Polymer Electrolytes for Lithium/Sulfur Batteries
Abstract
:1. Introduction
2. Dry Solid Polymer Electrolytes in Li/S Batteries
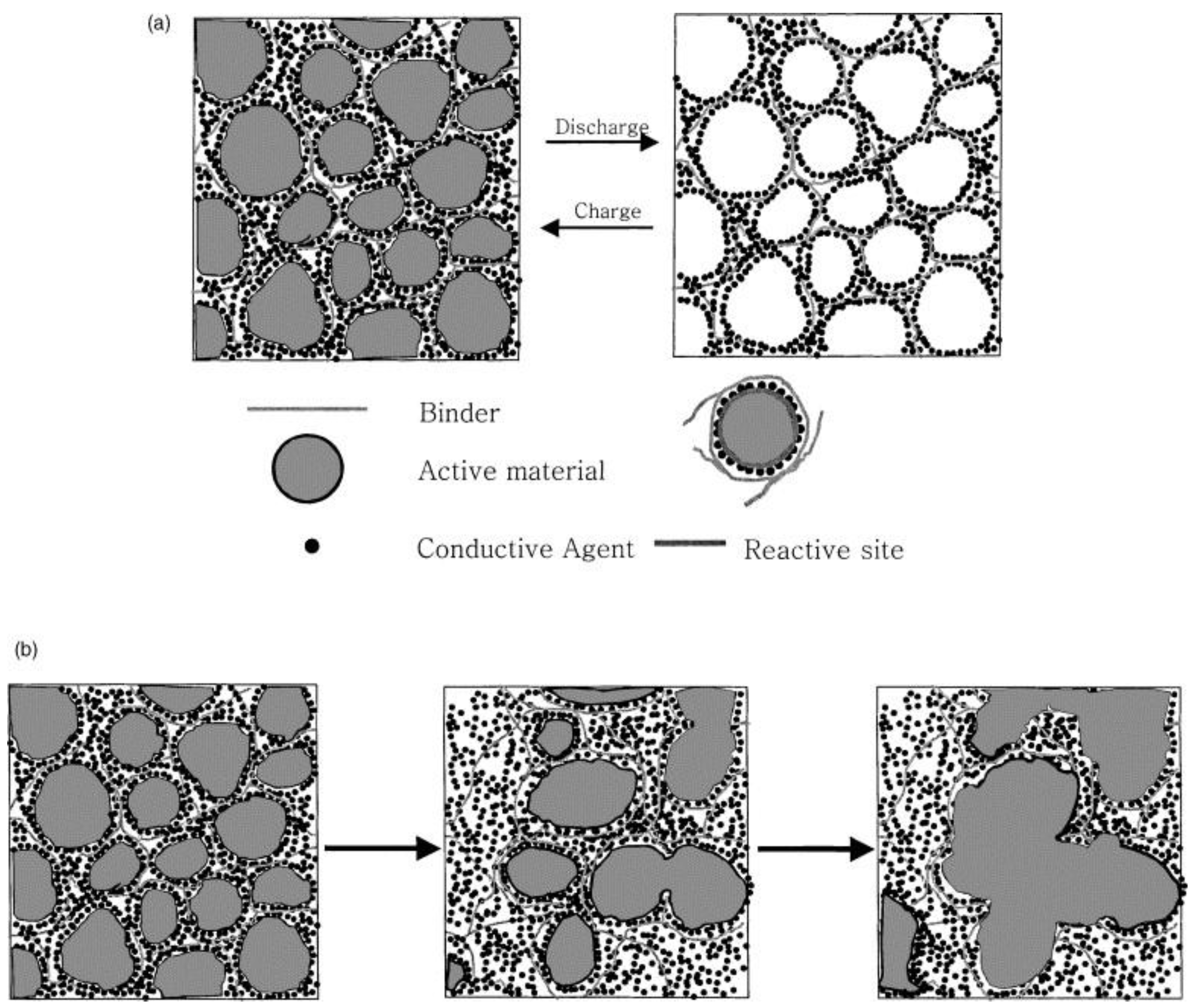
3. Gel Polymer Electrolytes in Li/S Batteries
3.1. PEO-Based Gel Polymer Electrolyte
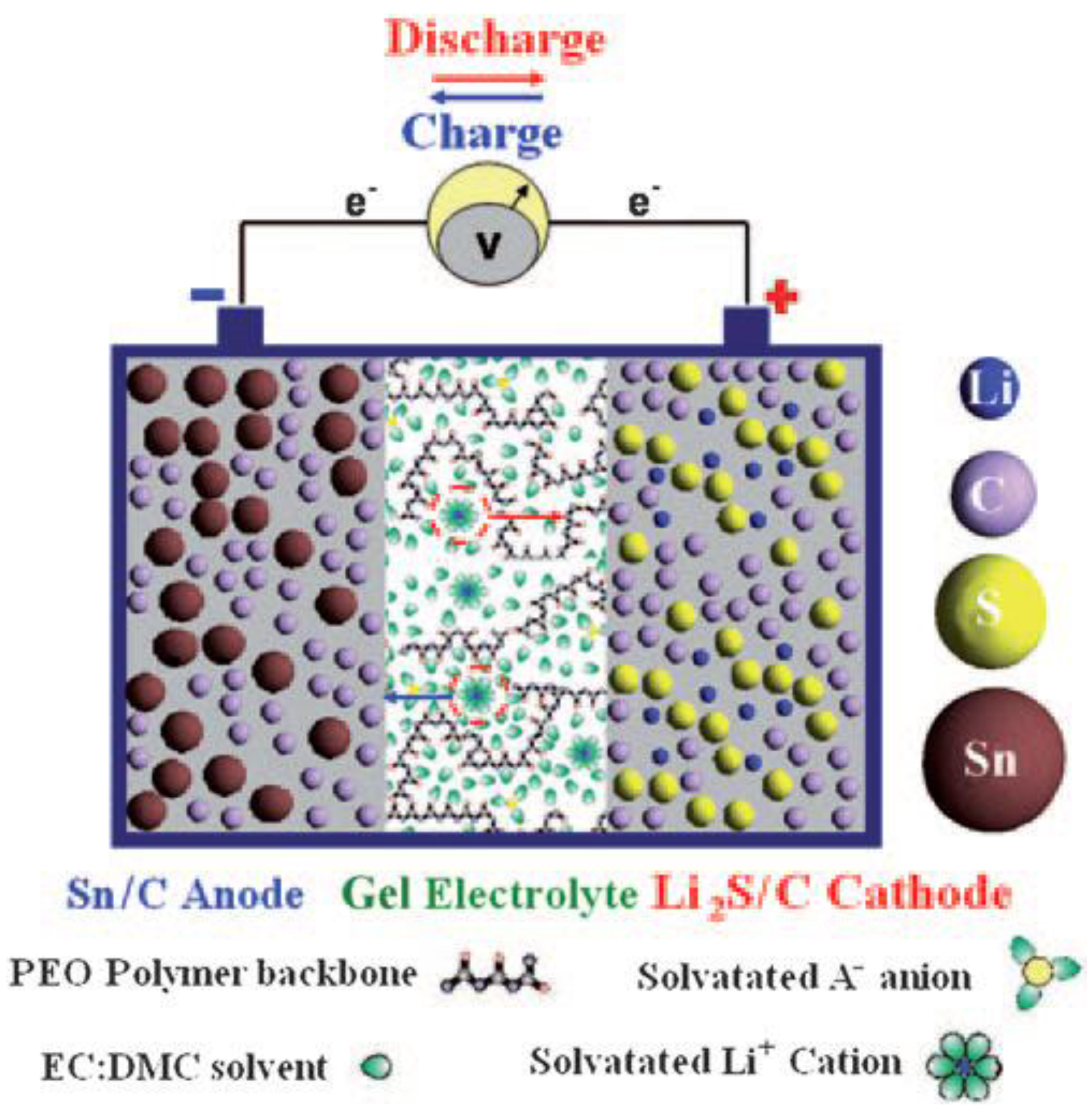
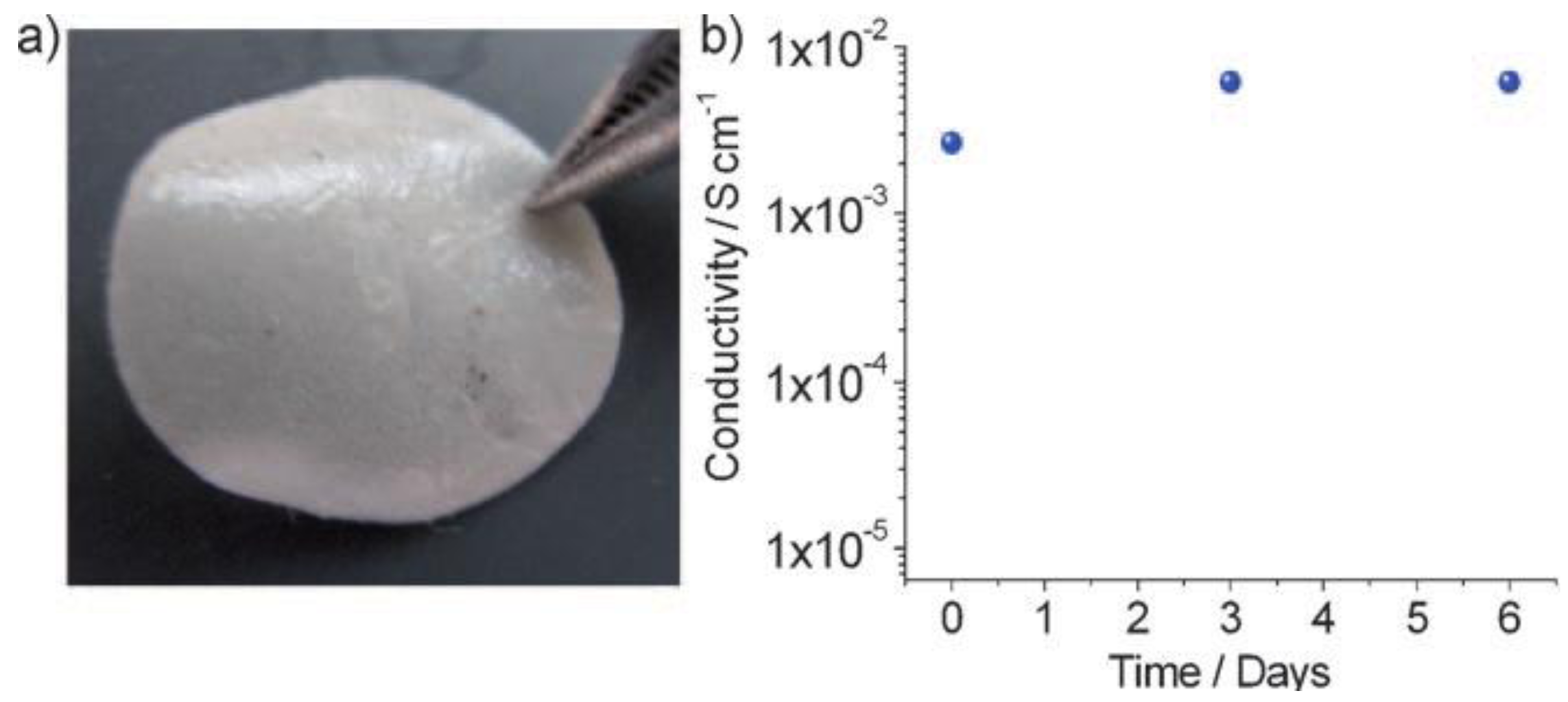
3.2. PVDF-Based Gel Polymer Electrolyte
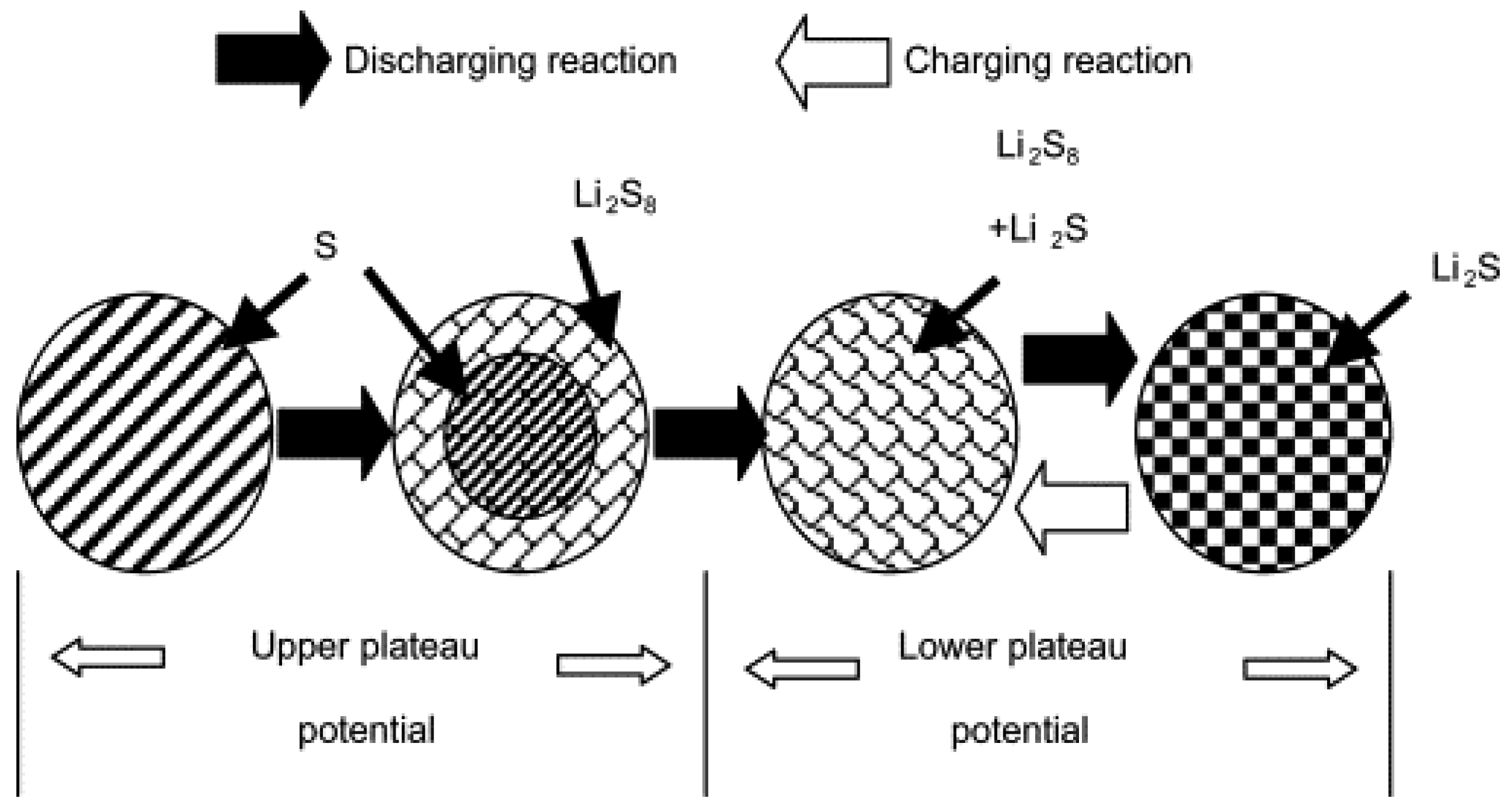
3.3. PVDF-HFP Based Gel Polymer Electrolyte
4. Conclusions
Acknowledgments
References
- Song, D.; Ikuta, H.; Uchida, T.; Wakihara, M. The spinel phases LiAlyMn2−yO4 (y = 0, 1/12, 1/9, 1/6, 1/3) and Li(Al,M)1/6Mn11/6O4 (M = Cr, Co) as the cathode for rechargeable lithium batteries. Solid State Ionics 1999, 117, 151–156. [Google Scholar] [CrossRef]
- Bakenov, Z.; Taniguchi, I. Electrochemical performance of nanostructured LiMxMn2−xO4 (M = Coand Al) powders at highcharge-discharge operations. Solid State Ionics 2005, 176, 1027–1034. [Google Scholar] [CrossRef]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nature 2009, 458, 190–193. [Google Scholar]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Ji, X.L.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode forlithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Sun, Y.K.; Myung, S.T.; Park, B.C.; Prakash, J.; Belharouak, I.; Amine, K. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 2009, 8, 320–324. [Google Scholar]
- Yang, Y.; McDowell, M.T.; Jackson, A.; Cha, J.J.; Hong, S.S.; Cui, Y. New nanostructured Li2S/silicon rechargeable battery with high specific energy. Nano Lett. 2010, 10, 1486–1491. [Google Scholar] [CrossRef]
- Tischer, R.P. The Sulfur Electrode; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Marmorstein, D.; Yu, T.H.; Striebel, K.A.; McLarnon, F.R.; Hou, J.; Cairns, E.J. Electrochemical performance of lithium/sulfur cells with three different polymer electrolytes. J. Power Sources 2000, 89, 219–226. [Google Scholar] [CrossRef]
- Yamin, H.; Peled, E. Electrochemistry of a nonaqueous lithium/sulfur cell. J. Power Sources 1983, 9, 281–287. [Google Scholar] [CrossRef]
- Rauh, R.D.; Abraham, K.M.; Pearson, G.F.; Surprenant, J.K.; Brummer, S.B. A lithium/dissolved sulfur battery with an organic electrolyte. J. Electrochem. Soc. 1979, 126, 523–527. [Google Scholar] [CrossRef]
- Yamin, H.; Gorenshtein, A.; Penciner, J.; Sternberg, Y.; Peled, E. Lithium sulfur battery. J. Electrochem. Soc. 1988, 135, 1045–1048. [Google Scholar] [CrossRef]
- Shim, J.; Striebel, K.A.; Cairns, E.J. The lithium/sulfur rechargeable cell. J. Electrochem. Soc. 2002, 149, A1321–A1325. [Google Scholar] [CrossRef]
- Cheon, S.E.; Ko, K.S.; Cho, J.H.; Kim, S.W.; Chin, E.Y.; Kim, H.T. Rechargeable lithium sulfur battery. J. Electrochem. Soc. 2003, 150, A796–A799. [Google Scholar]
- Wang, J.L.; Yang, J.; Xie, J.Y.; Xu, N.X.; Li, Y. Sulfur-carbon nano-composite as cathode for rechargeable lithium battery based on gel electrolyte. Electrochem. Commun. 2002, 4, 499–502. [Google Scholar] [CrossRef]
- Han, S.C.; Song, M.S.; Lee, H.; Kim, H.S.; Ahn, H.J.; Lee, J.Y. Effect of multiwalled carbon nanotubes on electrochemical properties of lithium/sulfur rechargeable batteries. J. Electrochem. Soc. 2003, 150, A889–A893. [Google Scholar]
- Zheng, W.; Liu, Y.W.; Hu, X.G.; Zhang, C.F. Novel Nanosized Adsorbing Sulfur Composite Cathode Materials for the Advanced Secondary Lithium Batteries. Electrochim. Acta 2006, 51, 1330–1335. [Google Scholar] [CrossRef]
- Yuan, L.X.; Yuan, H.P.; Qiu, X.P.; Chen, L.Q.; Zhu, W.T. Improvement of cycle property of sulfur-coated multi-walled carbon nanotubes composite cathode for lithium/sulfur batteries. J. Power Sources 2009, 189, 1141–1146. [Google Scholar] [CrossRef]
- Chen, S.R.; Zhai, Y.P.; Xu, G.L.; Jiang, Y.X.; Zhao, D.Y.; Li, J.T.; Huang, L.; Sun, S.G. Ordered mesoporous carbon/sulfur nanocomposite of high performances as cathode for lithium-sulfur battery. Electrochem. Acta 2011, 56, 9549–9555. [Google Scholar] [CrossRef]
- Wang, J.; Chew, S.Y.; Zhao, Z.W.; Ashraf, S.; Wexler, D.; Chen, J.; Ng, S.H.; Chou, S.L.; Liu, H.K. Sulfur-mesoporous carbon composites in conjunction with a novel ionic liquid electrolyte for lithium rechargeable batteries. Carbon 2008, 46, 229–235. [Google Scholar]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Environ. Sci. 2010, 3, 1531–1537. [Google Scholar]
- Zhang, B.; Lai, C.; Zhou, Z.; Gao, X.P. Preparation and electrochemical properties of sulfur-acetylene black composites as cathode materials. Electrochim. Acta 2009, 54, 3708–3713. [Google Scholar] [CrossRef]
- Wang, H.L.; Yang, Y.; Liang, Y.Y.; Robinson, J.T.; Li, Y.G.; Jackson, A.; Cui, Y.; Dai, H.J. Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar]
- Wang, J.L.; Yang, J.; Wan, C.R.; Du, K.; Xie, J.Y.; Xu, N.X. Sulfur composite cathode materials for rechargeable Lithium batteries. Adv. Funct. Mater. 2003, 13, 487–492. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Konstantinov, K.; Zhao, L.; Ng, S.H.; Wang, G.X.; Guo, Z.P.; Liu, H.K. Sulfur-polypyrrole composite positive electrode materials for rechargeable lithium batteries. Electrochim. Acta 2006, 51, 4634–4638. [Google Scholar]
- Sun, M.M.; Zhang, S.C.; Jiang, T.; Zhang, L.; Yu, J.H. Nano-wire networks of sulfurpolypyrrole composite cathode materials for rechargeable lithium batteries. Electrochem. Commun 2008, 10, 1819–1822. [Google Scholar]
- Liang, X.; Liu, Y.; Wen, Z.Y.; Huang, L.Z.; Wang, X.Y.; Zhang, H. A nano-structured and highly ordered polypyrrole-sulfur cathode for lithium-sulfur batteries. J. Power Sources 2011, 196, 6951–6955. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Bakenov, Z.; Zhao, Y.; Konarov, A.; Doan, T.N.L.; Malik, M.; Paron, T.; Chen, P. One-step synthesis of branched sulfur/polypyrrole nanocomposite cathode for lithium rechargeable batteries. J. Power Sources 2012, 208, 1–8. [Google Scholar] [CrossRef]
- Liang, X.; Wen, Z.; Liu, Y.; Zhang, H.; Huang, L.; Jin, J. Highly dispersed sulfur in ordered mesoporous carbon sphere as a composite cathode for rechargeable polymer Li/S battery. J. Power Sources 2011, 196, 3655–3658. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Liang, X.; Wen, Z.; Liu, Y.; Wu, M.; Jin, J.; Zhang, H.; Wu, X. Improved cycling performances of lithium sulfur batteries with LiNO3− modified electrolyte. J. Power Sources 2011, 196, 9839–9843. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, J.K.; Cheruvally, G.; Ahn, J.H.; Ahn, H.J.; Kim, K.W. Rechargeable lithium/sulfur battery with suitable mixed liquid electrolytes. Electrochimica. Acta 2007, 52, 2075–2082. [Google Scholar] [CrossRef]
- Chang, D.R.; Lee, S.H.; Kim, S.W.; Kim, H.T. Binary electrolyte based on tetra(ethylene glycol) dimethyl ether and 1,3-dioxolane for lithium-sulfur battery. J. Power Sources 2002, 112, 452–460. [Google Scholar] [CrossRef]
- Kim, S.; Jung, Y.; Park, S.J. Effects of imidazolium salts on discharge performance of rechargeable lithium-sulfur cells containing organic solvent electrolytes. J. Power Sources 2005, 152, 272–277. [Google Scholar] [CrossRef]
- Choi, J.W.; Cheruvally, G.; Kim, D.S.; Ahn, J.H.; Kim, K.W.; Ahn, H.J. Rechargeable lithium/sulfur battery with liquid electrolytes containing toluene as additive. J. Power Sources 2008, 183, 441–445. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar]
- Shriver, D.F.; Bruce, P.G. Solid State Electrochemistry; Cambridge University Press: Cambridge, UK, 1995; p. 95. [Google Scholar]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Gray, F.M. Solid Polymer Electrolytes-Fundamentals and Technological Applications; VCH: New York, NY, USA, 1991. [Google Scholar]
- Scrosati, B. Applications of Electroactive Polymers; Chapman Hall: London, UK, 1993. [Google Scholar]
- Gray, F.M. Polymer Electrolytes; The Royal Society of Chemistry: Canterbury, UK, 1997. [Google Scholar]
- MacCallum, J.R.; Vincent, C.A. Polymer Electrolytes Reviews-I; Elsevier: London, UK, 1987. [Google Scholar]
- MacCallum, J.R.; Vincent, C.A. Polymer Electrolytes Reviews-II; Elsevier: London, UK, 1989. [Google Scholar]
- Idris, N.H.; Rahman, M.M.; Wang, J.Z.; Liu, H.K. Microporous gel polymer electrolytes for lithium rechargeable battery application. J. Power Sources 2012, 201, 294–300. [Google Scholar] [CrossRef]
- Kim, K.M.; Park, N.G.; Ryu, K.S.; Chang, S.H. Characteristics of PVdF-HFP/TiO2 composite membrane electrolytes prepared by phase inversion and conventional casting methods. Electrochim. Acta 2006, 51, 5636–5644. [Google Scholar] [CrossRef]
- Weston, J.E.; Steele, B.C.H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ionics 1982, 7, 75–79. [Google Scholar] [CrossRef]
- Jeon, B.H.; Yeon, J.H.; Kim, K.M.; Chung, I.J. Preparation and electrochemical properties of lithium-sulfur polymer batteries. J. Power Sources 2002, 109, 89–97. [Google Scholar] [CrossRef]
- Croce, F.; Persi, L.; Scrosati, B.; Serraino-Fiory, F.; Plichta, E.; Hendrickson, M.A. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim. Acta 2001, 46, 2457–2461. [Google Scholar] [CrossRef]
- Dissanayake, M.A.K.L.; Jayathilake, P.A.R.D.; Bokalawela, R.S.P.; Albinsson, I.; Mellander, B.E. Effect of concentration and grain size of alumina filler on the ionic conductivity enhancement of the (PEO)9LiCF3SO3:Al2O3 composite polymer electrolyte. J. Power Sources 2003, 119–121, 409–414. [Google Scholar]
- Ahn, J.H.; Wang, G.X.; Liu, H.K.; Dou, S.X. Nanoparticle-dispersed PEO polymer electrolytes for Li batteries. J. Power Sources 2003, 119–121, 422–426. [Google Scholar]
- Appetecchi, G.B.; Croce, F.; Persi, L.; Ronci, F.; Scrosati, B. Transport and interfacial properties of composite polymer electrolyte. Electrochim. Acta 2000, 45, 1481–1490. [Google Scholar] [CrossRef]
- Jayathilake, P.A.R.D.; Dissanayake, M.A.K.L.; Albinsson, I.; Mellander, B.E. Effect of nano-porous Al2O3 on thermal, dielectric and transport properties of the (PEO)9LiTFSI polymer electrolyte system. Electrochim. Acta 2002, 47, 3257–3268. [Google Scholar]
- Lin, C.W.; Hung, C.L.; Venkateswarlu, M.; Hwang, B.J. Influence of TiO2 nano-particles on the transport properties of composite polymer electrolyte for lithium-ion batteries. J. Power Sources 2005, 146, 397–401. [Google Scholar] [CrossRef]
- Xi, J.; Qiu, X.; Ma, X.; Cui, M.; Yang, J.; Tang, X.; Zhu, W.; Chen, L. Composite polymer electrolyte doped with mesoporous silica SBA-15 for lithium polymer battery. Solid State Ionics 2005, 176, 1249–1260. [Google Scholar] [CrossRef]
- Chung, S.H.; Wang, Y.; Persi, L.; Croce, F.; Greenbaum, S.G.; Scrosati, B.; Plichta, E. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides. J. Power Sources 2001, 97–98, 644–648. [Google Scholar] [CrossRef]
- Croce, F.; Settimi, L.; Scrosati, B. Superacid ZrO2-added, composite polymer electrolytes with improved transport properties. Electrochem. Commun. 2006, 8, 364–368. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, K.W.; Ahn, H.J.; Ahn, J.H. Electrochemical properties and interfacial stability of (PEO)10LiCF3SO3-TinO2n-1 composite polymer electrolytes for lithium/sulfur battery. Mater. Sci. Eng. B 2002, 95, 148–156. [Google Scholar] [CrossRef]
- Jeong, S.S.; Lim, Y.T.; Choi, Y.J.; Cho, G.B.; Kim, K.W.; Ahn, H.J.; Cho, K.K. Electrochemical properties of lithium sulfur cells using PEO polymer electrolytes prepared under three different mixing conditions. J. Power Sources 2007, 174, 745–750. [Google Scholar] [CrossRef]
- Zhu, X.; Wen, Z.; Gu, Z.; Lin, Z. Electrochemical characterization and performance improvement of lithium/sulfur polymer batteries. J. Power Sources 2005, 139, 269–273. [Google Scholar] [CrossRef]
- Agrawa, R.C.; Pandey, G.P. Solid polymer electrolytes: Materialdesigning and all-solid-state batteryapplications: An overview. J. Phys. D Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Gray, F.M. Polymer Electrolytes: Fundamentals and Technological Applications; VCH: New York, NY, USA, 1991. [Google Scholar]
- Wu, Y.P.; Zhang, H.P.; Wu, F.; Li, Z.H. Polymer Lithium-Ion Batteries; Chemical Industry Press: Beijing, China, 2007. [Google Scholar]
- Xu, J.J.; Ye, H. Polymer gel electrolytes based on oligomeric polyether/cross-linked PMMA blends prepared via in situ polymerization. Electrochem. Commun. 2005, 7, 829–835. [Google Scholar] [CrossRef]
- Oliver, M. Blended Polymer Gel Electrolytes. U.S. Patent 5,658,685, 19 August 1997. [Google Scholar]
- Hassoun, J.; Scrosati, B. A high-performance polymer tin sulfur lithium ion battery. Angew. Chem. Int. Ed. 2010, 49, 2371–2374. [Google Scholar] [CrossRef]
- Scrosati, B. Lithium polymer electrolytes. In Advances in Lithium Ion Batteries; Scrosati, S., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 251–266. [Google Scholar]
- Appetecchi, G.B.; Romagnoli, P.; Scrosati, B. Composite gel membranes: A new class of improved polymer electrolytes for lithium batteries. Electrochem. Commun. 2001, 3, 281–284. [Google Scholar] [CrossRef]
- MacDonald, J.R.; Barsoukov, E. Impedance Spectroscopy, Theory, Experiment and Applications, 2nd ed; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membrane Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Watanabe, M.; Kanba, M.; Matsuda, H.; Mizoguchi, K.; Shinohara, I.; Tsuchida, E.; Tsunemi, K. High lithium ionic conductivity of polymeric solid electrolytes. Makromol. Chem Rapid Commun. 1981, 2, 741–744. [Google Scholar]
- Ryu, H.S.; Ahn, H.J.; Kim, K.W.; Ahn, J.H.; Lee, J.Y. Discharge process of Li/PVdF/S cells at room temperature. J. Power Sources 2006, 153, 360–364. [Google Scholar] [CrossRef]
- Stephan, A.M.; Nahm, K.S.; Kulandainathan, M.A.; Ravi, G.; Wilson, J. Poly(vinylidene fluoride-hexafluoropropylene) (PVdF-HFP) based composite electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 1728–1734. [Google Scholar]
- Stephan, M.A.; Dale, T. Charge-discharge studies on a lithium cell composed of PVdF-HFP polymer membranes prepared by phase inversion technique with a nanocomposite cathode. J. Power Sources 2003, 119–121, 460–467. [Google Scholar] [CrossRef]
- Stephan, M.A.; Dale, T. Characterization of PVdF-HFP polymer membranes prepared by phase inversion technique I: Morphology and charge discharge studies. Electrochim. Acta 2003, 48, 2143–2148. [Google Scholar] [CrossRef]
- Shin, J.H.; Jung, S.S.; Kim, K.W.; Ahn, H.J. Preparation and characterization of plasticized polymer electrolytes based on the PVdF-HFP copolymer for lithium/sulfur battery. J. Mater. Sci. Mater. Electron. 2002, 13, 727–733. [Google Scholar] [CrossRef]
- Li, G.C.; Li, Z.H.; Zhang, P.; Zhang, H.P.; Wu, Y.P. Research on a gel polymer electrolyte for Li-ionbatteries. Pure Appl. Chem. 2008, 80, 2553–2563. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, Y.; Zhang, Y.; Gosselink, D.; Doan, T.N.L.; Sadhu, M.; Cheang, H.-J.; Chen, P. Polymer Electrolytes for Lithium/Sulfur Batteries. Membranes 2012, 2, 553-564. https://doi.org/10.3390/membranes2030553
Zhao Y, Zhang Y, Gosselink D, Doan TNL, Sadhu M, Cheang H-J, Chen P. Polymer Electrolytes for Lithium/Sulfur Batteries. Membranes. 2012; 2(3):553-564. https://doi.org/10.3390/membranes2030553
Chicago/Turabian StyleZhao, Yan, Yongguang Zhang, Denise Gosselink, The Nam Long Doan, Mikhail Sadhu, Ho-Jae Cheang, and Pu Chen. 2012. "Polymer Electrolytes for Lithium/Sulfur Batteries" Membranes 2, no. 3: 553-564. https://doi.org/10.3390/membranes2030553
APA StyleZhao, Y., Zhang, Y., Gosselink, D., Doan, T. N. L., Sadhu, M., Cheang, H.-J., & Chen, P. (2012). Polymer Electrolytes for Lithium/Sulfur Batteries. Membranes, 2(3), 553-564. https://doi.org/10.3390/membranes2030553



