A Study on the Performance of Vacuum Membrane Distillation in Treating Acidic, Simulated, Low-Level Radioactive Liquid Waste
Abstract
1. Introduction
2. Materials and Methods
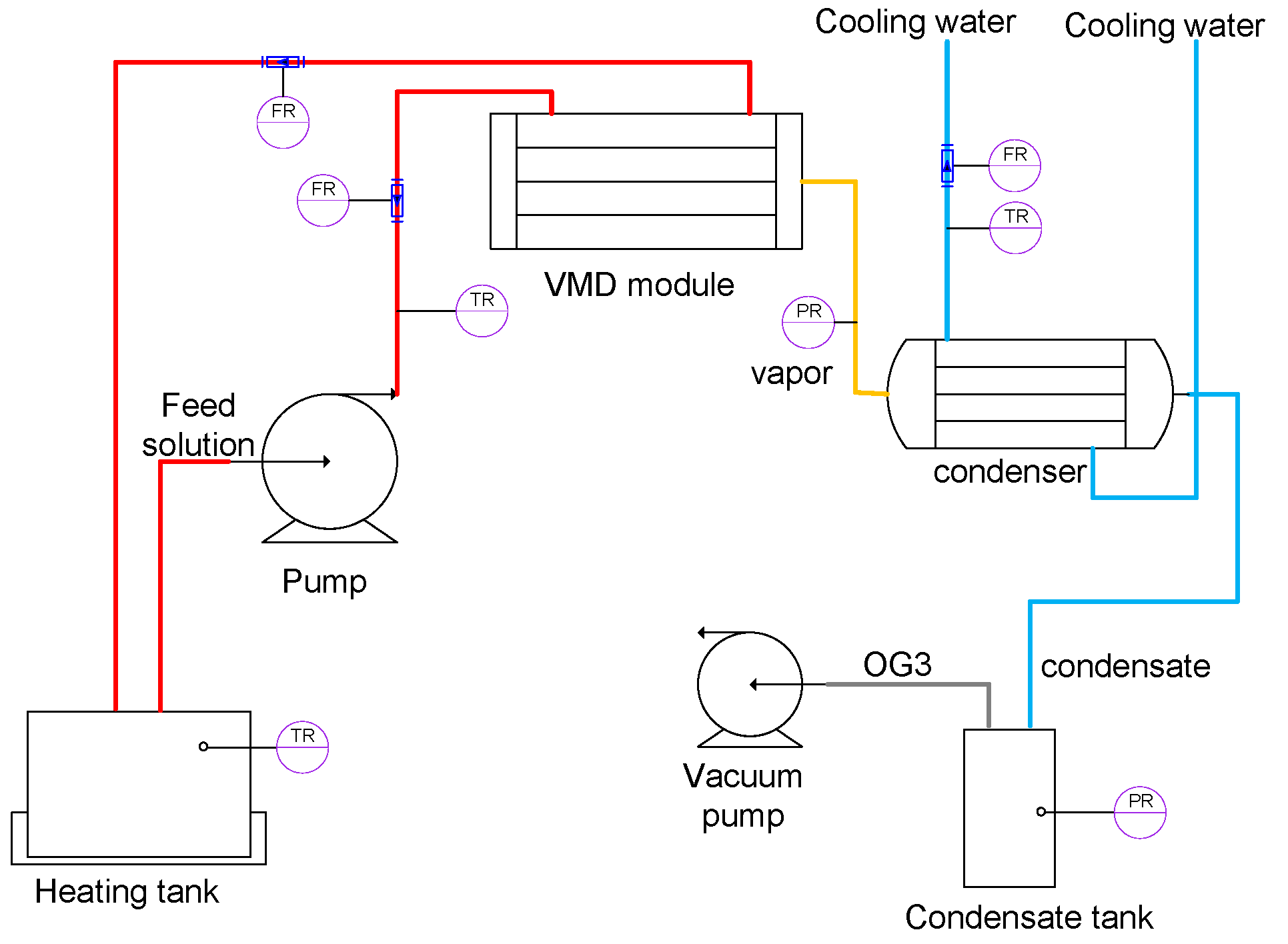
2.1. Experimental Device and Materials
- A hot-side distillation source system, including a water bath heating tank, a high-temperature corrosion-resistant variable frequency pump (0.6T/H-12M, LiYu Energy Saving Equipment Co., Ltd., Foshan, China), a high-temperature corrosion-resistant flow meter (LZM-6T, Jintai Instrument Co., Ltd., Yuyao, China), a corrosion-resistant temperature probe and a multi-channel temperature recorder(TCP-500XL-8, Huipu Instrument Co., Ltd., Zhongshan, China), pipes, valves, and insulation.
- A cold-side condensation system, including a condenser tube, a chilled water circulation loop with thermal insulation, and a condensate collection vessel.
- A vacuum generation system, comprising a water-circulating vacuum pump (SHB-III, Zhengzhou Great wall Scientific Industrial & Trade Co., Ltd., Zhengzhou, China).
- A metering system equipped with a digital transmission electronic scale (RS232 signal interface, OHAUS, Parsippany, NJ, USA).
- Effective membrane surface area: 0.3 m2;
- Membrane dimensions: 1.2 mm inner diameter and 2.3 mm outer diameter;
- Pore size distribution: 0.1 μm nominal pore diameter;
- Pressure: 0.1 MPa bubble point pressure.
2.2. Experimental and Analytical Methods
2.3. Orthogonal Test Design
- Factor A (feed solution temperature): 40 °C, 50 °C, 60 °C, 70 °C.
- Factor B (vacuum pressure): 5 kPa, 15 kPa, 25 kPa, 30 kPa.
- Factor C (feed flow rate): 200 L·h−1, 300 L·h−1, 400 L·h−1, 500 L·h−1.
3. Results and Discussion
3.1. Selection of Operating Conditions for VMD
- Feed temperature: 70 °C (A4);
- Vacuum pressure: 15 kPa (B2);
- Feed flow rate: 500 L·h−1 (C4).
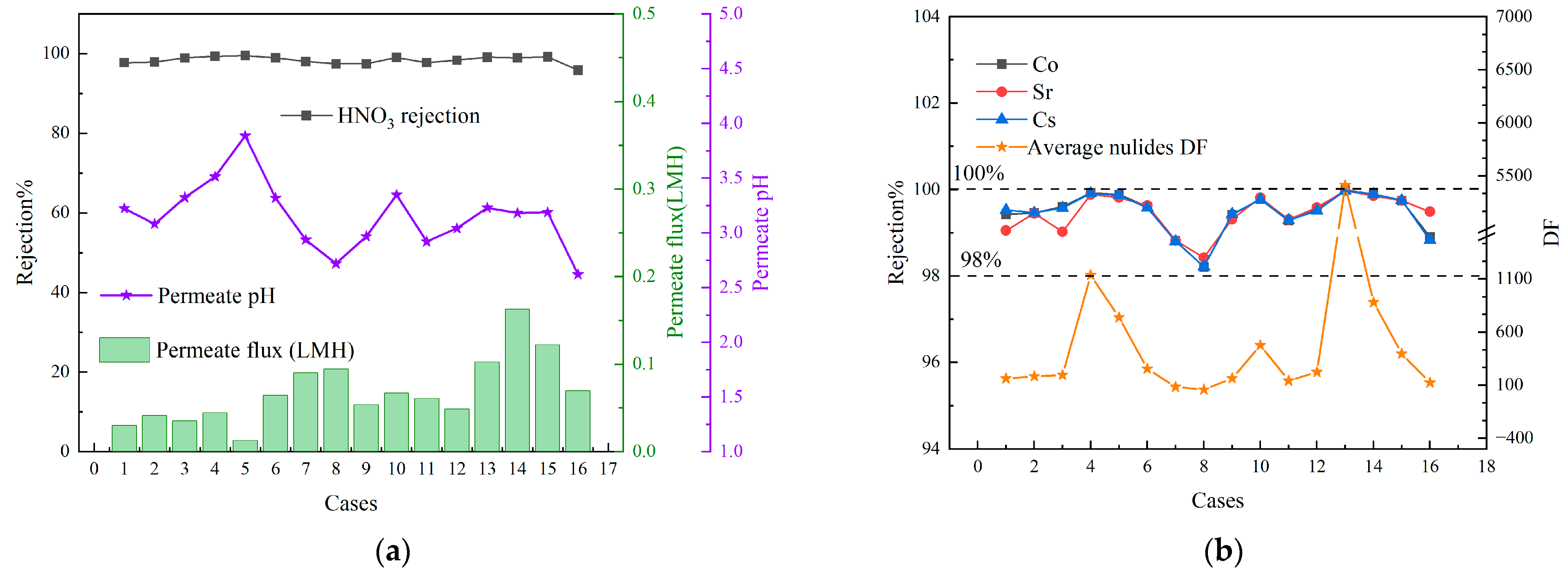
3.2. Orthogonal Experiment Results Analysis
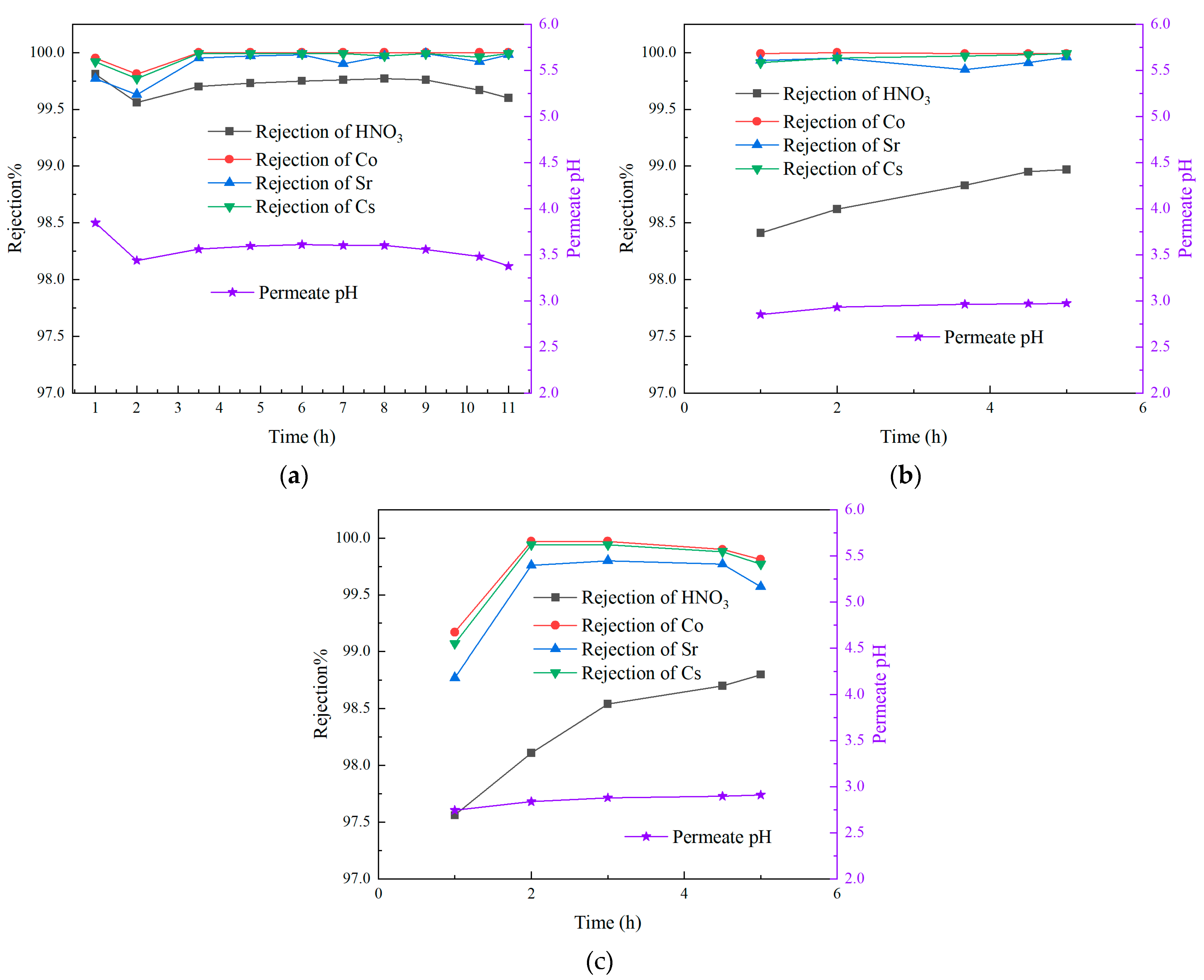
3.3. Effect of Different Nuclide Feed Concentrations
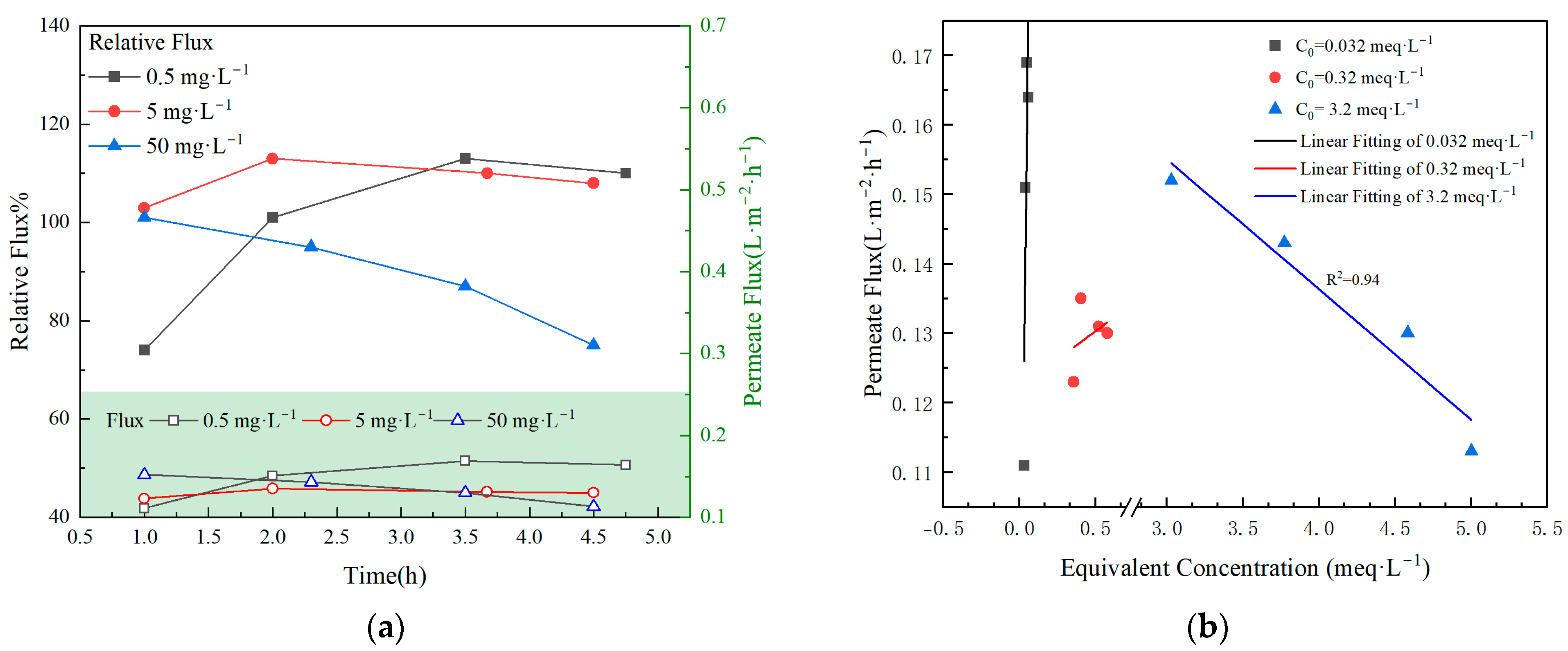
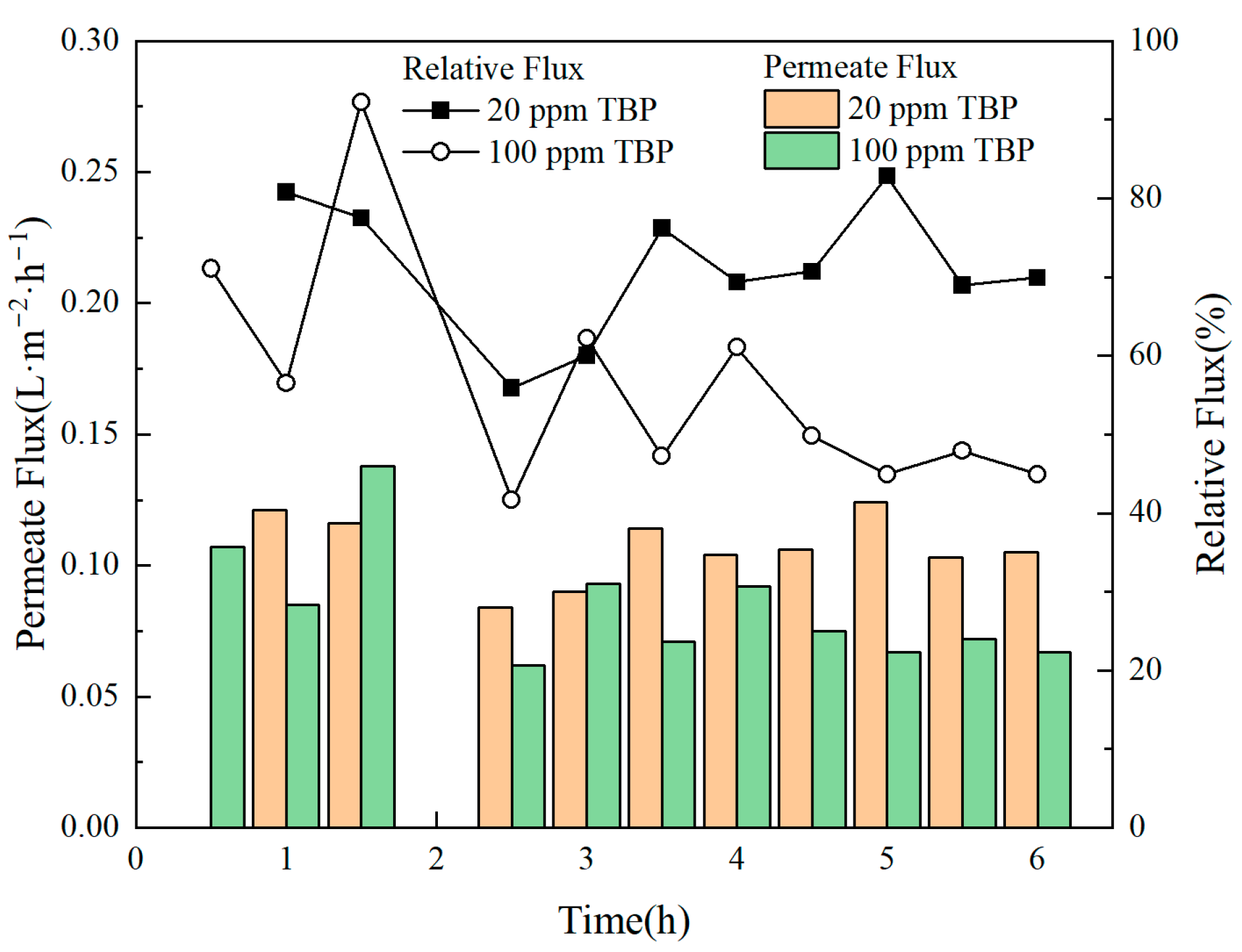
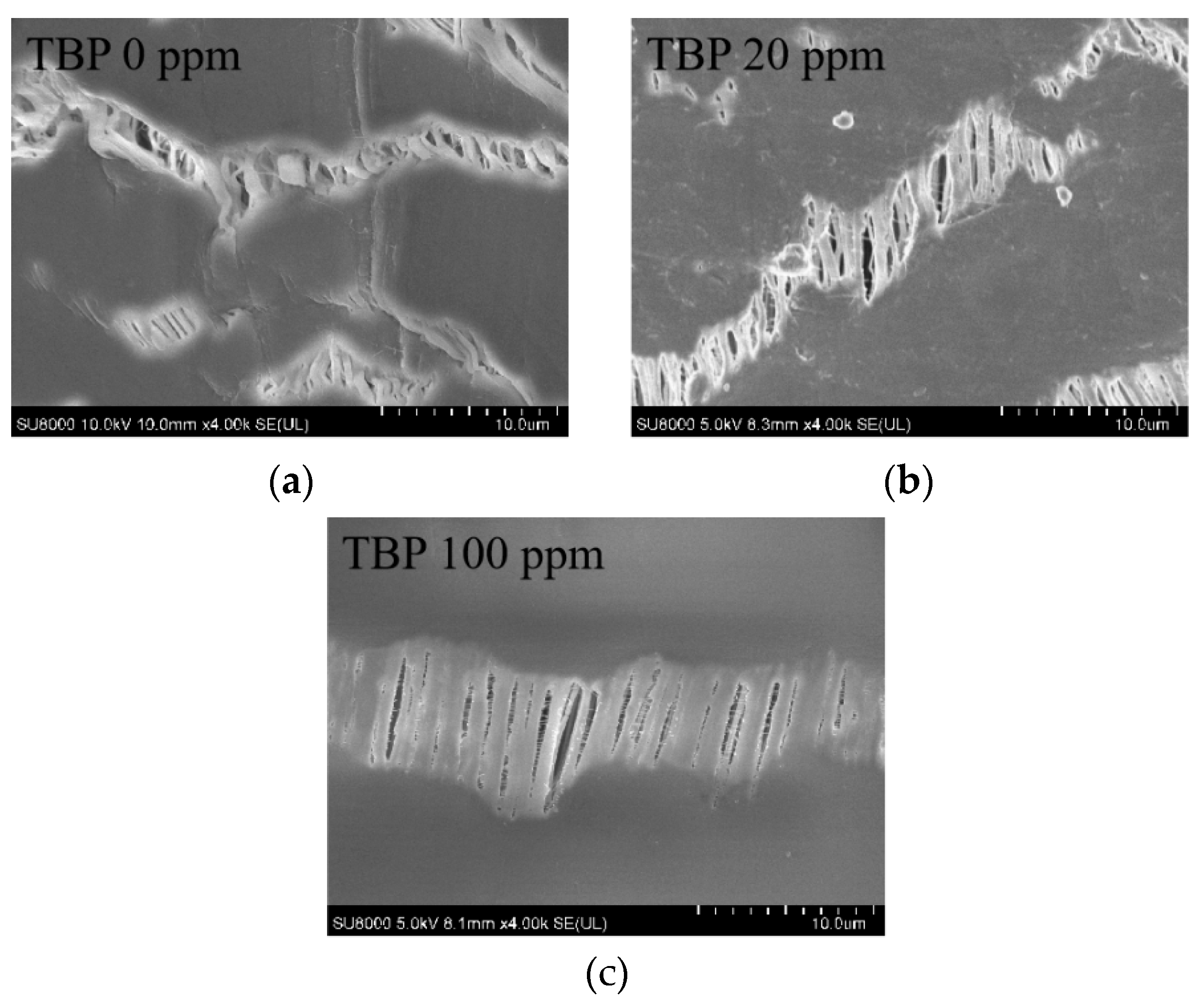
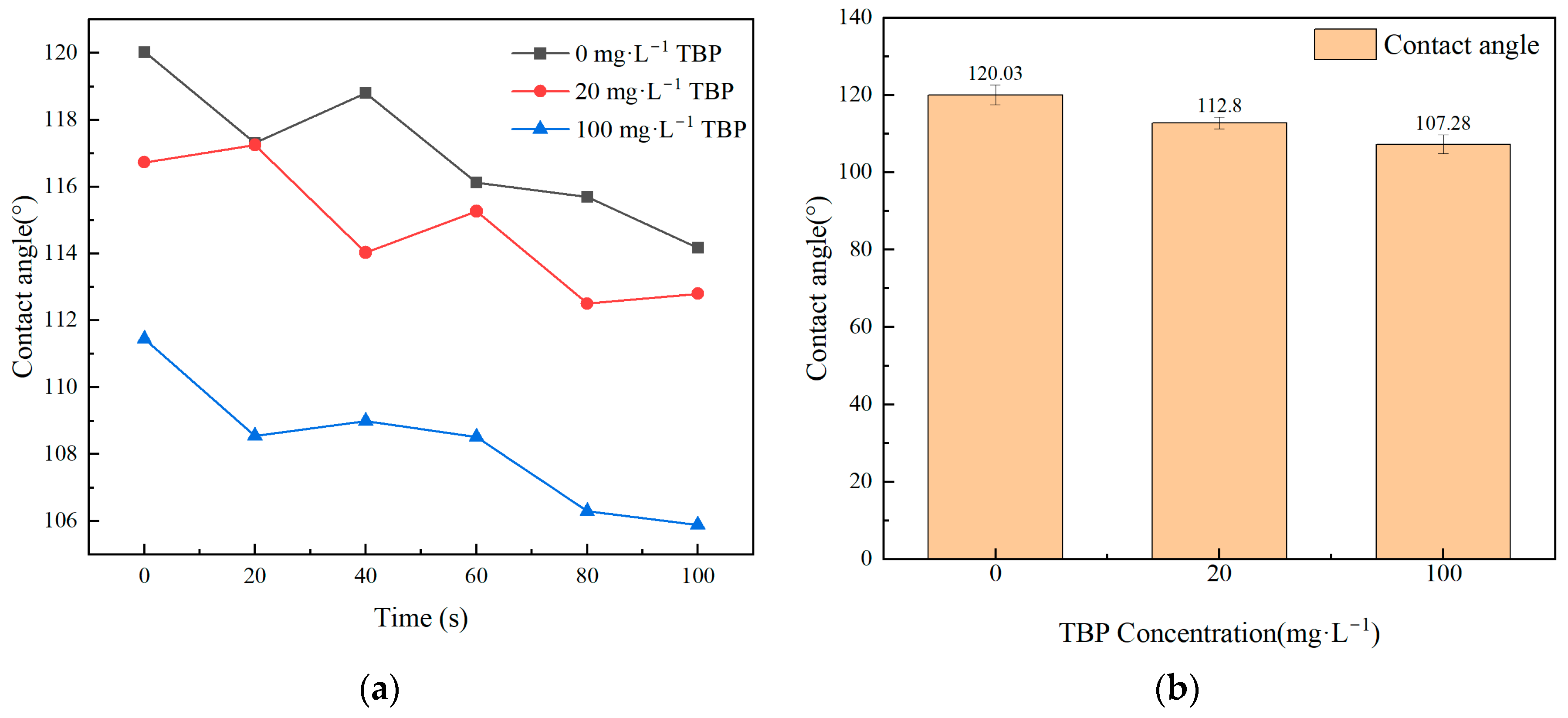
3.4. Effect of TBP Concentration on Feed
4. Conclusions
- Orthogonal experimental analysis revealed the optimal operational parameters for the VMD process as follows: a feed temperature of 70 °C, a permeate-side vacuum pressure of 90 kPa, and a feed flow rate of 500 L·h−1.
- The VMD process demonstrated a good performance in retaining nuclides and remains unaffected by variations in feed concentration. The rejection rate was consistently maintained at over 99.7%, with a DF exceeding 300. Additionally, the rejection rate of nitric acid remained above 98%. However, as the concentration of nuclides in the feed increased, the rejection rate exhibited a tendency to decrease.
- Under optimized operational parameters, the VMD process maintained a membrane flux of 0.9 L·m−2·h−1. At low nuclide concentrations in the feed solution, no significant negative effect on membrane flux was observed.
- The presence of TBP in the feed solution could lead to the contamination of the membrane material, thereby causing a reduction in membrane flux and a decline in DF. This issue was likely to become more obvious as the concentration of TBP increased.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- GB/T 13695-1992; Authorized Limits for Normalized Releases of Radioactive Effluents from Nuclear Fuel Cycle. State Bureau of Technical Supervision, Standards Press of China: Beijing, China, 1993.
- Bond, G.; Eccles, H.; Emmott, J.D. Separation of cations from nitric acid solutions using commercially available ion exchange Resins. J. Chem. Eng. Process Technol. 2019, 10, 1. [Google Scholar] [CrossRef]
- Van Wagner, E.M.; Sagle, A.C.; Sharma, M.M.; Freeman, B.D. Effect of crossflow testing conditions, including feed pH and continuous feed filtration, on commercial reverse osmosis membrane performance. J. Membr. Sci. 2009, 345, 97–109. [Google Scholar] [CrossRef]
- Tan, H.; Chen, Z.; Liu, T.; Wang, M.; Pan, Q. Study on the acid and alkali resistance of commercial seawater desalination RO Membranes. Technol. Water Treat. 2016, 42, 70–74. [Google Scholar]
- Jiang, M.; Fang, Z.; Liu, Z.; Huang, X.; Wei, H.; Yu, C.Y. Application of membrane distillation for purification of radioactive liquid. Clean. Eng. Technol. 2023, 12, 100589. [Google Scholar] [CrossRef]
- Wen, X.; Li, F.; Zhao, X. Filtering of low-level radioactive wastewater by means of vacuum membrane distillation. Nucl. Technol. 2016, 194, 379–386. [Google Scholar] [CrossRef]
- Jia, X.; Lan, L.; Zhang, X.; Wang, T.; Wang, Y.; Ye, C.; Lin, J. Pilot-scale vacuum membrane distillation for decontamination of simulated radioactive wastewater: System design and performance evaluation. Sep. Purif. Technol. 2021, 275, 119129. [Google Scholar] [CrossRef]
- Li, S.; He, Z.; Xiao, D.; Guo, L. Study on the treatment of radioactive wastewater by non-contact membrane distillation. Sep. Purif. Technol. 2022, 290, 120766. [Google Scholar] [CrossRef]
- Jia, F.; Li, J.; Wang, J.; Sun, Y. Removal of strontium ions from simulated radioactive wastewater by vacuum membrane distillation. Ann. Nucl. Energy 2017, 103, 363–368. [Google Scholar] [CrossRef]
- Jia, F.; Yin, Y.; Wang, J. Removal of cobalt ions from simulated radioactive wastewater by vacuum membrane distillation. Prog. Nucl. Energy 2018, 103, 20–27. [Google Scholar] [CrossRef]
- Wen, X.; Li, F.; Jiang, B.; Zhang, X.; Zhao, X. Effect of surfactants on the treatment of radioactive laundry wastewater by direct contact membrane distillation. J. Chem. Technol. Biotechnol. 2018, 93, 2252–2261. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, P.; Su, C. Engineering application of membrane distillation in spend acid treatment. Sulphuric Acid Industry. 2022, 11, 24–28. [Google Scholar]
- Kesieme, U.K.; Milne, N.; Cheng, C.Y.; Aral, H.; Duke, M. Recovery of water and acid from leach solutions using direct contact membrane distillation. Water Sci. Technol. 2014, 69, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Thiruvenkatachari, R.; Manickam, M.; Ouk Kwon, T.; Shik Moon, I.; Woo Kim, J. Separation of water and nitric acid with porous hydrophobic membrane by air gap membrane distillation (AGMD). Sep. Sci. Technol. 2006, 41, 3187–3199. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Liu, R. A Multiple-Effect Membrane Distillation Process for Deep Concentrating Aqueous Solution of Hydrochloric Acid. Master’s Dissertation, Tianjin University, Tianjin, China, 2012. [Google Scholar]
- SINOPEC Shanghai Engineering Co., Ltd. Chemical Process Design Handbook, 5th ed.; Chemical Industry Press: Beijing, China, 2018; ISBN 978-7-122-30906-8. [Google Scholar]
- Li, J.; Guan, Y.; Cheng, F.; Liu, Y. Treatment of high salinity brines by direct contact membrane distillation: Effect of membrane characteristics and salinity. Chemosphere 2015, 140, 143–149. [Google Scholar] [CrossRef]
- Yang, H. Study of the Membrane Module and System for Solar Heated Vacuum Membrane Distillation. Master’s Dissertation, Zhejiang University, Hangzhou, China, 2008. [Google Scholar]
- Safavi, M.; Mohammadi, T. High-salinity water desalination using VMD. Chem. Eng. J. 2009, 149, 191–195. [Google Scholar] [CrossRef]
- Gryta, M. Fouling in direct contact membrane distillation process. J. Membr. Sci. 2008, 325, 383–394. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Gryta, M.; Morawski, A.W. Study on the concentration of acids by membrane distillation. J. Membr. Sci. 1995, 102, 113–122. [Google Scholar] [CrossRef]
- Elkina, I.B.; Gilman, A.B.; Ugrozov, V.V.; Volkov, V.V. Separation of mineral acid solutions by membrane distillation and thermopervaporation through porous and nonporous membranes. Ind. Eng. Chem. Res. 2013, 52, 8856–8863. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Treatment of high salinity solutions: Application of air gap membrane distillation. Desalination 2012, 287, 55–60. [Google Scholar] [CrossRef]
- Wen, X.; Li, F.; Zhao, X. Removal of nuclides and boron from highly saline radioactive wastewater by direct contact membrane distillation. Desalination 2016, 394, 101–107. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Muhammad, M.; Ren, L.; Shao, J. Research progress of novel membranes for membrane distillation against wetting, fouling and scaling. Membr. Sci. Technol. 2024, 44, 157–167. [Google Scholar]
- Yu, J.; Li, X.; Wang, J.; Zhao, F.; Wang, L.; Sha, Q. Physical properties of the two phases in tributyl phosphate extraction system-density, viscosity and surface tension. J. Nucl. Radiochem. 1981, 2, 65–76. [Google Scholar]


| Cases | Testing Scheme | Factors | ||
|---|---|---|---|---|
| Feed Temperature (°C) | Vacuum Degree (kPa) | Flow Rate (L·h−1) | ||
| 1 | A1B1C1 | 40 | 5 | 200 |
| 2 | A1B2C2 | 40 | 15 | 300 |
| 3 | A1B3C3 | 40 | 25 | 400 |
| 4 | A1B4C4 | 40 | 30 | 500 |
| 5 | A2B1C2 | 50 | 5 | 300 |
| 6 | A2B2C1 | 50 | 15 | 200 |
| 7 | A2B3C4 | 50 | 25 | 500 |
| 8 | A2B4C3 | 50 | 30 | 400 |
| 9 | A3B1C3 | 60 | 5 | 400 |
| 10 | A3B2C4 | 60 | 15 | 500 |
| 11 | A3B3C1 | 60 | 25 | 200 |
| 12 | A3B4C2 | 60 | 30 | 300 |
| 13 | A4B1C4 | 70 | 5 | 500 |
| 14 | A4B2C3 | 70 | 15 | 400 |
| 15 | A4B3C2 | 70 | 25 | 300 |
| 16 | A4B4C1 | 70 | 30 | 200 |
| Parameters | Factors | K | T | Range | Rank | Optimum Operation Parameters |
|---|---|---|---|---|---|---|
| Feed temperature (°C) | A1 | 0.1505 | 0.0376 | 0.0766 | Feed temperature > Vacuum degree > Flow rate | A4B2C3 |
| A2 | 0.2611 | 0.0653 | ||||
| A3 | 0.2298 | 0.0574 | ||||
| A4 | 0.4570 | 0.1143 | ||||
| Vacuum degree (kPa) | B1 | 0.1985 | 0.0496 | 0.0342 | ||
| B2 | 0.3353 | 0.0838 | ||||
| B3 | 0.3076 | 0.0769 | ||||
| B4 | 0.2569 | 0.0642 | ||||
| Flow rate (L·h−1) | C1 | 0.2247 | 0.0562 | 0.0303 | ||
| C2 | 0.2243 | 0.0561 | ||||
| C3 | 0.3457 | 0.0864 | ||||
| C4 | 0.3037 | 0.0759 |
| Parameters | Factors | K | T | Range | Rank | Optimum Operation Parameters |
|---|---|---|---|---|---|---|
| Feed temperature (°C) | A1 | 3.9398 | 0.9849 | 0.0035 | Flow rate > Vacuum degree > Feed temperature | A2B2C4 |
| A2 | 3.9403 | 0.9851 | ||||
| A3 | 3.9263 | 0.9816 | ||||
| A4 | 3.9319 | 0.9830 | ||||
| Vacuum degree (kPa) | B1 | 3.9392 | 0.9848 | 0.0096 | ||
| B2 | 3.9490 | 0.9873 | ||||
| B3 | 3.9396 | 0.9849 | ||||
| B4 | 3.9104 | 0.9776 | ||||
| Flow rate (L·h−1) | C1 | 3.9035 | 0.9759 | 0.0132 | ||
| C2 | 3.9503 | 0.9876 | ||||
| C3 | 3.9283 | 0.9821 | ||||
| C4 | 3.9561 | 0.9890 |
| Parameters | Factors | K | T | Range | Rank | Optimum Operation Parameters |
|---|---|---|---|---|---|---|
| Feed temperature (°C) | A1 | 398.1039 | 99.5260 | 0.5302 | Flow rate > Vacuum degree > Feed temperature | A2B2C4 |
| A2 | 396.5675 | 99.1419 | ||||
| A3 | 398.0212 | 99.5053 | ||||
| A4 | 398.6885 | 99.6721 | ||||
| Vacuum degree (kPa) | B1 | 398.5627 | 99.6407 | 0.4776 | ||
| B2 | 398.7356 | 99.6839 | ||||
| B3 | 397.2574 | 99.3143 | ||||
| B4 | 396.8253 | 99.2063 | ||||
| Flow rate (L·h−1) | C1 | 397.3109 | 99.3277 | 0.4129 | ||
| C2 | 398.6146 | 99.6537 | ||||
| C3 | 396.9630 | 99.2407 | ||||
| C4 | 398.4925 | 99.6231 |
| Cases | Flux (L·m−2·h−1) | HNO3 Rejection (%) | Permeate pH |
|---|---|---|---|
| 1 | 0.953 | 99.62 | 3.44 |
| 2 | 0.913 | 99.71 | 3.56 |
| 3 | 0.930 | 98.91 | 3.16 |
| Average | 0.932 ± 0.0163 | 99.41 ± 0.358 | 3.39 ± 0.168 |
| Feed Composition | HNO3 DF | Co DF | Sr DF | Cs DF |
|---|---|---|---|---|
| 0 mg·L−1 TBP | 247 | 41,733 | 5853 | 10,317 |
| 20 mg·L−1 TBP | 135 | 1332 | 3010 | 822 |
| 100 mg·L−1 TBP | 66 | 482 | 572 | 383 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Xu, Y.; Wu, Y.; Lu, Y.; Weng, Z.; Tao, Y.; Liu, J.; Jiang, B. A Study on the Performance of Vacuum Membrane Distillation in Treating Acidic, Simulated, Low-Level Radioactive Liquid Waste. Membranes 2025, 15, 213. https://doi.org/10.3390/membranes15070213
Chen S, Xu Y, Wu Y, Lu Y, Weng Z, Tao Y, Liu J, Jiang B. A Study on the Performance of Vacuum Membrane Distillation in Treating Acidic, Simulated, Low-Level Radioactive Liquid Waste. Membranes. 2025; 15(7):213. https://doi.org/10.3390/membranes15070213
Chicago/Turabian StyleChen, Sifan, Yan Xu, Yuyong Wu, Yizhou Lu, Zhan Weng, Yaoguang Tao, Jianghai Liu, and Baihua Jiang. 2025. "A Study on the Performance of Vacuum Membrane Distillation in Treating Acidic, Simulated, Low-Level Radioactive Liquid Waste" Membranes 15, no. 7: 213. https://doi.org/10.3390/membranes15070213
APA StyleChen, S., Xu, Y., Wu, Y., Lu, Y., Weng, Z., Tao, Y., Liu, J., & Jiang, B. (2025). A Study on the Performance of Vacuum Membrane Distillation in Treating Acidic, Simulated, Low-Level Radioactive Liquid Waste. Membranes, 15(7), 213. https://doi.org/10.3390/membranes15070213





