2.1. Supported Lipid Bilayer Micropatterning
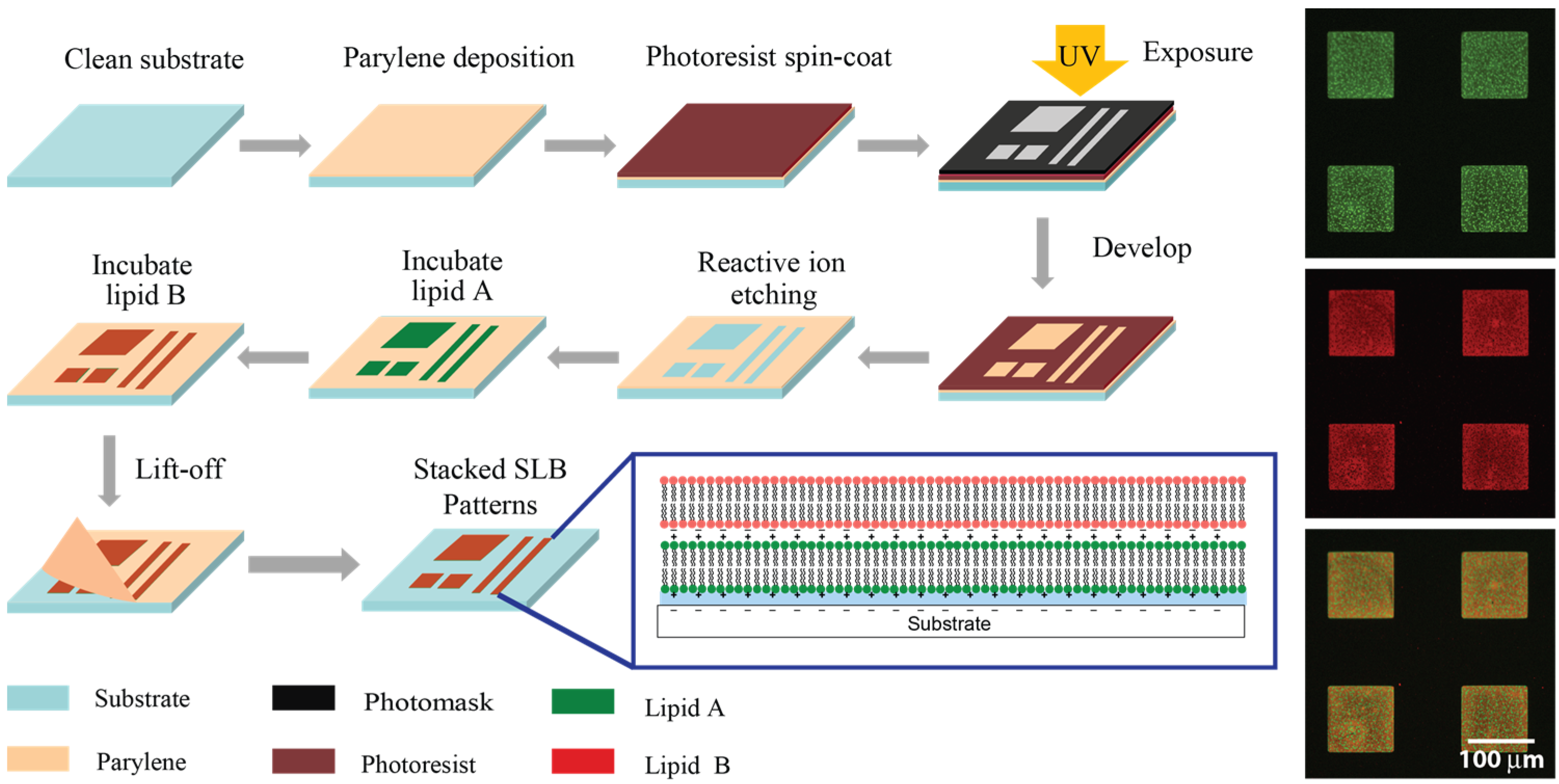
Supported Lipid Bilayer stacks were deposited into micron-sized patterns through the use of Polymer Stencil Lift-Off (PSLO,
Figure 1). In this process, Parylene was first deposited through chemical vapour deposition onto SiO
2 surfaces to form a 500–1000 nm thick, conformal, pinhole-free film. Parylene films were then patterned thorough standard photolithography and reactive ion etching processes. This resulted in polymer stencils with arrays of features with 2–200 μm critical dimensions, which were subsequently cleaned with acetone, UV-O
3, and 100 mM sodium hydroxide solution to remove any photoresist and polymer residue in the opening areas, producing smooth surfaces suitable for SLB formation through vesicle fusion. By incubating small unilamellar lipid vesicles (SUVs) on the substrates under the appropriate temperature, SLBs formed within the Parylene stencil openings. For single lipid bilayer patterning, the stencil could be removed after the first incubation step, while for multiple SLB stack formation the stencil remained in place until after the last bilayer was deposited. The Parylene stencil served as a stable physical barrier preventing the SLBs from spreading and remained intact during the whole SLB stacking process, which was conducted under aqueous conditions. The deposited lipids formed micron-sized single and multiple SLBs domains, demonstrating the suitability of PSLO for patterning stacked SLBs.
Figure 1.
Illustration of the PSLO patterning process for stacked SLBs. Parylene film is deposited onto a clean substrate, and photoresist is patterned using photolithography. After reactive ion etching of Parylene and removal of photoresist, small unilamellar lipid vesicles are deposited on the stencils and incubated to form SLBs. The final step is to lift off the stencil, revealing stacked SLB patterns. Inset: side view illustration of a double lipid bilayer with cationic and anionic lipids, and fluorescence images of a two-bilayer stack.
Figure 1.
Illustration of the PSLO patterning process for stacked SLBs. Parylene film is deposited onto a clean substrate, and photoresist is patterned using photolithography. After reactive ion etching of Parylene and removal of photoresist, small unilamellar lipid vesicles are deposited on the stencils and incubated to form SLBs. The final step is to lift off the stencil, revealing stacked SLB patterns. Inset: side view illustration of a double lipid bilayer with cationic and anionic lipids, and fluorescence images of a two-bilayer stack.
2.2. Homogeneous SLB Stacks
The ability to pattern SLB stacks through the PSLO technique was first tested using lipid mixtures that formed homogenous bilayers. Two kinds of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) vesicle solutions were prepared: one was doped with 10% cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), and the other with 10% anionic lipid 1,2-dimyristoyl-sn-glycero-3-phospho-L-serine (DMPS). As the SiO
2 substrate was negatively charged under slightly basic buffer conditions, a positively-charged SLB containing DOTAP was formed first by vesicle fusion directly on the substrate. With the first cationic bilayer formed, the anionic lipid vesicles were then incubated to form the second SLB. The electrostatic interactions between the oppositely-charged lipids promoted the fusion of anionic vesicles on top of the underlying cationic bilayer. By alternating the charge of the subsequent layers, three- and four-bilayer stacks could be similarly formed. We demonstrated the formation of up to four homogenous SLBs with DOPC as the main component through this bilayer-by-bilayer approach. However, it must be noted that any number of bilayers is possible provided the thickness of the resulting stack does not exceed the stencil thickness. The stacked SLB patterns were visualized by fluorescence microscopy during the stack formation (
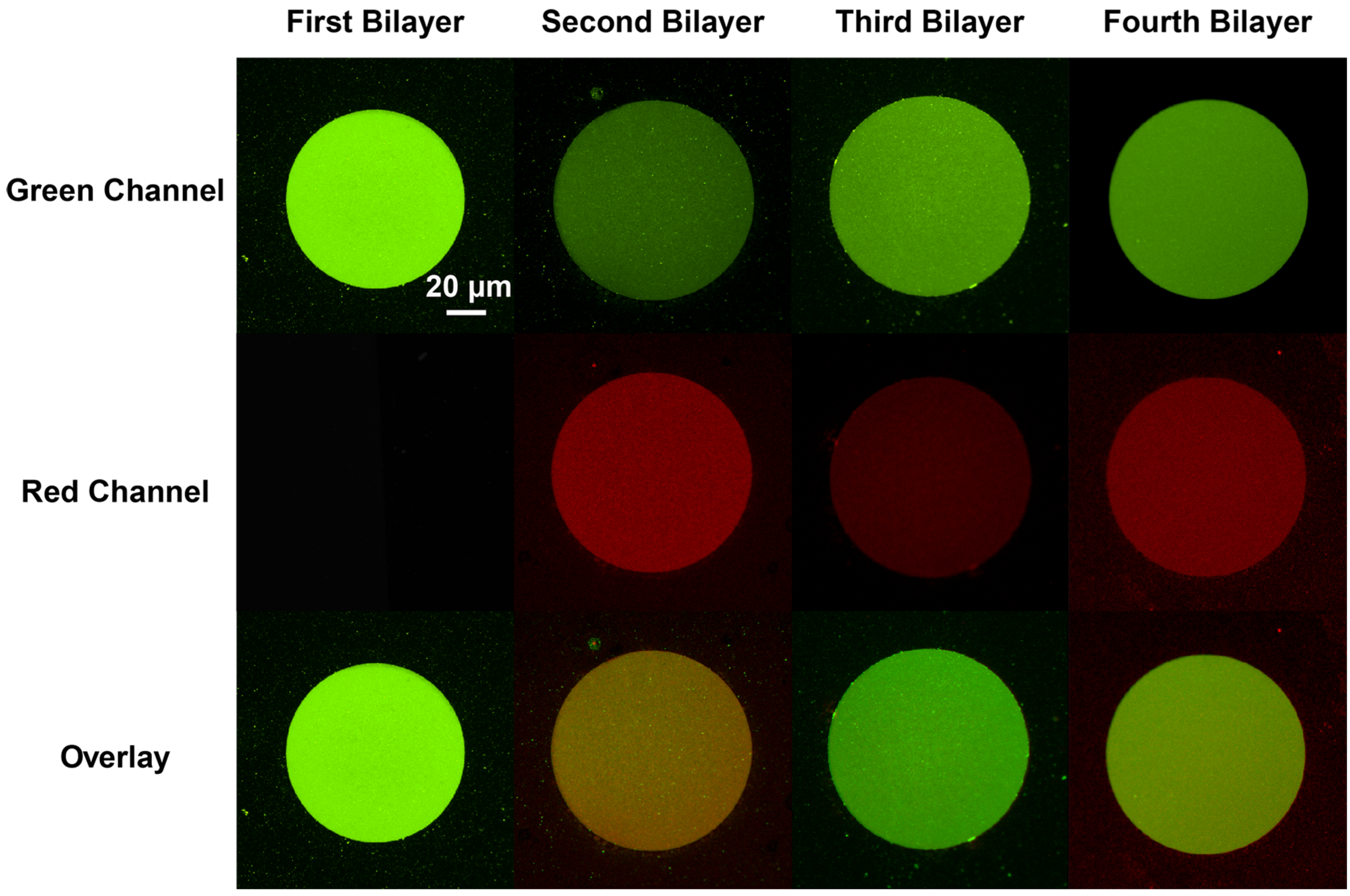
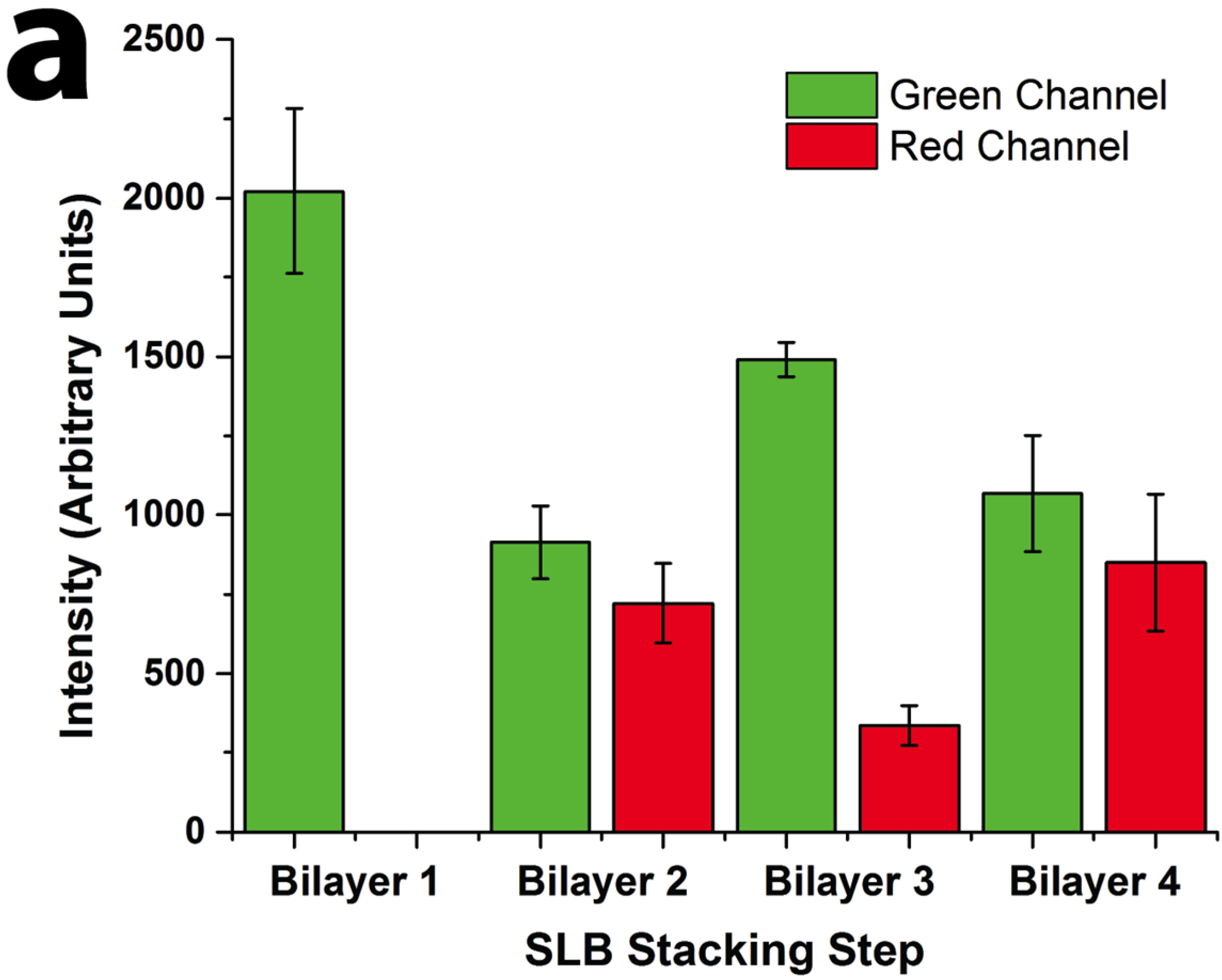
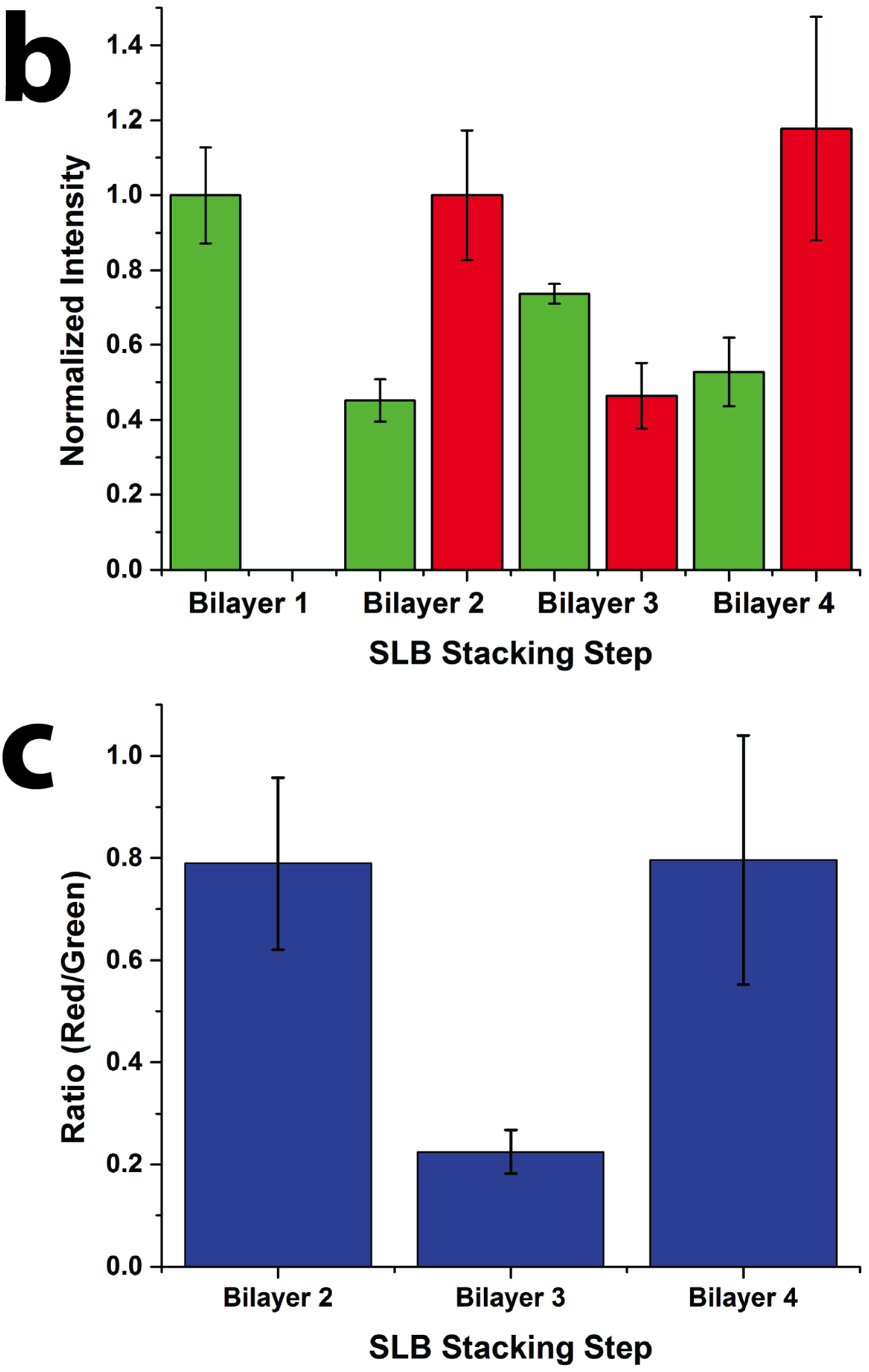
Figure 2). Oregon Green labeled 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (DHPE-OG) and Lissamine Rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (DHPE-LR) were added to the vesicles at low concentrations (0.1% molar ratio) to label the SLBs, and were imaged in the green and red fluorescence channels, respectively. The images show that fluorescence intensity of both channels changed with the addition of each new bilayer. Specifically, after the second bilayer (labeled with DHPE-LR) and third bilayers were formed, the intensity of DHPE-OG in the first bilayer and the DHPE-LR in the second bilayer greatly decreased, a change ascribed to fluorescence resonance energy transfer (FRET) or collisional quenching mechanisms, respectively. On one hand, in FRET, Oregon Green, as a donor chromophore, initially in its electronic excited state, transferred energy through non-radiative dipole–dipole coupling to the acceptor chromophore, Lissamine Rhodamine (LR), resulting in decreased green channel intensity; on the other hand, the addition of a third bilayer and the close proximity of DHPE-OG molecules to DHPE-LR could cause collisional quenching, leading to reduced intensities in the red channel, too. Quantitative fluorescence analysis was conducted by measuring the absolute and relative fluorescence intensities of each bilayer (relative intensity normalized to the intensity of the first bilayer added in each channel), as shown in
Figure 3. The ratio of red over the green channel raw intensities (
Figure 3c) increases with the deposition of LR labeled SLBs, and decreases with the addition of OG-labeled SLBs. The trend for fluorescence intensity change was in good agreement with expected intensity variations based on the successive addition of fluorescent bilayers, taking into account FRET and quenching effects, further confirming the formation of patterned SLB stacks. In addition, fluorescence recovery after photobleaching (FRAP) experiments were performed on each successive bilayer added (
Figure S1), where the observation of homogeneous recovery indicated that each bilayer of the stack retained good mobility at room temperature. The fluorescence background observed outside the patterns in bilayers 2 and 3 of
Figure 3 was generated by vesicles adhered on the Parylene stencil, as the stencil was not lifted off until all four bilayers had been deposited. The fluorescence images, fluorescence intensity analysis, and FRAP tests demonstrated that fully-mobile SLB stacks could be formed by layer-by layer deposition and easily patterned using the PSLO approach.
Figure 2.
Epifluorescence images of four-bilayer homogeneous stacked SLB micropatterns. The first and third bilayers were composed of DOPC:DOTAP:DHPE-OG and can be observed in green channel. Second and fourth bilayers were composed of DOPC:DMPS:DHPE-LR, and can be observed in red channel (middle). The bottom row presents an overlay of both red and green channels. All images were acquired at the same magnification.
Figure 2.
Epifluorescence images of four-bilayer homogeneous stacked SLB micropatterns. The first and third bilayers were composed of DOPC:DOTAP:DHPE-OG and can be observed in green channel. Second and fourth bilayers were composed of DOPC:DMPS:DHPE-LR, and can be observed in red channel (middle). The bottom row presents an overlay of both red and green channels. All images were acquired at the same magnification.
Figure 3.
Quantitative fluorescence intensity analysis of stacked SLB formation. (a) Raw fluorescence intensity of green (DHPE-OG) and red (DHPE-LR) channels of the four-bilayer SLB patterns after each stacking step; (b) normalized intensity of each stacking step (green channel normalized to bilayer 1 and red channel normalized to bilayer 2 values); and (c) ratio of the raw intensities of red to green channels at each stacking step.
Figure 3.
Quantitative fluorescence intensity analysis of stacked SLB formation. (a) Raw fluorescence intensity of green (DHPE-OG) and red (DHPE-LR) channels of the four-bilayer SLB patterns after each stacking step; (b) normalized intensity of each stacking step (green channel normalized to bilayer 1 and red channel normalized to bilayer 2 values); and (c) ratio of the raw intensities of red to green channels at each stacking step.
2.3. Stacked SLBs Containing Phase-Segregated Domains
The same bilayer-by-bilayer deposition and PSLO approach was applied to create stacked SLB patterns containing phase-segregating lipid compositions. The anionic and cationic lipids used in these experiments (DOPS, DOTAP) partition preferentially into the liquid-disordered phase due to their unsaturated carbon chains, which result in lower transition temperatures (
Tm = −11 °C and −11.9 °C, respectively). Phase segregation was achieved by introducing 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC, a fully saturated lipid with
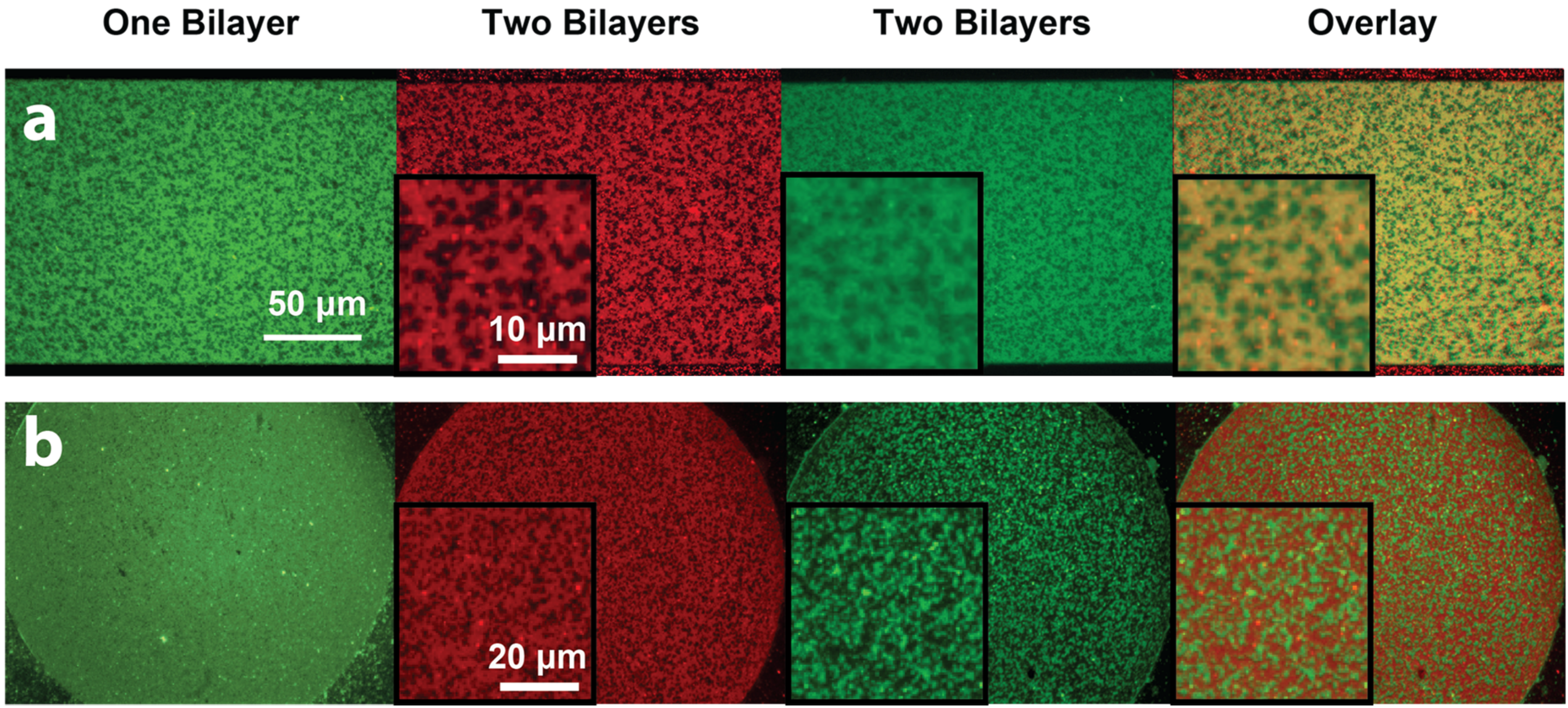
Tm = 55 °C) into the DOPC:DOPS/DOTAP:DHPE-OG/LR lipid mixtures. Homogeneous, fully-mixed bilayers were successfully formed at 60°C (above the DSPC transition temperature), after which cooling to room temperature (21 °C) resulted in the formation of visible phase-segregated lipid domains. Since the DHPE-OG/LR dyes preferentially partition into the liquid-disordered phase (primarily composed of DOPC), the gel phase consisting mostly of DSPC would be observed as dark domains. First, we demonstrated the ability to form two-bilayer stacks composed of a phase-segregated bilayer on top of a homogeneous one (
Figure 4). As expected, the first lipid bilayer deposited, with composition DOPC:DOTAP:DHPE-OG, was uniformly fluorescent in the green channel. However, upon formation of a second phase-segregated SLB, with composition DOPC:DSPC:DOPS:DHPE-LR, it was observed that the green channel now showed a complementary pattern to the red channel, as shown in the overlay image of
Figure 4. This phenomenon can be readily explained by the close proximity and interaction between the fluorescent probes in each of the bilayers within the stack, which results in FRET. Since FRET could only occur between DHPE-OG molecules (green) in the first bilayer (homogeneous) that came into close proximity with DHPE-LR molecules (red) in the second bilayer (phase-segregated), and given that the DHPE-LR molecules partitioned preferentially to the fluid phase, areas of the first bilayer overlapping the gel domains in the second bilayer appeared brighter than the surrounding areas. This phenomenon directly probes the interactions between the two bilayers, and demonstrates that the stacked bilayers are in close proximity. Additionally, the observation of phase segregation in the second bilayer, coupled with FRAP measurements on both homogenous and phase-segregated layers in this experiment (
Figure S2), confirmed the formation of fully-mobile heterogeneous SLB stacks through the PSLO technique.
Figure 4.
Epifluorescence images showing patterns of two-bilayer SLB stacks consisting of a homogeneous bottom bilayer and a phase-segregated top bilayer. The first lipid bilayer (composed of DOPC: DOTAP: DHPE-OG) is observed in the green channel, while the second lipid bilayer (composed of DOPC: DSPC: DOPS: DHPE-LR) can be observed in the red channel. The formation of the second bilayer leads to significant fluorescence intensity changes in the first bilayer. The overlaid image of the two bilayers shows the fluorescence patterns are complimentary, due to FRET.
Figure 4.
Epifluorescence images showing patterns of two-bilayer SLB stacks consisting of a homogeneous bottom bilayer and a phase-segregated top bilayer. The first lipid bilayer (composed of DOPC: DOTAP: DHPE-OG) is observed in the green channel, while the second lipid bilayer (composed of DOPC: DSPC: DOPS: DHPE-LR) can be observed in the red channel. The formation of the second bilayer leads to significant fluorescence intensity changes in the first bilayer. The overlaid image of the two bilayers shows the fluorescence patterns are complimentary, due to FRET.
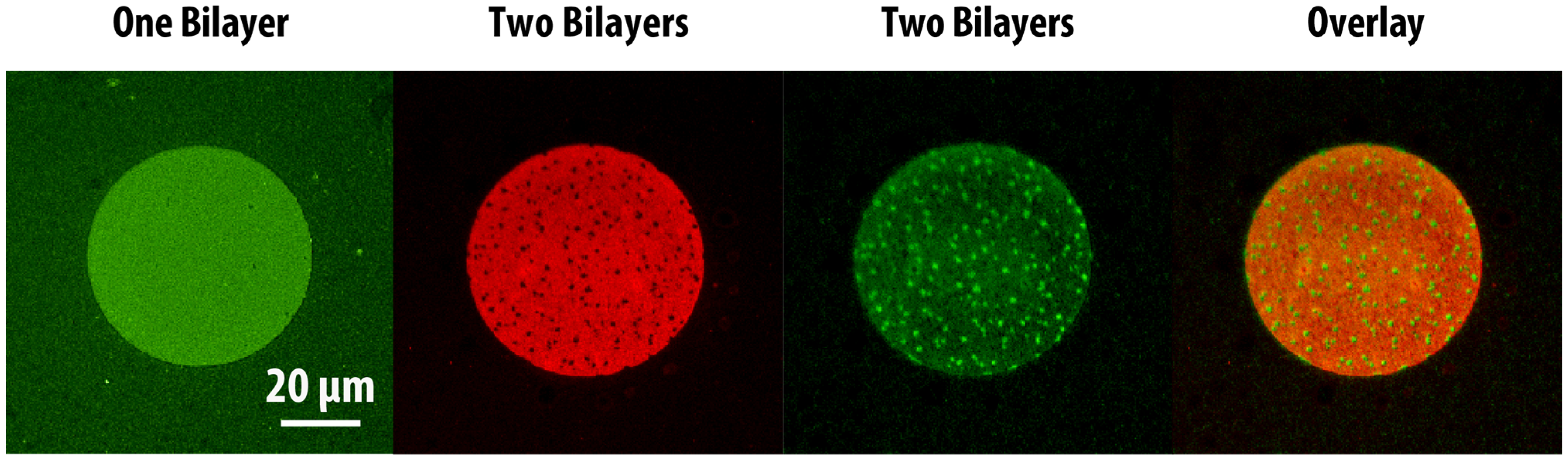
Next, we explored the possibility of patterning two-bilayer stacks where both bilayers would phase-segregate, and investigated the phase behaviour of the stacked bilayers. Small unilamellar lipid vesicles composed of DOPC:DSPC:DOTAP:DHPE-OG were deposited and incubated at 60 °C to form the first bilayer. It was observed that when the substrate was properly cleaned (
i.e., the bilayer mobility was appropriate), micron-scale gel domains formed on the cationic bilayer upon slow cooling. As expected, the DHPE-OG molecules preferentially partitioned into the liquid disordered phase, leaving the gel phase dark (
Figure 5a, green channel). A second anionic phase-segregated bilayer (with composition DOPC:DSPC:DOPS:DHPE-LR,
Figure 5a red channel) could then be formed on top by incubating the lipid vesicles at 60 °C followed by slow cooling. The formation of this second bilayer also led to decreased fluorescence intensity from the first bilayer due to FRET. When both lipid bilayers phase-segregated, it was observed that the gel domains aligned between the two bilayers (
Figure 5a, overlay), indicating coupling between the phase-segregation processes of both bilayers. Tayebi and collaborators [
1] have previously reported the long-range alignment of phase-segregated lipid bilayer domains across hundreds of membrane lamellae. They postulated that such alignment originated from the surface tension associated with differences in the network of hydrogen-bonded water molecules at the hydrated interfaces between the domains and the surrounding phases. In our case, in addition to surface tension effects, the alignment could also be promoted by charges within the lipid bilayer, since the cationic and anionic lipids both partition preferentially into the liquid disordered phase. On the other hand, when the first cationic bilayer was not highly mobile, due to improper cleaning or cooling rate, it did not show visible phase segregation and the fluorescence intensity remained homogeneous (
Figure 5b, green channel). Under this scenario, formation of the second anionic lipid bilayer led to phase segregation only on the second layer, where gel phase domains appeared dark (
Figure 5b, red channel). In this case FRET between the two layers caused the green channel intensity from the first bilayer to show a complementary pattern to the phase segregation of the second bilayer (
Figure 5b, overlaid image), resembling the results obtained from patterned SLB stacks where the first bilayer was homogeneous and the second one was phase segregating. These experimental results demonstrate that it is possible to pattern lipid bilayer stacks with heterogeneous lipid bilayers through the bilayer-by-bilayer and PSLO approach, and observe interactions between phase-segregating lipid mixtures across lipid lamellae. However, it must be noted that sample preparation and substrate cleanliness are key to obtaining highly-mobile lipid bilayers that enable such interactions.
Figure 5.
Epifluorescence images of stacked phase-segregated SLB patterns. The first lipid bilayer was composed of DOPC:DSPC:DOTAP:DHPE-OG (green channel), while the second lipid bilayer was composed of DOPC:DSPC:DOPS:DHPE-LR (red channel). Insets show zoomed-in areas of the patterned bilayers. (a) When the first bilayer showed good phase segregation (green), the second lipid bilayer would phase segregate in the same pattern (red). The fluorescence intensity of first bilayer would decrease due to FRET, and the overlaid image of the two channels showed alignment of gel phase domains in the two bilayers; (b) When the first bilayer did not phase-segregate, the second SLB would still show gel-phase domains. The first bilayer appeared dimmer in areas that overlapped with the liquid disordered phase of the second bilayer. Image contrast has been adjusted to facilitate the visualization of the overlapping patterns in the stacked lipid bilayers.
Figure 5.
Epifluorescence images of stacked phase-segregated SLB patterns. The first lipid bilayer was composed of DOPC:DSPC:DOTAP:DHPE-OG (green channel), while the second lipid bilayer was composed of DOPC:DSPC:DOPS:DHPE-LR (red channel). Insets show zoomed-in areas of the patterned bilayers. (a) When the first bilayer showed good phase segregation (green), the second lipid bilayer would phase segregate in the same pattern (red). The fluorescence intensity of first bilayer would decrease due to FRET, and the overlaid image of the two channels showed alignment of gel phase domains in the two bilayers; (b) When the first bilayer did not phase-segregate, the second SLB would still show gel-phase domains. The first bilayer appeared dimmer in areas that overlapped with the liquid disordered phase of the second bilayer. Image contrast has been adjusted to facilitate the visualization of the overlapping patterns in the stacked lipid bilayers.