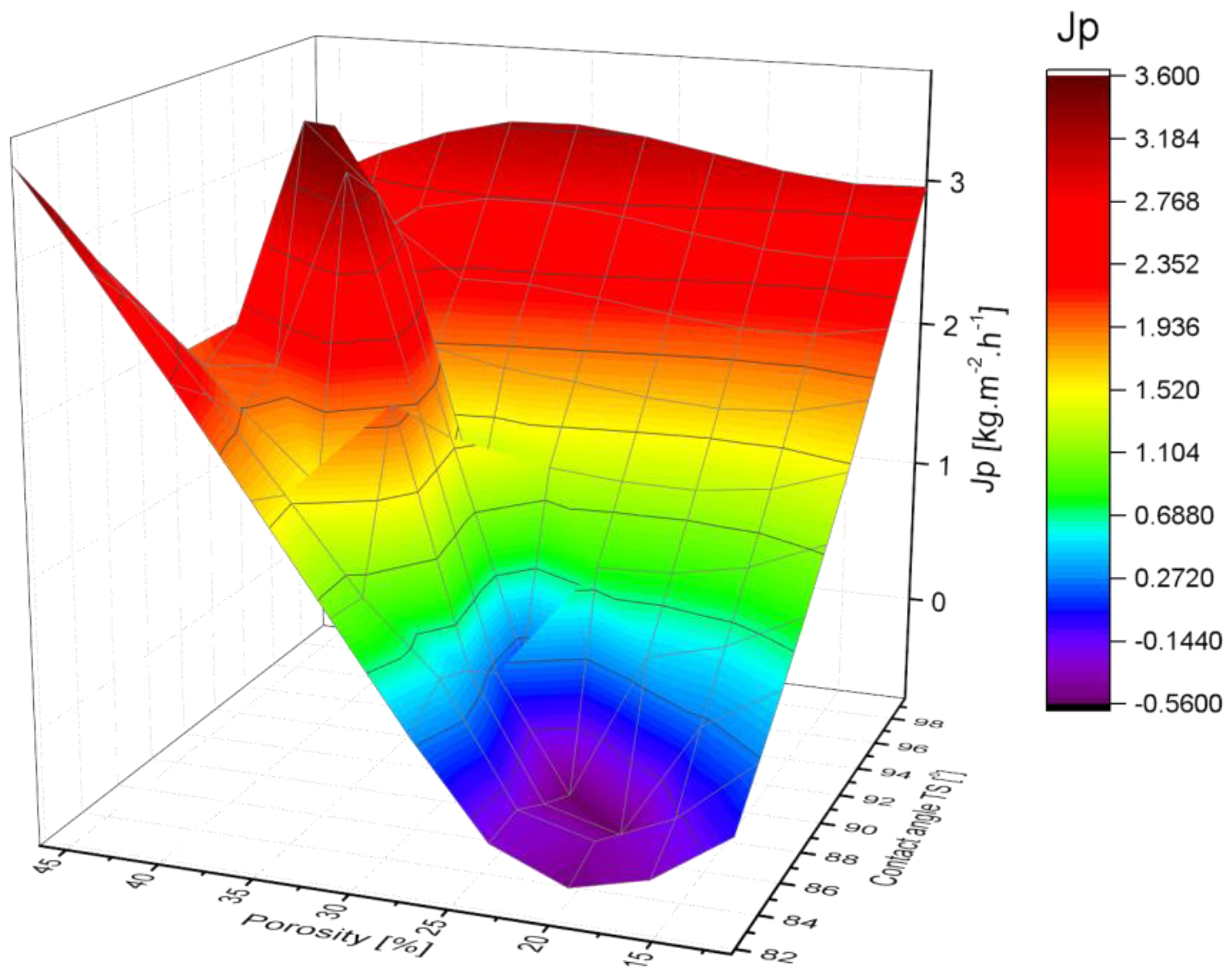
3.3. Membrane Pure Water Permeability
4. Conclusions
- Addition of PVDF-co-HFP copolymer, increasing the overall PVDF content, and use of mixed-solvent improved mechanical properties of the membranes.
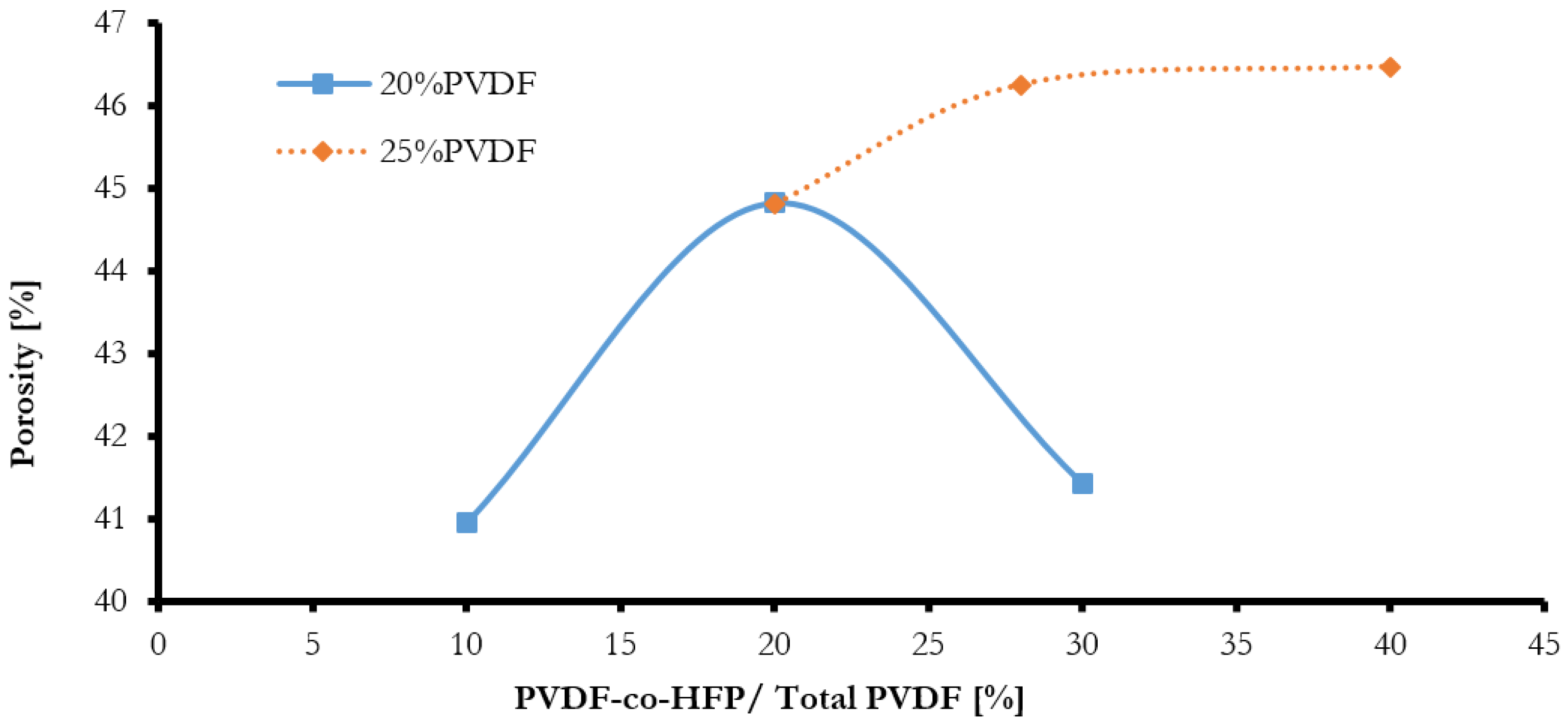
- The porosity of the elaborated membranes increased with the increase in the overall content of PVDF and with the addition of PVDF-co-HFP copolymer.
- At 20% overall PVDF content, the addition of copolymer increased hydrophobicity and the opposite was true at 25% overall PVDF content. Mixed solvent had an adverse effect on hydrophobicity.
- The AFM results show that the prepared membranes have a relatively good smoothness.
- Permeability of the membranes increased with the increase in overall content of PVDF. Mixed-solvents significantly improved permeability.
- The results of the DCMD experiment demonstrate that the obtained membrane does not meet the required target to be used in the MD process without pore forming agent.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdel-Aziz, M.H.; Bassyouni, M.; Gutub, S.A.; El-Ashtoukhy, E.S.Z.; Abdel-Hamid, S.M.S.; Sedahmed, G.H. Removal of cyanide from liquid waste by electrochemical oxidation in a new cell design employing a graphite anode. Chem. Eng. Commun. 2016, 32, 1045–1052. [Google Scholar] [CrossRef]
- Bassyouni, M.; Gutub, S.A.; Abdel-Hamid, S.M.S. Dissolved solids adsorption of freshwater using synthesized bio-foam composite. Life Sci. J. 2013, 10, 464–471. [Google Scholar]
- Abdel-Aziz, M.H.; El-Ashtoukhy, E.S.; Sh Zoromba, M.; Bassyouni, M. Oil-in-water emulsion breaking by electrocoagulation in a modified electrochemical cell. Int. J. Electrochem. Sci. 2016, 11, 9634–9643. [Google Scholar] [CrossRef]
- Sharma, P.P.; Swati, G.; Vaibhav, K. One pot synthesis of pvdf based copolymer proton conducting membrane by free radical polymerization for electro-chemical energy applications. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 520, 239–245. [Google Scholar] [CrossRef]
- Ameduri, B. From vinylidene fluoride (vdf) to the applications of vdf-containing polymers and copolymers: recent developments and future trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.Z.; Wang, P.; Yang, Y.N. Rheological and thermodynamic variation in polysulfone solution by PEG introduction and its effect on kinetics of membrane formation via phase-inversion process. J. Membr. Sci. 2006, 279, 230–237. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Zheng, Q. Preparation and properties of polysulfone/TiO2 composite ultrafiltration membranes. J. Polym. Sci. B Polym. Phys. 2006, 44, 879–887. [Google Scholar] [CrossRef]
- Handbook of Industrial Membranes. Available online: https://www.elsevier.com/books/handbook-of-industrial-membranes/scott/978-1-85617-233-2 (accessed on 21 March 2017).
- Akthakul, A.; McDonald, W.F.; Mayes, A.M. Noncircular pores on the surface of asymmetric polymer membranes: Evidence of pore formation via spinodal demixing. J. Membr. Sci. 2002, 208, 147–155. [Google Scholar] [CrossRef]
- Soliman, M.F.; Abdel-Aziz, M.H.; Bamaga, O.A.; Gzara, L.; Sharaf, F.; Al-Sharif, M.; Bassyouni, Z.; Ahmad, R. Performance evaluation of blended PVDF membranes for desalination of seawater RO brine using direct contact membrane distillation. Desalination.Water. Treat. 2017, 63, 6–14. [Google Scholar] [CrossRef]
- Young, T.H.; Chen, L.W. Pore formation mechanism of membranes from phase inversion process. Desalination. 1995, 103, 233–247. [Google Scholar] [CrossRef]
- Young, T.H.; Chen, L.W.; Cheng, L.P. Membranes with a microparticulate morphology. Polymer. 1996, 37, 1305–1310. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Zereshki, S.; Mansourpanah, Y. Preparation and characterization of modified nano-porous PVDF membrane with high antifouling property using UV photo-grafting. Appl. Surf. Sci. 2009, 255, 7455–7461. [Google Scholar] [CrossRef]
- Javaid, U.; Khan, Z.M.; Khan, M.B.; Bassyouni, M.; Abdel-Hamid, S.S.; Abdel-Aziz, M.H.; ul Hasan, S.W. Fabrication and thermo-mechanical characterization of glass fiber/vinyl ester wind turbine rotor blade. Compos. Part B: Eng. 2016, 91, 257–266. [Google Scholar] [CrossRef]
- Hassan, A.; Niazi, M.B.K.; Hussain, A.; Farrukh, S.; Ahmad, T. Development of anti-bacterial pva/starch based hydrogel membrane for wound dressing. J. Polym. Environ. 2017, 26, 1–9. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.S.; Xiang, C.B. Preparation of poly(vinylidene fluoride)(pvdf) ultrafiltration membrane modified by nano-sized alumina (Al2O3) and its antifouling research. Polymer 2005, 46, 7701–7706. [Google Scholar] [CrossRef]
- Hou, D.; Wang, J.; Sun, X.; Ji, Z.; Luan, Z. Preparation and properties of PVDF composite hollow fiber membranes for desalination through direct contact membrane distillation. J. Membr. Sci. 2012, 406, 185–200. [Google Scholar] [CrossRef]
- Bassyouni, M.; Gutub, S.A. Materials selection strategy and surface treatment of polymer composites for wind turbine blades fabrication. Polym. Polym. Compos. 2013, 21, 463. [Google Scholar]
- Abdel-Mohsen, A.M.; Aly, A.S.; Hrdina, R.; Montaser, A.S.; Hebeish, A. Eco-synthesis of PVA/chitosan hydrogels for biomedical application. J. Polym. Environ. 2011, 19, 1005–1012. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Alfadul, S.M.; Al-Romaih, H.S.; Al Shabouna, F.S.; Al-Harbi, O.A.; Drioli, E. Effect of selected spinning parameters on PVDF hollow fiber morphology for potential application in desalination by VMD. Desalination 2014, 344, 28–35. [Google Scholar] [CrossRef]
- Tomaszewska, M. Preparation and properties of flat-sheet membranes from poly(vinylidene fluoride) for membrane distillation. Desalination 1996, 104, 1–11. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.S.; Munari, A.T. Solubility parameters of poly(vinylidene fluoride). J. Polym. Sci. B Polym. Phys. 1988, 26, 785–794. [Google Scholar] [CrossRef]
- Tao, M.; Liu, F.; Ma, B.; Xue, L. Effect of solvent power on PVDF membrane polymorphism during phase inversion. Desalination 2013, 316, 137–145. [Google Scholar] [CrossRef]
- Nejati, S.; Boo, C.; Osuji, C.O.; Elimelech, M. Engineering flat sheet microporous PVDF films for membrane distillation. J. Membr. Sci. 2015, 492, 355–363. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhu, B.K.; Ma, X.T.; Xu, Y.Y. Porous membranes modified by hyperbranched polymers: Preparation and characterization of PVDF membrane using hyperbranched polyglycerol as additive. J. Membr. Sci. 2007, 290, 222–229. [Google Scholar] [CrossRef]
- Wang, D.; Li, K.; Teo, W.K. Porous PVDF asymmetric hollow fiber membranes prepared with the use of small molecular additives. J. Membr. Sci. 2000, 178, 13–23. [Google Scholar] [CrossRef]
- Fontananova, E.; Jansen, J.C.; Cristiano, A.; Curcio, E.; Drioli, E. Effect of additives in the casting solution on the formation of PVDF membranes. Desalination 2006, 192, 190–197. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.L.; Yu, L.Y. Effects of mixed solvents and PVDF types on performances of PVDF microporous membranes. J. Appl. Polym. Sci. 2010, 115, 2277–2287. [Google Scholar] [CrossRef]
- Prince, J.A.; Singh, G.; Rana, D.; Matsuura, T.; Anbharasi, V.; Shanmugasundaram, T.S. Preparation and characterization of highly hydrophobic poly (vinylidene fluoride)-Clay nanocomposite nanofiber membranes (PVDF–clay NNMs) for desalination using direct contact membrane distillation. J. Membr. Sci. 2012, 397–398, 80–86. [Google Scholar] [CrossRef]
- Li, N.N.; Fane, A.G.; Ho, W.S.W.; Matsuura, T. Advanced Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 297–370. [Google Scholar]
- Taniguchi, M.; Belfort, G. Correcting for surface roughness: Advancing and receding contact angles. Langmuir 2002, 18, 6465–6467. [Google Scholar] [CrossRef]
- Figoli, A.; Simone, S.; Criscuoli, A.; AL-Jlil, S.A.; Al Shabouna, F.S.; Al-Romaih, H.S.; Di Nicolò, E.; Al-Harbi, O.A.; Drioli, E. Hollow fibers for seawater desalination from blends of PVDF with different molecular weights: Morphology, properties and VMD performance. Polymer. 2014, 55, 1296–1306. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, K. Membrane Distillation: Principles and Applications, 1st ed.; Elsevier: Amersterdam, The Netherlands, 2011; pp. 249–294. [Google Scholar]
- Cui, Z.L. Poly(vinylidene fluoride) membrane preparation with an environmental diluent via thermally induced phase separation. J. Membr. Sci. 2013, 444, 223–236. [Google Scholar] [CrossRef]
- Ji, G.L.; Zhu, L.P.; Zhu, B.K.; Zhang, C.F.; Xu, Y.Y. Structure formation and characterization of PVDF hollow fiber membrane prepared via TIPS with diluent mixture. J. Membr. Sci. 2008, 319, 264–270. [Google Scholar] [CrossRef]
- Chen, Z.; Rana, D.; Matsuura, T.; Meng, D.; Lan, C.Q. Study on structure and vacuum membrane distillation performance of PVDF membranes: II. influence of molecular weight. Chem. Eng. J. 2015, 276, 174–184. [Google Scholar] [CrossRef]
- German, R.M. Handbook of Mathematical Relations in Particulate Materials Processing; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 267–270. [Google Scholar]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Chen, Z.; Rana, D.; Matsuura, T.; Yang, Y.; Lan, C.Q. Study on the structure and vacuum membrane distillation performance of PVDF composite membranes: I. influence of blending. Sep. Purif. Technol. 2014, 133, 303–312. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Heat transport and membrane distillation coefficients in direct contact membrane distillation. J. Membr. Sci. 2003, 212, 177–193. [Google Scholar] [CrossRef]
- Yun, Y.; Ma, R.; Zhang, W.; Fane, A.G.; Li, J. Direct contact membrane distillation mechanism for high concentration NaCl solutions. Desalination 2006, 188, 251–262. [Google Scholar] [CrossRef]
- Srisurichan, S.; Jiraratananon, R.; Fane, A.G. Mass transfer mechanisms and transport resistances in direct contact membrane distillation process. J. Membr. Sci. 2006, 277, 186–194. [Google Scholar] [CrossRef]
- Ding, Z.; Ma, R.; Fane, A.G. A new model for mass transfer in direct contact membrane distillation. Desalination 2003, 151, 217–227. [Google Scholar] [CrossRef]
- Kim, E.S.; wang, G.H.; El-Din, M.G.; Liu, Y. Development of nanosilver and multi-walled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J. Membr. Sci. 2012, 394–395, 37–48. [Google Scholar] [CrossRef]
- Hassankiadeh, N.T.; Cui, Z.; Kim, J.H.; Shin, D.W.; Sanguineti, A.; Arcella, V.; Lee, Y.M.; Drioli, E. PVDF hollow fiber membranes prepared from green diluent via thermally induced phase separation: Effect of PVDF molecular weight. J. Membr. Sci. 2014, 471, 237–246. [Google Scholar] [CrossRef]
- Chen, C.; Tang, L.; Liu, B.; Zhang, X.; Crittenden, J.; Chen, K.L.; Chen, Y. Forming mechanism study of unique pillar-like and defect-free PVDF ultrafiltration membranes with high flux. J. Membr. Sci. 2015, 487, 1–11. [Google Scholar] [CrossRef]
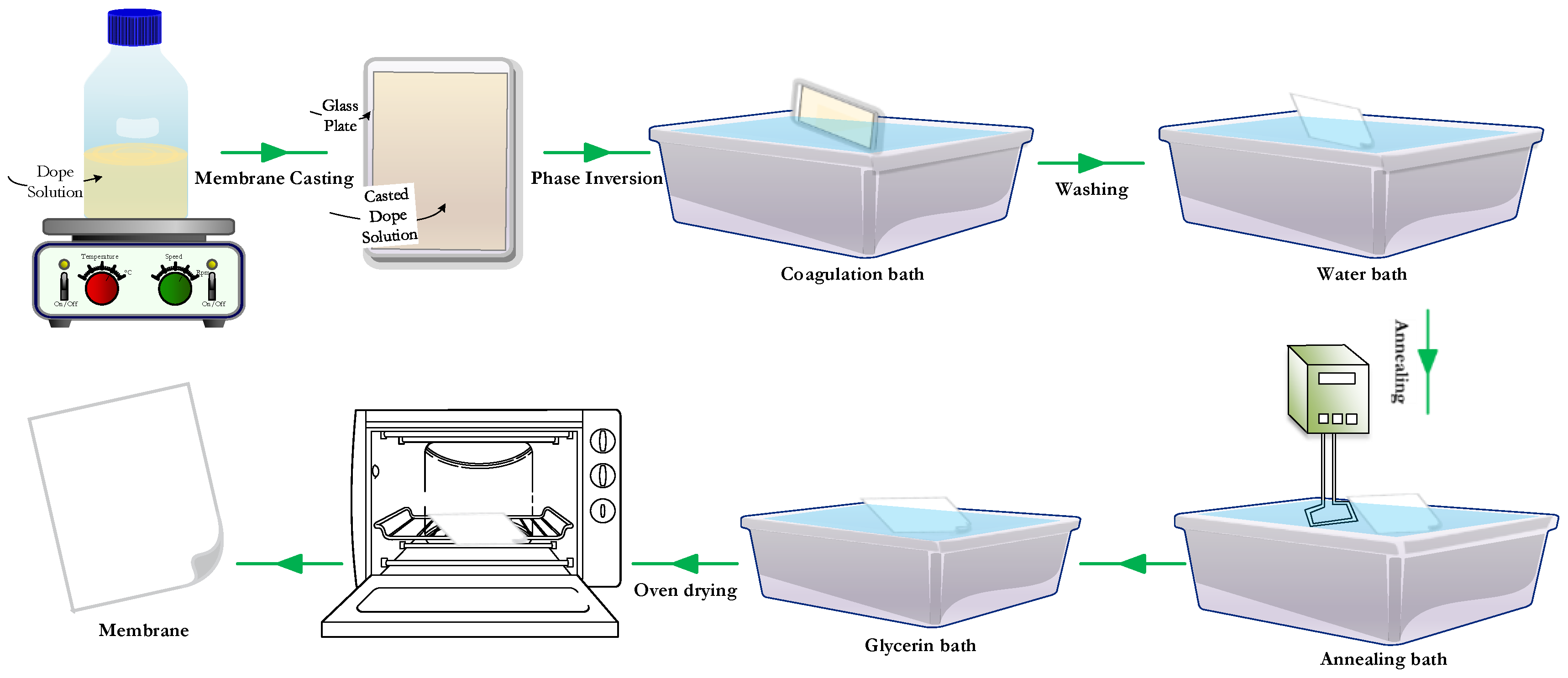




| Membrane | PVDF Homopolymer | PVDF-co-HFP | NMP | THF | DMF |
|---|---|---|---|---|---|
| PR-01 | 18 | 2 | 80 | - | - |
| PR-02 | 16 | 4 | 80 | - | - |
| PR-03 | 14 | 6 | 80 | - | - |
| PR-04 | 20 | 5 | 75 | - | - |
| PR-05 | 18 | 7 | 75 | - | - |
| PR-06 | 15 | 10 | 75 | - | - |
| PR-07 | 15 | 10 | - | 40 | 35 |
| PR-08 | 15 | 10 | 40 | 35 | - |
| PR-09 | 15 | 10 | 40 | - | 35 |
| PR-10 | 15 | 10 | 25 | 25 | 25 |
| Sr. No. | Parameter | Value |
|---|---|---|
| 1. | Casting knife thickness | 400 µm |
| 2. | Casting knife speed | 10 mm·s−1 |
| 3. | Time before immersion | 90 s |
| 4. | Coagulation bath | 30% isopropanol in water |
| 5. | Coagulation bath | 180 s at 10 °C |
| 6. | Washing bath (water) | 15 min |
| 7. | Annealing bath | 30 min at 70 °C |
| 8. | Glycerin bath | 30 min |
| 9. | Oven | 3 h at 60 °C |
| 10. | Indoor relative humidity | 60% |
| Membrane | Thickness [μm] | Yield Strength [MPa] | Fracture Strength [MPa] | Young’s Modulus [MPa] | Elongation at Break [%] |
|---|---|---|---|---|---|
| PR-01 | 130.5 (±4.7) | 2.56 | 3.01 | 50.77 | 26.86 |
| PR-02 | 148.3 (±6.4) | 1.78 | 1.82 | 76.42 | 9.11 |
| PR-03 | 126.0 (±2.2) | 2.08 | 2.72 | 66.03 | 17.21 |
| PR-04 | 150.0 (±4.8) | 4.01 | 4.05 | 58.19 | 29.32 |
| PR-05 | 138.3 (±3.0) | 3.17 | 3.94 | 50.88 | 34.77 |
| PR-06 | 161.0 (±5.4) | 4.01 | 4.41 | 25.25 | 51.33 |
| PR-07 | 94.5 (±1.9) | 5.22 | 7.37 | 60.33 | 55.32 |
| PR-08 | 114.3 (±2.2) | 5.53 | 9.62 | 82.26 | 62.36 |
| PR-09 | 140.0 (±3.4) | 6.13 | 10.56 | 87.12 | 69.44 |
| PR-10 | 105.5 (±4.2) | 3.59 | 4.22 | 45.52 | 36.21 |
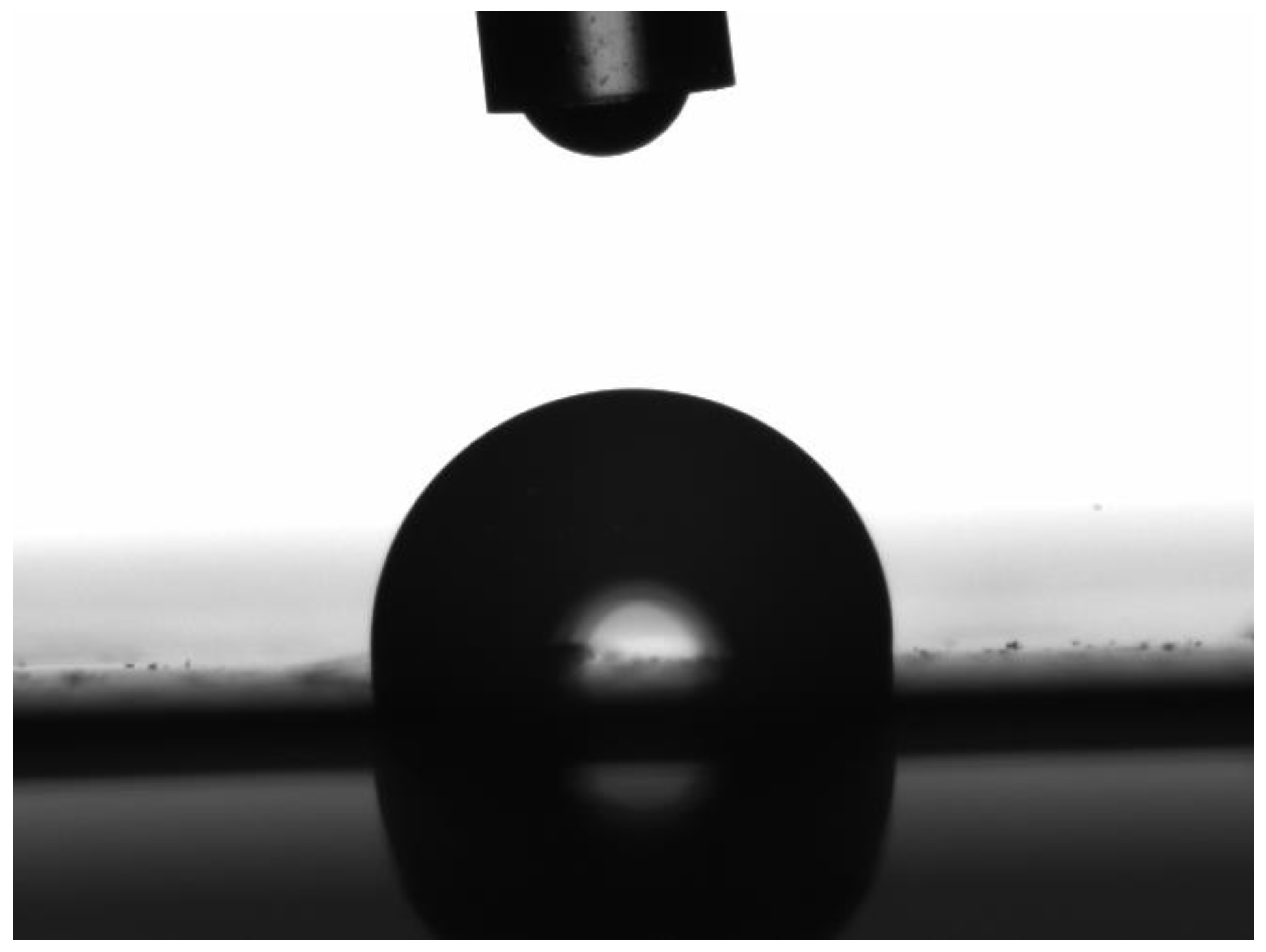
| Membrane | Porosity [%] | Mean Flow Pore (MFP) Size | Contact Angle (Topside) [°] (±Std. Dev.) | Roughness (Topside) Ra [nm] | Roughness (Bottom Side) Ra [nm] |
|---|---|---|---|---|---|
| PR-01 | 40.95 (±0.70) | 0.02 | 91.39 (±1.52) | 3.49 | 39.49 |
| PR-02 | 44.83 (±0.80) | - | 99.70 (±1.04) | 2.05 | 08.93 |
| PR-03 | 41.43 (±0.27) | 0.08 | 99.33 (±0.93) | 3.28 | 14.34 |
| PR-04 | 44.82 (±0.70) | 0.05 | 96.33 (±0.86) | 3.43 | 18.49 |
| PR-05 | 46.25 (±0.00) | - | 97.44 (±0.36) | 3.13 | 13.74 |
| PR-06 | 46.47 (±0.33) | 0.02 | 90.11 (±0.13) | 2.78 | 17.68 |
| PR-07 | 11.22 (±0.05) | - | 83.46 (±0.10) | 4.28 | 33.13 |
| PR-08 | 25.04 (±0.08) | - | 81.71 (±0.50) | 3.21 | 23.63 |
| PR-09 | 43.63 (±0.50) | 0.02 | 91.85 (±1.01) | 3.15 | 18.42 |
| PR-10 | 26.74 (±0.22) | 0.02 | 88.33 (±1.45) | - | 14.23 |
| Membrane | Water Permeability Lp [L·h−1·m−2·bar−1] (R2) | Permeate Vapor Flux [kg·m−2·h−1] (±Std. dev.) |
|---|---|---|
| PR-01 | 11.91 (0.977) | 2.916 (±0.006) |
| PR-02 | 14.41 (0.923) | 2.892 (±0.006) |
| PR-03 | 12.12 (0.998) | 2.871 (±0.006) |
| PR-04 | 9.59 (0.994) | 1.524 (±0.004) |
| PR-05 | 5.79 (0.997) | 1.652 (±0.005) |
| PR-06 | 22.09 (0.982) | 1.833 (±0.005) |
| PR-07 | 35.31 (0.976) | 0.219 (±0.002) |
| PR-08 | 25.85 (0.969) | 0.004 (±0.002) |
| PR-09 | 21.34 (0.997) | 1.361 (±0.004) |
| PR-10 | 21.78 (0.977) | 0.083 (±0.002) |
| Mean Pore Size [μm] | Thickness [μm] | Feed Velocity [m·s−1] | Tf [°C] | Permeate Flux [kg·m−2·h−1] | Reference |
|---|---|---|---|---|---|
| 0.22 | 126 | 0.1 | 40–70 | 3.6–16.2 | [40] |
| 0.22 | 120 | 0.145 | 36–66 | 5.4–36 | [41] |
| 0.22 | 110 | 0.23 | 40–70 | 7–33 | [42] |
| 0.2 | 100 | 1.2 L·min−1 | 30–46 | 7–41 | [43] |
| 0.02–0.08 | 95–161 | 1.7 L·min−1 | 70 | 0.004–2.9 | Present study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.; Bamaga, O.A.; Gzara, L.; Bassyouni, M.; Abdel-Aziz, M.H.; Soliman, M.F.; Drioli, E.; Albeirutty, M. Assessment of Blend PVDF Membranes, and the Effect of Polymer Concentration and Blend Composition. Membranes 2018, 8, 13. https://doi.org/10.3390/membranes8010013
Ali I, Bamaga OA, Gzara L, Bassyouni M, Abdel-Aziz MH, Soliman MF, Drioli E, Albeirutty M. Assessment of Blend PVDF Membranes, and the Effect of Polymer Concentration and Blend Composition. Membranes. 2018; 8(1):13. https://doi.org/10.3390/membranes8010013
Chicago/Turabian StyleAli, Imtiaz, Omar A. Bamaga, Lassaad Gzara, M. Bassyouni, M. H. Abdel-Aziz, M. F. Soliman, Enrico Drioli, and Mohammed Albeirutty. 2018. "Assessment of Blend PVDF Membranes, and the Effect of Polymer Concentration and Blend Composition" Membranes 8, no. 1: 13. https://doi.org/10.3390/membranes8010013
APA StyleAli, I., Bamaga, O. A., Gzara, L., Bassyouni, M., Abdel-Aziz, M. H., Soliman, M. F., Drioli, E., & Albeirutty, M. (2018). Assessment of Blend PVDF Membranes, and the Effect of Polymer Concentration and Blend Composition. Membranes, 8(1), 13. https://doi.org/10.3390/membranes8010013