Effect of Cholesterol on the Structure of a Five-Component Mitochondria-Like Phospholipid Membrane
Abstract
:1. Introduction
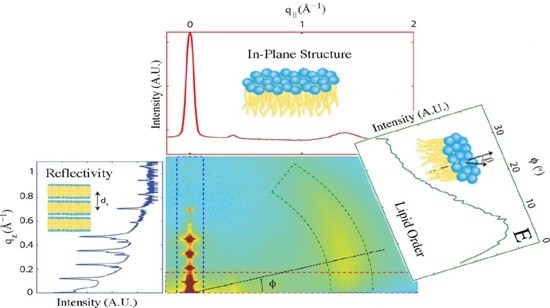
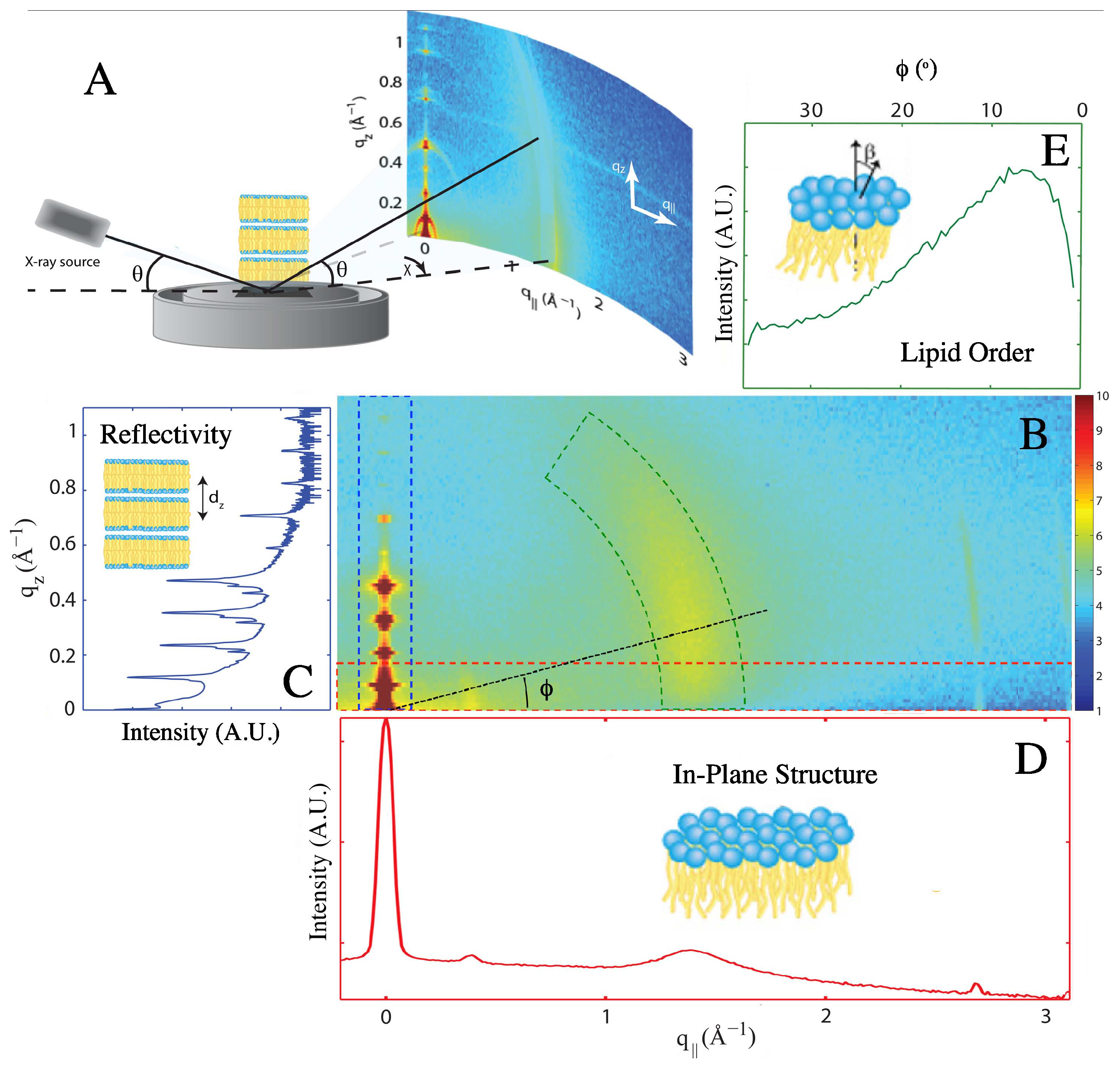
2. Results
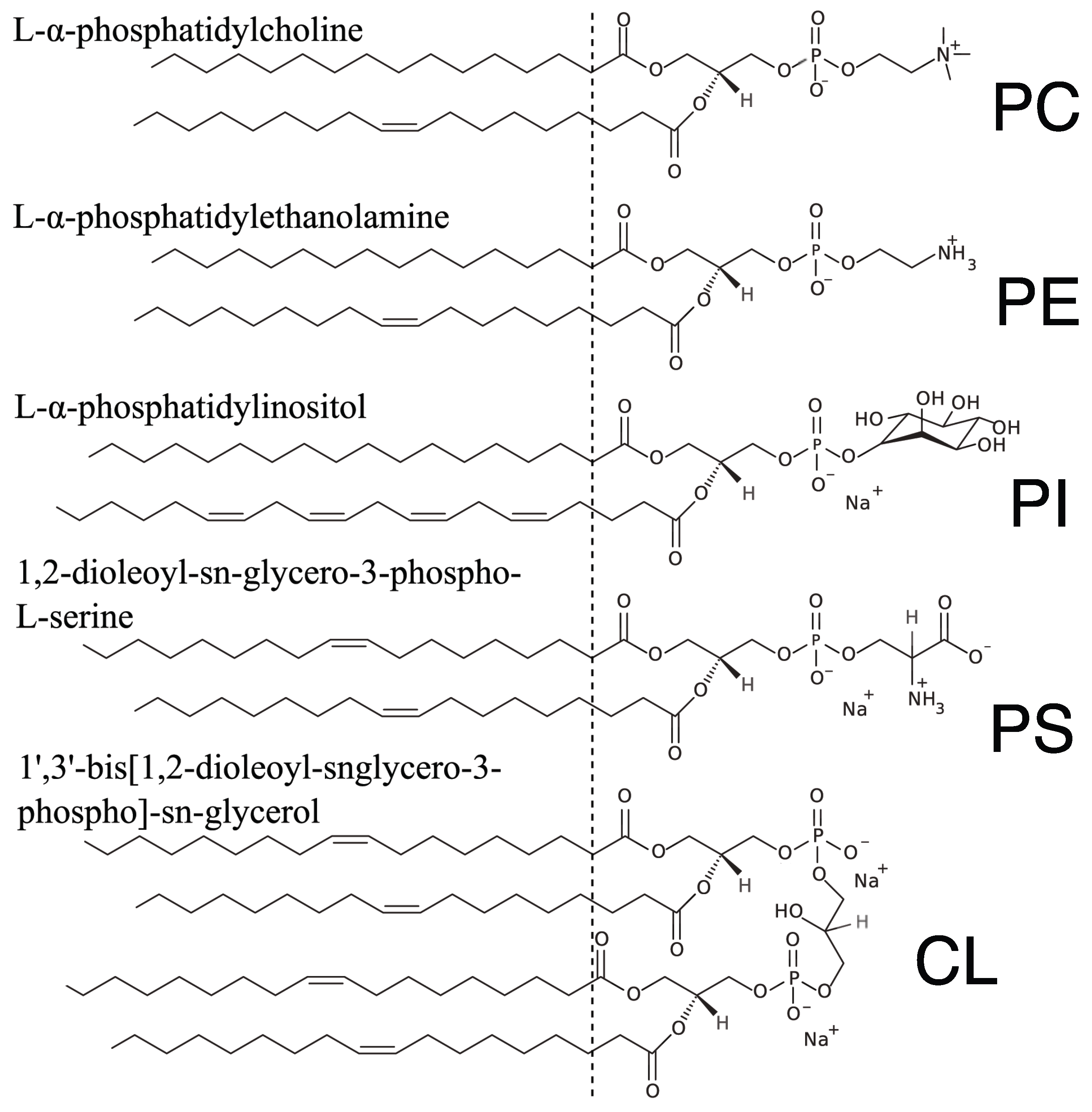
2.1. Structure of Multi-Component Mitochondria-Like Membranes
| Sample | (Å) | (Å) | (Å) | (Å) | |||
|---|---|---|---|---|---|---|---|
| POPC | 23.40 ± 0.04 | 23.4 | 0.36 | 53.2 ± 0.8 | 39.1 ± 0.5 | 9.7 | 7.7 |
| Mitochondria-like | 23.31 ± 0.06 | 23.3 | 0.26 | 50.3 ± 0.2 | 39.9 ± 0.5 | 8.5 | 6.7 |
| Mitochondria-like (10% cholesterol ) | 24.1 ± 0.2 | 23.2 | 0.39 | 50.7 ± 0.2 | 40.3 ± 0.5 | 9.0 | 7.6 |
| Mitochondria-like (20% cholesterol) | 24.6 ± 0.1 | 22.8 | 0.54 | 51.4 ± 0.3 | 42.6 ± 0.5 | 9.9 | 10.2 |
| Mitochondria-like (30% cholesterol) | 24.8 ± 0.2 | 21.8 | 0.48 | 53.9 ± 0.7 | 43.1± 0.5 | 10.8 | 10.6 |
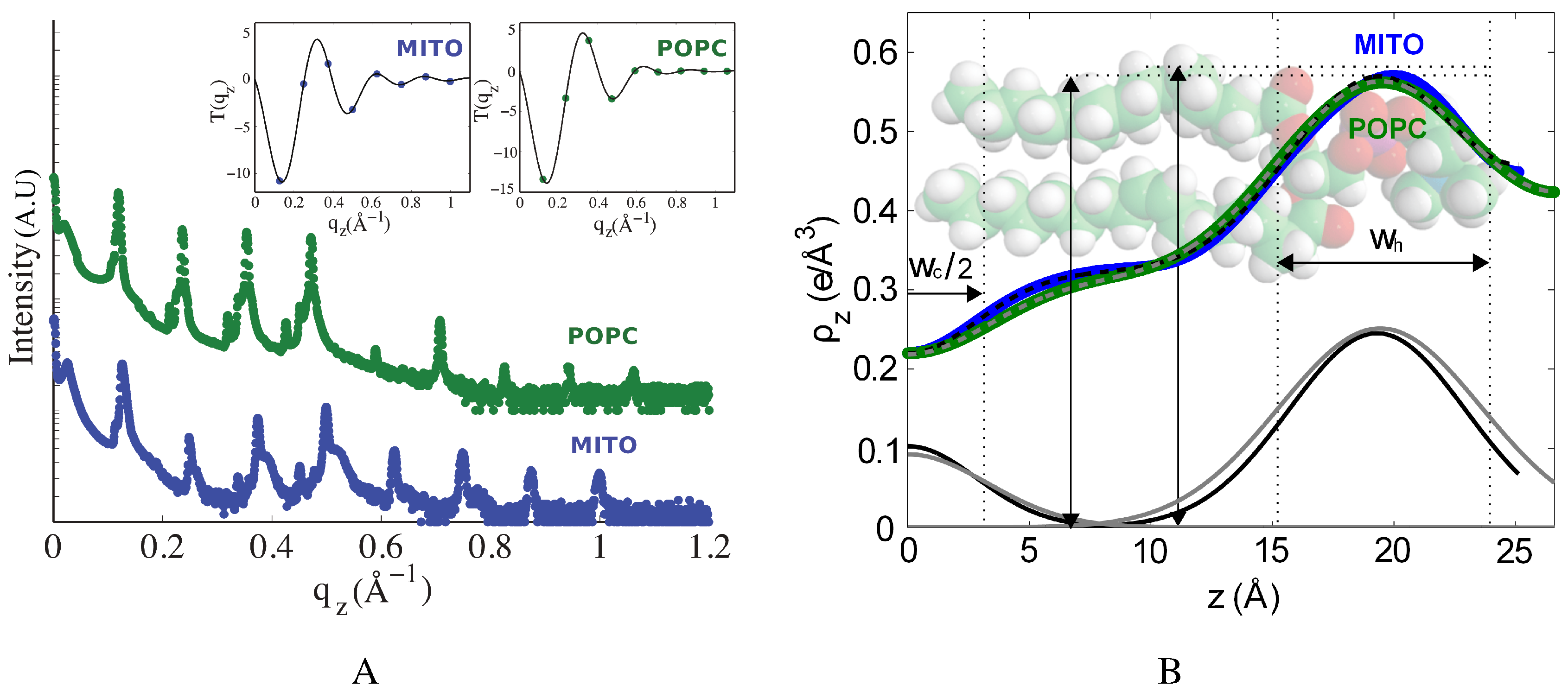
2.2. The Effect of Cholesterol on Mitochondria-Like Membranes
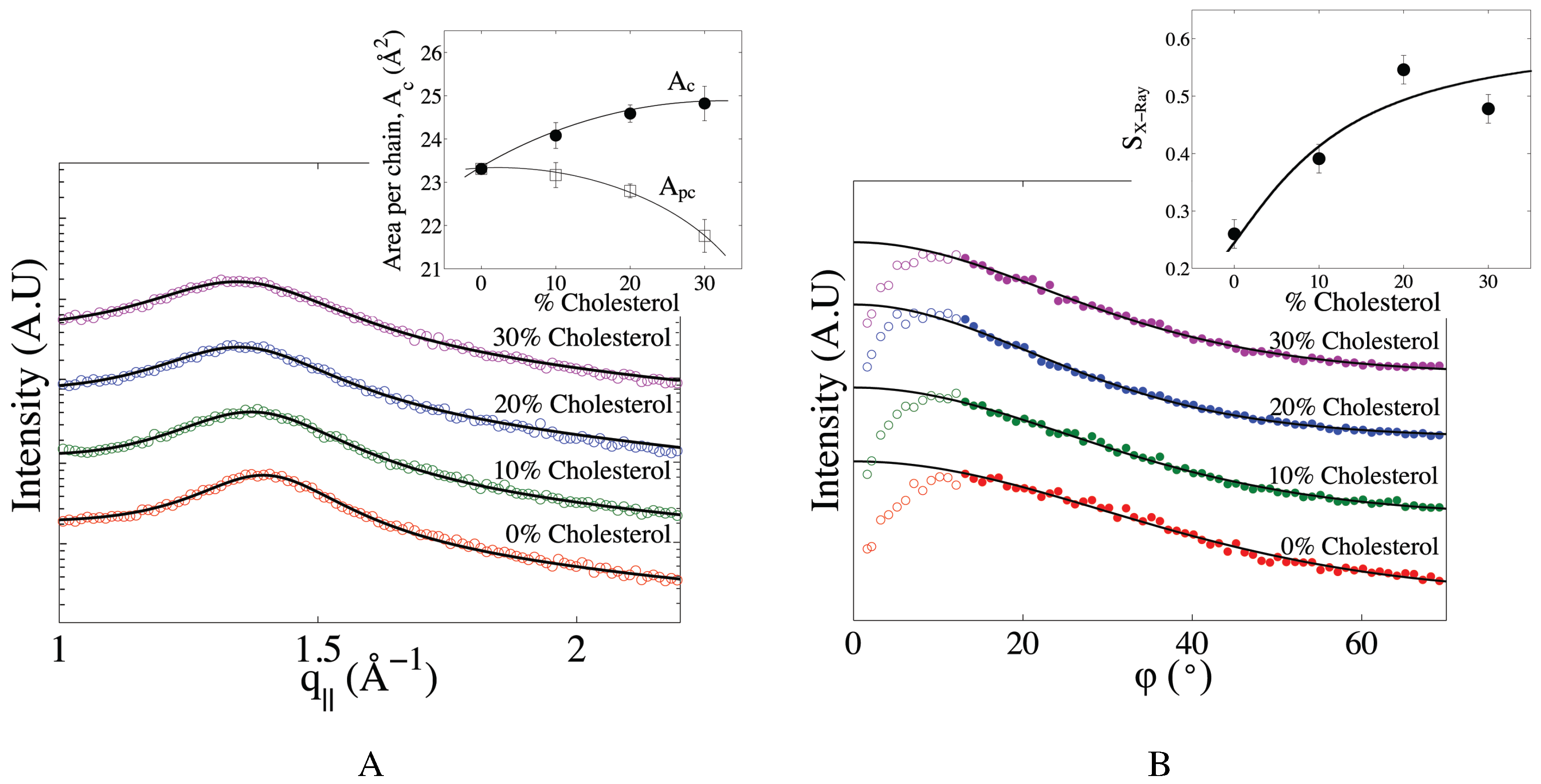
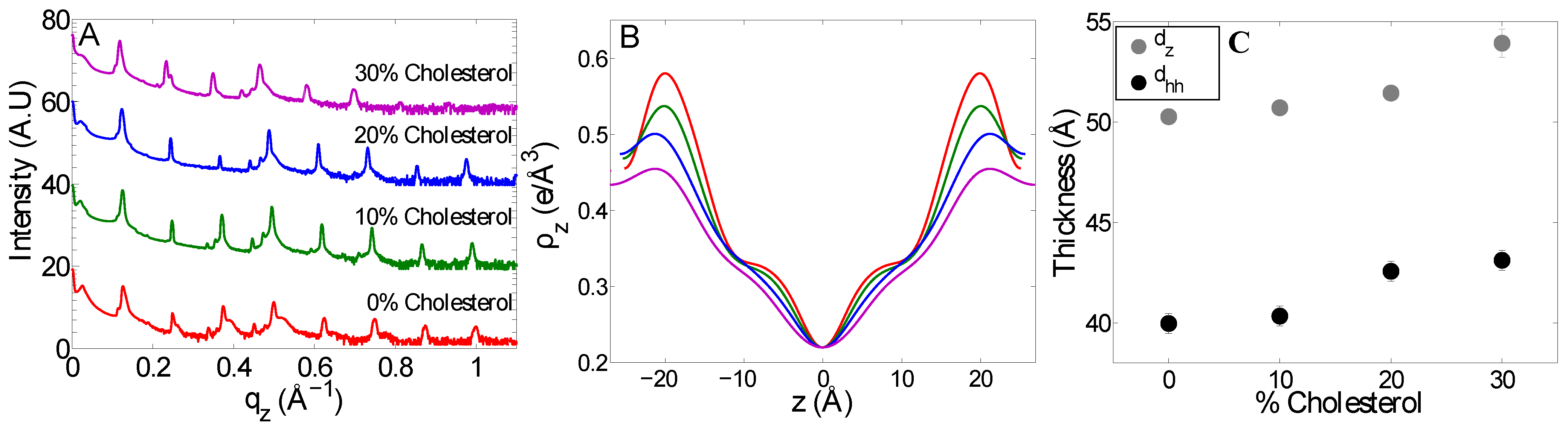
3. Discussion
3.1. How Different Is the Multi-Component Mitochondria-Like Membrane from a POPC Membrane?
3.2. Effect of Cholesterol on the Multi-Component Mitochondria-Like Membrane
3.3. Implications for Mitochondria with Higher than Normal Cholesterol Content
4. Experimental Section
4.1. Lipids and Reagents
| Sample | POPC (mol%) | Egg PC (mol%) | Egg PE (mol%) | Liver PI (mol%) | DOPS (mol%) | TOCL (mol%) | Cholesterol (mol%) | ||
|---|---|---|---|---|---|---|---|---|---|
| POPC | 100 | – | – | – | – | – | – | 7 | 245 |
| Mitochondria-like | – | 48 | 28 | 10 | 10 | 4 | 0 | 4.5 | 235.4 |
| Mitochondria-like | – | 38 | 28 | 10 | 10 | 4 | 10 | 4.5 | 235.9 |
| 10% cholesterol | |||||||||
| Mitochondria-like | – | 28 | 28 | 10 | 10 | 4 | 20 | 4.5 | 236.4 |
| 20% cholesterol | |||||||||
| Mitochondria-like | – | 18 | 28 | 10 | 10 | 4 | 30 | 4.5 | 236.9 |
| 30% cholesterol |
4.2. Preparation of Highly-Oriented Membrane Stacks
4.3. X-Ray Scattering Experiments
4.4. Chain Packing and Chain Order Parameter
4.5. Electron Density Profile
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Cullis, P.R.; de Kruijff, B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochem. Biophys. Acta Rev. Biomembr. 1979, 559, 399–420. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, A.; Fujiwara, T.K.; Chadda, R.; Xie, M.; Tsunoyama, T.A.; Kalay, Z.; Kasai, R.S.; Suzuki, K.G. Dynamic organizing principles of the plasma membrane that regulate signal transduction: Commemorating the fortieth anniversary of Singer and Nicolson’s fluid-mosaic model. Annu. Rev. Cell Dev. Biol. 2012, 28, 215–250. [Google Scholar] [CrossRef] [PubMed]
- Boesze-Battaglia, K.; Schimmel, R. Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. J. Exp. Biol. 1997, 200, 2927–2936. [Google Scholar] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G.; Zuckermann, M.J. What’s so special about cholesterol? Lipids 2004, 39, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, T.J. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochem. Biophys. Acta Rev. Biomembr. 1978, 513, 43–58. [Google Scholar] [CrossRef]
- Hui, S.; He, N.B. Molecular organization in cholesterol-lecithin bilayers by X-ray and electron diffraction measurements. Biochemistry 1983, 22, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, J.H.; Mouritsen, O.G.; Bloom, M. Relationships between lipid membrane area, hydrophobic thickness, and acyl-chain orientational order. The effects of cholesterol. Biophys. J. 1990, 57, 405–412. [Google Scholar] [CrossRef]
- Nezil, F.A.; Bloom, M. Combined influence of cholesterol and synthetic amphiphillic peptides upon bilayer thickness in model membranes. Biophys. J. 1992, 61, 1176–1183. [Google Scholar] [CrossRef]
- Smondyrev, A.M.; Berkowitz, M.L. Structure of dipalmitoylphosphatidylcholine/cholesterol bilayer at low and high cholesterol concentrations: molecular dynamics simulation. Biophys. J. 1999, 77, 2075–2089. [Google Scholar] [CrossRef]
- Kučerka, N.; Pencer, J.; Nieh, M.P.; Katsaras, J. Influence of cholesterol on the bilayer properties of monounsaturated phosphatidylcholine unilamellar vesicles. Eur. Phys. J. E 2007, 23, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.; Chapman, D. Dynamics of lipids in membranes: heterogeneity and the role of cholesterol. FEBS Lett. 1972, 23, 285–297. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Brown, D.A.; London, E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: Physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry 1997, 36, 10944–10953. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; London, E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry 2000, 39, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.L.; Marquardt, D.; Dies, H.; Kučerka, N.; Yamani, Z.; Harroun, T.A.; Katsaras, J.; Shi, A.C.; Rheinstädter, M.C. The Observation of Highly Ordered Domains in Membranes with Cholesterol. PLoS ONE 2013, 8, e66162. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, S.; Vink, R.L.C.; Schmid, F. Monolayer curvature stabilizes nanoscale raft domains in mixed lipid bilayers. Proc. Natl. Acad. Sci. USA 2013, 110, 4476–4481. [Google Scholar] [CrossRef] [PubMed]
- Rheinstädter, M.C.; Mouritsen, O.G. Small-scale structure in fluid cholesterol-lipid bilayers. Curr. Opin. Colloid Interface Sci. 2013, 18, 440–447. [Google Scholar] [CrossRef]
- Lovell, J.F.; Billen, L.P.; Bindner, S.; Shamas-Din, A.; Fradin, C.; Leber, B.; Andrews, D.W. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 2008, 135, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Satsoura, D.; Kučerka, N.; Shivakumar, S.; Pencer, J.; Griffiths, C.; Leber, B.; Andrews, D.W.; Katsaras, J.; Fradin, C. Interaction of the full-length Bax protein with biomimetic mitochondrial liposomes: A small-angle neutron scattering and fluorescence study. Biochem. Biophys. Acta Rev. Biomembr. 2012, 1818, 384–401. [Google Scholar] [CrossRef] [PubMed]
- Shamas-Din, A.; Bindner, S.; Zhu, W.; Zaltsman, Y.; Campbell, C.; Gross, A.; Leber, B.; Andrews, D.W.; Fradin, C. tBid undergoes multiple conformational changes at the membrane required for Bax activation. J. Biol. Chem. 2013, 288, 22111–22127. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, S.; Kurylowicz, M.; Hirmiz, N.; Manan, Y.; Friaa, O.; Shamas-Din, A.; Masoudian, P.; Leber, B.; Andrews, D.W.; Fradin, C. The Proapoptotic Protein tBid Forms Both Superficially Bound and Membrane-Inserted Oligomers. Biophys. J. 2014, 106, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef] [PubMed]
- Daum, G. Lipids of mitochondria. Biochem. Biophys. Acta Rev. Biomembr. 1985, 822, 1–42. [Google Scholar] [CrossRef]
- Baggetto, L.G.; Clottes, E.; Vial, C. Low mitochondrial proton leak due to high membrane cholesterol content and cytosolic creatine kinase as two features of the deviant bioenergetics of Ehrlich and AS30-D tumor cells. Cancer Res. 1992, 52, 4935–4941. [Google Scholar] [PubMed]
- Crain, R.C.; Clark, R.W.; Harvey, B.E.; Mitochondria, M.H.; Grain, R.C. Role of Lipid Transfer Proteins in the Abnormal Lipid Content of Morris Hepatoma Mitochondria and Microsomes. Cancer Res. 1983, 42, 3197–3202. [Google Scholar]
- Feo, F.; Canuto, R.A.; Bertone, G.; Garcea, R.; Pani, P. Cholesterol and phospholipid composition of mitochondria and microsomes isolated from morris hepatoma 5123 and rat liver. FEBS Lett. 1973, 33, 229–232. [Google Scholar] [CrossRef]
- Parlo, R.A.; Coleman, S. Enhanced Rate of Citrate Export from Cholesterol-rich Hepatoma Mitochondria. J. Biol. Chem. 1984, 259, 9997–10000. [Google Scholar] [PubMed]
- Montero, J.; Morales, A.; Llacuna, L.; Lluis, J.M.; Terrones, O.; Basañez, G.; Antonsson, B.; Prieto, J.; García-Ruiz, C.; Colell, A.; Fernández-Checa, J.C. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer Res. 2008, 68, 5246–5256. [Google Scholar] [CrossRef] [PubMed]
- Lucken-Ardjomande, S.; Montessuit, S.; Martinou, J.C. Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in mitochondrial membranes. Cell Death Diff. 2008, 15, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.; Merlin, S.; Saito, M.; Schlesinger, P. Cholesterol effects on BAX pore activation. J. Mol. Biol. 2008, 381, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- Shamas-Din, A.; Bindner, S.; Chi, X.; Leber, B.; Andrews, D.; Fradin, C. Distinct lipid effects on tBid and Bim activation of membrane permeabilization by pro-apoptotic Bax. Biochem. J. 2015, 467, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Hristova, K.; White, S.H. Determination of the Hydrocarbon Core Structure of Fluid Dioleoylphosphocholine (DOPC) Bilayers by X-ray Diffraction Using Specific Bromination of the Double-Bonds: Effect of Hydration. Biophys. J. 1998, 74, 2419–2433. [Google Scholar] [CrossRef]
- Dies, H.; Toppozini, L.; Rheinstädter, M.C. The Interaction between Amyloid-β Peptides and Anionic Lipid Membranes Containing Cholesterol and Melatonin. PLoS ONE 2014, 9, e99124. [Google Scholar] [CrossRef] [PubMed]
- Wiener, M.C.; White, S.H. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of X-ray and neutron diffraction data. III. Complete structure. Biophys. J. 1992, 61, 434–447. [Google Scholar] [CrossRef]
- Nagle, J.; Zhang, R.; Tristram-Nagle, S.; Sun, W.; Petrache, H.; Suter, R. X-ray structure determination of fully hydrated Lα phase dipalmitoylphosphatidylcholine bilayers. Biophys. J. 1996, 70, 1419–1431. [Google Scholar] [CrossRef]
- Fragneto, G.; Rheinstädter, M. Structural and dynamical studies from bio-mimetic systems: An overview. Comptes Rendus Phys. 2007, 8, 865–883. [Google Scholar] [CrossRef]
- Pabst, G.; Kučerka, N.; Nieh, M.P.; Rheinstädter, M.; Katsaras, J. Applications of neutron and X-ray scattering to the study of biologically relevant model membranes. Chem. Phys. Lipids 2010, 163, 460–479. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.A.; Zheng, S.; Roshankar, G.; Alsop, R.J.; Belanger, R.K.; Huynh, C.; Kučerka, N.; Rheinstädter, M.C. Interaction of Aspirin (Acetylsalicylic Acid) with Lipid Membranes. PLoS ONE 2012, 7, e34357. [Google Scholar] [CrossRef] [PubMed]
- Toppozini, L.; Armstrong, C.L.; Barrett, M.A.; Zheng, S.; Luo, L.; Nanda, H.; Garcia Sakai, V.; Rheinstädter, M. Partitioning of ethanol into lipid membranes and its effect on fluidity and permeability as seen by X-ray and neutron scattering. Soft Matter 2012, 8, 11839–11849. [Google Scholar] [CrossRef]
- Tristram-Nagle, S.; Liu, Y.; Legleiter, J.; Nagle, J.F. Structure of gel phase DMPC determined by X-ray diffraction. Biophys. J. 2002, 83, 3324–3335. [Google Scholar] [CrossRef]
- Mills, T.T.; Toombes, G.E.S.; Tristram-Nagle, S.; Smilgies, D.M.; Feigenson, G.W.; Nagle, J.F. Order Parameters and Areas in Fluid-Phase Oriented Lipid Membranes Using Wide Angle X-ray Scattering. Biophys. J. 2008, 95, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Kucerka, N.; Liu, Y.; Chu, N.; Petrache, H.I.; Tristram-Nagle, S.; Nagle, J.F. Structure of fully hydrated fluid phase DMPC and DLPC lipid bilayers using X-ray scattering from oriented multilamellar arrays and from unilamellar vesicles. Biophys. J. 2005, 88, 2626–37. [Google Scholar] [CrossRef] [PubMed]
- Fidorra, M.; Duelund, L.; Leidy, C.; Simonsen, A.C.; Bagatolli, L. Absence of fluid-ordered/fluid-disordered phase coexistence in ceramide/POPC mixtures containing cholesterol. Biophys. J. 2006, 90, 4437–4451. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.; Rowat, A.C.; Brief, E.; Hsueh, Y.; Thewalt, J.; Zuckermann, M.; Ipsen, J.H. Universal behavior of membranes with sterols. Biophys. J. 2006, 90, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Jójárt, B.; Martinek, T.A. Performance of the general amber force field in modeling aqueous POPC membrane bilayers. J. Comput. Chem. 2007, 28, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Gawrisch, K.; Gaede, H.C.; Mihailescu, M.; White, S.H. Hydration of POPC bilayers studied by 1H-PFG-MAS-NOESY and neutron diffraction. Eur. Biophys. J. 2007, 36, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.A.; Chiu, S.W.; Jakobsson, E.; Grama, A.; Scott, H. Cholesterol surrogates: A comparison of cholesterol and 16: 0 ceramide in POPC bilayers. Biophys. J. 2007, 92, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Deserno, M. A systematically coarse-grained solvent-free model for quantitative phospholipid bilayer simulations. J. Phys. Chem. B 2010, 114, 11207–11220. [Google Scholar] [CrossRef] [PubMed]
- Armen, R.S.; Uitto, O.D.; Feller, S.E. Phospholipid component volumes: Determination and application to bilayer structure calculations. Biophys. J. 1998, 75, 734–744. [Google Scholar] [CrossRef]
- Edholm, O.; Nagle, J.F. Areas of Molecules in Membranes Consisting of Mixtures. Biophys. J. 2005, 89, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Hyslop, P.A.; Morel, B.; Sauerheber, R.D. Organization and interaction of cholesterol and phosphatidylcholine in model bilayer membranes. Biochemistry 1990, 29, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Mills, T.T.; Tristram-Nagle, S.; Nagle, J.F. Cholesterol Perturbs Lipid Bilayers Nonuniversally. Phys. Rev. Lett. 2008, 100, 198103. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, N.; McLeod, R.; Vance, J.; Vance, D.E. Biochemistry of Lipids, Lipoproteins and Membranes; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Avanti Polar Lipids Inc. Phase Transition Temperatures for Glycerophospholipids. 2014. Available online: http://www.avantilipids.com/index.php?option=com_content&id=1700&itemid=419 (accessed on 3 August 2014).
- Rose, M.; Hirmiz, N.; Moran-Mirabal, J.; Fradin, C. Lipid diffusion in supported lipid bilayers: A comparison between line-scanning fluorescence correlation spectroscopy and single-particle tracking. Membranes 2015. to be submitted. [Google Scholar]
- Wang, Z.J.; Deserno, M. Systematic implicit solvent coarse-graining of bilayer membranes: Lipid and phase transferability of the force field. New J. Phys. 2010, 12, 095004. [Google Scholar] [CrossRef] [PubMed]
- Petrache, H.; Dodd, S.; Brown, M. Area per lipid and acyl length distributions in fluid phosphatidylcholines determined by (2)H NMR spectroscopy. Biophys. J. 2000, 79, 3172–3192. [Google Scholar] [CrossRef]
- Pan, D.; Wang, W.; Liu, W.; Yang, L.; Huang, H.W. Chain packing in the inverted hexagonal phase of phospholipids: A study by X-ray anomalous diffraction on bromine-labeled chains. J. Am. Chem. Soc. 2006, 128, 3800–3807. [Google Scholar] [CrossRef] [PubMed]
- McConnell, H.M.; Radhakrishnan, A. Condensed complexes of cholesterol and phospholipids. Biochem. Biophys. Acta Rev. Biomembr. 2003, 1610, 159–173. [Google Scholar] [CrossRef]
- De Meyer, F.; Smit, B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Natl. Acad. Sci. USA 2009, 106, 3654–3658. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, F.J.M.; Benjamini, A.; Rodgers, J.M.; Misteli, Y.; Smit, B. Molecular Simulation of the DMPC-Cholesterol Phase Diagram. J. Phys. Chem. B 2010, 114, 10451–10461. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.C.; Lee, M.T.; Chen, F.Y.; Huang, H.W. The condensing effect of cholesterol in lipid bilayers. Biophys. J. 2007, 92, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Gershfeld, N.L. Equilibrium studies of lecithin-cholesterol interactions: I. Stoichiometry of lecithin-cholesterol complexes in bulk systems. Biophys. J. 1978, 22, 469–488. [Google Scholar] [CrossRef]
- Barrett, M.; Zheng, S.; Toppozini, L.; Alsop, R.; Dies, H.; Wang, A.; Jago, N.; Moore, M.; Rheinstädter, M. Solubility of cholesterol in lipid membranes and the formation of immiscible cholesterol plaques at High cholesterol concentrations. Soft Matter 2013, 9, 9342–9351. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Rawicz, W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett. 1990, 64, 2094. [Google Scholar] [CrossRef] [PubMed]
- Fradin, C.; Satsoura, D.; Andrews, D.W. Punching holes in membranes: how oligomeric pore-forming proteins and lipids cooperate to form aqueous channels in membranes. In Biomembrane Frontiers; Springer: New York, NY, USA, 2009; pp. 223–262. [Google Scholar]
- Tristram-Nagle, S.A. Preparation of oriented, fully hydrated lipid samples for structure determination using X-ray scattering. Methods Mol. Biol. 2007, 400, 63–75. [Google Scholar] [PubMed]
- Mills, T.T.; Tristram-Nagle, S.; Heberle, F.A.; Morales, N.F.; Zhao, J.; Wu, J.; Toombes, G.E.; Nagle, J.F.; Feigenson, G.W. Liquid-liquid domains in bilayers detected by wide angle X-ray scattering. Biophys. J. 2008, 95, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Buerger, M. The correction of X-ray diffraction intensities for Lorentz and polarization factors. Proc. Natl. Acad. Sci. USA 1940, 26, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T. A new method for determining the phase in the X-ray diffraction structure analysis of phosphatidylcholine/alcohol. Chem. Phys. Lipids 2000, 107, 93–97. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cathcart, K.; Patel, A.; Dies, H.; Rheinstädter, M.C.; Fradin, C. Effect of Cholesterol on the Structure of a Five-Component Mitochondria-Like Phospholipid Membrane. Membranes 2015, 5, 664-684. https://doi.org/10.3390/membranes5040664
Cathcart K, Patel A, Dies H, Rheinstädter MC, Fradin C. Effect of Cholesterol on the Structure of a Five-Component Mitochondria-Like Phospholipid Membrane. Membranes. 2015; 5(4):664-684. https://doi.org/10.3390/membranes5040664
Chicago/Turabian StyleCathcart, Kelly, Amit Patel, Hannah Dies, Maikel C. Rheinstädter, and Cécile Fradin. 2015. "Effect of Cholesterol on the Structure of a Five-Component Mitochondria-Like Phospholipid Membrane" Membranes 5, no. 4: 664-684. https://doi.org/10.3390/membranes5040664
APA StyleCathcart, K., Patel, A., Dies, H., Rheinstädter, M. C., & Fradin, C. (2015). Effect of Cholesterol on the Structure of a Five-Component Mitochondria-Like Phospholipid Membrane. Membranes, 5(4), 664-684. https://doi.org/10.3390/membranes5040664