Potable Water Recovery for Space Habitation Systems Using Hybrid Life Support Systems: Biological Pretreatment Coupled with Reverse Osmosis for Humidity Condensate Recovery
Abstract
1. Introduction
2. Materials and Method
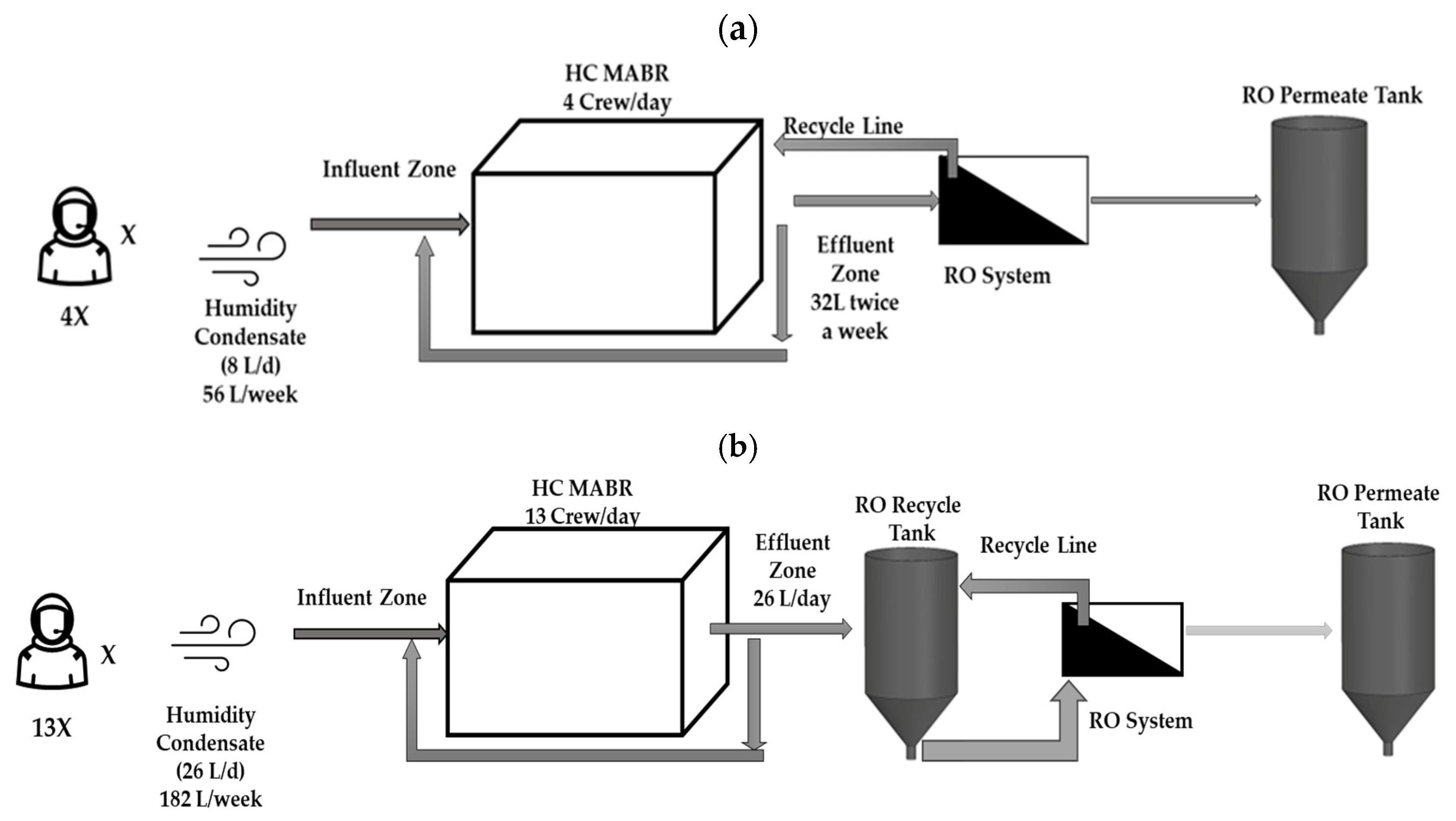
2.1. Configuration and Process of the HC MABR and BWRO System
2.1.1. Attributes of the Humidity Condensate Membrane Aerated Biological Reactor
2.1.2. Attributes of Brackish Water Reverse Osmosis (BWRO) Unit
2.1.3. Humidity Condensates Stream Composition and Feeding Regime
2.2. BWRO Treatment Process Configuration Type
2.2.1. Configuration 1
2.2.2. Configuration 2
2.3. Testing and Evaluation of Treatment System
3. Results
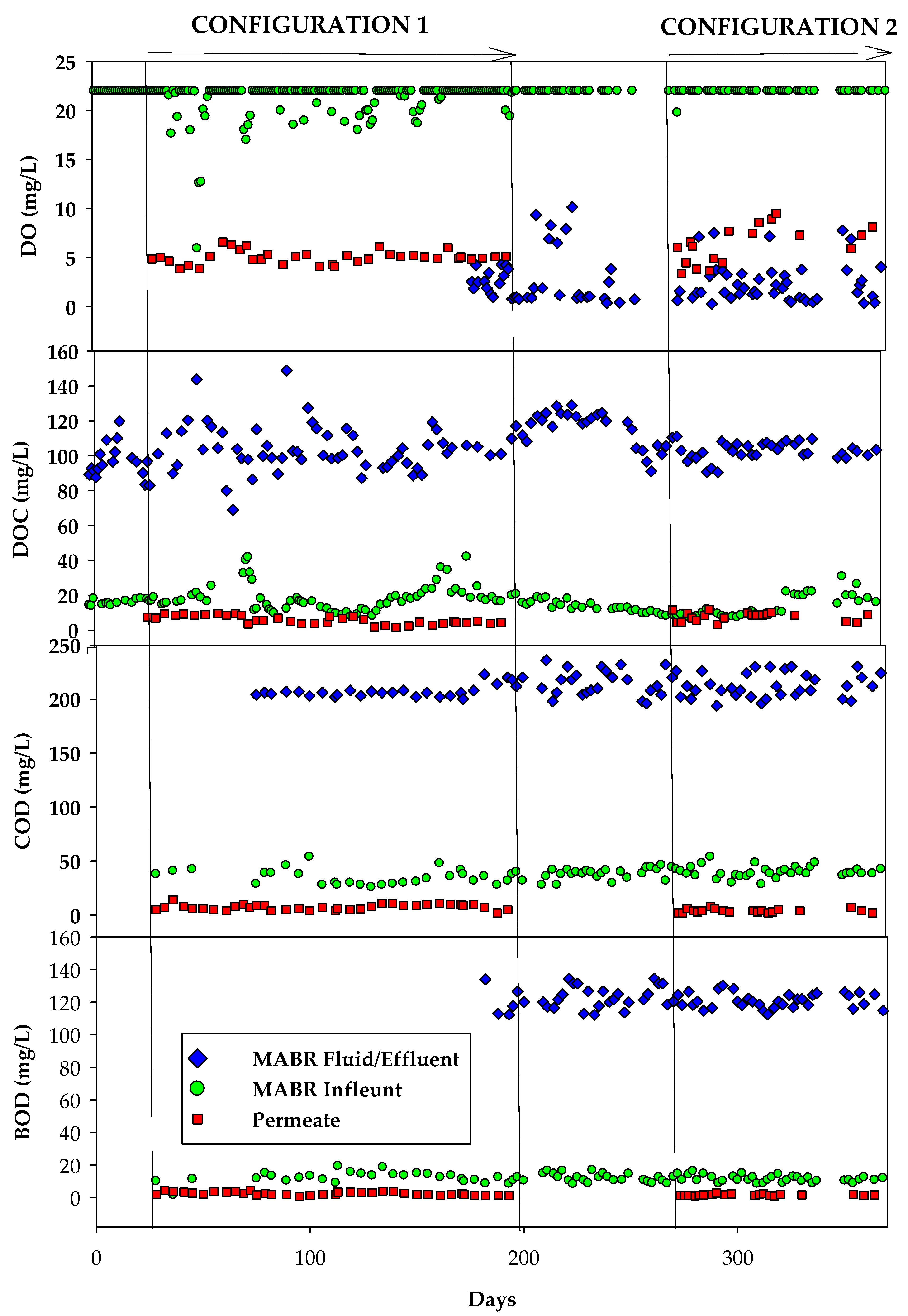
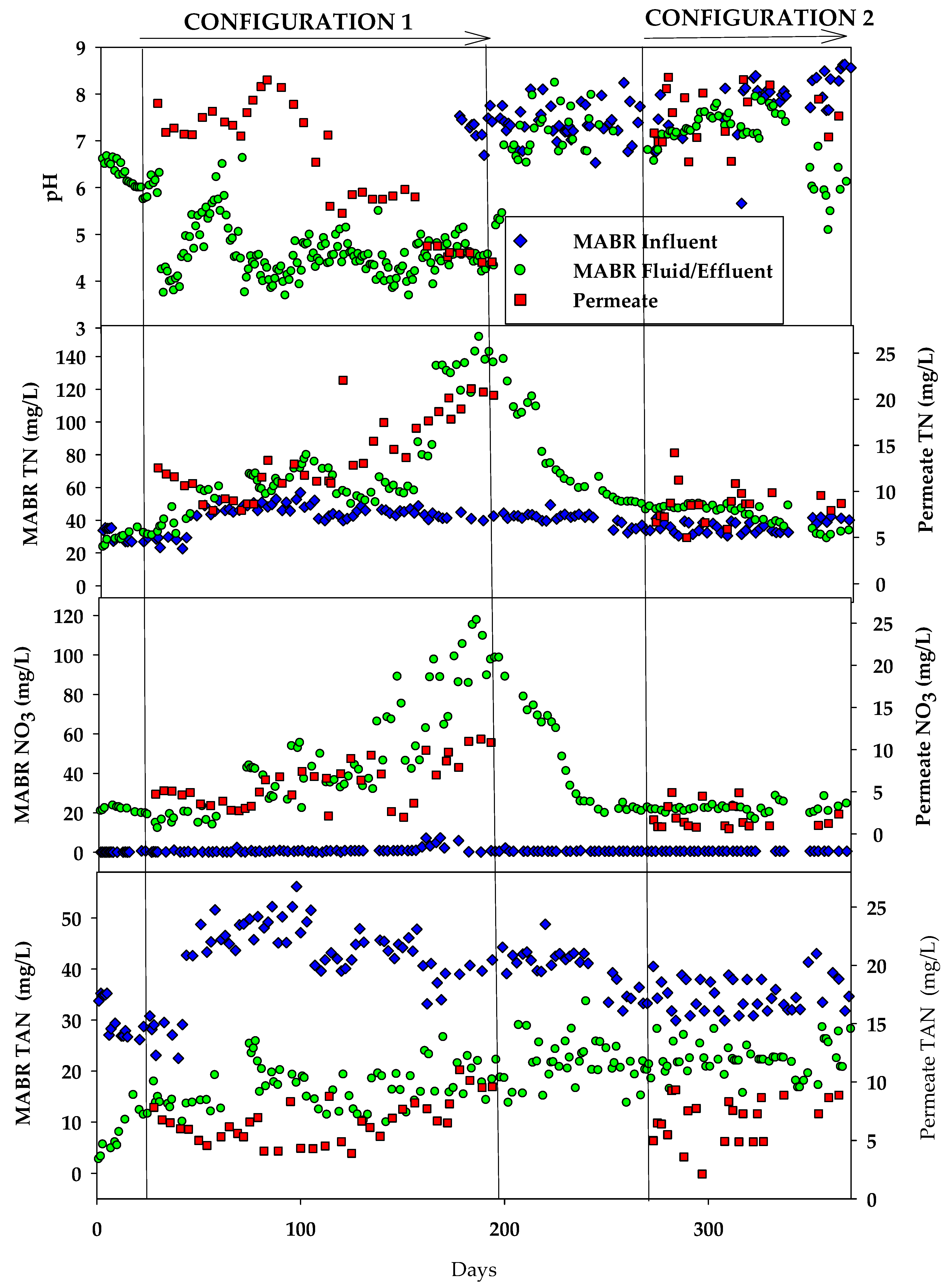
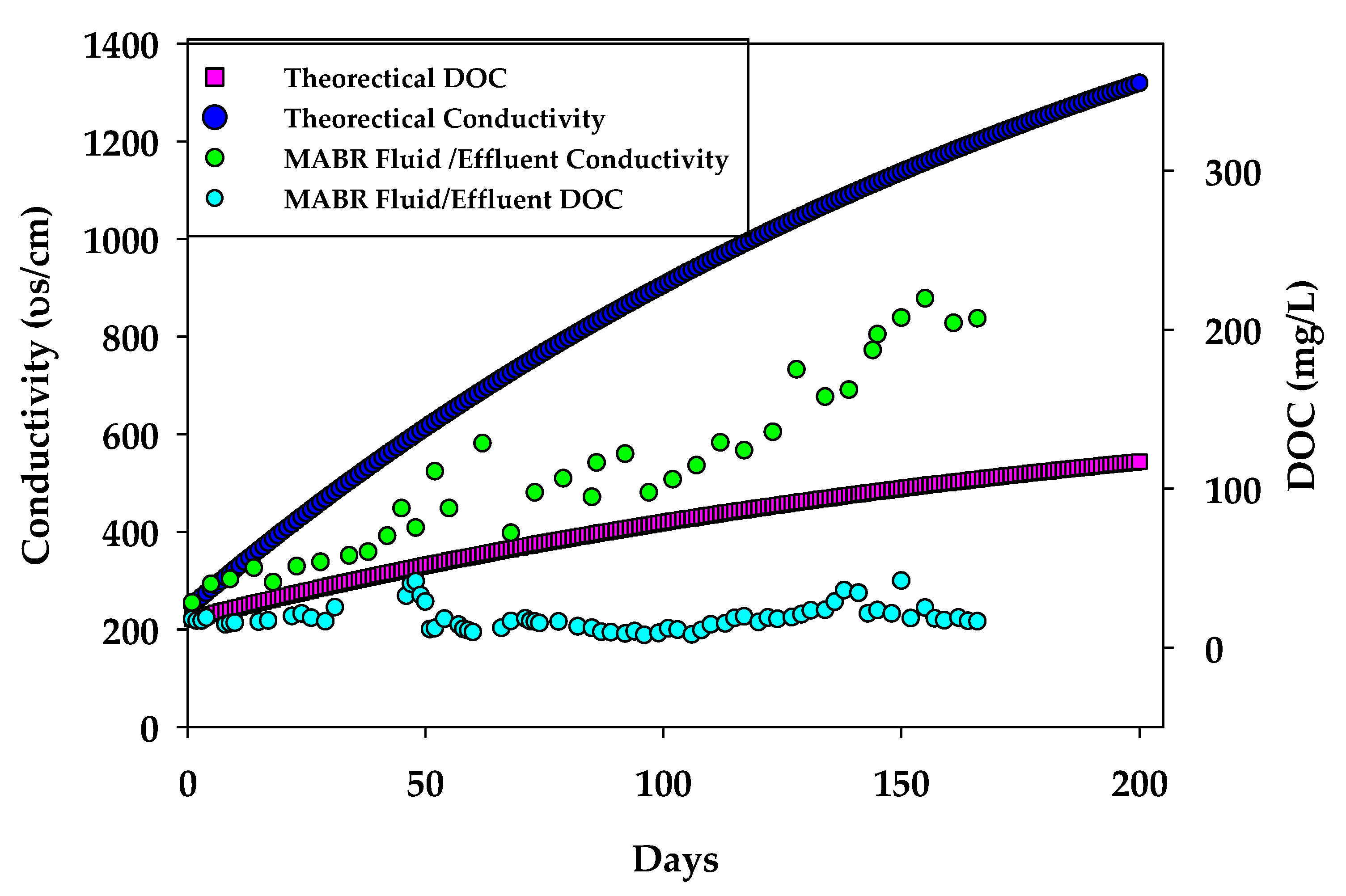
3.1. Organic, Nutrient, and Inorganic Salt Removal in the HC MABR-BWRO System
3.1.1. Configuration 1
3.1.2. Configuration 2
3.2. Permeate Flux
3.2.1. Configuration 1
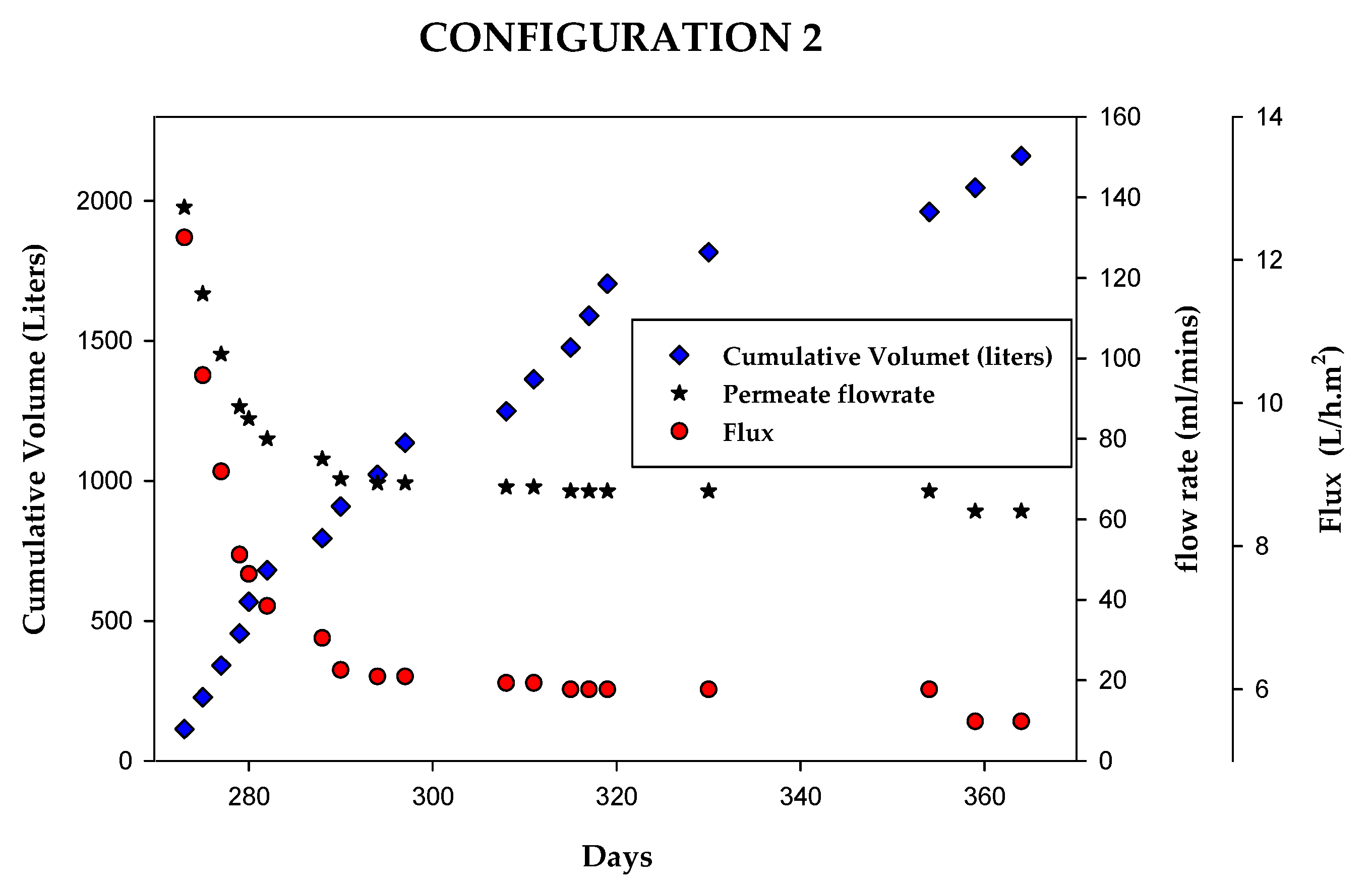
3.2.2. Configuration 2
4. Discussion
- Brine Management and Long-Term Stability:
- Scalability and Modular Design:
- Operational Risks and System Robustness:
- Maintenance Requirements and Consumables:
- Compatibility with Existing ISS Systems:
- System Integration Outlook:
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary of Acronyms
| BWRO | brackish water reverse osmosis |
| DOC | dissolved organic carbon |
| ECLSS | environmental control and life support system |
| GW | greywater |
| HC | humidity condensate |
| HRT | hydraulic retention time |
| ISS | international space station |
| NASA | National Aeronautics and Space Administration (USA) |
| MABR | membrane-aerated biological reactor |
| PGH | partial gravity habitation |
| RO | reverse osmosis |
| TAN | total ammoniacal nitrogen |
| TDS | total dissolved solids |
| THC | temperature and humidity control |
| TN | total nitrogen |
| TOC | total organic carbon |
| WPA | water process assembly |
| WRS | water recovery system |
References
- Duda, K.R.; Newman, D.J.; Zhang, J.; Meirhaeghe, N.; Zhou, H.L. Human Side of Space Exploration and Habitation. In Handbook of Human Factors and Ergonomics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 1480–1511. [Google Scholar]
- Meyer, C.; Schneider, W. NASA Advanced Explorations Systems: 2018 Advancements in Life Support Systems. In Proceedings of the 2018 AIAA SPACE and Astronautics Forum and Exposition, Orlando, FL, USA, 17–19 September 2018; p. 5151. [Google Scholar]
- Grigoriev, A.; Sinyak, Y.E.; Samsonov, N.; Bobe, L.; Protasov, N.; Andreychuk, P. Regeneration of water at space stations. Acta Astronaut. 2011, 68, 1567–1573. [Google Scholar] [CrossRef]
- Lee, J.S.; Yelderman, J.; Cleaver, G.B. Water Management Considerations for a Self-Sustaining Moonbase. arXiv 2024, arXiv:2405.14100. [Google Scholar]
- Muirhead, D.L.; Moller, S.L.; Adam, N.; Callahan, M.R. A Review of Baseline Assumptions and Ersatz Waste Streams for Partial Gravity Habitats and Orbiting Micro-gravity Habitats. In Proceedings of the International Conference on Environmental Systems, St. Paul, MN, USA, 10–14 July 2022. [Google Scholar]
- Zapparrata, W.R.D. Innovative Microgravity Condenser for Future Fully Closed Environmental Control and Life Support Systems. Ph.D. Thesis, Politecnico di Torino, Turin, Italy, 2019. [Google Scholar]
- Williamson, J.; Wilson, J.P.; Luong, H. Status of ISS Water Management and Recovery. In Proceedings of the 53rd International Conference on Environmental Systems (ICES), Louisville, KY, USA, 21–25 July 2024. [Google Scholar]
- Bobe, L.; Kochetkov, A.; Romanov, S.; Andreychuk, P.; Tsygankov, A.; Korobkov, A.; Zeleznyakov, A.; Sinyak, J. Design and operation of water recovery systems for space stations. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10 July 2016. [Google Scholar]
- Salmaso, M.; Wang, Z.; Duenas-Osorio, L.; Jernigan, M. Sustainable Space Logistics for Artemis Missions and Deep Space Exploration. In Proceedings of the AIAA SCITECH 2025 Forum, Orlando, FL, USA, 6–10 January 2025; p. 1479. [Google Scholar]
- Jones, H.W. Would current international space station (iss) recycling life support systems save mass on a mars transit? In Proceedings of the International Conference on Environmental Systems (ICES), Charleston, SC, USA, 16–20 July 2017. [Google Scholar]
- Sanchez, M.; Voss, J. From ISS to the Moon, Mars and Beyond-Applying Lessons Learned. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 10–13 January 2005; p. 705. [Google Scholar]
- Bullard, T.; Smith, A.; Delgado-Navarro, M.; Uman, A.; Hoque, B.; Bair, R.; Yeh, D.; Long, P.; Pickett, M.; Roberson, L. A prototype early planetary organic processor assembly (OPA) based on dual-stage anaerobic membrane bioreactor (AnMBR) for fecal and food waste treatment and resource recovery. In Proceedings of the 50th International Conference on Environmental Systems, Virtual, 12–15 July 2021. [Google Scholar]
- Prieto, A.L.; Futselaar, H.; Lens, P.N.; Bair, R.; Yeh, D.H. Development and start up of a gas-lift anaerobic membrane bioreactor (Gl-AnMBR) for conversion of sewage to energy, water and nutrients. J. Membr. Sci. 2013, 441, 158–167. [Google Scholar] [CrossRef]
- Gao, D.-W.; Zhang, T.; Tang, C.-Y.Y.; Wu, W.-M.; Wong, C.-Y.; Lee, Y.H.; Yeh, D.H.; Criddle, C.S. Membrane fouling in an anaerobic membrane bioreactor: Differences in relative abundance of bacterial species in the membrane foulant layer and in suspension. J. Membr. Sci. 2010, 364, 331–338. [Google Scholar] [CrossRef]
- Drexler, I.L.; Yeh, D.H. Membrane applications for microalgae cultivation and harvesting: A review. Rev. Environ. Sci. Bio/Technol. 2014, 13, 487–504. [Google Scholar] [CrossRef]
- Hooshyari, G.; Bose, A.; Jackson, W.A. Integration of Full-Size Graywater Membrane-Aerated Biological Reactor with Reverse Osmosis System for Space-Based Wastewater Treatment. Membranes 2024, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Pourbavarsad, M.S.; Jalalieh, B.J.; Harkins, C.; Sevanthi, R.; Jackson, W.A. Nitrogen oxidation and carbon removal from high strength nitrogen habitation wastewater with nitrification in membrane aerated biological reactors. J. Environ. Chem. Eng. 2021, 9, 106271. [Google Scholar] [CrossRef]
- Li, X.; Bao, D.; Zhang, Y.; Xu, W.; Zhang, C.; Yang, H.; Ru, Q.; Wang, Y.-f.; Ma, H.; Zhu, E. Development and application of membrane aerated biofilm reactor (MABR)—A review. Water 2023, 15, 436. [Google Scholar] [CrossRef]
- Meyer, C.E.; Pensinger, S.; Adam, N.; Pickering, K.D.; Barta, D.; Shull, S.A.; Vega, L.M.; Lange, K.; Christenson, D.; Jackson, W.A. Results of the alternative water processor test, a novel technology for exploration wastewater remediation. In Proceedings of the International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016. [Google Scholar]
- Zhou, Y.; Li, R.; Guo, B.; Zhang, L.; Zou, X.; Xia, S.; Liu, Y. Greywater treatment using an oxygen-based membrane biofilm reactor: Formation of dynamic multifunctional biofilm for organics and nitrogen removal. Chem. Eng. J. 2020, 386, 123989. [Google Scholar] [CrossRef]
- Veleva, I.; Van Weert, W.; Van Belzen, N.; Cornelissen, E.; Verliefde, A.; Vanoppen, M. Petrochemical condensate treatment by membrane aerated biofilm reactors: A pilot study. Chem. Eng. J. 2022, 428, 131013. [Google Scholar] [CrossRef]
- Nemeth, A.; Casey, E.; Syron, E. Mitigating Nitrous Oxide Emission from a Lab-Scale Membrane-Aerated Biofilm Reactor. Water 2025, 17, 500. [Google Scholar] [CrossRef]
- Childress, A.E. Innovative Treatment Technologies for Natural Waters and Wastewaters; University of Nevada: Reno, NV, USA, 2011. [Google Scholar]
- Wei, X.; Sanders, K.T.; Childress, A.E. Reclaiming wastewater with increasing salinity for potable water reuse: Water recovery and energy consumption during reverse osmosis desalination. Desalination 2021, 520, 115316. [Google Scholar] [CrossRef]
- Childress, A. Membrane Processes to Address the Global Challenge of Desalination; Frontiers of Engineering, The National Academies Press: Washington, DC, USA, 2008; pp. 125–134. [Google Scholar]
- Shah, M.P.; Rodriguez-Couto, S. Membrane-Based Hybrid Processes for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Wang, Q.; Shi, P.; Sun, Z.; Wang, J.; Li, B. Advanced treatment of municipal reverse osmosis concentrate by a hybrid ozone-membrane aerated biofilm reactor system. J. Water Process Eng. 2023, 53, 103923. [Google Scholar] [CrossRef]
- Hou, J.; Zhu, Y.; Meng, F.; Ni, B.-J.; Chen, X. Impact of comammox process on membrane-aerated biofilm reactor for autotrophic nitrogen removal. Water Res. X 2025, 28, 100318. [Google Scholar] [CrossRef]
- Nalette, T.; Snowdon, D.; Pickering, K.; Callahan, M. The ISS Water Processor Catalytic Reactor as a Post Processor for Advanced Water Reclamation Systems 07ICES-39. In Proceedings of the International Conference on Environmental Systems, Chicago, IL, USA, 9–12 July 2007. [Google Scholar]
- Kayatin, M.J.; Carter, D.L.; Schunk, R.G.; Pruitt, J.M. Upgrades to the ISS water recovery system. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016. [Google Scholar]




| SI NO | Ingredient | Concentration mg/L |
|---|---|---|
| 1 | Ethanol | 67 |
| 2 | Propylene Glycol | 27 |
| 3 | Methanol | 6.5 |
| 4 | Benzyl Alcohol | 15 |
| 5 | Ethylene Glycol | 4.5 |
| 6 | Acetone | 2.6 |
| 7 | Caprolactam | 2.3 |
| 8 | 2-Propanol (Isopropanol) | 1.0 |
| 9 | Benzoic acid | 2.0 |
| 10 | 2-Phenoxyethanol | 2.0 |
| 11 | 2-(2-Butoxyethoxy) ethanol | 2.0 |
| 12 | N, N-Dimethyl acetamide | 0.9 |
| 13 | Diethylphathalate | 1.2 |
| 14 | Trimethylsilanol | 0.41 |
| 15 | Acetaldehyde | 0.16 |
| 16 | Formaldehyde | 0.081 |
| 17 | Zinc Acetate | 15 |
| 18 | Nickel Acetate | 5.9 |
| 19 | Ammonium Bicarbonate | 198.4 |
| 20 | Ammonium Acetate | 14.8 |
| 21 | Ammonium Formate | 2.9 |
| 22 | Ammonium Fluoride | 1.4 |
| 23 | Monopotassium Phosphate | 0.6 |
| 24 | Calcium Bicarbonate | 1.4 |
| 25 | Sodium Bicarbonate | 0.3 |
| Test Type | Configuration 1 | |||||
| Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation | |
| Influent | Effluent | Permeate | ||||
| DOC (mg/L) | 105 | 12.2 | 15.9 | 6.83 | 6.53 | 2.64 |
| TN (mg/L) | 39.6 | 7.13 | 64.4 | 29.9 | 12.1 | 4.38 |
| Conductivity (mg/L) | 188 | 24.7 | 398 | 166 | 93.6 | 38.6 |
| Test Type | Configuration 2 | |||||
| Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation | |
| Influent | Effluent | Permeate | ||||
| DOC (mg/L) | 99.1 | 3.91 | 22.1 | 5.22 | 6.06 | 2.54 |
| TN (mg/L) | 39.2 | 3.24 | 32.6 | 2.58 | 8.73 | 0.815 |
| Conductivity (mg/L) | 183 | 29.9 | 246 | 28.3 | 39.8 | 9.53 |
| Metric | Config 1 Final Permeate | Config 2 Final Permeate | ISS WPA Final Produced Water [29,30] | Graywater MABR-RO Final Permeate [16] |
|---|---|---|---|---|
| DOC Removal (%) | ~94% | ~94% | ~98% | ~90% |
| Conductivity reduction (%) | Moderate (~50%) | High (~78%) | ~60% | ~60% |
| pH of permeate | 4–6 | 6–8 | 6.5–8.5 | ~7 |
| Operational days (crew-days) | ~500 | >1000 | Continuous | 200–1000 |
| Biofouling control | High | High | None | Very High |
| Consumable mass (Kg/year/crew) | <1 | <1 | N/A | ~2–3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adu, S.; Walker, W.S.; Jackson, W.A. Potable Water Recovery for Space Habitation Systems Using Hybrid Life Support Systems: Biological Pretreatment Coupled with Reverse Osmosis for Humidity Condensate Recovery. Membranes 2025, 15, 212. https://doi.org/10.3390/membranes15070212
Adu S, Walker WS, Jackson WA. Potable Water Recovery for Space Habitation Systems Using Hybrid Life Support Systems: Biological Pretreatment Coupled with Reverse Osmosis for Humidity Condensate Recovery. Membranes. 2025; 15(7):212. https://doi.org/10.3390/membranes15070212
Chicago/Turabian StyleAdu, Sunday, William Shane Walker, and William Andrew Jackson. 2025. "Potable Water Recovery for Space Habitation Systems Using Hybrid Life Support Systems: Biological Pretreatment Coupled with Reverse Osmosis for Humidity Condensate Recovery" Membranes 15, no. 7: 212. https://doi.org/10.3390/membranes15070212
APA StyleAdu, S., Walker, W. S., & Jackson, W. A. (2025). Potable Water Recovery for Space Habitation Systems Using Hybrid Life Support Systems: Biological Pretreatment Coupled with Reverse Osmosis for Humidity Condensate Recovery. Membranes, 15(7), 212. https://doi.org/10.3390/membranes15070212






