Effects of Low Concentrations of Docosahexaenoic Acid on the Structure and Phase Behavior of Model Lipid Membranes
Abstract
:1. Introduction
2. Experimental Section

3. Results and Discussion
3.1. Phase Behavior of DPPC/DHA-PE Mixtures
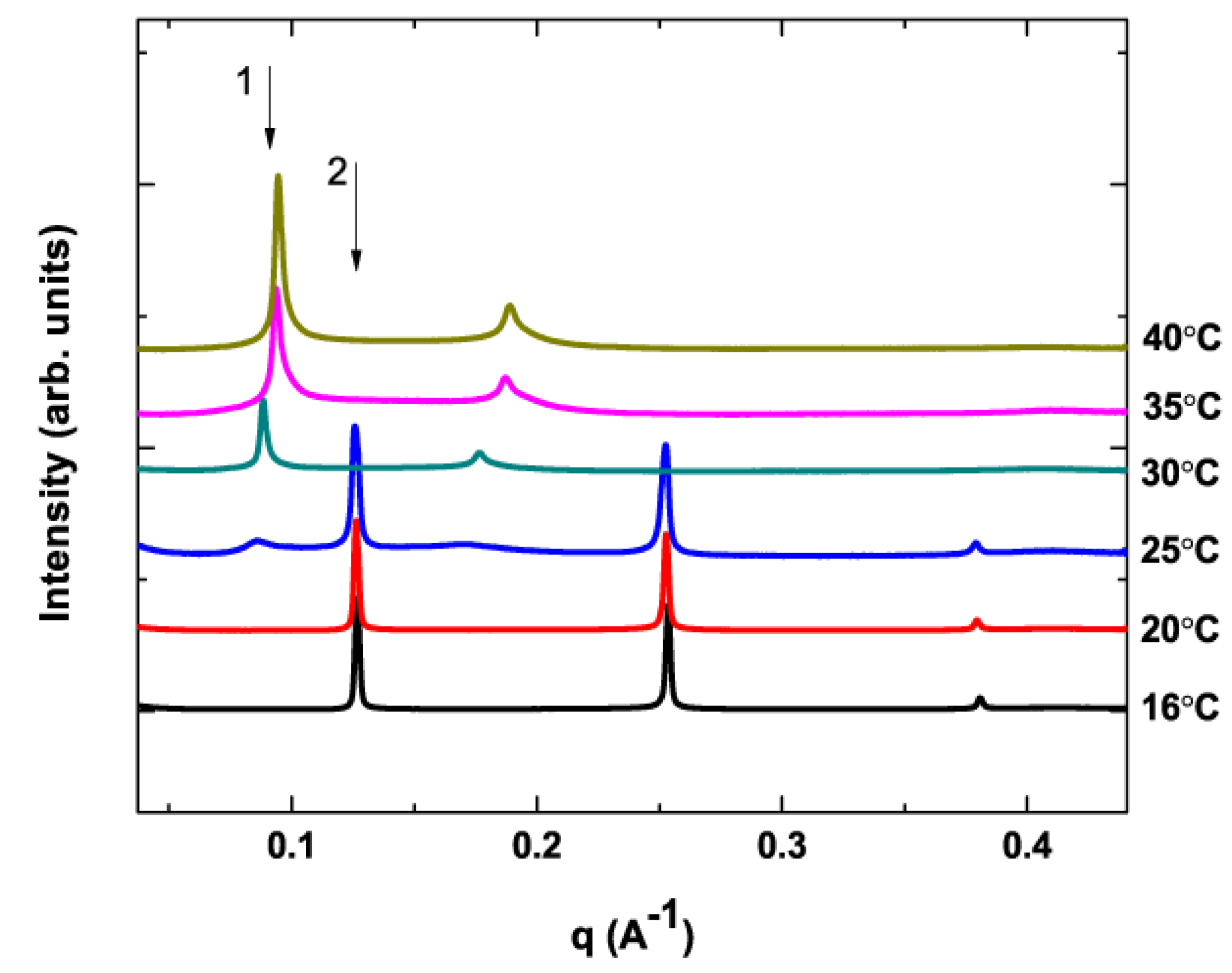
3.2. No X-ray Beam Damage
3.3. Phase Diagram of DPPC/DHA-PE

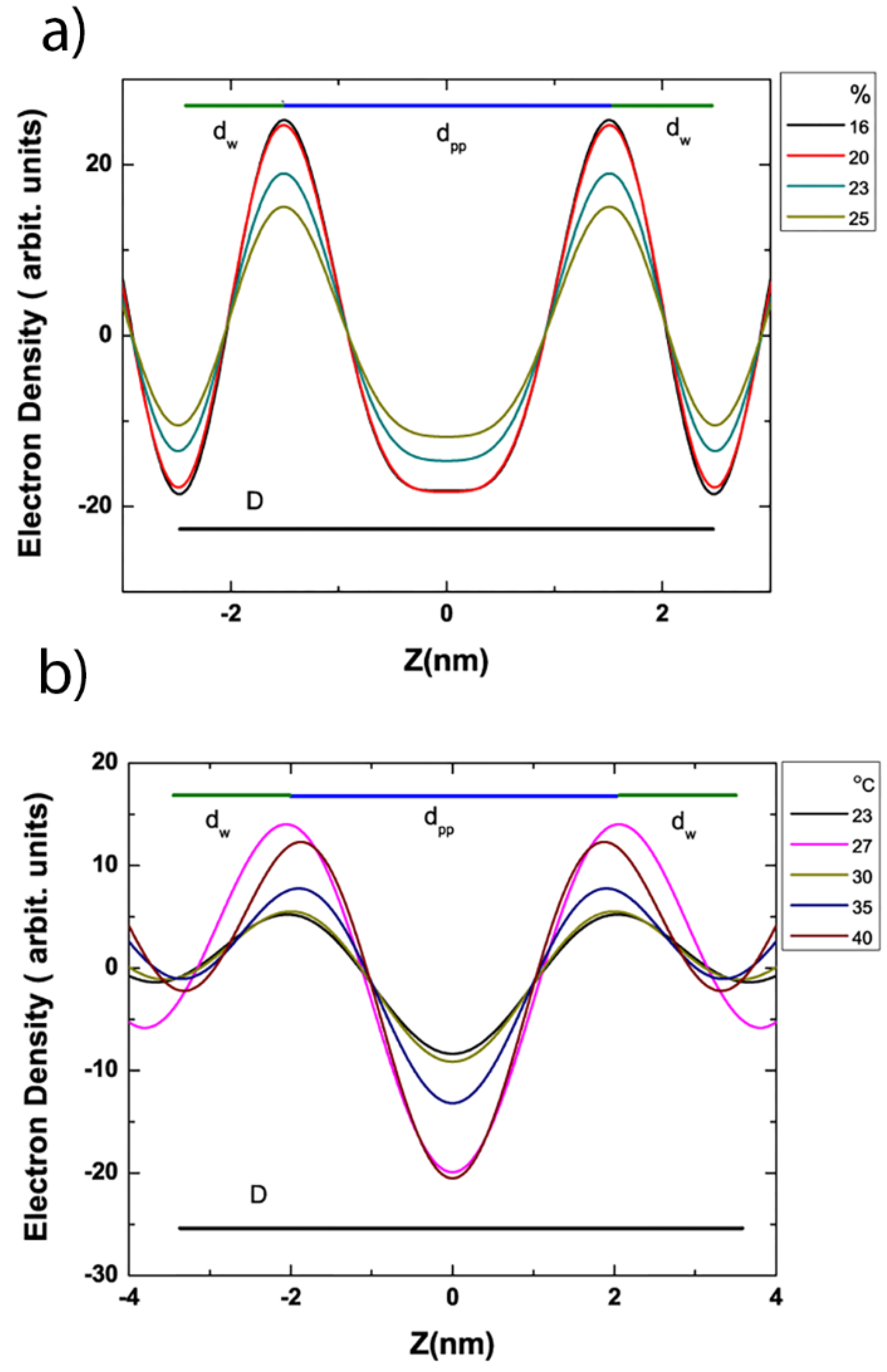
3.4. Electron Density Profile Calculations
3.5. Bilayer d-Spacing Plots
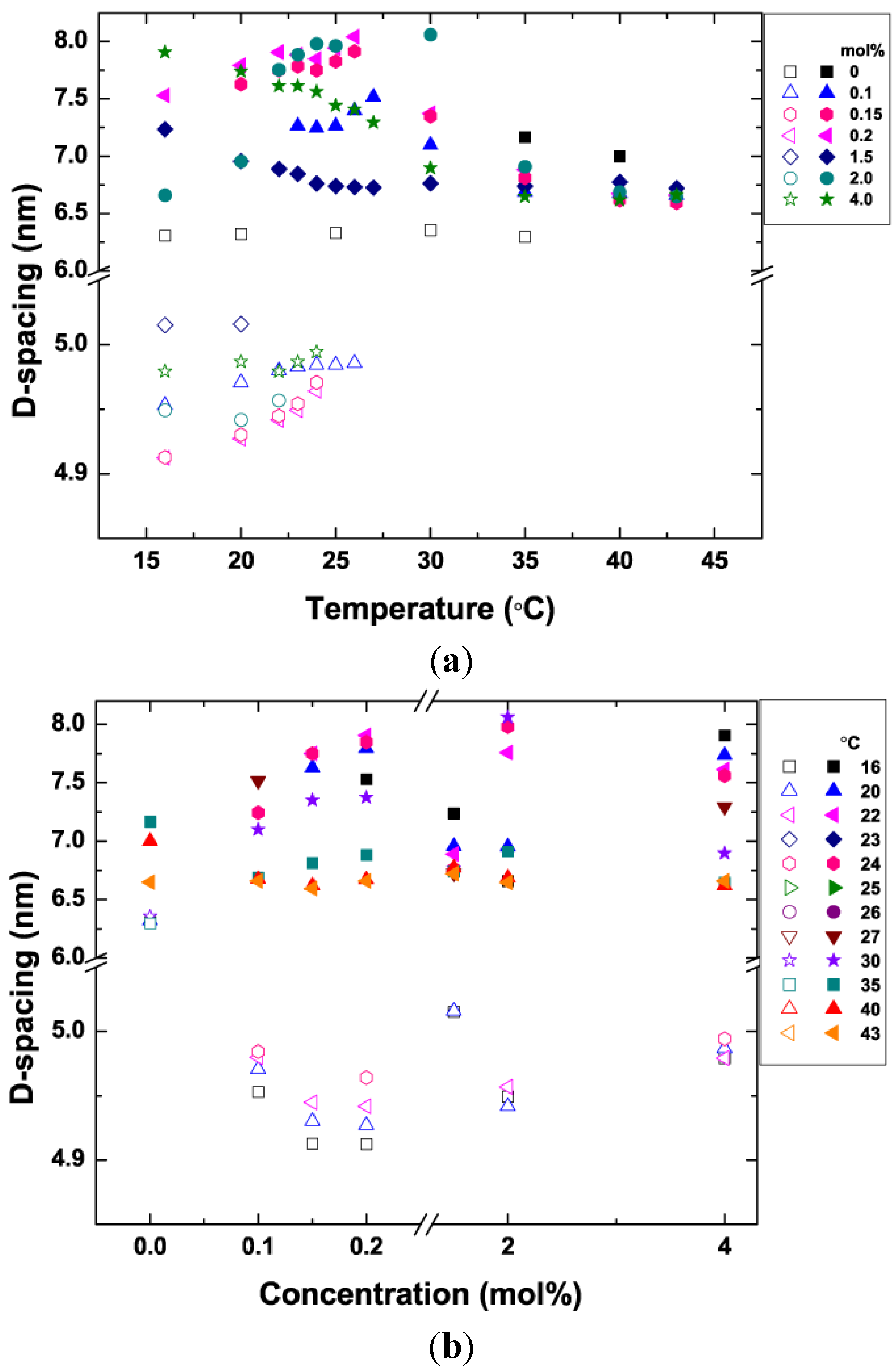
3.6. Proposed Cause of Decreased Bilayer Thickness in Lβ′
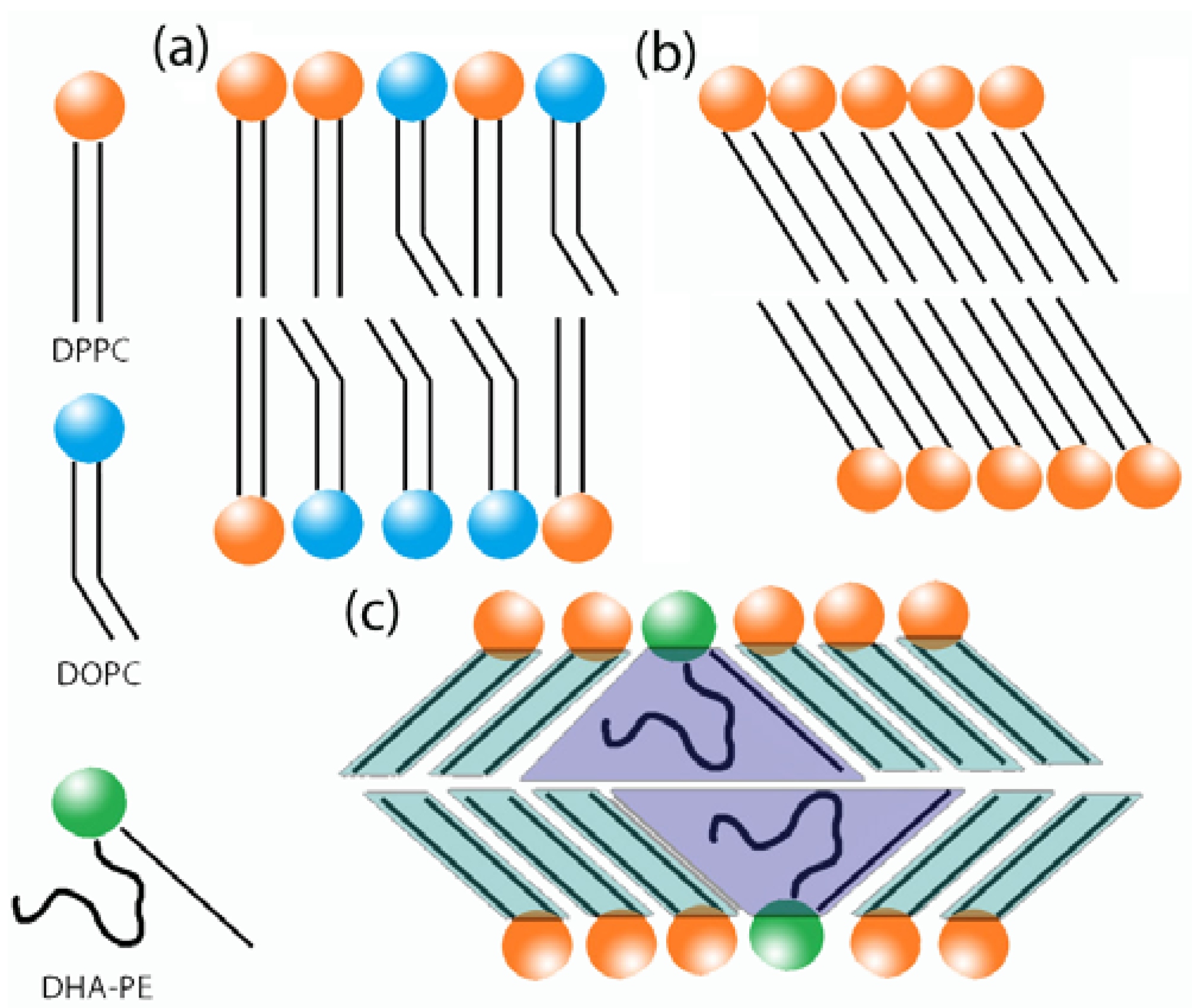
3.6.1. Increase in Tilt Angle
3.6.2. Interdigitation
3.6.3. Change in Water Layer Thickness
3.6.4. Presence of α-Tocopherol
3.6.5. Influence from Oxidized DHA-PE
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bang, H.O.; Dyerberg, J.; Hjoorne, N. The composition of food consumed by greenland eskimos. Acta Med. Scand. 1976, 200, 69–73. [Google Scholar] [CrossRef] [PubMed]
- McLennan, P.; Howe, P.; Abeywardena, M.; Muggli, R.; Raederstorff, D.; Mano, M.; Rayner, T.; Head, R. The cardiovascular protective role of docosahexaenoic acid. Eur. J. Pharm. 1996, 300, 83–89. [Google Scholar] [CrossRef]
- Carroll, K.K. Dietary fats and cancer. Am. J. Clin. Nutr. 1991, 53, S1064–S1067. [Google Scholar]
- Zerouga, M.; Stillwell, W.; Stone, J.; Powner, A.; Jenski, L.J. Phospholipid class as a determinant in docosahexaenoic acid's effect on tumor cell viability. Anitcancer Res. 1996, 16, 2863–2868. [Google Scholar]
- Kremer, J.M.; Lawrence, D.A.; Jubiz, W.; DiGiacomo, R.; Rynes, R.; Bartholomew, L.E.; Sherman, M. Dietary fish oil and olive oil supplementation in patients with rheumatoid-arthritis-clinical and immunological effects. Arthritis Rheum. 1990, 33, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Salem, N. Dietary polyunsaturated fatty-acids and depression—When cholesterol does not satisfy. Am. J. Clin. Nutr. 1995, 62, 1–9. [Google Scholar] [PubMed]
- Itua, I.; Naderali, E.K. Omega-3 and memory function: To eat or not to eat. Am. J. Alzheimers Dis. Other Demen. 2010, 25, 479–482. [Google Scholar] [CrossRef] [PubMed]
- International Society for the Study of Fatty Acids and Lipids (ISSFAL). ISSFAL Board Statement: Recommendations for the essential fatty acid requirement for infant formulas. J. Am. Coll. Nutr. 1995, 14, 213–214. [Google Scholar]
- Salem, N.J.; Kim, H.Y.; Yergey, J.A.; Simopoulos, A.P.; Kifer, R.R.; Martin, R.E. Omega-3 Fatty Acids in Growth and Development; Academic Press: New York, NY, USA, 1986; pp. 319–351. [Google Scholar]
- Vanmeter, A.R.; Ehringer, W.D.; Stillwell, W.; Blumenthal, E.; Jenski, L.J. Aged lymphocyte-proliferation following incorporation and retention of dietary omega-3-fatty acids. Mech. Ageing Dev. 1994, 75, 95–114. [Google Scholar] [CrossRef]
- Church, M.W.; Jen, K.L.C.; Jackson, D.A.; Adams, B.R.; Hotra, J.W. Abnormal neurological responses in young adult offspring caused by excess omega-3 fatty acid (fish oil) consumption by the mother during pregnancy and lactation. Neurotoxicol. Teratol. 2009, 31, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Essential fatty acid metabolism during early development. In Biology of Metabolism in Growing Animals; Burrin, D.G., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2005; pp. 235–274. [Google Scholar]
- Martin, R.E.; Elliott, M.H.; Brush, R.S.; Anderson, R.E. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes. Investig. Opthalmol. Vis. Sci. 2005, 46, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Miljanich, G.P.; Sklar, L.A.; White, D.L.; Dratz, E.A. Disaturated and dipolyunsaturated phospholipids in the bovine retinal rod outer segment disk membrane. Biochem. Biophys. Acta 1979, 552, 294–306. [Google Scholar] [CrossRef]
- Bell, M.V.; Dick, J.R.; Buda, C. Molecular speciation of fish sperm phospholipids: Large amounts of dipolyunsaturated phosphatidylserine. Lipids 1997, 32, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R.; Xu, L.; Tateno, S.; Guo, M.; Colvin, R.B. Suppression of autoimmune-disease by dietary n-3 fatty-acids. J. Lipid Res. 1993, 34, 1435–1444. [Google Scholar] [PubMed]
- Huster, D.; Arnold, K.; Gawrisch, K. Influence of docosahexaenoic acid and cholesterol on lateral lipid organization in phospholipid mixtures. Biochemistry 1998, 37, 17299–17308. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C.; Litman, B.J. Molecular order and dynamics in bilayers consisting of highly polyunsaturated phospholipids. Biophys. J. 1998, 74, 879–891. [Google Scholar] [CrossRef]
- Rajamoorthi, K.; Petrache, H.I.; McIntosh, J.T.; Brown, M.F. Packing and viscoelasticity of polyunsaturated omega-3 and omega-6 lipid bilayers as seen by H-2 NMR and X-ray diffraction. J. Am. Chem. Soc. 2005, 127, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Salem, N., Jr.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.J.; Nicolson, G.L. Fluid mosaic model of structure of cell-membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Karmaker, S.; Raghunathan, V.A.; Mayor, S. Phase behavior of dipalmitoyl phosphatidylcholine (DPPC)-cholesterol membranes. J. Phys. Condens. Matter 2005, 17, S1177–S1182. [Google Scholar] [CrossRef]
- De Lange, M.J.L.; Bonn, M.; Muller, M. Direct measurement of phase coexistence in DPPC/cholesterol vesicles using Raman spectroscopy. Chem. Phys. Lipids 2007, 146, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Miles, K.; Cheng, K.H.; Huang, J. Lateral distribution of cholesterol in dioleoylphosphatidycholine lipid bilayers: Cholesterol-phospholipid interactions at high cholesterol limit. Biophys J. 2004, 86, 1532–1544. [Google Scholar] [CrossRef]
- Schmidt, M.L.; Ziani, L.; Bourdeau, M.; Davis, J.H. Phase equilibria in DOPC/DPPC: Conversion from gel to subgel in two component mixtures. J. Chem. Phys. 2009, 131. [Google Scholar] [CrossRef] [PubMed]
- Wassall, S.R.; Stillwell, W. Docosahexaenoic acid domains: The ultimate non-raft membrane domain. Chem. Phys. Lipids 2008, 153, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; LoCascio, D.S.; Liu, Y.; Williams, J.A.; Bittman, R.; Stillwell, W.; Wassall, S.R. Docosahexaenoic acid enchances segregation of lipids between raft and nonraft domains: H-2-NMR study. Biophys. J. 2008, 95, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Batten, S.E.; Harris, M.; Rockett, B.D.; Shaikh, S.R.; Stillwell, W.; Wassall, S.R. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys. J. 2012, 103, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Dumaual, A.C.; Castillo, A.; LoCascio, D.; Siddiqui, R.A.; Stillwell, W.; Wassall, S.R. Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: A comparative NMR, DSC, AFM, and detergent extraction study. Biophys. J. 2004, 87, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Wassall, S.R.; Shaikh, S.R.; Brzustowicz, M.R.; Cherezov, V.; Siddiqui, R.A.; Caffrey, M.; Stillwell, W. Interaction of polyunsaturated fatty acids with cholesterol: A role in lipid raft phase separation. Macromol. Symp. 2005, 219, 73–83. [Google Scholar] [CrossRef]
- Shaikh, S.R.; LoCascio, D.S.; Soni, S.P.; Wassall, S.R.; Stillwell, W. Oleic and docosahexaenoic acid containing phosphatidylethanolamines differentially phase separate from sphingomyelin. Biochim. Biophys. Acta 2009, 1788, 2421–2426. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Cherezov, V.; Caffrey, M.; Soni, S.P.; LoCascio, D.S.; Stillwell, W.; Wassall, S.R. Molecular organization of cholesterol in unsaturated phosphatidylethanolamines: X-ray diffraction and solid state H-2 NMR reveal difference with phosphotidylcholines. J. Am. Chem. Soc. 2006, 128, 5375–5383. [Google Scholar] [CrossRef] [PubMed]
- Thid, D.; Benkoski, J.J.; Syedhem, S.; Kasemo, B.; Gold, J. DHA-induced changes of supported lipid membrane morphology. Langmuir 2007, 23, 5878–5881. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Cho, N. Spectrum of membrane morphological responses to antibacterial fatty acids and related surfactants. Langmuir 2015, 31, 10223–10232. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commum. 2005, 338, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Fujimoto, K.; Miyazawa, T. Polyunsaturated (n-3) Fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of fats fed docosahexaenoic acid-containing oils. J. Nutr. 2000, 130, 3028–3033. [Google Scholar] [PubMed]
- Garcia, Y.J.; Rodriguez-Malaver, A.J.; Penaloza, N. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J. Neurosci. Methods. 2005, 144, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Rael, L.T.; Thomas, G.W.; Craun, M.L.; Curtis, C.G.; Bar-Or, R.; Bar-Or, D. Lipid peroxidation and the thiobarbituric acid assay: Standardization of the assay when using saturated and unsaturated fatty acids. J. Biochem. Mol. Biol. 2004, 37, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Kiss, A.; Pramudya, Y.H.; Nguyen, L.T.; Hirst, L.S. Solution synchrotron X-ray diffraction reveals structural details of lipid domains in ternary mixtures. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2009, 79. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, L.; Ma, Y.; Vashaee, D.; Chen, G.; Sinha, S.K.; Parikh, A.N. Long-range interlayer alignment of intralayer domains in stacked lipid bilayers. Nat. Mater. 2012, 12, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. Lateral chain packing in lipids and membranes. J. Lipid Res. 1984, 25, 1490–1500. [Google Scholar] [PubMed]
- Garcia-Manyes, S.; Oncins, G.; Sans, F. Effect of temperature on the nanomechanics of lipid bilayers studied by force spectroscopy. Biophys. J. 2005, 89, 4261–4274. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Marsh, D. Phospholipid Bilayers. Physical Principles and Models; Wiley-Interscience: New York, NY, USA, 1987. [Google Scholar]
- Quinn, P.J.; Wolf, C. Hydrocarbon chains dominate coupling and phase coexistence in bilayers of natural phosphatidylcholines and sphingomyelin. Biochim. Biophys. Acta 2009, 1788, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Torbet, J.; Wiklins, M.H.F. X-ray diffraction studies of lecithin bilayers. J. Theor. Biol. 1976, 62, 447–458. [Google Scholar] [CrossRef]
- Nagle, J.F.; Tristram-Nagle, S. Lipid bilayer structure. Biochim. Biophys. Acta 2000, 1469, 159–195. [Google Scholar] [CrossRef]
- Mills, T.T.; Huang, J.; Feigenson, G.W.; Nagle, J.F. Effects of cholesterol and unsaturated DOPC lipid on chain packing of saturated gel-phase DPPC bilayers. Gen. Physiol. Biophys. 2009, 28, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Sullan, R.M.A.; Li, J.K.; Hao, C.; Walker, G.c.; Zou, S. Cholesterol-dependent nanomechanical stability of phase-segregated multicomponent lipid bilayes. Biophys. J. 2010, 99, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Leekumjorn, S.; Sum, A.K. Molecular studies of the gel to liquid-crystalline phase transition for fully hydrated DPPC and DPPE bilayers. Biochim. Biophys. Acta 2007, 1768, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Katsaras, J.; Yang, D.S.C.; Epand, R.M. Fatty-acid chain tilt angels and directions in dipalmitoyl phosphatidylcholine bilayers. Biophys. J. 1992, 63, 1170–1175. [Google Scholar] [CrossRef]
- Smith, E.A.; Dea, P.K. Differential Scanning Calorimetry Studies of Phospholipid Membranes: The Interdigitated Gel Phase, Applications of Calorimetry in a Wide Context-Differential Scanning Calorimetry, Isothermal Titration Calorimetry and Microcalorimetry; Elkordy, A.A., Ed.; InTech: Rijeka, Croatia, 2013; pp. 407–444. [Google Scholar]
- Sun, W.J.; Suter, R.M.; Knewtson, M.A.; Worthington, C.R.; Trsitram-Nagle, S.; Zhang, R.; Nagle, J.F. Order and disorder in fully hydrated unoriented bilayers of gel phase dipalmitolyphosphatidylcholine. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. 1994, 49, 4665–4676. [Google Scholar]
- Atkinson, J.; Harroun, T.; Wassall, S.R.; Stillwell, W.; Katsaras, J. The location and behavior of α-tocopherol in membranes. Mol. Nutr. Food Res. 2010, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Raghunathan, V.A. Modulated phases of phospholipid bilayers induced by tocopherols. Biochim. Biophys. Acta 2012, 1818, 2486–2493. [Google Scholar] [CrossRef] [PubMed]
- Wallgren, M.; Beranova, L.; Pham, Q.D.; Linh, K.; Lidman, M.; Procek, J.; Cyprych, K.; Kinnunen, P.K.J.; Hof, M.; Grobner, G. Impact of oxidized phospholipids on the structural and dynamic organization of phospholipid membranes: A combined DSC and solid state NMR study. Faraday Discuss. 2013, 161, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Khandelia, H.; Mouritsen, O.G. Lipid Gymnastics: Evidence of complete acyl chain reversal in oxidized phospholipids from molecular simulations. Biophys. J. 2009, 96, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Wong-Wkkabut, J.; Wu, Z.; Triampo, W.; Tang, I.M.; Tieleman, D.P. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophys. J. 2007, 90, 4488–4499. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lor, C.; Hirst, L.S. Effects of Low Concentrations of Docosahexaenoic Acid on the Structure and Phase Behavior of Model Lipid Membranes. Membranes 2015, 5, 857-874. https://doi.org/10.3390/membranes5040857
Lor C, Hirst LS. Effects of Low Concentrations of Docosahexaenoic Acid on the Structure and Phase Behavior of Model Lipid Membranes. Membranes. 2015; 5(4):857-874. https://doi.org/10.3390/membranes5040857
Chicago/Turabian StyleLor, Chai, and Linda S. Hirst. 2015. "Effects of Low Concentrations of Docosahexaenoic Acid on the Structure and Phase Behavior of Model Lipid Membranes" Membranes 5, no. 4: 857-874. https://doi.org/10.3390/membranes5040857
APA StyleLor, C., & Hirst, L. S. (2015). Effects of Low Concentrations of Docosahexaenoic Acid on the Structure and Phase Behavior of Model Lipid Membranes. Membranes, 5(4), 857-874. https://doi.org/10.3390/membranes5040857





