Improving Balance Between Oxygen Permeability and Stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Through High-Entropy Design
Abstract
1. Introduction
2. Materials and Methods
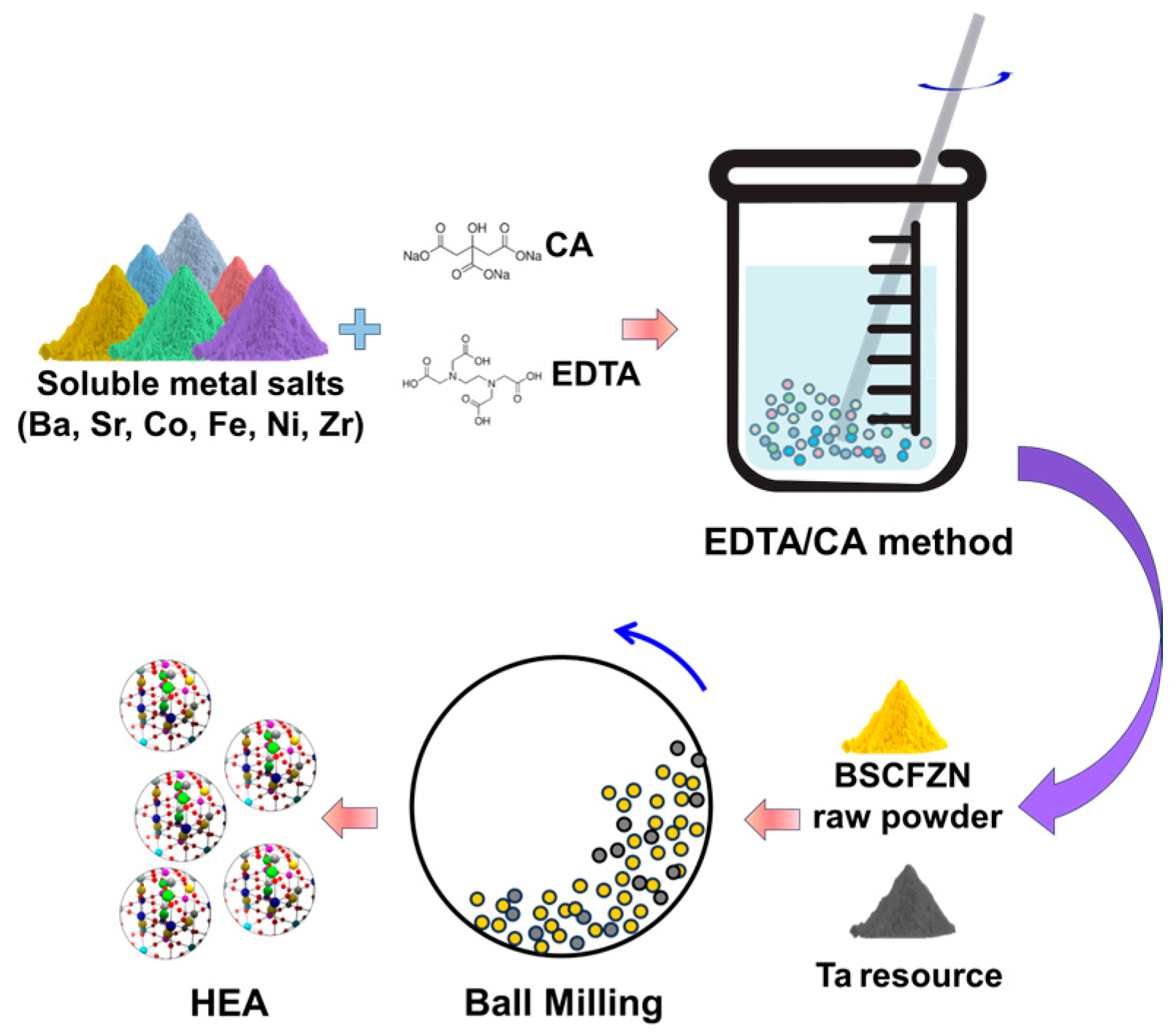
2.1. Synthesis of Materials
2.2. Membrane Testing
2.3. Characterization
2.4. Formatting of Mathematical Components
3. Results and Discussion
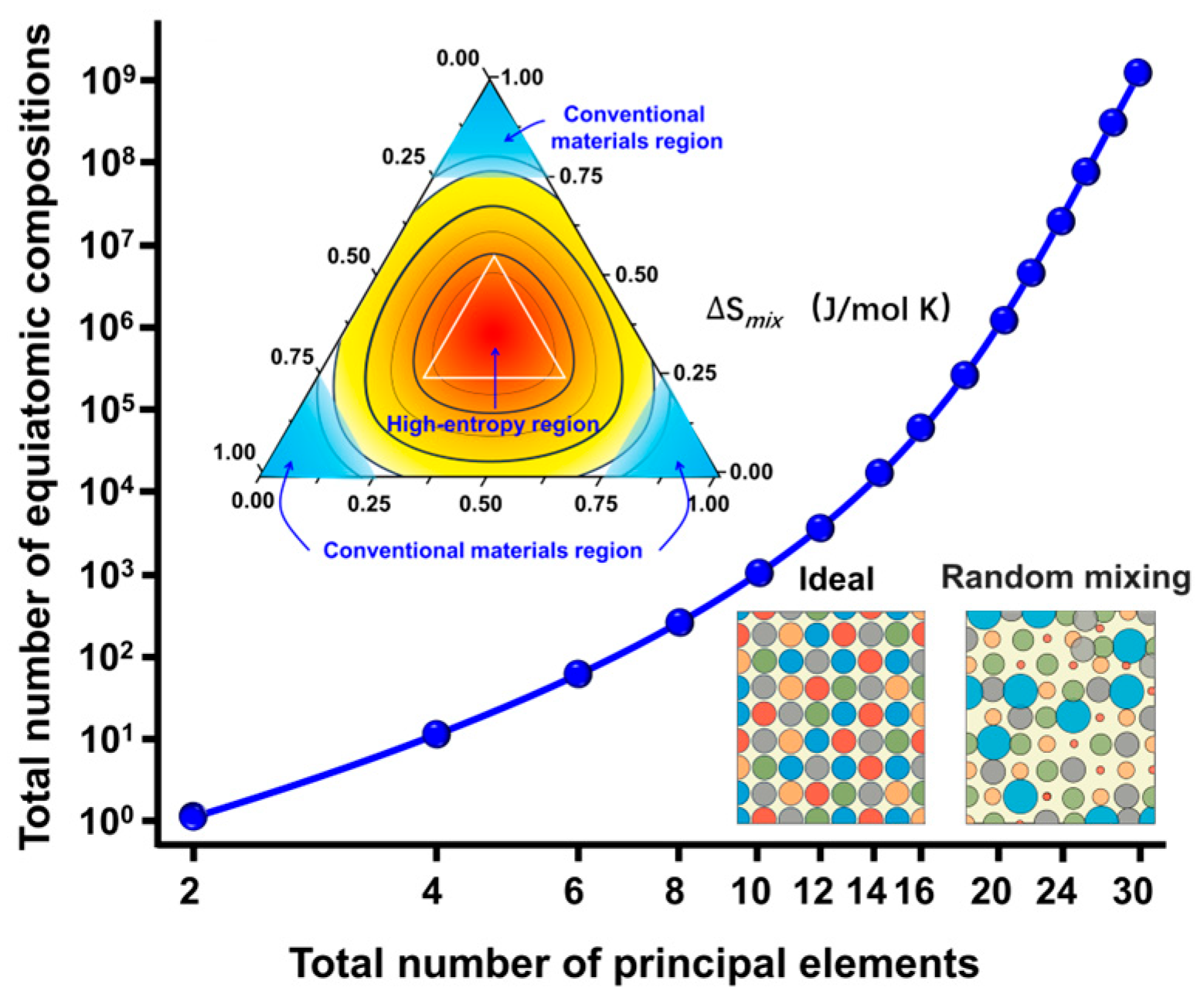
3.1. Material Design
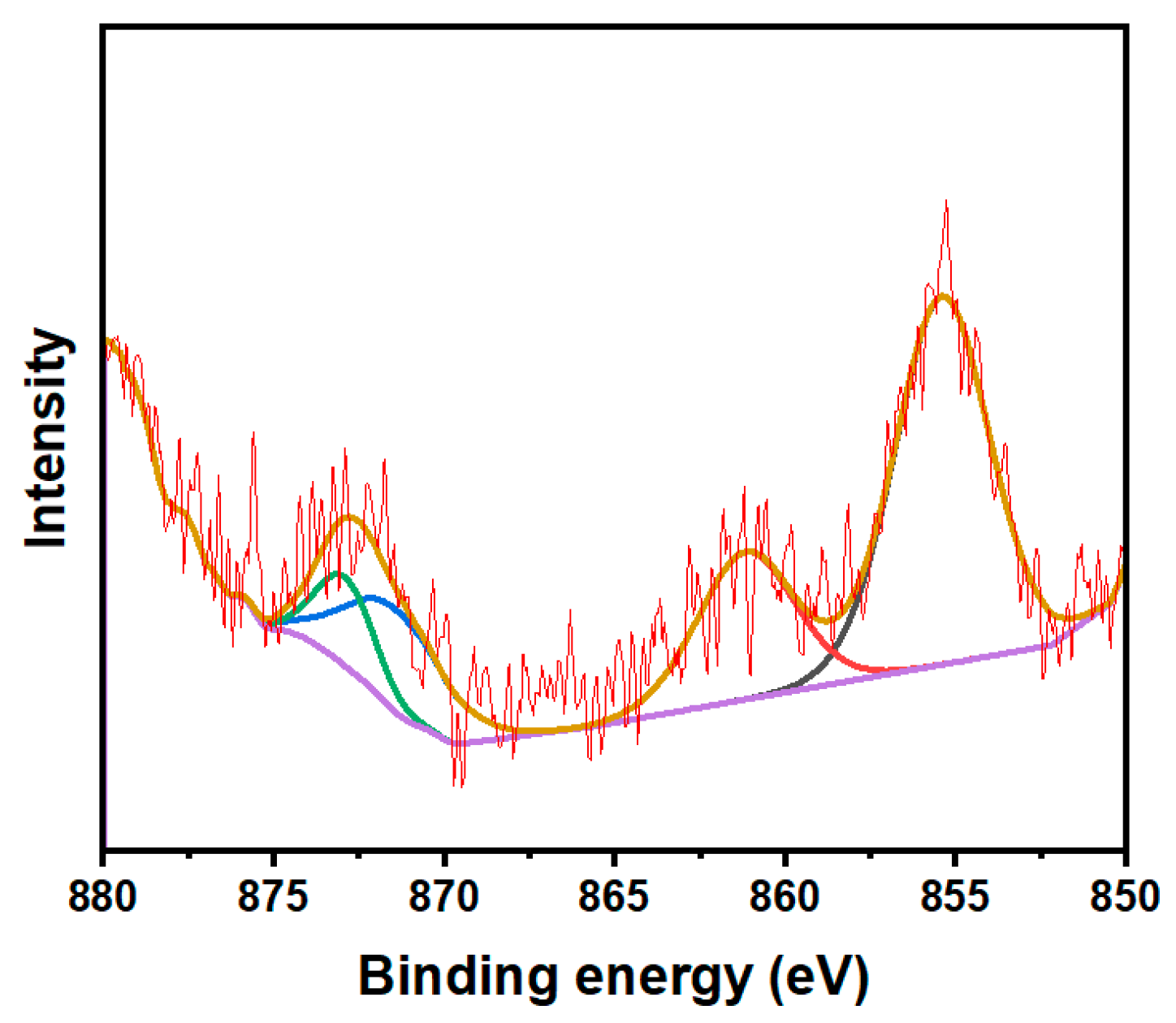
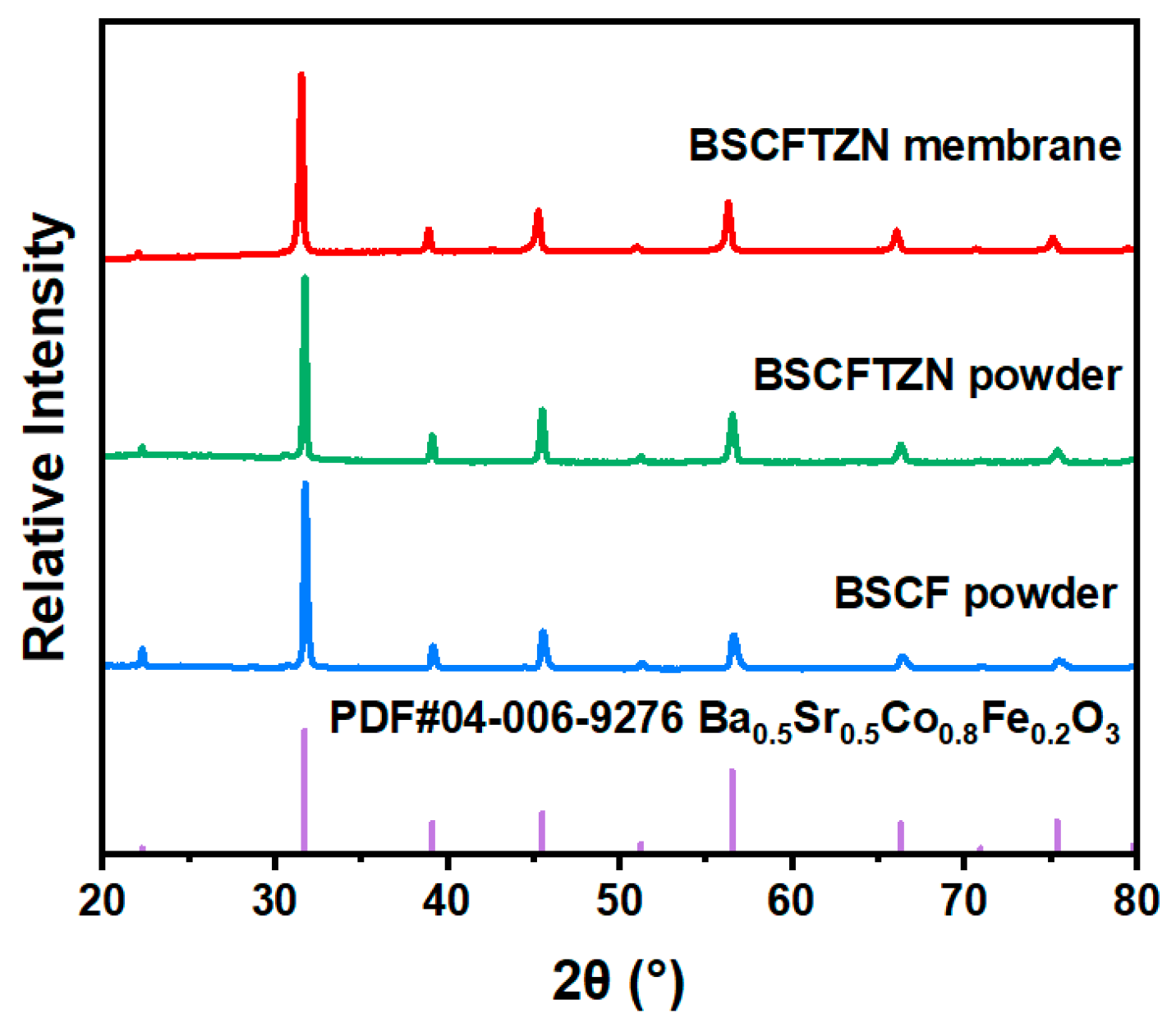
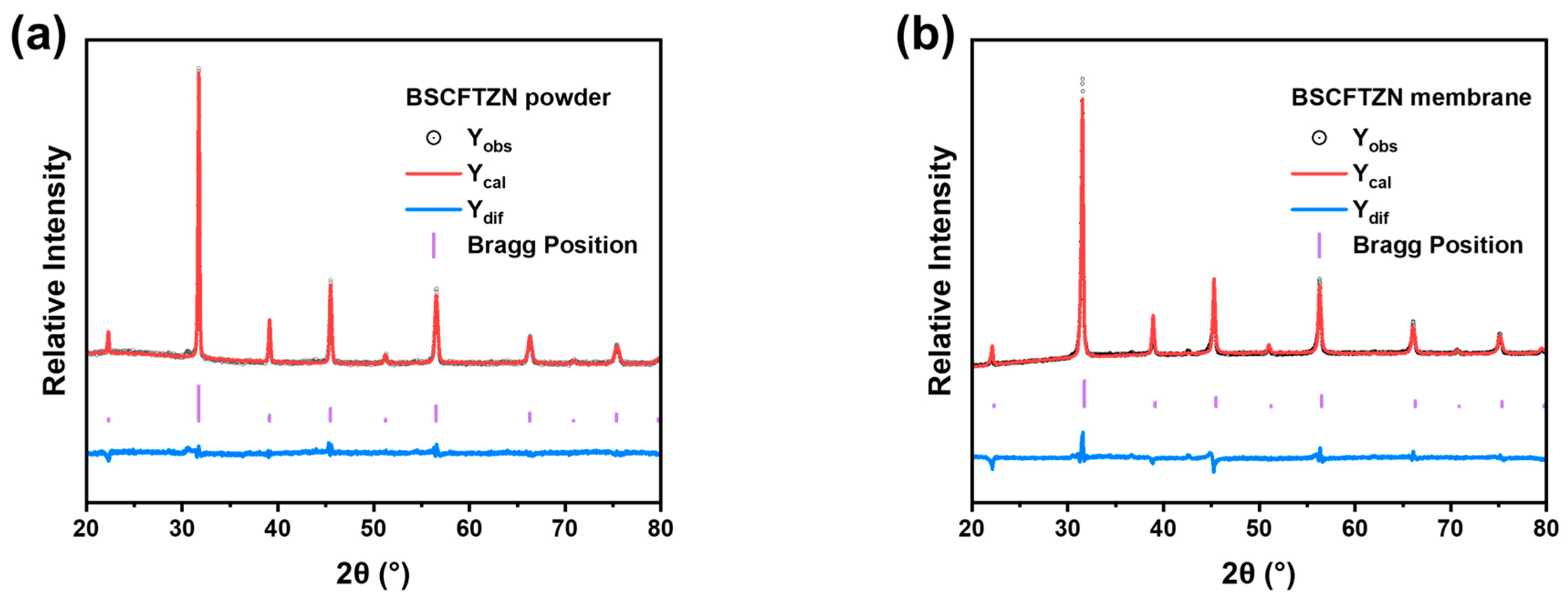
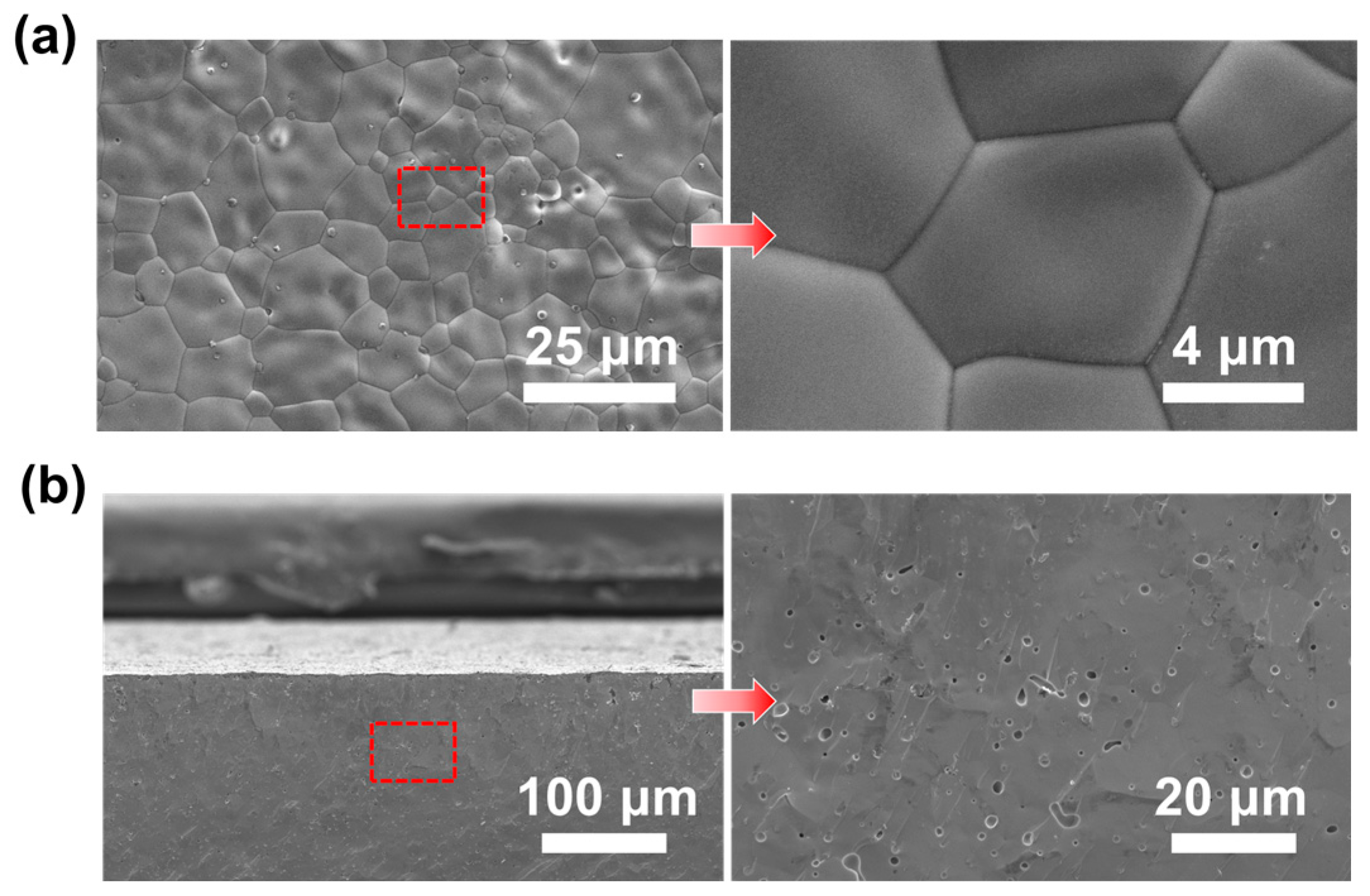
3.2. Crystal Phase and Microstructure
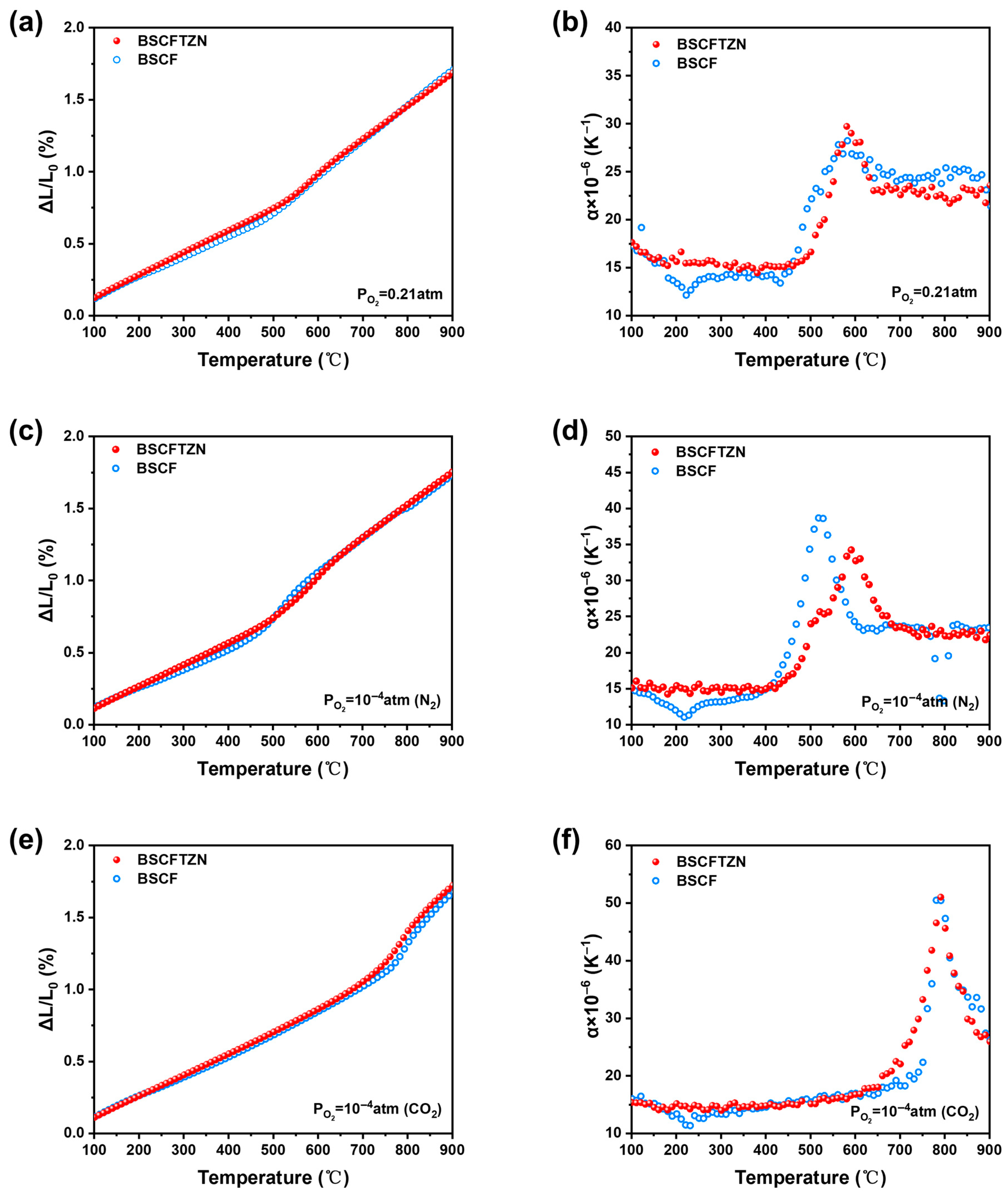
3.3. Thermal Expansion Behavior Analysis
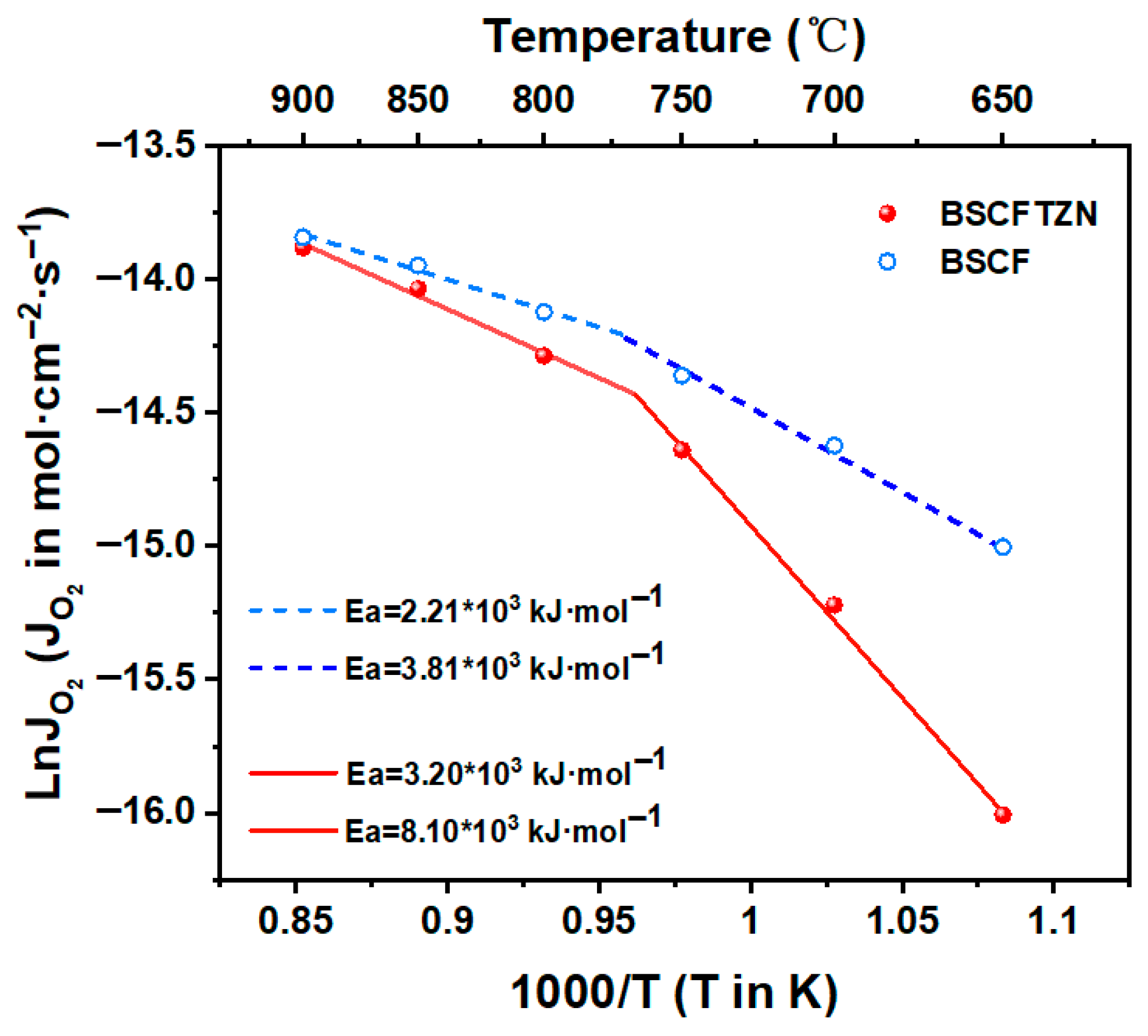
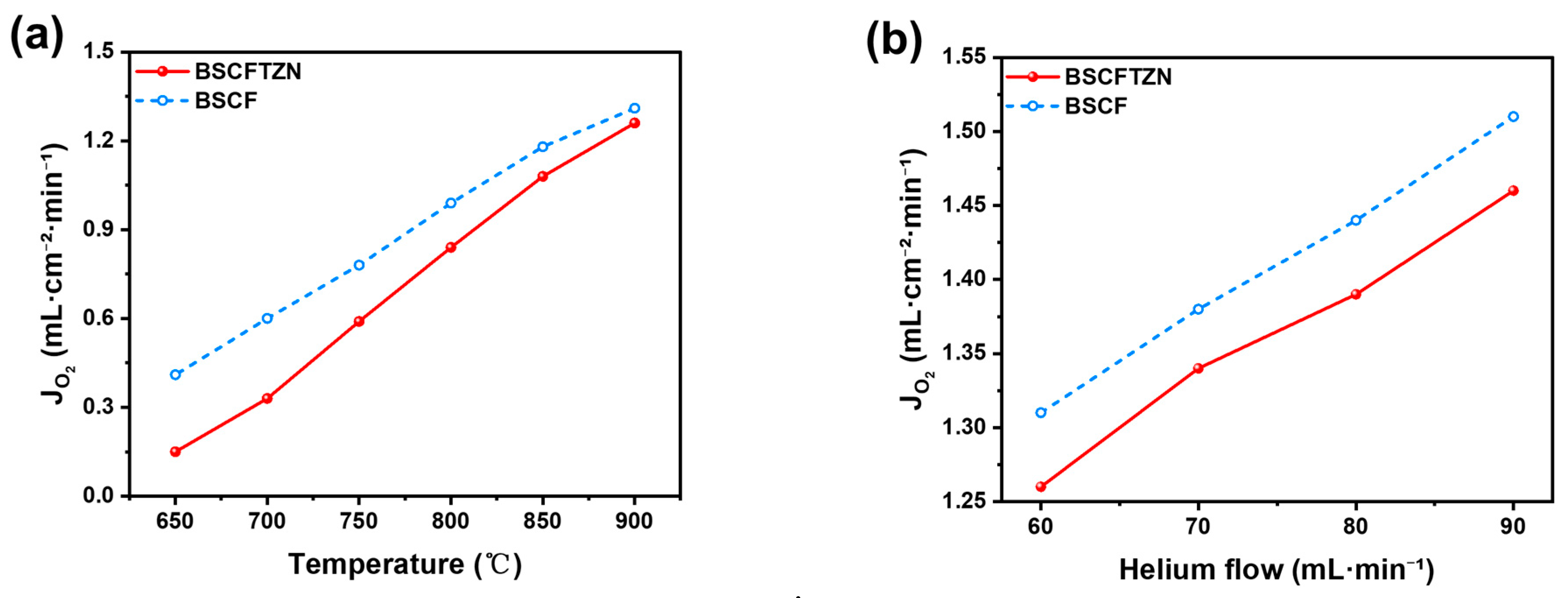
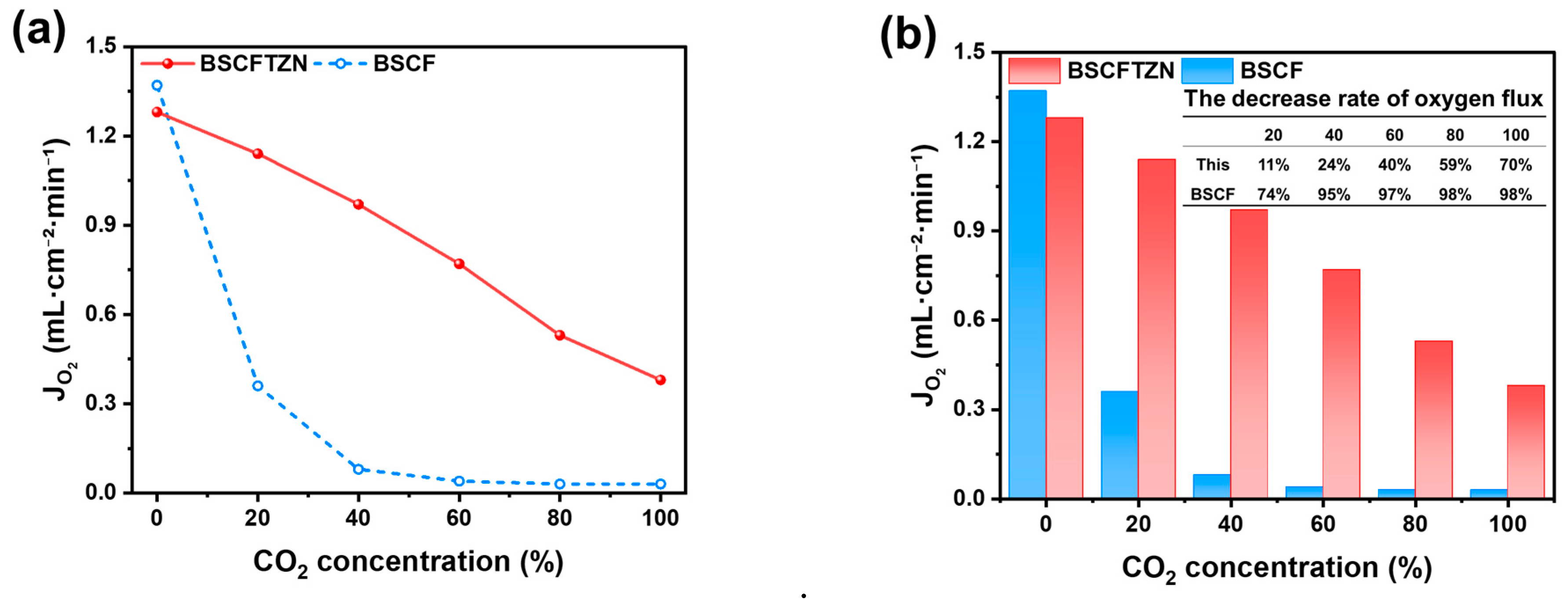
3.4. Oxygen Permeation Test
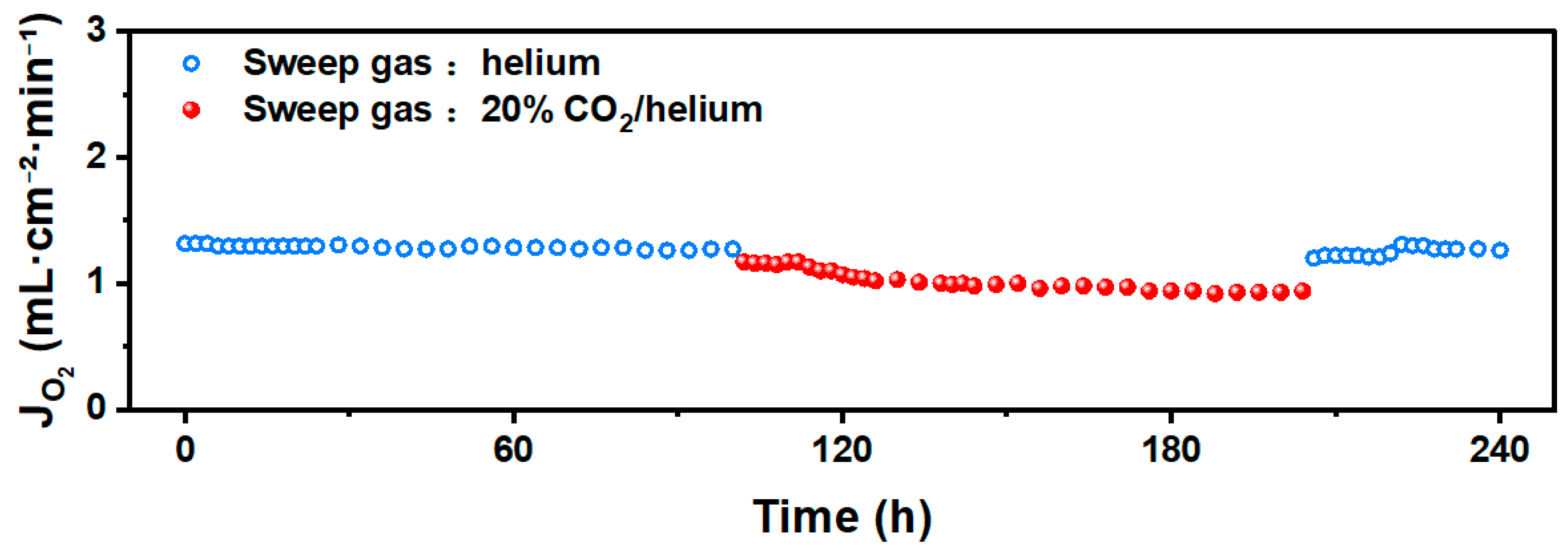
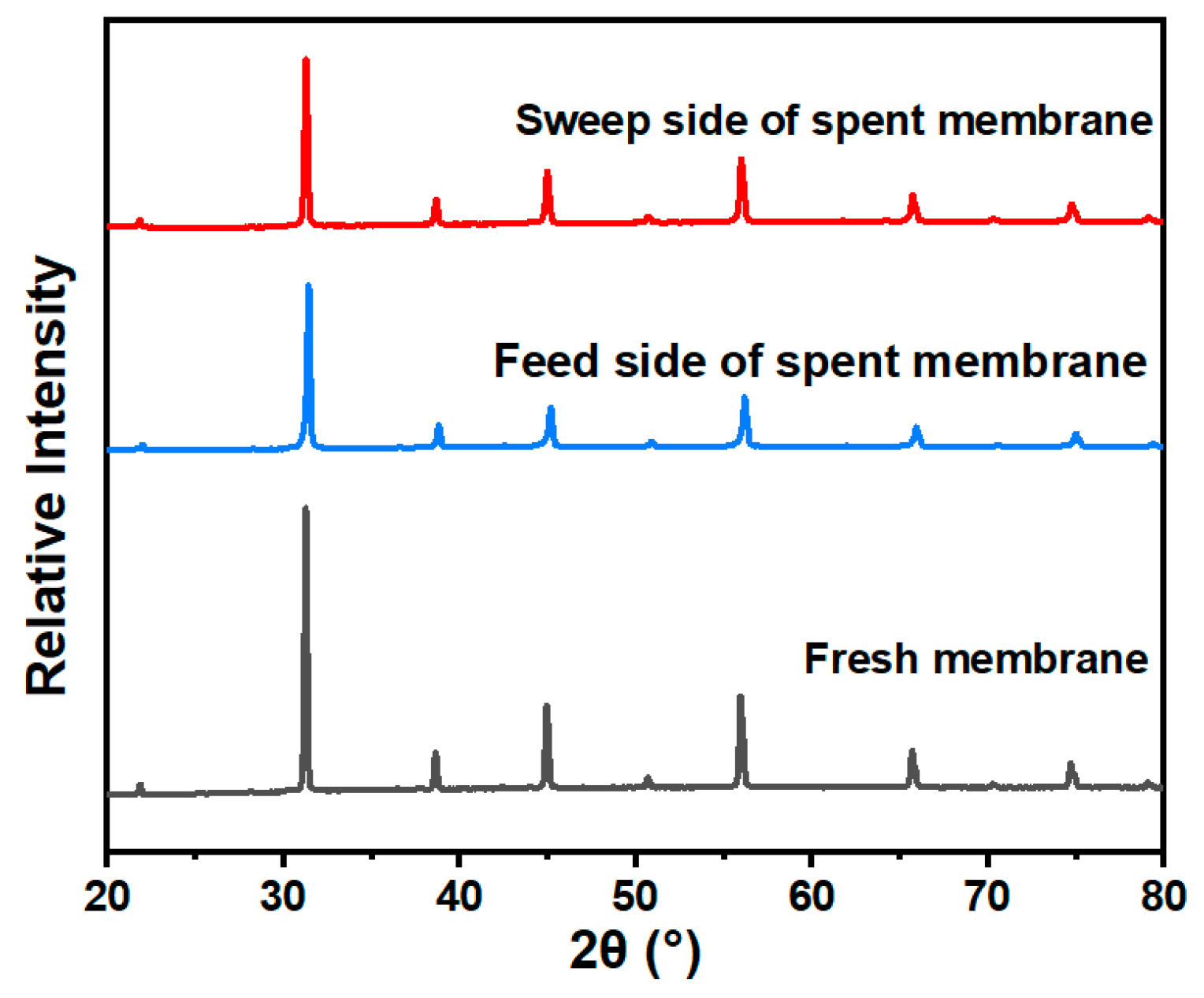
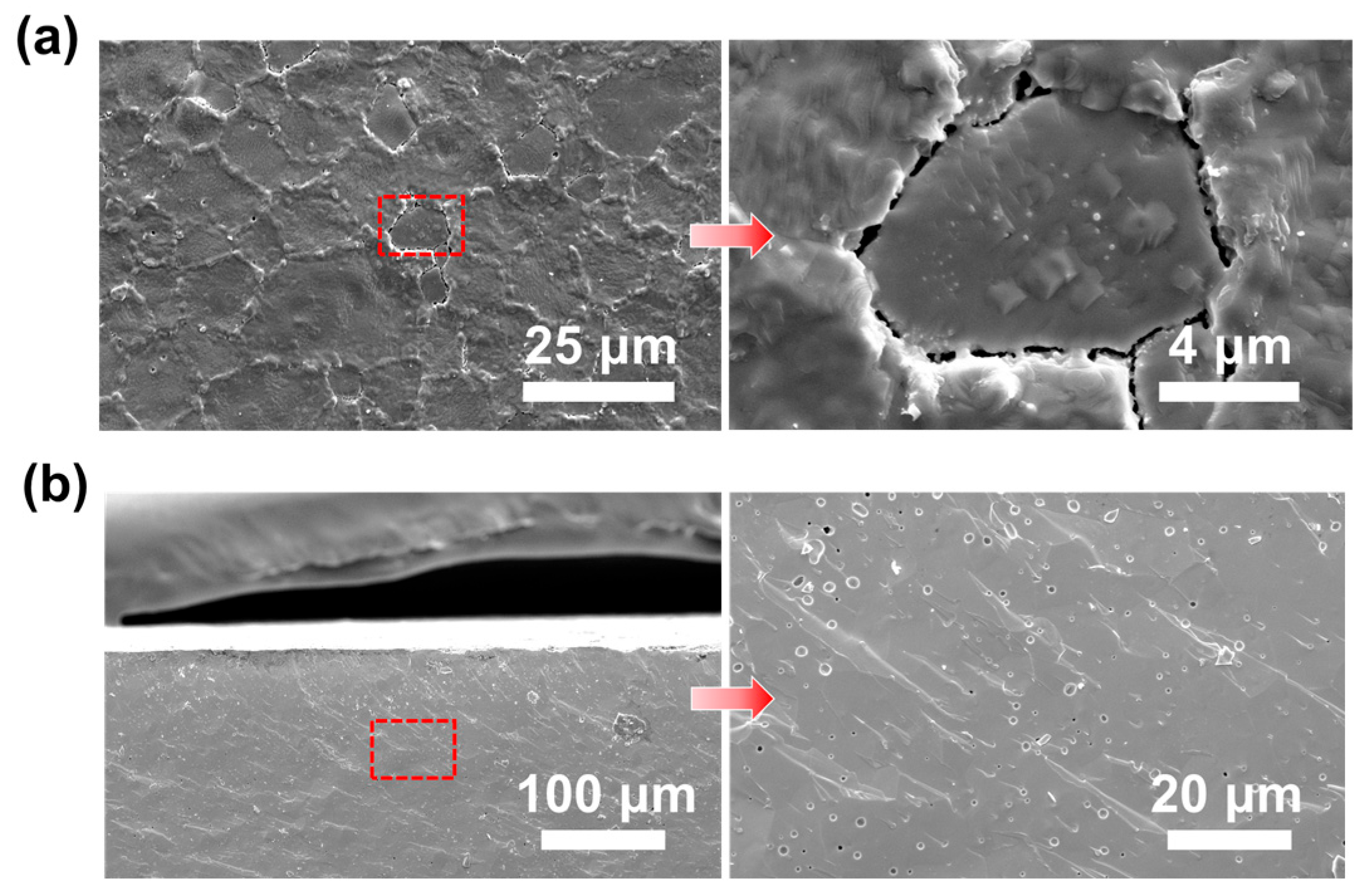
3.5. Long-Term Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| BSCF | Titration times and average values | δ | 3 − δ |
| 1 | 0.5297 | 2.4701 | |
| 2 | 0.5312 | ||
| 3 | 0.5288 | ||
| Average values | 0.5299 | ||
| BSCFTZN | Titration times and average values | δ | 3 − δ |
| 1 | 0.4947 | 2.5030 | |
| 2 | 0.4886 | ||
| 3 | 0.5078 | ||
| Average values | 0.4970 |
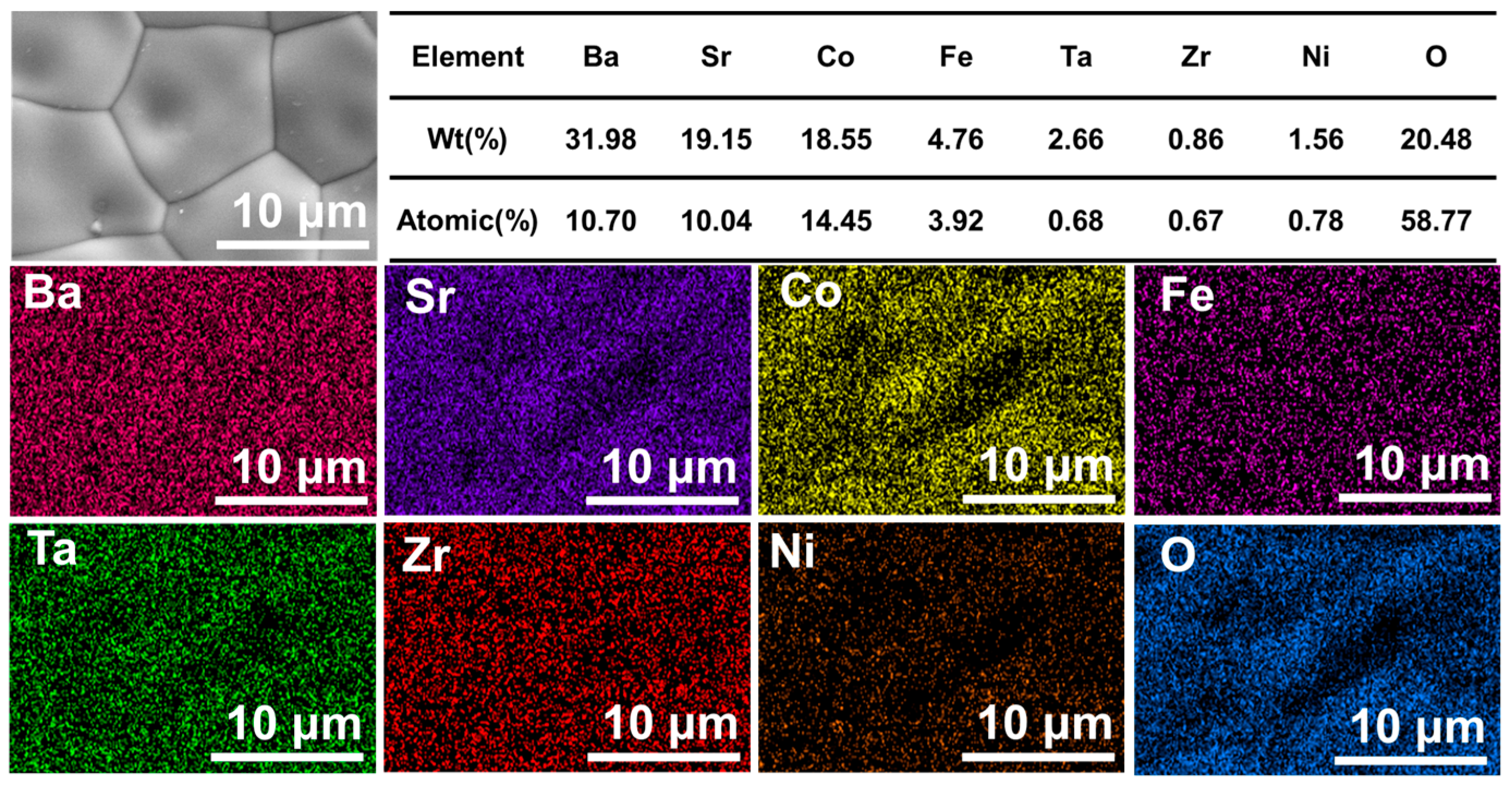
| Elements | Wt cal (%) | At cal (%) | Wt obs (%) | At obs (%) | X Wt | X At |
|---|---|---|---|---|---|---|
| Ba | 30.75 | 10.00 | 31.98 | 10.70 | 4.00 | 7.00 |
| Sr | 19.61 | 10.00 | 19.15 | 10.04 | 2.34 | 4.00 |
| Co | 18.72 | 14.20 | 18.55 | 14.45 | 0.91 | 1.76 |
| Fe | 5.00 | 4.00 | 4.76 | 3.92 | 4.80 | 2.00 |
| Ta | 2.43 | 0.60 | 2.66 | 0.68 | 9.5 | 13.33 |
| Zr | 0.79 | 0.60 | 0.86 | 0.78 | 8.86 | 11.66 |
| Ni | 1.23 | 0.60 | 1.56 | 0.67 | 26.82 | 30.00 |
| O | 21.49 | 60.00 | 20.48 | 58.77 | 4.93 | 2.05 |
References
- Wu, X.-Y.; Ghoniem, A.F. Mixed ionic-electronic conducting (MIEC) membranes for thermochemical reduction of CO2: A review. Prog. Energy Combust. Sci. 2019, 74, 1–30. [Google Scholar] [CrossRef]
- Alami, A.H.; Alashkar, A.; Abdelkareem, M.A.; Rezk, H.; Masdar, M.S.; Olabi, A.G. Perovskite Membranes: Advancements and Challenges in Gas Separation, Production, and Capture. Membranes 2023, 13, 661. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, M.; Liu, J.; Jia, D.; Tan, J.; Zhang, G.; Liu, Z.; Liu, G.; Jin, W. Binary-halogen doped BSCF oxide provides a highly oxygen permeable membrane. J. Membr. Sci. 2025, 717, 123654. [Google Scholar] [CrossRef]
- Markov, A.A.; Merkulov, O.V.; Suntsov, A.Y. Development of Membrane Reactor Coupling Hydrogen and Syngas Production. Membranes 2023, 13, 626. [Google Scholar] [CrossRef]
- Chadwick, A.; Smith, D.; Hodrien, C.; Hovorka, S.; Mackay, E.; Mathias, S.; Lovell, B.; Kalaydjian, F.; Sweeney, G.; Benson, S.; et al. The realities of storing carbon dioxide - A response to CO2 storage capacity issues raised by Ehlig-Economides & Economides. Nature Precedings 2010. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Lei, J.; Liu, J.; Tan, J.K.; Zhang, G.R.; Liu, Z.K.; Jin, W.Q. Fabrication of CO2-tolerant SrFe0.8Nb0.2O3-δ/SrCo0.9Nb0.1O3-δ dual-layer 7-channel hollow fiber membrane by co-spinning and one-step thermal process. J. Membr. Sci. 2023, 670, 121346. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Wang, W. Zirconium and Yttrium Co-Doped BaCo0.8Zr0.1Y0.1O3-δ: A New Mixed-Conducting Perovskite Oxide-Based Membrane for Efficient and Stable Oxygen Permeation. Membranes 2022, 12, 831. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, W.; Xu, N. Design and Fabrication of Ceramic Catalytic Membrane Reactors for Green Chemical Engineering Applications. Engineering 2018, 4, 848–860. [Google Scholar] [CrossRef]
- Hashim, S.S.; Mohamed, A.R.; Bhatia, S. Oxygen separation from air using ceramic-based membrane technology for sustainable fuel production and power generation. Renew. Sustain. Energy Rev. 2011, 15, 1284–1293. [Google Scholar] [CrossRef]
- Shao, Z.; Yang, W.; Cong, Y.; Dong, H.; Tong, J.; Xiong, G. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen membrane. J. Membr. Sci. 2000, 172, 177–188. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.; Dong, F.; Shao, Z. Efficient and CO2-tolerant oxygen transport membranes prepared from high-valence B-site substituted cobalt-free SrFeO3−δ. J. Membr. Sci. 2015, 495, 187–197. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, M.; Zhou, W.; Tan, J.; Zhang, Z.; Zhang, G.; Liu, Z.; Liu, G.; Jin, W. Low chemical-expansion and self-catalytic nickel-substituted strontium cobaltite perovskite four-channel hollow fibre membrane for partial oxidation of methane. J. Membr. Sci. 2025, 715, 123454. [Google Scholar] [CrossRef]
- Chen, Z.; Ran, R.; Zhou, W.; Shao, Z.; Liu, S. Assessment of Ba0.5Sr0.5Co1−yFeyO3−δ (y=0.0–1.0) for prospective application as cathode for IT-SOFCs or oxygen permeating membrane. Electrochim. Acta 2007, 52, 7343–7351. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, J.; Liu, Z.; Liu, G.; Jin, W. Design of CO2-Resistant High-Entropy Perovskites Based on Ba0.5Sr0.5Co0.8Fe0.2O3-δ Materials. Materials 2024, 17, 4672. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Feng, S.; Zuo, Y.; Liu, W.; Chen, C. Oxygen Permeability and Stability of Sr0.95Co0.8Fe0.2O3-δ in a CO2- and H2O-Containing Atmosphere. Chem. Mater. 2005, 17, 5856–5861. [Google Scholar] [CrossRef]
- Ge, L.; Zhou, W.; Ran, R.; Liu, S.; Shao, Z.; Jin, W.; Xu, N. Properties and performance of A-site deficient (Ba0.5Sr0.5)1−xCo0.8Fe0.2O3−δ for oxygen permeating membrane. J. Membr. Sci. 2007, 306, 318–328. [Google Scholar] [CrossRef]
- Falkenstein-Smith, R.; Zeng, P.; Ahn, J. Investigation of oxygen transport membrane reactors for oxy-fuel combustion and carbon capture purposes. Proc. Combust. Inst. 2017, 36, 3969–3976. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Y.; Huang, L.; Wang, H. Chlorine-doped Ba0.5Sr0.5Co0.8Fe0.2O3-δ as an oxygen-permeable membrane at intermediate temperature. Funct. Mater. Lett. 2012, 04, 261–264. [Google Scholar] [CrossRef]
- Sahini, M.G.; Mwankemwa, B.S.; Kanas, N. BaxSr1-xCoyFe1-yO3-delta (BSCF) mixed ionic-electronic conducting (MIEC) materials for oxygen separation membrane and SOFC applications: Insights into processing, stability, and functional properties. Ceram. Int. 2022, 48, 2948–2964. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Yang, W.S.; Caro, J.; Wang, H.H. Dense ceramic oxygen permeable membranes and catalytic membrane reactors. Chem. Eng. J. 2013, 220, 185–203. [Google Scholar] [CrossRef]
- Popov, M.P.; Starkov, I.A.; Bychkov, S.F.; Nemudry, A.P. Improvement of Ba0.5Sr0.5Co0.8Fe0.2O3−δ functional properties by partial substitution of cobalt with tungsten. J. Membr. Sci. 2014, 469, 88–94. [Google Scholar] [CrossRef]
- Shubnikova, E.V.; Bragina, O.A.; Nemudry, A.P. Mixed conducting molybdenum doped BSCF materials. J. Ind. Eng. Chem. 2018, 59, 242–250. [Google Scholar] [CrossRef]
- Xiang, H.; Xing, Y.; Dai, F.-Z.; Wang, H.; Su, L.; Miao, L.; Zhang, G.; Wang, Y.; Qi, X.; Yao, L.; et al. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Biesuz, M.; Fu, S.; Dong, J.; Jiang, A.; Ke, D.; Xu, Q.; Zhu, D.; Bortolotti, M.; Reece, M.J.; Hu, C.; et al. High entropy Sr((Zr0.94Y0.06)0.2Sn0.2Ti0.2Hf0.2Mn0.2)O3−x perovskite synthesis by reactive spark plasma sintering. J. Asian Ceram. Soc. 2019, 7, 127–132. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Cao, Z.; Zhu, X.; Yang, W. Oxygen transport kinetics of BSCF-based high entropy perovskite membranes. Sep. Purif. Technol. 2023, 309, 123093. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Z.; Zhu, X.; Yang, W. Improving intermediate-temperature stability of BSCF by constructing high entropy perovskites. J. Membr. Sci. Lett. 2022, 2, 100026. [Google Scholar] [CrossRef]
- Zhao, Z.; Rehder, L.; Steinbach, F.; Feldhoff, A. High-Entropy Perovskites Pr1−xSrx(Cr, Mn, Fe, Co, Ni)O3−δ (x=0–0.5): Synthesis and Oxygen Permeation Properties. Membranes 2022, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Dean, J.A. Chemical Thermodynamic Properties. In Lange’s Handbook of Chemistry, 2nd ed.; McGRAW-HILL, Inc.: New York, NY, USA, 1999; p. 6. [Google Scholar]
- Zhu, X.; Yang, W. Mixed Conducting Ceramic Membianes; Springer Nature: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Wang, Y.; Liu, J.; Song, Y.; Yu, J.; Tian, Y.; Robson, M.J.; Wang, J.; Zhang, Z.; Lin, X.; Zhou, G.; et al. High-Entropy Perovskites for Energy Conversion and Storage: Design, Synthesis, and Potential Applications. Small Methods 2023, 7, 2201138. [Google Scholar] [CrossRef]
- Zhu, J.W.; Guo, S.B.; Chu, Z.Y.; Jin, W.Q. CO2-tolerant oxygen-permeable perovskite-type membranes with high permeability. J. Mater. Chem. A 2015, 3, 22564–22573. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.; Guo, S.; Jin, W. Influence of permeation modes on oxygen permeability of the multichannel mixed-conducting hollow fibre membrane. Chem. Eng. Sci. 2015, 122, 614–621. [Google Scholar] [CrossRef]
- Fang, S.M.; Chen, C.S.; Winnubst, L. Effect of microstructure and catalyst coating on the oxygen permeability of a novel CO2-resistant composite membrane. Solid State Ion. 2011, 190, 46–52. [Google Scholar] [CrossRef]
- Li, K.; Zhao, H.; Lu, Y.; Ma, Y.; Du, Z.; Zhang, Z. High CO2 tolerance oxygen permeation membranes BaFe0.95Ca0.05TixO3-δ. J. Membr. Sci. 2018, 550, 302–312. [Google Scholar] [CrossRef]
- Hu, R.S.; Bai, Y.Q.; Du, H.Y.; Zhang, H.M.; Du, Y.F.; Zhang, J.G.; Zhou, Q.H. Surface structure and catalytic performance of Sr-doped La2NiAlO6 double perovskite catalysts for methane combustion. J. Rare Earth. 2015, 33, 1284. [Google Scholar] [CrossRef]
- Rosa, P.; Vıctor, M.G.-D.; Juan, P.H.; Caballero, A. Synthesis and characterization of a LaNiO3 perovskite as precursor for methane reforming reactions catalysts. Appl. Catal. B Environ. 2010, 93, 346–353. [Google Scholar] [CrossRef]
| Reagent | Purity | Manufacturer |
|---|---|---|
| Ba(NO3)2 | 99.999% | Macklin, Shanghai, China |
| Sr(NO3)2, | 99.999% | Macklin, Shanghai, China |
| Co(NO3)2·6H2O | 99.999% | Macklin, Shanghai, China |
| Fe(NO3)2·9H2O | 99.999% | Macklin, Shanghai, China |
| ZrO(NO3)2·xH2O | 99.999% | Macklin, Shanghai, China |
| Ni(NO3)2·6H2O | 99.999% | Macklin, Shanghai, China |
| Ta2O5 | 99% | Sinopharm Chemical Reagent Co., Ltd. Sunzhou, China |
| Materials | t | δ(RA) | δ(RB) | ΔSmix |
|---|---|---|---|---|
| BSCFTZN | 1.0 | 5.11% | 2.99% | 1.57R |
| Materials | Thickness (mm) | Temperature (°C) | (mL·cm−2·min−1) | CO2 Concentration | Ref. |
|---|---|---|---|---|---|
| Sm0.2Ce0.8O2−δ−La0.7Ca0.3CrO3−δ | 1 | 900 | 0.17 | 20% CO2 | [35] |
| BaFe0.8Ga0.05Ti0.15O3−δ | 1 | 900 | 0.40 | 30% CO2 | [36] |
| SrFe0.8Zr0.2O3−δ | 1 | 900 | 0.39 | 10% CO2 | [11] |
| La0.1Pr0.2Nd0.2Ba0.2Sr0.2Co0.7Fe0.2Ni0.1O3−δ | 1 | 900 | 0.29 | 20% CO2 | [14] |
| BSCFTZN | 1 | 900 | 0.92 | 20% CO2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wu, M.; Zhang, G.; Liu, Z.; Liu, G. Improving Balance Between Oxygen Permeability and Stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Through High-Entropy Design. Membranes 2025, 15, 232. https://doi.org/10.3390/membranes15080232
Zhu Y, Wu M, Zhang G, Liu Z, Liu G. Improving Balance Between Oxygen Permeability and Stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Through High-Entropy Design. Membranes. 2025; 15(8):232. https://doi.org/10.3390/membranes15080232
Chicago/Turabian StyleZhu, Yongfan, Meng Wu, Guangru Zhang, Zhengkun Liu, and Gongping Liu. 2025. "Improving Balance Between Oxygen Permeability and Stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Through High-Entropy Design" Membranes 15, no. 8: 232. https://doi.org/10.3390/membranes15080232
APA StyleZhu, Y., Wu, M., Zhang, G., Liu, Z., & Liu, G. (2025). Improving Balance Between Oxygen Permeability and Stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Through High-Entropy Design. Membranes, 15(8), 232. https://doi.org/10.3390/membranes15080232








