Visualization of Film Formation Process of Copolyesteramide Containing Phthalazine Moieties During Interfacial Polymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
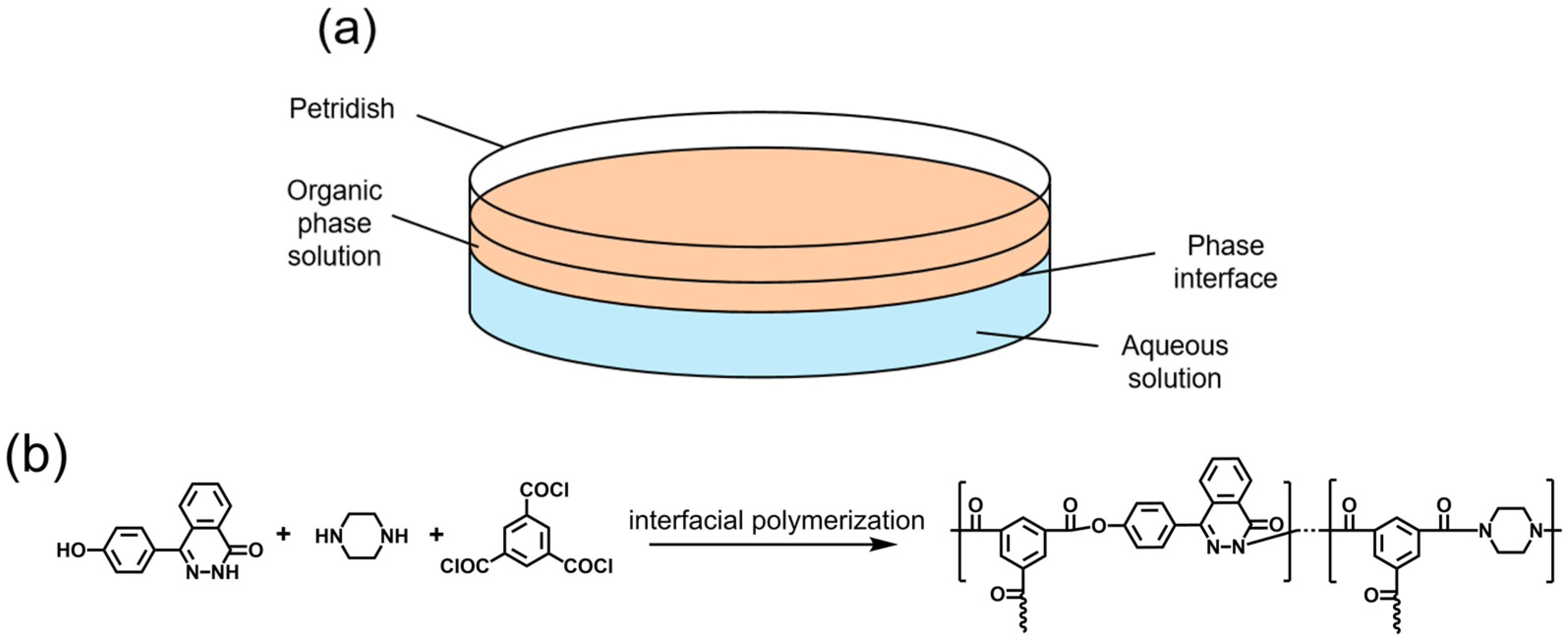
2.2. Formation and Characterization of IP Film
2.3. Observation of IP Film Morphology
3. Results and Discussion
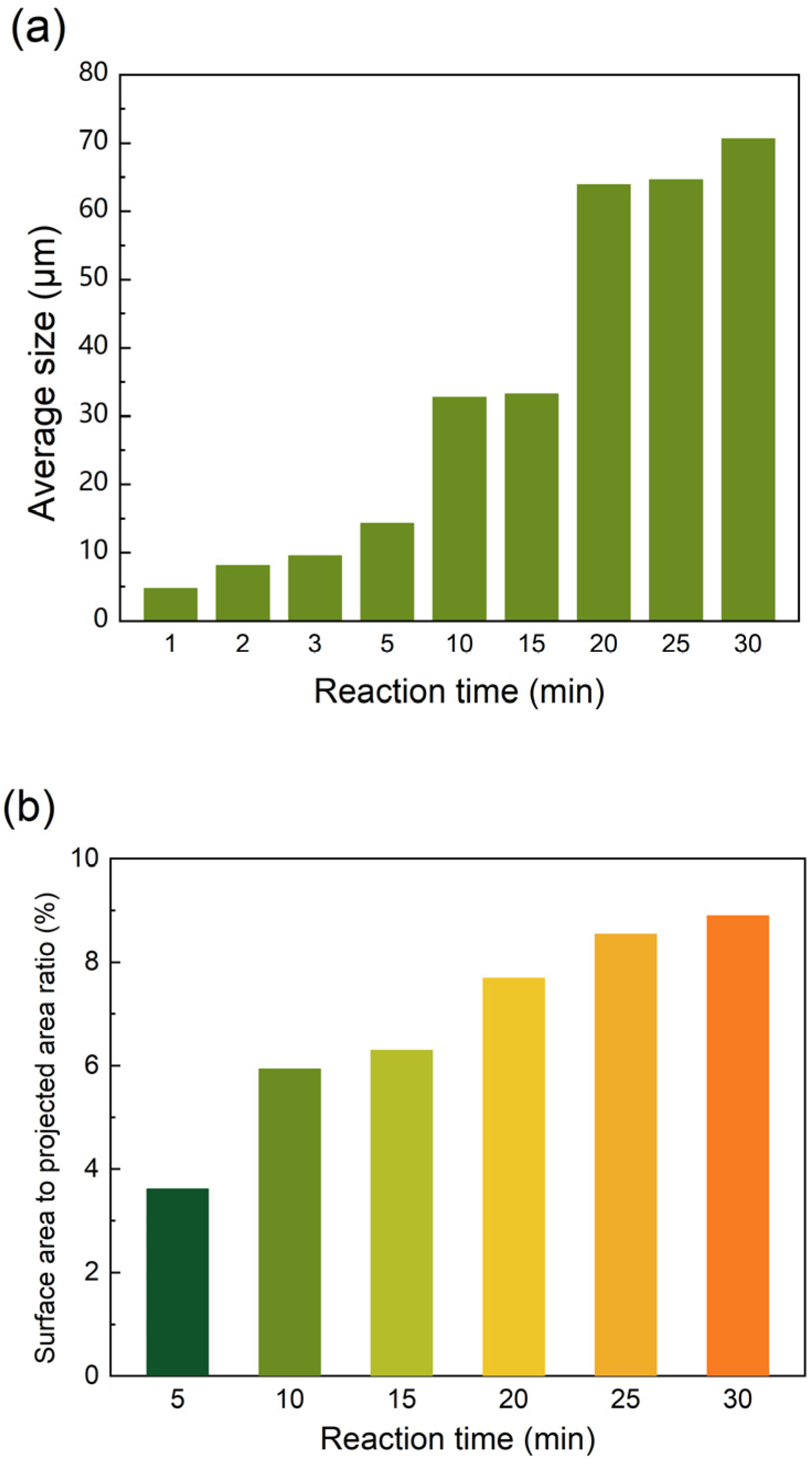
3.1. The Formation Process of Polymer Films
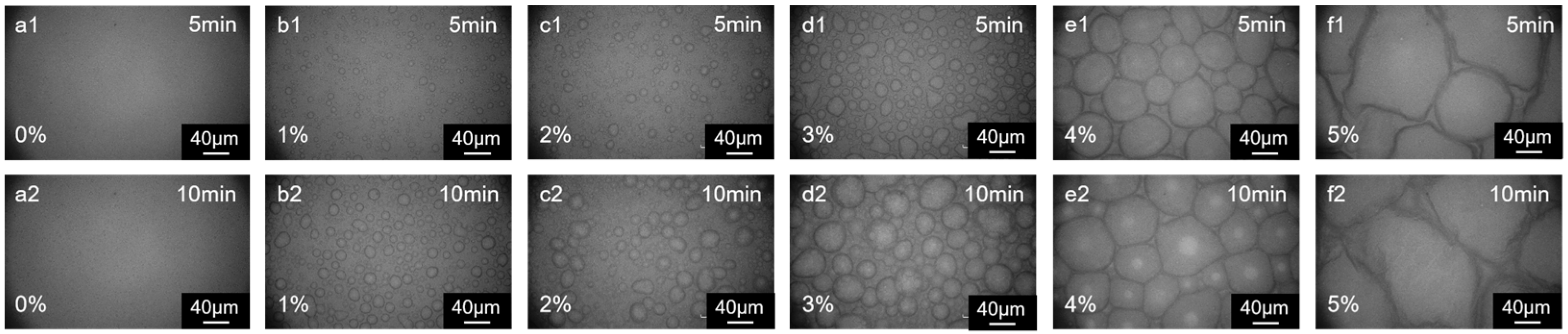
3.2. Aqueous Phase Monomer Ratio
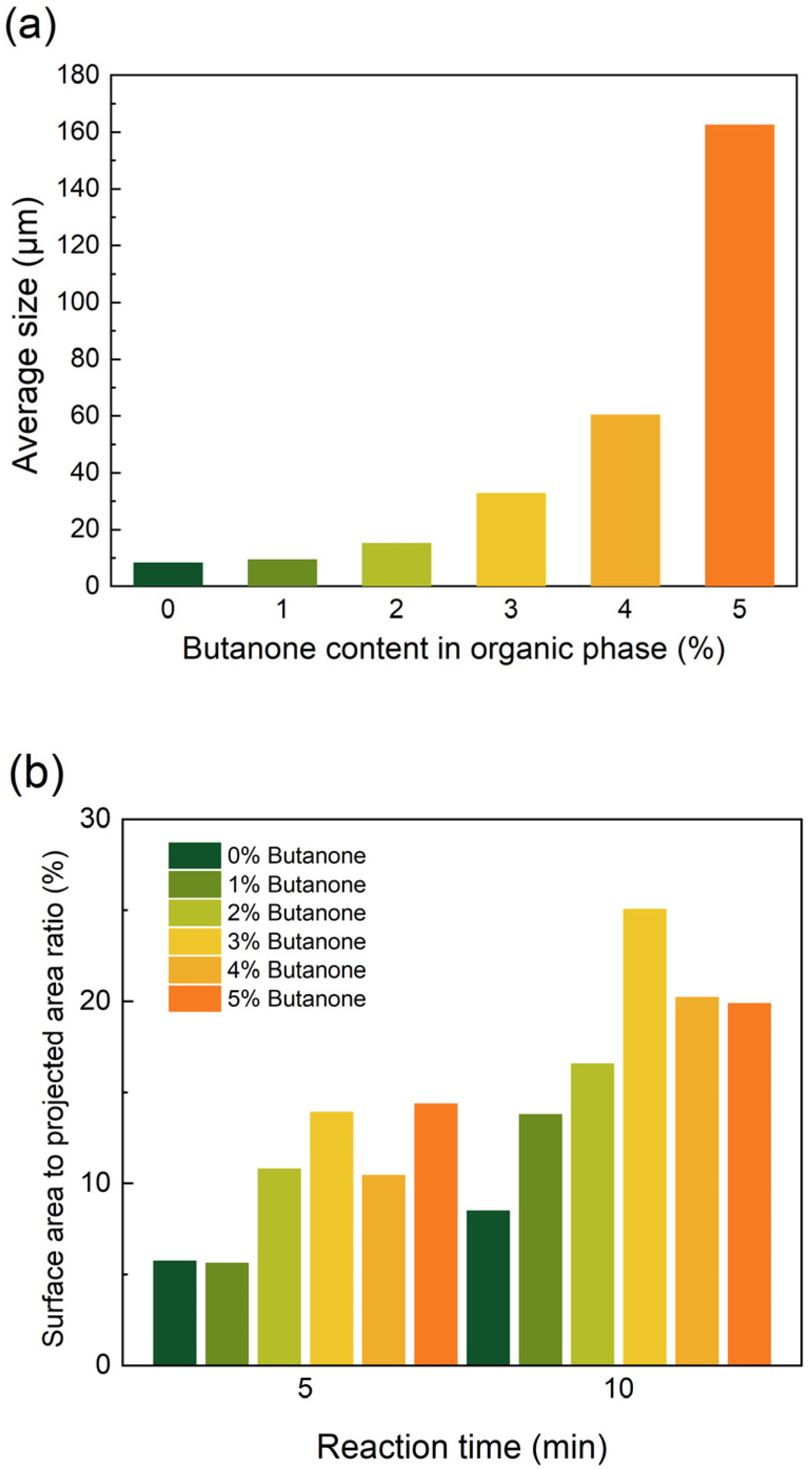
3.3. Co-Solvent
3.4. Phase Transfer Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Fan, K.; Liu, Y.; Xia, S. A review on polyester and polyester-amide thin film composite nanofiltration membranes: Synthesis, characteristics and applications. Sci. Total Environ. 2023, 858, 159922. [Google Scholar] [CrossRef]
- Rabbani, E.M.; Aghapour, A.S.; Zoheir, D.; Mostafa, D.F.; Ahmad, R.; Joyner, E.; Isabel, C.E.; Mojtaba, A.; Lauren, F.G.; Amirsalar, R.E.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic Membranes for Water Purification: Status and Future. Angew. Chem. Int. Ed. 2015, 54, 3368–3386. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Han, X.; Liu, Y.; Wang, C.; Yan, F.; Wang, J. Regulating the interfacial polymerization process toward high-performance polyamide thin-film composite reverse osmosis and nanofiltration membranes: A review. J. Membr. Sci. 2021, 640, 119765. [Google Scholar] [CrossRef]
- Lloyd, D.R. Evolution of Composite Reverse Osmosis Membranes. Mater. Sci. Synth. Membr. 1985, 12, 273–294. [Google Scholar]
- Zhao, S.; Liao, Z.; Fane, A.; Li, J.; Tang, C.; Zheng, C.; Lin, J.; Kong, L. Engineering antifouling reverse osmosis membranes: A review. Desalination 2021, 499, 114857. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, S.; Kang, Y.; Wang, Z.; Xia, Y.; Yang, J.; Wang, H.; Zhang, X. Manipulating interfacial polymerization for polymeric nanofilms of composite separation membranes. Prog. Polym. Sci. 2021, 122, 101450. [Google Scholar] [CrossRef]
- Chai, G.-Y.; Krantz, W.B. Formation and characterization of polyamide membranes via interfacial polymerization. J. Membr. Sci. 1994, 93, 175–192. [Google Scholar] [CrossRef]
- Khare, V.P.; Greenberg, A.R.; Krantz, W.B. Development of pendant drop mechanical analysis as a technique for determining the stress–relaxation and water-permeation properties of interfacially polymerized barrier layers. J. Appl. Polym. Sci. 2003, 90, 2618–2628. [Google Scholar] [CrossRef]
- Khare, V.P.; Greenberg, A.R.; Krantz, W.B. Investigation of the viscoelastic and transport properties of interfacially polymerized barrier layers using pendant drop mechanical analysis. J. Appl. Polym. Sci. 2004, 94, 558–568. [Google Scholar] [CrossRef]
- Roh, I.J.; Kim, J.-J.; Park, S.Y. Mechanical properties and reverse osmosis performance of interfacially polymerized polyamide thin films. J. Membr. Sci. 2002, 197, 199–210. [Google Scholar] [CrossRef]
- Fields, S.D.; Thomas, E.L.; Ottino, J.M. Visualization of interfacial urethane polymerizations by means of a new microstage reactor. Polymer 1986, 27, 1423–1432. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Z.; Yu, X.; Wei, Z.; Li, S.; Wang, J.; Wang, S. Visualization of the Formation of Interfacially Polymerized Film by an Optical Contact Angle Measuring Device. J. Phys. Chem. C 2012, 116, 11496–11506. [Google Scholar] [CrossRef]
- Xu, P.; Liu, Z.; Liu, Q.; Li, H.; Xu, S.; Zhang, Y.; Wang, H.; Zhang, S.; Jian, X. Visualizing the formation process of interfacial polymerized (Co)polyarylate films by an optical three-dimensional microscope. Chem. Eng. Sci. 2023, 278, 118882. [Google Scholar] [CrossRef]
- Kong, C.; Shintani, T.; Kamada, T.; Freger, V.; Tsuru, T. Co-solvent-mediated synthesis of thin polyamide membranes. J. Membr. Sci. 2011, 384, 10–16. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, J.; Lin, Z.; Sun, R.; Chen, L.; Jiang, Z.; Pang, J. Rigid twisted structured PA membranes for organic solvent nanofiltration via co-solvent assisted interfacial polymerization. J. Membr. Sci. 2023, 666, 121179. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, H.; Jiang, S.; Chen, J.; Wang, J.; Liang, H.; Li, G.; Tang, X. An efficient co-solvent tailoring interfacial polymerization for nanofiltration: Enhanced selectivity and mechanism. J. Membr. Sci. 2023, 677, 121615. [Google Scholar] [CrossRef]
- Lee, J.; Wang, R.; Bae, T.-H. A comprehensive understanding of co-solvent effects on interfacial polymerization: Interaction with trimesoyl chloride. J. Membr. Sci. 2019, 583, 70–80. [Google Scholar] [CrossRef]
- Esfandian, F.; Peyravi, M.; Ghoreyshi, A.A.; Jahanshahi, M.; Rad, A.S. Fabrication of TFC nanofiltration membranes via co-solvent assisted interfacial polymerization for lactose recovery. Arab. J. Chem. 2019, 12, 5325–5338. [Google Scholar] [CrossRef]
- Hailemariam, R.H.; Choi, J.-S.; Damtie, M.M.; Rho, H.; Park, K.-D.; Lee, J.; Woo, Y.C. Enhancing performances of polyamide thin film composite membranes via co-solvent assisted interfacial polymerization. Desalination 2022, 524, 115481. [Google Scholar] [CrossRef]
- Kamada, T.; Ohara, T.; Shintani, T.; Tsuru, T. Controlled surface morphology of polyamide membranes via the addition of co-solvent for improved permeate flux. J. Membr. Sci. 2014, 467, 303–312. [Google Scholar] [CrossRef]
- Gozzi, D.; Tomellini, M.; Lazzarini, L.; Latini, A. High-temperature determination of surface free energy of copper nanoparticles. J. Phys. Chem. C 2010, 114, 12117–12124. [Google Scholar] [CrossRef]
- Siegal, M.P.; Kaatz, F.H.; Graham, W.R. Formation of epitaxial yttrium silicide on (111) silicon. J. Appl. Phys. 1989, 66, 2999–3006. [Google Scholar] [CrossRef]
- Shen, J.; Giergiel, J.; Schmid, A.K.; Kirschner, J. Surface alloying and pinhole formation in ultra-thin Fe/Cu(100) films. Surf. Sci. 1995, 328, 32–46. [Google Scholar] [CrossRef]
- Keavney, D.J.; Fullerton, E.E.; Bader, S.D. Perpendicular conductance and magnetic coupling in epitaxial Fe/MgO/Fe(100) trilayers. J. Appl. Phys. 1997, 81, 795–798. [Google Scholar] [CrossRef]
- Fischer, A.E.M.J.; Slijkerman, W.F.J.; Nakagawa, K.; Smith, R.J.; Vanderveen, J.F.; Bullelieuwma, C.W.T. Growth of uniform epitaxial CoSi2 films on Si(111). J. Appl. Phys. 1988, 64, 3005–3013. [Google Scholar] [CrossRef]
- O’Donnell, J.L.; Keefe, M.H.; Hupp, J.T. Interfacial polymerization of molecular squares: Thin microporous membranes featuring size selective transport. Mater. Res. Soc. Symp. Proc. 2002, 734, 3–14. [Google Scholar] [CrossRef]
- Pacheco, F.; Sougrat, R.; Reinhard, M.; Leckie, J.O.; Pinnau, I. 3D visualization of the internal nanostructure of polyamide thin films in RO membranes. J. Membr. Sci. 2016, 501, 33–44. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S. Instability of cast film and its relevance to membrane morphology. Chem. Eng. Sci. 1999, 54, 1243–1251. [Google Scholar] [CrossRef]
- Jiang, C.; Tian, L.; Hou, Y.; Niu, Q.J. Nanofiltration membranes with enhanced microporosity and inner-pore interconnectivity for water treatment: Excellent balance between permeability and selectivity. J. Membr. Sci. 2019, 586, 192–201. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.-L.; Cheng, D.; Li, J.; Wang, M.; Duan, Q.; Gong, G.; Hu, Y. Thin film composite polyesteramide membrane with the incorporation of rigidly-contorted binaphthol-based monomer for enhanced nanofiltration separation performance. Sep. Purif. Technol. 2023, 322, 124376. [Google Scholar] [CrossRef]
- Kamada, T.; Ohara, T.; Shintani, T.; Tsuru, T. Optimizing the preparation of multi-layered polyamide membrane via the addition of a co-solvent. J. Membr. Sci. 2014, 453, 489–497. [Google Scholar] [CrossRef]
- Scriven, L.E.; Sternling, C.V. The Marangoni effect. Nature 1960, 127, 186–188. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Zhang, S.; Liu, Q.; Wang, Z.; Wang, D.; Xu, P.; Xu, S.; Jiang, H.; Jian, X. A novel polyester-amide containing phthalazinone moieties nanofiltration membrane prepared by co-solvent assisted interfacial polymerization. J. Membr. Sci. 2025, 733, 124297. [Google Scholar] [CrossRef]
- Fu, W.; Deng, L.; Hu, M.; Mai, Z.; Xu, G.; Shi, Y.; Guan, K.; Gonzales, R.R.; Matsuoka, A.; Matsuyama, H. Polyamide composite membrane with 3D honeycomb-like structure via acetone-regulated interfacial polymerization for high-efficiency organic solvent nanofiltration. J. Membr. Sci. 2023, 679, 121711. [Google Scholar] [CrossRef]
- Chehardoli, G.; Bahmani, A. The role of crown ethers in drug delivery. Supramol. Chem. 2019, 31, 221–238. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, H.; Liu, Q.; Wang, Z.; Wang, D.; Xu, P.; Jian, X.; Zhang, S. Visualization of Film Formation Process of Copolyesteramide Containing Phthalazine Moieties During Interfacial Polymerization. Membranes 2025, 15, 233. https://doi.org/10.3390/membranes15080233
Liu Z, Li H, Liu Q, Wang Z, Wang D, Xu P, Jian X, Zhang S. Visualization of Film Formation Process of Copolyesteramide Containing Phthalazine Moieties During Interfacial Polymerization. Membranes. 2025; 15(8):233. https://doi.org/10.3390/membranes15080233
Chicago/Turabian StyleLiu, Zeyuan, Hailong Li, Qian Liu, Zhaoqi Wang, Danhui Wang, Peiqi Xu, Xigao Jian, and Shouhai Zhang. 2025. "Visualization of Film Formation Process of Copolyesteramide Containing Phthalazine Moieties During Interfacial Polymerization" Membranes 15, no. 8: 233. https://doi.org/10.3390/membranes15080233
APA StyleLiu, Z., Li, H., Liu, Q., Wang, Z., Wang, D., Xu, P., Jian, X., & Zhang, S. (2025). Visualization of Film Formation Process of Copolyesteramide Containing Phthalazine Moieties During Interfacial Polymerization. Membranes, 15(8), 233. https://doi.org/10.3390/membranes15080233






