Adsorption of Myelin Basic Protein on Model Myelin Membranes Reveals Weakening of van der Waals Interactions in a Lipid Ratio-Dependent Manner
Abstract
1. Introduction
2. Experimental
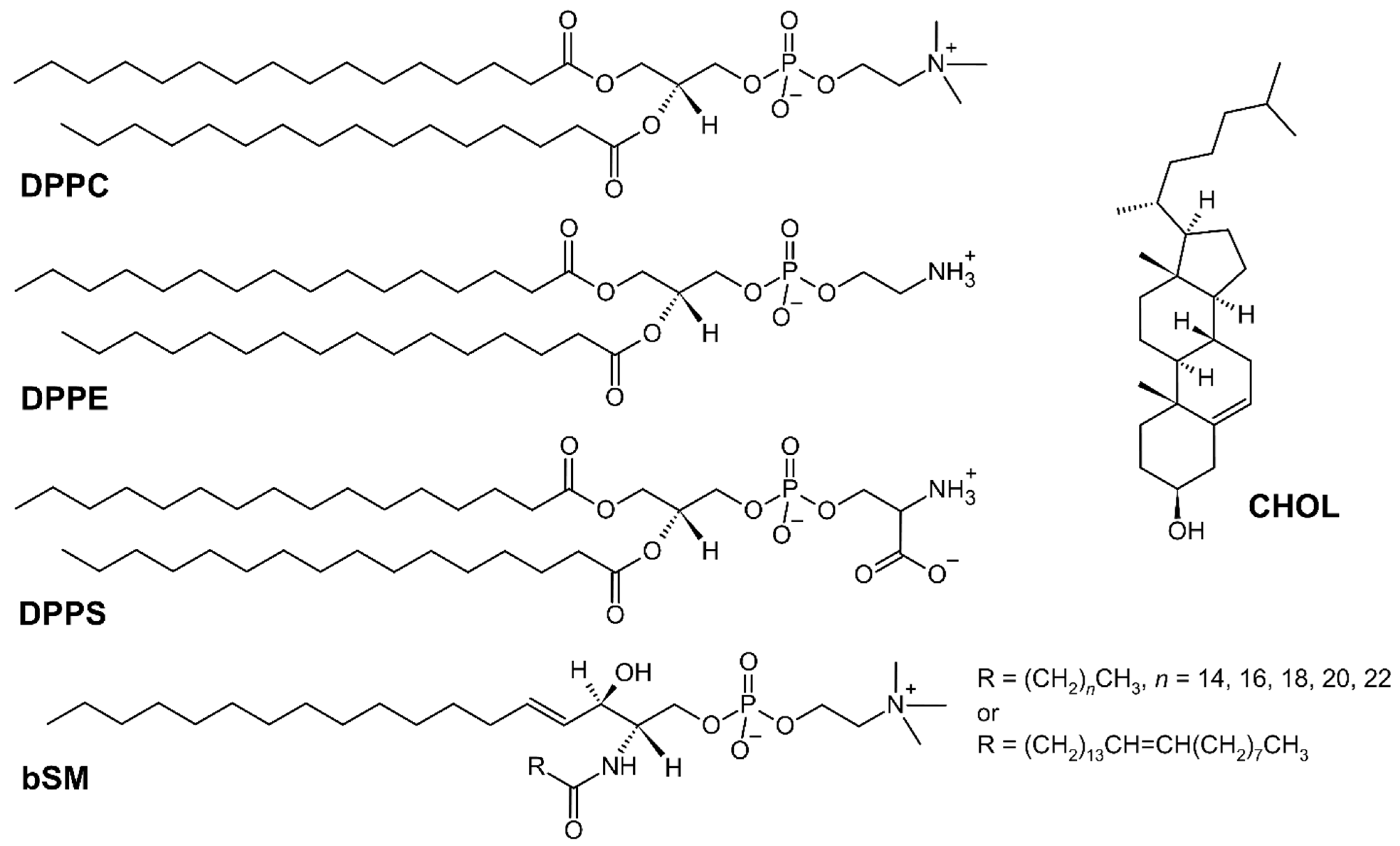
2.1. Chemicals and MMM Preparation
2.2. Dynamic and Electrophoretic Light Scattering (DLS and ELS) of LUVs: Measurements and Data Analysis
2.3. Differential Scanning Calorimetry (DSC) of MMMs: Data Acquisition and Curve Analysis
2.4. FTIR Spectra of MMMs: Data Acquisition and Band Analysis
2.5. CD Spectra of MBP Adsorbed on MMMs: Measurements and Data Analysis
3. Results and Discussion
3.1. The Size and Aggregation of MMMs Depend on the Lipid Composition and the Presence of MBP
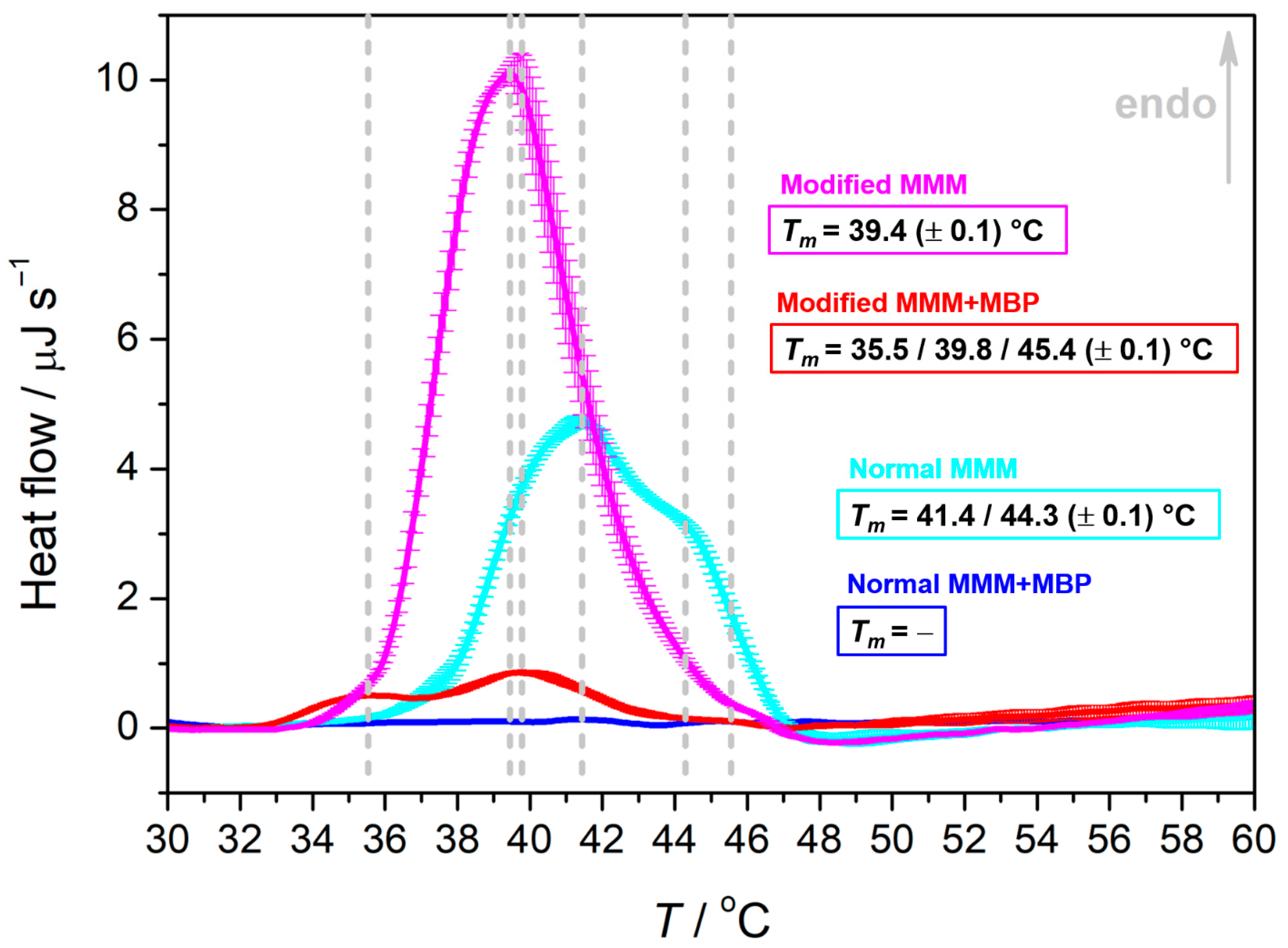
3.2. MBP Modulates Thermotropic Properties of Normal and Modified MMMs in a Qualitatively Different Manner
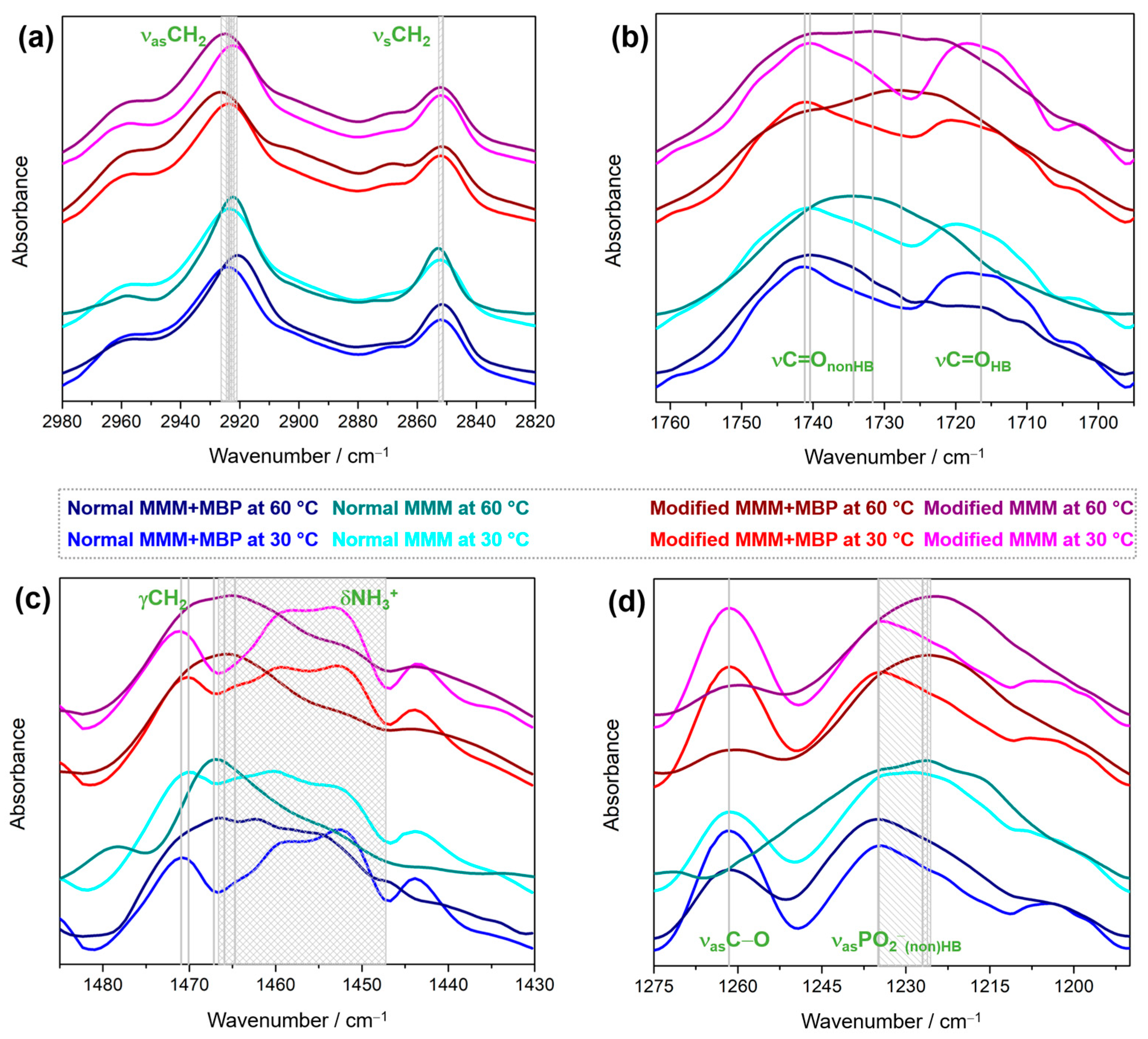
3.3. MBP-Induced Weakening of van der Waals Interactions Between Hydrocarbon Chains Depends on Lipid Composition
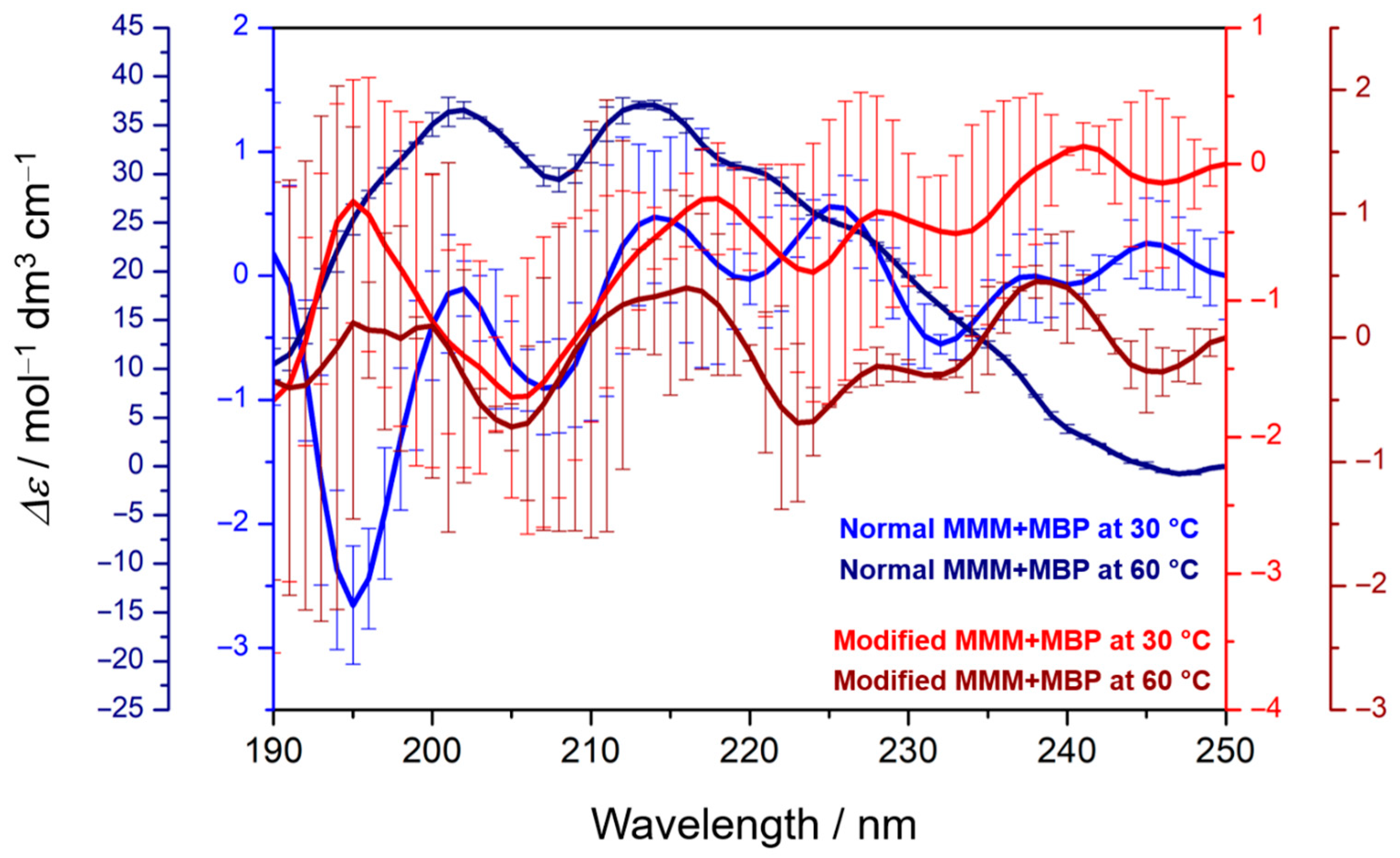
3.4. Secondary Structure of MBP Depends on MMM Composition and the Phase
3.5. Implications of MBP Adsorption on the Changes in Lipid Membrane Fluidity and Aggregation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caprariello, A.V.; Rogers, J.A.; Morgan, M.L.; Hoghooghi, V.; Plemel, J.R.; Koebel, A.; Tsutsui, S.; Dunn, J.F.; Kotra, L.P.; Ousman, S.S.; et al. Biochemically Altered Myelin Triggers Autoimmune Demyelination. Proc. Natl. Acad. Sci. USA 2018, 115, 5528–5533. [Google Scholar] [CrossRef]
- Snaidero, N.; Simons, M. Myelination at a Glance. J. Cell Sci. 2014, 127, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Quarles, R.H. Myelin Lipids and Proteins: Structure, Function, and Roles in Neurological Disorders; Sibley, D.R., Hanin, I., Kuhar, M., Skolnick, P., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Kristiansen, K.; Boggs, J.M.; Husted, C.; Zasadzinski, J.A.; Israelachvili, J. Interaction Forces and Adhesion of Supported Myelin Lipid Bilayers Modulated by Myelin Basic Protein. Proc. Natl. Acad. Sci. USA 2009, 106, 3154–3159. [Google Scholar] [CrossRef]
- Palavicini, J.P.; Wang, C.; Chen, L.; Ahmar, S.; Higuera, J.D.; Dupree, J.L.; Han, X. Novel Molecular Insights into the Critical Role of Sulfatide in Myelin Maintenance/Function. J. Neurochem. 2016, 139, 40–54. [Google Scholar] [CrossRef]
- Träger, J.; Meister, A.; Hause, G.; Harauz, G.; Hinderberger, D. Shaping Membrane Interfaces in Lipid Vesicles Mimicking the Cytoplasmic Leaflet of Myelin through Variation of Cholesterol and Myelin Basic Protein Contents. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184179. [Google Scholar] [CrossRef] [PubMed]
- Raasakka, A.; Kursula, P. Flexible Players within the Sheaths: The Intrinsically Disordered Proteins of Myelin in Health and Disease. Cells 2020, 9, 470. [Google Scholar] [CrossRef]
- Harauz, G.; Ishiyama, N.; Hill, C.M.D.; Bates, I.R.; Libich, D.S.; Farès, C. Myelin Basic Protein—Diverse Conformational States of an Intrinsically Unstructured Protein and Its Roles in Myelin Assembly and Multiple Sclerosis. Micron 2004, 35, 503–542. [Google Scholar] [CrossRef]
- Beniac, D.R.; Luckevich, M.D.; Czarnota, G.J.; Tompkins, T.A.; Ridsdale, R.A.; Ottensmeyer, F.P.; Moscarello, M.A.; Harauz, G. Three-Dimensional Structure of Myelin Basic Protein: I. Reconstruction via Angular Reconstitution of Randomly Oriented Single Particles. J. Biol. Chem. 1997, 272, 4261–4268. [Google Scholar] [CrossRef]
- Mac Millan, S.V.; Ishiyama, N.; White, G.F.; Palaniyar, N.; Hallett, F.R.; Harauz, G. Myelin Basic Protein Component C1 in Increasing Concentrations Can Elicit Fusion, Aggregation, and Fragmentation of Myelin-like Membranes. Eur. J. Cell Biol. 2000, 79, 327–335. [Google Scholar] [CrossRef]
- Harauz, G.; Boggs, J.M. Myelin Management by the 18.5-kKDa and 21.5-kDa Classic Myelin Basic Protein Isoforms. J. Neurochem. 2013, 125, 334–361. [Google Scholar] [CrossRef]
- Mahajan, K.R.; Herman, D.; Zheng, Y.; Androjna, C.; Thoomukuntla, B.; Ontaneda, D.; Nakamura, K.; Trapp, B.D. Neurodegeneration and Demyelination in the Multiple Sclerosis Spinal Cord: Clinical, Pathological, and 7T MRI Perspectives. Neurology 2025, 104, e210259. [Google Scholar] [CrossRef]
- Osorio-Querejeta, I.; Sáenz-Cuesta, M.; Muñoz-Culla, M.; Otaegui, D. Models for Studying Myelination, Demyelination and Remyelination. Neuromol. Med. 2017, 19, 181–192. [Google Scholar] [CrossRef]
- Osorio-Querejeta, I.; Alberro, A.; Muñoz-Culla, M.; Mäger, I.; Otaegui, D. Therapeutic Potential of Extracellular Vesicles for Demyelinating Diseases; Challenges and Opportunities. Front. Mol. Neurosci. 2018, 11, 434. [Google Scholar] [CrossRef]
- Widder, K.; Harauz, G.; Hinderberger, D. Myelin Basic Protein (MBP) Charge Variants Show Different Sphingomyelin-Mediated Interactions with Myelin-like Lipid Monolayers. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183077. [Google Scholar] [CrossRef]
- Desai, R.A.; Davies, A.L.; Tachrount, M.; Kasti, M.; Laulund, F.; Golay, X.; Smith, K.J. Cause and Prevention of Demyelination in a Model Multiple Sclerosis Lesion. Ann. Neurol. 2016, 79, 591–604. [Google Scholar] [CrossRef]
- Rodriguez, M. Effectors of Demyelination and Remyelination in the CNS: Implications for Multiple Sclerosis. Brain Pathol. 2007, 17, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Situ, A.J.; Ulmer, T.S. Structural and Thermodynamic Basis of Proline-Induced Transmembrane Complex Stabilization. Sci. Rep. 2016, 6, 29809. [Google Scholar] [CrossRef] [PubMed]
- Widder, K.; Träger, J.; Kerth, A.; Harauz, G.; Hinderberger, D. Interaction of Myelin Basic Protein with Myelin-like Lipid Monolayers at Air-Water Interface. Langmuir 2018, 34, 6095–6108. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.S.B.; Færgeman, N.J. Sphingolipids: Membrane Microdomains in Brain Development, Function and Neurological Diseases. Open Biol. 2017, 7, 170069. [Google Scholar] [CrossRef]
- Lee, D.W.; Banquy, X.; Kristiansen, K.; Kaufman, Y.; Boggs, J.M.; Israelachvili, J.N. Lipid Domains Control Myelin Basic Protein Adsorption and Membrane Interactions between Model Myelin Lipid Bilayers. Proc. Natl. Acad. Sci. USA 2014, 111, E768–E775. [Google Scholar] [CrossRef]
- Weil, M.T.; Möbius, W.; Winkler, A.; Ruhwedel, T.; Wrzos, C.; Romanelli, E.; Bennett, J.L.; Enz, L.; Goebels, N.; Nave, K.A.; et al. Loss of Myelin Basic Protein Function Triggers Myelin Breakdown in Models of Demyelinating Diseases. Cell Rep. 2016, 16, 314–322. [Google Scholar] [CrossRef]
- Don, A.S.; Hsiao, J.H.T.; Bleasel, J.M.; Couttas, T.A.; Halliday, G.M.; Kim, W.S. Altered Lipid Levels Provide Evidence for Myelin Dysfunction in Multiple System Atrophy. Acta Neuropathol. Commun. 2014, 2, 150. [Google Scholar] [CrossRef] [PubMed]
- Gasecka, P.; Jaouen, A.; Bioud, F.Z.; de Aguiar, H.B.; Duboisset, J.; Ferrand, P.; Rigneault, H.; Balla, N.K.; Debarbieux, F.; Brasselet, S. Lipid Order Degradation in Autoimmune Demyelination Probed by Polarized Coherent Raman Microscopy. Biophys. J. 2017, 113, 1520–1530. [Google Scholar] [CrossRef]
- Shaharabani, R.; Ram-On, M.; Avinery, R.; Aharoni, R.; Arnon, R.; Talmon, Y.; Beck, R. Structural Transition in Myelin Membrane as Initiator of Multiple Sclerosis. J. Am. Chem. Soc. 2016, 138, 12159–12165. [Google Scholar] [CrossRef]
- Shaharabani, R.; Ram-On, M.; Talmon, Y.; Beck, R. Pathological Transitions in Myelin Membranes Driven by Environmental and Multiple Sclerosis Conditions. Proc. Natl. Acad. Sci. USA 2018, 115, 11156–11161. [Google Scholar] [CrossRef]
- Raasakka, A.; Ruskamo, S.; Kowal, J.; Barker, R.; Baumann, A.; Martel, A.; Tuusa, J.; Myllykoski, M.; Bürck, J.; Ulrich, A.S.; et al. Membrane Association Landscape of Myelin Basic Protein Portrays Formation of the Myelin Major Dense Line. Sci. Rep. 2017, 7, 4974. [Google Scholar] [CrossRef] [PubMed]
- Raasakka, A.; Jones, N.C.; Hoffmann, S.V.; Kursula, P. Ionic Strength and Calcium Regulate Membrane Interactions of Myelin Basic Protein and the Cytoplasmic Domain of Myelin Protein Zero. Biochem. Biophys. Res. Commun. 2019, 511, 7–12. [Google Scholar] [CrossRef]
- Keniry, M.A.; Smith, R. Circular dichroic analysis of the secondary structure of myelin basic protein and derived peptides bound to detergents and to lipid vesicles. Biochim. Biophys. Acta 1979, 578, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Smith, R. The Basic Protein of CNS Myelin: Its Structure and Ligand Binding. J. Neurochem. 1992, 59, 1589–1608. [Google Scholar] [CrossRef]
- Polverini, E.; Fasano, A.; Zito, F.; Riccio, P.; Cavatorta, P. Conformation of Bovine Myelin Basic Protein Purified with Bound Lipids. Eur. Biophys. J. 1999, 28, 351–355. [Google Scholar] [CrossRef]
- Maleš, P.; Brkljača, Z.; Crnolatac, I.; Petrov, D.; Bakarić, D. Phase-Dependent Adsorption of Myelin Basic Protein to Phosphatidylcholine Lipid Bilayers. Membranes 2024, 14, 15. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and Phase Transitions of the Phosphatidylcholines. Biochim. Biophys. Acta Rev. Biomembr. 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and Phase Transitions of the Sphingolipids. Biochim. Biophys. Acta 1995, 1255, 213–236. [Google Scholar] [CrossRef]
- Marsh, D. Structural and Thermodynamic Determinants of Chain-Melting Transition Temperatures for Phospholipid and Glycolipids Membranes. Biochim. Biophys. Acta Biomembr. 2010, 1798, 40–51. [Google Scholar] [CrossRef]
- Schmitt, S.; Castelvetri, L.C.; Simons, M. Metabolism and Functions of Lipids in Myelin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 999–1005. [Google Scholar] [CrossRef]
- Di Gioacchino, M.; Bianconi, A.; Burghammer, M.; Ciasca, G.; Bruni, F.; Campi, G. Myelin Basic Protein Dynamics from Out-of-Equilibrium Functional State to Degraded State in Myelin. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183256. [Google Scholar] [CrossRef]
- Frid, K.; Einstein, O.; Friedman-Levi, Y.; Binyamin, O.; Ben-Hur, T.; Gabizon, R. Aggregation of MBP in Chronic Demyelination. Ann. Clin. Transl. Neurol. 2015, 2, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An Overview of Liposome Lyophilization and Its Future Potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Maleš, P.; Brkljača, Z.; Crnolatac, I.; Bakarić, D. Application of MCR-ALS with EFA on FT-IR Spectra of Lipid Bilayers in the Assessment of Phase Transition Temperatures: Potential for Discernment of Coupled Events. Colloids Surf. B Biointerfaces 2021, 201, 111645. [Google Scholar] [CrossRef] [PubMed]
- Menges, F. Spectragryph—Optical Spectroscopy Software. Available online: https://www.effemm2.de/spectragryph/ (accessed on 28 May 2025).
- Pašalić, L.; Pem, B.; Bakarić, D. Lamellarity-Driven Differences in Surface Structural Features of DPPS Lipids: Spectroscopic, Calorimetric and Computational Study. Membranes 2023, 13, 83. [Google Scholar] [CrossRef]
- Pem, B.; Brkljača, Z.; Philippe, A.; Schaumann, G.E.; Vazdar, M.; Bakarić, D. FTIR Spectroscopy and Molecular Level Insight of Diluted Aqueous Solutions of Acetic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 302, 123135. [Google Scholar] [CrossRef]
- BeStSel. BeStSelTM (2014–2023)—ELTE Eötvös Loránd University, Budapest, Hungary. Available online: https://bestsel.elte.hu/index.php (accessed on 8 May 2025).
- Bamm, V.V.; De Avila, M.; Smith, G.S.T.; Ahmed, M.A.M.; Harauz, G. Structured Functional Domains of Myelin Basic Protein: Cross Talk between Actin Polymerization and Ca2+-Dependent Calmodulin Interaction. Biophys. J. 2011, 101, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Levine, Z.A.; Larini, L.; LaPointe, N.E.; Feinstein, S.C.; Shea, J.E. Regulation and Aggregation of Intrinsically Disordered Peptides. Proc. Natl. Acad. Sci. USA 2015, 112, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manyes, S.; Oncins, G.; Sanz, F. Effect of Ion-Binding and Chemical Phospholipid Structure on the Nanomechanics of Lipid Bilayers Studied by Force Spectroscopy. Biophys. J. 2005, 89, 1812–1826. [Google Scholar] [CrossRef]
- De Vries, A.H.; Yefimov, S.; Mark, A.E.; Marrink, S.J. Molecular Structure of the Lecithin Ripple Phase. Proc. Natl. Acad. Sci. USA 2005, 102, 5392–5396. [Google Scholar] [CrossRef]
- Böckmann, R.A.; Hac, A.; Heimburg, T.; Grubmüller, H. Effect of Sodium Chloride on a Lipid Bilayer. Biophys. J. 2003, 85, 1647–1655. [Google Scholar] [CrossRef]
- Šegota, S.; Vojta, D.; Pletikapić, G.; Baranović, G. Ionic Strength and Composition Govern the Elasticity of Biological Membranes. A Study of Model DMPC Bilayers by Force- and Transmission IR Spectroscopy. Chem. Phys. Lipids 2015, 186, 17–29. [Google Scholar] [CrossRef]
- Cevc, G. Polymorphism of the Bilayer Membranes in the Ordered Phase and the Molecular Origin of the Lipid Pretransition and Rippled Lamellae. Biochim. Biophys. Acta Biomembr. 1991, 1062, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Heimburg, T. A Model for the Lipid Pretransition: Coupling of Ripple Formation with the Chain-Melting Transition. Biophys. J. 2000, 78, 1154–1165. [Google Scholar] [CrossRef]
- Mantsch, H.H.; Madec, C.; Lewis, R.N.; McElhaney, R.N. Thermotropic Phase Behavior of Model Membranes Composed of Phosphatidylcholines Containing Iso-Branched Fatty Acids. 2. Infrared and 31P NMR Spectroscopy Studies. Biochemistry 1985, 24, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.N.A.H.; McElhaney, R.N. Membrane Lipid Phase Transitions and Phase Organization Studied by Fourier Transform Infrared Spectroscopy. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Casal, H.L.; Mantsch, H.H. The Thermotropic Phase Behavior of N-Methylated Dipalmitoylphosphatidylethanolamines. Biochim. Biophys. Acta Biomembr. 1983, 735, 387–396. [Google Scholar] [CrossRef]
- Cameron, D.G.; Casal, H.L.; Mantsch, H.H. Characterization of the Pretransition in 1,2-Dipalmitoyl-sn-Glycero-3-Phosphocholine by Fourier Transform Infrared Spectroscopy. Biochemistry 1980, 19, 3665–3672. [Google Scholar] [CrossRef]
- Pašalić, L.; Pem, B.; Domazet Jurašin, D.; Vazdar, M.; Bakarić, D. Interaction of Guanidinium and Ammonium Cations with Phosphatidylcholine and Phosphatidylserine Lipid Bilayers—Calorimetric, Spectroscopic and Molecular Dynamics Simulations Study. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184122. [Google Scholar] [CrossRef] [PubMed]
- Maleš, P.; Munivrana, J.; Pašalić, L.; Pem, B.; Bakarić, D. Reorientation of Interfacial Water Molecules during Melting of Brain Sphingomyelin Is Associated with the Phase Transition of Its C24:1 Sphingomyelin Lipids. Chem. Phys. Lipids 2024, 264, 105434. [Google Scholar] [CrossRef]
- Maleš, P.; Nikšić-Franjić, I.; Wang, A.; Pem, B.; Bakarić, D. Optical and Molecular Features of Negatively Curved Surfaces Created by POPE Lipids: A Crucial Role of the Initial Conditions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 317, 124462. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N.; Pohle, W.; Mantsch, H.H. Components of the Carbonyl Stretching Band in the Infrared Spectra of Hydrated 1,2-Diacylglycerolipid Bilayers: A Reevaluation. Biophys. J. 1994, 67, 2367–2375. [Google Scholar] [CrossRef]
- Steinbauer, B.; Mehnert, T.; Beyer, K. Hydration and Lateral Organization in Phospholipid Bilayers Containing Sphingomyelin: A 2H-NMR Study. Biophys. J. 2003, 85, 1013–1024. [Google Scholar] [CrossRef][Green Version]
- Schmid, F. Physical Mechanisms of Micro- and Nanodomain Formation in Multicomponent Lipid Membranes. Biochim. Biophys. Acta Biomembr. 2017, 1859, 509–528. [Google Scholar] [CrossRef]
- Bagatolli, L.A. To See or Not to See: Lateral Organization of Biological Membranes and Fluorescence Microscopy. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1541–1556. [Google Scholar] [CrossRef] [PubMed]
- Ramstedt, B.; Slotte, J.P. Membrane Properties of Sphingomyelins. FEBS Lett. 2002, 531, 33–37. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N. Vibrational Spectroscopy of Lipids. In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 3447–3464. ISBN 9780470027325. [Google Scholar]
- Csiszár, A.; Koglin, E.; Meier, R.J.; Klumpp, E. The Phase Transition Behavior of 1,2-Dipalmitoyl-sn-Glycero-3-Phosphocholine (DPPC) Model Membrane Influenced by 2,4-Dichlorophenol—An FT-Raman Spectroscopy Study. Chem. Phys. Lipids 2006, 139, 115–124. [Google Scholar] [CrossRef]
- Arsov, Z.; Quaroni, L. Detection of Lipid Phase Coexistence and Lipid Interactions in Sphingomyelin/Cholesterol Membranes by ATR-FTIR Spectroscopy. Biochim. Biophys. Acta Biomembr. 2008, 1778, 880–889. [Google Scholar] [CrossRef]
- Jiménez-Rojo, N.; García-Arribas, A.B.; Sot, J.; Alonso, A.; Goñi, F.M. Lipid Bilayers Containing Sphingomyelins and Ceramides of Varying N-Acyl Lengths: A Glimpse into Sphingolipid Complexity. Biochim. Biophys. Acta Biomembr. 2014, 1838, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Alavizargar, A.; Gass, M.; Krahn, M.P.; Heuer, A. Elucidating the Membrane Binding Process of a Disordered Protein: Dynamic Interplay of Anionic Lipids and the Polybasic Region. ACS Phys. Chem. Au 2024, 4, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Rispoli, P.; Carzino, R.; Svaldo-Lanero, T.; Relini, A.; Cavalleri, O.; Fasano, A.; Liuzzi, G.M.; Carlone, G.; Riccio, P.; Gliozzi, A.; et al. A Thermodynamic and Structural Study of Myelin Basic Protein in Lipid Membrane Models. Biophys. J. 2007, 93, 1999–2010. [Google Scholar] [CrossRef][Green Version]
- Min, Y.; Alig, T.F.; Lee, D.W.; Boggs, J.M.; Israelachvili, J.N.; Zasadzinski, J.A. Critical and Off-Critical Miscibility Transitions in Model Extracellular and Cytoplasmic Myelin Lipid Monolayers. Biophys. J. 2011, 100, 1490–1498. [Google Scholar] [CrossRef]
- Benesch, M.G.K.; Lewis, R.N.A.H.; McElhaney, R.N. A Calorimetric and Spectroscopic Comparison of the Effects of Cholesterol and Its Sulfur-Containing Analogs Thiocholesterol and Cholesterol Sulfate on the Thermotropic Phase Behavior and Organization of Dipalmitoylphosphatidylcholine Bilayer Membranes. Biochim. Biophys. Acta Biomembr. 2016, 1858, 168–180. [Google Scholar] [CrossRef]
- Chatterley, A.S.; Laity, P.; Holland, C.; Weidner, T.; Woutersen, S.; Giubertoni, G. Broadband Multidimensional Spectroscopy Identifies the Amide II Vibrations in Silkworm Films. Molecules 2022, 27, 6275. [Google Scholar] [CrossRef]
- de la Arada, I.; González-Ramírez, E.J.; Alonso, A.; Goñi, F.M.; Arrondo, J.L.R. Exploring Polar Headgroup Interactions between Sphingomyelin and Ceramide with Infrared Spectroscopy. Sci. Rep. 2020, 10, 17606. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Thyparambil, A.A.; Latour, R.A. Protein Helical Structure Determination Using CD Spectroscopy for Solutions with Strong Background Absorbance from 190 to 230 Nm. Biochim. Biophys. Acta Proteins Proteom. 2014, 1844, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Paradoxes and Wonders of Intrinsic Disorder: Stability of Instability. Intrinsically Disord. Proteins 2017, 5, e1327757. [Google Scholar] [CrossRef]
- Inoue, R.; Oda, T.; Nakagawa, H.; Tominaga, T.; Ikegami, T.; Konuma, T.; Iwase, H.; Kawakita, Y.; Sato, M.; Sugiyama, M. Revealing an Origin of Temperature-Dependent Structural Change in Intrinsically Disordered Proteins. Biophys. J. 2025, 124, 540–548. [Google Scholar] [CrossRef]
- Balleza, D.; Mescola, A.; Marín–Medina, N.; Ragazzini, G.; Pieruccini, M.; Facci, P.; Alessandrini, A. Complex Phase Behavior of GUVs Containing Different Sphingomyelins. Biophys. J. 2019, 116, 503–517. [Google Scholar] [CrossRef]
- Krugmann, B.; Radulescu, A.; Appavou, M.S.; Koutsioubas, A.; Stingaciu, L.R.; Dulle, M.; Förster, S.; Stadler, A.M. Membrane Stiffness and Myelin Basic Protein Binding Strength as Molecular Origin of Multiple Sclerosis. Sci. Rep. 2020, 10, 16691. [Google Scholar] [CrossRef]
- Has, C.; Sivadas, P.; Das, S.L. Insights into Membrane Curvature Sensing and Membrane Remodeling by Intrinsically Disordered Proteins and Protein Regions. J. Membr. Biol. 2022, 255, 237–259. [Google Scholar] [CrossRef]
- Akbayrak, I.Y.; Caglayan, S.I.; Ozcan, Z.; Uversky, V.N.; Coskuner-Weber, O. Current Challenges and Limitations in the Studies of Intrinsically Disordered Proteins in Neurodegenerative Diseases by Computer Simulations. Curr. Alzheimer Res. 2020, 17, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Gil Pineda, L.I.; Milko, L.N.; He, Y. Performance of CHARMM36m with Modified Water Model in Simulating Intrinsically Disordered Proteins: A Case Study. Biophys. Rep. 2020, 6, 80–87. [Google Scholar] [CrossRef]
- Wang, W. Recent Advances in Atomic Molecular Dynamics Simulation of Intrinsically Disordered Proteins. Phys. Chem. Chem. Phys. 2021, 23, 777–784. [Google Scholar] [CrossRef]
- Majewski, J.; Jones, E.M.; Vander Zanden, C.M.; Biernat, J.; Mandelkow, E.; Chi, E.Y. Lipid Membrane Templated Misfolding and Self-Assembly of Intrinsically Disordered Tau Protein. Sci. Rep. 2020, 10, 13324. [Google Scholar] [CrossRef]
- El Mammeri, N.; Gampp, O.; Duan, P.; Hong, M. Membrane-Induced Tau Amyloid Fibrils. Commun. Biol. 2023, 6, 467. [Google Scholar] [CrossRef] [PubMed]
- Ivankov, O.; Murugova, T.N.; Ermakova, E.V.; Kondela, T.; Badreeva, D.R.; Hrubovčák, P.; Soloviov, D.; Tsarenko, A.; Rogachev, A.; Kuklin, A.I.; et al. Amyloid-Beta Peptide (25–35) Triggers a Reorganization of Lipid Membranes Driven by Temperature Changes. Sci. Rep. 2021, 11, 21990. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Doktorova, M.; Molugu, T.R.; Heberle, F.A.; Scott, H.L.; Dzikovski, B.; Nagao, M.; Stingaciu, L.R.; Standaert, R.F.; Barrera, F.N.; et al. How Cholesterol Stiffens Unsaturated Lipid Membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 21896–21905. [Google Scholar] [CrossRef] [PubMed]
- Träger, J.; Widder, K.; Kerth, A.; Harauz, G.; Hinderberger, D. Effect of Cholesterol and Myelin Basic Protein (MBP) Content on Lipid Monolayers Mimicking the Cytoplasmic Membrane of Myelin. Cells 2020, 9, 529. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maleš, P.; Pem, B.; Petrov, D.; Mangiarotti, A.; Dimova, R.; Bakarić, D. Adsorption of Myelin Basic Protein on Model Myelin Membranes Reveals Weakening of van der Waals Interactions in a Lipid Ratio-Dependent Manner. Membranes 2025, 15, 279. https://doi.org/10.3390/membranes15090279
Maleš P, Pem B, Petrov D, Mangiarotti A, Dimova R, Bakarić D. Adsorption of Myelin Basic Protein on Model Myelin Membranes Reveals Weakening of van der Waals Interactions in a Lipid Ratio-Dependent Manner. Membranes. 2025; 15(9):279. https://doi.org/10.3390/membranes15090279
Chicago/Turabian StyleMaleš, Petra, Barbara Pem, Dražen Petrov, Agustín Mangiarotti, Rumiana Dimova, and Danijela Bakarić. 2025. "Adsorption of Myelin Basic Protein on Model Myelin Membranes Reveals Weakening of van der Waals Interactions in a Lipid Ratio-Dependent Manner" Membranes 15, no. 9: 279. https://doi.org/10.3390/membranes15090279
APA StyleMaleš, P., Pem, B., Petrov, D., Mangiarotti, A., Dimova, R., & Bakarić, D. (2025). Adsorption of Myelin Basic Protein on Model Myelin Membranes Reveals Weakening of van der Waals Interactions in a Lipid Ratio-Dependent Manner. Membranes, 15(9), 279. https://doi.org/10.3390/membranes15090279





