Antifouling and Desalination Enhancement of Forward Osmosis-Based Thin Film Composite Membranes via Functionalized Multiwalled Carbon Nanotubes Mixed Matrix Polyethersulfone Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Fabrication of Thin-Film Composite (TFC) Membrane
2.3. Characterization
2.3.1. Field Emission Scanning Electron Microscopy (FE-SEM)
2.3.2. Brunauer-Emmett-Teller (BET)
2.3.3. Attenuated Total Reflectance Fourier-Transform Infrared (ATR-FTIR) Analysis
2.3.4. X-Ray Photoelectron Spectroscopy (XPS)
2.3.5. X-Ray Diffraction Spectroscopy (XRD)
2.3.6. Water Contact Angle (WCA)
2.3.7. Mechanical Property
2.3.8. Thermal Stability
2.3.9. Zeta Potential Measurement
2.3.10. Atomic Force Microscopy (AFM)
2.4. Determination of Porosity and Pore Size of Membrane Support
2.5. FO Experiments
2.6. Evaluation of Membrane Separation Parameters
2.7. Fouling Testing Protocol
3. Results and Discussion
3.1. Characterization of COOH-MWCNTs
3.2. Characterization of Membrane Support
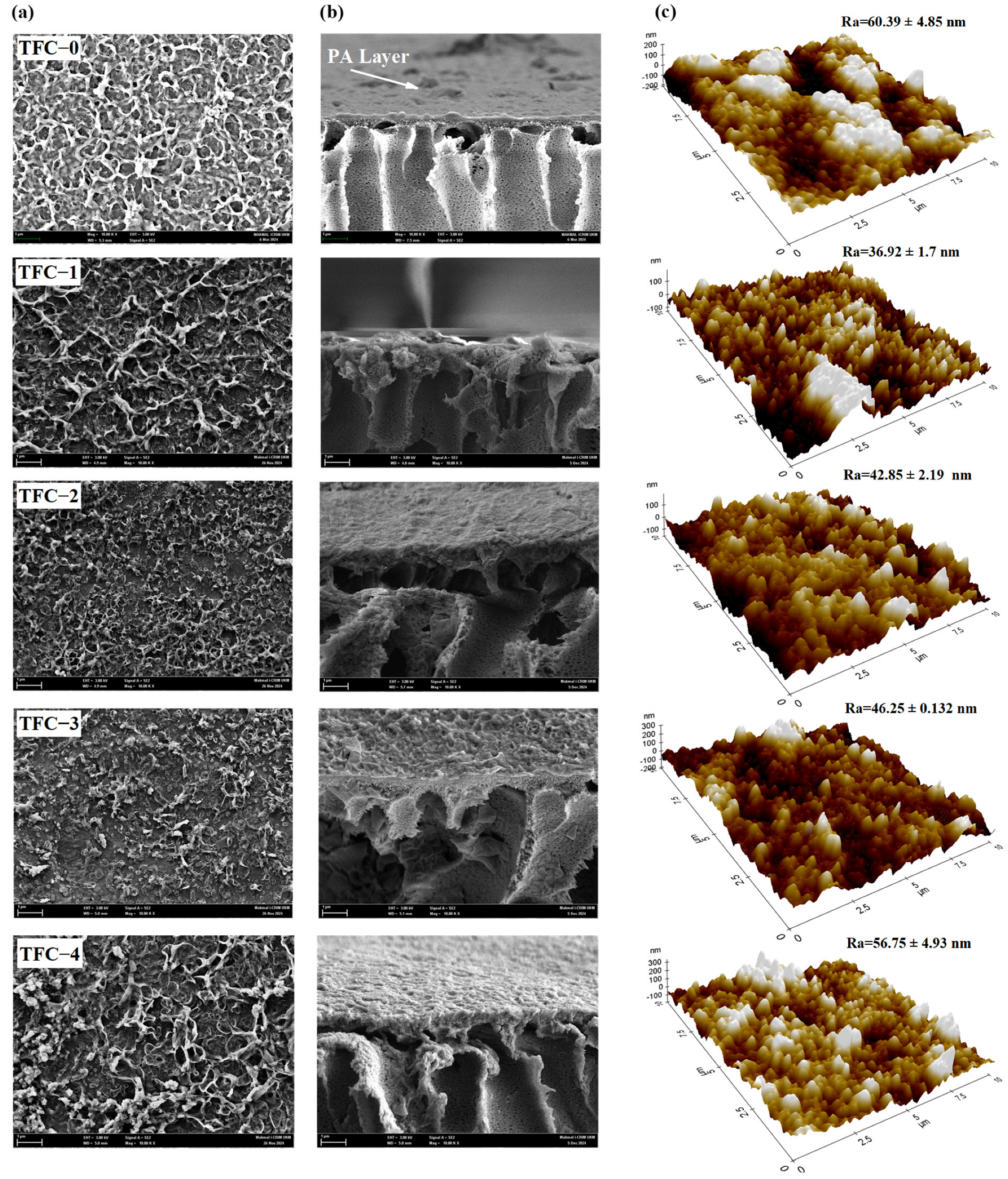
3.3. Characterization of TFC Membranes
3.4. Effect of COOH-MWCNTs Loading on FO Performance
3.5. Effect of Incorporating COOH-MWCNTs on Membrane Separation Properties
| Membranes | A (LMH/bar) | R2 (Jw) | B (LMH) | R2 (Js) | R (%) | S (μm) | Ew% |
|---|---|---|---|---|---|---|---|
| TFC-0 | 0.1169 | 0.99 | 0.7884 | 0.99 | 99.60 | 1029.31 | 20.21 |
| TFC-1 | 0.2078 | 0.95 | 2.1113 | 0.99 | 98.98 | 166.75 | 13.79 |
| TFC-2 | 0.1578 | 0.94 | 2.1863 | 0.99 | 98.96 | 235 | 27.64 |
| TFC-3 | 0.2211 | 0.97 | 2.4255 | 0.99 | 98.94 | 116.55 | 13.72 |
| TFC-4 | 0.2644 | 0.98 | 2.5723 | 0.99 | 99.01 | 162.5 | 16.24 |
3.6. Fouling Resistance
3.7. Flux Stability During Real Seawater Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| ALDS | Active Layer Facing Draw Solution Mode |
| ALFS | Active Layer Facing Feed Solution Mode |
| BET | Brunauer-Emmett-Teller |
| CA | Contact Angle |
| CNM | Charcoal-based Carbon Nanomaterial |
| CNPs | Carbon Nano-Particles |
| COOH-MWCNTs | Carboxyl-Functionalized Multiwalled Carbon Nanotubes |
| DI | Deionized Water |
| DS | Draw Solution |
| f-CNFs | Functionalized Carboxyl Carbon Nanofibers |
| fMWCNTs | Functionalized Multi-Wall Carbon Nanotubes |
| FE-SEM | Field Emission Scanning Electron Microscopy |
| FO | Forward Osmosis |
| FRR | Flux Recovery Ratio |
| FS | Feed Solution |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| GNPs | Graphene Nanoplatelets |
| GO | Graphene Oxide |
| HCDs | Hydrophobic Carbon Dots |
| ICP | Internal Concentration Polarization |
| IP | Interfacial Polymerization |
| Js | Reverse Solute Flux |
| Jw | Water Flux |
| LMH | Liters per Square Meter per Hour |
| MMM | Mixed Matrix Membrane |
| MPD | m-Phenylenediamine |
| MWCNTs | Multiwalled Carbon Nanotubes |
| NaCl | Sodium Chloride |
| NMP | N-Methyl-2-pyrrolidone |
| PA | Polyamide |
| pCN | Protonated Carbon Nitride |
| PEG | Polyethylene Glycol |
| PES | Polyethersulfone |
| pDA | Polydopamine |
| PSf | Polysulfone |
| PVA | Polyvinyl Alcohol |
| PVP | Polyvinylpyrrolidone |
| RO | Reverse Osmosis |
| RT | Room Temperature |
| Rt | Total Flux Decline Ratio |
| SA | Sodium Alginate |
| SGO | Sulfonated Graphene Oxide |
| SRSF | Specific Reverse Solute Flux |
| σ | Tensile Strength |
| TFC | Thin-Film Composite |
| TGA | Thermogravimetric Analysis |
| TMC | Trimesoyl Chloride |
| WCA | Water Contact Angle |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
| ZP | Zeta Potential |
References
- Karmakar, S.; Dechnik, J.; Janiak, C.; De, S. Aluminium fumarate metal-organic framework: A super adsorbent for fluoride from water. J. Hazard. Mater. 2016, 303, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, A.; Peyravi, M.; Arjmandi, M.; Altaee, A. Exploring the use of cheap natural raw materials to reduce the internal concentration polarization in thin-film composite forward osmosis membranes. Chem. Eng. J. 2020, 398, 125483. [Google Scholar] [CrossRef]
- Criscuoli, A.; Figoli, A. Pressure-driven and thermally-driven membrane operations for the treatment of arsenic-contaminated waters: A comparison. J. Hazard. Mater. 2019, 370, 147–155. [Google Scholar] [CrossRef]
- Hou, J.; Liu, P.; Jiang, M.; Yu, L.; Li, L.; Tang, Z. Olefin/paraffin separation through membranes: From mechanisms to critical materials. J. Mater. Chem. A Mater. 2019, 7, 23489–23511. [Google Scholar] [CrossRef]
- Flanagan, M.F.; Escobar, I.C. Novel charged and hydrophilized polybenzimidazole (PBI) membranes for forward osmosis. J. Membr. Sci. 2013, 434, 85–92. [Google Scholar] [CrossRef]
- Dabaghian, Z.; Rahimpour, A. Carboxylated carbon nanofibers as hydrophilic porous material to modification of cellulosic membranes for forward osmosis desalination. Chem. Eng. Res. Des. 2015, 104, 647–657. [Google Scholar] [CrossRef]
- Hausman, R.; Digman, B.; Escobar, I.C.; Coleman, M.; Chung, T.S. Functionalization of polybenzimidizole membranes to impart negative charge and hydrophilicity. J. Membr. Sci. 2010, 363, 195–203. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, N.; Dou, P.; Lin, S.; He, T. Structure parameter as an intrinsic property of thin-film composite forward osmosis membrane. Desalination 2024, 581, 117616. [Google Scholar] [CrossRef]
- Almansouri, H.E.; Edokali, M.; Seman, M.N.A. Flat sheet thin film composite forward osmosis membranes for water treatment and purification: A review on modification techniques and concepts. Desalination Water Treat. 2024, 320, 100815. [Google Scholar] [CrossRef]
- Xu, G.-R.; Xu, J.-M.; Feng, H.-J.; Zhao, H.-L.; Wu, S.-B. Tailoring structures and performance of polyamide thin film composite (PA-TFC) desalination membranes via sublayers adjustment-a review. Desalination 2017, 417, 19–35. [Google Scholar] [CrossRef]
- Yasukawa, M.; Mishima, S.; Tanaka, Y.; Takahashi, T.; Matsuyama, H. Thin-film composite forward osmosis membrane with high water flux and high pressure resistance using a thicker void-free polyketone porous support. Desalination 2017, 402, 1–9. [Google Scholar] [CrossRef]
- Shen, L.; Xiong, S.; Wang, Y. Graphene oxide incorporated thin-film composite membranes for forward osmosis applications. Chem. Eng. Sci. 2016, 143, 194–205. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Guan, C.-Y.; Liu, C.-X.; Lang, W.-Z.; Wang, Y. Construction of SiO2@MWNTs incorporated PVDF substrate for reducing internal concentration polarization in forward osmosis. J. Membr. Sci. 2018, 564, 328–341. [Google Scholar] [CrossRef]
- Xue, Q.; Lim, Y.J.; Zhang, K. Engineering multi-channel water transport in surface-porous MXene nanosheets for high-performance thin-film nanocomposite membranes. J. Membr. Sci. 2025, 728, 124151. [Google Scholar] [CrossRef]
- Xue, Q.; Zhu, J.; Meng, W.; Zhang, K. Effect of MXene Nanosheet Dispersed Phases on the Fabrication of Polyamide Nanofiltration Membranes. ACS Appl. Eng. Mater. 2023, 1, 679–689. [Google Scholar] [CrossRef]
- Sallehudin, T.A.T.; Seman, M.N.A.; Chik, S.M.S.T. Preparation and Characterization Silver Nanoparticle Embedded Polyamide Nanofiltration (NF) Membrane. MATEC Web. Conf. 2018, 150, 02003. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Golgoli, M.; Khiadani, M.; Najafi, M.; Suwaileh, W.; Razmjou, A.; Zargar, M. Recent advances in surface tailoring of thin film forward osmosis membranes: A review. Chemosphere 2024, 346, 140493. [Google Scholar] [CrossRef] [PubMed]
- Rastgar, M.; Shakeri, A.; Bozorg, A.; Salehi, H.; Saadattalab, V. Impact of nanoparticles surface characteristics on pore structure and performance of forward osmosis membranes. Desalination 2017, 421, 179–189. [Google Scholar] [CrossRef]
- Widjojo, N.; Chung, T.-S.; Weber, M.; Maletzko, C.; Warzelhan, V. A sulfonated polyphenylenesulfone (sPPSU) as the supporting substrate in thin film composite (TFC) membranes with enhanced performance for forward osmosis (FO). Chem. Eng. J. 2013, 220, 15–23. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Esteves, C.M.P.; Figueiredo, J.L.; Silva, A.M.T. Thin-film composite forward osmosis membranes based on polysulfone supports blended with nanostructured carbon materials. J. Membr. Sci. 2016, 520, 326–336. [Google Scholar] [CrossRef]
- Darabi, R.R.; Hosseini, S.P.; Peyravi, M.; Jahanshahi, M. Thin-Film Nanocomposite Forward Osmosis Membranes Incorporated with Hydrophilic TiO2/Fe3O4 Nanoparticles: Toward Alleviated ICP. Arab. J. Sci. Eng. 2025, 50, 3971–3986. [Google Scholar] [CrossRef]
- Sharma, S.K.; Deka, B.J. Engineering Novel Thin-Film Composite Membrane for Efficient Forward Osmosis Desalination: Fabrication and Performance Evaluation. ACS ES&T Water 2024, 4, 5913–5924. [Google Scholar] [CrossRef]
- Sirinupong, T.; Youravong, W.; Tirawat, D.; Lau, W.J.; Lai, G.S.; Ismail, A.F. Synthesis and characterization of thin film composite membranes made of PSF-TiO2/GO nanocomposite substrate for forward osmosis applications. Arab. J. Chem. 2018, 11, 1144–1153. [Google Scholar] [CrossRef]
- Salehi, T.M.; Peyravi, M.; Jahanshahi, M.; Lau, W.-J.; Rad, A.S. Impacts of zeolite nanoparticles on substrate properties of thin film nanocomposite membranes for engineered osmosis. J. Nanoparticle Res. 2018, 20, 113. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Qi, S.; Zhao, Y.; Gao, Y.; Tang, C.Y. Nanocomposite substrates for controlling internal concentration polarization in forward osmosis membranes. J. Membr. Sci. 2013, 441, 54–62. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Riazi, H.; Almasi, D.; Ismail, A.F. Minimizing structural parameter of thin film composite forward osmosis membranes using polysulfone/halloysite nanotubes as membrane substrates. Desalination 2016, 377, 152–162. [Google Scholar] [CrossRef]
- Tavakol, I.; Hadadpour, S.; Shabani, Z.; Tofighy, M.A.; Mohammadi, T.; Sahebi, S. Synthesis of novel thin film composite (TFC) forward osmosis (FO) membranes incorporated with carboxylated carbon nanofibers (CNFs). J. Environ. Chem. Eng. 2020, 8, 104614. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Ge, Q.; Wang, H.; Xu, T. Preparation of polyethersulfone/carbon nanotube substrate for high-performance forward osmosis membrane. Desalination 2013, 330, 70–78. [Google Scholar] [CrossRef]
- Son, M.; Choi, H.; Liu, L.; Celik, E.; Park, H.; Choi, H. Efficacy of carbon nanotube positioning in the polyethersulfone support layer on the performance of thin-film composite membrane for desalination. Chem. Eng. J. 2015, 266, 376–384. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, C.; Hou, L.A. Comparison of performance and biofouling resistance of thin-film composite forward osmosis membranes with substrate/active layer modified by graphene oxide. RSC Adv. 2019, 9, 6502–6509. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.-M.; Chen, G.; Chung, W.-J.; Shon, H.K. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Lu, P.; Liang, S.; Qiu, L.; Gao, Y.; Wang, Q. Thin film nanocomposite forward osmosis membranes based on layered double hydroxide nanoparticles blended substrates. J. Membr. Sci. 2016, 504, 196–205. [Google Scholar] [CrossRef]
- Kahrizi, M.; Gonzales, R.R.; Kong, L.; Matsuyama, H.; Lu, P.; Lin, J.; Zhao, S. Significant roles of substrate properties in forward osmosis membrane performance: A review. Desalination 2022, 528, 115615. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; Zheng, J.; Wang, X.; Xia, S.; Van der Bruggen, B. A critical review on thin-film nanocomposite membranes enabled by nanomaterials incorporated in different positions and with diverse dimensions: Performance comparison and mechanisms. J. Membr. Sci. 2022, 661, 120952. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jegal, J.; Kim, W.-N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Silva, T.L.S.; Pastrana-Martínez, L.M.; Brandão, A.T.S.C.; Figueiredo, J.L.; Silva, A.M.T. Modification of the surface chemistry of single- and multi-walled carbon nanotubes by HNO3 and H2SO4 hydrothermal oxidation for application in direct contact membrane distillation. Phys. Chem. Chem. Phys. 2014, 16, 12237–12250. [Google Scholar] [CrossRef]
- Ihsanullah. Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019, 209, 307–337. [Google Scholar] [CrossRef]
- Elsehly, E.M.; Chechenin, N.G. The Performance of Functionalized Multi-Walled Carbon Nanotube-Based Filters for Water Treatment Applications. In Carbon Nanotubes-Recent Advances, Perspectives and Applications; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Barrejón, M.; Prato, M. Carbon Nanotube Membranes in Water Treatment Applications. Adv. Mater. Interfaces 2022, 9, 2101260. [Google Scholar] [CrossRef]
- Lee, B.; Kim, C. Innovative membrane technology for water treatment solutions: Current status and future prospects of carbon nanotube membranes. Environ. Eng. Res. 2024, 29, 240104. [Google Scholar] [CrossRef]
- Goh, K.; Setiawan, L.; Wei, L.; Jiang, W.; Wang, R.; Chen, Y. Fabrication of novel functionalized multi-walled carbon nanotube immobilized hollow fiber membranes for enhanced performance in forward osmosis process. J. Membr. Sci. 2013, 446, 244–254. [Google Scholar] [CrossRef]
- Noy, A.; Park, H.G.; Fornasiero, F.; Holt, J.K.; Grigoropoulos, C.P.; Bakajin, O. Nanofluidics in carbon nanotubes. Nano Today 2007, 2, 22–29. [Google Scholar] [CrossRef]
- Das, R.; Hamid, S.B.A.; Ali, M.E.; Ismail, A.F.; Annuar, M.S.M.; Ramakrishna, S. Multifunctional carbon nanotubes in water treatment: The present, past and future. Desalination 2014, 354, 160–179. [Google Scholar] [CrossRef]
- Kar, S.; Bindal, R.C.; Tewari, P.K. Carbon nanotube membranes for desalination and water purification: Challenges and opportunities. Nano Today 2012, 7, 385–389. [Google Scholar] [CrossRef]
- Rashed, A.O.; Esawi, A.M.K.; Ramadan, A.R. Novel Polysulfone/Carbon Nanotube-Polyamide Thin Film Nanocomposite Membranes with Improved Water Flux for Forward Osmosis Desalination. ACS Omega 2020, 5, 14427–14436. [Google Scholar] [CrossRef]
- Jia, Y.X.; Li, H.L.; Wang, M.; Wu, L.Y.; Hu, Y.D. Carbon nanotube: Possible candidate for forward osmosis. Sep. Purif. Technol. 2010, 75, 55–60. [Google Scholar] [CrossRef]
- Choi, H.; Son, M.; Choi, H. Integrating seawater desalination and wastewater reclamation forward osmosis process using thin-film composite mixed matrix membrane with functionalized carbon nanotube blended polyethersulfone support layer. Chemosphere 2017, 185, 1181–1188. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Wu, D. Recent advances in innovative osmotic membranes for resource enrichment and energy production in wastewater treatment. Sci. Total Environ. 2024, 927, 172153. [Google Scholar] [CrossRef]
- ASTM D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2012. [CrossRef]
- Vrasna, D.K.; Goh, P.S.; Ahmad, N.A.; Gonzales, R.R.; Wong, K.C.; Lim, J.W.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Matsuyama, H. Thin film nanocomposite forward osmosis membrane with exfoliated layered double hydroxide nanosheets embedded support for fouling-resistant microalgae dewatering. J. Water Process Eng. 2024, 58, 104800. [Google Scholar] [CrossRef]
- Pardeshi, P.M.; Mungray, A.K.; Mungray, A.A. Polyvinyl chloride and layered double hydroxide composite as a novel substrate material for the forward osmosis membrane. Desalination 2017, 421, 149–159. [Google Scholar] [CrossRef]
- Shafie, N.A.; Seman, M.N.A.; Saufi, S.M.; Mohammad, A.W. Influence of Polyethersulfone substrate properties on the performance of thin film composite forward osmosis membrane: Effect of additive concentration, polymer concentration and casting thickness. Chem. Eng. Res. Des. 2023, 200, 186–201. [Google Scholar] [CrossRef]
- Aziz, A.A.; Wong, K.C.; Goh, P.S.; Ismail, A.F.; Azelee, I.W. Tailoring the surface properties of carbon nitride incorporated thin film nanocomposite membrane for forward osmosis desalination. J. Water Process Eng. 2020, 33, 101005. [Google Scholar] [CrossRef]
- Liu, X.; Ng, H.Y. Fabrication of layered silica–polysulfone mixed matrix substrate membrane for enhancing performance of thin-film composite forward osmosis membrane. J. Membr. Sci. 2015, 481, 148–163. [Google Scholar] [CrossRef]
- Edokali, M.; Bocking, R.; Mehrabi, M.; Massey, A.; Harbottle, D.; Menzel, R.; Hassanpour, A. Chemical modification of reduced graphene oxide membranes: Enhanced desalination performance and structural properties for forward osmosis. Chem. Eng. Res. Des. 2023, 199, 659–675. [Google Scholar] [CrossRef]
- Akbari, S.; Peyravi, M. Improving water flux and salt rejection by a tradeoff between hydrophilicity and hydrophobicity of sublayer in TFC FO membrane. Chem. Eng. Res. Des. 2020, 162, 94–106. [Google Scholar] [CrossRef]
- Kim, B.; Gwak, G.; Hong, S. Review on methodology for determining forward osmosis (FO) membrane characteristics: Water permeability (A), solute permeability (B), and structural parameter (S). Desalination 2017, 422, 5–16. [Google Scholar] [CrossRef]
- Aziz, S.N.S.A.; Rahman, A.F.H.A.; Seman, M.N.A.; Hilal, N. Comparison of the intrinsic parameters (A, B and S) of a forward osmosis membrane using pressurized and non-pressurized methods. Desalination Water Treat. 2018, 129, 14–23. [Google Scholar] [CrossRef]
- Kim, W.-J.; Heldman, D.R. A mathematical estimation of the structural parameter for prediction of Forward Osmosis (FO) performance. J. Water Process Eng. 2021, 39, 101719. [Google Scholar] [CrossRef]
- Aziz, S.N.S.A.; Seman, M.N.A.; Saufi, S.M.; Mohammad, A.W.; Khayet, M. Effect of Methacrylic Acid Monomer on UV-Grafted Polyethersulfone Forward Osmosis Membrane. Membranes 2023, 13, 232. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Straub, A.P.; Castrillon, S.R.-V.; Elimelech, M. A method for the simultaneous determination of transport and structural parameters of forward osmosis membranes. J. Membr. Sci. 2013, 444, 523–538. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Y.N.; Wang, R. Synthesis and characterization of novel high-performance thin film nanocomposite (TFN) FO membranes with nanofibrous substrate reinforced by functionalized carbon nanotubes. Desalination 2015, 370, 79–86. [Google Scholar] [CrossRef]
- Edokali, M.; Mehrabi, M.; Cespedes, O.; Sun, C.; Collins, S.M.; Harbottle, D.; Menzel, R.; Hassanpour, A. Antifouling and stability enhancement of electrochemically modified reduced graphene oxide membranes for water desalination by forward osmosis. J. Water Process Eng. 2024, 59, 104809. [Google Scholar] [CrossRef]
- Rastgar, M.; Shakeri, A.; Bozorg, A.; Salehi, H.; Saadattalab, V. Highly-efficient forward osmosis membrane tailored by magnetically responsive graphene oxide/Fe3O4 nanohybrid. Appl. Surf. Sci. 2018, 441, 923–935. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Bayrami, A.; Shekari, Z.; Amini, M. High-performance thin-film nanocomposite (TFN) forward osmosis (FO) membranes incorporated with porous hydrophobic-core/hydrophilic-shell nanoparticles. Desalination 2021, 515, 115181. [Google Scholar] [CrossRef]
- Konni, M.; Dadhich, A.S.; Mukkamala, S.B. Evaluation of surface changes at the interface between TiO2 nanoparticles and COOH-MWCNTs on hydrogen adsorption capability. Nano-Struct. Nano-Objects 2019, 18, 100304. [Google Scholar] [CrossRef]
- Su, X.; Jiang, L.; Xu, Z.; Liu, Y.; Yu, Z.; Zhang, L.; Chen, X.; Yu, S.; Xian, M.; Xu, C. Synthesis of carboxyl-modified multi-walled carbon nanotubes for efficient adsorption of furfurylamine. J. Taiwan Inst. Chem. Eng. 2023, 153, 105212. [Google Scholar] [CrossRef]
- Al-Hobaib, A.S.; Al-Sheetan, K.M.; Shaik, M.R.; Al-Suhybani, M.S. Modification of thin-film polyamide membrane with multi-walled carbon nanotubes by interfacial polymerization. Appl. Water Sci. 2017, 7, 4341–4350. [Google Scholar] [CrossRef]
- Huang, Y.; Lee, C.; Tai, N. Support-free multi-walled carbon nanotubes-based membrane for forward osmosis applications. J. Water Process Eng. 2023, 54, 104022. [Google Scholar] [CrossRef]
- Chao, M.; Li, Y.; Wu, G.; Zhou, Z.; Yan, L. Functionalized Multiwalled Carbon Nanotube-Reinforced Polyimide Composite Films with Enhanced Mechanical and Thermal Properties. Int. J. Polym. Sci. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Liang, S.; Li, G.; Tian, R. Multi-walled carbon nanotubes functionalized with a ultrahigh fraction of carboxyl and hydroxyl groups by ultrasound-assisted oxidation. J. Mater. Sci. 2016, 51, 3513–3524. [Google Scholar] [CrossRef]
- Alimohammady, M.; Jahangiri, M.; Kiani, F.; Tahermansouri, H. Preparation and characterization of functionalized MWCNTs-COOH with 3-amino-5-phenylpyrazole as an adsorbent and optimization study using central composite design. Carbon Lett. 2019, 29, 1–20. [Google Scholar] [CrossRef]
- Bayrami, A.; Bagherzadeh, M.; Navi, H.; Chegeni, M.; Hosseinifard, M.; Amini, M. Thin-film nanocomposite membranes containing aspartic acid-modified MIL-53-NH2 (Al) for boosting desalination and anti-fouling performance. Desalination 2022, 521, 115386. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Shamsabadi, A.A.; Aktij, S.A.; Seyedpour, S.F.; Sharifian Gh., M.; Rahimpour, A.; Esfahani, M.R.; Ulbricht, M.; Soroush, M. Exploiting Synergetic Effects of Graphene Oxide and a Silver-Based Metal–Organic Framework to Enhance Antifouling and Anti-Biofouling Properties of Thin-Film Nanocomposite Membranes. ACS Appl. Mater. Interfaces 2018, 10, 42967–42978. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Ali, Z.; Yaqoob, S.; Yu, J.; D’Amore, A. Critical review on the characterization, preparation, and enhanced mechanical, thermal, and electrical properties of carbon nanotubes and their hybrid filler polymer composites for various applications. Compos. Part C Open Access 2024, 13, 100434. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J.-M. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Dabaghian, Z.; Rahimpour, A.; Jahanshahi, M. Highly porous cellulosic nanocomposite membranes with enhanced performance for forward osmosis desalination. Desalination 2016, 381, 117–125. [Google Scholar] [CrossRef]
- Fadaie, N.; Sheikhi, M.; Mohammadi, T.; Tofighy, M.A.; Rajabzadeh, S.; Sahebi, S. Novel Plasma Functionalized Graphene Nanoplatelets (GNPs) incorporated in forward osmosis substrate with improved performance and tensile strength. J. Environ. Chem. Eng. 2021, 9, 105708. [Google Scholar] [CrossRef]
- Butt, A.S.; Qaiser, A.A.; Abid, N.; Mahmood, U. Novel polyaniline–polyethersulfone nanofiltration membranes: Effect of in situ polymerization time on structure and desalination performance. RSC Adv. 2022, 12, 33889–33898. [Google Scholar] [CrossRef]
- Farnam, M.; Mukhtar, H.; Shariff, A. Analysis of the Influence of CMS Variable Percentages on Pure PES Membrane Gas Separation Performance. Procedia Eng. 2016, 148, 1206–1212. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Z.; Wu, J.; Zhao, S.; Wang, J.; Wang, S. Enhancing the flux of brackish water TFC RO membrane by improving support surface porosity via a secondary pore-forming method. J. Membr. Sci. 2016, 498, 227–241. [Google Scholar] [CrossRef]
- Celik, E.; Park, H.; Choi, H.; Choi, H. Carbon nanotube blended polyethersulfone membranes for fouling control in water treatment. Water Res. 2011, 45, 274–282. [Google Scholar] [CrossRef]
- Son, M.; Park, H.; Liu, L.; Choi, H.; Kim, J.H.; Choi, H. Thin-film nanocomposite membrane with CNT positioning in support layer for energy harvesting from saline water. Chem. Eng. J. 2016, 284, 68–77. [Google Scholar] [CrossRef]
- Samieirad, S.; Mousavi, S.M.; Saljoughi, E. Alignment of functionalized multiwalled carbon nanotubes in forward osmosis membrane support layer induced by electric and magnetic fields. Powder Technol. 2020, 364, 538–552. [Google Scholar] [CrossRef]
- Seyedpour, S.F.; Rahimpour, A.; Shamsabadi, A.A.; Soroush, M. Improved performance and antifouling properties of thin-film composite polyamide membranes modified with nano-sized bactericidal graphene quantum dots for forward osmosis. Chem. Eng. Res. Des. 2018, 139, 321–334. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Nikkhoo, M.; Ahadian, M.M.; Amini, M. Novel Thin-Film Nanocomposite Forward Osmosis Membranes Modified with WS2/CuAl LDH Nanocomposite to Enhance Desalination and Anti-fouling Performance. J. Inorg. Organomet. Polym. Mater. 2023, 33, 956–968. [Google Scholar] [CrossRef]
- Lee, W.J.; Goh, P.S.; Lau, W.J.; Wong, K.C.; Suzaimi, N.D.; Ismail, A.F. Tailoring the permeability and flux stability of forward osmosis membrane with tert-butylamine functionalized carbon nanotubes for paracetamol removal. J. Environ. Chem. Eng. 2022, 10, 107977. [Google Scholar] [CrossRef]
- He, M.; Wang, L.; Lv, Y.; Wang, X.; Zhu, J.; Zhang, Y.; Liu, T. Novel polydopamine/metal organic framework thin film nanocomposite forward osmosis membrane for salt rejection and heavy metal removal. Chem. Eng. J. 2020, 389, 124452. [Google Scholar] [CrossRef]
- Sun, P.-F.; Yang, Z.; Song, X.; Lee, J.H.; Tang, C.Y.; Park, H.-D. Interlayered Forward Osmosis Membranes with Ti3C2Tx MXene and Carbon Nanotubes for Enhanced Municipal Wastewater Concentration. Environ. Sci. Technol. 2021, 55, 13219–13230. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhou, S.; Zhou, X. A low-cost and high-performance thin-film composite forward osmosis membrane based on an SPSU/PVC substrate. Sci. Rep. 2018, 8, 10022. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-F.; Huang, X.; Zhang, X.; Li, K.; Ji, Y.-L.; Yu, C.-Y.; Gao, C.-J. Modification of polyamide TFC nanofiltration membrane for improving separation and antifouling properties. RSC Adv. 2018, 8, 15102–15110. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Salehi, H.; Ghorbani, F.; Amini, M.; Naslhajian, H. Polyoxometalate based thin film nanocomposite forward osmosis membrane: Superhydrophilic, anti-fouling, and high water permeable. J. Colloid. Interface Sci. 2019, 536, 328–338. [Google Scholar] [CrossRef]
- Lu, P.; Liang, S.; Zhou, T.; Mei, X.; Zhang, Y.; Zhang, C.; Umar, A.; Wang, Q. Layered double hydroxide/graphene oxide hybrid incorporated polysulfone substrate for thin-film nanocomposite forward osmosis membranes. RSC Adv. 2016, 6, 56599–56609. [Google Scholar] [CrossRef]
- Azelee, I.W.; Goh, P.; Lau, W.; Ismail, A.; Rezaei-DashtArzhandi, M.; Wong, K.; Subramaniam, M. Enhanced desalination of polyamide thin film nanocomposite incorporated with acid treated multiwalled carbon nanotube-titania nanotube hybrid. Desalination 2017, 409, 163–170. [Google Scholar] [CrossRef]
- Khorshidi, B.; Thundat, T.; Pernitsky, D.; Sadrzadeh, M. A parametric study on the synergistic impacts of chemical additives on permeation properties of thin film composite polyamide membrane. J. Membr. Sci. 2017, 535, 248–257. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, S.; Liu, C.-X.; Shen, L.; Ding, C.; Guan, C.-Y.; Wang, Y. Confining migration of amine monomer during interfacial polymerization for constructing thin-film composite forward osmosis membrane with low fouling propensity. Chem. Eng. Sci. 2019, 207, 54–68. [Google Scholar] [CrossRef]
- Bendoy, A.P.; Zeweldi, H.G.; Park, M.J.; Shon, H.K.; Kim, H.; Chung, W.-J.; Nisola, G.M. Silicene nanosheets as support fillers for thin film composite forward osmosis membranes. Desalination 2022, 536, 115817. [Google Scholar] [CrossRef]
- Rastgar, M.; Bozorg, A.; Shakeri, A. Novel Dimensionally Controlled Nanopore Forming Template in Forward Osmosis Membranes. Environ. Sci. Technol. 2018, 52, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Q.; Li, X.; Liu, Y.; Xie, J. Template-Assisted Fabrication of Thin-Film Composite Forward-Osmosis Membrane with Controllable Internal Concentration Polarization. Ind. Eng. Chem. Res. 2016, 55, 5327–5334. [Google Scholar] [CrossRef]
- Lu, P.; Li, W.; Yang, S.; Wei, Y.; Zhang, Z.; Li, Y. Layered double hydroxides (LDHs) as novel macropore-templates: The importance of porous structures for forward osmosis desalination. J. Membr. Sci. 2019, 585, 175–183. [Google Scholar] [CrossRef]
- Fang, L.-F.; Cheng, L.; Jeon, S.; Wang, S.-Y.; Takahashi, T.; Matsuyama, H. Effect of the supporting layer structures on antifouling properties of forward osmosis membranes in AL-DS mode. J. Membr. Sci. 2018, 552, 265–273. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Yu, M.; Wang, Y.; Gao, T.; Yang, F. Conductive thin film nanocomposite forward osmosis membrane (TFN-FO) blended with carbon nanoparticles for membrane fouling control. Sci. Total Environ. 2019, 697, 134050. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, E.; Park, H.; Choi, H. Anti-biofouling effect of a thin film nanocomposite membrane with a functionalized-carbon-nanotube-blended polymeric support for the pressure-retarded osmosis process. RSC Adv. 2020, 10, 5697–5703. [Google Scholar] [CrossRef] [PubMed]
- Bui, N.N.; Lind, M.L.; Hoek, E.M.V.; McCutcheon, J.R. Electrospun nanofiber supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2011, 385–386, 10–19. [Google Scholar] [CrossRef]
- Arena, J.T.; McCloskey, B.; Freeman, B.D.; McCutcheon, J.R. Surface modification of thin film composite membrane support layers with polydopamine: Enabling use of reverse osmosis membranes in pressure retarded osmosis. J. Membr. Sci. 2011, 375, 55–62. [Google Scholar] [CrossRef]
- Hadadpour, S.; Tavakol, I.; Shabani, Z.; Mohammadi, T.; Tofighy, M.A.; Sahebi, S. Synthesis and characterization of novel thin film composite forward osmosis membrane using charcoal-based carbon nanomaterials for desalination application. J. Environ. Chem. Eng. 2021, 9, 104880. [Google Scholar] [CrossRef]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The Critical Need for Increased Selectivity, Not Increased Water Permeability, for Desalination Membranes. Environ. Sci. Technol. Lett. 2016, 3, 112–120. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Sun, D.D. Nano Gives the Answer: Breaking the Bottleneck of Internal Concentration Polarization with a Nanofiber Composite Forward Osmosis Membrane for a High Water Production Rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. Relating performance of thin-film composite forward osmosis membranes to support layer formation and structure. J. Membr. Sci. 2011, 367, 340–352. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Liu, S.; Hao, X.; Li, L.; Zou, G.; Hou, H.; Ji, X. Channel regulation of TFC membrane with hydrophobic carbon dots in forward osmosis. Chin. Chem. Lett. 2021, 32, 2882–2886. [Google Scholar] [CrossRef]
- Pan, Y.-H.; Zhao, Q.-Y.; Gu, L.; Wu, Q.-Y. Thin film nanocomposite membranes based on imologite nanotubes blended substrates for forward osmosis desalination. Desalination 2017, 421, 160–168. [Google Scholar] [CrossRef]
- Xiong, S.; Zuo, J.; Ma, Y.G.; Liu, L.; Wu, H.; Wang, Y. Novel thin film composite forward osmosis membrane of enhanced water flux and anti-fouling property with N-[3-(trimethoxysilyl) propyl] ethylenediamine incorporated. J. Membr. Sci. 2016, 520, 400–414. [Google Scholar] [CrossRef]
- Rastgar, M.; Bozorg, A.; Shakeri, A.; Sadrzadeh, M. Substantially improved antifouling properties in electro-oxidative graphene laminate forward osmosis membrane. Chem. Eng. Res. Des. 2019, 141, 413–424. [Google Scholar] [CrossRef]
- Motsa, M.M.; Mamba, B.B.; D’Haese, A.; Hoek, E.M.V.; Verliefde, A.R.D. Organic fouling in forward osmosis membranes: The role of feed solution chemistry and membrane structural properties. J. Membr. Sci. 2014, 460, 99–109. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Chemical and physical aspects of organic fouling of forward osmosis membranes. J. Membr. Sci. 2008, 320, 292–302. [Google Scholar] [CrossRef]
- Lee, S.; Boo, C.; Elimelech, M.; Hong, S. Comparison of fouling behavior in forward osmosis (FO) and reverse osmosis (RO). J. Membr. Sci. 2010, 365, 34–39. [Google Scholar] [CrossRef]
- Lay, W.C.L.; Chong, T.H.; Tang, C.Y.; Fane, A.G.; Zhang, J.; Liu, Y. Fouling propensity of forward osmosis: Investigation of the slower flux decline phenomenon. Water Sci. Technol. 2010, 61, 927–936. [Google Scholar] [CrossRef]
- Shen, L.; Tian, L.; Zuo, J.; Zhang, X.; Sun, S.; Wang, Y. Developing high-performance thin-film composite forward osmosis membranes by various tertiary amine catalysts for desalination. Adv. Compos. Hybrid Mater. 2019, 2, 51–69. [Google Scholar] [CrossRef]
- Xie, M.; Gray, S.R. Gypsum scaling in forward osmosis: Role of membrane surface chemistry. J. Membr. Sci. 2016, 513, 250–259. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Gypsum Scaling and Cleaning in Forward Osmosis: Measurements and Mechanisms. Environ. Sci. Technol. 2010, 44, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Wang, X.; Huang, X.; Waite, T.D.; Wen, X. Combined effect of membrane and foulant hydrophobicity and surface charge on adsorptive fouling during microfiltration. J. Membr. Sci. 2011, 373, 140–151. [Google Scholar] [CrossRef]
- Parsamehr, P.S.; Zahed, M.; Tofighy, M.A.; Mohammadi, T.; Rezakazemi, M. Preparation of novel cross-linked graphene oxide membrane for desalination applications using (EDC and NHS)-activated graphene oxide and PEI. Desalination 2019, 468, 114079. [Google Scholar] [CrossRef]
- Adha, R.S.; Nguyen, T.T.; Lee, C.; Jang, J.; Kim, I.S. An improved perm-selectivity prediction of forward osmosis membrane by incorporating the effect of the surface charge on the solute partitioning. J. Membr. Sci. 2021, 629, 119303. [Google Scholar] [CrossRef]
- Samsami, S.; Sarrafzadeh, M.-H.; Ahmadi, A. Surface modification of thin-film nanocomposite forward osmosis membrane with super-hydrophilic MIL-53 (Al) for doxycycline removal as an emerging contaminant and membrane antifouling property enhancement. Chem. Eng. J. 2022, 431, 133469. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yangali-Quintanilla, V.; Valladares-Linares, R.; Li, Q.; Zhan, T.; Amy, G. Flux patterns and membrane fouling propensity during desalination of seawater by forward osmosis. Water Res. 2012, 46, 195–204. [Google Scholar] [CrossRef]
- Ibrar, I.; Naji, O.; Sharif, A.; Malekizadeh, A.; Alhawari, A.; Alanezi, A.A.; Altaee, A. A Review of Fouling Mechanisms, Control Strategies and Real-Time Fouling Monitoring Techniques in Forward Osmosis. Water 2019, 11, 695. [Google Scholar] [CrossRef]
- Yu, J.; Jing, W.; Liu, E.; Du, S.; Cai, H.; Du, H.; Wang, J. Sulfonated graphene oxide modified polysulfone-polyamide forward osmosis membrane and its application in fluorine-containing wastewater treatment. Mater. Chem. Phys. 2024, 313, 128757. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, X.-H.; Tang, C.Y. Recent development of novel membranes for desalination. Desalination 2018, 434, 37–59. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Seyedpour, S.F.; Aktij, S.A.; Giagnorio, M.; Bazrafshan, N.; Mollahosseini, A.; Samadi, F.; Ahmadalipour, S.; Firouzjaei, F.D.; Esfahani, M.R.; et al. Recent advances in functionalized polymer membranes for biofouling control and mitigation in forward osmosis. J. Membr. Sci. 2020, 596, 117604. [Google Scholar] [CrossRef]







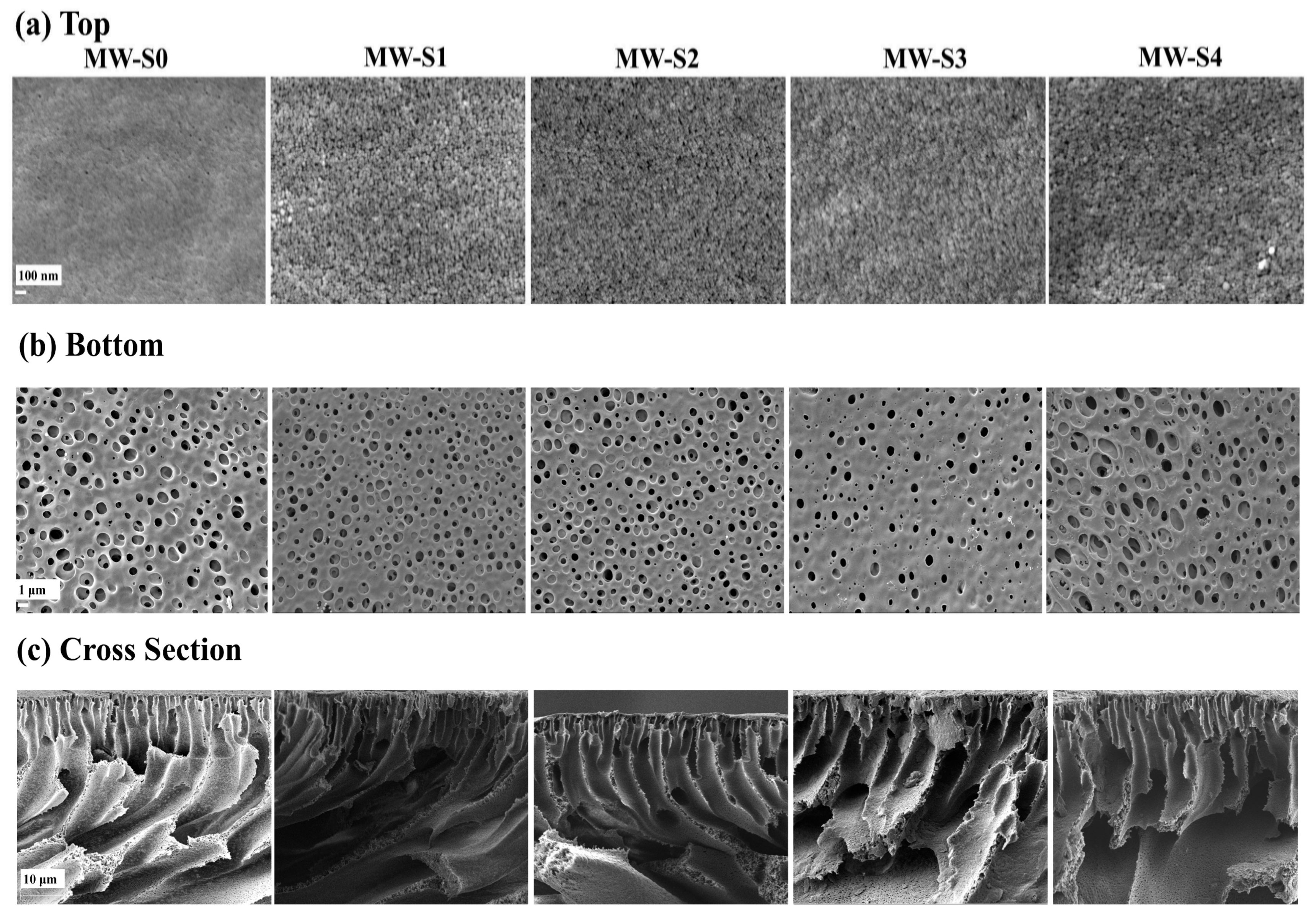




| Substrate | Formulation of Dope Solution (PES/PVP/CNTs, wt.%) |
|---|---|
| MW-S0 | 15/5/0 |
| MW-S1 | 15/5/0.1 |
| MW-S2 | 15/5/0.25 |
| MW-S3 | 15/5/0.5 |
| MW-S4 | 15/5/0.75 |
| Membranes Support | Porosity (By Gravimetric Method) (%) | Pore Size (By Guerout-Elford-Ferry Theory) (nm) | Thickness (By FE-SEM) (μm) |
|---|---|---|---|
| MW-S0 | 66.45 | 111.90 | 214.16 |
| MW-S1 | 69.90 | 74.54 | 131.60 |
| MW-S2 | 70.18 | 59.82 | 142.29 |
| MW-S3 | 73 | 58.03 | 129.61 |
| MW-S4 | 72.43 | 79.77 | 128.68 |
| Membranes | C (%) | N (%) | O (%) | O/N |
|---|---|---|---|---|
| TFC-0 | 75 | 11.3 | 13.5 | 1.194 |
| TFC-1 | 74.1 | 3.3 | 18.1 | 5.48 |
| TFC-2 | 71 | 10.7 | 17.8 | 1.663 |
| TFC-3 | 75.1 | 7.2 | 16.6 | 2.305 |
| TFC-4 | 74.7 | 6.7 | 18.6 | 2.776 |
| Active Layer Support Layer | Optimum Loading | Jw (LMH) | Js/Jw (g/L) | R (%) | S (μm) | FS DS | Anti-Fouling Performance | Ref. |
|---|---|---|---|---|---|---|---|---|
| PA | 2 wt.% COOH-MWCNTs | ~12 | - | ~95% * | 2042 | 0.01 M NaCl | - | [29] |
| PES | 2.0 M glucose | |||||||
| PA | 0.5 wt.% fMWCNTs | 11.98 | 0.6427 | 90.46% | 387 | DI water | High | [48] |
| PES | 0.6 M NaCl | |||||||
| PA | 0.5 wt.% GO | ~11 | ~0.318 | 90.1% | 420 | DI water | - | [24] |
| PSF | 2.0 M NaCl | |||||||
| PA | 4 wt.% CNPs | 14 | 0.357 | - | - | DI water | High | [105] |
| PES | 1.0 M NaCl | |||||||
| PA | 0.15 wt.% GO | 28.5 | 0.421 | 90.5% | - | DI water | - | [31] |
| PSF | 2.0 M NaCl | |||||||
| PA | 0.5 wt.% pCN | 4.24 | 0.01297 | 97.89% | 2960 | DI water | - | [53] |
| PSF | 1.0 M NaCl | |||||||
| PA | 0.3 wt.% f-CNFs | 13.08 | 0.24 | 94.5% | 788.2 | DI water | - | [28] |
| PSF | 1.0 M NaCl | |||||||
| PA | 10 wt.% HCDs | 15.47 | 0.1874 | 94.4% | 188 | DI water | - | [113] |
| PAN | 1.0 M NaCl | |||||||
| PA | 0.5 wt.% GNPs | 19.97 | 0.5172 | 95.50% | 449 | DI water | - | [81] |
| PSF | 1.0 M NaCl | |||||||
| PA | 0.5 wt.% CNM | 12.08 | 0.2458 | 91.3% | 883.4 | DI water | High | [109] |
| PES | 1.0 M NaCl | |||||||
| PA | 3 wt.% SGO | 38.12 | ~0.0041 | - | - | DI water | - | [131] |
| PSF | 2.0 M NaCl | |||||||
| PA | 0.5 wt.% COOH-MWCNTs | 7.48 | 8.80 | 98.94% * | 116.5 | DI water | High | Present work |
| PES | 1.0 M NaCl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almansouri, H.E.; Edokali, M.; Abu Seman, M.N.; Ndia Ntone, E.P.; Che Ku Yahya, C.K.M.F.; Mohammad, A.W. Antifouling and Desalination Enhancement of Forward Osmosis-Based Thin Film Composite Membranes via Functionalized Multiwalled Carbon Nanotubes Mixed Matrix Polyethersulfone Substrate. Membranes 2025, 15, 240. https://doi.org/10.3390/membranes15080240
Almansouri HE, Edokali M, Abu Seman MN, Ndia Ntone EP, Che Ku Yahya CKMF, Mohammad AW. Antifouling and Desalination Enhancement of Forward Osmosis-Based Thin Film Composite Membranes via Functionalized Multiwalled Carbon Nanotubes Mixed Matrix Polyethersulfone Substrate. Membranes. 2025; 15(8):240. https://doi.org/10.3390/membranes15080240
Chicago/Turabian StyleAlmansouri, Hamza E., Mohamed Edokali, Mazrul N. Abu Seman, Ellora Priscille Ndia Ntone, Che Ku Mohammad Faizal Che Ku Yahya, and Abdul Wahab Mohammad. 2025. "Antifouling and Desalination Enhancement of Forward Osmosis-Based Thin Film Composite Membranes via Functionalized Multiwalled Carbon Nanotubes Mixed Matrix Polyethersulfone Substrate" Membranes 15, no. 8: 240. https://doi.org/10.3390/membranes15080240
APA StyleAlmansouri, H. E., Edokali, M., Abu Seman, M. N., Ndia Ntone, E. P., Che Ku Yahya, C. K. M. F., & Mohammad, A. W. (2025). Antifouling and Desalination Enhancement of Forward Osmosis-Based Thin Film Composite Membranes via Functionalized Multiwalled Carbon Nanotubes Mixed Matrix Polyethersulfone Substrate. Membranes, 15(8), 240. https://doi.org/10.3390/membranes15080240






