Novel Dual-Layer Zwitterionic Modification of Electrospun Nanofibrous Membrane for Produced Water Treatment and Reclamation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
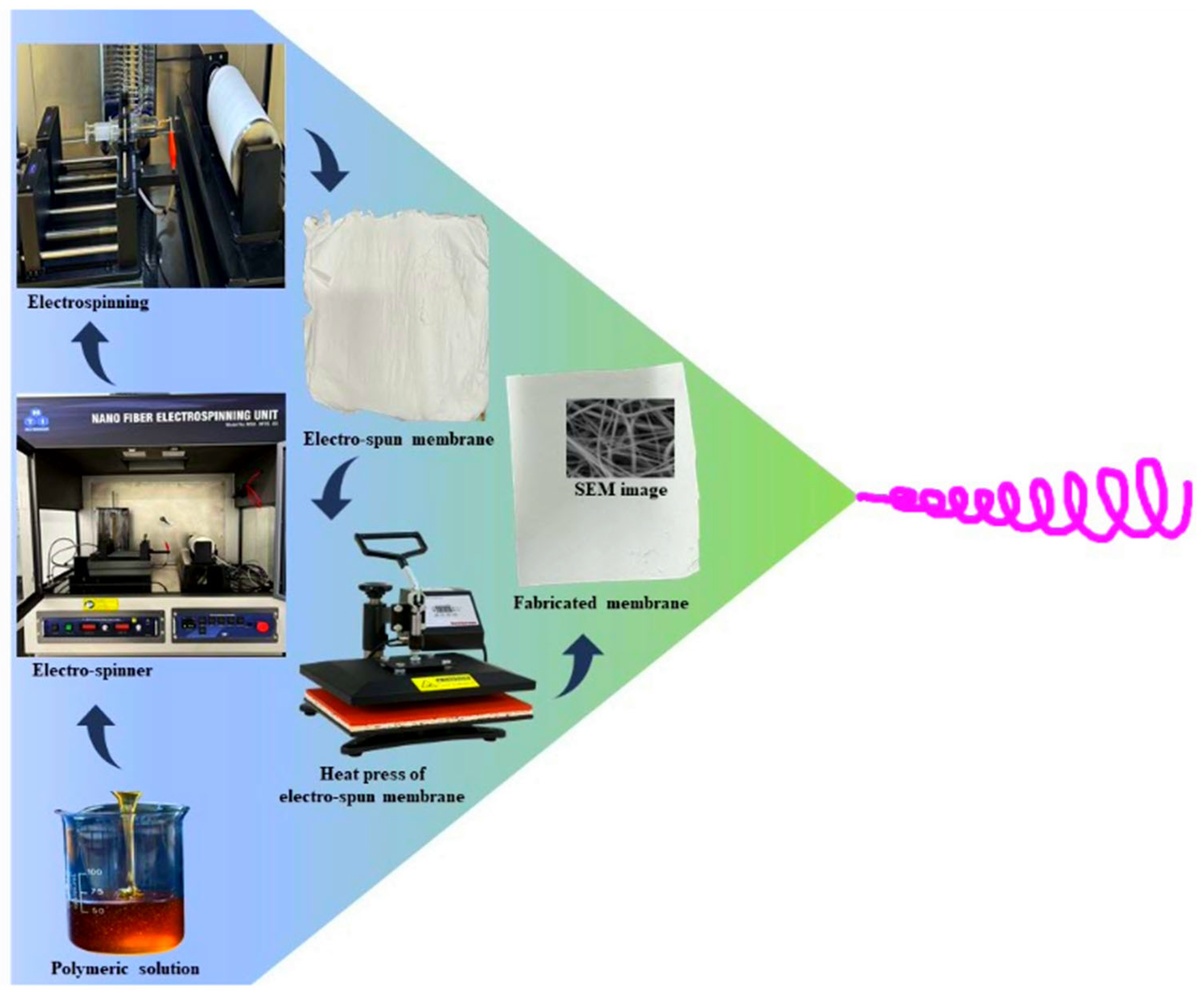
2.2. Membrane Fabrication by Electrospinning
2.3. Membrane Modification
2.4. Synthetic Produced Water Preparation
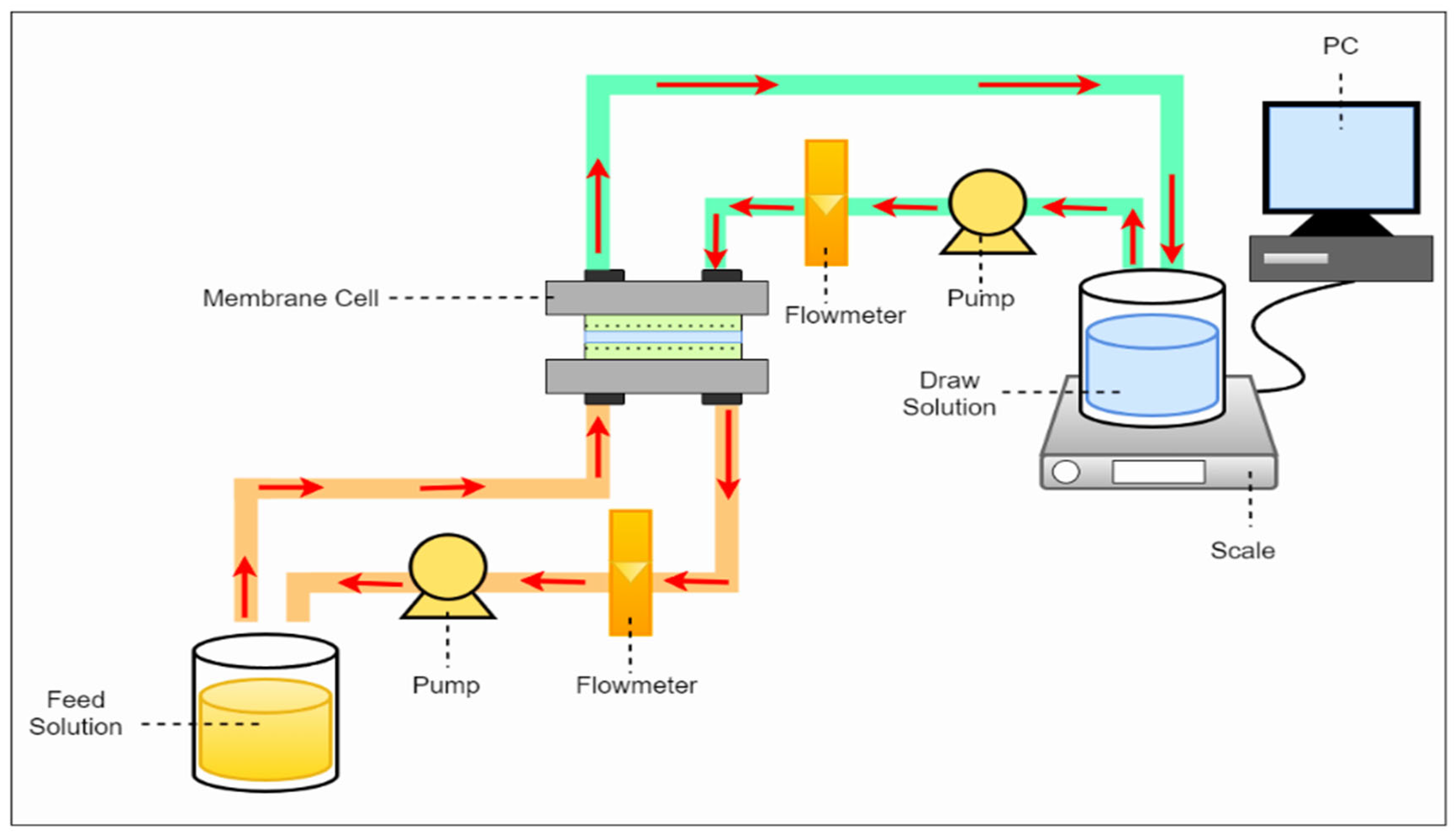
2.5. Forward Osmosis
3. Results and Discussion
3.1. Membrane Characterization
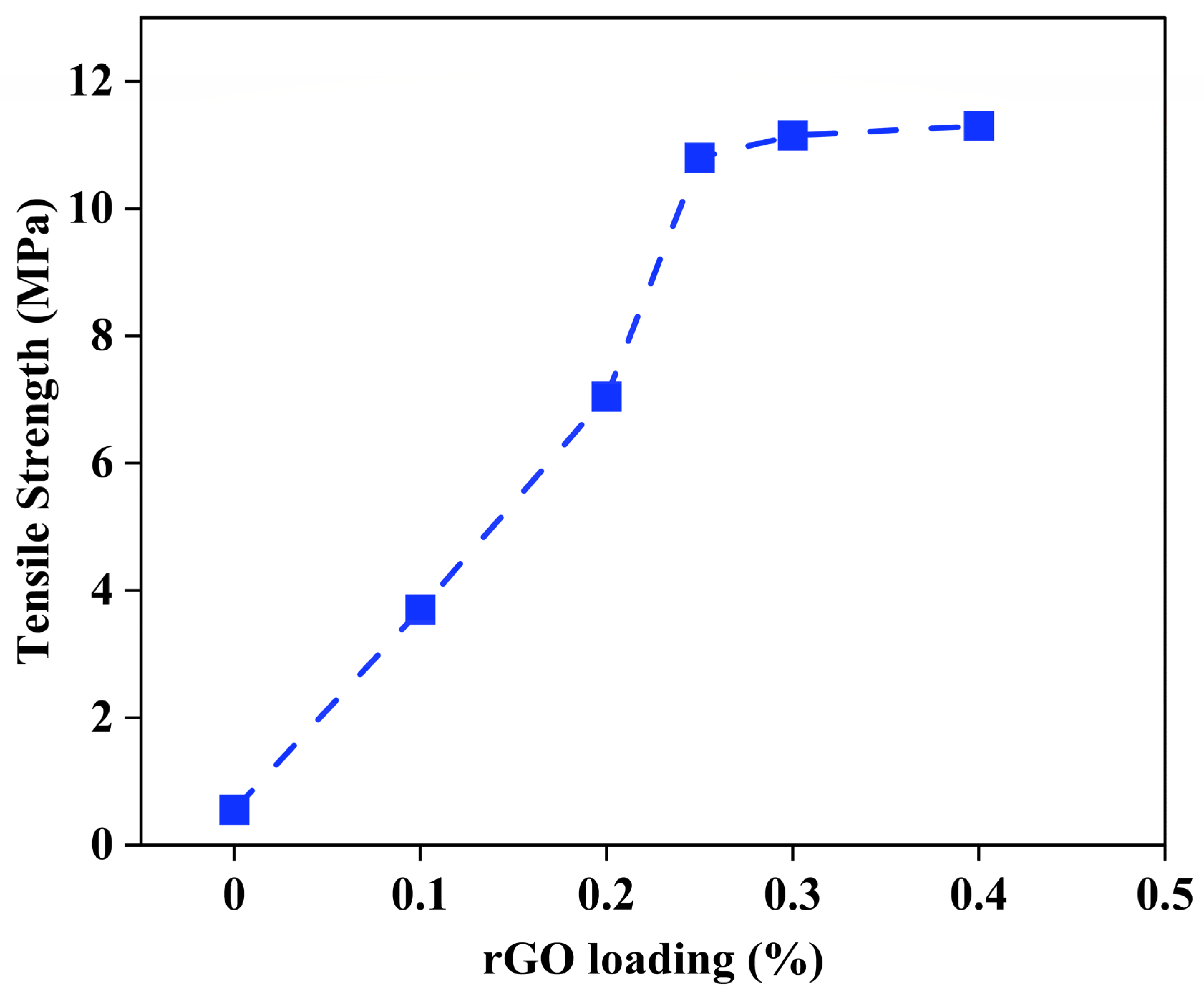
3.1.1. Optimization of rGO Loading (%)
3.1.2. Mechanical Properties
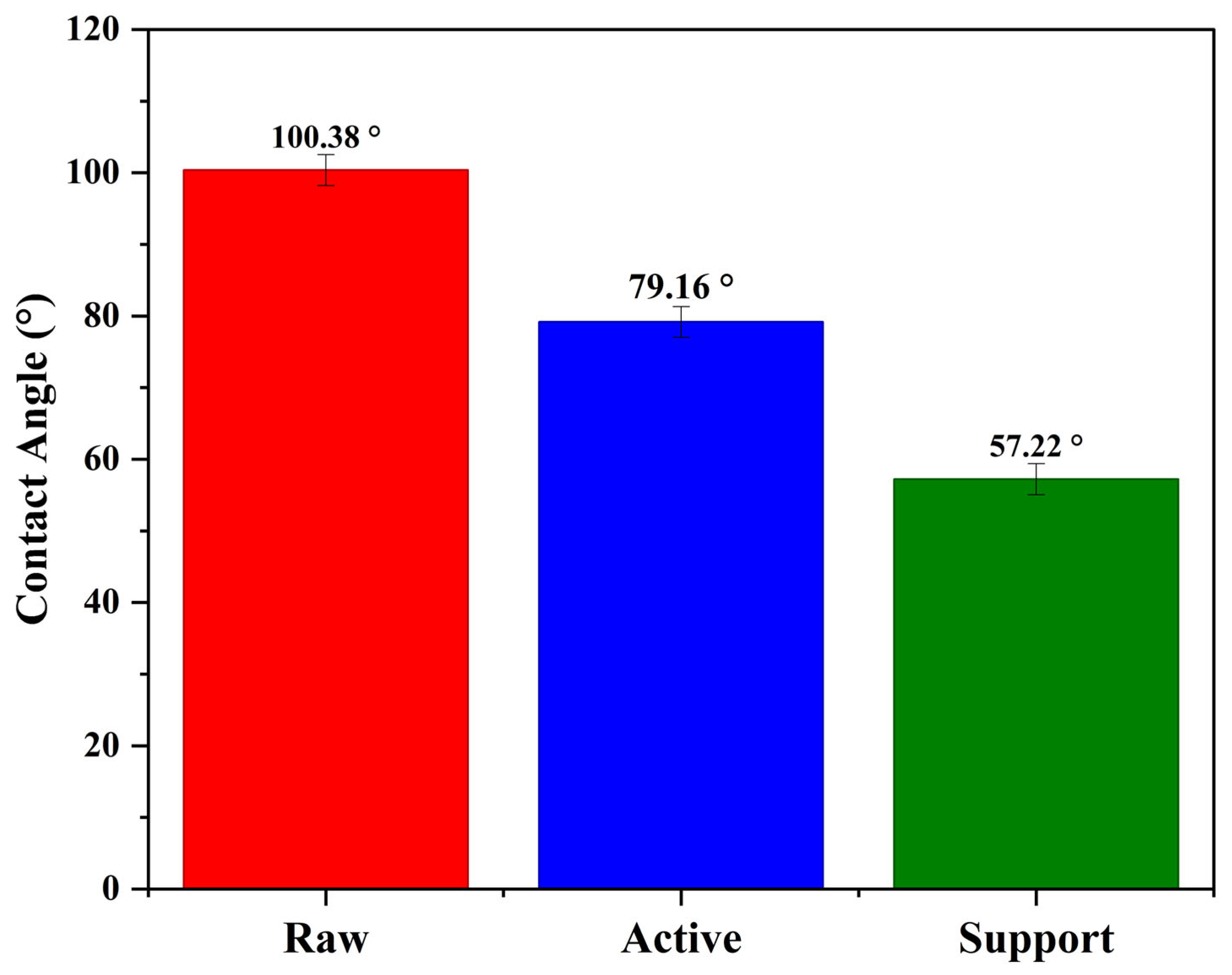
3.1.3. Contact Angle
- -
- θ = contact angle
- -
- H= height of droplet
- -
- R = radius of droplet’s base
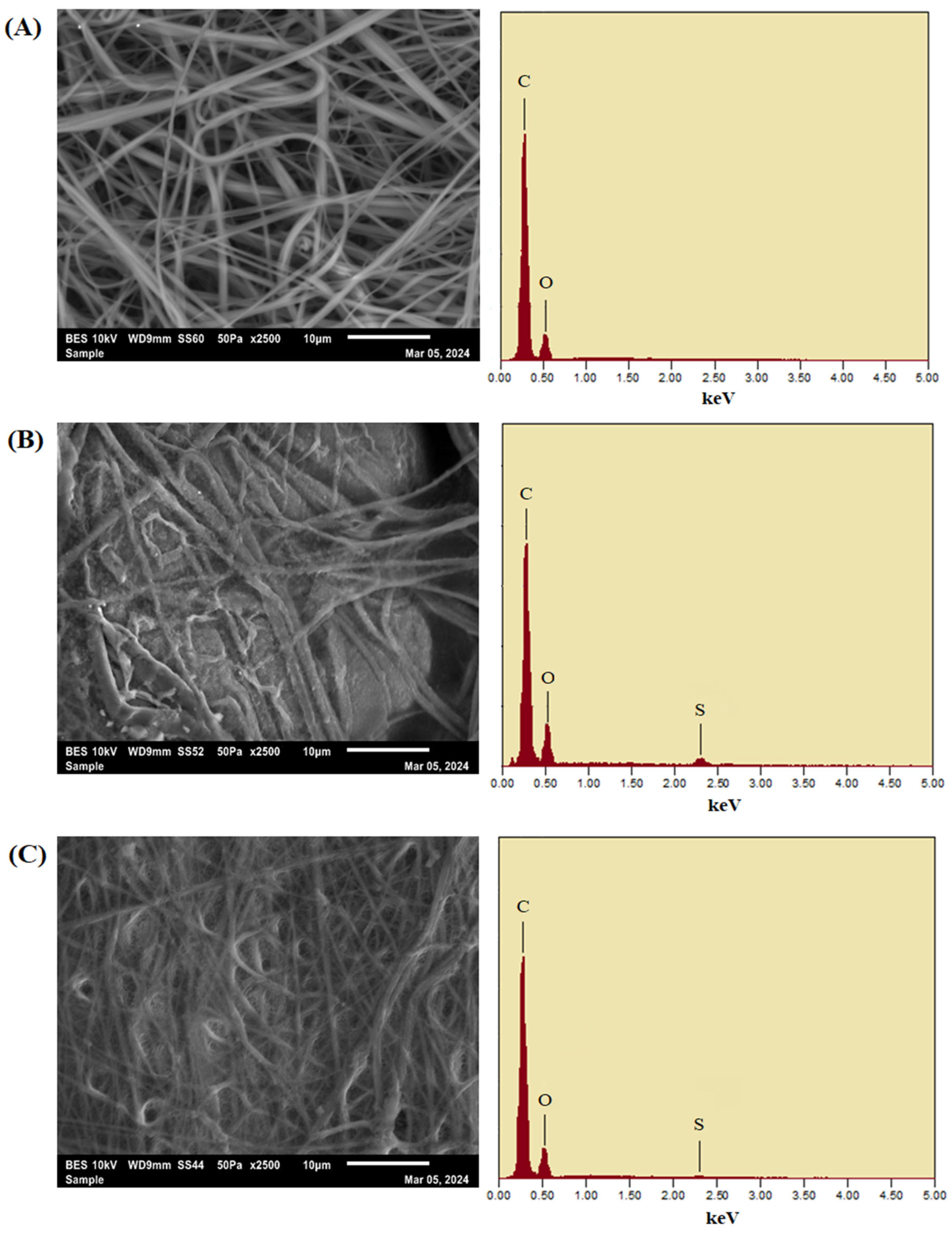
3.1.4. Scanning Electron Microscopy
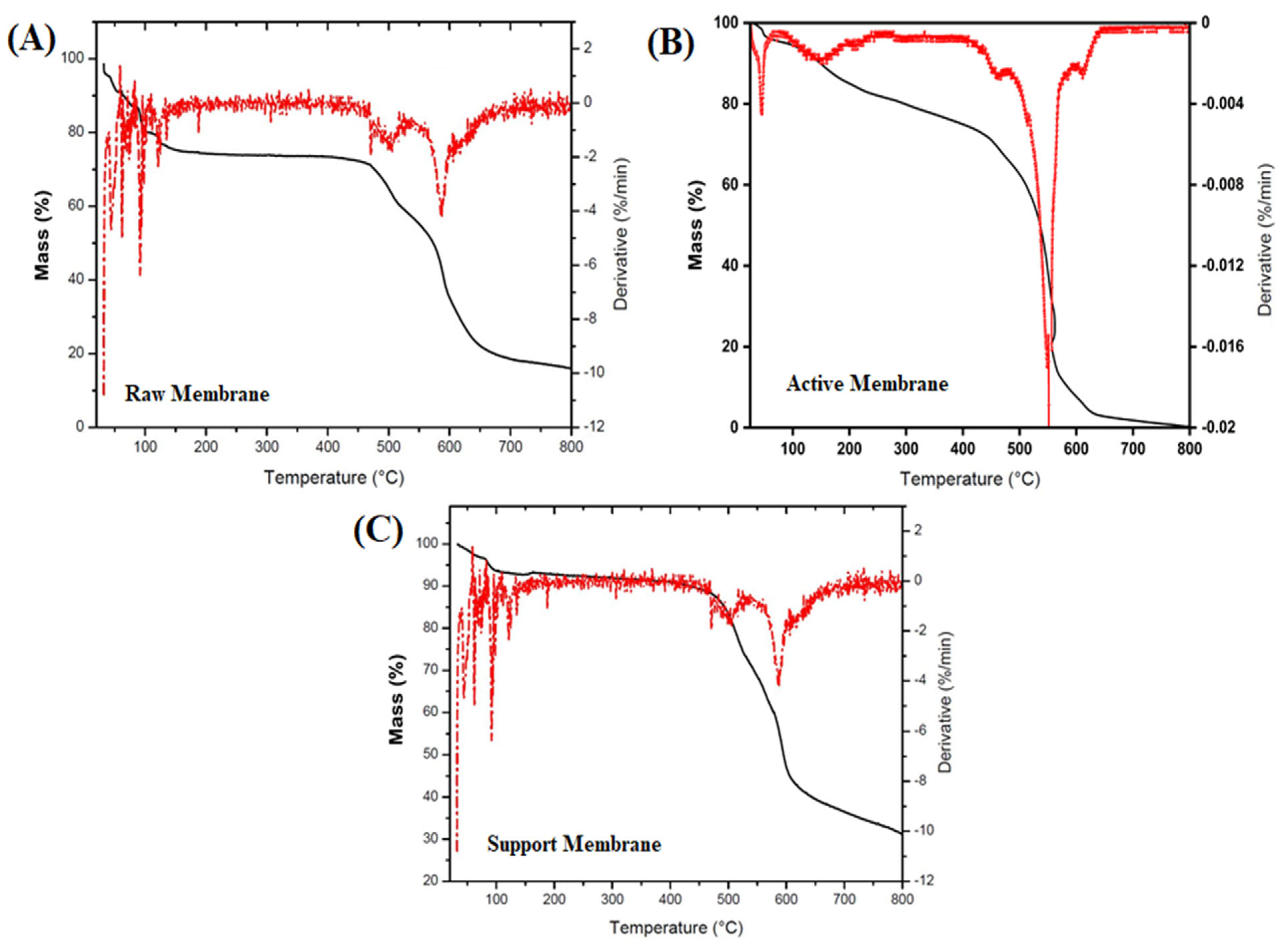
3.1.5. Thermogravimetric Analysis
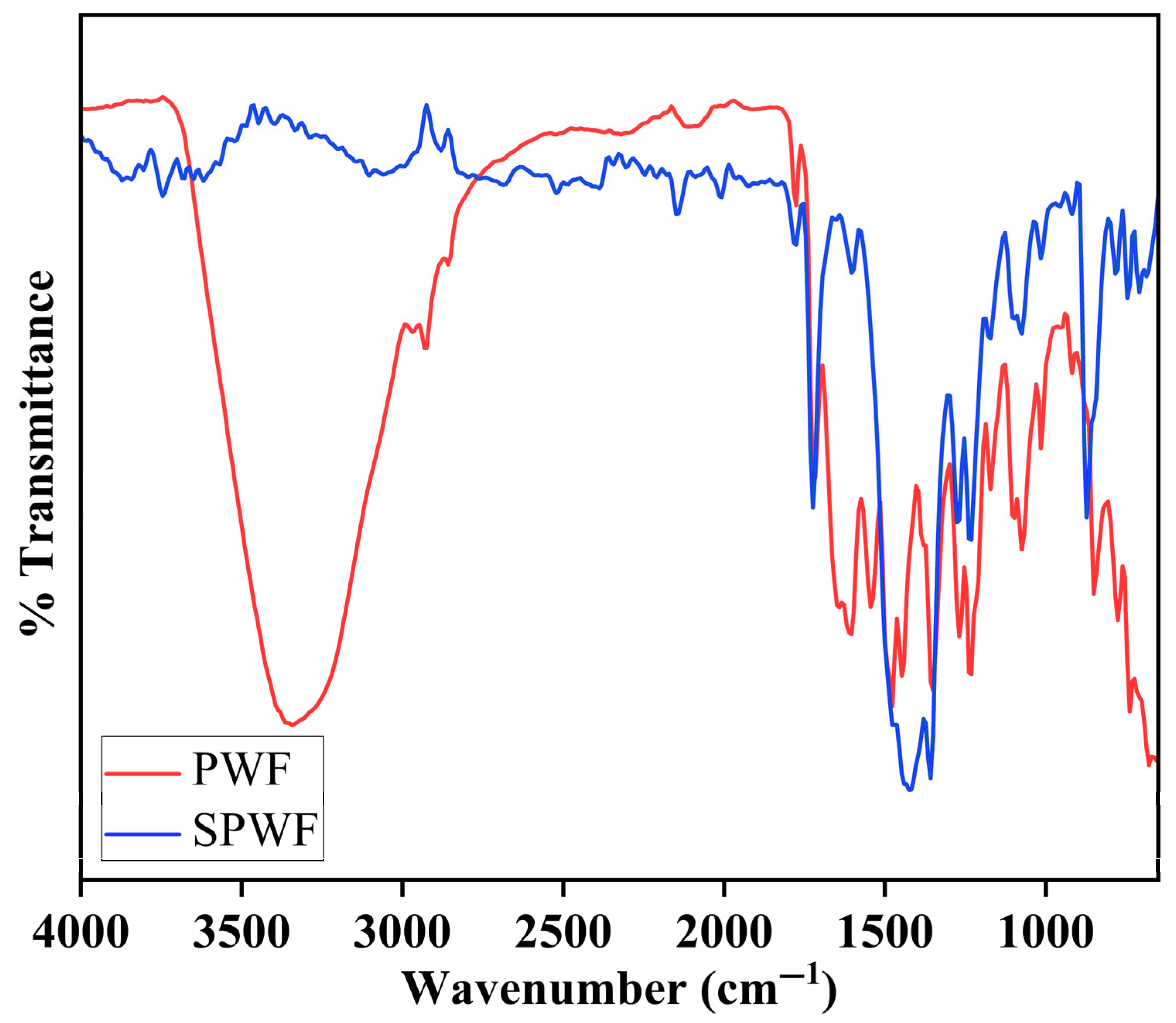
3.1.6. Fourier Transform Infrared Spectroscopy
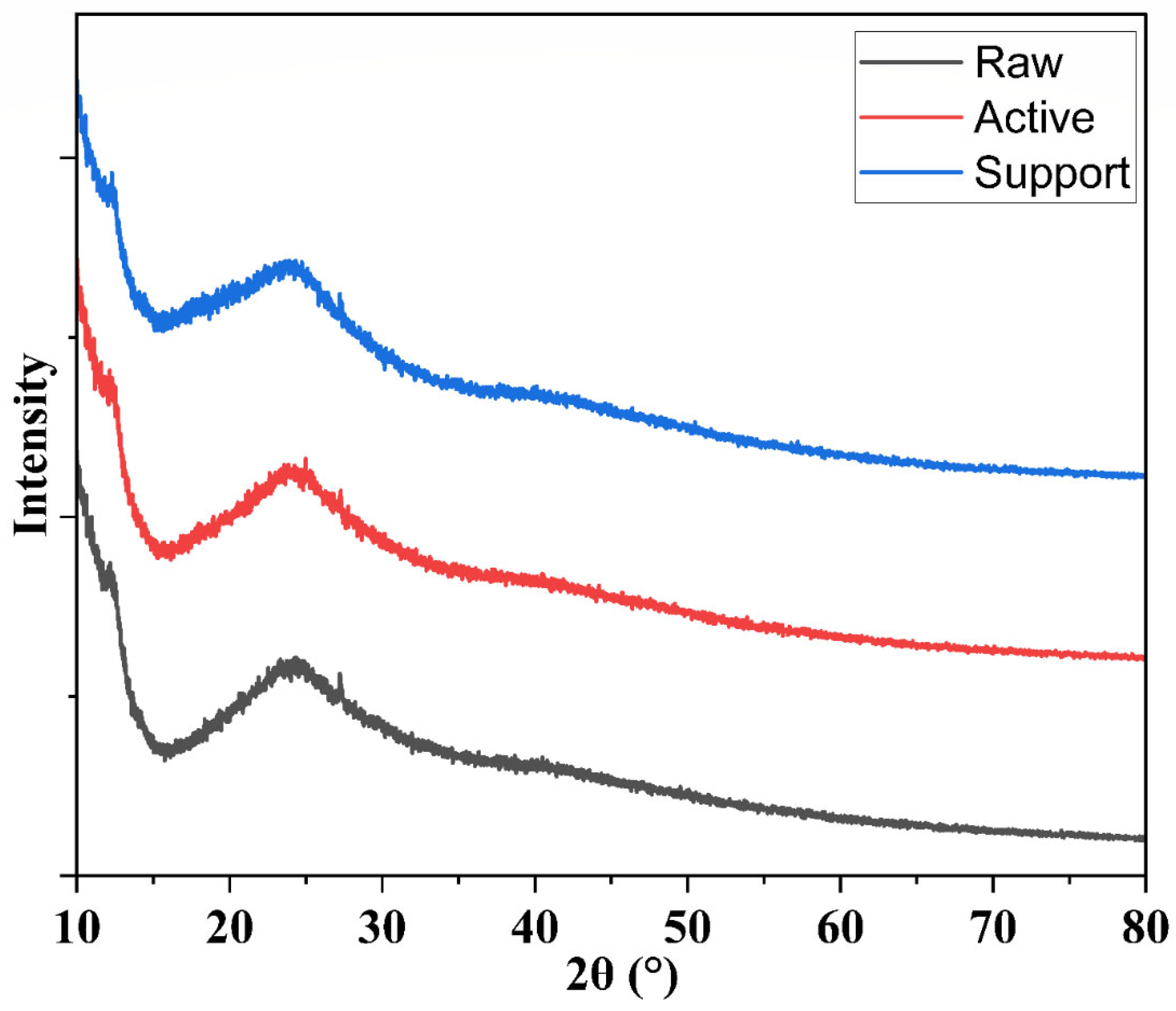
3.1.7. X-Ray Diffraction
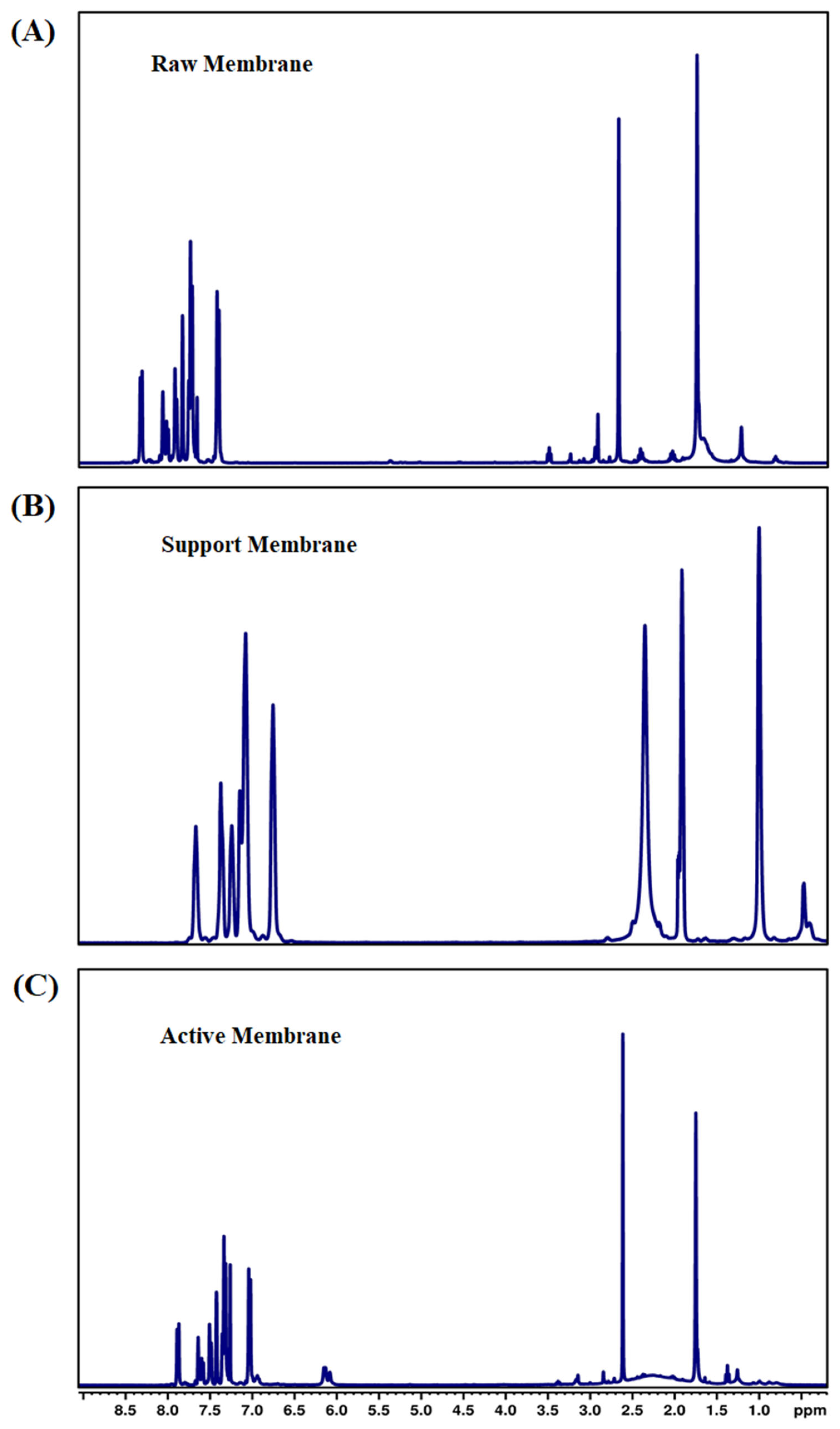
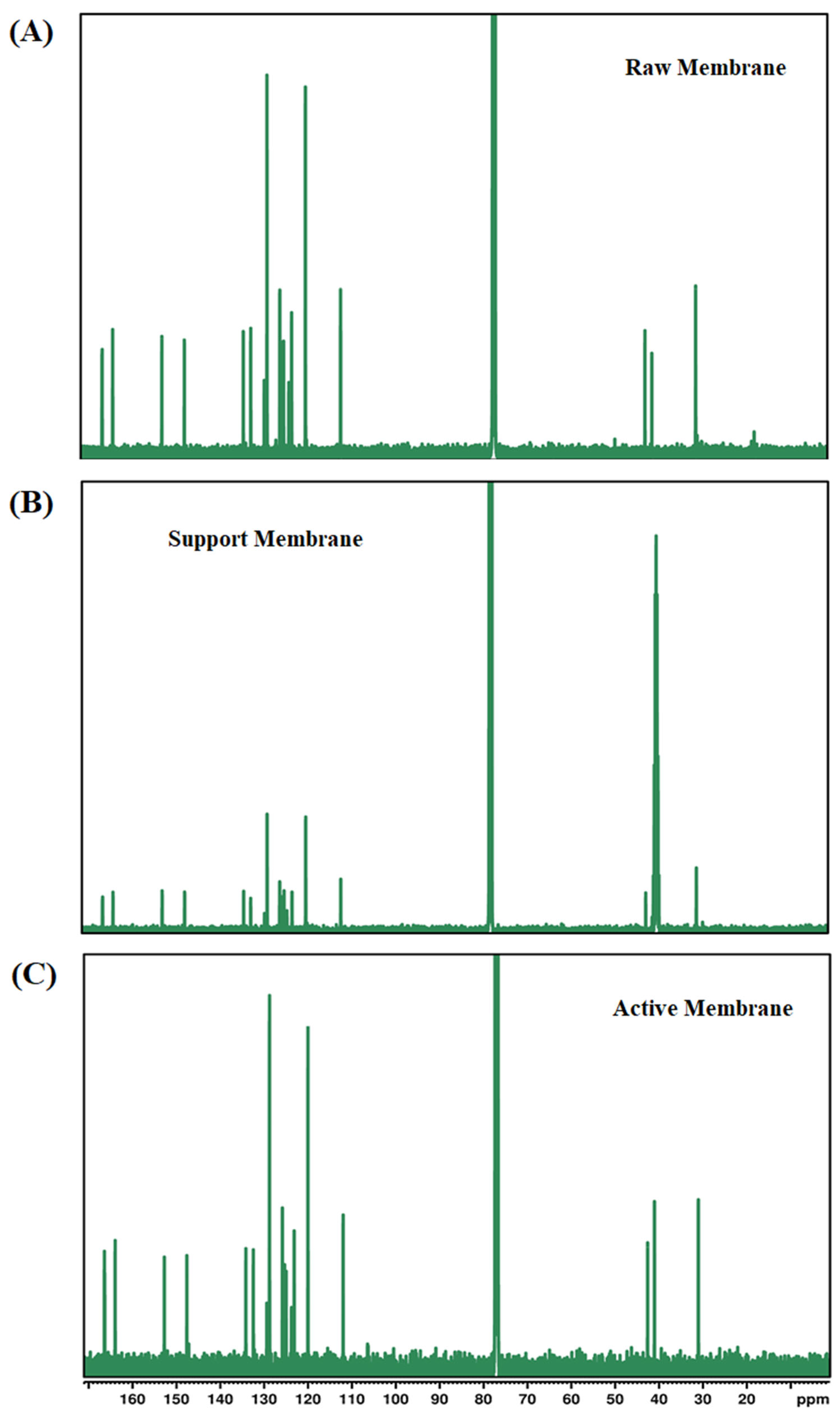
3.1.8. Nuclear Magnetic Resonance Spectroscopy
3.1.9. FTIR Analysis of Post-Operational FO Membranes
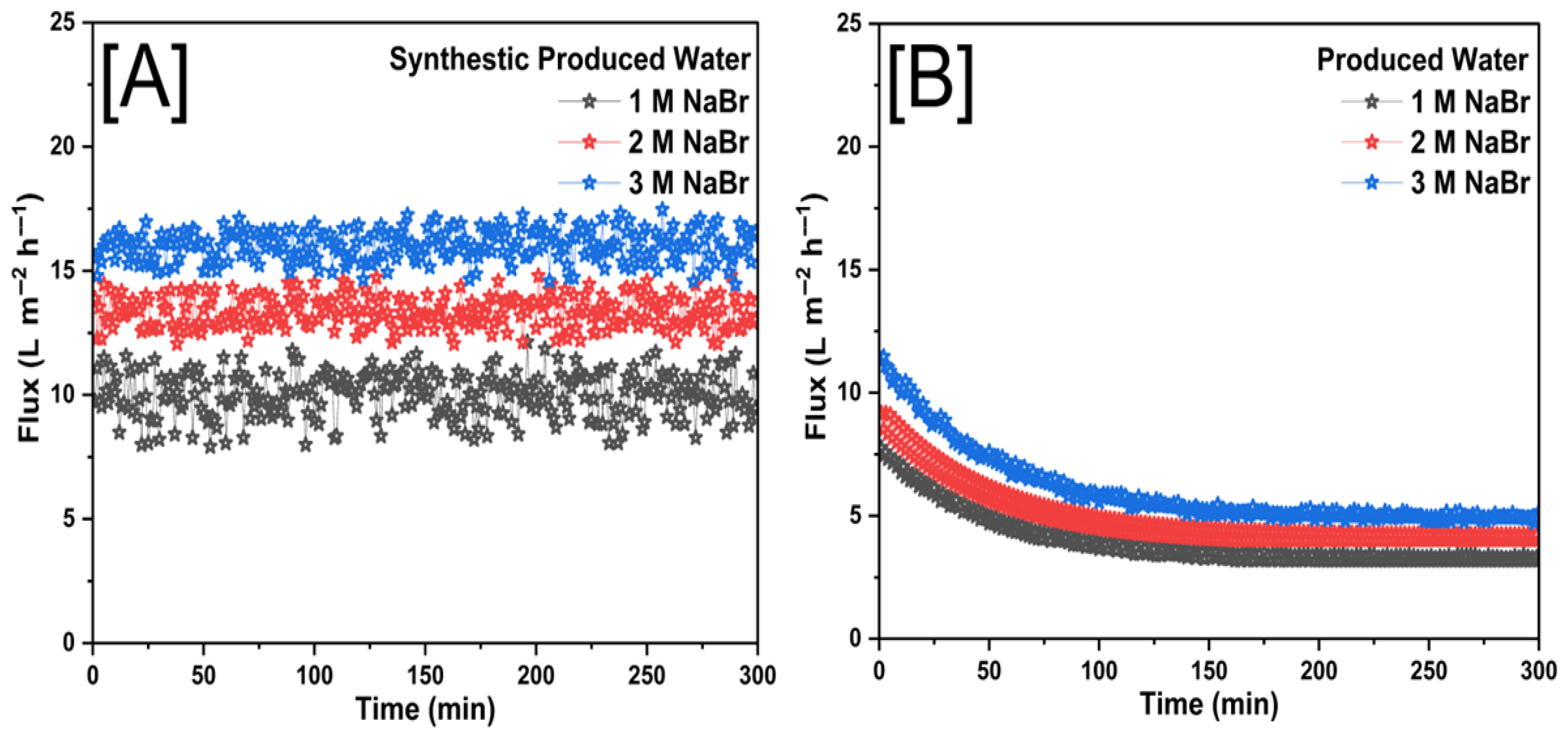
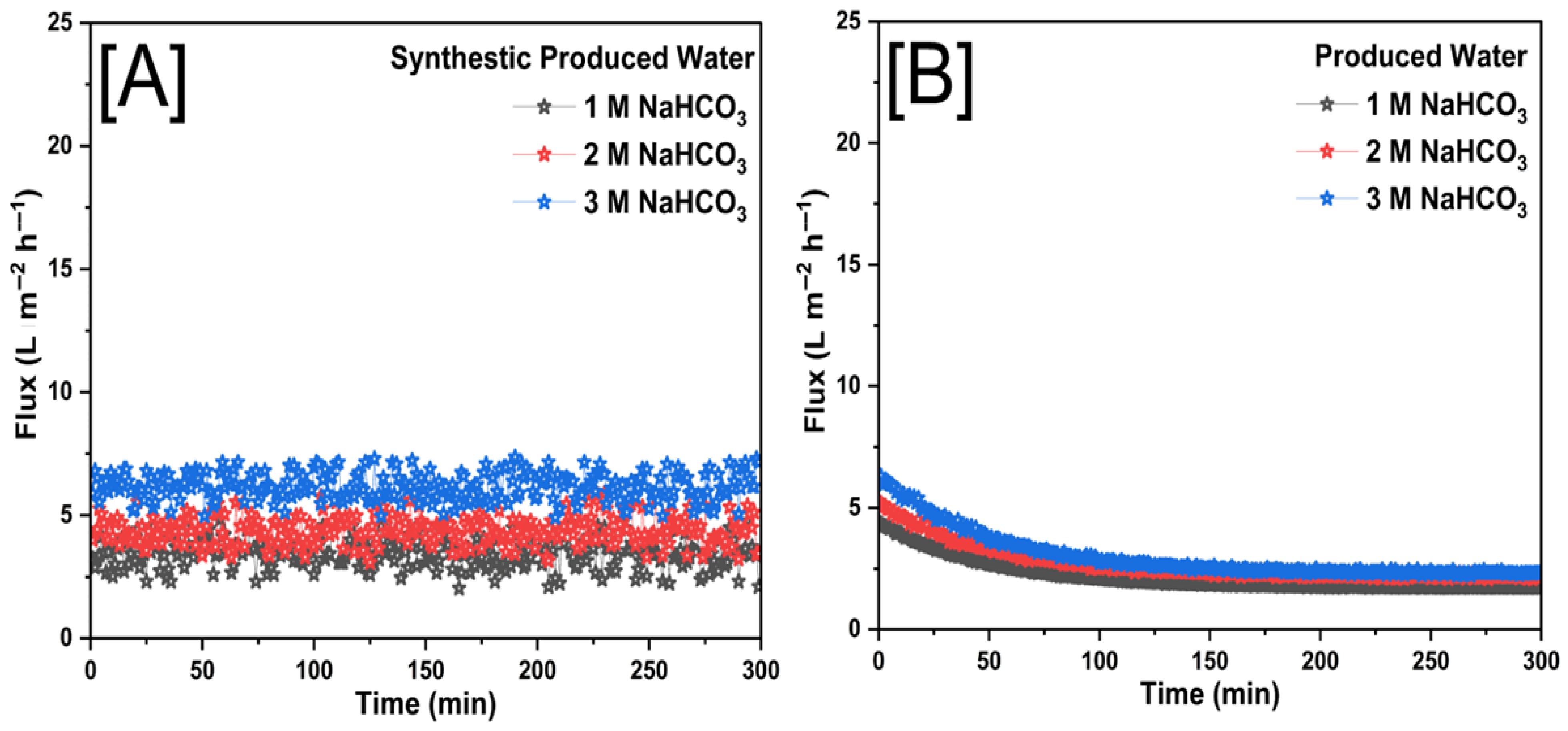
3.2. Membrane Performance with Forward Osmosis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gul Zaman, H.; Baloo, L.; Pendyala, R.; Singa, P.K.; Ilyas, S.U.; Kutty, S.R.M. Produced Water Treatment with Conventional Adsorbents and MOF as an Alternative: A Review. Materials 2021, 14, 7607. [Google Scholar] [CrossRef] [PubMed]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’nA, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Alomar, T.S.; Hameed, B.; Usman, M.; Almomani, F.; Ba-Abbad, M.; Khraisheh, M. Recent advances on the treatment of oil fields produced water by adsorption and advanced oxidation processes. J. Water Process Eng. 2022, 49, 103034. [Google Scholar] [CrossRef]
- Siagian, U.W.R.; Lustiyani, L.; Khoiruddin, K.; Ismadji, S.; Wenten, I.; Adisasmito, S. From waste to resource: Membrane technology for effective treatment and recovery of valuable elements from oilfield produced water. Environ. Pollut. 2024, 340, 122717. [Google Scholar] [CrossRef]
- Mehler, W.T.; Snihur, K.N.; Zhang, Y.; Li, H.; Alessi, D.S.; Goss, G.G. A complex bioaccumulation story in flowback and produced water from hydraulic fracturing: The role of organic compounds in inorganic accumulation in Lumbriculus variegatus. J. Hazard. Mater. 2021, 414, 125525. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Xu, G.R.; Xu, K.; Zhao, H.L.; Wu, Y.Q.; Su, H.C.; Xu, J.M.; Das, R. Electrospun nanofibrous membranes in membrane distillation: Recent developments and future perspectives. Sep. Purif. Technol. 2019, 221, 44–63. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef]
- Mobarak, M.H.; Siddiky, A.Y.; Islam, A.; Hossain, A.; Rimon, I.H.; Oliullah, S.; Khan, J.; Rahman, M.; Hossain, N.; Chowdhury, M.A. Progress and prospects of electrospun nanofibrous membranes for water filtration: A comprehensive review. Desalination 2024, 574, 117285. [Google Scholar] [CrossRef]
- Nain, A.; Sangili, A.; Hu, S.-R.; Chen, C.-H.; Chen, Y.-L.; Chang, H.-T. Recent progress in nanomaterial-functionalized membranes for removal of pollutants. iScience 2022, 25, 104616. [Google Scholar] [CrossRef] [PubMed]
- Nayak, V.; Shivanna, J.M.; Ramu, S.; Radoor, S.; Balakrishna, R.G. Efficacy of Electrospun Nanofiber Membranes on Fouling Mitigation: A Review. ACS Omega 2022, 7, 43346–43363. [Google Scholar] [CrossRef] [PubMed]
- Darrell, H.R.; Iksoo, C. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Tan, X.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride). Polymers 2019, 11, 1160. [Google Scholar] [CrossRef]
- Zou, D.; Nunes, S.P.; Vankelecom, I.F.J.; Figoli, A.; Lee, Y.M. Recent advances in polymer membranes employing non-toxic solvents and materials. Green Chem. 2021, 23, 9815–9843. [Google Scholar] [CrossRef]
- Kulkarni, D.; Musale, S.; Panzade, P.; Paiva-Santos, A.C.; Sonwane, P.; Madibone, M.; Choundhe, P.; Giram, P.; Cavalu, S. Surface Functionalization of Nanofibers: The Multifaceted Approach for Advanced Biomedical Applications. Nanomaterials 2022, 12, 3899. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, S.; Kaner, P.; Thomas, D.; Cebe, P.; Asatekin, A. Hydrophobic Antifouling Electrospun Mats from Zwitterionic Amphiphilic Copolymers. ACS Appl. Mater. Interfaces 2018, 10, 18300–18309. [Google Scholar] [CrossRef]
- Salimi, P.; Aroujalian, A.; Iranshahi, D. Graft copolymerization of zwitterionic monomer on the polyethersulfone membrane surface by corona air plasma for separation of oily wastewater. Sep. Purif. Technol. 2021, 258, 117939. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Ramanan, S.N.; Fister, D.; Park, E.; Venna, S.R.; Sun, H.; Cheng, C.; Lin, H. Facile Grafting of Zwitterions onto the Membrane Surface To Enhance Antifouling Properties for Wastewater Reuse. Ind. Eng. Chem. Res. 2017, 56, 9202–9212. [Google Scholar] [CrossRef]
- Saleem, H.; Trabzon, L.; Kilic, A.; Zaidi, S.J. Recent advances in nanofibrous membranes: Production and applications in water treatment and desalination. Desalination 2020, 478, 114178. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Jing, X.; Zhao, R.; Zhu, G.; Wang, C. Bio-inspired functionalization of electrospun nanofibers with anti-biofouling property for efficient uranium extraction from seawater. Chem. Eng. J. 2023, 465, 142844. [Google Scholar] [CrossRef]
- Mokhtari, F.; Samadi, A.; Rashed, A.O.; Li, X.; Razal, J.M.; Kong, L.; Varley, R.J.; Zhao, S. Recent progress in electrospun polyvinylidene fluoride (PVDF)-based nanofibers for sustainable energy and environmental applications. Prog. Mater. Sci. 2025, 148, 101376. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Tarboush, B.J.A.; Rana, D.; Matsuura, T.; Arafat, H.; Narbaitz, R. Preparation of thin-film-composite polyamide membranes for desalination using novel hydrophilic surface modifying macromolecules. J. Membr. Sci. 2008, 325, 166–175. [Google Scholar] [CrossRef]
- Zhao, L.-M.; Yan, B. Novel polymer–inorganic hybrid materials fabricated with in situ composition and luminescent properties. J. Non-Cryst. Solids 2007, 353, 4654–4659. [Google Scholar] [CrossRef]
- Zhang, M.; Sabatini, C.; Chen, K.; Makowka, S.; Hu, R.; Swihart, M.; Cheng, C. Novel polymer/halloysite composites with high halloysite content and remarkable mechanical strength. RSC Appl. Interfaces 2024, 2, 410–419. [Google Scholar] [CrossRef]
- Su, H.-P.; Hwang, S.-J. A novel approach to fabricate high performance electrically tunable fiber device based on well-aligned liquid crystal- infiltrated hollow core fiber. Opt. Laser Technol. 2023, 164, 109416. [Google Scholar] [CrossRef]
- Liu, W.; Huang, H.; Peng, K.; Zhu, L.; Jin, F.; Liu, Z. A novel approach to manufacturing high-performance reclaimed carbon fiber composites via convergent flow-induced discontinuous fiber aligned placement (CFi-DFAP). Compos. Part B Eng. 2024, 271, 111178. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Tang, Y.; Huang, X.; Pang, H. Recent advances and challenges of metal–organic framework/graphene-based composites. Compos. Part B Eng. 2022, 230, 109532. [Google Scholar] [CrossRef]
- Abdollahi, F.; Khosravi, A.; Karagöz, S.; Keshavarz, A. A systematic review of recent advances in the application of machine learning in membrane-based gas separation technologies. Appl. Energy 2025, 381, 125203. [Google Scholar] [CrossRef]
- Jafari, M.; Tzirtzipi, C.; Castro-Dominguez, B. Applications of artificial intelligence for membrane separation: A review. J. Water Process Eng. 2024, 68, 106532. [Google Scholar] [CrossRef]
- Ibraheem, B.M.; Al Aani, S.; Alsarayreh, A.A.; Alsalhy, Q.F.; Salih, I.K. Forward Osmosis Membrane: Review of Fabrication, Modification, Challenges and Potential. Membranes 2023, 13, 379. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X. Forward osmosis technology for water treatment: Recent advances and future perspectives. J. Clean. Prod. 2021, 280, 124354. [Google Scholar] [CrossRef]
- Duan, J.; Litwiller, E.; Choi, S.-H.; Pinnau, I. Evaluation of sodium lignin sulfonate as draw solute in forward osmosis for desert restoration. J. Membr. Sci. 2014, 453, 463–470. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Chakraborty, S.; Naskar, S.; Rajabalaya, R. 3—Techniques and methods used for the fabrication of bionanocomposites. In Bionanocomposites in Tissue Engineering and Regenerative Medicine; Ahmed, S., Annu, Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 17–43. [Google Scholar] [CrossRef]
- Odularu, A.T. Basic Principles of Electrospinning, Mechanisms, Nanofibre Production, and Anticancer Drug Delivery. J. Chem. 2022, 2022, 9283325. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, T.; Tian, J.; Xu, H.; Zuo, L.; Zhang, W.; Zuo, X.; Zhang, Y. Oscillating evaporation of pendant droplets under the action of ionic wind. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129579. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.-W.; Wong, S.-C.; Abtahi, M.; Chen, P. Electrospinning of polymer nanofibers: Effects on oriented morphology, structures and tensile properties. Compos. Sci. Technol. 2010, 70, 703–718. [Google Scholar] [CrossRef]
- Bisht, G.; Nesterenko, S.; Kulinsky, L.; Madou, M. A Computer-Controlled Near-Field Electrospinning Setup and Its Graphic User Interface for Precision Patterning of Functional Nanofibers on 2D and 3D Substrates. SLAS Technol. 2012, 17, 302–308. [Google Scholar] [CrossRef]
- Gadkari, S. Influence of Polymer Relaxation Time on the Electrospinning Process: Numerical Investigation. Polymers 2017, 9, 501. [Google Scholar] [CrossRef]
- Šimko, M.; Lukáš, D. Mathematical modeling of a whipping instability of an electrically charged liquid jet. Appl. Math. Model. 2016, 40, 9565–9583. [Google Scholar] [CrossRef]
- Long, Y.-Z.; Yan, X.; Wang, X.-X.; Zhang, J.; Yu, M. Chapter 2—Electrospinning: The Setup and Procedure. In Electrospinning: Nanofabrication and Applications; Ding, B., Wang, X., Yu, J., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 21–52. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Zhang, Y.; Yu, H.; Tan, Z. Near-field electrospinning for 2D and 3D structuring: Fundamentals, methods, and applications. Mater. Today Adv. 2024, 21, 100461. [Google Scholar] [CrossRef]
- Deng, S.; Bai, R.; Chen, J.; Jiang, Z.; Yu, G.; Zhou, F.; Chen, Z. Produced water from polymer flooding process in crude oil extraction: Characterization and treatment by a novel crossflow oil–water separator. Sep. Purif. Technol. 2002, 29, 207–216. [Google Scholar] [CrossRef]
- Dumitriu, S.; Popa, V. Polymeric Biomaterials; CRC Press: Boca Raton, FA, USA, 2013; Volume 2. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Kao, Y.-C.; Chuang, S.-H.; Wang, J.-S.; Lai, J.-Y.; Tsai, H.-C. Thermosensitive double network of zwitterionic polymers for controlled mechanical strength of hydrogels. RSC Adv. 2019, 9, 24241–24247. [Google Scholar] [CrossRef]
- Nuñez, S.A.; Hickner, M.A. Quantitative 1H NMR Analysis of Chemical Stabilities in Anion-Exchange Membranes. ACS Macro Lett. 2013, 2, 49–52. [Google Scholar] [CrossRef] [PubMed]
- White, D.M.; Takekoshi, T.; Williams, F.J.; Relles, H.M.; Donahue, P.E.; Klopfer, H.J.; Loucks, G.R.; Manello, J.S.; Matthews, R.O.; Schluenz, R.W. Polyetherimides via nitro-displacement polymerization: Monomer synthesis and 13C-NMR analysis of monomers and polymers. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 1635–1658. [Google Scholar] [CrossRef]
- Choi, J.; Kim, I.-K.; Kwak, S.-Y. Synthesis and characterization of a series of star-branched poly(ε-caprolactone)s with the variation in arm numbers and lengths. Polymer 2005, 46, 9725–9735. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Sun, D.D. Nano Gives the Answer: Breaking the Bottleneck of Internal Concentration Polarization with a Nanofiber Composite Forward Osmosis Membrane for a High Water Production Rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Hoover, L.A.; Schiffman, J.D.; Elimelech, M. Nanofibers in thin-film composite membrane support layers: Enabling expanded application of forward and pressure retarded osmosis. Desalination 2013, 308, 73–81. [Google Scholar] [CrossRef]
- Tian, M.; Qiu, C.; Liao, Y.; Chou, S.; Wang, R. Preparation of polyamide thin film composite forward osmosis membranes using electrospun polyvinylidene fluoride (PVDF) nanofibers as substrates. Sep. Purif. Technol. 2013, 118, 727–736. [Google Scholar] [CrossRef]
- Tian, E.L.; Zhou, H.; Ren, Y.W.; Mirza, Z.; Wang, X.Z.; Xiong, S.W. Novel design of hydrophobic/hydrophilic interpenetrating network composite nanofibers for the support layer of forward osmosis membrane. Desalination 2014, 347, 207–214. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Hydrophilic nylon 6,6 nanofibers supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2014, 457, 162–169. [Google Scholar] [CrossRef]
- Miao, L.; Jiang, T.; Lin, S.; Jin, T.; Hu, J.; Zhang, M.; Tu, Y.; Liu, G. Asymmetric forward osmosis membranes from p-aramid nanofibers. Mater. Des. 2020, 191, 108591. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Tajik, S. Fabrication of thin film composite forward osmosis membrane using electrospun polysulfone/polyacrylonitrile blend nanofibers as porous substrate. Desalination 2018, 425, 68–76. [Google Scholar] [CrossRef]
- Han, C.; Zhang, X.; Ding, C.; Xiong, S.; Yu, X.; Wang, Y. Improved performance of thin-film composite membrane supported by aligned nanofibers substrate with slit-shape pores for forward osmosis. J. Membr. Sci. 2020, 612, 118447. [Google Scholar] [CrossRef]
- Obaid, M.; Kang, Y.; Wang, S.; Yoon, M.-H.; Kim, C.-M.; Song, J.-H.; Kim, I.S. Fabrication of highly permeable thin-film nanocomposite forward osmosis membranes via the design of novel freestanding robust nanofiber substrates. J. Mater. Chem. 2018, 6, 11700–11713. [Google Scholar] [CrossRef]
- Shibuya, M.; Park, M.J.; Lim, S.; Phuntsho, S.; Matsuyama, H.; Shon, H.K. Novel CA/PVDF nanofiber supports strategically designed via coaxial electrospinning for high performance thin-film composite forward osmosis membranes for desalination. Desalination 2018, 445, 63–74. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Sengupta, A.; Chen, S.-T.; Huang, S.-H.; Hu, C.-C.; Hung, W.-S.; Chang, Y.; Qian, X.; Wickramasinghe, S.R.; Lee, K.-R.; et al. Zwitterion augmented polyamide membrane for improved forward osmosis performance with significant antifouling characteristics. Sep. Purif. Technol. 2019, 212, 316–325. [Google Scholar] [CrossRef]
- Goh, K.; Setiawan, L.; Wei, L.; Jiang, W.; Wang, R.; Chen, Y. Fabrication of novel functionalized multi-walled carbon nanotube immobilized hollow fiber membranes for enhanced performance in forward osmosis process. J. Membr. Sci. 2013, 446, 244–254. [Google Scholar] [CrossRef]


| Draw Solution | Average Flux (L m−2 h−1) | % Total Solids Rejection | |||
|---|---|---|---|---|---|
| SPW | PW | SPW | PW | ||
| NaCl | 1 M | 7.89 | 2.54 | 92.26 | 79.86 |
| 2 M | 10.94 | 3.54 | 84.79 | 66.50 | |
| 3 M | 12.26 | 4.46 | 77.80 | 54.52 | |
| NaBr | 1 M | 9.99 | 3.96 | 96.02 | 88.90 |
| 2 M | 13.33 | 4.89 | 88.15 | 74.45 | |
| 3 M | 16.05 | 6.00 | 81.36 | 59.04 | |
| NaHCO3 | 1 M | 2.61 | 2.15 | 82.12 | 65.89 |
| 2 M | 4.41 | 2.66 | 75.89 | 57.32 | |
| 3 M | 6.22 | 2.97 | 66.36 | 48.08 | |
| Electrospun Membrane Materials | Flux (L m−2 h−1) | Salt Flux/Salt Rejection | Reference |
|---|---|---|---|
| PES | Water = 37.8 | 97% | [51] |
| ePET/PSf | Water =1.13 | 0.23 (g/L) | [52] |
| PVDF | Water = 30.4 | 0.21 (g/L) | [53] |
| PET/PVA | Water = 47.2 | 9.5 (gMH) | [54] |
| Nylon 6, 6 | Water = 27 | 0.44 (g/L) | [55] |
| p-aramid nanofibers | Water = 19.9 | 98.6% | [56] |
| PAN/PSf | Water = 38.3 | 0.27 (g/L) | [57] |
| PAN | Water = 62.9 | 0.132 (g/L) | [58] |
| PVDF-GO | Water = 54.6 | 0.42 (g/L) | [59] |
| CA/PVDF | Water = 31.3 | 0.03 (g/L) | [60] |
| Zwitterionic TFC | Water = 15 | 95.7% | [61] |
| Hollow fiber | Water = 13.0 | 87.8% | [62] |
| PAI substrate | |||
| PEI-Zwitterionic | Average water | This study | |
| dual layered | SPW = 16.05 | 96.02% | |
| PW = 6.00 | 88.90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madduri, S.B.; Kommalapati, R.R. Novel Dual-Layer Zwitterionic Modification of Electrospun Nanofibrous Membrane for Produced Water Treatment and Reclamation. Membranes 2025, 15, 244. https://doi.org/10.3390/membranes15080244
Madduri SB, Kommalapati RR. Novel Dual-Layer Zwitterionic Modification of Electrospun Nanofibrous Membrane for Produced Water Treatment and Reclamation. Membranes. 2025; 15(8):244. https://doi.org/10.3390/membranes15080244
Chicago/Turabian StyleMadduri, Sunith B., and Raghava R. Kommalapati. 2025. "Novel Dual-Layer Zwitterionic Modification of Electrospun Nanofibrous Membrane for Produced Water Treatment and Reclamation" Membranes 15, no. 8: 244. https://doi.org/10.3390/membranes15080244
APA StyleMadduri, S. B., & Kommalapati, R. R. (2025). Novel Dual-Layer Zwitterionic Modification of Electrospun Nanofibrous Membrane for Produced Water Treatment and Reclamation. Membranes, 15(8), 244. https://doi.org/10.3390/membranes15080244






