Corrosion Protection of Al/Au/ZnO Anode for Hybrid Cell Application
Abstract
:1. Introductions
2. Material and Methods
3. Results and Discussion
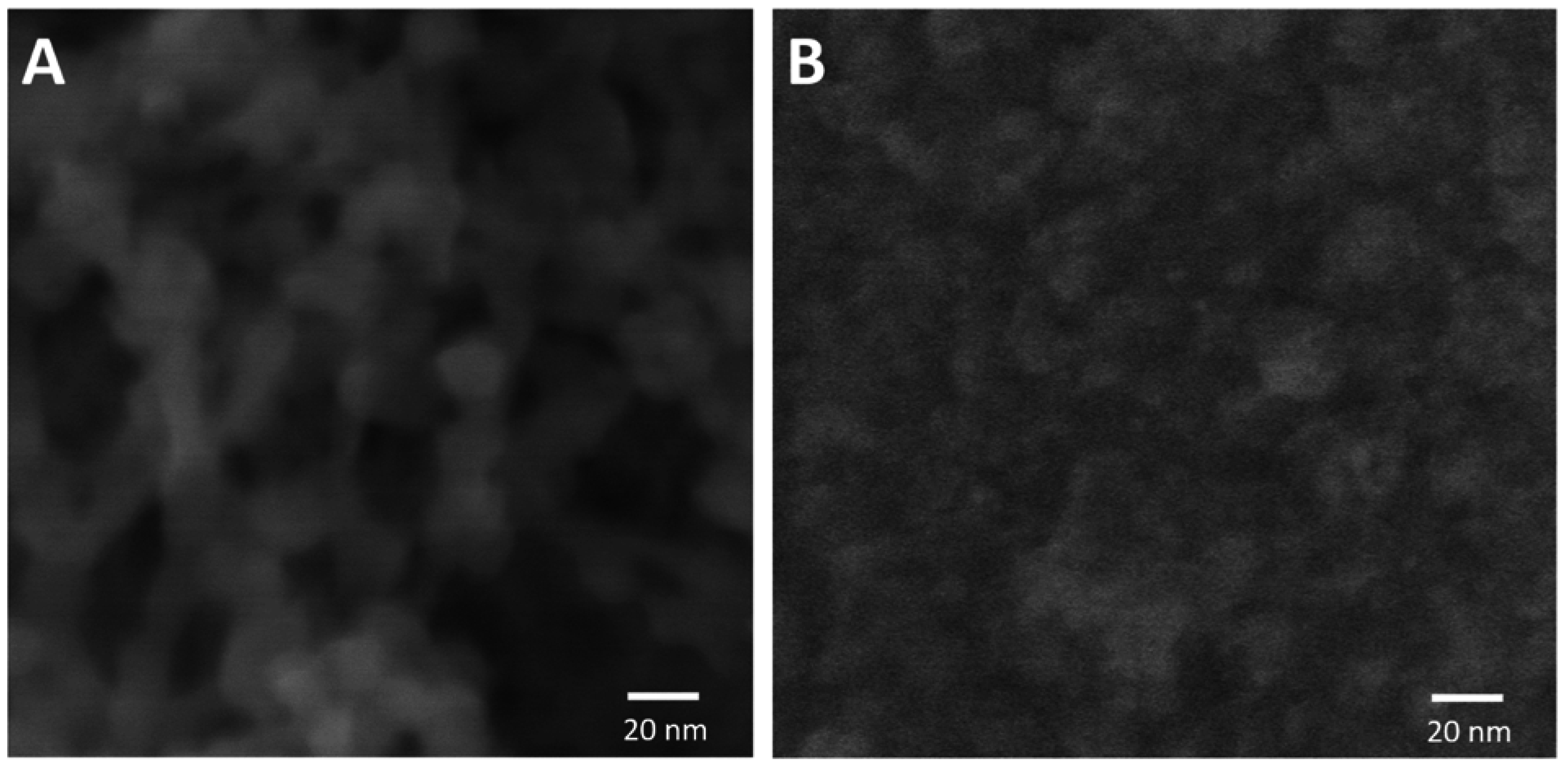
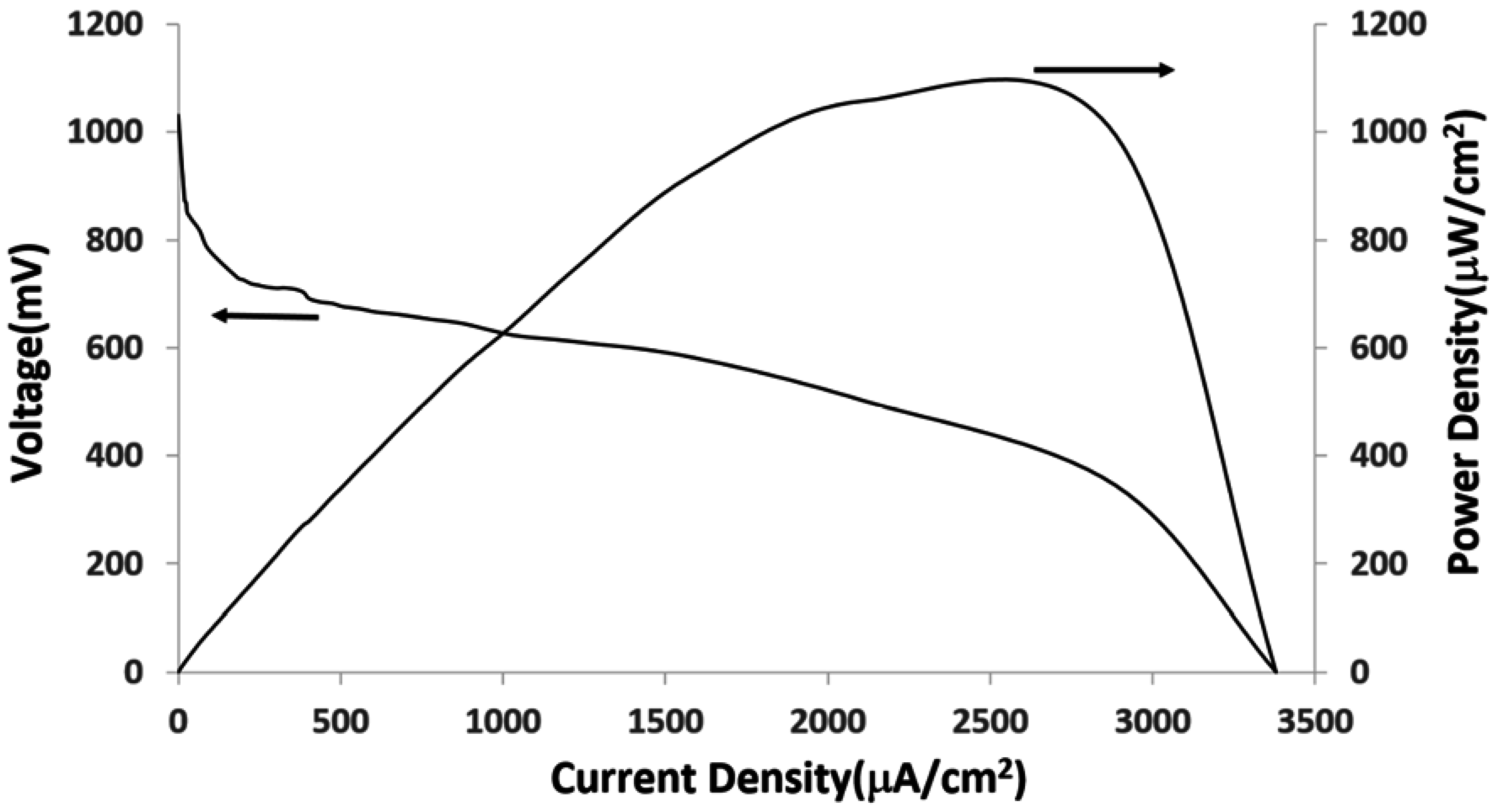
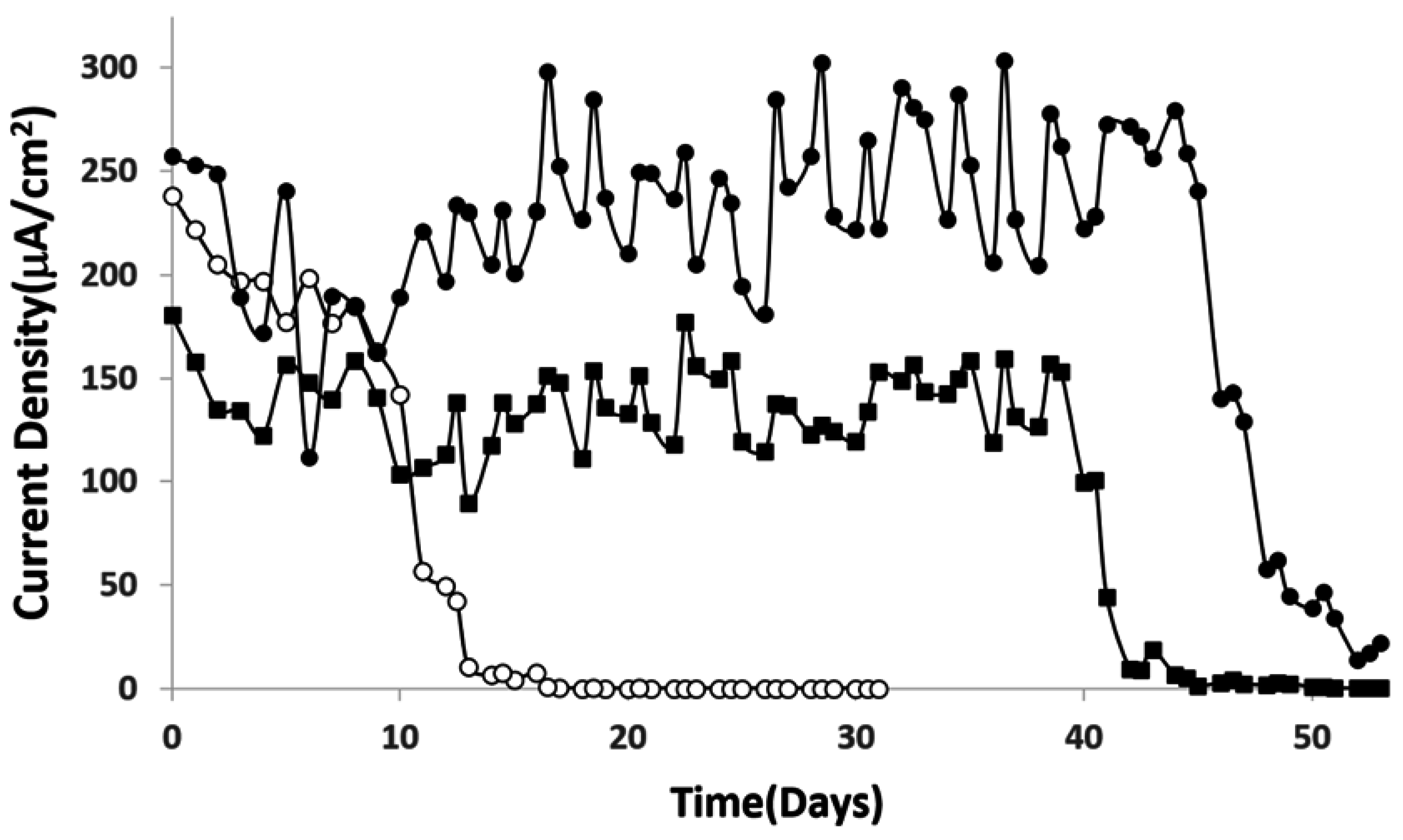

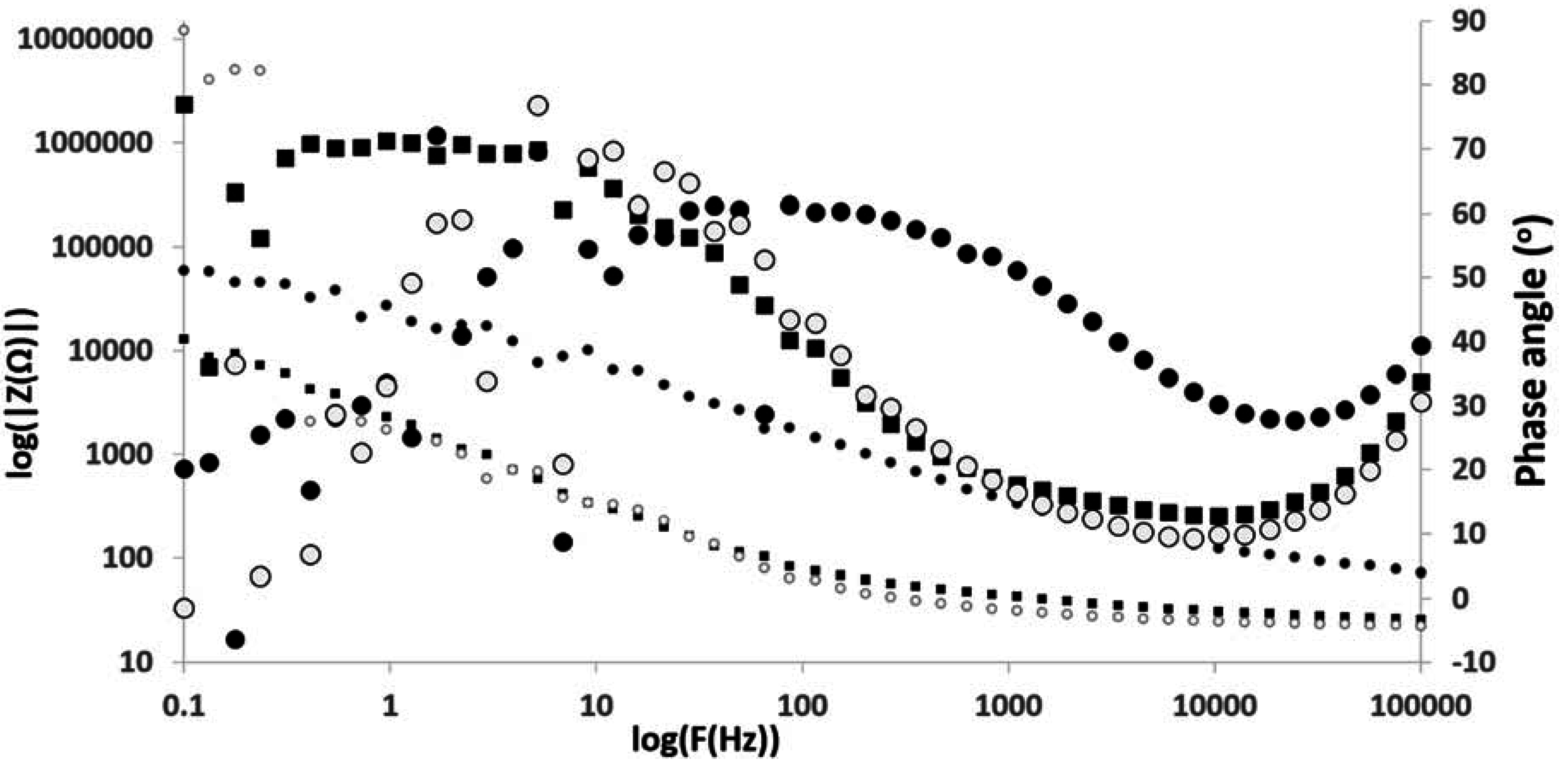

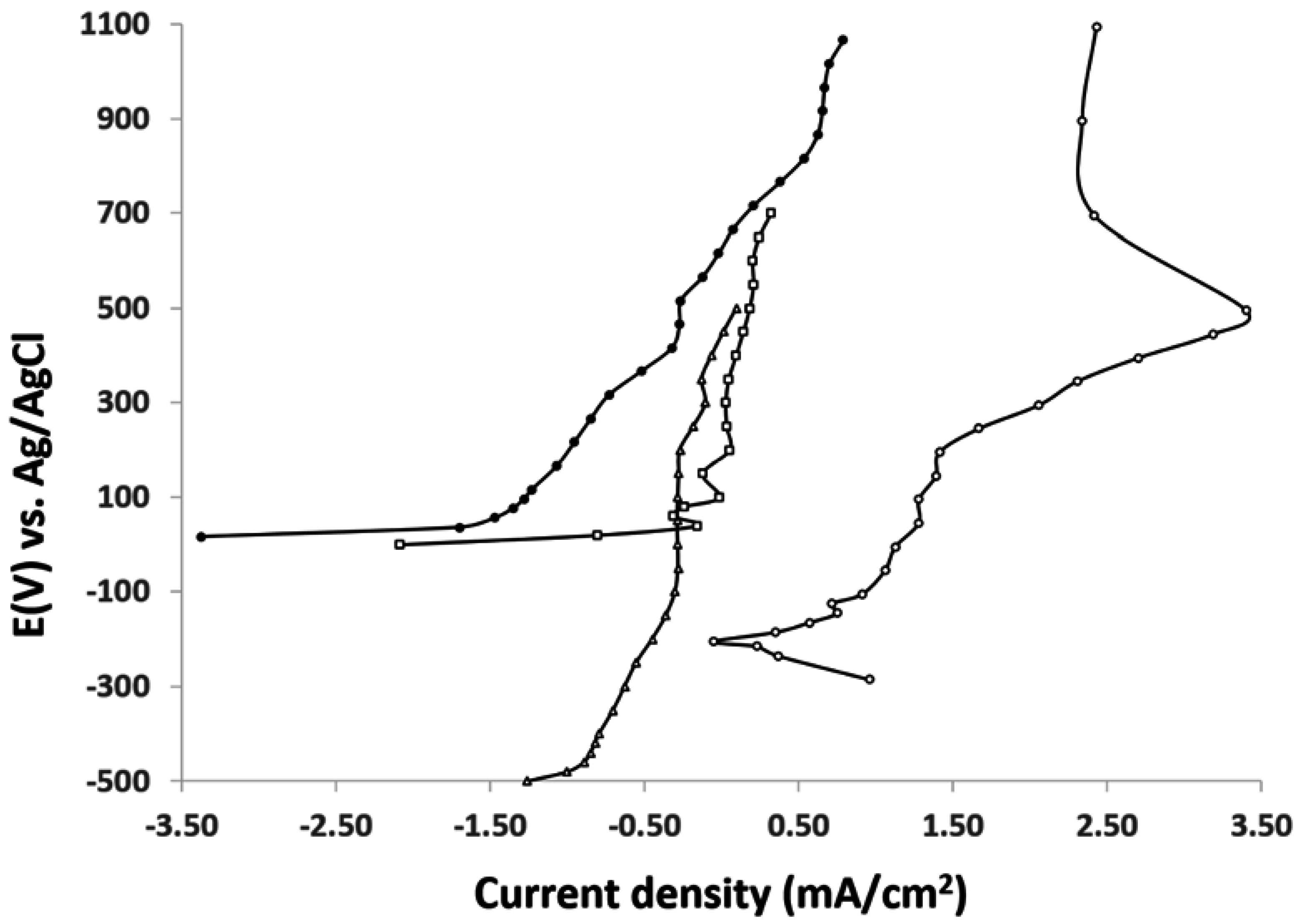
4. Conclusion
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Jiang, G.; Zhou, D. Technology advances and challenges in hermetic packaging for implantable medical devices. In Implantable Neural Prostheses 2; Springer: New York, NY, USA, 2010; pp. 27–61. [Google Scholar]
- Yun, Y.; Dong, Z.; Lee, N.; Liu, Y.; Xue, D.; Guo, X.; Kuhlmann, J.; Doepke, A.; Halsall, B.; Heineman, W.; et al. Revolutionizing biodegradable metals. Mater. Today 2009, 12, 22–32. [Google Scholar] [CrossRef]
- Samani, M.; Mahnam, A.; Hosseini, N. An Arbitrary Waveform Wearable Neuro-stimulator System for Neurophysiology Research on Freely Behaving Animals. J. Med. Signals Sens. 2014, 4, 94–102. [Google Scholar] [PubMed]
- Bennet, D.; Kim, S. Implantable microdevice for peripheral nerve regeneration: materials and fabrications. J. Mater. Sci. 2011, 46, 4723–4740. [Google Scholar] [CrossRef]
- Okamoto, E.; Inoue, T.; Watanabe, K.; Hashimoto, T.; Iwazawa, E.; Abe, Y.; Chinzei, T.; Isoyama, T.; Kobayashi, S.; Saito, I.; et al. Development of an Implantable High-Energy and Compact Battery System for Artificial Heart. Artif. Organs 2003, 27, 184–188. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, J.F.; Beard, R.; Carim, H.; Dubin, S. Polarization and Corrosion Studies of Porous and Solid Anodes for Implantable Power-Generating Electrodes. IEEE Trans. Biomed. Eng. 1973, 5, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Herweg, B. New Technology for Implantable Cardioverter Defibrillators. Card. Electrophysiol. Clin. 2014, 6, 261–267. [Google Scholar] [CrossRef]
- Chanlen, T.; Hongeng, S.; Nasongkla, N. Tri-component copolymer rods as an implantable reservoir drug delivery system for constant and controllable drug release rate. J. Polym. Res. 2012, 19, 1–12. [Google Scholar] [CrossRef]
- Gensler, H.; Sheybani, R.; Li, P.; Mann, R.; Meng, E. An implantable MEMS micropump system for drug delivery in small animals. Biomed. Microdevices 2012, 14, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Juanola-Feliu, E.; Miribel-Català, P.; Páez Avilés, C.; Colomer-Farrarons, J.; González-Piñero, M.; Samitier, J. Design of a customized multipurpose nano-enabled implantable system for in vivo theranostics. Sensors 2014, 14, 19275–19306. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.T.; Yuan, F.; Reichert, W.M. Macrophage embedded fibrin gels: An in vitro platform for assessing inflammation effects on implantable glucose sensors. Biomaterials 2014, 35, 9563–9572. [Google Scholar] [CrossRef] [PubMed]
- Vaddiraju, S.; Legassey, A.; Qiang, L.; Wang, Y.; Burgess, D.; Papadimitrakopoulos, F. Enhancing the Sensitivity of Needle-Implantable Electrochemical Glucose Sensors via Surface Rebuilding. J. Diabetes Sci. Technol. 2013, 7, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Morais, R.; Frias, C.; Silva, N.; Azevedo, J.; Serôdio, C.; Silva, P.; Ferreira, J.; Simões, J.; Reis, M. An activation circuit for battery-powered biomedical implantable systems. Sens. Actuators A Phys. 2009, 156, 229–236. [Google Scholar] [CrossRef]
- Slaughter, G.; Sunday, J. A membraneless single compartment abiotic glucose fuel cell. J. Power Sources 2014, 261, 332–336. [Google Scholar] [CrossRef]
- Slaughter, G.; Sunday, J.; Stevens, B. Energy conversion from aluminium and phosphate rich solution via ZnO activation of aluminium. Mater. Chem. Phys. 2015, 163, 245–252. [Google Scholar] [CrossRef]
- Williams, D. The Williams Dictionary of Biomaterials; Liverpool University Press: Liverpool, UK, 1999. [Google Scholar]
- Williams, D. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, G. Improving the Biocompatibility of Implantable Bioelectronics Devices. In Implantable Bioelectronics; Katz, E., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 265–283. [Google Scholar]
- Anderson, J. Biological responses to materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Nail-plates, F. Corrosion of orthopaedic implants. J. Bone Jt. Surg. 1959, 41B, 810–820. [Google Scholar]
- Williams, D. Tissue-biomaterial interactions. J. Mater. Sci. 1987, 22, 3421–3445. [Google Scholar] [CrossRef]
- Aksakal, B.; Yildirim, Ö.S.; Gul, H. Metallurgical failure analysis of various implant materials used in orthopedic applications. J. Fail. Anal. Prev. 2004, 4, 17–23. [Google Scholar] [CrossRef]
- Reclaru, L.; Lerf, R.; Eschler, P.-Y.; Meyer, J.-M. Corrosion behavior of a welded stainless-steel orthopedic implant. Biomaterials 2001, 22, 269–279. [Google Scholar] [CrossRef]
- Drago, D.; Bettella, M.; Bolognin, S.; Cendron, L.; Scancar, J.; Milacic, R.; Ricchelli, F. Potential pathogenic role of β-amyloid 1–42–aluminum complex in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2008, 40, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Zhang, R.; Tian, H.; Li, X. Studies on the interactions of Copper and Zinc ions with β-Amyloid peptides by a surface plasmon resonance biosensor. Int. J. Mol. Sci. 2012, 13, 11832–11843. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (USEPA). Nitrates and Nitrites: TEACH Chemical Summary; USEPA: Washington, DC, USA, 2007.
- Kouisni, L.; Azzi, M.; Zertoubi, M.; Dalard, F.; Maximovitch, S. Phosphate coatings on magnesium alloy AM60 part 1: Study of the formation and the growth of zinc phosphate films. Surf. Coat. Technol. 2004, 185, 58–67. [Google Scholar] [CrossRef]
- Shin, W.; Lee, J.; Kim, Y.; Steinfink, H.; Heller, A. Ionic Conduction in Zn3(PO4)2·4H2O Enables Efficient Discharge of the Zinc Anode in Serum. J. Am. Chem. Soc. 2005, 127, 14590–14591. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Lee, J.; Lee, J.; Park, Y.; Heller, A. Zinc Anode Operating in Physiological Condition. In Proceedings of the 215th The Electrochemical Society (ECS) Meeting, San Francisco, CA, USA, 24–29 May 2009; Abstract #242. pp. 242–242.
- Heller, A. Potentially implantable miniature batteries. Anal. Bioanal. Chem. 2006, 385, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, K.; Kizling, M.; Majdecka, D.; Żelechowska, K.; Biernat, J.; Rogalski, J.; Bilewicz, R. Biobatteries and biofuel cells with biphenylated carbon nanotubes. J. Power Sources 2014, 249, 263–269. [Google Scholar] [CrossRef]
- Skunik-Nuckowska, M.; Grzejszczyk, K.; Stolarczyk, K.; Bilewicz, R.; Kulesza, P. Integration of supercapacitors with enzymatic biobatteries toward more effective pulse-powered use in small-scale energy harvesting devices. J. Appl. Electrochem. 2014, 44, 497–507. [Google Scholar] [CrossRef]
- Nguyen, T.; Waterman, J.; Staiger, M.; Woodfield, T. Controlling in vitro corrosion rate of pure Mg with rough surface texture via biomimetic coating systems. Corros. Eng. Sci. Technol. 2012, 47, 358–364. [Google Scholar] [CrossRef]
- Waterman, J.; Birbilis, N.; Dias, G.J.; Woodfield, T.B.F.; Staiger, M.P. Improving in vitro corrosion resistance of biomimetic calcium phosphate coatings for Mg substrates using calcium hydroxide layer. Corros. Eng. Sci. Technol. 2012, 47, 340–345. [Google Scholar] [CrossRef]
- Xia, W.; Lindahl, C.; Engqvist, H.; Lausmaa, J. Biomimetic hydroxyapatite deposition on titanium oxide surfaces for biomedical application. In Advances in Biomimetics; INTECH Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar]
- Raoufi, D.; Raoufi, T. The effect of heat treatment on the physical properties of sol–gel derived ZnO thin films. Appl. Surf. Sci. 2009, 255, 5812–5817. [Google Scholar] [CrossRef]
- Barton, S.C. Handbook of Fuel Cells: Advances in Electrocatalysis, Materials, Diagnostics and Durability; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Minteer, S.; Liaw, B.; Cooney, M. Enzyme-based biofuel cells. Curr. Opin. Biotechnol. 2007, 18, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Zebda, A.; Cosnier, S.; Alcaraz, J.; Holzinger, M.; Le Goff, A.; Gondran, C.; Bouche, F. Single glucose biofuel cells implanted in rats power electronic devices. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Hussein, L.; Urban, G. An Abiotically Catalyzed Glucose Fuel Cell Based on Decorated Buckypaper. In Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Groningen, The Netherlands, 3–7 October 2010.
- Oncescu, V.; Erickson, D. A microfabricated low cost enzyme-free glucose fuel cell for powering low-power implantable devices. J. Power Sources 2011, 196, 9169–9175. [Google Scholar] [CrossRef]
- Halámková, L.; Halámek, J.; Bocharova, V.; Szczupak, A.; Alfonta, L.; Katz, E. Implanted biofuel cell operating in a living snail. J. Am. Chem. Soc. 2012, 134, 5040–5043. [Google Scholar] [CrossRef] [PubMed]
- Szczupak, A.; Halámek, J.; Halámková, L.; Bocharova, V.; Alfonta, L.; Katz, E. Living battery—Biofuel cells operating in vivo in clams. Energy Environ. Sci. 2012, 5, 8891–8895. [Google Scholar] [CrossRef]
- MacVittie, K.; Halámek, J.; Halámková, L.; Southcott, M.; Jemison, W.; Lobel, R.; Katz, E. From “cyborg” lobsters to a pacemaker powered by implantable biofuel cells. Energy Environ. Sci. 2013, 6, 81–86. [Google Scholar] [CrossRef]
- Cinquin, P.; Gondran, C.; Giroud, F.; Mazabrard, S.; Pellissier, A.; Boucher, F.; Alcaraz, J.-P. A glucose biofuel cell implanted in rats. PLoS One 2010, 5, e10476. [Google Scholar] [CrossRef] [PubMed]
- Dahn, J.; Jiang, J.; Mushurchak, L.; Buhrmester, C.; Wang, R. The drugstore Li-ion cell. Electrochem. Soc. Interface 2005, 14, 27–31. [Google Scholar]
- Dahn, J.; Jiang, J.; Moshurchak, L.; Fleischauer, M.; Buhrmester, C.; Krause, L. High-rate overcharge protection of LiFePO4-based Li-ion cells using the redox shuttle additive 2, 5-ditertbutyl-1, 4-dimethoxybenzene. J. Electrochem. Soc. 2005, 152, A1283–A1289. [Google Scholar] [CrossRef]
- Chen, X-B.; Zhou, X.; Abbott, T.; Easton, M.; Birbilis, N. Double-layered manganese phosphate conversion coating on magnesium alloy AZ91D: Insights into coating formation, growth and corrosion resistance. Surf. Coat. Technol. 2013, 217, 147–155. [Google Scholar]
- Jegannathan, S.; Narayanan, T.S.N.; Ravichandran, N.; Rajeswari, S. Performance of zinc phosphate coatings obtained by cathodic electrochemical treatment in accelerated corrosion tests. Electrochim. Acta 2005, 51, 247–256. [Google Scholar] [CrossRef]
- Jegannathan, S.; Narayanan, T.S.N.; Ravichandran, N.; Rajeswari, S. Formation of zinc phosphate coating by anodic electrochemical treatment. Surf. Coat. Technol. 2006, 200, 6014–6021. [Google Scholar] [CrossRef]
- Tsuji, N.; Nozawa, K.; Aramaki, K. Ultrathin protective films prepared by modification of an N,N-dimethylalkylamine monolayer with chlorosilanes for preventing corrosion of iron. Corros. Sci. 2000, 42, 1523–1538. [Google Scholar] [CrossRef]
- Li, G.Y.; Lian, J.S.; Niu, L.Y.; Jiang, Z.H.; Jiang, Q. Growth of zinc phosphate coatings on AZ91D magnesium alloy. Surf. Coat. Technol. 2006, 201, 1814–1820. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slaughter, G.; Stevens, B. Corrosion Protection of Al/Au/ZnO Anode for Hybrid Cell Application. Membranes 2015, 5, 739-751. https://doi.org/10.3390/membranes5040739
Slaughter G, Stevens B. Corrosion Protection of Al/Au/ZnO Anode for Hybrid Cell Application. Membranes. 2015; 5(4):739-751. https://doi.org/10.3390/membranes5040739
Chicago/Turabian StyleSlaughter, Gymama, and Brian Stevens. 2015. "Corrosion Protection of Al/Au/ZnO Anode for Hybrid Cell Application" Membranes 5, no. 4: 739-751. https://doi.org/10.3390/membranes5040739
APA StyleSlaughter, G., & Stevens, B. (2015). Corrosion Protection of Al/Au/ZnO Anode for Hybrid Cell Application. Membranes, 5(4), 739-751. https://doi.org/10.3390/membranes5040739






