A Review of Polylactic Acid (PLA) and Poly(3-hydroxybutyrate) (PHB) as Bio-Sourced Polymers for Membrane Production Applications
Abstract
1. Introduction
2. Methodology
3. Biodegradable and Bio-Sourced Polymers
4. Polylactic Acid (PLA) Production from Bio-Sources
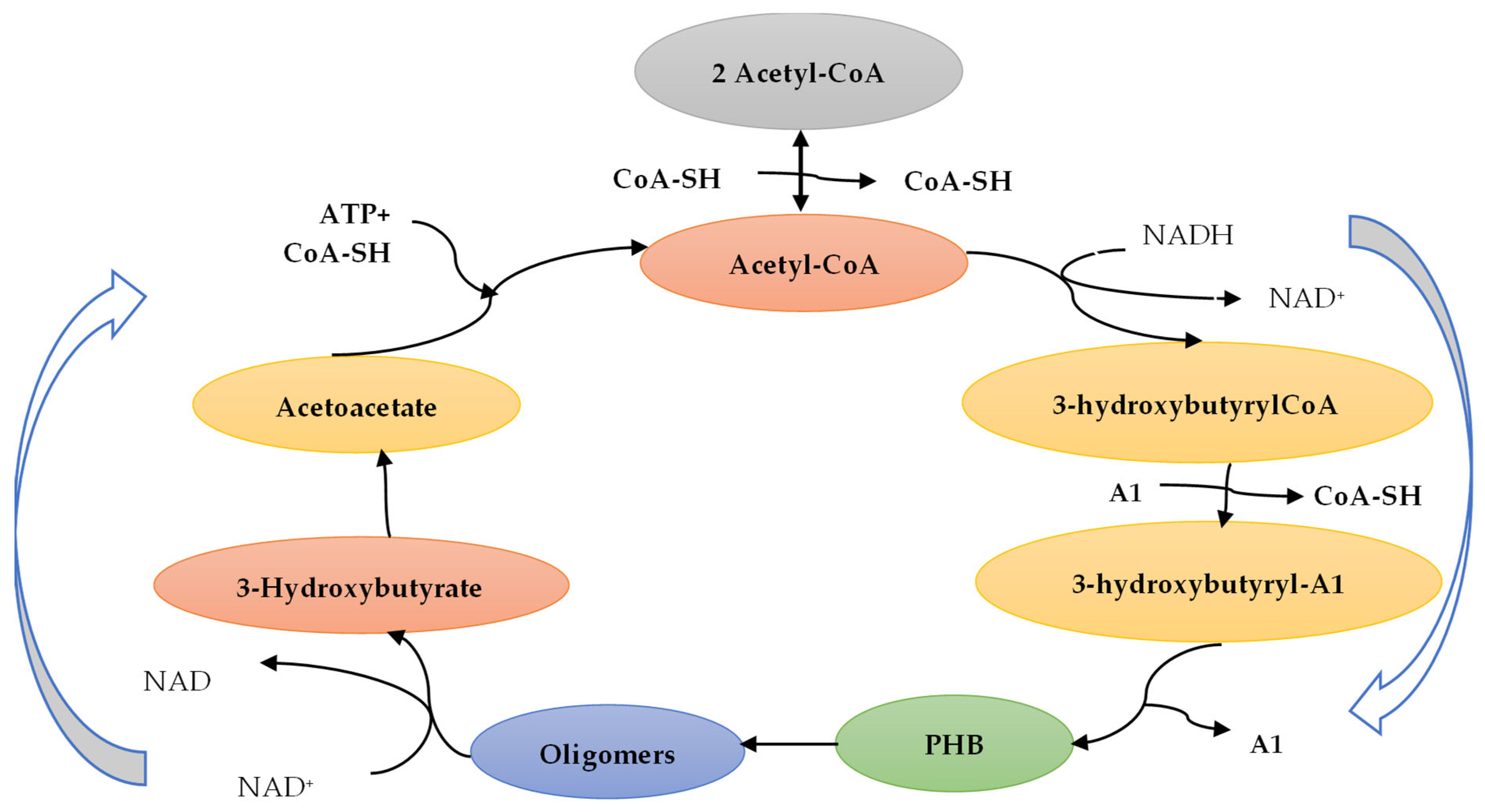
5. Poly(3-hydroxybutyrate) (PHB) Production from Bio-Sources
6. Characteristics of PLA and PHB
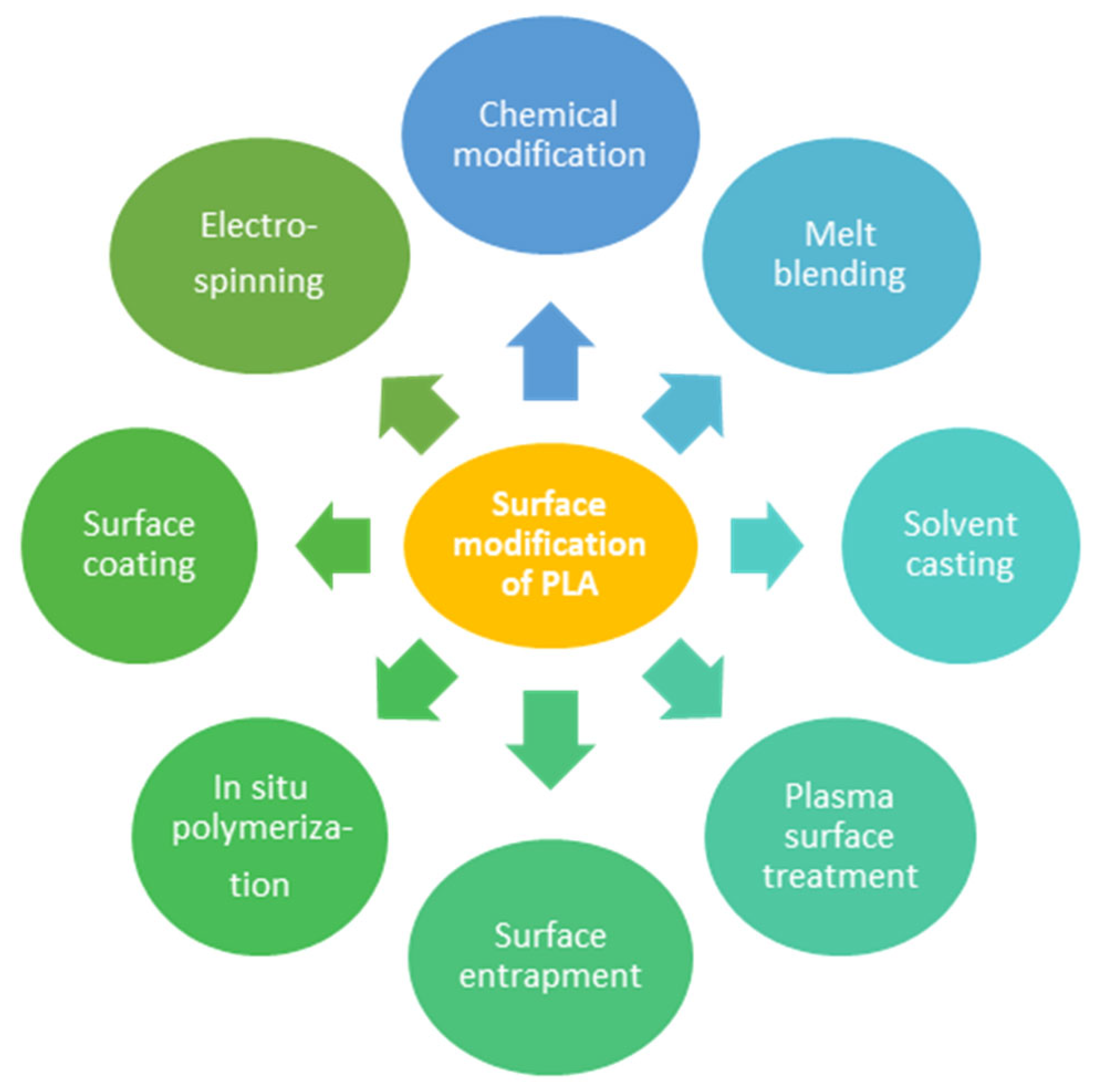
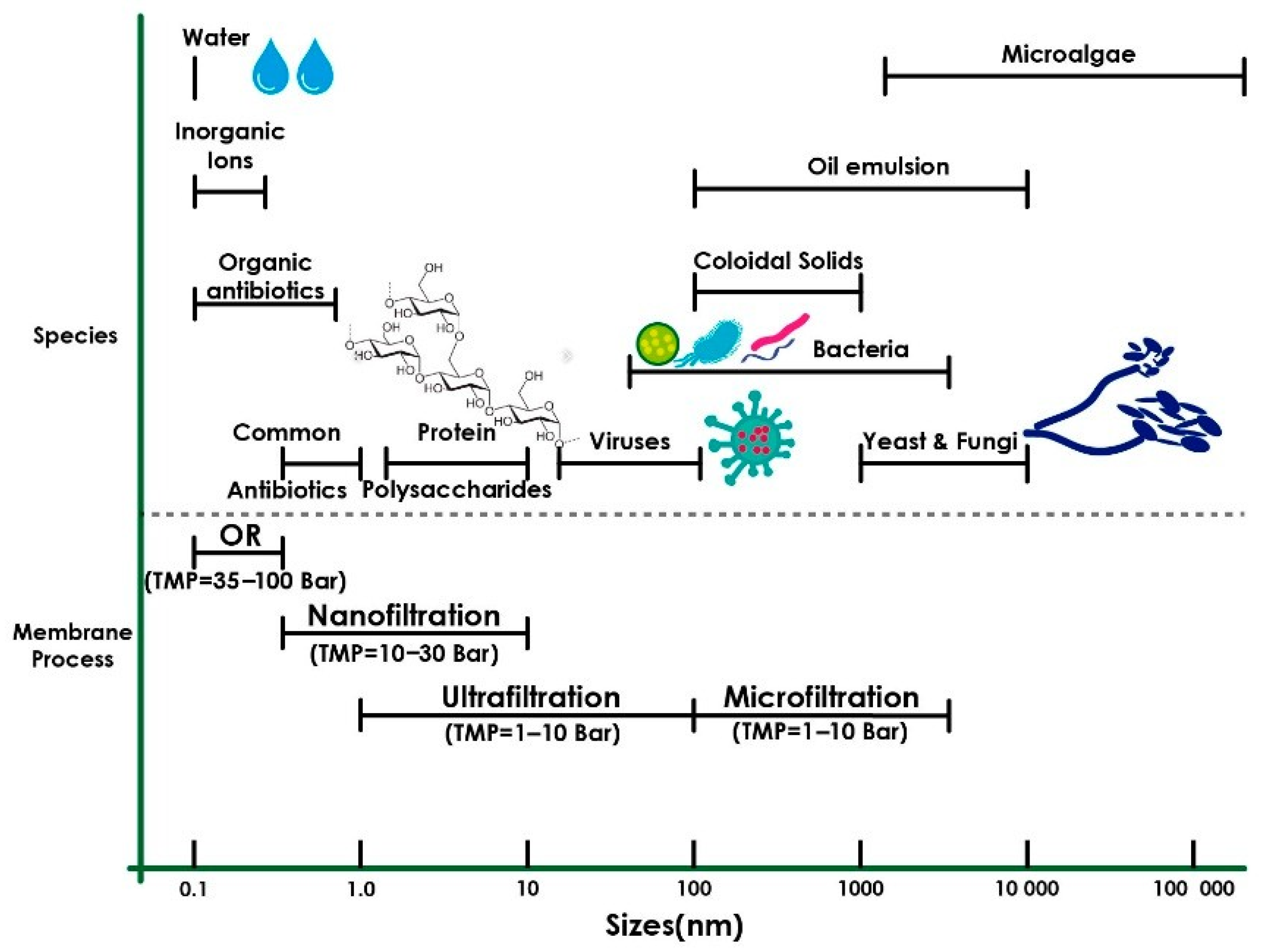
7. Membrane Preparation from PLA and PHB
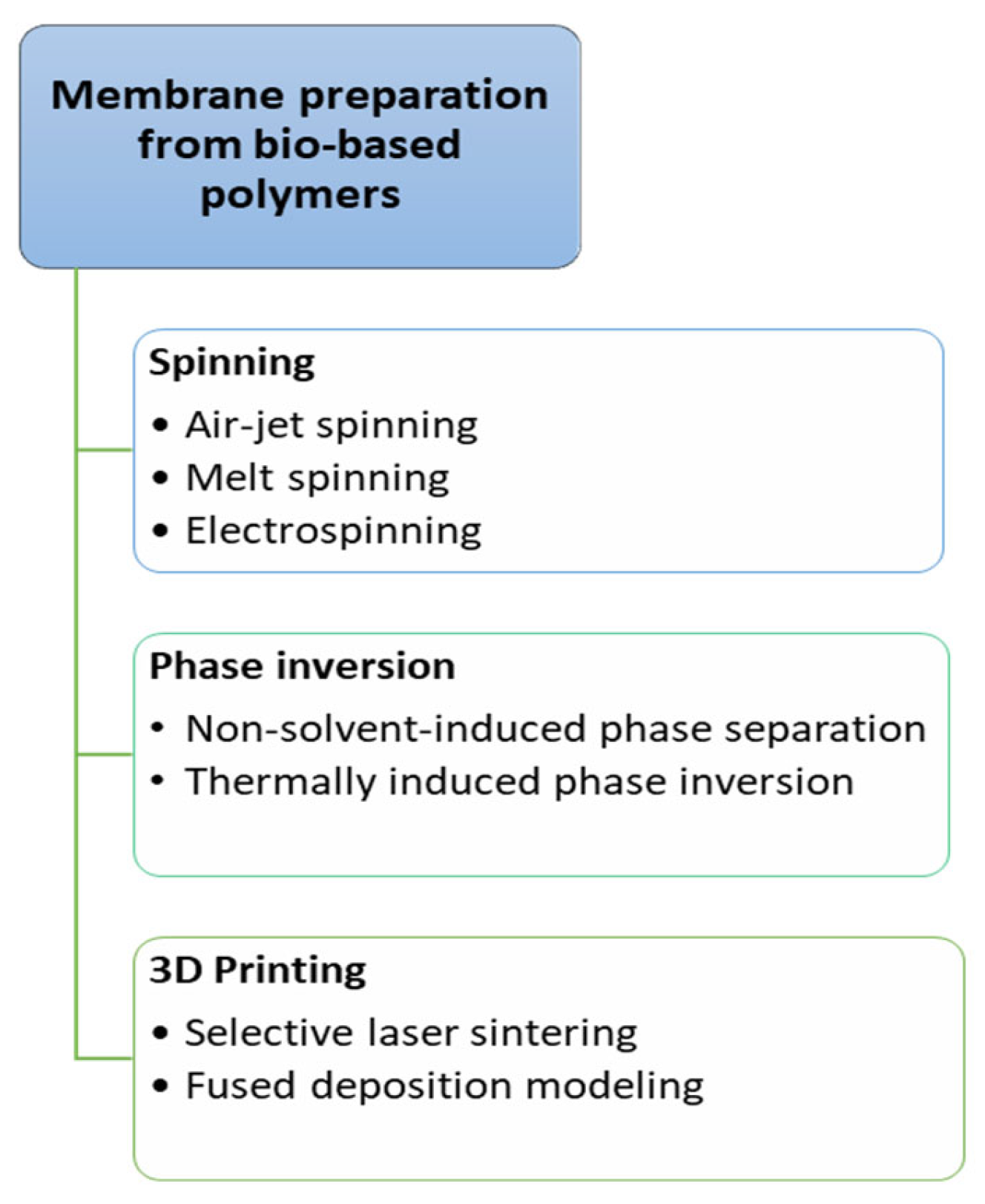
7.1. Preparation Methods of Bio-Based Polymer Membranes
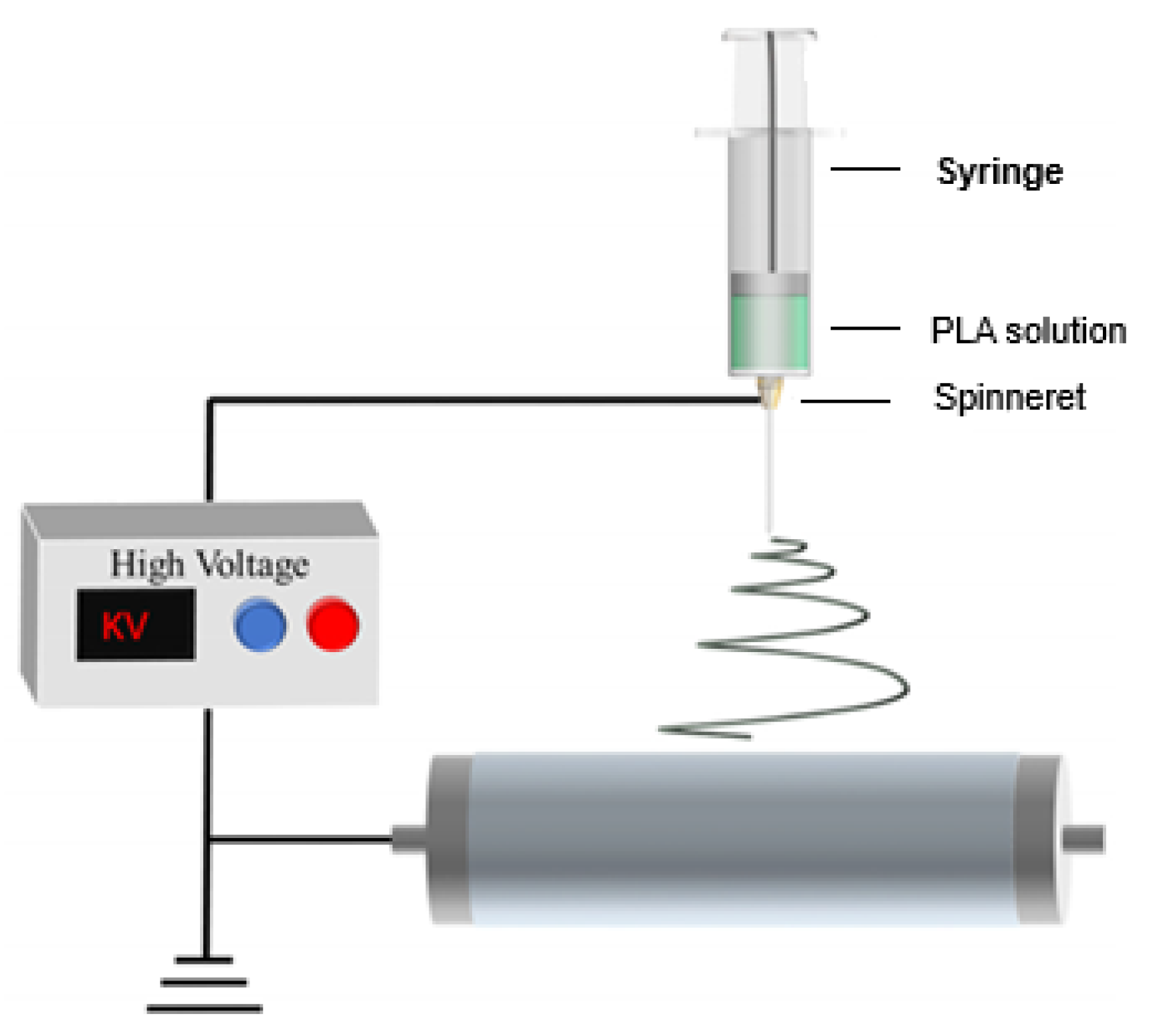
7.1.1. Spinning
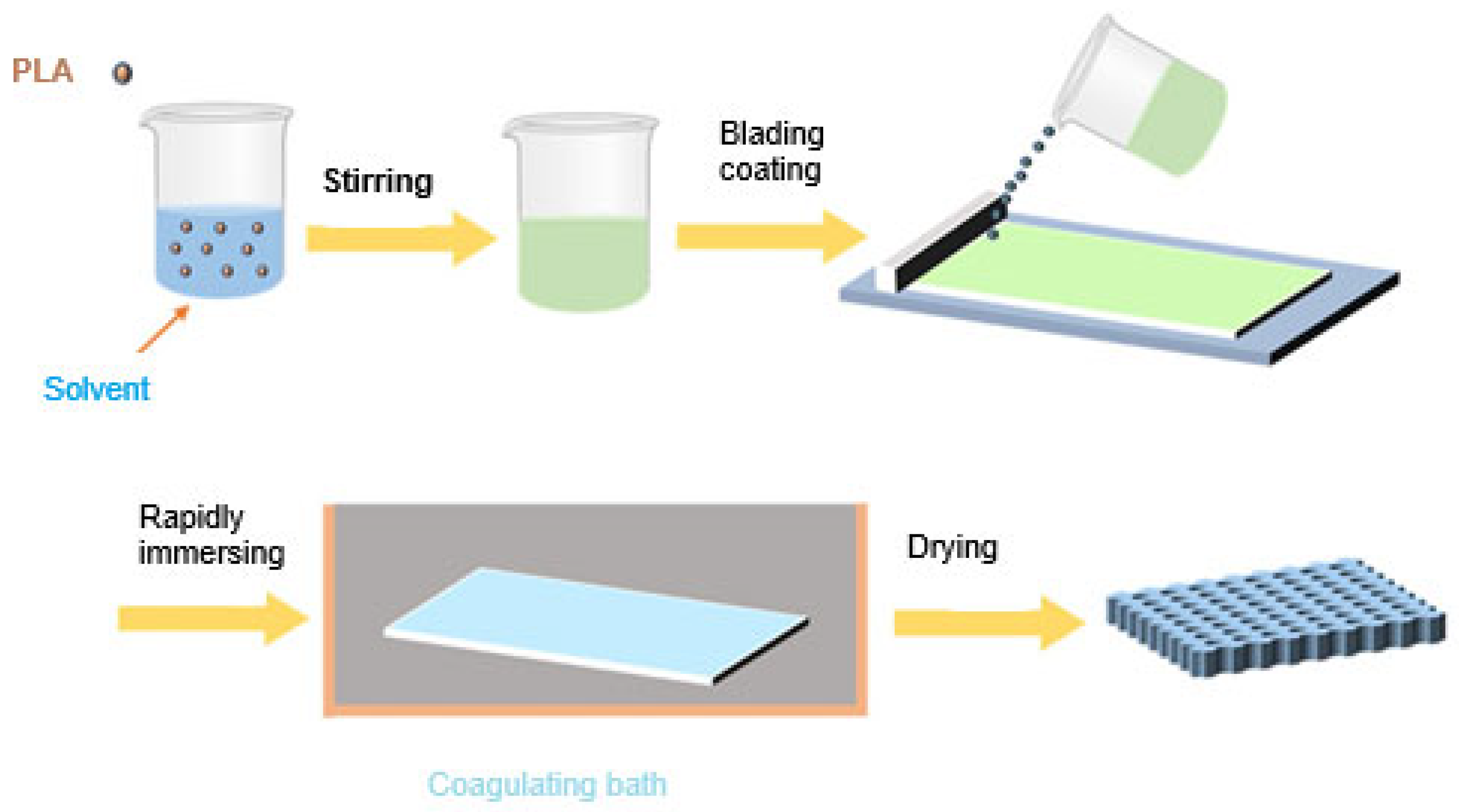
7.1.2. Phase Inversion
7.1.3. 3D Printing
7.2. Materials Used for Membrane Preparation
7.2.1. Membrane Preparation from PLA
7.2.2. Membrane Preparation from PLA–PHB Blends
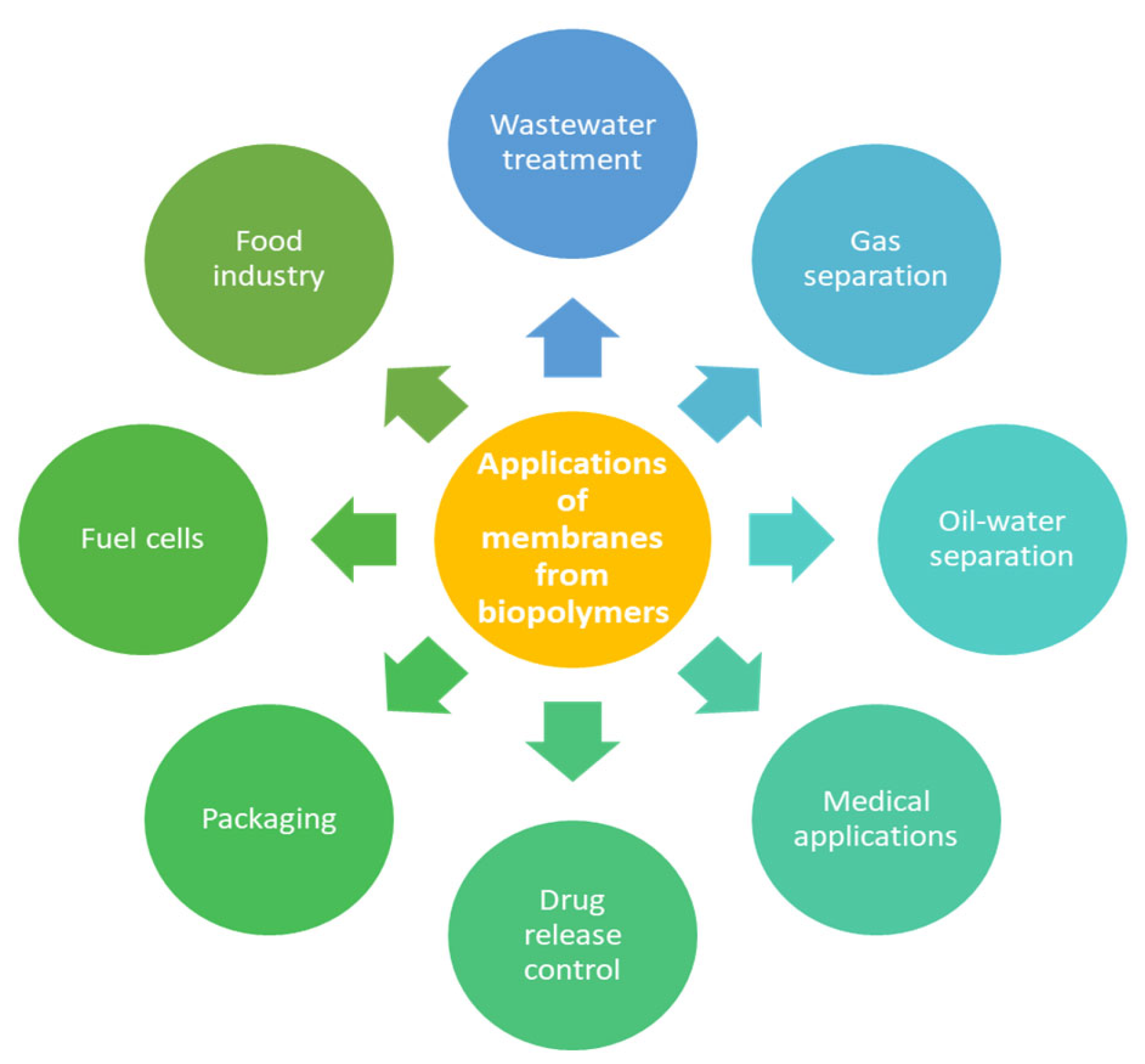
8. Applications, Advantages, and Limitations of Membranes Produced from Bio-Sourced Polymers
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PLA | Polylactic acid |
| PHB | Poly(3-hydroxybutyrate) |
| PHAs | Polyhydroxyalkanoates |
| PVC | Poly(vinyl chloride) |
| PVDF | Polyvinylidene fluoride |
| PVDF HFP | Poly(vinylidene fluoride-co-hexafluoropropylene) |
| PHV | Polyhydroxyvalerate |
| PCL | Polycaprolactone |
| CA | Cellulose acetate |
| CTA | Cellulose triacetate |
| LAB | Lactic acid bacteria |
| PDLA | Poly(D-lactic acid) |
| PLLA | Poly(L-lactic acid) |
| PDLLA | Poly(DL-lactic acid) |
| DW | Dry weight |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| TEP | Triethylene phosphate |
| ML | Methyl lactate |
| PHA | Polyhydroxyalkanoate |
| NIPS | Non-solvent-induced phase separation |
| TIPS | Thermally induced phase inversion |
| FDM | Fused deposition modeling |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| SEM | Scanning Electron Microscopy |
| XRD | X-ray diffraction |
| TGA | Thermogravimetric analysis |
| DSC | Differential Scanning Calorimetry |
| sc-PLA | Stereocomplex-type polylactide |
| scl-PHA | Short-chain-length polyhydroxyalkanoate |
| mcl-PHA | Medium-chain-length polyhydroxyalkanoate |
| lcl-PHA | Long-chain-length polyhydroxyalkanoate |
| L-LDH | L-lactate dehydrogenase |
| D-LDH | D-lactate dehydrogenase |
| CALB | Candida antarctica lipase B |
| CRL | Candida rugosa lipase |
| LA | Lactic acid |
| C/N | Carbon-to-nitrogen |
| CO2 | Carbon dioxide |
| Tm | Melting temperature |
| Tg | Glass transition temperature |
| PEO | Polyethyleneoxide |
| TEGDA | Triethylene glycol diacetate |
| ATBC | Acetyl tributyl citrate |
| SLS | Selective laser sintering |
| CHAp | Carbonated hydroxyapatite |
| ABS | Acrylonitrile butadiene styrene |
| HAp | Hydroxyapatite |
| PBS | Poly(butylene succinate |
| CNWs | Cellulose nanowhisker |
| MOF | Metal–organic framework |
| PIMs | Polymer inclusion membranes |
| GO | Graphene oxide |
| MF | Microfiltration |
| UF | Ultrafiltration |
| NF | Nanofiltration |
| RO | Reverse osmosis |
References
- Kolya, H.; Kang, C.-W. Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration. Polymers 2023, 15, 3421. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Neag, E.; Tanaselia, C.; Senila, L. Removal of Cesium and Strontium Ions from Aqueous Solutions by Thermally Treated Natural Zeolite. Materials 2023, 16, 2965. [Google Scholar] [CrossRef] [PubMed]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Senila, M. Polymer Inclusion Membranes (PIMs) for Metal Separation—Toward Environmentally Friendly Production and Applications. Polymers 2025, 17, 725. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Development Update 2024. Available online: https://www.european-bioplastics.org/market/ (accessed on 6 May 2025).
- Rogovina, S.; Zhorina, L.; Gatin, A.; Prut, E.; Kuznetsova, O.; Yakhina, A.; Olkhov, A.; Samoylov, N.; Grishin, M.; Iordanskii, A.; et al. Biodegradable Polylactide-Poly(3-Hydroxybutyrate) Compositions Obtained via Blending under Shear Deformations and Electrospinning: Characterization and Environmental Application. Polymers 2020, 12, 1088. [Google Scholar] [CrossRef]
- D’Amico, D.A.; Iglesias Montes, M.L.; Manfredi, L.B.; Cyras, V.P. Fully bio-based and biodegradable polylactic acid/poly(3-hydroxybutirate) blends: Use of a common plasticizer as performance improvement strategy. Polym. Test. 2016, 49, 22–28. [Google Scholar] [CrossRef]
- Senila, L.; Kovacs, E.; Resz, M.A.; Senila, M.; Becze, A.; Roman, C. Life Cycle Assessment (LCA) of Bioplastics Production from Lignocellulosic Waste (Study Case: PLA and PHB). Polymers 2024, 16, 3330. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Senila, L.; Gal, E.; Kovacs, E.; Cadar, O.; Dan, M.; Senila, M.; Roman, C. Poly(3-hydroxybutyrate) Production from Lignocellulosic Wastes Using Bacillus megaterium ATCC 14581. Polymers 2023, 15, 4488. [Google Scholar] [CrossRef]
- Butt, F.I.; Muhammad, N.; Hamid, A.; Moniruzzaman, M.; Sharif, F. Recent progress in the utilization of biosynthesized polyhydroxyalkanoates for biomedical applications–Review. Int. J. Biol. Macromol. 2018, 120, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. Electric Phenomenon: A Disregarded Tool in Tissue Engineering and Regenerative Medicine. Trends Biotechnol. 2020, 38, 24–49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yang, X.; Che, X.; Yang, M.; Zhai, G. Biomedical application and controlled drug release of electrospun fibrous materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly(lactide) and amorphous poly([R,S]-3-hydroxy butyrate)–morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Anuar, F.H.; Abdullah, I. The contribution of eco-friendly bio-based blends on enhancing the thermal stability and biodegradability of Poly(lactic acid). J. Clean. Prod. 2018, 198, 987–995. [Google Scholar] [CrossRef]
- Keawsupsak, K.; Jaiyu, A.; Pannoi, J.; Somwongsa, P.; Wanthausk, N.; Sueprasita, P.; Eamchotchawalit, C. Poly (lactic acid)/biodegradable polymer blend for the preparation of flat-sheet membrane. J. Teknol. 2014, 69, 99–102. [Google Scholar] [CrossRef]
- Findrik Balogova, A.; Hudak, R.; Toth, T.; Schnitzer, M.; Feranc, J.; Bakos, D.; Zivcak, J. Determination of geometrical and viscoelastic properties of PLA/PHB samples made by additive manufacturing for urethral substitution. J. Biotechnol. 2018, 284, 123–130. [Google Scholar] [CrossRef]
- Pavan, F.A.; Junqueira, T.L.; Watanabe, M.D.B.; Bonomi, A.; Quines, L.K.; Schmidell, W.; de Aragao, G.M.F. Economic analysis of polyhydroxybutyrate production by Cupriavidus necator using different routes for product recovery. Biochem. Eng. J. 2019, 146, 97–104. [Google Scholar] [CrossRef]
- Okolie, O.; Kumar, A.; Edwards, C.; Lawton, L.A.; Oke, A.; McDonald, S.; Thakur, V.K.; Njuguna, J. Bio-Based Sustainable Polymers and Materials: From Processing to Biodegradation. J. Compos. Sci. 2023, 7, 213. [Google Scholar] [CrossRef]
- Clarivate. Available online: https://www.webofscience.com/ (accessed on 3 July 2025).
- Kim, J.F.; Thi, H.Y.N. Biodegradable Synthetic Polymers. In Encyclopedia of Green Chemistry, 1st ed.; Török, B., Ed.; Elsevier: Oxford, UK, 2025; pp. 131–143. [Google Scholar]
- Lestido-Cardama, A.; Barbosa-Pereira, L.; Sendon, R.; Bustos, J.; Paseiro Losada, P.; Rodriguez Bernaldo de Quiros, A. Chemical safety and risk assessment of bio-based and/or biodegradable polymers for food contact: A review. Food Res. Int. 2025, 202, 115737. [Google Scholar] [CrossRef] [PubMed]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic acid (PLA): Properties, synthesis, and biomedical applications—A review of the literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Rahmayetty; Whulanza, Y.; Sukirno; Rahman, S.F.; Suyono, E.A.; Yohda, M.; Gozan, M. Use of Candida rugosa lipase as a biocatalyst for L-lactide ring-opening polymerization and polylactic acid production. Biocatal. Agric. Biotechnol. 2018, 16, 683–691. [Google Scholar] [CrossRef]
- de Albuquerque, T.L.; Marques Júnior, J.E.; de Queiroz, L.P.; Ricardo, A.D.S.; Rocha, M.V.P. Polylactic acid production from biotechnological routes: A review. Int. J. Biol. Macromol. 2021, 186, 933–951. [Google Scholar] [CrossRef]
- Marques Junior, J.E.; de Queiroz, L.P.; de Albuquerque, T.L.; de Souza Zampieri, D.; Melo, V.M.M.; Rocha, M.V.P. Lactic acid production from cashew apple bagasse, an agro-industrial waste, and its application in the enzymatic synthesis of polylactic acid. Biocatal. Agric. Biotechnol. 2024, 56, 102987. [Google Scholar] [CrossRef]
- Mou, L.; Li, J.; Lu, Y.; Li, G.; Li, J. Polylactic acid: A future universal biobased polymer with multifunctional performance—From monomer synthesis, and processing to applications: A review. J. Hazard. Mater. Adv. 2025, 18, 100757. [Google Scholar] [CrossRef]
- Tan, C.; Tao, F.; Xu, P. Direct carbon capture for the production of high-performance biodegradable plastics by cyanobacterial cell factories. Green Chem. 2022, 24, 4470–4483. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Kim, C.-H.; Ryu, M.; Park, J.; Kim, T.-K.; Yoo, Y.; Park, H.; Lee, J.-H.; Cho, Y.H. Exploring green solvents for the sustainable fabrication of bio-based polylactic acid membranes using nonsolvent-induced phase separation. J. Clean. Prod. 2024, 467, 142905. [Google Scholar] [CrossRef]
- Yashavanth, P.R.; Das, M.; Maiti, S.K. Recent progress and challenges in cyanobacterial autotrophic production of polyhydroxybutyrate (PHB), a bioplastic. J. Environ. Chem. Eng. 2021, 9, 105379. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Ng, I.S. Biofabrication of polyhydroxybutyrate (PHB) in engineered Cupriavidus necator H16 from waste molasses. J. Taiwan Inst. Chem. Eng. 2025, 167, 105843. [Google Scholar] [CrossRef]
- Kranert, L.; Kok, R.; Neumann, A.S.; Kienle, A.; Duvigneau, S. Estimation of PHA concentrations from cell density data in Cupriavidus necator. Appl. Microbiol. Biotechnol. 2025, 109, 11. [Google Scholar] [CrossRef] [PubMed]
- Fink, P.; Menzel, C.; Kwon, J.H.; Forchhammer, K. A novel recombinant PHB production platform in filamentous cyanobacteria avoiding nitrogen starvation while preserving cell viability. Microb. Cell Fact. 2025, 24, 43. [Google Scholar] [CrossRef]
- Russo, G.; Scocca, P.; Gelosia, M.; Fabbrizi, G.; Giannoni, T.; Urbani, S.; Esposto, S.; Nicolini, A. Poly(3-hydroxybutyrate) production for food packaging from biomass derived carbohydrates by cupriavidus necator DSM 545. Enzym. Microb. Technol. 2024, 181, 110516. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wang, R.; Yan, X.; Wang, J.; Wang, X.; Chen, S.; Bai, F.; He, Q.; Yang, S. Metabolic engineering ofZymomonas mobilisfor continuous co-production of bioethanol and poly-3-hydroxybutyrate (PHB). Green Chem. 2022, 24, 2588–2601. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Tang, X.; Thankappan, S.K.; Lee, P.; Fard, S.E.; Harmon, M.D.; Tran, K.; Yu, X. Chapter 21—Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 351–371. [Google Scholar]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. 4—Chemical Properties of Poly(lactic Acid). In Polylactic Acid; Sin, L.T., Rahmat, A.R., Rahman, W.A.W.A., Eds.; William Andrew Publishing: Oxford, UK, 2013; pp. 143–176. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications —A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef]
- Roohi; Zaheer, M.R.; Kuddus, M. PHB (poly-β-hydroxybutyrate) and its enzymatic degradation. Polym. Adv. Technol. 2017, 29, 30–40. [Google Scholar] [CrossRef]
- Domenek, S.; Ducruet, V. Characteristics and Applications of PLA. In Biodegradable and Biobased Polymers for Environmental and Biomedical Applications; Scrivener Publishing LLC.: Beverly, MA, USA, 2016; pp. 171–224. [Google Scholar]
- Rajan, K.P.; Thomas, S.P.; Gopanna, A.; Chavali, M. Polyhydroxybutyrate (PHB): A Standout Biopolymer for Environmental Sustainability. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–23. [Google Scholar]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Chapter 1—Polymer Synthesis and Processing. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 1–31. [Google Scholar]
- Zainuddin, M.Z.; Abu Bakar, A.A.; Adam, A.N.; Abdullah, S.M.; Tamchek, N.; Alauddin, M.S.; Mahat, M.M.; Wiwatcharagoses, N.; Alforidi, A.; Ghazali, M.I. Mechanical and Structural Properties of Polyhydroxybutyrate as Additive in Blend Material in Additive Manufacturing for Medical Applications. Polymers 2023, 15, 1849. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Ramezani Dana, H. Poly lactic acid (PLA) polymers: From properties to biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 1117–1130. [Google Scholar] [CrossRef]
- Avérous, L. 9—Synthesis, Properties, Environmental and Biomedical Applications of Polylactic Acid. In Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 171–188. [Google Scholar]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Kervran, M.; Vagner, C.; Cochez, M.; Ponçot, M.; Saeb, M.R.; Vahabi, H. Thermal degradation of polylactic acid (PLA)/polyhydroxybutyrate (PHB) blends: A systematic review. Polym. Degrad. Stab. 2022, 201, 109995. [Google Scholar] [CrossRef]
- Hong, S.-G.; Hsu, H.-W.; Ye, M.-T. Thermal properties and applications of low molecular weight polyhydroxybutyrate. J. Therm. Anal. Calorim. 2013, 111, 1243–1250. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.J.; Moon, M.; Lee, J.-S.; Min, K. Recent progress and challenges in microbial polyhydroxybutyrate (PHB) production from CO2 as a sustainable feedstock: A state-of-the-art review. Bioresour. Technol. 2021, 339, 125616. [Google Scholar] [CrossRef] [PubMed]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Ran, X.; Nie, W.; Semeniuk, I.; Koretska, N. Synthesis, Structure, and Properties of Polyhydroxybutyrate Derived from Azotobacter Vinelandii N-15. ChemistryOpen 2025, e2500150. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Lawley, M.D.; Boon, D.; Stein, L.Y.; Sauvageau, D. Switchable Solvents for the Reversible Dissolution of Poly(3-hydroxybutyrate). ACS Sustain. Chem. Eng. 2022, 10, 2602–2608. [Google Scholar] [CrossRef]
- Chaber, P.; Kwiecień, M.; Zięba, M.; Sobota, M.; Adamus, G. The heterogeneous selective reduction of PHB as a useful method for preparation of oligodiols and surface modification. RSC Adv. 2017, 7, 35096–35104. [Google Scholar] [CrossRef]
- Vaid, R.; Yildirim, E.; Pasquinelli, M.A.; King, M.W. Hydrolytic Degradation of Polylactic Acid Fibers as a Function of pH and Exposure Time. Molecules 2021, 26, 7554. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.L.; Yu, J. Abiotic Hydrolysis of Poly[(R)-3-hydroxybutyrate] in Acidic and Alkaline Media. Macromol. Symp. 2005, 224, 35–46. [Google Scholar] [CrossRef]
- Tapadiya, A.; Vasanthan, N. Crystallization and alkaline hydrolysis of poly(3-hydroxybutyrate) films probed by thermal analysis and infrared spectroscopy. Int. J. Biol. Macromol. 2017, 102, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ma, H.-L.; Du, Z.-H.; Yang, B.; Wu, J.; Wang, R.; Zhang, X.-Q. Hydrophilic and Antibacterial Modification of Poly(lactic acid) Films by γ-ray Irradiation. ACS Omega 2019, 4, 21439–21445. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Liu, S. The production, recovery, and valorization of polyhydroxybutyrate (PHB) based on circular bioeconomy. Biotechnol. Adv. 2024, 72, 108340. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Akoulina, E.A.; Chesnokova, D.V.; Wenhao, Y.; Makhina, T.K.; Demyanova, I.V.; Zhuikova, Y.V.; Voinova, V.V.; Belishev, N.V.; Surmenev, R.A.; et al. The Growth of 3T3 Fibroblasts on PHB, PLA and PHB/PLA Blend Films at Different Stages of Their Biodegradation In Vitro. Polymers 2020, 13, 108. [Google Scholar] [CrossRef]
- Attallah, O.A.; Mojicevic, M.; Garcia, E.L.; Azeem, M.; Chen, Y.; Asmawi, S.; Brenan Fournet, M. Macro and Micro Routes to High Performance Bioplastics: Bioplastic Biodegradability and Mechanical and Barrier Properties. Polymers 2021, 13, 2155. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Passanha, P.; Esteves, S.; Schönherr, H.; Jhurry, D. Biomineralization potential and cellular response of PHB and PHBV blends with natural anionic polysaccharides. Mater. Sci. Eng. C 2017, 76, 13–24. [Google Scholar] [CrossRef]
- Akdoğan, E.; Şirin, H.T.; Şahal, G.; Deniz, Z.; Kaya, A.; Serdaroğlu, D.Ç. Accelerating the environmental biodegradation of poly-3-hydroxybutyrate (PHB) via plasma surface treatment. Bioresour. Technol. Rep. 2023, 25, 101719. [Google Scholar] [CrossRef]
- Arrieta, M.; Samper, M.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- More, N.; Avhad, M.; Utekar, S.; More, A. Polylactic acid (PLA) membrane—Significance, synthesis, and applications: A review. Polym. Bull. 2022, 80, 1117–1153. [Google Scholar] [CrossRef]
- Vatanpour, V.; Dehqan, A.; Paziresh, S.; Zinadini, S.; Zinatizadeh, A.A.; Koyuncu, I. Polylactic acid in the fabrication of separation membranes: A review. Sep. Purif. Technol. 2022, 296, 121433. [Google Scholar] [CrossRef]
- Xix-Rodriguez, C.; Varguez-Catzim, P.; Alonzo-García, A.; Rodriguez-Fuentes, N.; Vázquez-Torres, H.; González-Diaz, A.; Aguilar-Vega, M.; González-Díaz, M.O. Amphiphilic poly(lactic acid) membranes with low fouling and enhanced hemodiafiltration. Sep. Purif. Technol. 2021, 259, 118124. [Google Scholar] [CrossRef]
- Ehsani, M.; Kalugin, D.; Doan, H.; Lohi, A.; Abdelrasoul, A. Bio-Sourced and Biodegradable Membranes. Appl. Sci. 2022, 12, 12837. [Google Scholar] [CrossRef]
- Granados-Hernandez, M.V.; Serrano-Bello, J.; Montesinos, J.J.; Alvarez-Gayosso, C.; Medina-Velazquez, L.A.; Alvarez-Fregoso, O.; Alvarez-Perez, M.A. In vitro and in vivo biological characterization of poly(lactic acid) fiber scaffolds synthesized by air jet spinning. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2435–2446. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Sheikh, F.A.; Lim, J.K. Air jet spinning of hydroxyapatite/poly(lactic acid) hybrid nanocomposite membrane mats for bone tissue engineering. Colloids Surf. B Biointerfaces 2013, 102, 635–643. [Google Scholar] [CrossRef]
- An Tran, N.H.; Brünig, H.; Hinüber, C.; Heinrich, G. Melt Spinning of Biodegradable Nanofibrillary Structures from Poly(lactic acid) and Poly(vinyl alcohol) Blends. Macromol. Mater. Eng. 2013, 299, 219–227. [Google Scholar] [CrossRef]
- Fryczkowski, R.; Fryczkowska, B.; Biniaś, W.; Janicki, J. Morphology of fibrous composites of PLA and PVDF. Compos. Sci. Technol. 2013, 89, 186–193. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, X.; Dong, Z.; Wang, B.; Jin, X.; Chen, Y.; Cui, M.; Wang, R.; Zhang, X. Regulation of polylactic acid using irradiation and preparation of PLA–SiO2–ZnO melt-blown nonwovens for antibacterial and air filtration. RSC Adv. 2023, 13, 7857–7866. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, M.; Ye, X.; Wei, S.; Huang, L.; Guo, H. High-Efficiency and Low-Resistance Melt-Blown/Electrospun PLA Composites for Air Filtration. Polymers 2025, 17, 424. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, X.; Pu, X.; Shen, Z.; Xu, W.; Yang, J. Preparation Method and Application of Porous Poly(lactic acid) Membranes: A Review. Polymers 2024, 16, 1846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.; Yan, L.; Mei, X.; Hou, Z. Combining emulsion electrospinning with surface functionalization to fabricate multistructural PLA/CS@ZIF-8 nanofiber membranes toward pH-responsive dual drug delivery. Int. J. Biol. Macromol. 2023, 253, 126506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ming, J.; Cai, S.; Wang, X.; Ning, X. One-step fabrication of polylactic acid (PLA) nanofibrous membranes with spider-web-like structure for high-efficiency PM0.3 capture. J. Hazard. Mater. 2023, 465, 133232. [Google Scholar] [CrossRef] [PubMed]
- Kahrs, C.; Schwellenbach, J. Membrane formation via non-solvent induced phase separation using sustainable solvents: A comparative study. Polymer 2020, 186, 122071. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Cano-Vicent, A.; Tambuwala, M.M.; Hassan, S.S.; Barh, D.; Aljabali, A.A.A.; Birkett, M.; Arjunan, A.; Serrano-Aroca, Á. Fused deposition modelling: Current status, methodology, applications and future prospects. Addit. Manuf. 2021, 47, 102378. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Jiang, H.B.; Ryu, J.-H.; Kang, H.; Kim, K.-M.; Kwon, J.-S. Comparing Properties of Variable Pore-Sized 3D-Printed PLA Membrane with Conventional PLA Membrane for Guided Bone/Tissue Regeneration. Materials 2019, 12, 1718. [Google Scholar] [CrossRef]
- Choi, W.J.; Hwang, K.S.; Kwon, H.J.; Lee, C.; Kim, C.H.; Kim, T.H.; Heo, S.W.; Kim, J.-H.; Lee, J.-Y. Rapid development of dual porous poly(lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater. Sci. Eng. C 2020, 110, 110693. [Google Scholar] [CrossRef]
- Yehia, H.M.; Hamada, A.; Sebaey, T.A.; Abd-Elaziem, W. Selective Laser Sintering of Polymers: Process Parameters, Machine Learning Approaches, and Future Directions. J. Manuf. Mater. Process. 2024, 8, 197. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, D.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Castro, N.J.; Lee, S.-J.; Noh, H.; Zhu, W.; Zhang, L.G. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale 2017, 9, 5055–5062. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, N.D.; Koumentakou, I.; Samiotaki, C.; Meimaroglou, D.; Varytimidou, D.; Karatza, A.; Kalantzis, Z.; Roussou, M.; Bikiaris, R.D.; Papageorgiou, G.Z. Recent Advances in the Investigation of Poly(lactic acid) (PLA) Nanocomposites: Incorporation of Various Nanofillers and their Properties and Applications. Polymers 2023, 15, 1196. [Google Scholar] [CrossRef]
- Ramezani Dana, H.; Ebrahimi, F. Synthesis, properties, and applications of polylactic acid-based polymers. Polym. Eng. Sci. 2022, 63, 22–43. [Google Scholar] [CrossRef]
- Ferreira, P.S.; Ribeiro, S.M.; Pontes, R.; Nunes, J. Production methods and applications of bioactive polylactic acid: A review. Environ. Chem. Lett. 2024, 22, 1831–1859. [Google Scholar] [CrossRef]
- Luo, N.; Stewart, M.J.; Hirt, D.E.; Husson, S.M.; Schwark, D.W. Surface modification of ethylene-co-acrylic acid copolymer films: Addition of amide groups by covalently bonded amino acid intermediates. J. Appl. Polym. Sci. 2004, 92, 1688–1694. [Google Scholar] [CrossRef]
- Wang, S.; Cui, W.; Bei, J. Bulk and surface modifications of polylactide. Anal. Bioanal. Chem. 2005, 381, 547–556. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Y.; Qin, S.; Zhang, B.; Luo, Y. Grafting reaction of poly(D,L)lactic acid with maleic anhydride and hexanediamine to introduce more reactive groups in its bulk. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74B, 476–480. [Google Scholar] [CrossRef]
- Tejada-Oliveros, R.; Fiori, S.; Gomez-Caturla, J.; Lascano, D.; Montanes, N.; Quiles-Carrillo, L.; Garcia-Sanoguera, D. Development and Characterization of Polylactide Blends with Improved Toughness by Reactive Extrusion with Lactic Acid Oligomers. Polymers 2022, 14, 1874. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- de Kort, G.W.; Bouvrie, L.H.C.; Rastogi, S.; Wilsens, C.H.R.M. Thermoplastic PLA-LCP Composites: A Route toward Sustainable, Reprocessable, and Recyclable Reinforced Materials. ACS Sustain. Chem. Eng. 2019, 8, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Darie-Niță, R.N.; Irimia, A.; Doroftei, F.; Stefan, L.M.; Iwanczuk, A.; Trusz, A. Bioactive and Physico-Chemical Assessment of Innovative Poly(lactic acid)-Based Biocomposites Containing Sage, Coconut Oil, and Modified Nanoclay. Int. J. Mol. Sci. 2023, 24, 3646. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.H.; de Nóbile Pires, L.; Selistre-de-Araujo, H.S.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Development and characterization of printable PLA/β-TCP bioactive composites for bone tissue applications. J. Appl. Polym. Sci. 2020, 138, 49759. [Google Scholar] [CrossRef]
- Mukaffa, H.; Asrofi, M.; Sujito; Asnawi; Hermawan, Y.; Sumarji; Qoryah, R.D.H.; Sapuan, S.M.; Ilyas, R.A.; Atiqah, A. Effect of alkali treatment of piper betle fiber on tensile properties as biocomposite based polylactic acid: Solvent cast-film method. Mater. Today Proc. 2022, 48, 761–765. [Google Scholar] [CrossRef]
- Stoll, L.; Rech, R.; Flôres, S.H.; Nachtigall, S.M.B.; de Oliveira Rios, A. Poly(acid lactic) films with carotenoids extracts: Release study and effect on sunflower oil preservation. Food Chem. 2019, 281, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Qasim, U.; Osman, A.I.; Al-Muhtaseb, A.H.; Farrell, C.; Al-Abri, M.; Ali, M.; Vo, D.-V.N.; Jamil, F.; Rooney, D.W. Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: A review. Environ. Chem. Lett. 2020, 19, 613–641. [Google Scholar] [CrossRef]
- Abdulkareem, A.; Kasak, P.; Nassr, M.G.; Mahmoud, A.A.; Al-Ruweidi, M.K.A.A.; Mohamoud, K.J.; Hussein, M.K.; Popelka, A. Surface Modification of Poly(lactic acid) Film via Cold Plasma Assisted Grafting of Fumaric and Ascorbic Acid. Polymers 2021, 13, 3717. [Google Scholar] [CrossRef]
- Nakagawa, M.; Teraoka, F.; Fujimoto, S.; Hamada, Y.; Kibayashi, H.; Takahashi, J. Improvement of cell adhesion on poly(L-lactide) by atmospheric plasma treatment. J. Biomed. Mater. Res. Part A 2006, 77A, 112–118. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Shockravi, A.; Vatanpour, V. High performance compatible thiazole-based polymeric blend cellulose acetate membrane as selective CO2 absorbent and molecular sieve. Carbohydr. Polym. 2021, 252, 117215. [Google Scholar] [CrossRef]
- Filippova, E.O.; Zhuravleva, A.D.; Gorbunova, E.A.; Ivanova, N.M. The influence of implantation of plasma-modified polylactic acid films on the structure of the cornea. AIP Conf. Proc. 2020, 2310, 020097. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Li, Y.; Luo, C.; Yang, C.; Shi, W.; Li, L. Covalent Immobilization of Polypeptides on Polylactic Acid Films and Their Application to Fresh Beef Preservation. J. Agric. Food Chem. 2020, 68, 10532–10541. [Google Scholar] [CrossRef] [PubMed]
- Quirk, R.A.; Davies, M.C.; Tendler, S.J.B.; Shakesheff, K.M. Surface Engineering of Poly(lactic acid) by Entrapment of Modifying Species. Macromolecules 2000, 33, 258–260. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, J.; Lin, R.; Gao, C.; Feng, L.; Shen, J. Surface engineering of poly(dl-lactic acid) by entrapment of alginate-amino acid derivatives for promotion of chondrogenesis. Biomaterials 2002, 23, 3141–3148. [Google Scholar] [CrossRef]
- Wang, C.; Mao, L.; Yao, J.; Zhu, H. Improving the active food packaging function of poly(lactic acid) film coated by poly(vinyl alcohol) based on proanthocyanidin functionalized layered clay. LWT 2023, 174, 114407. [Google Scholar] [CrossRef]
- Danafar, H.; Rostamizadeh, K.; Davaran, S.; Hamidi, M. Drug-conjugated PLA–PEG–PLA copolymers: A novel approach for controlled delivery of hydrophilic drugs by micelle formation. Pharm. Dev. Technol. 2016, 22, 947–957. [Google Scholar] [CrossRef]
- Hazarika, D.; Kumar, A.; Katiyar, V. Structural evolution of in situ polymerized poly(L-lactic acid) nanocomposite for smart textile application. Sci. Rep. 2022, 12, 14724. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Nazeer, M.A.; Onder, O.C.; Sevgili, I.; Yilgor, E.; Kavakli, I.H.; Yilgor, I. 3D printed poly(lactic acid) scaffolds modified with chitosan and hydroxyapatite for bone repair applications. Mater. Today Commun. 2020, 25, 101515. [Google Scholar] [CrossRef]
- Saniei, H.; Mousavi, S. Surface modification of PLA 3D-printed implants by electrospinning with enhanced bioactivity and cell affinity. Polymer 2020, 196, 122467. [Google Scholar] [CrossRef]
- Imani, F.; Karimi-Soflou, R.; Shabani, I.; Karkhaneh, A. PLA electrospun nanofibers modified with polypyrrole-grafted gelatin as bioactive electroconductive scaffold. Polymer 2021, 218, 123487. [Google Scholar] [CrossRef]
- Canales, D.A.; Reyes, F.; Saavedra, M.; Peponi, L.; Leonés, A.; Palza, H.; Boccaccini, A.R.; Grünewald, A.; Zapata, P.A. Electrospun fibers of poly (lactic acid) containing bioactive glass and magnesium oxide nanoparticles for bone tissue regeneration. Int. J. Biol. Macromol. 2022, 210, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Ma, P.; Spoelstra, A.B.; Schmit, P.; Lemstra, P.J. Toughening of poly (lactic acid) by poly (β-hydroxybutyrate-co-β-hydroxyvalerate) with high β-hydroxyvalerate content. Eur. Polym. J. 2013, 49, 1523–1531. [Google Scholar] [CrossRef]
- Jasek, V.; Fucik, J.; Ivanova, L.; Vesely, D.; Figalla, S.; Mravcova, L.; Sedlacek, P.; Krajcovic, J.; Prikryl, R. High-Pressure Depolymerization of Poly(lactic acid) (PLA) and Poly(3-hydroxybutyrate) (PHB) Using Bio-Based Solvents: A Way to Produce Alkyl Esters Which Can Be Modified to Polymerizable Monomers. Polymers 2022, 14, 5236. [Google Scholar] [CrossRef] [PubMed]
- Khajavian, M.; Salehi, E.; Vatanpour, V. Chitosan/polyvinyl alcohol thin membrane adsorbents modified with zeolitic imidazolate framework (ZIF-8) nanostructures: Batch adsorption and optimization. Sep. Purif. Technol. 2020, 241, 116759. [Google Scholar] [CrossRef]
- Shokri, E.; Khanghahi, B.; Esmizadeh, E.; Etemadi, H. Biopolymer-based adsorptive membrane for simultaneous removal of cationic and anionic heavy metals from water. Int. J. Environ. Sci. Technol. 2021, 19, 4167–4180. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Lu, Z.; Khawar, M.T.; Song, J.; Gong, H.; Meng, J.; Li, Z.; Li, J. Porous poly(L-lactic acid)/chitosan nanofibres for copper ion adsorption. Carbohydr. Polym. 2020, 227, 115343. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Meng, J.; Xin, Z.; Gong, H.; Li, J. Polydopamine-assisted grafting of chitosan on porous poly (L-lactic acid) electrospun membranes for adsorption of heavy metal ions. Int. J. Biol. Macromol. 2021, 167, 1479–1490. [Google Scholar] [CrossRef]
- Mohammad, N.; Atassi, Y. TiO2/PLLA Electrospun Nanofibers Membranes for Efficient Removal of Methylene Blue Using Sunlight. J. Polym. Environ. 2020, 29, 509–519. [Google Scholar] [CrossRef]
- Modolon, H.B.; Teixeira, L.B.; Mazur, L.P.; Santos, P.H.; Camani, P.H.; Mei, L.H.I.; Wermuth, T.B.; Montedo, O.R.K.; Zimmermann, M.V.G.; Arcaro, S.; et al. Electrospun adsorbent membrane of PLA containing chitosan for toxic metal ions removal from aqueous solution: Effect of chitosan incorporation. Int. J. Biol. Macromol. 2025, 297, 139435. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Nasef, M.M.; Fouad, H.; Karim, Z. Poly(lactic acid)/poly(butylene succinate) dual-layer membranes with cellulose nanowhisker for heavy metal ion separation. Int. J. Biol. Macromol. 2021, 192, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Prazeres Mazur, L.; Reis Ferreira, R.; Felix da Silva Barbosa, R.; Henrique Santos, P.; Barcelos da Costa, T.; Gurgel Adeodato Vieira, M.; da Silva, A.; dos Santos Rosa, D.; Helena Innocentini Mei, L. Development of novel biopolymer membranes by electrospinning as potential adsorbents for toxic metal ions removal from aqueous solution. J. Mol. Liq. 2024, 395, 123782. [Google Scholar] [CrossRef]
- Mokoena, L.S.; Mofokeng, J.P. Preparation of poly(lactic acid) (PLA)/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/graphene oxide (GO) polymeric composites for the selective removal of lead ions (Pb(II)) in water. Polym. Compos. 2024, 45, 8527–8542. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Tan, B.; Bektaş, T.E.; Karakoca, B. A comparative study on removal of boron via pervaporation and vacuum membrane distillation using zirconium metal–organic framework-loaded poly(lactic acid) membrane. Appl. Water Sci. 2025, 15, 94. [Google Scholar] [CrossRef]
- Bożejewicz, D.; Witt, K.; Kaczorowska, M.A. Influence of the type of polymer and plasticizer on the properties and efficiency of membranes containing acetylacetone carrier for the removal of Cd(II) ions from aqueous solutions. Desalination Water Treat. 2023, 316, 483–492. [Google Scholar] [CrossRef]
- Hammadi, M.H.; Kerakra, S.; Bey, S.; Sellami, F.; Djermoune, A.; Habi, A. Advancements in Cr(VI) Removal from Aqueous Solution Using PLA/PBAT/GO/Cloisite 30b Hybrid Nanocomposite Polymer Inclusion Membranes. Water Air Soil Pollut. 2024, 235, 730. [Google Scholar] [CrossRef]
- Matei, E.; Covaliu, C.I.; Coman, G.; Negroiu, M.; Rapa, M.; Predescu, A.-M.; Berbecaru, A.-C.; Predescu, C. Nonwoven Bio-Based Membranes for Removal of Micropollutants from Aqueous Water. Mater. Plast. 2021, 57, 34–44. [Google Scholar] [CrossRef]
- Cairone, S.; Hegab, H.M.; Khalil, H.; Nassar, L.; Wadi, V.S.; Naddeo, V.; Hasan, S.W. Novel eco-friendly polylactic acid nanocomposite integrated membrane system for sustainable wastewater treatment: Performance evaluation and antifouling analysis. Sci. Total Environ. 2023, 912, 168715. [Google Scholar] [CrossRef]
- Morales-Jimenez, M.; Palacio, D.A.; Palencia, M.; Melendrez, M.F.; Rivas, B.L. Bio-Based Polymeric Membranes: Development and Environmental Applications. Membranes 2023, 13, 625. [Google Scholar] [CrossRef]
- Lehermeier, H.J.; Dorgan, J.R.; Way, J.D. Gas permeation properties of poly(lactic acid). J. Membr. Sci. 2001, 190, 243–251. [Google Scholar] [CrossRef]
- Iulianelli, A.; Algieri, C.; Donato, L.; Garofalo, A.; Galiano, F.; Bagnato, G.; Basile, A.; Figoli, A. New PEEK-WC and PLA membranes for H2 separation. Int. J. Hydrogen Energy 2017, 42, 22138–22148. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Wang, X.; Duan, Z.; Lu, P.; Li, S.; Ji, D.; Wang, Z.; Li, G.; Yu, D.; et al. High-hydrophobic ZIF-8@PLA composite aerogel and application for oil-water separation. Sep. Purif. Technol. 2021, 270, 118794. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, Y.; Zheng, W.; Yu, H.; Liu, Y.; Xu, L. Asymmetric Sc-PLA Membrane with Multi-scale Microstructures: Wettability, Antifouling, and Oil-Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 55520–55526. [Google Scholar] [CrossRef]
- Jain, A.; Kunduru, K.R.; Basu, A.; Mizrahi, B.; Domb, A.J.; Khan, W. Injectable formulations of poly(lactic acid) and its copolymers in clinical use. Adv. Drug Deliv. Rev. 2016, 107, 213–227. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Salah Eldin, R.E.; Abu Serea, E.S.; Gomaa, N.M.; AboElmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Shalan, A.E. Advances in nanotechnology and antibacterial properties of biodegradable food packaging materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Yang, W.; Hao, J.; Wu, Y.; Zhou, Y.; Mi, L.; Hu, Y. Electrostatic spinning construction of GA/HP-β-CD functionalized COS/PLA composite membrane for cherry tomato preservation applications. Food Packag. Shelf Life 2025, 49, 101495. [Google Scholar] [CrossRef]
- Dong, H.; Tong, L.; Cheng, M.; Hou, S. Utilizing electrospun molecularly imprinted membranes for food industry: Opportunities and challenges. Food Chem. 2024, 460, 140695. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Han, Z.; Sun, Y.; Chen, H.; Li, H.; Wang, R.; Ji, Y.; He, B. A novel, green method for the preparation of biodegradable PLA membranes without pore-forming agent. J. Water Process. Eng. 2025, 74, 107841. [Google Scholar] [CrossRef]
- do Nascimento, N.N.; Paraíso, C.M.; Molina, L.C.A.; Dzyazko, Y.S.; Bergamasco, R.; Vieira, A.M.S. Innovative Trends in Modified Membranes: A Mini Review of Applications and Challenges in the Food Sector. Membranes 2024, 14, 209. [Google Scholar] [CrossRef]
- Dibdiakova, J.; Matic, J.; Wubshet, S.G.; Uhl, W.; Manamperuma, L.D.; Rusten, B.; Vik, E.A. Membrane Separation of Chicken Byproduct Hydrolysate for Up-Concentration of Bioactive Peptides. Membranes 2024, 14, 28. [Google Scholar] [CrossRef]
- Stoyanova, Y.; Lazarova-Zdravkova, N.; Peshev, D. Is Membrane Filtration Applicable for the Recovery of Biologically Active Substances from Spent Lavender? Membranes 2025, 15, 21. [Google Scholar] [CrossRef] [PubMed]
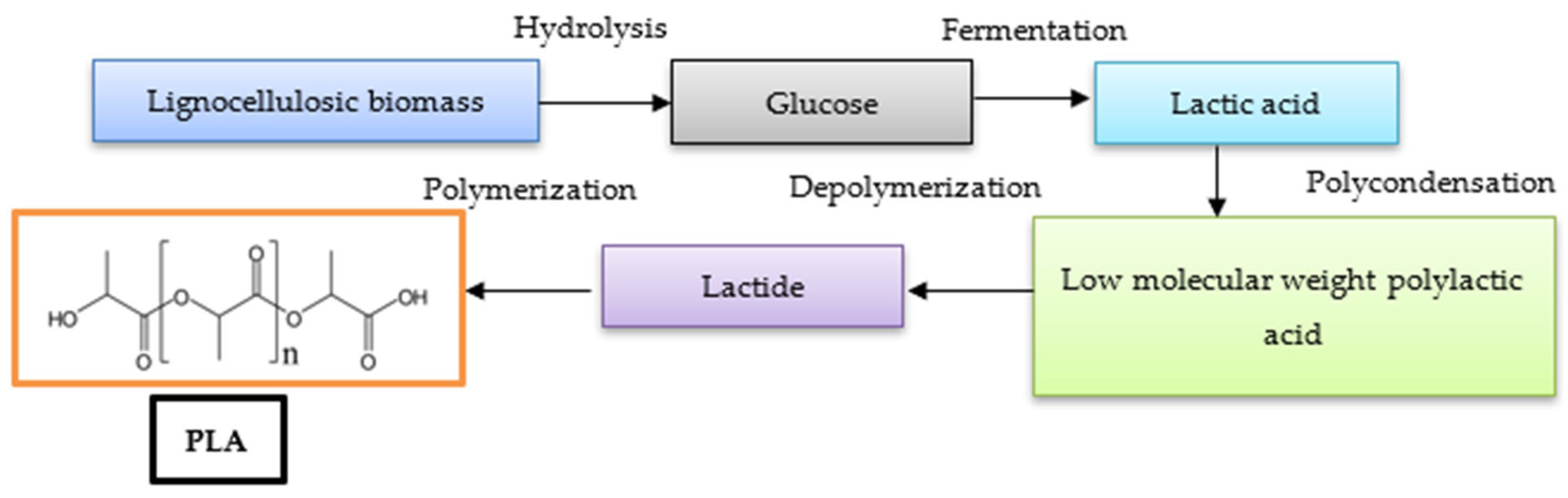
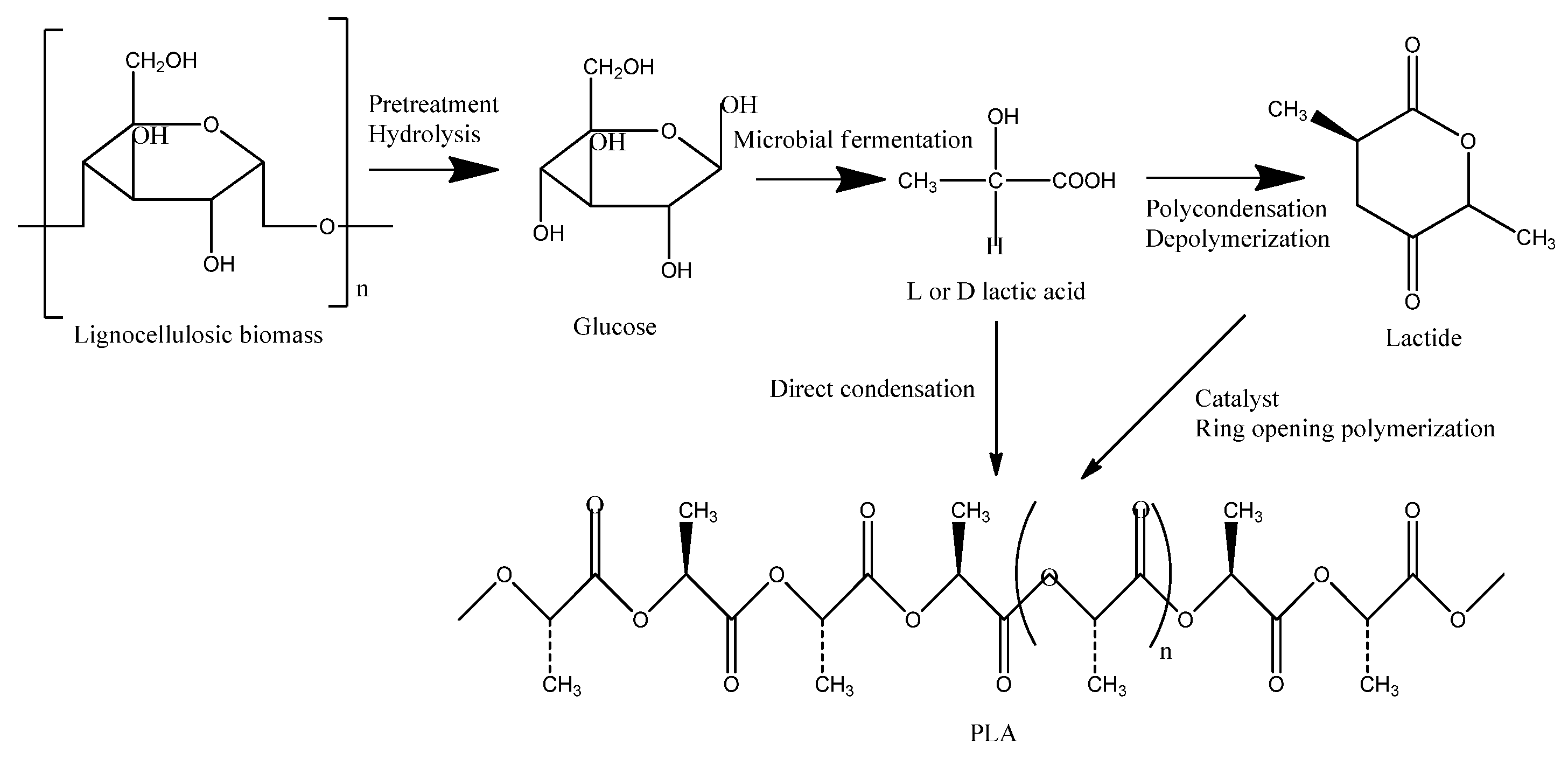
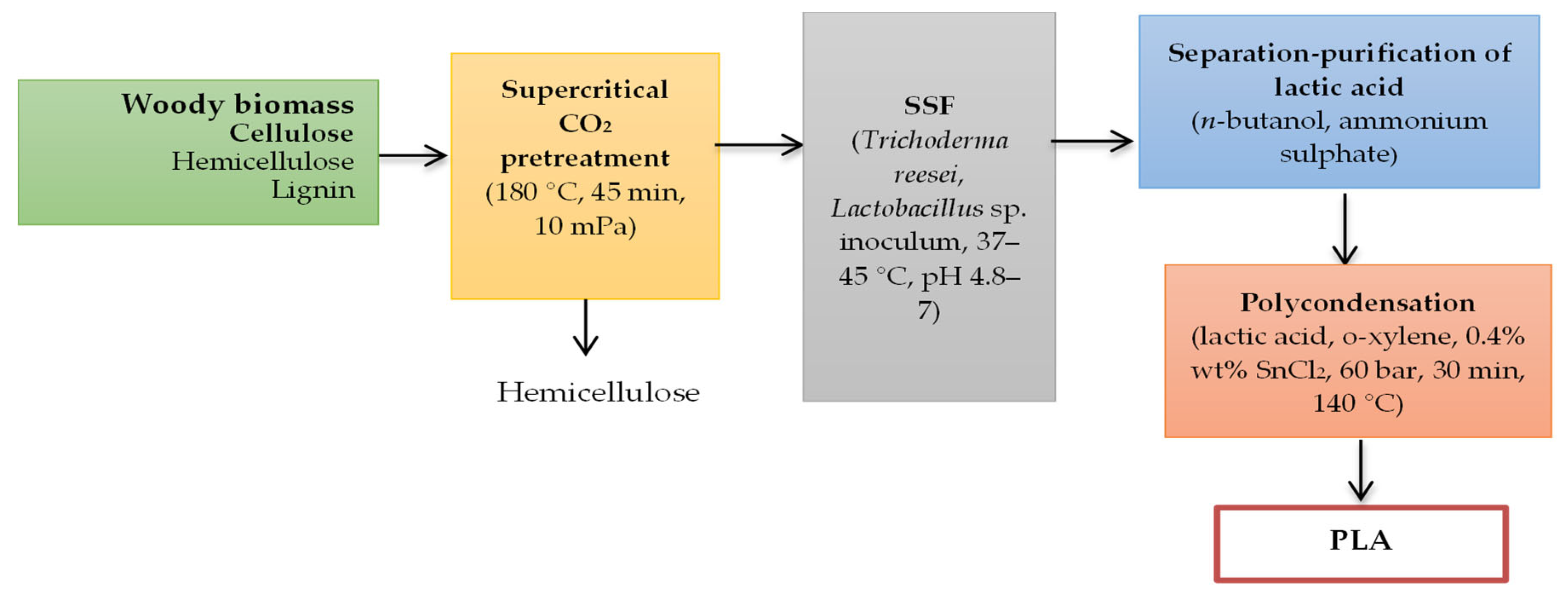
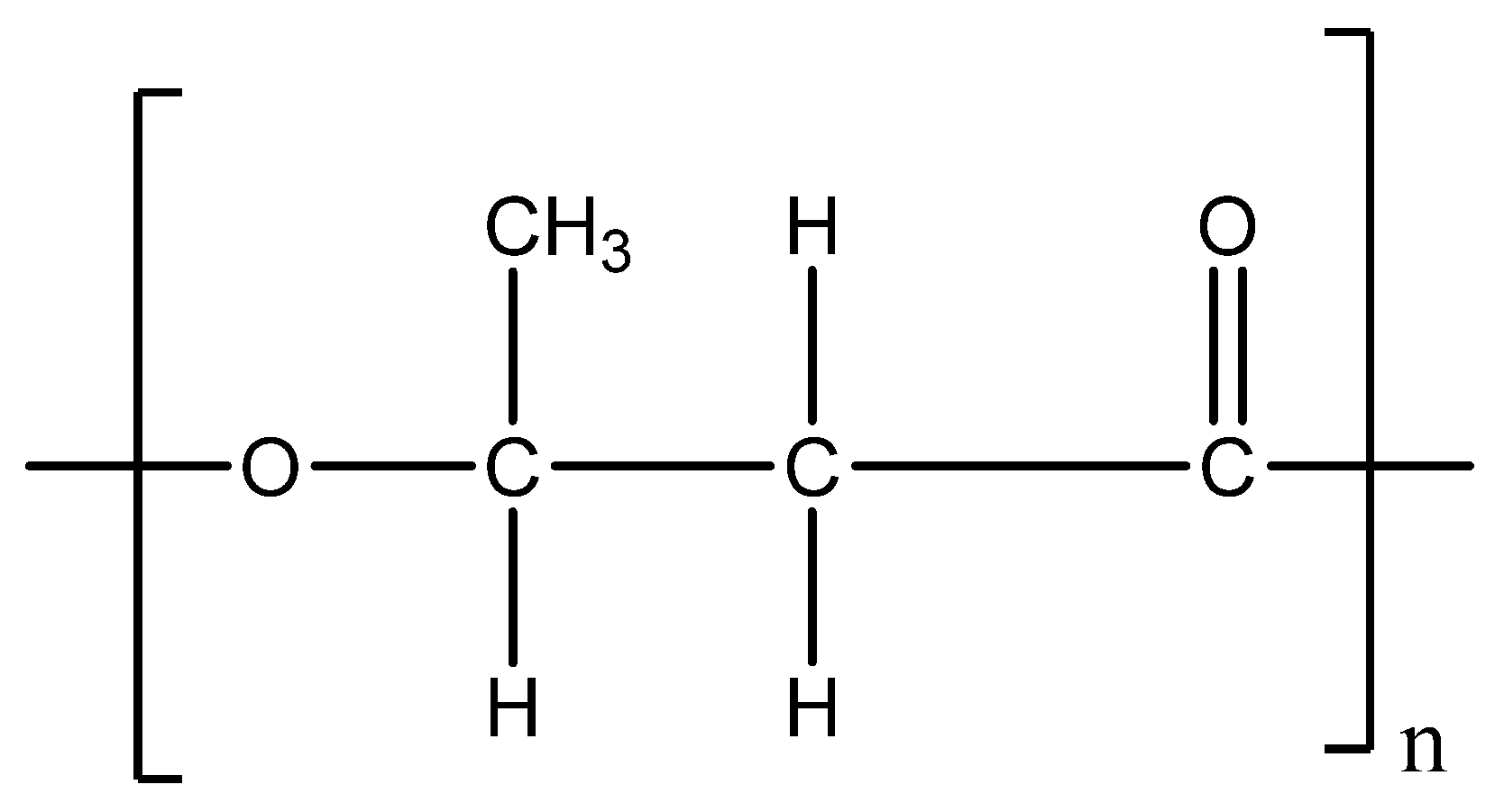

| Characteristics | PLA | PHB |
|---|---|---|
| Physico-chemical and mechanical | ||
| Structure | Linear aliphatic polyester (levorotatory (L-), dextrorotatory (D-), meso (combination of L- and D-)) [40,41] | Microbial polyester, composed of 3-hydroxybutyrate monomers [38] |
| Functional groups | Ester (−COO−) linkages [42] | Methyl (–CH3) and ester (–COOR) groups [38] |
| Density | 1.21–1.25 g/cm3 [43,44] 1.24–1.25 g/cm3 [45] | 1.23–1.25 g/cm3 [44] 1.25 g/cm3 [43,46] |
| Crystallinity | 37% [25,44,47] | 50–60% [38] 60% [43,44,46] |
| Tensile strength | 21–60 MPa [42,43,44] 45–70 MPa [25] 32–68 MPa [45] | 20–40 MPa [38,48] 31 MPa [45] 40 MPa [46,47] 43 MPa [44] |
| Elongation at break | <10% [42] 2.5–6% [43,44] | 6% [46] 7% [45] 5–10% [38] |
| Thermal | ||
| Melting temperature (Tm) | 150–162 °C [42,43,44] 160 °C [49] 170–183 °C [25] 173–178 °C [47] 130–180 °C [45,50] | 160–180 °C [39] 168–182 °C [42] 177 °C [43,46] 175 °C [47] 165–175 °C [38] 171–182 °C [44] |
| Glass transition temperature (Tg) | 45–60 °C [42,43,44] 60 °C [51] 55–65 °C [25,49] 60–65 °C [47] 50–80 °C [50] 60–80 °C [45] | 2 °C [43,46,47] 2–15 °C [44] 5–9 °C [38] 15.0–5.0 °C [42] |
| Thermal degradation | 200 °C [42,52] 230–260 °C [50] 215 °C [45] | 180 °C [53] 220 and 290 °C [52] |
| Barrier | ||
| Oxygen permeability | 15.0–25.0 mL mm/m2 day atm [43] 1.94–2.30 m3 m/m2 s Pa [45] | 2.0–10.0 mL mm/m2 day atm [43] |
| Water vapor permeability | 5.0–7.0 g mm/m2 day [43] 1.2–2.2 kg m/m2 s Pa [45] | 1.0–5.0 g mm/m2 day [43] |
| Other | ||
| Biodegradability | Hydrolitic, enzymatic [43,45] | Microbial [46,54] |
| Hydrophilicity | Hydrophobic, water contact angle 70–80° [42,55] | Hydrophobic, water contact angle of 80–105° [56] |
| Solubility | Soluble in chloroform, methylene chloride, acetonitrile, 1,1,2-trichloroethane and dichloroacetic acid, dioxane [49,57] Insoluble in alcohols, water, linear hydrocarbons [57] | Soluble in chloroform, dichloromethane and chlorinated hydrocarbons [56,58] Insoluble in water, alcohols, organic solvents [59] |
| pH stability | Stable in neutral and acidic pH, degrades in alkaline pH [60] | Stable in neutral and more resistant in acidic pH, rapid hydrolysis in alkaline pH [61,62] |
| Property | PLA | PHB |
|---|---|---|
| Origin | Bio-based (corn, sugarcane, wheat, cassava, and maize) and lignocellulosic biomass | Bio-based (carbon sources) |
| Production | Sugar extraction, lactic acid fermentation, polymerization, processing | Carbon source preparation, fermentation, PBB accumulation, extraction, purification and drying |
| Biodegradability | Biodegradable and compostable | Completely biodegradable |
| Degradation time | Months, years in natural environments | Weeks to months in soil or water |
| Degradation mechanism | Hydrolytic cleavage of ester to lactic acid | Hydrolytic and enzymatic cleavage to 3-hydroxybutyric acid |
| Environmental impact | Low, slower degradation | Very low, rapid degradation |
| Transparency | Transparent | Opaque |
| Thermal properties | Low thermal resistance | Higher than PLA, better heat resistance |
| Processability | Easy to process | Difficult to process (hard and brittle thermoplastic) |
| Mechanical properties | High tensile strengths | Good mechanical properties, comparable with polypropylene |
| Application | Packaging, agriculture, medical, textiles, fibers, etc. | Packaging, biomedical, textiles, agriculture, etc. |
| Cost | Lower production costs, widely available | Higher production cost, less commercially available |
| Method | Spinning | Phase Inversion | Three-Dimensional Printing |
|---|---|---|---|
| Basic Principle |
|
|
|
| Suitability for Biodegradable Polymers |
|
|
|
| Structural Control |
|
|
|
| Scalability and Cost |
|
|
|
| Environmental Impact |
|
|
|
| Application Suitability |
|
|
|
| Challenges |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senila, L.; Kovacs, E.; Senila, M. A Review of Polylactic Acid (PLA) and Poly(3-hydroxybutyrate) (PHB) as Bio-Sourced Polymers for Membrane Production Applications. Membranes 2025, 15, 210. https://doi.org/10.3390/membranes15070210
Senila L, Kovacs E, Senila M. A Review of Polylactic Acid (PLA) and Poly(3-hydroxybutyrate) (PHB) as Bio-Sourced Polymers for Membrane Production Applications. Membranes. 2025; 15(7):210. https://doi.org/10.3390/membranes15070210
Chicago/Turabian StyleSenila, Lacrimioara, Eniko Kovacs, and Marin Senila. 2025. "A Review of Polylactic Acid (PLA) and Poly(3-hydroxybutyrate) (PHB) as Bio-Sourced Polymers for Membrane Production Applications" Membranes 15, no. 7: 210. https://doi.org/10.3390/membranes15070210
APA StyleSenila, L., Kovacs, E., & Senila, M. (2025). A Review of Polylactic Acid (PLA) and Poly(3-hydroxybutyrate) (PHB) as Bio-Sourced Polymers for Membrane Production Applications. Membranes, 15(7), 210. https://doi.org/10.3390/membranes15070210







