Abstract
ThermoTRP channels (thermoTRPs) define a subfamily of the transient receptor potential (TRP) channels that are activated by changes in the environmental temperature, from noxious cold to injurious heat. Acting as integrators of several stimuli and signalling pathways, dysfunction of these channels contributes to several pathological states. The surface expression of thermoTRPs is controlled by both, the constitutive and regulated vesicular trafficking. Modulation of receptor surface density during pathological processes is nowadays considered as an interesting therapeutic approach for management of diseases, such as chronic pain, in which an increased trafficking is associated with the pathological state. This review will focus on the recent advances trafficking of the thermoTRP channels, TRPV1, TRPV2, TRPV4, TRPM3, TRPM8 and TRPA1, into/from the plasma membrane. Particularly, regulated membrane insertion of thermoTRPs channels contributes to a fine tuning of final channel activity, and indeed, it has resulted in the development of novel therapeutic approaches with successful clinical results such as disruption of SNARE-dependent exocytosis by botulinum toxin or botulinomimetic peptides.
1. Introduction
Transient receptor potential (TRP) channels consist of a large family of ion channels that play a wide diversity of physiological functions [1,2,3]. Expressed in a large number of tissues from nerve to epithelial cells, genetic studies have linked mutations in these ion channels to human diseases [3,4]. The majority of TRP channels are non-selective cation channels that permeate Na+ and K+, and most of them with significant Ca2+ selectivity. In mammals, this family consists of 28 different TRP members grouped in 7 subfamilies: TRPC (classical or canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin-like), TRPPP (polycysteine), TRPML (mucolipin) and the TRPN (no mechanoreceptor potential C (NOMPC) [1,2,3], while the TRPY is present in yeast.
Thermosensory channels, also named “thermoTRPs”, define a subfamily of the TRP channels that are activated by changes in the environmental temperature, from noxious cold (<15 °C) to injurious heat (>42 °C) (Figure 1). Acting as integrators of several stimuli and signalling pathways, dysfunction of these channels, for instance, contributes to thermal hyperalgesia and allodynia under pathological painful conditions such as inflammation or cancer [4,5,6,7]. For this reason, thermoTRPs have become pivotal drug targets, and the development of therapeutic compounds for pharmacological intervention is actively pursued by the academy and the industry [8,9].
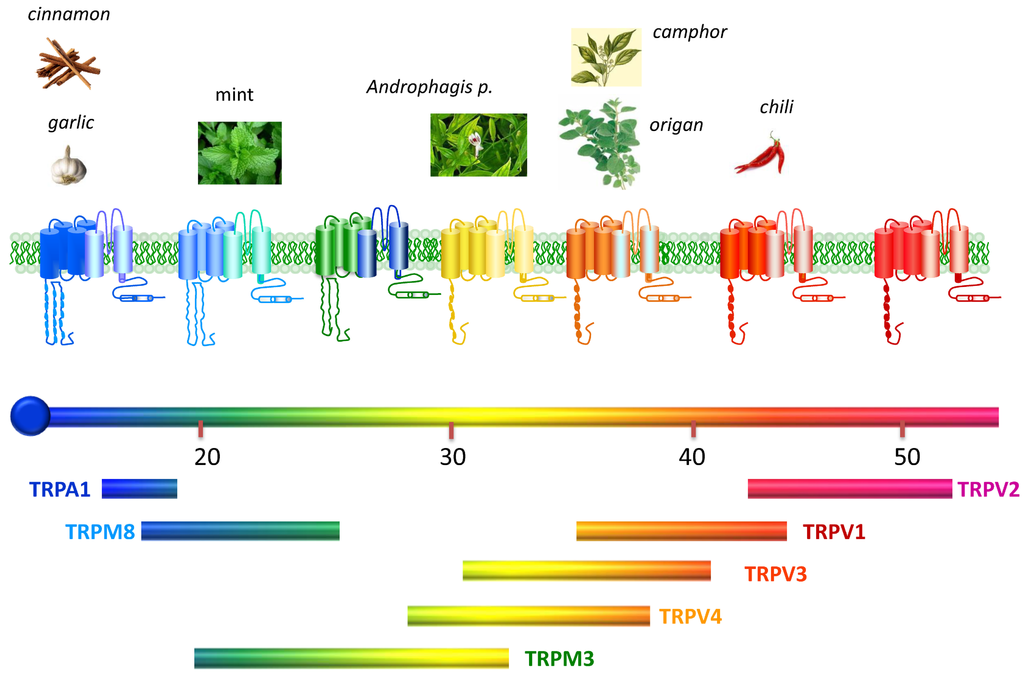
Figure 1.
Thermotransient receptor potential (TRP) channels. Structurally thermoTRP are tetramers and each subunit contains six transmembrane domains (S1–S6), a hydrophobic pore loop linking transmembrane S5 and S6, and large cytoplasmic N- and C-terminals (NB not drawn to scale). All thermoTRPs have a variable number of ankyrin repeat domains in the N-terminus, except TRPM8 which has none and instead contains TRPM homology region. ThermoTRPs display distinct thermal thresholds from very noxious cold (TRPA1) to harmful hot (TRPV2). Each thermoTRP is also activated by specific natural or synthetic compounds, known to induce the relevant thermal and pain sensations in humans. Adapted from [10].
The cellular activity of polymodal thermoTRP channels is subjected to a complex modulation encompassing from posttraductional modification, usually by phosphorylation/dephosphorylation, to regulation of their membrane expression [11]. Notably, recruitment of thermoTRP channels to the cell surface has been revealed as a pivotal mechanism for algesic sensitization of sensory neurons [12]. Furthermore, yeast-screen assays have identified a large number of proteins implicated in vesicular trafficking as partners of thermoTRP channels [13]. Accordingly, the cellular and molecular mechanisms involved in the transport and mobilization of these channels to the cell membrane are currently being an area of intense investigation.
Cumulative evidence suggest that thermoTRP channels are actively transported by both constitutive and regulated pathways depending on the cell type and cellular environment. Here, we will cover the recent advances in the membrane trafficking of the classical thermosensory channels, namely TRPV1, TRPV2, TRPV4, TRPM8, TRPM3 and TRPA1. It should be mention that TRPV3 is also one of the thermoTRPs; however, we will not discuss this channel since until now there are no data describing any finding on its trafficking. This review will focus on the progress carried out on this fascinating topic and will highlight the tenet that regulated membrane insertion of thermoTRPs channels contribute to a fine tuning of channel activity. The clear contribution of this mechanism to the final channel function has opened exciting research lines driven to the development of novel therapeutic approaches with successful clinical results in the treatment of several painful pathologies.
2. ThermoTRP Channels
ThermoTRPs are expressed as homotetramers, although heterotetramers have been also proposed for some of them [3]. All of them present six transmembrane domains (S1–S6), with a pore region between S5 and S6, and with N- and C-terminal cytosolic domain. The N-terminus has several ankyrin repeats or TRPM homology regions, while the C-terminus contains motives for multimerization, protein consensus sequences for kinases, and the transient receptor potential (TRP) domain important for channel gating.
2.1. TRPV1
Transient receptor potential vanilloid 1 (TRPV1) is the archetypal and the most studied thermoTRP channel. TRPV1 is a 95 kD protein with 838 aa [14] whose putative structure has been recently proposed by cryo-electron microscopy [15,16,17]. Functionally TRPV1 exists as homotetramer [16] and there are at least six ankyrin repeats in the N-terminus [18,19] essential for channel function and numerous protein-protein interactions [20,21]. The C-terminal contains subdomains involved in different channel functions: (i) determinants for subunit tetramerization [22]; (ii) the TRP domain [23,24]; and (iii) consensus sequences for phosphoinositides [25] and protein kinases [26].
This polymodal channel can be activated by noxious heat (>42 °C), acidic pH (pH < 5.9), voltage and numerous chemical ligands [27,28]. Capsaicin is the main known natural agonist of TRPV1, but it can be also activated by others pungent extract, toxins or even some endogenous ligands such as anandamide (known as endo-vanilloid) [29,30]. TRPV1 shows equal selectivity for Na+, K+, Li+, Cs+ and Rb+, but moderate for divalent cations such as Ca2+ and Mg2+ [14,31,32].
TRPV1 is subjected to complex regulation, from gene expression to post-translational modification as well as subcellular compartmentalisation and association with regulatory proteins [33,34]. The activity of TRPV1 can be rapidly regulated by phosphorylation of several key residues leading to an increased sensitivity to both chemical and thermal stimuli. Kinases such as protein kinase C (PKC), protein kinase A (PKA), calmodulin-dependent kinase (CaM) and Src-kinase increase TRPV1 activity, while calcineurin-mediated dephosophorylation at the same sites produces TRPV1 desentitization [11]. As consequence, the formation of signalplexes or the physical assembly of signalling molecules into discrete macromolecular entities is an essential pathway that influences TRPV1 activity [11].
This thermoTRP channel is widely distributed in neuronal as well as non-neuronal tissues. In the peripheral nervous system, TRPV1 is highly expressed in dorsal root (DRG), trigeminal (TG) and nodose (ND) ganglia [14]. Mainly present in small and medium peptidergic neurons, and to a lesser extent in non-peptidergic nociceptors [29,35]. Strongly localized on lamina I and outer portion of II in the dorsal horn [36,37,38], the presence of TRPV1 in sensory nerves and in areas involved in detection, transmission and regulation of pain reveals its key role in nociception and neurogenic inflammation [39]. In the central nervous system, although with some initial controversy on its brain distribution [40,41,42], the role of TRPV1 in synaptic transmission, neurotransmitter release and plasticity is already described by several groups [43,44], while a potential contribution in cognition, perception and neuropsychiatric disorders is being proposed [45].
There is an extensive list of non-neuronal tissues expressing TRPV1 channel [46,47]. For instance, TRPV1 participates in many aspects of skin biology [48,49], joint homeostasis and pathophysiology [50,51,52], produces vasodilatation and extravasation through the release of neuropeptides from the innervating sensory neurons [53,54], but it can directly evoke constriction [55]. Other physiological mechanisms can be modulated by TRPV1 such as cough [56] or to control bladder function and overactivity [57].
Nevertheless, the main known role of TRPV1 is as thermosensor transducing physical, chemical and thermal noxius stimuli. With a prominent role during inflammation, TRPV1 is responsible for thermal development and maintenance. In fact, both pharmacological and knockout studies have demonstrated its pivotal role on pain. TRPV1 is overexpressed in several chronic painful pathologies [58] such as rheumatoid arthritis [59,60], osteoarthritis [61], bone cancer-induced pain [62] and several neuropathies [63,64] among others. All these evidences promoted TRPV1 as an interesting pharmacological target to develop new analgesic treatments especially in diseases with an inflammatory component. In this regard, clinical trials have already shown a hyperthermic response upon treatment with some TRPV1 antagonists [65]. It is now known that TRPV1 is involved in the maintenance of normal body temperature [66], and recent studies propose that antagonists that do not block activation of TRPV1 by acidic solution apparently do not induced hyperthermia [67].
2.2. TRPV2
Transient receptor potential vanilloid 2 (TRPV2) is a thermoTRP channel activated by high temperatures (≈52 °C). Cloning of human and rat TRPV2 was made based on their homology to TRPV1, hence it has been also referred to as vanilloid receptor like protein 1 (VRL-1) or osm-9-like TRP channel 2 (OTRPC2). Structurally, TRPV2 consists of 740 aa with six ankyrin repeats domains (ARD) in its large N-terminus. The membrane proximal domain is an important region that connects TRPV2-ARD to the S1 and apparently modulates temperature sensitivity [68]. The C-terminus contains the TRP domain important for oligomerization [23], as well as domains for phosphotidylinositol 4,5- bisphospate (PIP2) [69] and CaM binding [70]. The N-glycosylation motif between S5–S6 loop is required for TRPV2 plasma membrane expression, since only the glycolsylated form reaches cell surface while the non-glycosylated remains in the cytosol [71].
TRPV2 is permeable to divalent cations with high selectivity for Ca2+ [72]. TRPV2 is strongly activated by 2-aminoethoxydiphenyl borate [73], diphenylyboronic anhydride, and derivatives of Cannabis sativa like cannabidiol, similar to other TRP channels such as TRPV1 or TRPA1. Probenecid is a potent activator of TRPV2 [74], while other endogenous activators like lysophosphotidyl choline can activate TRPV2 as well [75].
Similar to TRPV1, TRPV2 function can be rapidly regulated by post-translational modifications such as phosphorylation/dephosphorylation by PKA or PI3-kinase [76,77] or desensitization by Ca2+. Though TRPV2 does not contain the binding sites for CaM, ATP, or PIP2, a recent novel Ca2+-dependent binding site for CaM in the C-terminal fragment has been proposed, but probably CaM binding may not be functionally coupled to TRPV2 desensitization [69].
Expressed in several tissues, TRPV2 shows different physiological functions. In the nervous system, it is highly expressed in sensory neurons, being present in large and medium diameter DRG neurons with higher heat threshold and slow activating currents [78], while in TG is also located in large diameter neurons [79]. The expression of TRPV2 in sensory neurons and its activation by noxius heat [72] suggested a role in nociception. Nevertheless, deletion of TRPV2 gene expression does not affect thermal or mechanical sensing in mice [80]. During peripheral chronic inflammation, TRPV2 expression is increased, [81] and contributes to noxious heat hyperalgesia, especially in the absence of TRPV1. However, since acute nociception and thermal hyperalgesia are not impaired [80], the role of TRPV2 in thermosensing still remains highly controversial. The presence of TRPV2 in spinal cord and in different brain areas reveals other roles for this channel such as axonal outgrowth [82] or modulation of astrocyte function [75].
Outside the nervous system, TRPV2 mediates oxytocin and vasopressin release [83], participates in cardiac contractility and Ca2+-regulation [84], acts as an important stretch sensor in myocytes [85] and contributes to osteoclastogenesis [86]. Interestingly, TRPV2 shows an important role in immune response being expressed in several immune cell types [87], and in several cancer processes, like urothelial carcinoma in bladder [88] or glioblastomamultiforme [89], with a relevant role on cell migration [90]. Notably, TRPV2 has been involved in some hereditary diseases, such as muscular dystrophy [91], being a player in the pathogenesis of myocyte degeneration, and cell stretch increases TRPV2 translocation to the sarcolemma leading to external Ca2+ overloading in animal models and patients [92].
2.3. TRPV4
Transient receptor potential vanilloid 4 (TRPV4), also recognized as TRP12, OTRPC4 or VR-OAC, was initially detected as a channel activated by hypotonicity [93]. All mammalian TRPV4 homologues share high degree of sequence identity (95%–98%) [94]. In the N-terminus, the 6 ankyrin repeated domains are involved in TRPV4 protein-protein interactions [95], and seem to be related to self-association of N-termini into the tetrameric structure [96], acting as molecular determinants of subunit assembly and subsequent processing of the channel [97]. In the N-terminus, TRPV4 contains a proline rich domain involved in mechano-sensitive properties [98]. Like other TRP channels, TRPV4 is subject to dual Ca2+-dependent regulation, with channel activity potentiated and inactivated during agonist-dependent activation in the presence of the divalent cation [99]. The C-terminus comprises several putative CaM binding sites basis of the Ca2+-dependent potentiation process [100].
Nowadays, TRPV4 is already defined as a polymodal channel activated by various stimuli ranging from physical stimuli to chemical activators, being considered as a mechano- or osmo-sensor and a moderate heat sensor (between 24 and 27 °C) [101]. Agonists of TRPV4 include the endocannabinoid anandamide, the metabolite arachidonic acid [102], bisandrographolide A [103], 4-alpha-phorbol 12,13-didecanoate [104], acetylcholine [105], apigenin [106], or dimethylallyl pyrophosphate [107].
This thermoTRP is a non-selective cationic channel with higher permeability to Ca2+ and Mg2+ that Na+ [108]. Apart from the complex Ca2+ dependent regulation of TRPV4 [99], gating of the channel can be modulated by other mechanisms. For instance, PIP2 interaction with N-terminus increases channel activation by hypotonicity and heat, while disruption of this interaction prevents channel activation or sensitization through the PLC pathway [109]. Gating of TRPV4 can be also regulated by both PKC-independent and dependent pathways [110,111,112].
TRPV4 shows a wide range expression and physiological functions, and it is involved in the development of several pathological conditions [94]. For instance, TRPV4 regulates cell-cell junction, maintains skin barrier homeostasis playing a role in cutaneous thermoregulation [113,114], is determinant in bone physiology, joint homeostasis, bone remodelling, and chondrocyte differentiation [115,116]. This channel is also present in airway smooth muscle cells [117], different types of renal cells [118], and aortic endothelial cells [119]. In the nervous system, TRPV4 is a key regulator of hippocampal neural excitability [120] and participating in astrocytes homeostasis [121]; however, TRPV4 presence in DRG, TG and ND neurons promoted its study on pain pathophysiology [122]. Thus, TRPV4 contributes to thermal and mechanical hyperalgesia [123,124], several peripheral preclinical and clinical neuropathies [125,126,127] and a preclinical model of migraine [128]. TRPV4 has been also implied in cystic fibrosis [129], human skin cancer [130], post burn pruritus, and liver and renal associated pathologies among other.
Interestingly, TRPV4 has gained medical and clinical interest since mutations in the TRPV4 gene can result in genetic disorders like Brachyolmia, Charco-Marie Tooth type 2C, spinal muscular artrophy and hereditary motor and sensory neuropathy type 2 [131]. The mutation of TRPV4 does not seem to modify directly the channel activity. However, it seems that it is the environment and the interaction of other factors that modulate oligomeraziation, trafficking and degradation of TRPV4, enhancing or reducing the activity of the channels [131].
2.4. TRPM3
Transient receptor potential melastatin-3 (TRPM3) is a recently included member of the thermoTRP family that belongs to the melastatin subfamily of TRPs. Intriguingly, TRPM3 is a heat sensitive channel with limited homology to the TRPV members [132]. Human TRPM3 has 57% sequence identity and 67% similarity with human TRPM1, and both genes encode a microRNA [133], therefore often TRPM3 is grouped with TRPM1 [134]. The cytosolic C-terminus contains the TRP-box and the typical coiled-coil domain of melastatin subfamily members, which is important for assembly for other TRPM, but not yet for TRPM3. Important domains for protein-protein interaction such as CaM and PIP2 binding domains are present in the N-terminus [135].
Several splice variants of TRPM3 are known which are divided in three groups depending on their first exon. TRPM3α (α1–α5) isoforms start with exon 1 and lack exon 2, while TRPM3β isoforms (β1–β17) start with exon 2. A third group is composed of isoforms starting with exon 3 [136]. This plethora of splice variants together with the microRNA seems to facilitate a fine tuning of the channel expression level. In contrast, alternative splicing in exon 24 leads to two different pores and influences cation selectivity [137].
TRPM3 channels are non-selective cationic channels activated by both physical (heat, voltage and hypotonic cell swelling) and chemical stimuli such as the neuroactive steroid pregnenolone sulfate, d-erythro-sphingosine or internal Ca2+ store depletion. However, less is known about the post-translation mechanisms that regulate TRPM3 channel activity. Indeed, there is no evidence of phosphorylation regulation through the well-known PKC and PKA, and there is limited information on the functional implication of calmodulin and PIP2 association.
The physiological and/or pathological role of TRPM3 still remains to be deeply elucidated since research on this channel has just started. Nevertheless, TRPM3 has recently drawn much attention since it has been described as a heat sensing channel expressed in nociceptors [132]. Expressed in a large number of small-diameter DRG and TG neurons, TRPM3 detects noxious heat, alike as TRPV1 but with more lower and broad range of temperatures, and is involved in heat-, but not cold-, mediated hyperalgesia [132,138]. Unlike with TRPV1, inhibition or knockout of TRPM3 does not affect the homeostasis of body temperature. Most importantly, TRPM3-deficient mice exhibit deficits in responses to noxious heat and development of inflammatory heat hyperalgesia [132,138].
TRPM3 expression is also found in the brain [139,140], eyes, reproductive system, smooth muscle cells, pituitary gland, adipose tissue, cardio vascular system [2], and pancreas [132,141].
2.5. TRPM8
In contrast to the above described thermoTRPs, transient receptor potential melastatin 8 (TRPM8), also known as Trp-p8, is a 1104 aa cold-sensitive ion channel [142]. While the C-terminus appears involved in temperature mediated gating [143] and contains the TRP-domain, the N-terminus harbours the four conserved regions with homology sequences (MHR) characteristic of the TRPM subfamily [144]. Serine residues on the S3–S4 extracellular linker are important for channel gating [145], and there are hints that S4 and the S4–S5 linker are part of the voltage sensor [146]. While the first 39 aa are not crucial for channel activity [144], the disulphide bond between Cys929 and Cys940 is essential [147]. Interestingly, residues 40–86 seem involved in localization of the TRPM8 tetramer in the plasma membrane and in vivo stabilization [144,148].
The typically observed temperature threshold for recombinant TRPM8 activation (17–25 °C) [148,149,150] does not account for its function in vivo where it responds to skin temperatures [151]. The original threshold is only recovered in primary sensory neurons but not in other neurons, that suggests a regulation via endogenous factors expressed solely in these neurons [152]. Activation of TRPM8 leads to a negative shift in the voltage-dependent activation that facilitates channel opening at negative potentials [153,154,155]. Apart from cold temperature, a broad range of natural and synthetic compounds activate TRPM8: menthol and derivatives, eucalyptol, icilin, hydroxycitronellal, heliotropyl acetone, helional, geraniol, and linalool [153,156,157].
TRPM8 can be regulated by various intracellular secondary messengers and signalling pathways that allow a fine tuning of channel activity by the cellular environment [158]. A well-known regulator of TRPM8 activity is PIP2, which directly interacts with the TRP domain and modulates cold, menthol or voltage activation [159,160]. Ca2+-dependent CaM provides a rapid desensitization mechanism of TRPM8 [161]. Besides, Ca2+ influx through TRPM8 activates PLC isoform which hidrolyzes PIP2 and decreases channel activity, being responsible of Ca2+-dependent tachyphylaxia [159,162,163]. Therefore, activation of any receptor that activates PLC and promotes PIP2 depletion may cause both inactivation and the desensitization of TRPM8, such as phorbol ester, bradykinin, or nerve growth factor (NGF) [164,165,166]. Additionally, although yet controversial, activation of GPCR/Gi followed by adenylate cyclase (AC)/cAMP/PKA or cPLA2 phopholipase2-arachidonic acid pathway results in a decrease in channel function [167]. On the contrary, positive regulation of TRPM8 channel activity can be achieved through Gs/AC/cAMP/PKA signalling pathway [168] or by the main products of iPLA2 [169]. In this regard, it should be mentioned that basal activity of TRPM8 depends on phosphorylation by PKA on residues Ser9 and Thr17 [168]. Other inhibitors of TRPM8 function include GCPR/Gαq, endovanilloid anandamide and several cannabinoids [170].
TRPM8-expressing fibres innervate tissues such as skin, mucosa or visceral organs like the colon [171,172]. For a long time, cooling or topical application of menthol has been used for their analgesic effects. This evidence, together with TRPM8 presence in DRG and TG neurons [173,174], suggests a potential role of TRPM8 in thermal sensing and nociception. In contrast to the pro-algesic activity of other ThermoTRPs, TRPM8 mediates analgesia in neuropathic and inflammatory pain models [175,176,177,178]. TRPM8 is a major integrator of cold and menthol sensitivity and allodynia, and it is required for behavioral responses to innocuous cool, noxious cold, injury-evoked cold hypersensitivity, cooling-mediated analgesia, and thermoregulation [179,180].
Other tissues expressing TRPM8 are skeletal and smooth muscle, epithelia of the prostate, lungs, bladder and urogenital tract [181,182], although the role of TRPM8 in these tissues is still not understood. Recent observations of TRPM8 expression in vagal neurons innervating bronchopulmonary tissue have brought up TRPM8 as a drug target for various respiratory disorders [183]. Because up-regulation of TRPM8 expression has been observed in various types of cancer including prostate, breast, lung and colon [184], this cold thermorTRP has been proposed as a marker for cancer tracing [185]. Other diseases where TRPM8 is involved include dry-eye-syndrome [186,187], amyloidotic polyneuropathy [188], and diseases of the urogenital tract like overactive bladder syndrome and pain bladder syndrome [177,181].
2.6. TRPA1
Transient Receptor Potential Ankyrin 1 (TPRA1) is the only member of the ankyrin subfamily found in mammals. Originally called ANKTM1, TRPA1 was identified by a homology search for ankyrin domains [189]. Its structure is distinct from other TRP channels as it is the only member with an extended ankyrin repeat domain in the N-terminus [190]. The N-terminus (half the size of the protein) contains between 14 and 18 ankyrin repeats that probably are important for protein-protein interactions and insertion of the channel into the plasma membrane [96,191]. Because of the unusual large N-terminal ankyrin repeat domain, it is also possible that TRPA1 is involved in mechanosensation, in which the N-terminal could act as a link between mechanical stimuli and channel gating [96]. The N-terminal region contains a large number of cysteines, some of which can form a complex network of protein disulphide bridges within and between monomers [96,192,193], targets for electrophilic TRPA1 activators, but cysteines outside the N-terminal region may also contribute to channel gating [194,195]. The N- and C-termini have been suggested to contain binding sites for Ca2+ that can both sensitize and desensitize TRPA1 [2,196,197,198,199,200].
TRPA1 is a non-selective cation channel permeable to Ca2+, Na+ and K+. TRPA1 was initially suggested to function as a detector of noxious cold, less than 17 °C [3,201], and to account for a component of cold sensitivity not mediated by TRPM8 [189]. Although this hypothesis remains controversial, current evidence suggest that TRPA1 plays, little, if any, role in acute cold sensation but more likely contributes to injury-evoked cold hypersensitivity [202,203,204,205]. Irrespective of this cold controversy, there is a widespread agreement that TRPA1 plays an important role in chemonociception by serving as a detector for chemical irritants that elicit acute and inflammatory pain [206,207,208]. TRPA1 is activated by a diverse assortment of pungent or irritating reactive chemical compounds including those found in mustard oil (MO, allyl isothiocyanate), cinnamon oil (cinnamaldehide), gas exhaust (acrolein), raw garlic and onions (allicin) and formalin (formaldehyde) [189,197,209,210,211,212] or endogenous compounds such as H2O2, the alkenyl aldehydes 4-hydroxynonena, 4-oxo-nonenal, 4 hydroxyhexenal and the cyclopentenone prostaglandin, 15-deoxy-δ-(12,14)-prostaglandin J(2) 15d-PGJ2 [213,214]. All of them activate the channel by covalent modification of cysteines and lysines in the N-terminus eliciting a painful burning or prickling sensation [215,216].
TRPA1 can be directly activated by Ca2+ which exerts dual effects on the channel, including initial activation or potentiation, followed by long-lasting inactivation [197,217]. It has been suggested that Ca2+ exerts its effects on TRPA1 by interacting directly with an intracellular domain(s) of the channel [200,218]; however, the underlying mechanism remains controversial [191,200]. In addition to activation by PLC-evoked release of Ca2+ from intracellular stores, TRPA1 may also amplify responses initiated by other Ca2+-permeable channels, such as TRPV1, further promoting sensitization to thermal or chemical stimuli [208,211].
Pro-inflammatory and pain producing agents, such as bradykinin, histamine, prostaglandins, and trypsin, acting on GPCRs stimulate via PLC or AC, directly or indirectly activate TRPA1 [219]. The downstream products of PLC-activation do not contribute to sensitization of TRPA1. Thus, the remaining possible mechanism is the consequence of membrane PIP2 hydrolysis by PLC activation. A similar mechanism was also found in B2R-induced potentiation of TRPA1 [217]. Although, recent pharmacological studies further suggest that some GPCRs, such as the chloroquine activated itch receptor MrgprA3, stimulates TRPA1 through a mechanism involving direct coupling to Gβγ subunits [220].
In sensory neurons from TG, DRG and ND, TRPA1 is expressed in both peptidergic and non-peptidergic neurons [206,221,222]. TRPA1 is mostly found in a subpopulation of TRPV1-positive neurons, but non-TRPV1-containing neurons expressing TRPA1 exist. TRPA1-positive C-fibres densely innervate the skin, airways and gastrointestinal tract. This location, along with the robust activation of TRPA1 by inflammatory mediators and the ability of the channel to promote inflammation, have implicated TRPA1 in pain perception, sensory hyperreactivity, or disease progression of arthritis, asthma, dermatitis, inflammatory bowel disease, and pancreatitis. Finally, TRPA1 has been also related in thermal and mechanical allodynia caused by many neurotoxic cancer chemotherapies together with TRPM8 and TRPV1 [2,211]. Outside sensory neurons, TRPA1 is found in epithelial cells, melanocytes, mast cells, fibroblasts, odontoblasts, and enterochromaffin cells and β-cells of the Langerhans islets. Notably, many of these cells have sensory properties and crosstalk with nearby nociceptors [2].
3. Trafficking of ThermoTRP
The function of any membrane protein can be up- or down-regulated by altering the number expressed at the cell surface. This type of regulation can involve synthesis of new protein or a change in the rate of degradation. However, a rapid change can be achieved by insertion into and retrieval from the surface of ready-synthesized molecules stored in intracellular compartments located beneath plasma membrane [223]. In general, like in all membrane proteins, surface expression of thermoTRP can be regulated in several manners with the final aim of keeping channel homeostasis (Figure 2). New channels are recruited to the cell surface when required, and the pre-existing channels are recycled by endocytosis/exocytosis, regulated by ubiquitination, internalized to proteasomal degradation [224] or degradated by lysosome pathway from multivesicular bodies [225].
There are two mechanisms involved in the exocytotic trafficking of any membrane receptor to the plasma membrane: the constitutive and the regulated exocytosis pathways. After folding, membrane proteins are assembled in the ER and Golgi cisternae, then sorted into vesicles and finally trafficked to the membrane. The constitutive route keeps membrane homeostasis and turnover through a fine tuning of delivery and retrieval of membrane components. This pathway does not respond to signals but can be regulated by a cascade of protein-protein interactions. On the contrary, regulated exocytosis is produced in response to stimuli that provokes mobilization, docking and fusion of intracellular pool of vesicles located near the plasma membrane. This pathway is commonly used in secretory and excitable cells for release of signalling molecules, and excellent reviews have been published on this topic [223,226,227,228,229,230].
Molecular mechanism involved in both types of exocytosis involved a cascade of protein-protein interactions [223,230]. Accordingly, it is important to better understand which interactions are present and how they affect channel expression and function. ThermoTRPs interact with a large number of signalling molecules, scaffolding and trafficking proteins [2,3,13]. Some of these interactions has been already shown essential for correct sorting, transport and delivery of the receptors to the membrane and for their functionality.
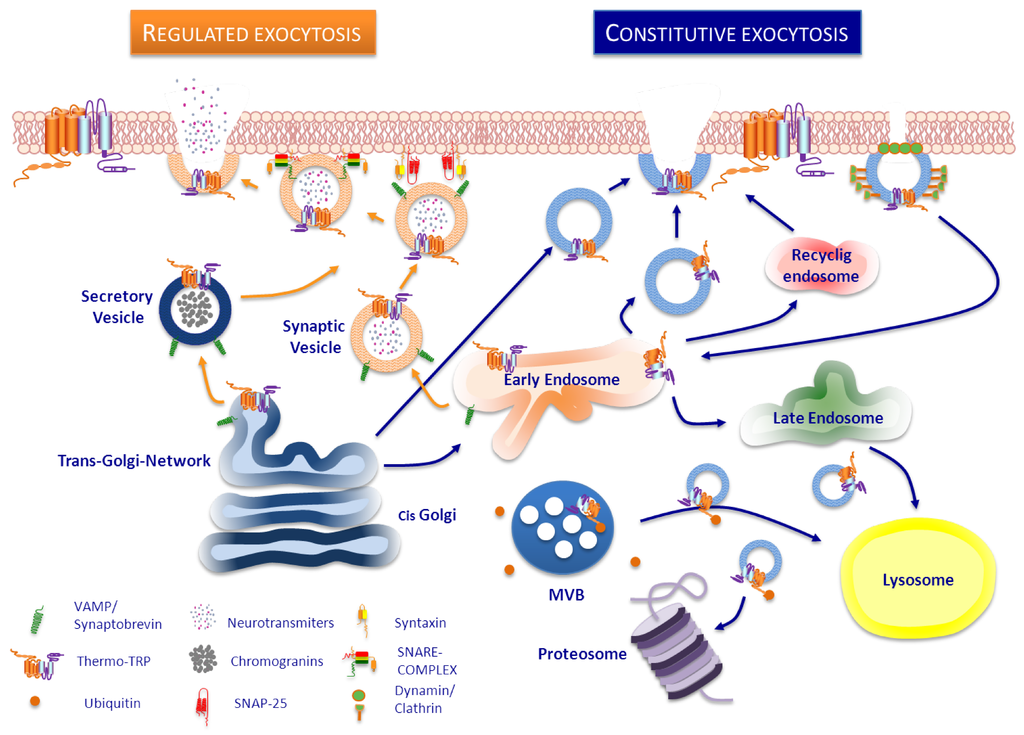
Figure 2.
Trafficking pathways of ThermoTRP channels to the plasma membrane. A neuron is illustrated here as example. (Left side). Regulated vesicles such as synaptic vesicles, filled with neurotransmitters, or secretory vesicles such as large dense core vesicles, that contain neuropeptides, can also store thermoTRP channels which are delivered to the cell surface upon stimulation by secretagogues. Docking, priming and fusion of these vesicles into the plasma membrane is a SNARE-complex and Ca2+-dependent process. (Right side). In the constitutive pathway, thermoTRP channels reach plasma membrane by a constant exocytosis from the trans-Golgi directly or via early endosomes (EE). Membrane levels of thermoTRP channels can be also regulated through the classic clathrin-dependent endocytosis pathway, in which dynamin form a ring around the neck of clathrin-coated pit, leading to formation of clathrin-coated vesicles which transport membrane proteins to EE. Once there, proteins could be sorted either into late endosomes and lysosomes or recycled to cell surface via recycling endosomes. ThermoTRP channels destined to degradation could be sorted into intraluminal vesicles of a multi vesicular body (MVB) and lead to lysosome or could be ubiquitinated and lead to proteasome.
3.1. TRPV1
With the raising interest of TRPV1 as therapeutic target, this channel was the first, and without any doubt, the most studied thermoTRP. Although, clinical studies are already ongoing on this target, there is still limited and fragmented available information on TRPV1 trafficking. Despite the increasing number of potential TRPV1 interaction partners identified using yeast two-hybrid screening, proteomic screening or immunoprecipitation followed mass spectrometry, the mechanisms and regulatory pathways that control TRPV1 traffic remain to be further elucidated. Among all these interactions, better knowledge of those interactions involved in TRPV1 trafficking could open new potential therapeutic approaches [2,3,13,231].
Ion channels such as TRPV1 are usually key components of macromolecular assemblies that act as functional protein networks [11]. One important component of the TRPV1 signal complex is the γ-amino butyric acid A-Type (GABAA) receptor associated protein (GABARAP). Associated with TRPV1 in HEK293 and rat DRGs, GABARAP enhances TRPV1 channel expression, traffic and clustering on the plasma membrane. In addition, it modulates TRPV1 functional activity at the level of channel gating and desensitization [232]. GABARAP slightly augments TRPV1 internalized fraction without affecting the kinetics decay, indicating that this anchor protein increases TRPV1 expression and regulates the receptor cycling between the plasma and the intracellular compartments without altering the receptor degradation rate. Additionally, the presence of GABARAP selectively increased the interaction of tubulin with the C-terminal domain of TRPV1, and both proteins are known to bind independently to microtubules [233,234]. Functionally, TRPV1 interaction with tubulin is increased drastically by GABARAP, which promotes and stabilizes the formation of the complex. Microtubule disruption and GABARAP had an attenuating effect on TRPV1 currents activated by capsaicin, and on the rate of desensitization. Thus, it seems that microtubule dynamics is important regulating these signalling complexes.
TRPV1 interacts physically with tubulin dimer as well as with polymerized microtubules [233], and shows multiple tubulin-binding sites [232,235]. There is a cross-talk between TRPV1 and microtubule cytoskeleton at various levels [236], although the exact mechanism remains still unclear. TRPV1 activation results in rapid disassembly of microtubules [235], while microtubule cytoskeleton helps to form TRPV1 tetramers from dimers at the membrane and preserves the functionality of TRPV1 [237]. Interestingly, upon disruption of microtubule dynamics and disassembly by nocodazole, the interaction with TRPV1 is lost inducing receptor self-aggregation with partial loss of activity, which may be a consequence of an interference with the vesicular trafficking of TRPV1.
Another protein recently described to regulate TRPV1 transport is the cyclin-dependent kinase 5 (Cdk5), which is involved in many cellular processes of the nervous system, including vesicle transport from the Golgi to neurites [238], and kinesin-driven motility [239]. Cdk5 positively controls TRPV1 membrane transport mediating KIF13B-TRPV1 association, without altering the total amount of TRPV1 [240]. Cdk5-dependent phosphorylation of KIF13B at Thr-506 is in part necessary for Cdk5-mediated motor-cargo association and contributes to efficient transport of TRPV1. Active Cdk5 promotes TRPV1 anterograde transport in vivo after inflammation [240], and regulates heat sensitivity and decreases of capsaicin-evoked calcium-influx in primary sensory neurons [241].
Finally, a new association of TRPV1 with Kvβ2 has been confirmed by co-immunoprecipitation assay in heterologous recombinant system in HEK as well as mouse DRGs. Kvβ2 exert chaperone-like effect resulting in an increased cell surface expression of TRPV1 associated with an enhancement in capsaicin sensitivity. These results suggest that this interaction may play a role in TRPV1 trafficking to the plasma membrane and could provide a structural basis of a spatially restricted repolarization pathway [231].
All of these reported protein-protein associations and signalling pathways evidence an enhanced exocytosis of TRPV1 to the plasma membrane; however, it is also possible to keep an increased surface expression of the channel by inhibiting its internalization. Persistent exposure to TRPV1 receptor agonists, such as the vanilloid capsaicin, makes cells partially or totally insensible to subsequent stimuli, a process known as desensitization. TRPV1 activation by capsaicin, temporary makes the channel unable to respond to the vanilloid or other agonists. Acute TRPV1 desensitization by capsaicin is a process completely dependent on Ca2+ and specific intracellular signalling cascades, mainly phosphorylation/dephosphorylation processes [242]. In contrast, capsaicin induces long-term receptor down-regulation by modulating the expression level of the channels promoting receptor endocytosis and degradation [243]. This process seems to be modulated by PKA-phosphorylation and it is mediated by clathrin- and dynamin- independent endocytosic mechanism.
Similarly, the E3-ubiquitin ligase Myc-binding protein-2 (MYCBP2, also known as PAM) regulates TRPV1 internalization through inhibition of P38MAPK signalling [244]. MYCBP2 is mainly expressed in peripheral and central nervous system, and it negatively controls neuronal growth and synaptic transmission [245]. Although loss of MYCBP2 seems not to cause fundamental changes in basal functions of sensory neurons, secondary inflammatory thermal hyperalgesia is significantly lower in MYCBP2 deficient mice [244]. There is an altered receptor trafficking in MYCBP2 deficient DRGs, and consecutive capsaicin-induced desensitization is not present in these nociceptors due to a decrease in TRPV1 internalization. Mechanistically, loss of MYCBP2 causes constitutive p38 MAPK activation that prevents activity-induced retrival of TRPV1 from the plasma membrane [246].
The above described protein-protein interactions mainly, but not exclusively, contribute to the constitutive trafficking of TRPV1. However, TRPV1 association with other proteins is involved in vesicle exocytosis upon stimulation by rapidly increasing the levels of this thermoTRP channel in the plasma membrane. In fact, this mechanism provides the basis for a rapid modulation of nociceptive TRPV1 responses and fine tuning of channel activity.
One of the first evidence was the finding that the N-terminus of TRPV1 associated with two well-known vesicular proteins, Snapin and Synaptotagmin IX, which bind to SNARE proteins and participate in neuronal regulated exocytosis [247]. Co-distribution of TRPV1 with Synaptotagmin IX and the vesicular protein synaptobrevin evidences TRPV1 located in vesicles near to the membrane surface. In this regard, PKC activation was able to induce a rapid delivery of functional TRPV1 channels to the plasma membrane from these vesicles, and the botulinum neurotoxin A (BoNT/A) blocked PKC-induced membrane translocation of this channel [247].
Subsequent findings demonstrate that TRPV1 membrane translocation can be induced by activation of several signalling cascades. For instance, activation of AC and PKA can lead also to transport of an intracellular pool of inactive TRPV1 monomers to the plasma membrane where they function as tetramers, without affecting total expression of the channel [248]. Another well-defined example is NGF that through activation of PI3K and Scr kinase, induces phosphorylation of TRPV1 and rapid insertion of new TRPV1 into the membrane surface [249]. This mechanism has been also shown for fibronectin-induced translocation of TRPV1 to the membrane of primary sensory neurons [250]. However, direct interaction of PI3K with TRPV1 can also promotes NGF-induced fast TRPV1 trafficking to the plasma membrane [251]. NGF is also able to promote long term up-regulation of TRPV1 translation and transport to the peripheral neuronal membrane through activation of P38MAKP pathway [252]. Interestingly, equivalent molecular mechanisms are shared by other stimuli such as insulin and insulin growth factor-1 (IGF-1) inducing enhancement of TRPV1 membrane trafficking through PI3K [253]. Based on these data, it is evident that activation of different signalling cascades promotes regulated exocytosis of a vesicle reservoir located near the plasma membrane. These vesicles contain new TRPV1 channels and they are transported to the membrane through SNARE-dependent mechanism highly sensitive to Ca2+. Consequently, it was found that TRPV1 membrane recruitment increased by several stimuli such as NGF, ATP or IGF-1 can be abolished by treatment with a botulinomimetic peptide, demonstrating how different signalling pathways convey only on regulated exocytosis [12].
In this line, the scaffolding protein A-kinase Ancoring Protein 79 (AKAP79, AKAP150 murine) allows the integration of several regulatory pathways. This anchor protein interacts with TRPV1 and has binding sites for PKA, PKC and calcineurin, forming a signalling complex that can regulate TRPV1 channel [254]. Overexpression of AKAP79/150 enhanced trafficking of TRPV1 to the membrane and conversely its down-regulation reduced TRPV1 membrane expression, although not completely [254]. Nevertheless, the main role of this association is on membrane trafficking of TRPV1 upon stimulation since the crossroad of several signalling cascades end up with TRPV1 translocation and phosphorylation of the channel. Interaction of AKAP79/150 has been also shown to other thermoTRP channels, especially TRPV4 but also TRPA1, TRPM8, TRPV2 or TRPV3.
Overall, it is important to note that there are two key features crucial for TRPV1 trafficking to the plasma membrane: one is phosphorylation and the other one is its mobilization through SNARE-dependent exocytosis.
Transport of TRPV1 in Neurons
TRPV1 is transported to the neuronal ends by different entities. The diffuse pattern of TRPV1 mostly matches well with the cytoplasmic and/or small vesicular distribution, but it can be also detected in structures with different and bigger size, mobility and recycling properties [255]. TRPV1 co-localizes with both pre- and post-synaptic proteins, and co-movements of TRPV1 with synaptic proteins, such as synaptophysin, can be observed. TRPV1 is detected in synaptic transport vesicles, and in transport packets within filopodia and neurites. Recycling and/or fusion of these vesicles can be rapidly modulated by TRPV1 activation, and as translocation of new TRPV1 channels to the plasma membrane of growth cones, can be measured immediately after addition of the endogenous TRPV1 agonist N-arachidonyl-dopamine [255,256]. This is mostly due to the translocation and subsequent fusion of readily available vesicles containing TRPV1. As a consequence it is also possible to define TRPV1 as a synaptic protein that regulates vesicle recycling [256,257].
It is well known that TRPV1 is expressed in peptidergic neurons that contain CGRP and SP. These neuropeptides are stored in LDCVs, and activation of TRPV1 induces release of both neuropeptides. Although still awaits demonstration, akin to other channels such as TRPV2 and DOR [258], it is reasonable to hypothesize that in peptidergic sensory neurons TRPV1 could be at least partially sorted into LDCVs. Therefore, as described for cortical neurons with synaptic vesicles [256], activation of TRPV1 not only would promote neuropeptides release but it also increase TRPV1 plasma membrane translocation. The enhancement of TRPV1 trafficking through a regulated exocytosis mechanism could be also the result of exogenous stimuli able to promote vesicle docking and fusion facilitating rapid modulation of neuronal excitability.
3.2. TRPV2
The analysis of protein-protein interactions with TRPV2 has revealed few insights in the mechanisms contributing to trafficking of this thermoreceptor. The only reported evidence is the association of TRPV2 with the recombinase gene activator protein (RGA) that tightly controls plasma membrane translocation of the channel in mast cells [259]. RGA is an intracellular transmembrane protein that transiently interacts with TRPV2 during cellular glycosylation. Although overexpression of RGA promotes an increase of basal TRPV2 in plasma membrane [260], RGA does not accompany TRPV2 to the cell surface. RGA is localized to a vesicular subcompartment of ER/Golgi apparatus functioning as a chaperone like and just promoting membrane trafficking of TRPV2 [259]. RGA seems to function as targeting protein during TRPV2 maturation controlling channel surface levels rather than a functional accessory subunit. Therefore, RGA interaction with this thermoTRP is a native complex determinant in biosynthesis and early trafficking of this channel [259,260].
An increased response of TRPV2 to certain factors has been studied through a mechanism that involves regulated access of new channels to the plasma membrane [260,261]. An elevation of cAMP is sufficient to drive TRPV2 to the plasma membrane of mast cells, thus any stimuli able to increase this secondary messenger may regulate TRPV2 trafficking. One of the first reported stimuli was IGF-1, which is able to promote TRPV2 recruitment to the membrane from the intracellular pools [262]. However, this is contradictory with the recent findings from Moiseenkova-Bell group, in which TRPV2 is reported to primarily reside in intracellular membranes and its subcellular distribution is not sensitive to IGF-1 treatment [263].
Another example is the exogenous application of insulin or high concentration of glucose which also stimulates translocation of TRPV2 to the plasma membrane in pancreatic beta cells [261]. The rapid exocytotic response induced by insulin is accompanied by membrane insertion of TRPV2 in a PI3K-dependent manner [261]. Similarly, depolarization of beta cells also induces translocation of TRPV2 to the membrane when exposed to K+ leading to increased entry of Ca2+ followed by enhanced insulin secretion [264]. Through a similar pathway, characterization of TRPV2 function and regulation in macrophages has revealed that TRPV2 translocation to the plasma membrane can be also promoted by the chemotactic peptide formyl Met-Leu-Phe, which is a PI3K-dependent mechanism sensible to pertussis toxin [265].
Alike TRPV1, but with lower affinity, TRPV2 interaction with the scaffold protein AKAP79/150 has been also reported [254]. Although until now no further research has been done on the functional implication of this association, we could speculate a comparable regulation as for TRPV1 whereby activity as well as translocation of the receptor to the membrane was modulated by this anchor protein.
The few data described above report that TRPV2 can be inserted to the plasma membrane though regulated exocytosis from an intracellular vesicular reservoir. This suggests that part of TRPV2 is partially sorted in appropriate competent vesicles. Unfortunately, there is still not enough evidence supporting this assertion, but recently TRPV2 has been associated to large-dense core vesicles from spinal cords homogenates by LC_MS and immunostaining. Further research is required to understand better the mechanism behind TRPV2 regulated exocytosis [258].
3.3. TRPV4
Mutation in TRPV4 gene results in some genetic disorders raising the medical and clinical interest of this thermoTRP channel. It seems that the environment and the interaction of other factors modulate oligomeraziation, trafficking and degradation of TRPV4, modifying channel activity [131].
The expression of TRPV4 in the plasma membrane depends on different factors. A complete and appropriate sequence of TRPV4 is required for channel membrane insertion. The C-terminal domain allows oligomerization of the channel and it is important not only for its trafficking and surface insertion but also for functional properties like selectivity and gating. In more detail, TRPV4 oligomerization promotes sorting from the ER to the cell surface, and complete deletion or site-specific mutations prevent membrane translocation resulting in partial or total retention of TRPV4 in the ER [266]. Consistently, new evidence further support the critical role of C-terminus in TRPV4 protein folding and trafficking. TRPV4 channels lacking 838–857 residues remain misfolded and fail to reach the Golgi apparatus [267]. These mutated channels suffer complex glycosylation and maturation, being subjected to degradation through the ER-associated degradation dependent pathway. On the contrary, mutation of Asn-651 into Gln shows an increase in constitutive membrane trafficking of TRPV4 without affecting overall channel expression in the cell. Since Asn-651 is located in the consensus N-linked glycosylation motif between S5 and S6, these results suggest that glycosylation of TRPV4 on this residue could influence membrane trafficking of the channel [268].
Several protein-protein interactions can tightly control the level and insertion of TRPV4 into the cell surface. For instance, the reticulum associated protein OS-9 interacts with the N-terminal tail of TRPV4 and facilitates proper folding and tetramer formation. OS-9 preferably binds to monomers and immature variants of TRPV4 and appears to protect TRPV4 from precocious ubiquitination and associated degradation. OS-9 acts as an auxiliary protein for TRPV4 maturation and impedes the release of the channel from the endoplasmic reticulum reducing its plasma membrane levels [269].
Another example is the novel association between the water channel AQP2 and TRPV4 that seems critical for Ca2+ entry during hypotonicity in renal cortical collecting duct cells [270]. TRPV4 plasma membrane expression is low under isotonic conditions, whereas hypotonic stimulation increases TRPV4 only in AQP2 expressing cells. TRPV1 membrane increase is inhibited by colchicine, a microtubule disrupting agent, showing the involvement of trafficking process. In this regard, TRPV4 is intimately associated with actin in CHO cells [271] through the C-terminus [272]. The phosphorylation in Ser-824 is a control point for its plasma insertion via the regulation of proper subcellular localization, being required for TRPV4 activity and protein stability [273]. Other researchers have previously reported that the TRPV4 phosphorylation at Tyr-110 residue is important in regulating its abundance at the plasma membrane [274]. Finally, TRPV4 can be also functionally down-regulated by WNK4 kinase via a decrease in the cell surface expression without affecting total abundance of TRPV4 [275]. Although a direct interaction between TRPV4 and WNK kinase has been not detected, a site mutation on WNK4 can abrogate the inhibitory effect on TRPV4 function and trafficking. However, it is necessary further investigation into the exact mechanism.
As mentioned for TRPV1, endocytosis can also modulate membrane expression of TRPV4 channels. The atrophin-interacting protein 4 (AIP-4) facilitates ubiquitination of TRPV4 and renders the channel available for endocytosis [276]. This process reduces TRPV4 surface expression and the internalized proteins are degraded by lysosomes. Mono or multi-ubiquinated TRPV4 channels are located in a vesicle pool below the plasma membrane, and overexpression of AIP-4 results in a reduction of TRPV4 basal activity due to an increased presence of TRPV2 in vesicles. On the contrary, interaction of TRPV4 with PACSIN 3 isoform regulates endocytosis enhancing the ratio of plasma membrane-associated versus cytosolic TRPV4 membrane [277]. In general, PACSINs are proteins implicated in synaptic vesicular membrane trafficking and regulation of dynamin-mediated endocytotic processes [278]. Co-expression of PACSIN 3 is suggested to increase cell surface expression of TRPV4 modulating its subcellular localization. A similar shift is also observable when dynamin-mediated endocytotic process is blocked. The interaction of PACSIN 3 is through the N-terminus of TRPV4 and it is required the specific proline-rich domain upstream of the ankyrin repeats. In conclusion, PACSIN 3 acts as a TRPV4 auxiliary protein that affects subcellular localization and modulates TRPV4 function in a stimulus-specific manner [98].
There are almost no data on stimulus-induced trafficking of homomeric TRPV4 channels. Xin Ma et al. [279] was the first group to identify the vesicular trafficking of heteromeric TRPV4-C1 channels to plasma membrane due to depletion of intracellular Ca2+ stores in HEK and vascular endothelial cells. Depletion by thapsigargin or by physiological agonists, such as bradykinin and ATP, caused an enhanced membrane translocation of TRPV4-C1 due to vesicular trafficking which was abolished by brefeldin A, but had little or no effect on TRPV4 or TRPC1 homomeric channels. One speculation could be that TRPV4-C1 heteromers are preferentially packaged into vesicles, thus their trafficking is more subjected to regulation by Ca2+ store depletion. In this regard, recent evidence indicates that the functional status of homomeric TRPV4 channels in the distal nephron is regulated by two distinct signalling pathways [110]: phosphorylation through PKC-dependent pathway increases the TRPV4 activity, while activation of the PKA-dependent cascade additionally promotes trafficking and translocation of TRPV4 to the apical membrane.
3.4. TRPM3
TRPM3 is a recently studied thermoTRP channel, thus until now there are only limited studies reporting some insights into the features and/or regulation of TRPM3 trafficking to the plasma membrane. Loss of 18 aa residues encoded by exon 13 partially diminishes channel insertion in the membrane [280]. This region, named the indispensable channel function (ICF), is required for TRPM3 channel function. Splicing variants within exon 13, such as TRPM3α7, are common in a variety of cell types and tissues. In fact, transcripts lacking ICF, are detectable in all TRPM3 expressing tissues, such as brain or DRG. These isoforms seem to interact, in a small degree, with other isoforms of TRPM3, possibly forming tetramers, but avoiding plasma membrane insertion. Apparently, ICF stabilizes interaction of TRPM3 subunits, essential for protein folding and allows cell surface insertion. It is interesting to mention that ICF region is also present in other members of the TRPM family such as TRPM8. Indeed, deletion of ICF also decreases expression of TRPM8 in cell surface, but in contrast the remaining channels in the plasma membrane are active.
3.5. TRPM8
Little is known so far about membrane trafficking of TRPM8. The only proposed mechanism reported that seem to regulate the transport and stabilization of TRPM8 to the plasma membrane are glycosylation and tetramerization [148].
Structurally, TRPM8 channels assemble as multimers using the putative coiled-coil region located in the C-terminus. Single-point mutation in this region, Leu-1089 into Pro, disrupts this interaction and reduces oligomerization and surface expression of TRPM8, without affecting total protein. Interestingly, although some monomers are able to reach plasma membrane, they are not able to form functional channels [148]. Within the N-terminal domain, two distinct regions have been recently identified, and they differentially contribute to channel activity and proper folding and assembly [281]. Deletion of region encompassing positions 40 to 60 aa is a key element in the proper folding and assembly of TRPM8, and augments responses to cold and menthol. In contrast, different deletions and site-directed mutations within this region rendered channels with an impaired function that are retained within the endoplasmic reticulum. Therefore, the initial region of the N-terminus is critical for the proper biogenesis of this thermoTRP channel.
N-glycosylation of TRPM8 takes place in Asn-934 in the extracellular loop between S5 and S6 near the pore region. First studies, reported that mutation of this residue into a glutamine reduced TRPM8 levels at the cell surface together with a concomitant reduction in channel activity [147,148]. These data also suggested that glycosylation was not an essential mechanism for TRPM8 to exit from the Golgi, since some channels still reach the plasma membrane. However, the impact of glycosylation on TRPM8 trafficking seems not so clear, since more recent reports have not been able to show any significant differences in the surface expression of this unglycosylated TRPM8 channels, although consistently temperature and menthol sensing is reduced [282,283].
Another proposed mechanism that could affect trafficking of TRPM8 is palmitoylation. Protein palmitoylation is a common post-traslational lipid modification which plays an important role in protein trafficking. TRPM8 has been identified to be efficiently palmitoylated by DHHC3 [284], although further research is needed to know whether palmitoylation plays some role and, if so, how it could affect membrane trafficking of the channel.
3.6. TRPA1
Many receptors and ion channels cycle between the plasma membrane and intracellular compartments, and the balance between membrane insertion and retrieval determines their surface abundance, and their activity [285,286]. Little is known about the TRPA1 trafficking, only Schmidt et al. [287] showed that the increased TRPA1 membrane availability observed upon MO application is at least partially dependent on SNARE-mediated vesicle-fusion and the other part might be via constitutive pathway. Only one work does reference to TRPA1 channel in the ubiquitination pathway showing that TRPA1 is a novel substrate for the de-ubiquitinating activity of the CYLD enzyme, and this de-ubiquitination causes a net increase in the cellular pool of TRPA1 proteins.
It is now believed that inflammation-mediated facilitation of trafficking of TRPV1 receptor channels plays an important role in the development and maintenance of inflammatory hyperalgesia. At the molecular level, it has been shown that TRPV1 surface expression could be regulated, in part, by SNARE-dependent exocytosis [12,247]. Wang et al. [206,217] reported that PKA and PLC signalling pathways sensitize MO-induced TRPA1 currents in vitro. Along this line, Schmidt et al. [288] showed that application of an activator of adenylyl cyclase and other of PLC-signalling, capsaicin, as well as activation of TRPA1 by MO significantly increased the levels of TRPA1 at the membrane of HEK cells and sensory neurons. The application of MO to DRG neurons induced an increase of the membrane capacitance which is indicative of the incorporation of new membrane channel into the neuronal surface. Tetanus toxin application selectively attenuated the response of cultured DRG to MO suggesting that the increased TRPA1 membrane availability observed upon MO application is at least partially dependent on SNARE-mediated vesicle-fusion. Recently, Burstein et al. [289,290] have proposed that, in addition to TRPV1, BoNT/A regulates SNARE-dependent cell-surface expression of TRPA1 channel.
As for TRPV1, the fact that TRPA1 trafficking to the plasma membrane can be induced by regulated exocytosis indirectly suggest the presence of this channel in secretory vesicles. In this regard, TRPA1 has been suggested to form a protein complex with secretogranin III [291]. Although the role of secretogranin III is poorly understood, it has been implicated in the biogenesis of secretory granules and this could indicate that TRPA1 might be stored in secretory vesicles.
4. A New Therapeutic Approach Targeting ThermoTRP Membrane Trafficking
The surface expression of ThermoTRP channels is controlled by constitutive and regulated vesicular trafficking. They are important mechanisms involved in assembly and trafficking of the channels to the plasma membrane and impact their function and regulation [285]. In fact, modulation of TRPV1 receptor surface density during pathological processes is nowadays considered as an interesting therapeutic approach for management of chronic pain, since an increase in trafficking is associated with the pathological state and does not seem to play a role in the physiological function of the channel [12,247].
Therefore, therapeutic strategies able to disrupt regulated SNARE-dependent exocytosis may have potential to treat pathologies with an associated increase in receptor membrane trafficking and vesicle exocytosis. The most evident and successful example is the use of botulinum neurotoxin (BoNT/A), known to disrupt SNARE complex formation by cleavage of the peripheral SNAP-25 protein, suppressing exocytosis of neurotransmitters, neuropeptides or receptors sorted in the vesicles [292,293]. BoNT/A modulates TRPV1 mobilization from the intracellular stores through regulated exocytosis [247,294]. Consistent with this, BoNT/A has been recently used to treat pain [295,296], showing beneficial effects in migraine [297,298], different neuropathic pain states [299,300], joint pain [301,302,303] and back pain [304]. This toxin reduces TRPV1 total expression inhibiting TRPV1 plasma membrane trafficking and renders TRPV1 vulnerable to ubiquitination and subsequent proteosomal degradation [289]. To improve the beneficial effects of BoNT/A, in the laboratory, protein engineering strategy has afforded several advances obtaining a potent, long-lasting and versatile inhibitor of exocytosis, a chimera toxin containing the protease of serotype E attached to the binding domain of serotype A with enhanced properties compared to native proteins, which could improve the treatment of chronic pain [305]. In this line, molecules that target regulated exocytosis and mimic botulinum neurotoxin effects offer an attractive therapeutic potential. Indeed, DD04107 is a palmitoylated peptide patterned after the N-terminus of SNAP-25 protein and is able to disrupt protein-protein interactions necessary to form SNARE-complex inhibiting regulated exocytosis [306]. This peptide is able to reduce inflammatory potentiation of TRPV1 in sensory neurons reducing TRPV1 membrane rapid translocation [12], and has demonstrated successful analgesic effect in animal models of inflammatory, neuropathic and bone-cancer induced pain [307].
5. Outlook
Modulation of the cellular expression of thermoTRP channels represents a fundamental cellular mechanism involved in the pathophysiology of these ion channels. The contribution of regulated exocytosis of these ion channels has been well documented for acute inflammatory sensitization of sensory neurons, although it remains yet elusive the underlying molecular details involved in the trafficking of these receptors to the cell membrane. Clearly, constitutive and regulated exocytotic routes coexist, at least in excitable cells, and appear to play in a concerted way to preserve the homeostasis of channel expression and to ensure fast recruitment of channels in response to an injury. However, the precise molecular components of both routes, as well as the type of vesicles used for the trafficking remain to be deciphered. Likewise, the contribution of the cellular context, which plays a key role defining the molecular composition of thermoTRP transport packets, remains largerly unknown. Furthermore, since the level of surface expression is finely tuned by the balance of exocytosis and endocytosis, understanding the molecular mechanism mediating receptor endocytosis appears also essential. Taken together, we have significantly progressed in this exciting field, although several questions are still requiring answer to fully understand the pathophysiological modulation of these ion channels and to identify novel targets for drug intervention that control their dysfunction by regulating the level of surface expression. A bright future in this research field is anticipated as the number of protein components potentially contributing to thermoTRP channel trafficking is still under intense scrutiny. The combination of complementary approaches, including in vivo life-imaging and systems biology will undoubtedly provide a comprehensive dynamical blueprint for the cellular trafficking of thermoTRPs and their modulation under different environmental conditions.
Acknowledgments
This work was supported by grants from el Ministerio de Economía y Competitividad (BFU2012-39092-C02-01, CONSOLIDER-INGENIO 2010 CSD2008-00005) and Generalitat Valenciana (PROMETEO/2010/046 and Santiago Grisolia Fellowship). The authors declare no competing financial interests.
Author Contributions
C.F.M., has revised the information on TRPA1, S.M., has revised the information on TRPV2 and TRPV4, C.J.W., has revised the information on TRPM3 and TRPM8, I.D., has revised the information on TRPV1 and has supervised the manuscript, and A.F.M. has supervised and edited the revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clapham, D.E.; Runnels, L.W.; Strubing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef]
- Flockerzi, V.; Nilius, B. TRPs: Truly Remarkable Proteins. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Nilius, B., Flockerzi, V., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin, Germany, 2014; Volume 222, pp. 1–12. [Google Scholar]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef]
- Latorre, R.; Brauchi, S.; Orta, G.; Zaelzer, C.; Vargas, G. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium 2007, 42, 427–438. [Google Scholar] [CrossRef]
- Schepers, R.J.; Ringkamp, M. Thermoreceptors and thermosensitive afferents. Neurosci. Biobehav. Rev. 2010, 34, 177–184. [Google Scholar] [CrossRef]
- Brederson, J.D.; Kym, P.R.; Szallasi, A. Targeting TRP channels for pain relief. Eur. J. Pharmacol. 2013, 716, 61–76. [Google Scholar] [CrossRef]
- Ferrer-Montiel, A.; Fernandez-Carvajal, A.; Planells-Cases, R.; Fernandez-Ballester, G.; Gonzalez-Ros, J.M.; Messeguer, A.; Gonzalez-Muniz, R. Advances in modulating thermosensory TRP channels. Expert Opin. Ther. Pat. 2012, 22, 999–1017. [Google Scholar] [CrossRef]
- Fernandez-Carvajal, A.; Fernandez-Ballester, G.; Devesa, I.; Gonzalez-Ros, J.M.; Ferrer-Montiel, A. New strategies to develop novel pain therapies: Addressing thermoreceptors from different points of view. Pharmaceuticals 2011, 5, 16–48. [Google Scholar] [CrossRef]
- Latorre, R.; Zaelzer, C.; Brauchi, S. Structure-functional intimacies of transient receptor potential channels. Q. Rev. Biophys. 2009, 42, 201–246. [Google Scholar] [CrossRef]
- Planells-Cases, R.; Valente, P.; Ferrer-Montiel, A.; Qin, F.; Szallasi, A. Complex regulation of TRPV1 and related thermo-TRPs: Implications for therapeutic intervention. Adv. Exp. Med. Biol. 2011, 704, 491–515. [Google Scholar] [CrossRef]
- Camprubi-Robles, M.; Planells-Cases, R.; Ferrer-Montiel, A. Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J. 2009, 23, 3722–3733. [Google Scholar] [CrossRef]
- Planells-Cases, R.; Ferrer-Montiel, A. TRP Channel Trafficking. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- De la Rosa, V.; Rangel-Yescas, G.E.; Ladron-de-Guevara, E.; Rosenbaum, T.; Islas, L.D. Coarse architecture of the transient receptor potential vanilloid 1 (TRPV1) ion channel determined by fluorescence resonance energy transfer. J. Biol. Chem. 2013, 288, 29506–29517. [Google Scholar]
- Kedei, N.; Szabo, T.; Lile, J.D.; Treanor, J.J.; Olah, Z.; Iadarola, M.J.; Blumberg, P.M. Analysis of the native quaternary structure of vanilloid receptor 1. J. Biol. Chem. 2001, 276, 28613–28619. [Google Scholar]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef]
- Tominaga, M.; Tominaga, T. Structure and function of TRPV1. Pflug. Arch. 2005, 451, 143–150. [Google Scholar] [CrossRef]
- Phelps, C.B.; Procko, E.; Lishko, P.V.; Wang, R.R.; Gaudet, R. Insights into the roles of conserved and divergent residues in the ankyrin repeats of TRPV ion channels. Channels 2007, 1, 148–151. [Google Scholar] [CrossRef]
- Lishko, P.V.; Procko, E.; Jin, X.; Phelps, C.B.; Gaudet, R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 2007, 54, 905–918. [Google Scholar] [CrossRef]
- Nagy, I.; Santha, P.; Jancso, G.; Urban, L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur. J. Pharmacol. 2004, 500, 351–369. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, S.; Yang, F.; Zheng, J.; Wang, K. Identification of a tetrameric assembly domain in the C terminus of heat-activated TRPV1 channels. J. Biol. Chem. 2011, 286, 15308–15316. [Google Scholar] [CrossRef]
- Garcia-Sanz, N.; Fernandez-Carvajal, A.; Morenilla-Palao, C.; Planells-Cases, R.; Fajardo-Sanchez, E.; Fernandez-Ballester, G.; Ferrer-Montiel, A. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J. Neurosci. 2004, 24, 5307–5314. [Google Scholar]
- Salazar, H.; Jara-Oseguera, A.; Hernandez-Garcia, E.; Llorente, I.; Arias-Olguin, I.I.; Soriano-Garcia, M.; Islas, L.D.; Rosenbaum, T. Structural determinants of gating in the TRPV1 channel. Nat. Struct. Mol. Biol. 2009, 16, 704–710. [Google Scholar] [CrossRef]
- Voets, T.; Nilius, B. Modulation of TRPs by PIPs. J. Physiol. 2007, 582, 939–944. [Google Scholar] [CrossRef]
- Cortright, D.N.; Szallasi, A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur. J. Biochem. 2004, 271, 1814–1819. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Caterina, M.J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R64–R76. [Google Scholar]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Cromer, B.A.; McIntyre, P. Painful toxins acting at TRPV1. Toxicon 2008, 51, 163–173. [Google Scholar] [CrossRef]
- Ahern, G.P.; Brooks, I.M.; Miyares, R.L.; Wang, X.B. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J. Neurosci. 2005, 25, 5109–5116. [Google Scholar] [CrossRef]
- Oh, U.; Hwang, S.W.; Kim, D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J. Neurosci. 1996, 16, 1659–1667. [Google Scholar]
- Planells-Cases, R.; Garcia-Sanz, N.; Morenilla-Palao, C.; Ferrer-Montiel, A. Functional aspects andmechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflug. Arch. 2005, 451, 151–159. [Google Scholar] [CrossRef]
- Devesa, I.; Planells-Cases, R.; Fernandez-Ballester, G.; Gonzalez-Ros, J.M.; Ferrer-Montiel, A.; Fernandez-Carvajal, A. Role of the transient receptor potential vanilloid 1 in inflammation and sepsis. J. Inflamm. Res. 2011, 4, 67–81. [Google Scholar]
- Price, T.J.; Flores, C.M. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J. Pain 2007, 8, 263–272. [Google Scholar] [CrossRef]
- Guo, A.; Vulchanova, L.; Wang, J.; Li, X.; Elde, R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 1999, 11, 946–958. [Google Scholar] [CrossRef]
- Szallasi, A.; Nilsson, S.; Farkas-Szallasi, T.; Blumberg, P.M.; Hokfelt, T.; Lundberg, J.M. Vanilloid (capsaicin) receptors in the rat: Distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res. 1995, 703, 175–183. [Google Scholar]
- Valtschanoff, J.G.; Rustioni, A.; Guo, A.; Hwang, S.J. Vanilloid receptor VR1 is both presynaptic and postsynaptic in the superficial laminae of the rat dorsal horn. J. Comp. Neurol. 2001, 436, 225–235. [Google Scholar] [CrossRef]
- Richardson, J.D.; Vasko, M.R. Cellular mechanisms of neurogenic inflammation. J. Pharmacol. Exp. Ther. 2002, 302, 839–845. [Google Scholar] [CrossRef]
- Mezey, E.; Toth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef]
- Cavanaugh, D.J.; Chesler, A.T.; Jackson, A.C.; Sigal, Y.M.; Yamanaka, H.; Grant, R.; O’Donnell, D.; Nicoll, R.A.; Shah, N.M.; Julius, D.; et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 2011, 31, 5067–5077. [Google Scholar] [CrossRef]
- Toth, A.; Boczan, J.; Kedei, N.; Lizanecz, E.; Bagi, Z.; Papp, Z.; Edes, I.; Csiba, L.; Blumberg, P.M. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res. Mol. Brain Res. 2005, 135, 162–168. [Google Scholar]
- Matta, J.A.; Ahern, G.P. TRPV1 and synaptic transmission. Curr. Pharm. Biotechnol. 2011, 12, 95–101. [Google Scholar] [CrossRef]
- Maione, S.; Cristino, L.; Migliozzi, A.L.; Georgiou, A.L.; Starowicz, K.; Salt, T.E.; Di Marzo, V. TRPV1 channels control synaptic plasticity in the developing superior colliculus. J. Physiol. 2009, 587, 2521–2535. [Google Scholar] [CrossRef]
- Starowicz, K.; Cristino, L.; Di Marzo, V. TRPV1 receptors in the central nervous system: Potential for previously unforeseen therapeutic applications. Curr. Pharm. Des. 2008, 14, 42–54. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar]
- Alawi, K.; Keeble, J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol. Ther. 2010, 125, 181–195. [Google Scholar] [CrossRef]
- Denda, M.; Tsutsumi, M. Roles of transient receptor potential proteins (TRPs) in epidermal keratinocytes. Adv. Exp. Med. Biol. 2011, 704, 847–860. [Google Scholar] [CrossRef]
- Stander, S.; Moormann, C.; Schumacher, M.; Buddenkotte, J.; Artuc, M.; Shpacovitch, V.; Brzoska, T.; Lippert, U.; Henz, B.M.; Luger, T.A.; et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp. Dermatol. 2004, 13, 129–139. [Google Scholar] [CrossRef]
- Basu, S.; Srivastava, P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5120–5125. [Google Scholar] [CrossRef]
- Hu, F.; Sun, W.W.; Zhao, X.T.; Cui, Z.J.; Yang, W.X. TRPV1 mediates cell death in rat synovial fibroblasts through calcium entry-dependent ROS production and mitochondrial depolarization. Biochem. Biophys. Res. Commun. 2008, 369, 989–993. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Torella, M.; Tortora, C.; Manzo, I.; Giordano, C.; Guida, F.; Luongo, L.; Papale, F.; Rosso, F.; et al. The genetic ablation or pharmacological inhibition of TRPV1 signalling is beneficial for the restoration of quiescent osteoclast activity in ovariectomized mice. Br. J. Pharmacol. 2014, 171, 2621–2630. [Google Scholar]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sorgard, M.; Di Marzo, V.; Julius, D.; Hogestatt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef]
- Kawasaki, H.; Takasaki, K.; Saito, A.; Goto, K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature 1988, 335, 164–167. [Google Scholar] [CrossRef]
- Kark, T.; Bagi, Z.; Lizanecz, E.; Pasztor, E.T.; Erdei, N.; Czikora, A.; Papp, Z.; Edes, I.; Porszasz, R.; Toth, A. Tissue-specific regulation of microvascular diameter: Opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol. Pharmacol. 2008, 73, 1405–1412. [Google Scholar] [CrossRef]
- Lee, L.Y.; Ni, D.; Hayes, D., Jr.; Lin, R.L. TRPV1 as a cough sensor and its temperature-sensitive properties. Pulm. Pharmacol. Ther. 2011, 24, 280–285. [Google Scholar] [CrossRef]
- Birder, L.A.; Nakamura, Y.; Kiss, S.; Nealen, M.L.; Barrick, S.; Kanai, A.J.; Wang, E.; Ruiz, G.; de Groat, W.C.; Apodaca, G.; et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 2002, 5, 856–860. [Google Scholar] [CrossRef]
- Brandt, M.R.; Beyer, C.E.; Stahl, S.M. TRPV1 Antagonists and Chronic Pain: Beyond Thermal Perception. Pharmaceuticals 2012, 5, 114–132. [Google Scholar] [CrossRef]
- Barton, N.J.; McQueen, D.S.; Thomson, D.; Gauldie, S.D.; Wilson, A.W.; Salter, D.M.; Chessell, I.P. Attenuation of experimental arthritis in TRPV1R knockout mice. Exp. Mol. Pathol. 2006, 81, 166–170. [Google Scholar] [CrossRef]
- Keeble, J.; Russell, F.; Curtis, B.; Starr, A.; Pinter, E.; Brain, S.D. Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum. 2005, 52, 3248–3256. [Google Scholar] [CrossRef]
- Engler, A.; Aeschlimann, A.; Simmen, B.R.; Michel, B.A.; Gay, R.E.; Gay, S.; Sprott, H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem. Biophys. Res. Commun. 2007, 359, 884–888. [Google Scholar] [CrossRef]
- Menendez, L.; Juarez, L.; Garcia, E.; Garcia-Suarez, O.; Hidalgo, A.; Baamonde, A. Analgesic effects of capsazepine and resiniferatoxin on bone cancer pain in mice. Neurosci. Lett. 2006, 393, 70–73. [Google Scholar] [CrossRef]
- Hong, S.; Wiley, J.W. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J. Biol. Chem. 2005, 280, 618–627. [Google Scholar] [CrossRef]
- Jhaveri, M.D.; Elmes, S.J.; Kendall, D.A.; Chapman, V. Inhibition of peripheral vanilloid TRPV1 receptors reduces noxious heat-evoked responses of dorsal horn neurons in naive, carrageenan-inflamed and neuropathic rats. Eur. J. Neurosci. 2005, 22, 361–370. [Google Scholar] [CrossRef]
- Gavva, N.R.; Bannon, A.W.; Hovland, D.N., Jr.; Lehto, S.G.; Klionsky, L.; Surapaneni, S.; Immke, D.C.; Henley, C.; Arik, L.; Bak, A.; et al. Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J. Pharmacol. Exp. Ther. 2007, 323, 128–137. [Google Scholar] [CrossRef]
- Jancso-Gabor, A.; Szolcsanyi, J.; Jancso, N. Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J. Physiol. 1970, 206, 495–507. [Google Scholar]
- Reilly, R.M.; McDonald, H.A.; Puttfarcken, P.S.; Joshi, S.K.; Lewis, L.; Pai, M.; Franklin, P.H.; Segreti, J.A.; Neelands, T.R.; Han, P.; et al. Pharmacology of modality-specific transient receptor potential vanilloid-1 antagonists that do not alter body temperature. J. Pharmacol. Exp. Ther. 2012, 342, 416–428. [Google Scholar] [CrossRef]
- Yao, J.; Liu, B.; Qin, F. Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proc. Natl. Acad. Sci. USA 2011, 108, 11109–11114. [Google Scholar]
- Mercado, J.; Gordon-Shaag, A.; Zagotta, W.N.; Gordon, S.E. Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2010, 30, 13338–13347. [Google Scholar] [CrossRef]
- Holakovska, B.; Grycova, L.; Bily, J.; Teisinger, J. Characterization of calmodulin binding domains in TRPV2 and TRPV5 C-tails. Amino Acids 2011, 40, 741–748. [Google Scholar] [CrossRef]
- Jahnel, R.; Bender, O.; Munter, L.M.; Dreger, M.; Gillen, C.; Hucho, F. Dual expression of mouse and rat VRL-1 in the dorsal root ganglion derived cell line F-11 and biochemical analysis of VRL-1 after heterologous expression. Eur. J. Biochem. 2003, 270, 4264–4271. [Google Scholar] [CrossRef]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Juvin, V.; Penna, A.; Chemin, J.; Lin, Y.L.; Rassendren, F.A. Pharmacological characterization and molecular determinants of the activation of transient receptor potential V2 channel orthologs by 2-aminoethoxydiphenyl borate. Mol. Pharmacol. 2007, 72, 1258–1268. [Google Scholar] [CrossRef]
- Bang, S.; Kim, K.Y.; Yoo, S.; Lee, S.H.; Hwang, S.W. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci. Lett. 2007, 425, 120–125. [Google Scholar] [CrossRef]
- Shibasaki, K.; Ishizaki, Y.; Mandadi, S. Astrocytes express functional TRPV2 ion channels. Biochem. Biophys. Res. Commun. 2013, 441, 327–332. [Google Scholar] [CrossRef]
- Penna, A.; Juvin, V.; Chemin, J.; Compan, V.; Monet, M.; Rassendren, F.A. PI3-kinase promotes TRPV2 activity independently of channel translocation to the plasma membrane. Cell Calcium 2006, 39, 495–507. [Google Scholar] [CrossRef]
- Stokes, A.J.; Shimoda, L.M.; Koblan-Huberson, M.; Adra, C.N.; Turner, H. A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J. Exp. Med. 2004, 200, 137–147. [Google Scholar] [CrossRef]
- Greffrath, W.; Binzen, U.; Schwarz, S.T.; Saaler-Reinhardt, S.; Treede, R.D. Co-expression of heat sensitive vanilloid receptor subtypes in rat dorsal root ganglion neurons. Neuroreport 2003, 14, 2251–2255. [Google Scholar] [CrossRef]
- Ichikawa, H.; Sugimoto, T. The co-expression of P2X3 receptor with VR1 and VRL-1 in the rat trigeminal ganglion. Brain Res. 2004, 998, 130–135. [Google Scholar]
- Park, U.; Vastani, N.; Guan, Y.; Raja, S.N.; Koltzenburg, M.; Caterina, M.J. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J. Neurosci. 2011, 31, 11425–11436. [Google Scholar] [CrossRef]
- Shimosato, G.; Amaya, F.; Ueda, M.; Tanaka, Y.; Decosterd, I.; Tanaka, M. Peripheral inflammation induces up-regulation of TRPV2 expression in rat DRG. Pain 2005, 119, 225–232. [Google Scholar] [CrossRef]
- Shibasaki, K.; Murayama, N.; Ono, K.; Ishizaki, Y.; Tominaga, M. TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J. Neurosci. 2010, 30, 4601–4612. [Google Scholar] [CrossRef]
- Wainwright, A.; Rutter, A.R.; Seabrook, G.R.; Reilly, K.; Oliver, K.R. Discrete expression of TRPV2 within the hypothalamo-neurohypophysial system: Implications for regulatory activity within the hypothalamic-pituitary-adrenal axis. J. Comp. Neurol. 2004, 474, 24–42. [Google Scholar] [CrossRef]
- Koch, S.E.; Gao, X.; Haar, L.; Jiang, M.; Lasko, V.M.; Robbins, N.; Cai, W.; Brokamp, C.; Varma, P.; Tranter, M.; et al. Probenecid: Novel use as a non-injurious positive inotrope acting via cardiac TRPV2 stimulation. J. Mol. Cell. Cardiol. 2012, 53, 134–144. [Google Scholar] [CrossRef]
- Muraki, K.; Iwata, Y.; Katanosaka, Y.; Ito, T.; Ohya, S.; Shigekawa, M.; Imaizumi, Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ. Res. 2003, 93, 829–838. [Google Scholar] [CrossRef]
- Kajiya, H.; Okamoto, F.; Nemoto, T.; Kimachi, K.; Toh-Goto, K.; Nakayana, S.; Okabe, K. RANKL-induced TRPV2 expression regulates osteoclastogenesis via calcium oscillations. Cell Calcium 2010, 48, 260–269. [Google Scholar] [CrossRef]
- Santoni, G.; Farfariello, V.; Liberati, S.; Morelli, M.B.; Nabissi, M.; Santoni, M.; Amantini, C. The role of transient receptor potential vanilloid type-2 ion channels in innate and adaptive immune responses. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Caprodossi, S.; Lucciarini, R.; Amantini, C.; Nabissi, M.; Canesin, G.; Ballarini, P.; Di Spilimbergo, A.; Cardarelli, M.A.; Servi, L.; Mammana, G.; et al. Transient receptor potential vanilloid type 2 (TRPV2) expression in normal urothelium and in urothelial carcinoma of human bladder: Correlation with the pathologic stage. Eur. Urol. 2008, 54, 612–620. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 2013, 34, 48–57. [Google Scholar] [CrossRef]
- Monet, M.; Lehen’kyi, V.; Gackiere, F.; Firlej, V.; Vandenberghe, M.; Roudbaraki, M.; Gkika, D.; Pourtier, A.; Bidaux, G.; Slomianny, C.; et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010, 70, 1225–1235. [Google Scholar] [CrossRef]
- Zanou, N.; Iwata, Y.; Schakman, O.; Lebacq, J.; Wakabayashi, S.; Gailly, P. Essential role of TRPV2 ion channel in the sensitivity of dystrophic muscle to eccentric contractions. FEBS Lett. 2009, 583, 3600–3604. [Google Scholar] [CrossRef]
- Iwata, Y.; Katanosaka, Y.; Arai, Y.; Komamura, K.; Miyatake, K.; Shigekawa, M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J. Cell Biol. 2003, 161, 957–967. [Google Scholar] [CrossRef]
- Liedtke, W.; Choe, Y.; Marti-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Everaerts, W.; Nilius, B.; Owsianik, G. The vanilloid transient receptor potential channel TRPV4: From structure to disease. Prog. Biophys. Mol. Biol. 2010, 103, 2–17. [Google Scholar] [CrossRef]
- Sedgwick, S.G.; Smerdon, S.J. The ankyrin repeat: A diversity of interactions on a common structural framework. Trends Biochem. Sci. 1999, 24, 311–316. [Google Scholar] [CrossRef]
- Gaudet, R. A primer on ankyrin repeat function in TRP channels and beyond. Mol. Biosyst. 2008, 4, 372–379. [Google Scholar] [CrossRef]
- Arniges, M.; Fernandez-Fernandez, J.M.; Albrecht, N.; Schaefer, M.; Valverde, M.A. Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J. Biol. Chem. 2006, 281, 1580–1586. [Google Scholar]
- D’hoedt, D.; Owsianik, G.; Prenen, J.; Cuajungco, M.P.; Grimm, C.; Heller, S.; Voets, T.; Nilius, B. Stimulus-specific modulation of the cation channel TRPV4 by PACSIN 3. J. Biol. Chem. 2008, 283, 6272–6280. [Google Scholar] [CrossRef]
- Watanabe, H.; Vriens, J.; Janssens, A.; Wondergem, R.; Droogmans, G.; Nilius, B. Modulation of TRPV4 gating by intra- and extracellular Ca2+. Cell Calcium 2003, 33, 489–495. [Google Scholar] [CrossRef]
- Strotmann, R.; Semtner, M.; Kepura, F.; Plant, T.D.; Schoneberg, T. Interdomain interactions control Ca2+-dependent potentiation in the cation channel TRPV4. PLoS One 2010, 5, e10580. [Google Scholar]
- Nilius, B.; Watanabe, H.; Vriens, J. The TRPV4 channel: Structure-function relationship and promiscuous gating behaviour. Pflug. Arch. 2003, 446, 298–303. [Google Scholar]
- Watanabe, H.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T.; Nilius, B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003, 424, 434–438. [Google Scholar] [CrossRef]
- Smith, P.L.; Maloney, K.N.; Pothen, R.G.; Clardy, J.; Clapham, D.E. Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J. Biol. Chem. 2006, 281, 29897–29904. [Google Scholar] [CrossRef]
- Watanabe, H.; Davis, J.B.; Smart, D.; Jerman, J.C.; Smith, G.D.; Hayes, P.; Vriens, J.; Cairns, W.; Wissenbach, U.; Prenen, J.; et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 2002, 277, 13569–13577. [Google Scholar] [CrossRef]
- Adapala, R.K.; Talasila, P.K.; Bratz, I.N.; Zhang, D.X.; Suzuki, M.; Meszaros, J.G.; Thodeti, C.K. PKCalpha mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H757–H765. [Google Scholar]
- Ma, X.; He, D.; Ru, X.; Chen, Y.; Cai, Y.; Bruce, I.C.; Xia, Q.; Yao, X.; Jin, J. Apigenin, a plant-derived flavone, activates transient receptor potential vanilloid 4 cation channel. Br. J. Pharmacol. 2012, 166, 349–358. [Google Scholar]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. Nociceptive and pro-inflammatory effects of dimethylallyl pyrophosphate via TRPV4 activation. Br. J. Pharmacol. 2012, 166, 1433–1443. [Google Scholar]
- Voets, T.; Prenen, J.; Vriens, J.; Watanabe, H.; Janssens, A.; Wissenbach, U.; Bodding, M.; Droogmans, G.; Nilius, B. Molecular determinants of permeation through the cation channel TRPV4. J. Biol. Chem. 2002, 277, 33704–33710. [Google Scholar] [CrossRef]
- Garcia-Elias, A.; Mrkonjic, S.; Pardo-Pastor, C.; Inada, H.; Hellmich, U.A.; Rubio-Moscardo, F.; Plata, C.; Gaudet, R.; Vicente, R.; Valverde, M.A. Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc. Natl. Acad. Sci. USA 2013, 110, 9553–9558. [Google Scholar] [CrossRef]
- Mamenko, M.; Zaika, O.L.; Boukelmoune, N.; Berrout, J.; O’Neil, R.G.; Pochynyuk, O. Discrete control of TRPV4 channel function in the distal nephron by protein kinases A and C. J. Biol. Chem. 2013, 288, 20306–20314. [Google Scholar]
- Peng, H.; Lewandrowski, U.; Muller, B.; Sickmann, A.; Walz, G.; Wegierski, T. Identification of a Protein Kinase C-dependent phosphorylation site involved in sensitization of TRPV4 channel. Biochem. Biophys. Res. Commun. 2010, 391, 1721–1725. [Google Scholar] [CrossRef]
- Gao, X.; Wu, L.; O’Neil, R.G. Temperature-modulated diversity of TRPV4 channel gating: Activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J. Biol. Chem. 2003, 278, 21729–27137. [Google Scholar]
- Kida, N.; Sokabe, T.; Kashio, M.; Haruna, K.; Mizuno, Y.; Suga, Y.; Nishikawa, K.; Kanamaru, A.; Hongo, M.; Oba, A.; et al. Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflug. Arch. 2012, 463, 715–725. [Google Scholar] [CrossRef]
- Chung, M.K.; Lee, H.; Caterina, M.J. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J. Biol. Chem. 2003, 278, 32037–32046. [Google Scholar] [CrossRef]
- Phan, M.N.; Leddy, H.A.; Votta, B.J.; Kumar, S.; Levy, D.S.; Lipshutz, D.B.; Lee, S.H.; Liedtke, W.; Guilak, F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009, 60, 3028–3037. [Google Scholar] [CrossRef]
- Itoh, Y.; Hatano, N.; Hayashi, H.; Onozaki, K.; Miyazawa, K.; Muraki, K. An environmental sensor, TRPV4 is a novel regulator of intracellular Ca2+ in human synoviocytes. Am. J. Physiol. Cell Physiol. 2009, 297, C1082–C1090. [Google Scholar]
- Jia, Y.; Wang, X.; Varty, L.; Rizzo, C.A.; Yang, R.; Correll, C.C.; Phelps, P.T.; Egan, R.W.; Hey, J.A. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L272–L278. [Google Scholar]
- Cohen, D.M. The transient receptor potential vanilloid-responsive 1 and 4 cation channels: Role in neuronal osmosensing and renal physiology. Curr. Opin. Nephrol. Hypertens. 2007, 16, 451–458. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Fisslthaler, B.; Suzuki, M.; Janssens, A.; Voets, T.; Morisseau, C.; Hammock, B.D.; Fleming, I.; Busse, R.; et al. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ. Res. 2005, 97, 908–915. [Google Scholar] [CrossRef]
- Shibasaki, K.; Suzuki, M.; Mizuno, A.; Tominaga, M. Effects of body temperature on neural activity in the hippocampus: Regulation of resting membrane potentials by transient receptor potential vanilloid 4. J. Neurosci. 2007, 27, 1566–1575. [Google Scholar] [CrossRef]
- Benfenati, V.; Amiry-Moghaddam, M.; Caprini, M.; Mylonakou, M.N.; Rapisarda, C.; Ottersen, O.P.; Ferroni, S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 2007, 148, 876–892. [Google Scholar] [CrossRef]
- Chung, M.K.; Jung, S.J.; Oh, S.B. Role of TRP channels in pain sensation. Adv. Exp. Med. Biol. 2011, 704, 615–636. [Google Scholar] [CrossRef]
- Todaka, H.; Taniguchi, J.; Satoh, J.; Mizuno, A.; Suzuki, M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J. Biol. Chem. 2004, 279, 35133–35138. [Google Scholar]
- Grant, A.D.; Cottrell, G.S.; Amadesi, S.; Trevisani, M.; Nicoletti, P.; Materazzi, S.; Altier, C.; Cenac, N.; Zamponi, G.W.; Bautista-Cruz, F.; et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 2007, 578, 715–733. [Google Scholar] [CrossRef]
- Fecto, F.; Shi, Y.; Huda, R.; Martina, M.; Siddique, T.; Deng, H.X. Mutant TRPV4-mediated toxicity is linked to increased constitutive function in axonal neuropathies. J. Biol. Chem. 2011, 286, 17281–17291. [Google Scholar] [CrossRef]
- Materazzi, S.; Fusi, C.; Benemei, S.; Pedretti, P.; Patacchini, R.; Nilius, B.; Prenen, J.; Creminon, C.; Geppetti, P.; Nassini, R. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflug. Arch. 2012, 463, 561–569. [Google Scholar] [CrossRef]
- Echaniz-Laguna, A.; Dubourg, O.; Carlier, P.; Carlier, R.Y.; Sabouraud, P.; Pereon, Y.; Chapon, F.; Thauvin-Robinet, C.; Laforet, P.; Eymard, B.; et al. Phenotypic spectrum and incidence of TRPV4 mutations in patients with inherited axonal neuropathy. Neurology 2014, 82, 1919–1926. [Google Scholar] [CrossRef]
- Wei, X.; Edelmayer, R.M.; Yan, J.; Dussor, G. Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia 2011, 31, 1595–1600. [Google Scholar] [CrossRef]
- Arniges, M.; Vazquez, E.; Fernandez-Fernandez, J.M.; Valverde, M.A. Swelling-activated Ca2+ entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J. Biol. Chem. 2004, 279, 54062–54068. [Google Scholar]
- Fusi, C.; Materazzi, S.; Minocci, D.; Maio, V.; Oranges, T.; Massi, D.; Nassini, R. Transient Receptor Potential Vanilloid 4 (TRPV4) Is Downregulated in Keratinocytes in Human Non-Melanoma Skin Cancer. J. Investig. Dermatol. 2014. [Google Scholar] [CrossRef]
- Verma, P.; Kumar, A.; Goswami, C. TRPV4-mediated channelopathies. Channels 2010, 4, 319–328. [Google Scholar]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S.; et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Meyer, J.; Borkhardt, A.; Tuschl, T. New microRNAs from mouse and human. RNA 2003, 9, 175–179. [Google Scholar] [CrossRef]
- Irie, S.; Furukawa, T. Trpm1. Handb. Exp. Pharmacol. 2014, 222, 387–402. [Google Scholar] [CrossRef]
- Holendova, B.; Grycova, L.; Jirku, M.; Teisinger, J. PtdIns(4,5)P2 interacts with CaM binding domains on TRPM3 N-terminus. Channels 2012, 6, 479–482. [Google Scholar] [CrossRef]
- Lee, N.; Chen, J.; Sun, L.; Wu, S.; Gray, K.R.; Rich, A.; Huang, M.; Lin, J.H.; Feder, J.N.; Janovitz, E.B.; et al. Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J. Biol. Chem. 2003, 278, 20890–20897. [Google Scholar] [CrossRef]
- Oberwinkler, J.; Lis, A.; Giehl, K.M.; Flockerzi, V.; Philipp, S.E. Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J. Biol. Chem. 2005, 280, 22540–22548. [Google Scholar]
- Straub, I.; Krugel, U.; Mohr, F.; Teichert, J.; Rizun, O.; Konrad, M.; Oberwinkler, J.; Schaefer, M. Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol. Pharmacol. 2013, 84, 736–750. [Google Scholar] [CrossRef]
- Zamudio-Bulcock, P.A.; Everett, J.; Harteneck, C.; Valenzuela, C.F. Activation of steroid-sensitive TRPM3 channels potentiates glutamatergic transmission at cerebellar Purkinje neurons from developing rats. J. Neurochem. 2011, 119, 474–485. [Google Scholar] [CrossRef]
- Hoffmann, A.; Grimm, C.; Kraft, R.; Goldbaum, O.; Wrede, A.; Nolte, C.; Hanisch, U.K.; Richter-Landsberg, C.; Bruck, W.; Kettenmann, H.; et al. TRPM3 is expressed in sphingosine-responsive myelinating oligodendrocytes. J. Neurochem. 2010, 114, 654–665. [Google Scholar] [CrossRef]
- Wagner, T.F.; Loch, S.; Lambert, S.; Straub, I.; Mannebach, S.; Mathar, I.; Dufer, M.; Lis, A.; Flockerzi, V.; Philipp, S.E.; et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat. Cell Biol. 2008, 10, 1421–1430. [Google Scholar]
- Stewart, A.P.; Egressy, K.; Lim, A.; Edwardson, J.M. AFM imaging reveals the tetrameric structure of the TRPM8 channel. Biochem. Biophys. Res. Commun. 2010, 394, 383–386. [Google Scholar] [CrossRef]
- Brauchi, S.; Orta, G.; Salazar, M.; Rosenmann, E.; Latorre, R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J. Neurosci. 2006, 26, 4835–4840. [Google Scholar] [CrossRef]
- Phelps, C.B.; Gaudet, R. The role of the N terminus and transmembrane domain of TRPM8 in channel localization and tetramerization. J. Biol. Chem. 2007, 282, 36474–36480. [Google Scholar] [CrossRef]
- Cao, C.; Yudin, Y.; Bikard, Y.; Chen, W.; Liu, T.; Li, H.; Jendrossek, D.; Cohen, A.; Pavlov, E.; Rohacs, T.; et al. Polyester modification of the mammalian TRPM8 channel protein: Implications for structure and function. Cell Rep. 2013, 4, 302–315. [Google Scholar] [CrossRef]
- Voets, T.; Owsianik, G.; Nilius, B. TRPM8. In Transient Receptor Potential (TRP) Channels; Flockerzi, V., Nilius, B., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin, Germany, 2007; Volume 179, pp. 329–344. [Google Scholar]
- Dragoni, I.; Guida, E.; McIntyre, P. The cold and menthol receptor TRPM8 contains a functionally important double cysteine motif. J. Biol. Chem. 2006, 281, 37353–37360. [Google Scholar] [CrossRef]
- Erler, I.; Al-Ansary, D.M.; Wissenbach, U.; Wagner, T.F.; Flockerzi, V.; Niemeyer, B.A. Trafficking and assembly of the cold-sensitive TRPM8 channel. J. Biol. Chem. 2006, 281, 38396–38404. [Google Scholar]
- Madrid, R.; Donovan-Rodriguez, T.; Meseguer, V.; Acosta, M.C.; Belmonte, C.; Viana, F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J. Neurosci. 2006, 26, 12512–12525. [Google Scholar] [CrossRef]
- Malkia, A.; Madrid, R.; Meseguer, V.; de la Pena, E.; Valero, M.; Belmonte, C.; Viana, F. Bidirectional shifts of TRPM8 channel gating by temperature and chemical agents modulate the cold sensitivity of mammalian thermoreceptors. J. Physiol. 2007, 581, 155–174. [Google Scholar] [CrossRef]
- Reid, G. ThermoTRP channels and cold sensing: What are they really up to? Pflug. Arch. 2005, 451, 250–263. [Google Scholar] [CrossRef]
- De la Pena, E.; Malkia, A.; Cabedo, H.; Belmonte, C.; Viana, F. The contribution of TRPM8 channels to cold sensing in mammalian neurones. J. Physiol. 2005, 567, 415–426. [Google Scholar] [CrossRef]
- Bodding, M.; Wissenbach, U.; Flockerzi, V. Characterisation of TRPM8 as a pharmacophore receptor. Cell Calcium 2007, 42, 618–628. [Google Scholar] [CrossRef]
- Brauchi, S.; Orio, P.; Latorre, R. Clues to understanding cold sensation: Thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc. Natl. Acad. Sci. USA 2004, 101, 15494–15499. [Google Scholar] [CrossRef]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef]
- Behrendt, H.J.; Germann, T.; Gillen, C.; Hatt, H.; Jostock, R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 2004, 141, 737–745. [Google Scholar]
- Voets, T.; Owsianik, G.; Janssens, A.; Talavera, K.; Nilius, B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat. Chem. Biol. 2007, 3, 174–182. [Google Scholar]
- Yudin, Y.; Rohacs, T. Regulation of TRPM8 channel activity. Mol. Cell. Endocrinol. 2012, 353, 68–74. [Google Scholar] [CrossRef]
- Rohacs, T.; Lopes, C.M.; Michailidis, I.; Logothetis, D.E. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 2005, 8, 626–634. [Google Scholar] [CrossRef]
- Zakharian, E.; Thyagarajan, B.; French, R.J.; Pavlov, E.; Rohacs, T. Inorganic polyphosphate modulates TRPM8 channels. PLoS One 2009, 4, e5404. [Google Scholar]
- Sarria, I.; Gu, J. Menthol response and adaptation in nociceptive-like and nonnociceptive-like neurons: Role of protein kinases. Mol. Pain 2010, 6. [Google Scholar] [CrossRef]
- Daniels, R.L.; Takashima, Y.; McKemy, D.D. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J. Biol. Chem. 2009, 284, 1570–1582. [Google Scholar] [CrossRef]
- Liu, B.; Qin, F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2005, 25, 1674–1681. [Google Scholar] [CrossRef]
- Abe, J.; Hosokawa, H.; Sawada, Y.; Matsumura, K.; Kobayashi, S. Ca2+-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci. Lett. 2006, 397, 140–144. [Google Scholar] [CrossRef]
- Linte, R.M.; Ciobanu, C.; Reid, G.; Babes, A. Desensitization of cold- and menthol-sensitive rat dorsal root ganglion neurones by inflammatory mediators. Exp. Brain Res. 2007, 178, 89–98. [Google Scholar] [CrossRef]
- Premkumar, L.S.; Raisinghani, M.; Pingle, S.C.; Long, C.; Pimentel, F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J. Neurosci. 2005, 25, 11322–11329. [Google Scholar] [CrossRef]
- Bavencoffe, A.; Kondratskyi, A.; Gkika, D.; Mauroy, B.; Shuba, Y.; Prevarskaya, N.; Skryma, R. Complex regulation of the TRPM8 cold receptor channel: Role of arachidonic acid release following M3 muscarinic receptor stimulation. J. Biol. Chem. 2011, 286, 9849–9855. [Google Scholar]
- Bavencoffe, A.; Gkika, D.; Kondratskyi, A.; Beck, B.; Borowiec, A.S.; Bidaux, G.; Busserolles, J.; Eschalier, A.; Shuba, Y.; Skryma, R.; et al. The transient receptor potential channel TRPM8 is inhibited via the alpha 2A adrenoreceptor signaling pathway. J. Biol. Chem. 2010, 285, 9410–9419. [Google Scholar] [CrossRef]
- Gentry, C.; Stoakley, N.; Andersson, D.A.; Bevan, S. The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol. Pain 2010, 6. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Vellani, V.; Schiano-Moriello, A.; Marini, P.; Magherini, P.C.; Orlando, P.; Di Marzo, V. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 2008, 325, 1007–1015. [Google Scholar] [CrossRef]
- Dhaka, A.; Earley, T.J.; Watson, J.; Patapoutian, A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J. Neurosci. 2008, 28, 566–575. [Google Scholar] [CrossRef]
- Takashima, Y.; Daniels, R.L.; Knowlton, W.; Teng, J.; Liman, E.R.; McKemy, D.D. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J. Neurosci. 2007, 27, 14147–14157. [Google Scholar] [CrossRef]
- Abe, J.; Hosokawa, H.; Okazawa, M.; Kandachi, M.; Sawada, Y.; Yamanaka, K.; Matsumura, K.; Kobayashi, S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res. Mol. Brain Res. 2005, 136, 91–98. [Google Scholar]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef]
- Knowlton, W.M.; McKemy, D.D. TRPM8: From cold to cancer, peppermint to pain. Curr. Pharm. Biotechnol. 2011, 12, 68–77. [Google Scholar] [CrossRef]
- Lashinger, E.S.; Steiginga, M.S.; Hieble, J.P.; Leon, L.A.; Gardner, S.D.; Nagilla, R.; Davenport, E.A.; Hoffman, B.E.; Laping, N.J.; Su, X. AMTB, a TRPM8 channel blocker: Evidence in rats for activity in overactive bladder and painful bladder syndrome. Am. J. Physiol. Ren. Physiol. 2008, 295, F803–F810. [Google Scholar]
- Proudfoot, C.J.; Garry, E.M.; Cottrell, D.F.; Rosie, R.; Anderson, H.; Robertson, D.C.; Fleetwood-Walker, S.M.; Mitchell, R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 2006, 16, 1591–1605. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J., Jr.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef]
- Mukerji, G.; Yiangou, Y.; Corcoran, S.L.; Selmer, I.S.; Smith, G.D.; Benham, C.D.; Bountra, C.; Agarwal, S.K.; Anand, P. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol. 2006, 6. [Google Scholar] [CrossRef]
- Stein, R.J.; Santos, S.; Nagatomi, J.; Hayashi, Y.; Minnery, B.S.; Xavier, M.; Patel, A.S.; Nelson, J.B.; Futrell, W.J.; Yoshimura, N.; et al. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J. Urol. 2004, 172, 1175–1178. [Google Scholar] [CrossRef]
- Xing, H.; Ling, J.X.; Chen, M.; Johnson, R.D.; Tominaga, M.; Wang, C.Y.; Gu, J. TRPM8 mechanism of autonomic nerve response to cold in respiratory airway. Mol. Pain 2008, 4. [Google Scholar] [CrossRef]
- Yee, N.S.; Zhou, W.; Lee, M. Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation in pancreatic adenocarcinoma. Cancer Lett. 2010, 297, 49–55. [Google Scholar] [CrossRef]
- Bai, V.U.; Murthy, S.; Chinnakannu, K.; Muhletaler, F.; Tejwani, S.; Barrack, E.R.; Kim, S.H.; Menon, M.; Veer Reddy, G.P. Androgen regulated TRPM8 expression: A potential mRNA marker for metastatic prostate cancer detection in body fluids. Int. J. Oncol. 2010, 36, 443–450. [Google Scholar]
- Kurose, M.; Meng, I.D. Dry eye modifies the thermal and menthol responses in rat corneal primary afferent cool cells. J. Neurophysiol. 2013, 110, 495–504. [Google Scholar] [CrossRef]
- Parra, A.; Madrid, R.; Echevarria, D.; del Olmo, S.; Morenilla-Palao, C.; Acosta, M.C.; Gallar, J.; Dhaka, A.; Viana, F.; Belmonte, C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat. Med. 2010, 16, 1396–1399. [Google Scholar] [CrossRef]
- Gasperini, R.J.; Hou, X.; Parkington, H.; Coleman, H.; Klaver, D.W.; Vincent, A.J.; Foa, L.C.; Small, D.H. TRPM8 and Nav1.8 sodium channels are required for transthyretin-induced calcium influx in growth cones of small-diameter TrkA-positive sensory neurons. Mol. Neurodegener. 2011, 6. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Nilius, B.; Prenen, J.; Owsianik, G. Irritating channels: The case of TRPA1. J. Physiol. 2011, 589, 1543–1549. [Google Scholar] [CrossRef]
- Cvetkov, T.L.; Huynh, K.W.; Cohen, M.R.; Moiseenkova-Bell, V.Y. Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J. Biol. Chem. 2011, 286, 38168–38176. [Google Scholar]
- Wang, L.; Cvetkov, T.L.; Chance, M.R.; Moiseenkova-Bell, V.Y. Identification of in vivo disulfide conformation of TRPA1 ion channel. J. Biol. Chem. 2012, 287, 6169–6176. [Google Scholar]
- Macpherson, L.J.; Xiao, B.; Kwan, K.Y.; Petrus, M.J.; Dubin, A.E.; Hwang, S.; Cravatt, B.; Corey, D.P.; Patapoutian, A. An ion channel essential for sensing chemical damage. J. Neurosci. 2007, 27, 11412–11415. [Google Scholar] [CrossRef]
- Takahashi, N.; Kuwaki, T.; Kiyonaka, S.; Numata, T.; Kozai, D.; Mizuno, Y.; Yamamoto, S.; Naito, S.; Knevels, E.; Carmeliet, P.; et al. TRPA1 underlies a sensing mechanism for O2. Nat. Chem. Biol. 2011, 7, 701–711. [Google Scholar]
- Doerner, J.F.; Gisselmann, G.; Hatt, H.; Wetzel, C.H. Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 2007, 282, 13180–13189. [Google Scholar] [CrossRef]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Hogestatt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Sura, L.; Zima, V.; Marsakova, L.; Hynkova, A.; Barvik, I.; Vlachova, V. C-terminal acidic clusteris involved in Ca2+-induced regulation of human transient receptor potential ankyrin 1 channel. J.Biol. Chem. 2012, 287, 18067–18077. [Google Scholar] [CrossRef]
- Zurborg, S.; Yurgionas, B.; Jira, J.A.; Caspani, O.; Heppenstall, P.A. Direct activation of the ion channel TRPA1 by Ca2+. Nat. Neurosci. 2007, 10, 277–279. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chang, R.B.; Waters, H.N.; McKemy, D.D.; Liman, E.R. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol. Chem. 2008, 283, 32691–32703. [Google Scholar] [CrossRef]
- Dhaka, A.; Viswanath, V.; Patapoutian, A. Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 2006, 29, 135–161. [Google Scholar] [CrossRef]
- Del Camino, D.; Murphy, S.; Heiry, M.; Barrett, L.B.; Earley, T.J.; Cook, C.A.; Petrus, M.J.; Zhao, M.; D’Amours, M.; Deering, N.; et al. TRPA1 contributes to cold hypersensitivity. J. Neurosci. 2010, 30, 15165–15174. [Google Scholar] [CrossRef]
- Knowlton, W.M.; Bifolck-Fisher, A.; Bautista, D.M.; McKemy, D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010, 150, 340–350. [Google Scholar] [CrossRef]
- Moran, M.M.; McAlexander, M.A.; Biro, T.; Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 601–620. [Google Scholar] [CrossRef]
- De Oliveira, C.; Garami, A.; Lehto, S.G.; Pakai, E.; Tekus, V.; Pohoczky, K.; Youngblood, B.D.; Wang, W.; Kort, M.E.; Kym, P.R.; et al. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J. Neurosci. 2014, 34, 4445–4452. [Google Scholar] [CrossRef]
- Andrade, E.L.; Meotti, F.C.; Calixto, J.B. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2012, 133, 189–204. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol 2013, 75, 181–200. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andre, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef]
- Hinman, A.; Chuang, H.H.; Bautista, D.M.; Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 2006, 103, 19564–19568. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef]
- Wang, S.; Dai, Y.; Fukuoka, T.; Yamanaka, H.; Kobayashi, K.; Obata, K.; Cui, X.; Tominaga, M.; Noguchi, K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: A molecular mechanism of inflammatory pain. Brain 2008, 131, 1241–1251. [Google Scholar]
- Nagata, K.; Duggan, A.; Kumar, G.; Garcia-Anoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005, 25, 4052–4061. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, S.; Tominaga, M.; Yamamoto, S.; Fukuoka, T.; Higashi, T.; Kobayashi, K.; Obata, K.; Yamanaka, H.; Noguchi, K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Investig. 2007, 117, 1979–1987. [Google Scholar] [CrossRef]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef]
- Hjerling-Leffler, J.; Alqatari, M.; Ernfors, P.; Koltzenburg, M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J. Neurosci. 2007, 27, 2435–2443. [Google Scholar] [CrossRef]
- Kim, Y.S.; Son, J.Y.; Kim, T.H.; Paik, S.K.; Dai, Y.; Noguchi, K.; Ahn, D.K.; Bae, Y.C. Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J. Comp. Neurol. 2010, 518, 687–698. [Google Scholar] [CrossRef]
- Royle, S.J.; Murrell-Lagnado, R.D. Constitutive cycling: A general mechanism to regulate cell surface proteins. Bioessays 2003, 25, 39–46. [Google Scholar] [CrossRef]
- Glickman, M.H.; Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar]
- Pizzirusso, M.; Chang, A. Ubiquitin-mediated targeting of a mutant plasma membrane ATPase, Pma1–7, to the endosomal/vacuolar system in yeast. Mol. Biol. Cell 2004, 15, 2401–2409. [Google Scholar] [CrossRef]
- Inoue, A.; Okabe, S. The dynamic organization of postsynaptic proteins: Translocating molecules regulate synaptic function. Curr. Opin. Neurobiol. 2003, 13, 332–340. [Google Scholar] [CrossRef]
- Kavalali, E.T. SNARE interactions in membrane trafficking: A perspective from mammalian central synapses. Bioessays 2002, 24, 926–936. [Google Scholar] [CrossRef]
- Jahn, R.; Lang, T.; Sudhof, T.C. Membrane fusion. Cell 2003, 112, 519–533. [Google Scholar] [CrossRef]
- Buckley, K.M.; Melikian, H.E.; Provoda, C.J.; Waring, M.T. Regulation of neuronal function by protein trafficking: A role for the endosomal pathway. J. Physiol. 2000, 525, 11–19. [Google Scholar] [CrossRef]
- Jahn, R.; Fasshauer, D. Molecular machines governing exocytosis of synaptic vesicles. Nature 2012, 490, 201–207. [Google Scholar] [CrossRef]
- Bavassano, C.; Marvaldi, L.; Langeslag, M.; Sarg, B.; Lindner, H.; Klimaschewski, L.; Kress, M.; Ferrer-Montiel, A.; Knaus, H.G. Identification of voltage-gated K(+) channel beta 2 (Kvbeta2) subunit as a novel interaction partner of the pain transducer Transient Receptor Potential Vanilloid 1 channel (TRPV1). Biochim. Biophys. Acta 2013, 1833, 3166–3175. [Google Scholar] [CrossRef]
- Lainez, S.; Valente, P.; Ontoria-Oviedo, I.; Estevez-Herrera, J.; Camprubi-Robles, M.; Ferrer-Montiel, A.; Planells-Cases, R. GABAA receptor associated protein (GABARAP) modulates TRPV1 expression and channel function and desensitization. FASEB J. 2010, 24, 1958–1970. [Google Scholar] [CrossRef]
- Goswami, C.; Kuhn, J.; Dina, O.A.; Fernandez-Ballester, G.; Levine, J.D.; Ferrer-Montiel, A.; Hucho, T. Estrogen destabilizes microtubules through an ion-conductivity-independent TRPV1 pathway. J. Neurochem. 2011, 117, 995–1008. [Google Scholar] [CrossRef]
- Wang, H.; Bedford, F.K.; Brandon, N.J.; Moss, S.J.; Olsen, R.W. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature 1999, 397, 69–72. [Google Scholar] [CrossRef]
- Goswami, C.; Hucho, T.B.; Hucho, F. Identification and characterisation of novel tubulin-binding motifs located within the C-terminus of TRPV1. J. Neurochem. 2007, 101, 250–262. [Google Scholar] [CrossRef]
- Goswami, C. TRPV1-tubulin complex: Involvement of membrane tubulin in the regulation of chemotherapy-induced peripheral neuropathy. J. Neurochem. 2012, 123, 1–13. [Google Scholar] [CrossRef]
- Storti, B.; Bizzarri, R.; Cardarelli, F.; Beltram, F. Intact microtubules preserve transient receptor potential vanilloid 1 (TRPV1) functionality through receptor binding. J. Biol. Chem. 2012, 287, 7803–7811. [Google Scholar]
- Ratner, N.; Bloom, G.S.; Brady, S.T. A role for cyclin-dependent kinase(s) in the modulation of fast anterograde axonal transport: Effects defined by olomoucine and the APC tumor suppressor protein. J. Neurosci. 1998, 18, 7717–7726. [Google Scholar]
- Paglini, G.; Caceres, A. The role of the Cdk5-p35 kinase in neuronal development. Eur. J. Biochem. 2001, 268, 1528–1533. [Google Scholar] [CrossRef]
- Xing, B.M.; Yang, Y.R.; Du, J.X.; Chen, H.J.; Qi, C.; Huang, Z.H.; Zhang, Y.; Wang, Y. Cyclin-dependent kinase 5 controls TRPV1 membrane trafficking and the heat sensitivity of nociceptors through KIF13B. J. Neurosci. 2012, 32, 14709–14721. [Google Scholar] [CrossRef]
- Pareek, T.K.; Keller, J.; Kesavapany, S.; Agarwal, N.; Kuner, R.; Pant, H.C.; Iadarola, M.J.; Brady, R.O.; Kulkarni, A.B. Cyclin-dependent kinase 5 modulates nociceptive signaling through direct phosphorylation of transient receptor potential vanilloid 1. Proc. Natl. Acad. Sci. USA 2007, 104, 660–665. [Google Scholar] [CrossRef]
- Koplas, P.A.; Rosenberg, R.L.; Oxford, G.S. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J. Neurosci. 1997, 17, 3525–3537. [Google Scholar]
- Sanz-Salvador, L.; Andres-Borderia, A.; Ferrer-Montiel, A.; Planells-Cases, R. Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J. Biol. Chem. 2012, 287, 19462–19471. [Google Scholar] [CrossRef]
- Holland, S.; Coste, O.; Zhang, D.D.; Pierre, S.C.; Geisslinger, G.; Scholich, K. The ubiquitin ligase MYCBP2 regulates transient receptor potential vanilloid receptor 1 (TRPV1) internalization through inhibition of p38 MAPK signaling. J. Biol. Chem. 2011, 286, 3671–3680. [Google Scholar] [CrossRef]
- Holland, S.; Scholich, K. Regulation of neuronal functions by the E3-ubiquitinligase protein associated with MYC (MYCBP2). Commun. Integr. Biol. 2011, 4, 513–515. [Google Scholar]
- Lewcock, J.W.; Genoud, N.; Lettieri, K.; Pfaff, S.L. The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron 2007, 56, 604–620. [Google Scholar] [CrossRef]
- Morenilla-Palao, C.; Planells-Cases, R.; Garcia-Sanz, N.; Ferrer-Montiel, A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J. Biol. Chem. 2004, 279, 25665–25672. [Google Scholar]
- Vetter, I.; Cheng, W.; Peiris, M.; Wyse, B.D.; Roberts-Thomson, S.J.; Zheng, J.; Monteith, G.R.; Cabot, P.J. Rapid, opioid-sensitive mechanisms involved in transient receptor potential vanilloid 1 sensitization. J. Biol. Chem. 2008, 283, 19540–19550. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef]
- Jeske, N.A.; Patwardhan, A.M.; Henry, M.A.; Milam, S.B. Fibronectin stimulates TRPV1 translocation in primary sensory neurons. J. Neurochem. 2009, 108, 591–600. [Google Scholar] [CrossRef]
- Stein, A.T.; Ufret-Vincenty, C.A.; Hua, L.; Santana, L.F.; Gordon, S.E. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J. Gen. Physiol. 2006, 128, 509–522. [Google Scholar] [CrossRef]
- Ji, R.R.; Samad, T.A.; Jin, S.X.; Schmoll, R.; Woolf, C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef]
- Van Buren, J.J.; Bhat, S.; Rotello, R.; Pauza, M.E.; Premkumar, L.S. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol. Pain 2005, 1. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; McNaughton, P.A. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008, 59, 450–461. [Google Scholar] [CrossRef]
- Goswami, C.; Rademacher, N.; Smalla, K.H.; Kalscheuer, V.; Ropers, H.H.; Gundelfinger, E.D.; Hucho, T. TRPV1 acts as a synaptic protein and regulates vesicle recycling. J. Cell Sci. 2010, 123, 2045–2057. [Google Scholar] [CrossRef]
- Goswami, C.; Schmidt, H.; Hucho, F. TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J. 2007, 274, 760–772. [Google Scholar] [CrossRef]
- Goswami, C. Structural and functional regulation of growth cone, filopodia and synaptic sites by TRPV1. Commun. Integr. Biol. 2010, 3, 614–618. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.B.; Lu, Y.J.; Hu, J.W.; Bao, L.; Zhang, X. Transport of receptors, receptor signaling complexes and ion channels via neuropeptide-secretory vesicles. Cell Res. 2011, 21, 741–753. [Google Scholar] [CrossRef]
- Barnhill, J.C.; Stokes, A.J.; Koblan-Huberson, M.; Shimoda, L.M.; Muraguchi, A.; Adra, C.N.; Turner, H. RGA protein associates with a TRPV ion channel during biosynthesis and trafficking. J. Cell. Biochem. 2004, 91, 808–820. [Google Scholar] [CrossRef]
- Stokes, A.J.; Wakano, C.; del Carmen, K.A.; Koblan-Huberson, M.; Turner, H. Formation of a physiological complex between TRPV2 and RGA protein promotes cell surface expression of TRPV2. J. Cell. Biochem. 2005, 94, 669–683. [Google Scholar] [CrossRef]
- Aoyagi, K.; Ohara-Imaizumi, M.; Nishiwaki, C.; Nakamichi, Y.; Nagamatsu, S. Insulin/phosphoinositide 3-kinase pathway accelerates the glucose-induced first-phase insulin secretion through TrpV2 recruitment in pancreatic beta-cells. Biochem. J. 2010, 432, 375–386. [Google Scholar]
- Kanzaki, M.; Zhang, Y.Q.; Mashima, H.; Li, L.; Shibata, H.; Kojima, I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat. Cell Biol. 1999, 1, 165–170. [Google Scholar]
- Cohen, M.R.; Huynh, K.W.; Cawley, D.; Moiseenkova-Bell, V.Y. Understanding the cellular function of TRPV2 channel through generation of specific monoclonal antibodies. PLoS One 2013, 8, e85392. [Google Scholar]
- Hisanaga, E.; Nagasawa, M.; Ueki, K.; Kulkarni, R.N.; Mori, M.; Kojima, I. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. Diabetes 2009, 58, 174–184. [Google Scholar] [CrossRef]
- Nagasawa, M.; Nakagawa, Y.; Tanaka, S.; Kojima, I. Chemotactic peptide fMetLeuPhe induces translocation of the TRPV2 channel in macrophages. J. Cell. Physiol. 2007, 210, 692–702. [Google Scholar] [CrossRef]
- Becker, D.; Muller, M.; Leuner, K.; Jendrach, M. The C-terminal domain of TRPV4 is essential for plasma membrane localization. Mol. Membr. Biol. 2008, 25, 139–151. [Google Scholar] [CrossRef]
- Lei, L.; Cao, X.; Yang, F.; Shi, D.J.; Tang, Y.Q.; Zheng, J.; Wang, K. A TRPV4 channel C-terminal folding recognition domain critical for trafficking and function. J. Biol. Chem. 2013, 288, 10427–10439. [Google Scholar]
- Xu, H.; Fu, Y.; Tian, W.; Cohen, D.M. Glycosylation of the osmoresponsive transient receptor potential channel TRPV4 on Asn-651 influences membrane trafficking. Am. J. Physiol. Ren. Physiol. 2006, 290, F1103–F1109. [Google Scholar]
- Wang, Y.; Fu, X.; Gaiser, S.; Kottgen, M.; Kramer-Zucker, A.; Walz, G.; Wegierski, T. OS-9 regulates the transit and polyubiquitination of TRPV4 in the endoplasmic reticulum. J. Biol. Chem. 2007, 282, 36561–36570. [Google Scholar] [CrossRef]
- Galizia, L.; Pizzoni, A.; Fernandez, J.; Rivarola, V.; Capurro, C.; Ford, P. Functional interaction between AQP2 and TRPV4 in renal cells. J. Cell. Biochem. 2012, 113, 580–589. [Google Scholar] [CrossRef]
- Ramadass, R.; Becker, D.; Jendrach, M.; Bereiter-Hahn, J. Spectrally and spatially resolved fluorescence lifetime imaging in living cells: TRPV4-microfilament interactions. Arch. Biochem. Biophys. 2007, 463, 27–36. [Google Scholar] [CrossRef]
- Goswami, C.; Kuhn, J.; Heppenstall, P.A.; Hucho, T. Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS One 2010, 5, e11654. [Google Scholar]
- Shin, S.H.; Lee, E.J.; Hyun, S.; Chun, J.; Kim, Y.; Kang, S.S. Phosphorylation on the Ser 824 residue of TRPV4 prefers to bind with F-actin than with microtubules to expand the cell surface area. Cell Signal. 2012, 24, 641–651. [Google Scholar] [CrossRef]
- Wegierski, T.; Lewandrowski, U.; Muller, B.; Sickmann, A.; Walz, G. Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J. Biol. Chem. 2009, 284, 2923–2933. [Google Scholar] [CrossRef]
- Fu, Y.; Subramanya, A.; Rozansky, D.; Cohen, D.M. WNK kinases influence TRPV4 channel function and localization. Am. J. Physiol. Ren. Physiol. 2006, 290, F1305–F1314. [Google Scholar]
- Wegierski, T.; Hill, K.; Schaefer, M.; Walz, G. The HECT ubiquitin ligase AIP4 regulates the cell surface expression of select TRP channels. EMBO J. 2006, 25, 5659–5669. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Grimm, C.; Oshima, K.; D’hoedt, D.; Nilius, B.; Mensenkamp, A.R.; Bindels, R.J.; Plomann, M.; Heller, S. PACSINs bind to the TRPV4 cation channel. PACSIN 3 modulates the subcellular localization of TRPV4. J. Biol. Chem. 2006, 281, 18753–18762. [Google Scholar] [CrossRef]
- Modregger, J.; Ritter, B.; Witter, B.; Paulsson, M.; Plomann, M. All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J. Cell Sci. 2000, 113, 4511–4521. [Google Scholar]
- Ma, X.; Cao, J.; Luo, J.; Nilius, B.; Huang, Y.; Ambudkar, I.S.; Yao, X. Depletion of intracellular Ca2+ stores stimulates the translocation of vanilloid transient receptor potential 4-c1 heteromeric channels to the plasma membrane. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2249–2255. [Google Scholar] [CrossRef]
- Fruhwald, J.; Camacho, L.J.; Dembla, S.; Mannebach, S.; Lis, A.; Drews, A.; Wissenbach, U.; Oberwinkler, J.; Philipp, S.E. Alternative splicing of a protein domain indispensable for function of transient receptor potential melastatin 3 (TRPM3) ion channels. J. Biol. Chem. 2012, 287, 36663–36672. [Google Scholar] [CrossRef]
- Pertusa, M.I.; Gonz, A.A.; Hardy, P.; Madrid, R.; Viana, F.E. Bidirectional Modulation of Thermal and Chemical Sensitivity of TRPM8 Channels by the Initial Region of the N-Terminal Domain. J. Biol. Chem. 2014, 289, 21828–21843. [Google Scholar] [CrossRef]
- Morenilla-Palao, C.; Pertusa, M.; Meseguer, V.; Cabedo, H.; Viana, F. Lipid raft segregation modulates TRPM8 channel activity. J. Biol. Chem. 2009, 284, 9215–9224. [Google Scholar] [CrossRef]
- Pertusa, M.; Madrid, R.; Morenilla-Palao, C.; Belmonte, C.; Viana, F. N-glycosylation of TRPM8 ion channels modulates temperature sensitivity of cold thermoreceptor neurons. J. Biol. Chem. 2012, 287, 18218–18229. [Google Scholar]
- Oku, S.; Takahashi, N.; Fukata, Y.; Fukata, M. In silico screening for palmitoyl substrates reveals a role for DHHC1/3/10 (zDHHC1/3/11)-mediated neurochondrin palmitoylation in its targeting to Rab5-positive endosomes. J. Biol. Chem. 2013, 288, 19816–19829. [Google Scholar] [CrossRef]
- Ambudkar, I.S. Trafficking of TRP channels: Determinants of channel function. In Transient Receptor Potential (TRP) Channels; Flockerzi, V., Nilius, B., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin, Germany, 2007; Volume 179, pp. 541–557. [Google Scholar]
- Shepherd, J.D.; Huganir, R.L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 2007, 23, 613–643. [Google Scholar] [CrossRef]
- Stokes, A.; Wakano, C.; Koblan-Huberson, M.; Adra, C.N.; Fleig, A.; Turner, H. TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cell Signal. 2006, 18, 1584–1594. [Google Scholar] [CrossRef]
- Schmidt, M.; Dubin, A.E.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 2009, 64, 498–509. [Google Scholar] [CrossRef]
- Shimizu, T.; Shibata, M.; Toriumi, H.; Iwashita, T.; Funakubo, M.; Sato, H.; Kuroi, T.; Ebine, T.; Koizumi, K.; Suzuki, N. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol. Dis. 2012, 48, 367–378. [Google Scholar] [CrossRef]
- Burstein, R.; Zhang, X.; Levy, D.; Aoki, K.R.; Brin, M.F. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: Therapeutic implications for migraine and other pains. Cephalalgia 2014. [Google Scholar] [CrossRef]
- Prasad, P.; Yanagihara, A.A.; Small-Howard, A.L.; Turner, H.; Stokes, A.J. Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J. Immunol. 2008, 181, 5024–5034. [Google Scholar] [CrossRef]
- De Paiva, A.; Ashton, A.C.; Foran, P.; Schiavo, G.; Montecucco, C.; Dolly, J.O. Botulinum A like type B and tetanus toxins fulfils criteria for being a zinc-dependent protease. J. Neurochem. 1993, 61, 2338–2341. [Google Scholar] [CrossRef]
- Qerama, E.; Fuglsang-Frederiksen, A.; Jensen, T.S. The role of botulinum toxin in management of pain: An evidence-based review. Curr. Opin. Anaesthesiol. 2010, 23, 602–610. [Google Scholar] [CrossRef]
- Dolly, J.O.; Aoki, K.R. The structure and mode of action of different botulinum toxins. Eur. J. Neurol. 2006, 13, 1–9. [Google Scholar] [CrossRef]
- Guo, B.L.; Zheng, C.X.; Sui, B.D.; Li, Y.Q.; Wang, Y.Y.; Yang, Y.L. A closer look to botulinum neurotoxin type A-induced analgesia. Toxicon 2013, 71, 134–139. [Google Scholar] [CrossRef]
- Naumann, M.; Dressler, D.; Hallett, M.; Jankovic, J.; Schiavo, G.; Segal, K.R.; Truong, D. Evidence-based review and assessment of botulinum neurotoxin for the treatment of secretory disorders. Toxicon 2013, 67, 141–152. [Google Scholar] [CrossRef]
- Aurora, S.K.; Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010, 30, 793–803. [Google Scholar] [CrossRef]
- Diener, H.C.; Dodick, D.W.; Aurora, S.K.; Turkel, C.C.; DeGryse, R.E.; Lipton, R.B.; Silberstein, S.D.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010, 30, 804–814. [Google Scholar] [CrossRef]
- Ghasemi, M.; Ansari, M.; Basiri, K.; Shaigannejad, V. The effects of intradermal botulinum toxin type a injections on pain symptoms of patients with diabetic neuropathy. J. Res. Med. Sci. 2014, 19, 106–111. [Google Scholar]
- Brown, E.A.; Schutz, S.G.; Simpson, D.M. Botulinum toxin for neuropathic pain and spasticity: An overview. Pain Manag. 2014, 4, 129–151. [Google Scholar]
- Singh, J.A.; Mahowald, M.L.; Kushnaryov, A.; Goelz, E.; Dykstra, D. Repeat injections of intra-articular botulinum toxin a for the treatment of chronic arthritis joint pain. J. Clin. Rheumatol. 2009, 15, 35–38. [Google Scholar] [CrossRef]
- Marchini, C.; Acler, M.; Bolognari, M.A.; Causero, A.; Volpe, D.; Regis, D.; Rizzo, A.; Rosa, R.; Eleopra, R.; Manganotti, P. Efficacy of botulinum toxin type A treatment of functional impairment of degenerative hip joint: Preliminary results. J. Rehabil. Med. 2010, 42, 691–693. [Google Scholar] [CrossRef]
- Singh, J.A.; Mahowald, M.L.; Noorbaloochi, S. Intra-articular botulinum toxin A for refractory shoulder pain: A randomized, double-blinded, placebo-controlled trial. Transl. Res. 2009, 153, 205–216. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.H.; Song, S.H. Clinical effectiveness of botulinum toxin A compared to a mixture of steroid and local anesthetics as a treatment for sacroiliac joint pain. Pain Med. 2010, 11, 692–700. [Google Scholar] [CrossRef]
- Dolly, J.O.; O’Connell, M.A. Neurotherapeutics to inhibit exocytosis from sensory neurons for the control of chronic pain. Curr. Opin. Pharmacol. 2012, 12, 100–108. [Google Scholar] [CrossRef]
- Blanes-Mira, C.; Merino, J.M.; Valera, E.; Fernandez-Ballester, G.; Gutierrez, L.M.; Viniegra, S.; Perez-Paya, E.; Ferrer-Montiel, A. Small peptides patterned after the N-terminus domain of SNAP25 inhibit SNARE complex assembly and regulated exocytosis. J. Neurochem. 2004, 88, 124–135. [Google Scholar]
- Ponsati, B.; Carreno, C.; Curto-Reyes, V.; Valenzuela, B.; Duart, M.J.; van den Nest, W.; Cauli, O.; Beltran, B.; Fernandez, J.; Borsini, F.; et al. An inhibitor of neuronal exocytosis (DD04107) displays long-lasting in vivo activity against chronic inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2012, 341, 634–645. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).