1. Introduction
In the last several years, olive mill wastewaters (OMWWs) have received increasing attention because of their high content of biophenols, which possess a high spectrum of biological functions, which include antioxidant, anti-inflammatory, antibacterial, and antiviral activities [
1,
2,
3]. The high polar nature of most olive oil biophenolic compounds result in their sizable loss with the wastewater during processing [
4]. Several approaches have been investigated for biophenol recovery for their potential reuse in functional foods, nutraceutical, and pharmaceutical products. Navarro
et al. [
5] used ultrafiltration and nanofiltration of OMWWs and successive spray drying with maltodextrin and acacia fiber as antiglycative ingredients for foods and pharmacological preparations. Petrotos
et al. [
6] clarified OMWWs by using membrane technology, and the recovered biophenols, after been processed by an reverse osmosis (RO) membrane technique followed by freeze-drying, were encapsulated for the enrichment of yogurt and other dairy products. Troisi
et al. [
7] evaluated the ability of biophenols obtained from OMWWs through ultrafiltration and successive spray drying in controlling the ultra-high-temperature milk treatment processing.
The activity and potential health benefits of the biophenolic compounds are influenced by their stability in end-product formulation and during storage (temperature, oxygen, light) as well as their bioavailability after administration (
i.e., insufficient gastric residence time, low permeability, and/or solubility within the gut). The delivery of these compounds therefore requires innovative productive strategies to provide protective mechanisms to preserve their active molecular form and/or to deliver them to target sites in the body. Microencapsulation technology has promoted the development of new products and the improvement of existing products by solving unique challenges, such as converting liquids to solids, separating reactive components, protecting ingredients from the environment, controlling release, or masking ingredients. There have been major advances in the development of delivery systems to encapsulate lipophilic bioactive components, while there is still a pressing need to develop novel manufacturing conditions and formulations to prepare effective delivery systems for hydrophilic bioactives such as biophenols [
8].
Major challenges in the design of delivery systems suitable for the encapsulation of bioactive compounds includes the control of particle size and surface properties, as well as the fundamental physicochemical phenomena associated with encapsulation and release (i.e., partitioning and mass transport release of active ingredients) for the production of particle-based end-products with target functionality.
Emulsion systems are essential components of food, cosmetics, and drugs, enhancing the bioavailability of hydrophilic or lipophilic active compounds dissolved in the dispersed water or oil phase, respectively. Monodisperse droplets gives better control over the dose and release behavior of the encapsulated active compounds and yields higher encapsulation efficiency and better biocompatibility. Conventional devices for preparing emulsions such as high pressure, ultrasonic homogenizers, colloid mills, rotor–stator systems, and microfluidizers require high energy input for the production of droplets, leading to droplets with a wide size distribution and a degradation of temperature/shear sensitive compounds that should be encapsulated. Membrane emulsification (ME) is a dispersion process to produce uniform droplets of one liquid phase (e.g., water) in a second immiscible liquid phase (e.g., oil) using a low energy per unit volume [
9,
10]. The shear stress is applied on the membrane surface, and the droplet size is controlled by the pore size of the membrane [
11].
In this work, for the first time, microparticle production, containing biophenols recovered from OMWWs by integrated membrane processes [
12], has been achieved. OMWWs used in the present work were produced from the biologic olive oil production (toxic pesticides are not used) and supplied by Olearia San Giorgio (San Giorgio Morgeto, Italy). The process used for the recovery and concentration of biophenols coming from OMWWs has been previously described [
12]. Briefly, after suspended solid removal by an acidification/microfiltration (MF) step, concentrated polyphenols where obtained by nanofiltration (NF) followed by osmotic distillation (OD). The enriched biophenolic fraction was used for the preparation of a water-in-oil (W/O) emulsion by ME. Pulsed back-and-forward ME was selected as a low shear encapsulation method because it is particularly attractive for the production of fragile particulate products, such as W/O microemulsions containing shear-sensitive ingredients [
13]. Moreover, considering that the bioactive molecule distribution between two phases plays an important role in determining its stability, retention, and release, the coefficient partition of both catechol, as a biomolecule model, and biophenols recovered and concentrated by an advanced membrane process [
12] was measured. The feasibility of valorizing biophenols coming from OMWWs in bio-functional particles has here been evaluated. This aspect is a crucial issue with a view to create a drug-controlled delivery system.
3. Materials and Methods
3.1. Materials
A water-in-oil emulsion was prepared using 15 wt % PVA (average MW 13,000–28,000 kDa, Sigma-Aldrich, Milan, Italy) as the dispersed phase and 2 wt % Span 80 (Sigma-Aldrich, Milan, Italy) in limonene (97%, Sigma-Aldrich, Milan, Italy) as the continuous water phase. Catechol (Sigma-Aldrich, Milan, Italy) has been selected as a biophenol molecule model because it was one of the most representative biophenols contained in OMWWs [
12] and it was dissolved in a PVA solution. Alternatively, a biophenol-concentrated solution obtained from OMWWs through integrated membrane operations [
12] was also used. Considering an initial OMWW volume of 1000 L, it was possible to obtain an enriched fraction of biophenol compounds of 87.5 g/L after taking NF and OD concentration steps. All solvents used for the high-performance liquid chromatography (HPLC) mobile phase preparation (acetonitrile, acetic acid, and methanol) were purchased from Sigma-Aldrich (Milan, Italy).
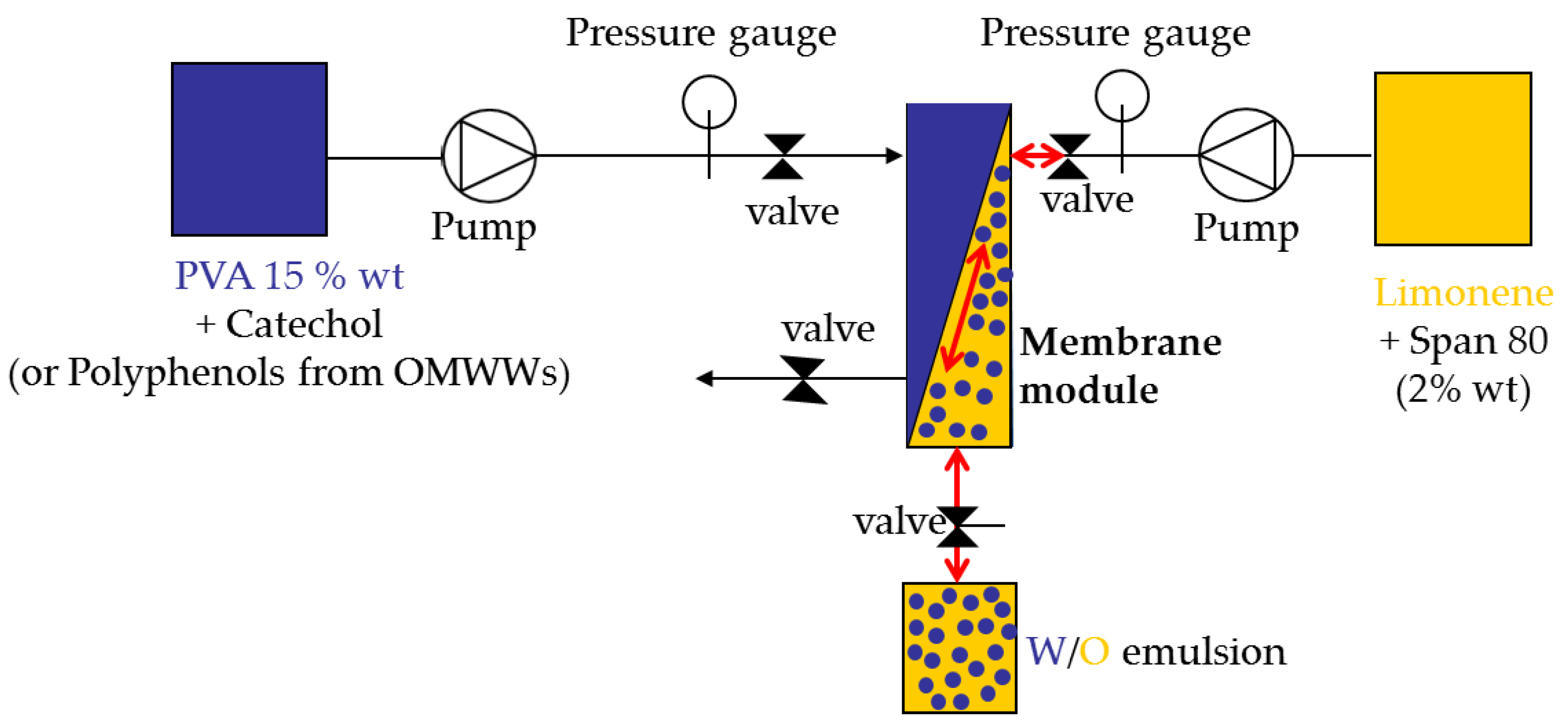
3.2. Preparation of Water-in-Oil Emulsions by Pulsed Back-and-Forward Cross-Flow Batch Membrane Emulsification
A SPG (Shirasu porous glass) tubular membrane (8.7 mm inner diameter × 0.65 mm wall thickness) with a nominal pore size of 3.1 μm from SPG Technology Co., Ltd. (Miyazaki, Japan) was used. The effective membrane area was 31.3 cm
2. The membrane was wetted in the continuous phase under vacuum and ultrasonic field before the installation. The schematic figure of the apparatus used for pulsed back-and-forward cross-flow batch membrane emulsification is illustrated in
Figure 6.
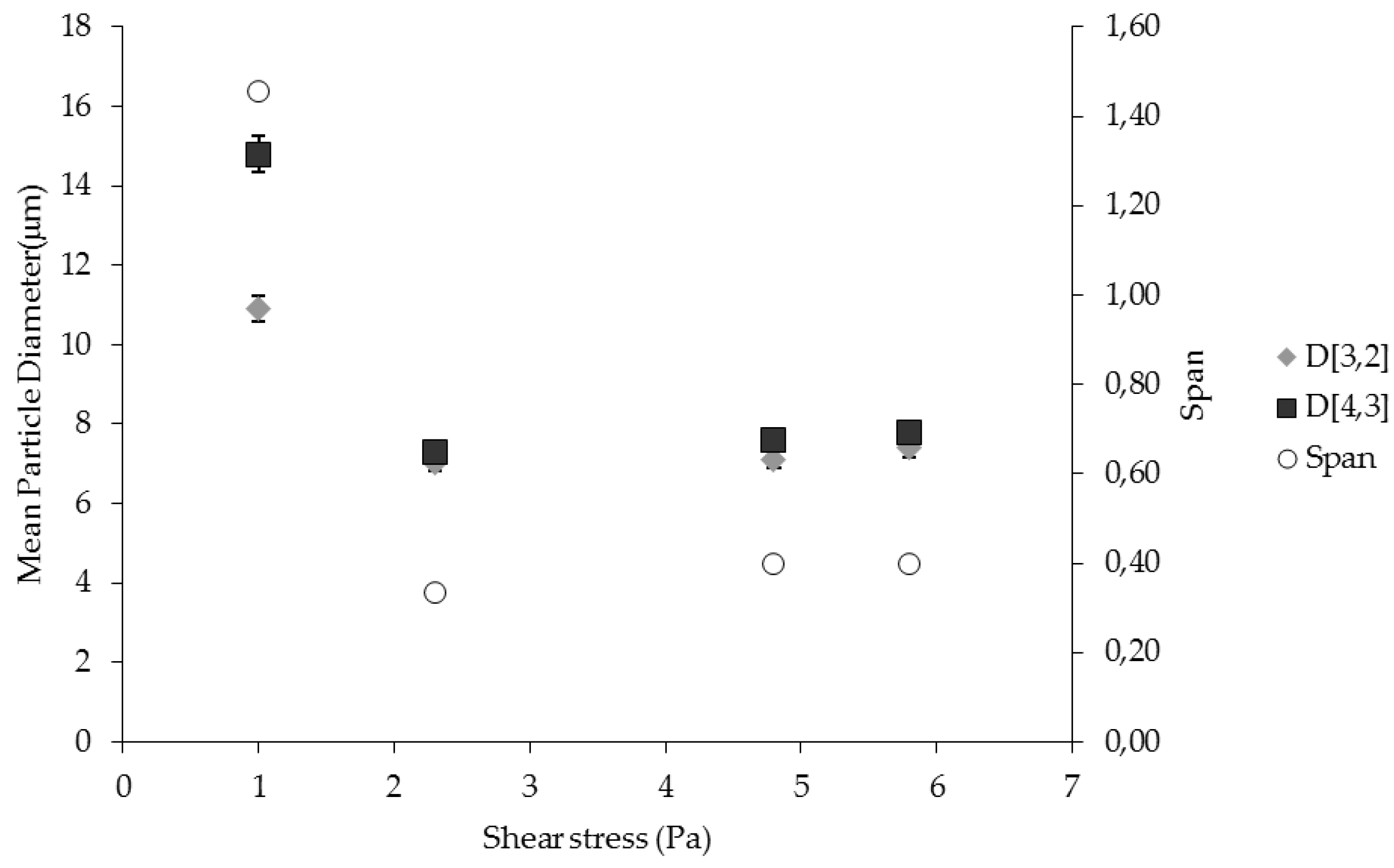
The dispersed phase was injected from the shell side of the membrane using a peristaltic pump (Gilson, Minipuls 3, 3V Chimica, Rome, Italy). The dispersed phase flux (Jd) was determined by volume upon the water consumption from the graduated feed cylinder. The effect of dispersed phase flux was investigated in the range between 2 to 20 L·h−1·m−2.
The continuous phase was agitated along the lumen side of the membrane by a programmable peristaltic pump (Digi-Staltic double-Y Masterflex
® Micropump, model GJ-N23.JF1SAB1; GENERALCONTROL S.p.A, Milan, Italy) at fixed amplitude and frequency. The maximum shear stress (τ
max) (Pa) is a function of the amplitude (a) and the frequency (f) of the pulsed flow according to the following equation:
The effect of shear stress was investigated in the range between 1 Pa and 5.8 Pa.
3.3. Experimental Setup and Procedure
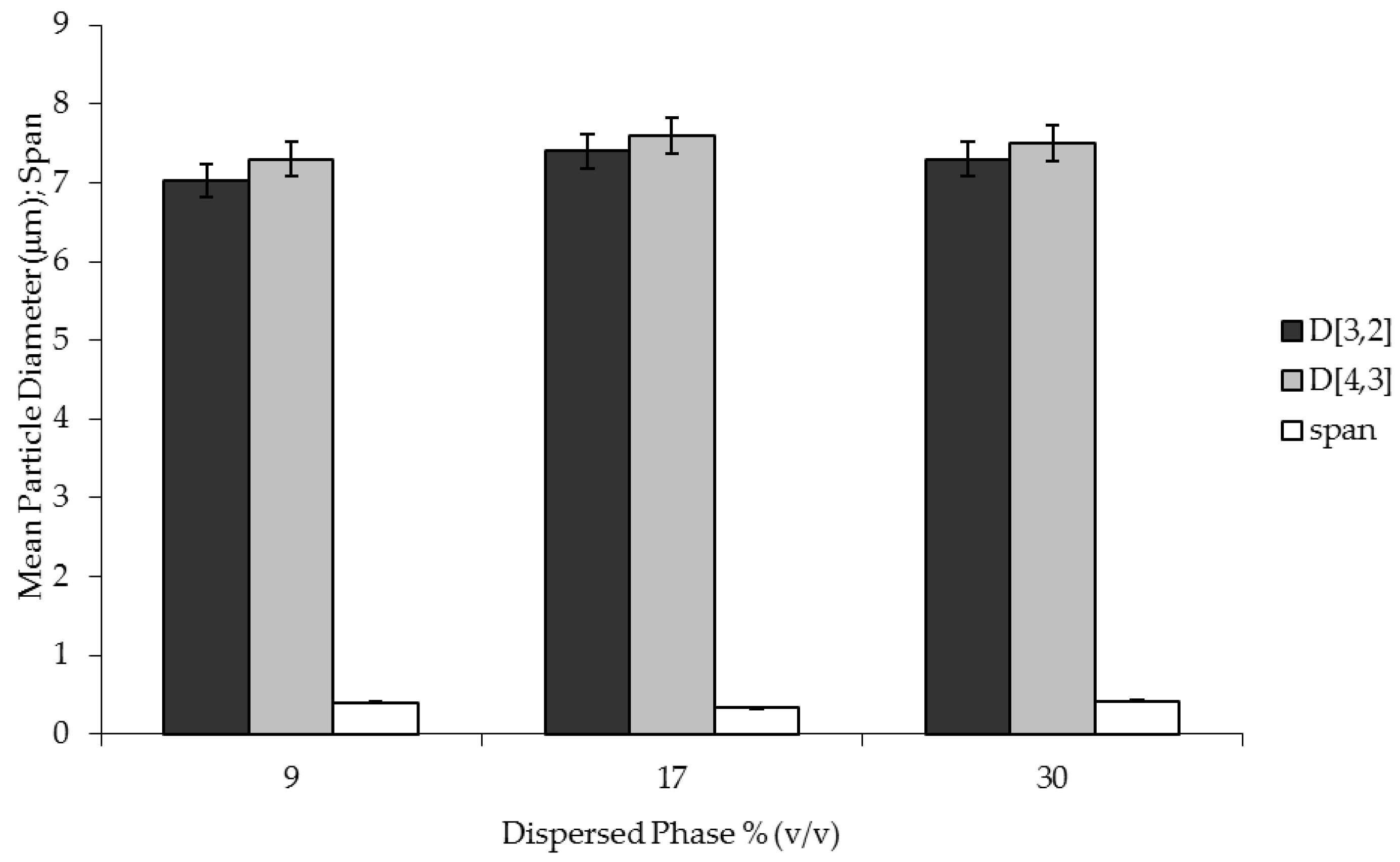
Preliminary experiments have been carried out in order to identify the appropriate process parameters (dispersed phase flux and shear stress) for membrane emulsification. The operating conditions providing the best uniformity of the droplets have been used for the production of W/O emulsion containing biophenols. Catechol and biophenols (recovered and concentrated from OMWWs) were dissolved in the aqueous dispersed phase. Catechol and biophenol concentration used were 10 g·L
−1 and 3 g·L
−1, respectively. The emulsification process was stopped when the dispersed phase percentage was 10% v/v. At the end of the emulsification, 10 mL of emulsion were centrifuged at 10,000 rpm for 10 min to promote phase separation, and the separated water phase was analyzed to measure the concentration of catechol and biophenols (according to the type of encapsulated materials used) via HPLC (Agilent Technologies Italia S.p.A., Milan, Italy) and UV spectrophotometry (Perckin Elmer, Monza, Italy), respectively. The encapsulation efficiency (
EE%) has been evaluated according to the following equation:
where
C0 and
Ci are the measured and initial concentration of catechol (or biophenols) in the aqueous dispersed phase, respectively.
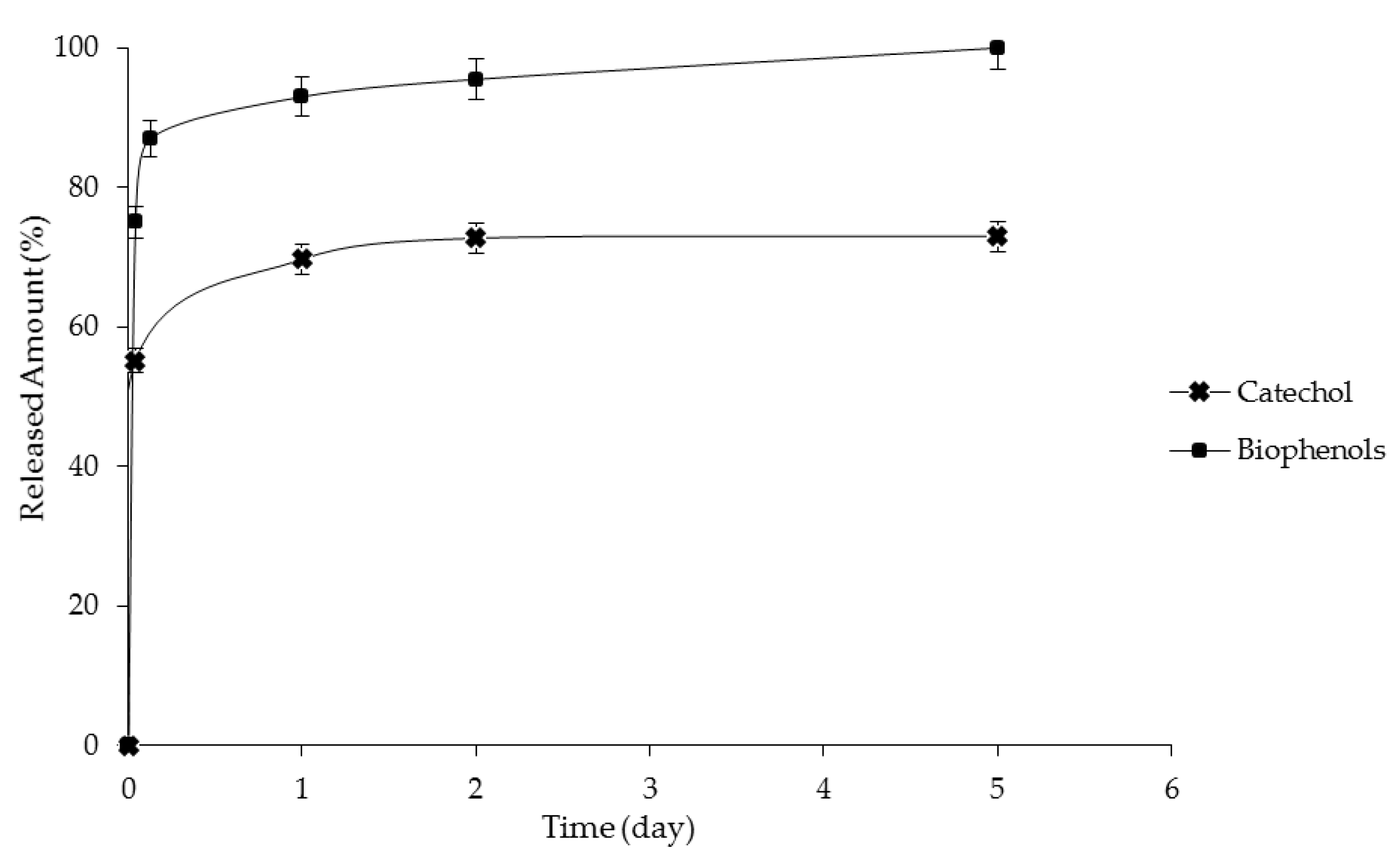
The experimental setup used for release experiments consisted of a cylindrical glass tube put in a thermostatic bath used to keep the temperature at 25 °C. Four milliliters of emulsion containing catechol or biophenols were filled in a dialysis bag (Spectra/Por Dialysis Membrane,
za < MWCO 15,000) and immersed in 18 mL of a receptor solution (ultrapure water). Aliquots of the receptor solution were withdrawn for the determination of catechol or biophenol concentration with an HPLC and UV spectrophotometer. The aliquot withdrawn was replaced with the same volume of pure water in order to maintain a constant volume of the receptor solution. The amount of catechol or biophenols released (
Cr) to the receptor solution is expressed as the ratio between the fraction of catechol (or biophenols) released and their initial encapsulated concentration during the time:
where
Ct and
CIN are the concentration of catechol (or biophenols) released at time
t and initially encapsulated in the aqueous dispersed phase, respectively.
3.4. Determination of the Partition Coefficient of Catechol between the Organic Solvent and Water
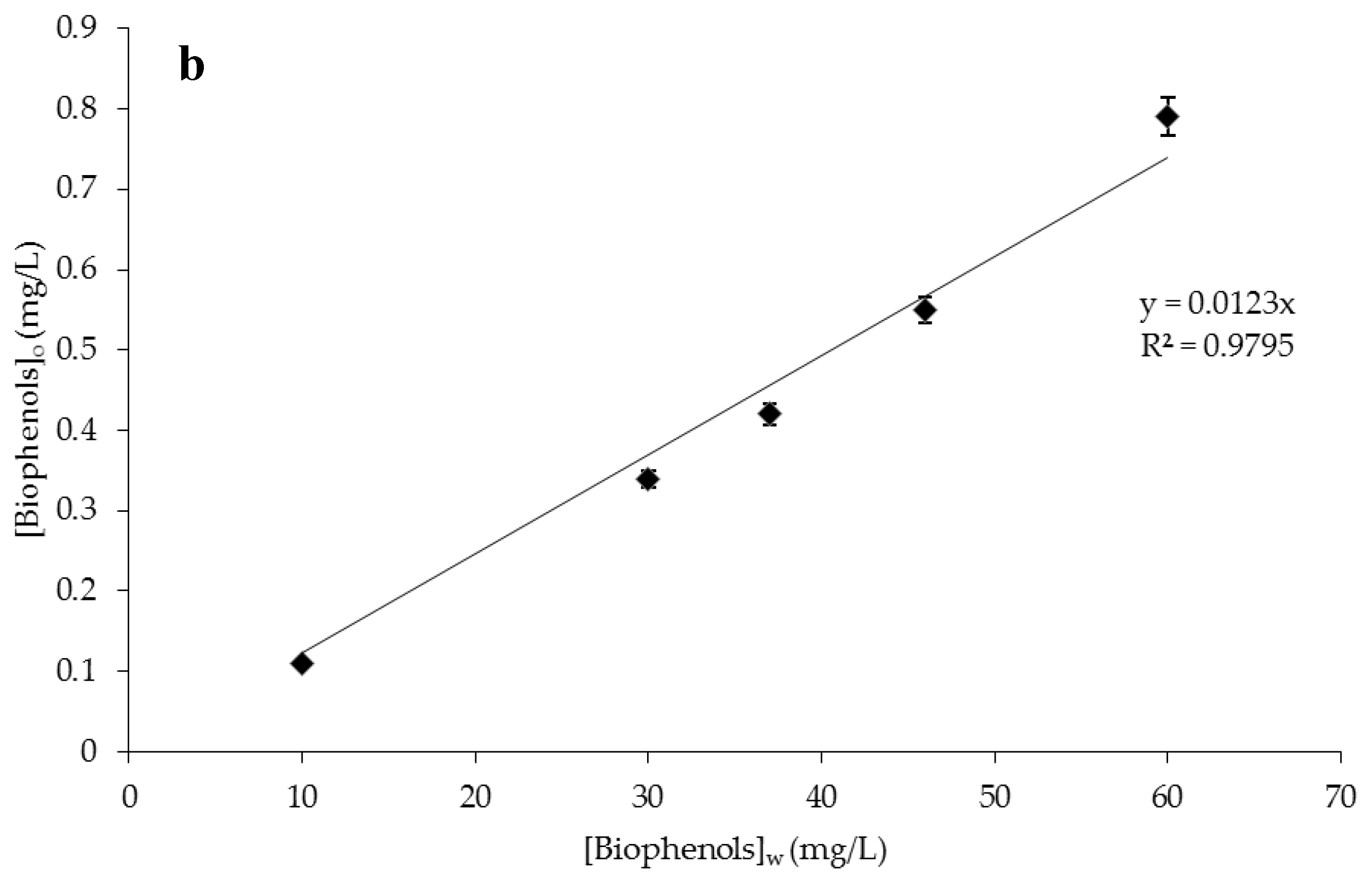
The bioactive partition between different phases depends on their relative thermodynamic affinity for each phase. Considering that the location of a bioactive within a delivery system plays an important role in determining its stability, retention, and release, the partition (or distribution) coefficient (KD) of catechol and biophenols was measured.
KD gives an indication of the substance solubility in the two phases and is defined by the equilibrium concentration ratio of the component A in the organic ([
A]
o) and aqueous phases ([
A]
w):
Another important parameter is the degree of extraction E defined as:
where
=
+
represents the initial moles of the component
A in the aqueous phase, and
represents the moles at equilibrium.
If
VW and
VO are the aqueous and organic phase volumes, then:
The methodology used to calculate the partition coefficients involved the formation of the limonene/water mixtures containing catechol or biophenols. In particular, catechol was dissolved in a known volume of distilled water at concentrations of 5 mg/L and then mixed with limonene; instead, the aqueous solution of biophenols comes from OMWWs. The flasks were immersed in a constant temperature bath (25 °C) and stirred for at least 4 h, long enough to approach equilibrium. After phase separation, catechol concentrations were determined quantitatively in both phases using HPLC. When biophenols from OMWWs were encapsulated, biophenols concentrations were determined by using the Folin-Ciocalteu method.
The distribution coefficient was calculated using different volume ratios of aqueous and organic phases (with a constant initial concentration of catechol in the aqueous phase) [
14].
The catechol concentration in limonene was plotted as a function of catechol concentration in the aqueous phase measured at equilibrium after each single extraction. The same procedure was used in the case of biophenols.
3.5. Determination of Particle Size and Particle Size Distribution
W/O emulsions were observed with an optical microscope (Zeiss, model Axiovert 25, Carl Zeiss S.p.A., Milan, Italy), equipped with a camera (JVC, model TK-C1481BEG, Carl Zeiss S.p.A., Milan, Italy). Pictures were analyzed by the Scion Image program that allows automatic counting and measurement of the droplets present in a selected area. From these measurements, the mean droplet size and size distribution were evaluated. For each sample, more than 900 droplets were counted and measured.
The mean particle size was expressed as the surface weighted mean diameter (or Sauter diameter), D [3,2], and as the volume weighted mean diameter (or the de Brouckere diameter), D [4,3]. D [3,2] and D [4,3] were determined, respectively, as follows:
where
Di = particle diameter of class, and
i and
ni = number of particle in class
i. The width of the droplet size distribution was expressed as a Span number, calculated by the following expression:
where
D[x0] is the diameter corresponding to
x0 vol % on a relative cumulative droplet size curve.
3.6. Analytical Measurements
The content of biophenols recovered and concentrated from OMWWs were determined by using the Folin-Ciocalteau method, while HPLC analysis was carried out to evaluate catechol concentration. A HPLC system equipped with an UV detector (Agilent 1200 Series, Agilent Technologies Italia S.p.A., Milan, Italy) and a reversed-phase Luna C18 column (Phenomenex, Torrance, CA, USA) were used. The analysis was carried out at a temperature of 25 °C, a pressure of 100 bar, and a wavelength of 280 nm.
A mixture of water/acetic acid (99/1, v/v) (75%, solvent A) and methanol/acetonitrile/acetic acid (90/9/1, v/v/v) (25%, solvent B) was used as the mobile phase. The analysis was made at a flow rate of 1.0 mL/min.
Total biophenols were estimated colorimetrically by using the Folin-Ciocalteu method. The absorbance was measured using a UV-visible spectrophotometer (Lamda EZ201; Perckin Elmer, Monza, Italy) at 765 nm.