Environmental Sustainability Assessment of a Filtration–Diafiltration Strategy for Recovering Savory Compounds from Mussel Cooking Water
Abstract
1. Introduction
2. Materials and Methods
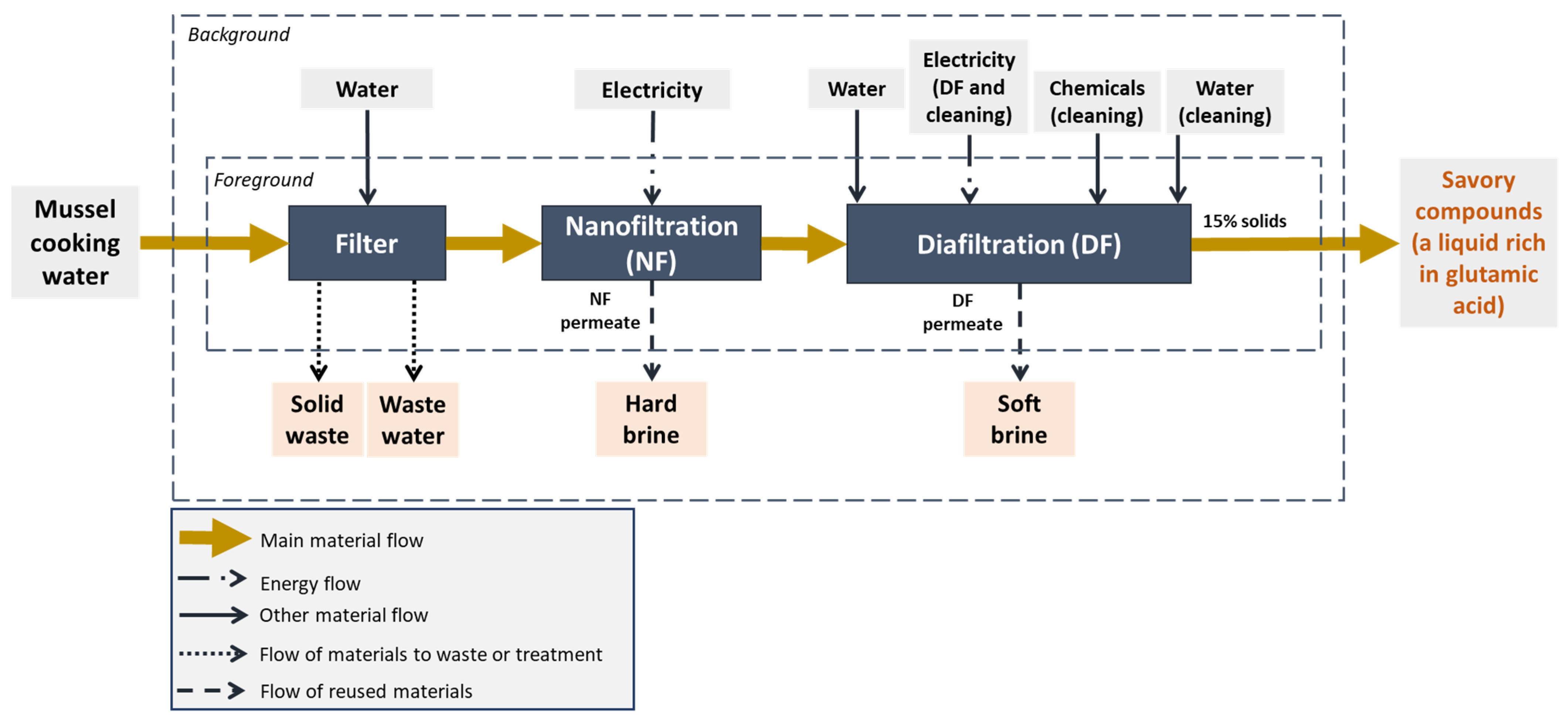
2.1. System Description
2.2. Concentration Process
2.3. Analytical Methods
2.4. Data Interpretation
2.5. Life Cycle Assessment
2.5.1. Goal and Scope
2.5.2. Life Cycle Inventory
2.5.3. Life Cycle Impact Assessment
2.5.4. Life Cycle Impact Interpretation
3. Results and Discussion
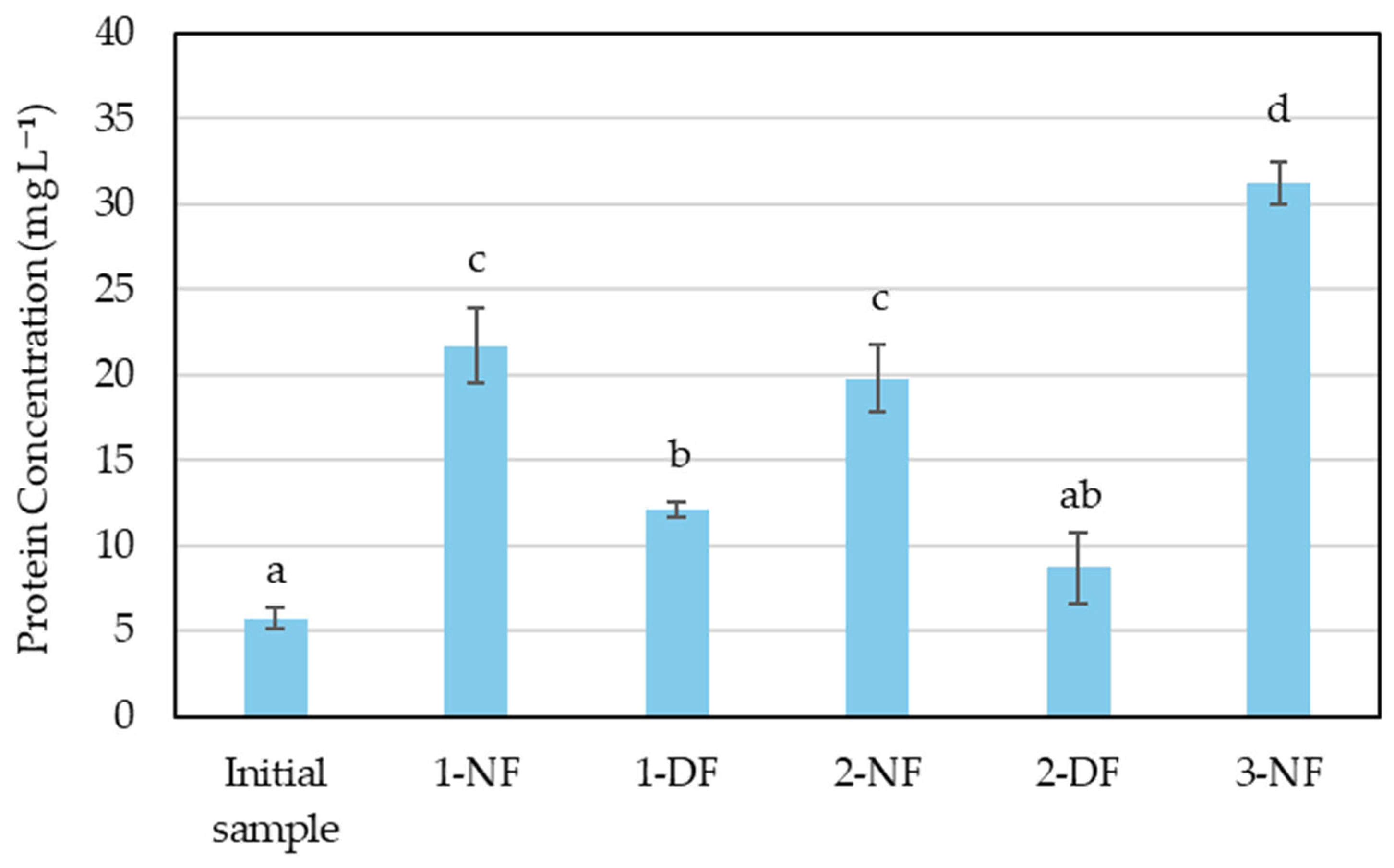
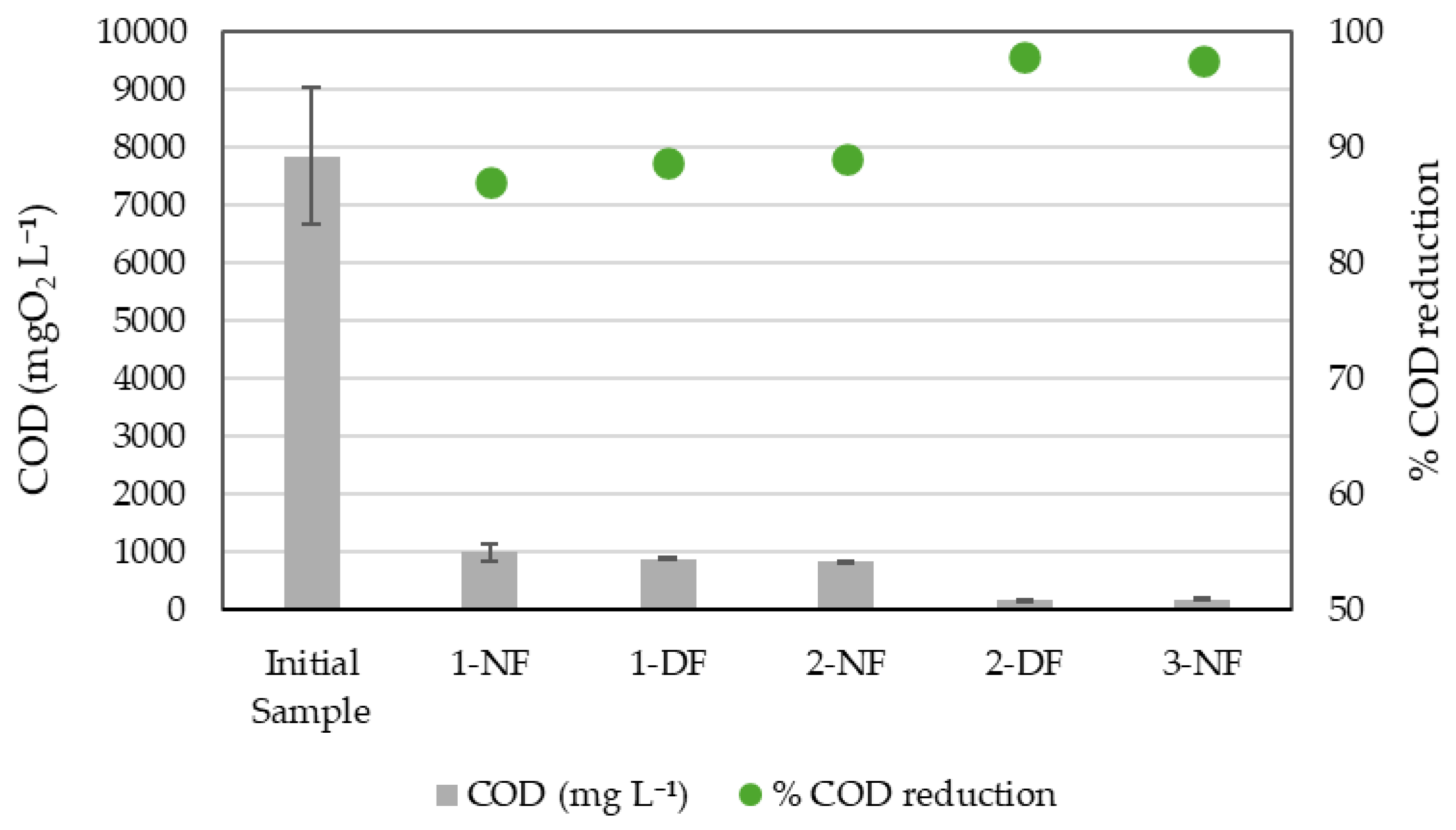
3.1. Concentration Processes
3.2. Effect of Membrane Processing on Glutamic Acid Recovery
3.3. Optimal Concentration Strategy of Proposed Nanofiltration System
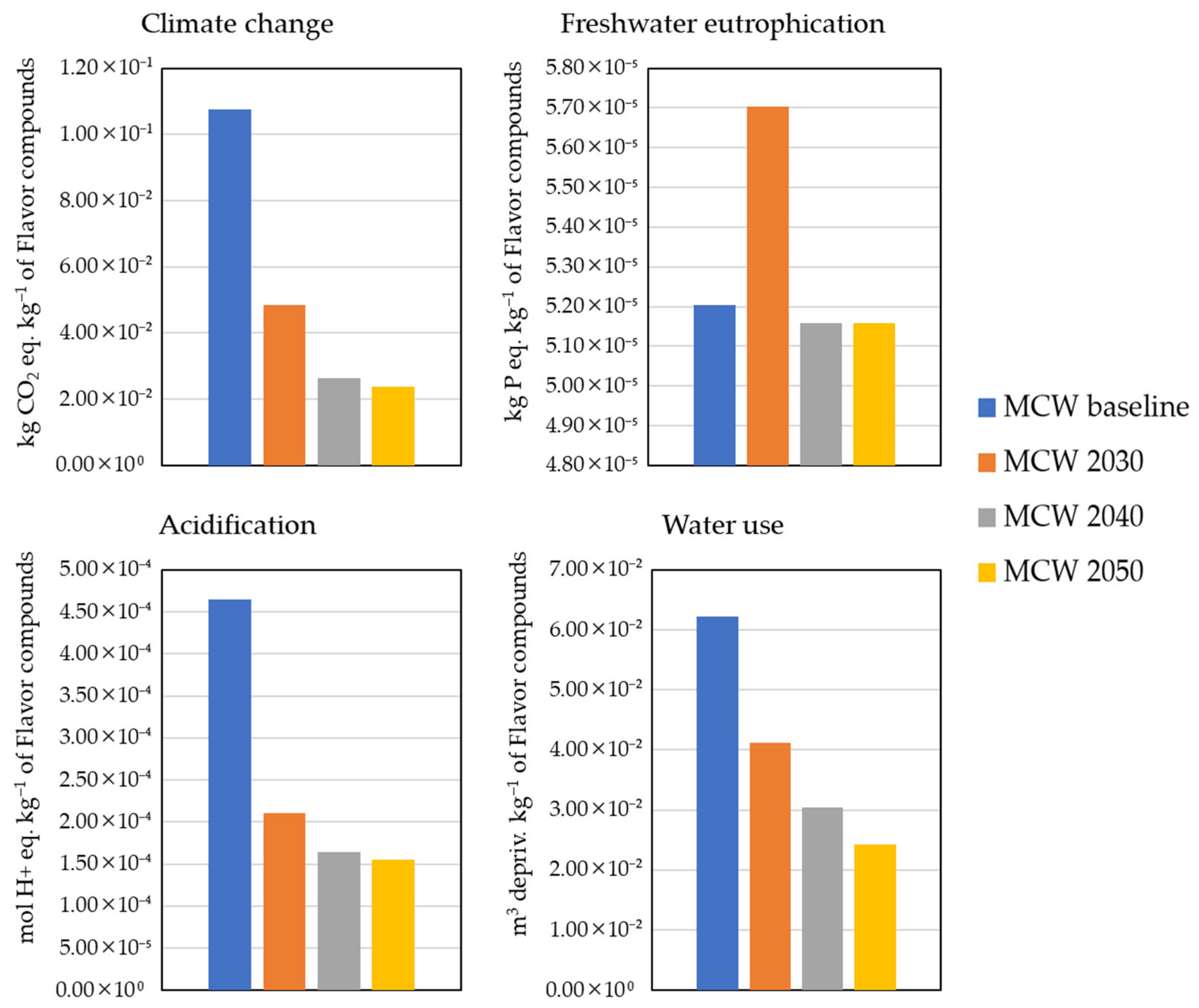
3.4. Life Cycle Assessment Results
3.4.1. Uncertainty Analysis
3.4.2. Sensitivity Analysis
3.5. Limitation of the Analysis and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aa | free amino acids |
| COD | Chemical Oxygen Demand |
| CV | coefficient of variation |
| DF | diafiltration |
| LCA | Life Cycle Assessment |
| MCW | mussel cooking water |
| MWCO | Molecular Weight Cut-Off |
| NF | nanofiltration |
| REMIND | Regional Model of Investments and Development |
| VCF | volumetric concentration factor |
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- EUMOFA. Organic Aquaculture in the EU: Current Situation, Drivers, Barriers and Potential for Growth; EUMOFA: Luxembourg, 2022. [Google Scholar]
- Avdelas, L.; Avdic-Mravlje, E.; Borges Marques, A.C.; Cano, S.; Capelle, J.J.; Carvalho, N.; Cozzolino, M.; Dennis, J.; Ellis, T.; Fernández Polanco, J.M. The Decline of Mussel Aquaculture in the European Union: Causes, Economic Impacts and Opportunities. Rev. Aquac. 2021, 13, 91–118. [Google Scholar] [CrossRef]
- Medina Uzcategui, L.U.; Vergara, K.; Martinez Bordes, G. Sustainable Alternatives for By-Products Derived from Industrial Mussel Processing: A Critical Review. Waste Manag. Res. 2022, 40, 123–138. [Google Scholar] [CrossRef]
- Barros, M.C.; Magán, A.; Valiño, S.; Bello, P.M.; Casares, J.J.; Blanco, J.M. Identification of Best Available Techniques in the Seafood Industry: A Case Study. J. Clean Prod. 2009, 17, 391–399. [Google Scholar] [CrossRef]
- Bugallo, P.M.B.; Stupak, A.; Andrade, L.C.; López, R.T. Material Flow Analysis in a Cooked Mussel Processing Industry. J. Food Eng. 2012, 113, 100–117. [Google Scholar] [CrossRef]
- Piercy, E.; Verstraete, W.; Ellis, P.R.; Banks, M.; Rockström, J.; Smith, P.; Witard, O.C.; Hallett, J.; Hogstrand, C.; Knott, G. A Sustainable Waste-to-Protein System to Maximise Waste Resource Utilisation for Developing Food-and Feed-Grade Protein Solutions. Green Chem. 2023, 25, 808–832. [Google Scholar] [CrossRef]
- Cros, S.; Ignot, B.L.; Razafintsalama, C.; Jaouen, P.; Ourseau, P.B. Electrodialysis Desalination and Reverse Osmosis Concentration of an Industrial Mussel Cooking Juice: Process Impact on Pollution Reduction and on Aroma Quality. J. Food Sci. 2004, 69, C435–C442. [Google Scholar] [CrossRef]
- Jundee, J.; Devahastin, S.; Chiewchan, N. Development and Testing of a Pilot-Scale Electrodialyser for Desalination of Fish Sauce. Procedia Eng. 2012, 32, 97–103. [Google Scholar] [CrossRef][Green Version]
- Cros, S.; Lignot, B.; Bourseau, P.; Jaouen, P. Reverse Osmosis for the Production of Aromatic Concentrates from Mussel Cooking Juices: A Technical Assessment. Desalination 2005, 180, 263–269. [Google Scholar] [CrossRef]
- Kumar, Y.; Khalangre, A.; Suhag, R.; Cassano, A. Applications of Reverse Osmosis and Nanofiltration Membrane Process in Wine and Beer Industry. Membranes 2025, 15, 140. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and Tight Ultrafiltration Membranes for the Recovery of Polyphenols from Agro-Food by-Products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef]
- Gavrilaș, S. Nanomembranes as Eco-Friendly Instruments for Modern Food Processing, from Filtration to Packaging. Membranes 2025, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, S.; Perreault, V.; Goulet, C.; Bouchard, M.; Dubé, P.; Boutin, Y.; Bazinet, L. Assessment of the Performance of Electrodialysis in the Removal of the Most Potent Odor-Active Compounds of Herring Milt Hydrolysate: Focus on Ion-Exchange Membrane Fouling and Water Dissociation as Limiting Process Conditions. Membranes 2020, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.D.; Bórquez, R. Nanofiltration of Wastewaters from the Fish Meal Industry. Desalination 2003, 151, 131–138. [Google Scholar] [CrossRef]
- Bourseau, P.; Vandanjon, L.; Jaouen, P.; Chaplain-Derouiniot, M.; Masse, A.; Guérard, F.; Chabeaud, A.; Fouchereau-Peron, M.; Le Gal, Y.; Ravallec-Plé, R. Fractionation of Fish Protein Hydrolysates by Ultrafiltration and Nanofiltration: Impact on Peptidic Populations. Desalination 2009, 244, 303–320. [Google Scholar] [CrossRef]
- Hilborn, R.; Banobi, J.; Hall, S.J.; Pucylowski, T.; Walsworth, T.E. The Environmental Cost of Animal Source Foods. Front. Ecol. Environ. 2018, 16, 329–335. [Google Scholar] [CrossRef]
- Michel, F.; Hartmann, C.; Siegrist, M. Consumers’ Associations, Perceptions and Acceptance of Meat and Plant-Based Meat Alternatives. Food Qual. Prefer. 2021, 87, 104063. [Google Scholar] [CrossRef]
- ISO 14040:2006; ISO-Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006.
- Xia, F.L.W.; Supri, S.; Djamaludin, H.; Nurdiani, R.; Seng, L.L.; Yin, K.W.; Rovina, K. Turning Waste into Value: Extraction and Effective Valorization Strategies of Seafood by-Products. Waste Manag. Bull. 2024, 2, 84–100. [Google Scholar] [CrossRef]
- Amponsah, L.; Chuck, C.; Parsons, S. Life Cycle Assessment of a Marine Biorefinery Producing Protein, Bioactives and Polymeric Packaging Material. Int. J. Life Cycle Assess 2024, 29, 174–191. [Google Scholar] [CrossRef]
- Iribarren, D.; Moreira, M.T.; Feijoo, G. Implementing By-Product Management into the Life Cycle Assessment of the Mussel Sector. Resour. Conserv. Recycl. 2010, 54, 1219–1230. [Google Scholar] [CrossRef]
- Ruiz-Salmón, I.; Laso, J.; Margallo, M.; Villanueva-Rey, P.; Rodríguez, E.; Quinteiro, P.; Dias, A.C.; Almeida, C.; Nunes, M.L.; Marques, A. Life Cycle Assessment of Fish and Seafood Processed Products–a Review of Methodologies and New Challenges. Sci. Total Environ. 2021, 761, 144094. [Google Scholar] [CrossRef]
- Cadena, E.; Kocak, O.; Dewulf, J.; Iñarra, B.; Bald, C.; Gutierrez, M.; San Martin, D.; Ibarruri, J.; Sørensen, A.-D.M.; Hyldig, G. Valorisation of Seafood Side-Streams through the Design of New Holistic Value Chains: WASEABI Project. Sustainability 2024, 16, 1846. [Google Scholar] [CrossRef]
- Gutierrez, M.; San Martin, D.; Ibarruri, J.; Foti, G.; Bald, C.; Goienetxea, N.; Zufia, J.; Iñarra, B. Recovery of Savory Compounds from Mussel Cooking Side Stream as Circular Economy Solution. Environ. Chall. 2024, 14, 100840. [Google Scholar] [CrossRef]
- Macedo, A.; Bilau, J.; Cambóias, E.; Duarte, E. Integration of Membrane Processes for By-Product Valorization to Improve the Eco-Efficiency of Small/Medium Size Cheese Dairy Plants. Foods 2021, 10, 1740. [Google Scholar] [CrossRef] [PubMed]
- Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 1997; ISBN 0935584676. [Google Scholar]
- APHA. Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association (APHA): Washington, DC, USA; American Water Works Association (AWWA): Denver, CO, USA; Water Environment Federation (WEF): Alexandria, VA, USA, 2012. [Google Scholar]
- Malaver Ortega, L.F.; Alméciga-Díaz, C.J.; Morales Monsalve, I.S.; Echeverri Peña, O.Y.; Guevara Morales, J.; Zuluaga Torres, E.; Córdoba Ruiz, H.A.; Barrera Avellaneda, L.A. Cuantificación de Aminoácidos En Plasma Empleando Cromatografía Líquida de Alta Eficiencia. Acta Bioquímica Clínica Latinoam. 2009, 43, 647–660. [Google Scholar]
- ISO 14044:2006; ISO-Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Geneva, Switzerland, 2006.
- Sacchi, R.; Terlouw, T.; Siala, K.; Dirnaichner, A.; Bauer, C.; Cox, B.; Mutel, C.; Daioglou, V.; Luderer, G. PRospective EnvironMental Impact AsSEment (Premise): A Streamlined Approach to Producing Databases for Prospective Life Cycle Assessment Using Integrated Assessment Models. Renew. Sustain. Energy Rev. 2022, 160, 112311. [Google Scholar] [CrossRef]
- Bordbar, B.; Khosravi, A.; Ahmadi Orkomi, A.; Peydayesh, M. Life Cycle Assessment of Hybrid Nanofiltration Desalination Plants in the Persian Gulf. Membranes 2022, 12, 467. [Google Scholar] [CrossRef]
- Steubing, B.; De Koning, D.; Haas, A.; Mutel, C.L. The Activity Browser—An Open Source LCA Software Building on Top of the Brightway Framework. Softw. Impacts 2020, 3, 100012. [Google Scholar] [CrossRef]
- Summa, D.; Turolla, E.; Lanzoni, M.; Tamisari, E.; Castaldelli, G.; Tamburini, E. Life Cycle Assessment (LCA) of Two Different Oyster (Crassostrea Gigas) Farming Strategies in the Sacca Di Goro, Northern Adriatic Sea, Italy. Resources 2023, 12, 62. [Google Scholar] [CrossRef]
- Kounina, A.; Margni, M.; Bayart, J.-B.; Boulay, A.-M.; Berger, M.; Bulle, C.; Frischknecht, R.; Koehler, A.; Milà i Canals, L.; Motoshita, M. Review of Methods Addressing Freshwater Use in Life Cycle Inventory and Impact Assessment. Int. J. Life Cycle Assess 2013, 18, 707–721. [Google Scholar] [CrossRef]
- Cadena, E.; Kocak, O.; Dewulf, J.; Undeland, I.; Abdollahi, M. Environmental Sustainability Assessment of PH-Shift Technology for Recovering Proteins from Diverse Fish Solid Side Streams. Sustainability 2025, 17, 323. [Google Scholar] [CrossRef]
- Baumstark, L.; Bauer, N.; Benke, F.; Bertram, C.; Bi, S.; Gong, C.C.; Dietrich, J.P.; Dirnaichner, A.; Giannousakis, A.; Hilaire, J. REMIND2. 1: Transformation and Innovation Dynamics of the Energy-Economic System within Climate and Sustainability Limits. Geosci. Model Dev. Discuss. 2021, 2021, 1–50. [Google Scholar]
- Mallakpour, S.; Azadi, E. Nanofiltration Membranes for Food and Pharmaceutical Industries. Emergent Mater. 2022, 5, 1329–1343. [Google Scholar] [CrossRef]
- Le-Clech, P. Protein Fouling Mechanisms. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1695–1696. ISBN 3662443244. [Google Scholar]
- Namla, D.; Oves, M.; Alshaeri, M.A.; Al-Maaqar, S.M.; Issa, H.N.Y.; Mangse, G. Nanofiltration as an Advanced Wastewater Treatment Technique: A Comprehensive Review. Discov. Appl. Sci. 2025, 7, 1–22. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Purification and Fractionation of Bioactive Peptides through Membrane Filtration: A Critical and Application Review. Trends Food Sci. Technol. 2023, 131, 118–128. [Google Scholar] [CrossRef]
- Saidi, S.; Ben Amar, R. Valorisation of Tuna Processing Waste Biomass for Recovery of Functional and Antioxidant Peptides Using Enzymatic Hydrolysis and Membrane Fractionation Process. Environ. Sci. Pollut. Res. 2016, 23, 21070–21085. [Google Scholar] [CrossRef]
- Gómez-Calvet, R.; Martínez-Duart, J.M.; Serrano-Calle, S. Current State and Optimal Development of the Renewable Electricity Generation Mix in Spain. Renew Energy 2019, 135, 1108–1120. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Energy. State of the Energy Union 2022—Snapshots per EU Country; European Commission: Brussels, Belgium, 2022. Available online: https://energy.ec.europa.eu/publications/state-energy-union-2022-snapshots-eu-country_en (accessed on 2 June 2025).
- Nikravan, M.; Firdous, R.; Stephan, D. Life Cycle Assessment of Alkali-Activated Materials: A Systematic Literature Review. Low-Carbon Mater. Green Constr. 2023, 1, 13. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S. Sodium Hydroxide for Clean Hydrogen Production. In Clean Hydrogen Production Methods; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–30. [Google Scholar]
- Burgess, A.A.; Brennan, D.J. Application of Life Cycle Assessment to Chemical Processes. Chem. Eng. Sci. 2001, 56, 2589–2604. [Google Scholar] [CrossRef]
- Garcia-Herrero, I.; Margallo, M.; Onandía, R.; Aldaco, R.; Irabien, A. Environmental Challenges of the Chlor-Alkali Production: Seeking Answers from a Life Cycle Approach. Sci. Total Environ. 2017, 580, 147–157. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. The Blue Water Footprint of Electricity from Hydropower. Hydrol. Earth Syst. Sci. 2012, 16, 179–187. [Google Scholar] [CrossRef]
- Bashiri, B.; Cropotova, J.; Kvangarsnes, K.; Gavrilova, O.; Vilu, R. Environmental and Economic Life Cycle Assessment of Enzymatic Hydrolysis-Based Fish Protein and Oil Extraction. Resources 2024, 13, 61. [Google Scholar] [CrossRef]
- Boukouvalas, C.; Kekes, T.; Oikonomopoulou, V.; Krokida, M. Life Cycle Assessment of Energy Production from Solid Waste Valorization and Wastewater Purification: A Case Study of Meat Processing Industry. Energies 2024, 17, 487. [Google Scholar] [CrossRef]
- do Nascimento, N.N.; Paraíso, C.M.; Molina, L.C.A.; Dzyazko, Y.S.; Bergamasco, R.; Vieira, A.M.S. Innovative Trends in Modified Membranes: A Mini Review of Applications and Challenges in the Food Sector. Membranes 2024, 14, 209. [Google Scholar] [CrossRef]
- Pini Pereira, P.; Pacola Gonçalves, I.; Molina, L.C.A.; Delcolle, R.; Dzyazko, Y.S.; Moser Paraiso, C.; Batista Neto, G.L.; Diório, A.; Marquetotti Salcedo Vieira, A.; Bergamasco, R. Membrane for Pressure-Driven Separation Prepared with a Method of 3D Printing: Performance in Concentrating Orange Peel Extract. Membranes 2025, 15, 105. [Google Scholar] [CrossRef]



| Assay nº | Nanofiltration Step | Diafiltration Step | ||
|---|---|---|---|---|
| VCF | Sample | VCF | Sample | |
| 1 | 10 | 1-NF | 10 | 1-DF |
| 2 | 10 | 2-NF | 20 | 2-DF |
| 3 | 20 | 3-NF | 20 | 3-DF |
| Inputs | Unit | Amount |
|---|---|---|
| Membrane module 1 | p | 1.00 × 100 |
| Process water | L | 1.91 × 101 |
| Cleaning water | L | 2.62 × 10−1 |
| Sodium hydroxide (NaOH) | kg | 7.40 × 10−4 |
| Citric acid | kg | 6.41 × 10−4 |
| Electricity for NF 2 | kWh | 1.98 × 10−1 |
| Electricity for DF 2 | kWh | 2.08 × 10−1 |
| Electricity (cleaning) 2 | kWh | 1.48 × 10−3 |
| Outputs | ||
| Savory compounds | kg | 1.00 × 100 |
| Wastewater (cleaning) | L | 3.81 × 10−1 |
| Solid waste | kg | 7.84 × 10−3 |
| Water recovery 3 | L | 2.74 × 101 |
| Filtration Step | Energy Consumption per m3 Treated (kWh m−3) | ||
|---|---|---|---|
| Assay 1 | Assay 2 | Assay 3 | |
| NF step | 8.43 | 8.35 | 21.51 |
| DF step | 7.99 | 8.77 | N.D. |
| Total energy per assay | 16.42 | 17.12 | 21.51 1 |
| Process Parameters | Assay 1 | Assay 2 | Assay 3 |
|---|---|---|---|
| NF process flowrate (L h−1) | 375 | 375 | 300 |
| NF permeate volume (L) | 900 | 900 | 950 |
| DF added water (L) | 900 | 900 | 950 * |
| DF process flowrate (L h−1) | 500 | 400 | 350 * |
| DF permeate volume (L) | 900 | 950 | 950 * |
| Final concentrate volume (L) | 100 | 50 | 50 * |
| Total permeate volume (L) | 1800 | 1850 | 1900 * |
| Total process time (h) | 4.67 | 5.17 | 6.19 * |
| Amino Acid | Initial Sample Aa (mg L−1) | Assay 1 Concentrates Aa (mg L−1) | Assay 2 Concentrates Aa (mg L−1) | ||
|---|---|---|---|---|---|
| NF 10× | DF 10× | NF 10× | DF 20× | ||
| Aspartic acid | 14.3 ± 0.1 | 283.9 ± 4.0 | 173.5 ± 1.1 | 365.5 ± 6.7 | 167.3 ± 3.7 |
| Glutamic acid | 81.8 ± 1.6 | 567.4 ± 8.2 | 366.0 ± 0.9 | 673.7 ± 5.4 | 441.4 ± 9.7 |
| Asparagine | 8.0 ± 0.1 | 89.5 ± 5.1 | 41.7 ± 0.3 | 98.4 ± 0.9 | 16.0 ± 0.6 |
| Serine | 5.0 ± 0.3 | 70.1 ± 2.2 | 54.1 ± 0.5 | 183.7 ± 2.3 | 27.2 ± 0.6 |
| Glutamine | 21.6 ± 1.0 | 227.2 ± 5.7 | 62.3 ± 0.4 | 190.9 ± 2.3 | 2.2 ± 0.3 |
| Histidine | 30.0 ± 0.1 | 121.3 ± 3.5 | 66.4 ± 1.0 | 94.6 ± 0.1 | 29.7 ± 1.1 |
| Glycine | 298.7 ± 2.8 | 566.3 ± 10.1 | 106.8 ± 0.1 | 650.9 ± 3.4 | 108.9 ± 2.2 |
| Threonine | 13.3 ± 1.0 | 96.3 ± 1.6 | 57.3 ± 0.0 | 69.0 ± 0.2 | 29.6 ± 0.2 |
| Arginine | 29.6 ± 1.1 | 60.5 ± 22.6 | 37.7 ± 0.7 | 317.1 ± 1.3 | 29.9 ± 8.8 |
| Alanine | 182.2 ± 4.4 | 417.8 ± 8.0 | 100.9 ± 0.6 | 477.8 ± 19.3 | 111.2 ± 1.9 |
| Tyrosine | 26.2 ± 2.4 | 239.2 ± 6.9 | 85.5 ± 0.4 | 124.8 ± 0.4 | 92.5 ± 1.2 |
| Cysteine | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Valine | 5.0 ± 0.3 | 52.2 ± 2.4 | 36.6 ± 0.4 | 51.3 ± 0.1 | 60.9 ± 1.5 |
| Methionine | 28.7 ± 5.8 | 48.4 ± 1.8 | 30.7 ± 0.2 | 40.2 ± 5.9 | 34.2 ± 5.7 |
| Norvaline | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Tryptophan | 24.1 ± 2.0 | 58.2 ± 3.8 | 38.0 ± 0.0 | 67.5 ± 5.4 | 16.1 ± 0.0 |
| Phenylalanine | 6.5 ± 1.5 | 53.7 ± 4.7 | 38.7 ± 0.4 | 38.3 ± 0.4 | 33.0 ± 1.4 |
| Isoleucine | 5.1 ± 0.1 | 37.6 ± 2.8 | 31.4 ± 0.3 | 40.5 ± 0.4 | 42.0 ± 1.9 |
| Leucine | 12.4 ± 0.9 | 48.0 ± 2.8 | 36.0 ± 0.3 | 68.2 ± 0.3 | 75.6 ± 1.2 |
| Lysine | 56.7 ± 0.8 | 356.3 ± 7.8 | 205.5 ± 7.4 | 388.5 ± 3.8 | 106.6 ± 3.3 |
| Hydroxyproline | 276.1 ± 11.6 | 148.3 ± 18.2 | 82.0 ± 3.4 | 21.6 ± 4.5 | 77.7 ± 7.1 |
| Sarcosine | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Proline | 19.0 ± 9.2 | 34.3 ± 11.1 | 17.6 ± 4.3 | 181.7 ± 2.1 | 98.3 ± 8.4 |
| Amino Acid | Initial Sample Aa (%) | Assay 1 Concentrates Aa (%) | Assay 2 Concentrates Aa (%) | ||
|---|---|---|---|---|---|
| NF 10× | DF 10× | NF 10× | DF 20× | ||
| Aspartic acid | 1.25 ± 0.05 | 7.94 ± 0.03 | 10.40 ± 0.13 | 8.76 ± 0.06 | 10.51 ± 0.04 |
| Glutamic acid | 7.15 ± 0.09 | 15.86 ± 0.07 | 21.94 ± 0.09 | 16.31 ± 0.06 | 27.72 ± 0.11 |
| Asparagine | 0.70 ± 0.02 | 2.50 ± 0.12 | 2.50 ± 0.00 | 2.38 ± 0.01 | 1.01 ± 0.01 |
| Serine | 0.44 ± 0.04 | 1.96 ± 0.04 | 3.24 ± 0.01 | 4.43 ± 0.00 | 1.71 ± 0.01 |
| Glutamine | 1.89 ± 0.03 | 6.35 ± 0.09 | 3.74 ± 0.00 | 4.60 ± 0.00 | 0.14 ± 0.02 |
| Histidine | 2.62 ± 0.09 | 3.39 ± 0.06 | 3.98 ± 0.03 | 2.31 ± 0.03 | 1.87 ± 0.02 |
| Glycine | 26.12 ± 0.60 | 15.83 ± 0.12 | 6.40 ± 0.05 | 15.81 ± 0.10 | 6.84 ± 0.04 |
| Threonine | 1.16 ± 0.05 | 2.69 ± 0.02 | 3.43 ± 0.02 | 1.68 ± 0.02 | 1.86 ± 0.03 |
| Arginine | 2.60 ± 0.18 | 1.70 ± 0.65 | 2.26 ± 0.05 | 7.71 ± 0.06 | 1.87 ± 0.50 |
| Alanine | 15.93 ± 0.13 | 11.68 ± 0.11 | 6.05 ± 0.00 | 11.19 ± 0.34 | 6.98 ± 0.06 |
| Tyrosine | 2.28 ± 0.14 | 6.69 ± 0.13 | 5.12 ± 0.01 | 3.06 ± 0.04 | 5.81 ± 0.08 |
| Cysteine | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Valine | 0.43 ± 0.01 | 1.46 ± 0.05 | 2.19 ± 0.01 | 1.25 ± 0.02 | 3.83 ± 0.01 |
| Methionine | 2.49 ± 0.43 | 1.35 ± 0.04 | 1.84 ± 0.00 | 1.13 ± 0.16 | 2.16 ± 0.41 |
| Norvaline | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Tryptophan | 2.11 ± 0.24 | 1.63 ± 0.09 | 2.28 ± 0.01 | 1.52 ± 0.11 | 0.52 ± 0.52 |
| Phenylalanine | 0.57 ± 0.15 | 1.50 ± 0.12 | 2.32 ± 0.04 | 0.92 ± 0.00 | 2.07 ± 0.03 |
| Isoleucine | 0.45 ± 0.01 | 1.05 ± 0.07 | 1.88 ± 0.03 | 0.98 ± 0.00 | 2.64 ± 0.05 |
| Leucine | 1.08 ± 0.04 | 1.34 ± 0.06 | 2.16 ± 0.00 | 1.67 ± 0.03 | 4.75 ± 0.05 |
| Lysine | 4.96 ± 0.09 | 9.96 ± 0.32 | 12.32 ± 0.37 | 9.39 ± 0.02 | 6.69 ± 0.04 |
| Hydroxyproline | 24.12 ± 0.24 | 4.15 ± 0.55 | 4.92 ± 0.23 | 0.42 ± 0.10 | 4.87 ± 0.32 |
| Sarcosine | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Proline | 1.63 ± 0.75 | 0.96 ± 0.30 | 1.05 ± 0.25 | 4.49 ± 0.10 | 6.17 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadena, E.; Dewulf, J.; San Martin, D.; Ibarruri, J.; Iñarra, B.; Gutierrez, M. Environmental Sustainability Assessment of a Filtration–Diafiltration Strategy for Recovering Savory Compounds from Mussel Cooking Water. Membranes 2025, 15, 242. https://doi.org/10.3390/membranes15080242
Cadena E, Dewulf J, San Martin D, Ibarruri J, Iñarra B, Gutierrez M. Environmental Sustainability Assessment of a Filtration–Diafiltration Strategy for Recovering Savory Compounds from Mussel Cooking Water. Membranes. 2025; 15(8):242. https://doi.org/10.3390/membranes15080242
Chicago/Turabian StyleCadena, Erasmo, Jo Dewulf, David San Martin, Jone Ibarruri, Bruno Iñarra, and Monica Gutierrez. 2025. "Environmental Sustainability Assessment of a Filtration–Diafiltration Strategy for Recovering Savory Compounds from Mussel Cooking Water" Membranes 15, no. 8: 242. https://doi.org/10.3390/membranes15080242
APA StyleCadena, E., Dewulf, J., San Martin, D., Ibarruri, J., Iñarra, B., & Gutierrez, M. (2025). Environmental Sustainability Assessment of a Filtration–Diafiltration Strategy for Recovering Savory Compounds from Mussel Cooking Water. Membranes, 15(8), 242. https://doi.org/10.3390/membranes15080242








