Effect of Tau Fragment and Membrane Interactions on Membrane Permeabilization and Peptide Aggregation
Abstract
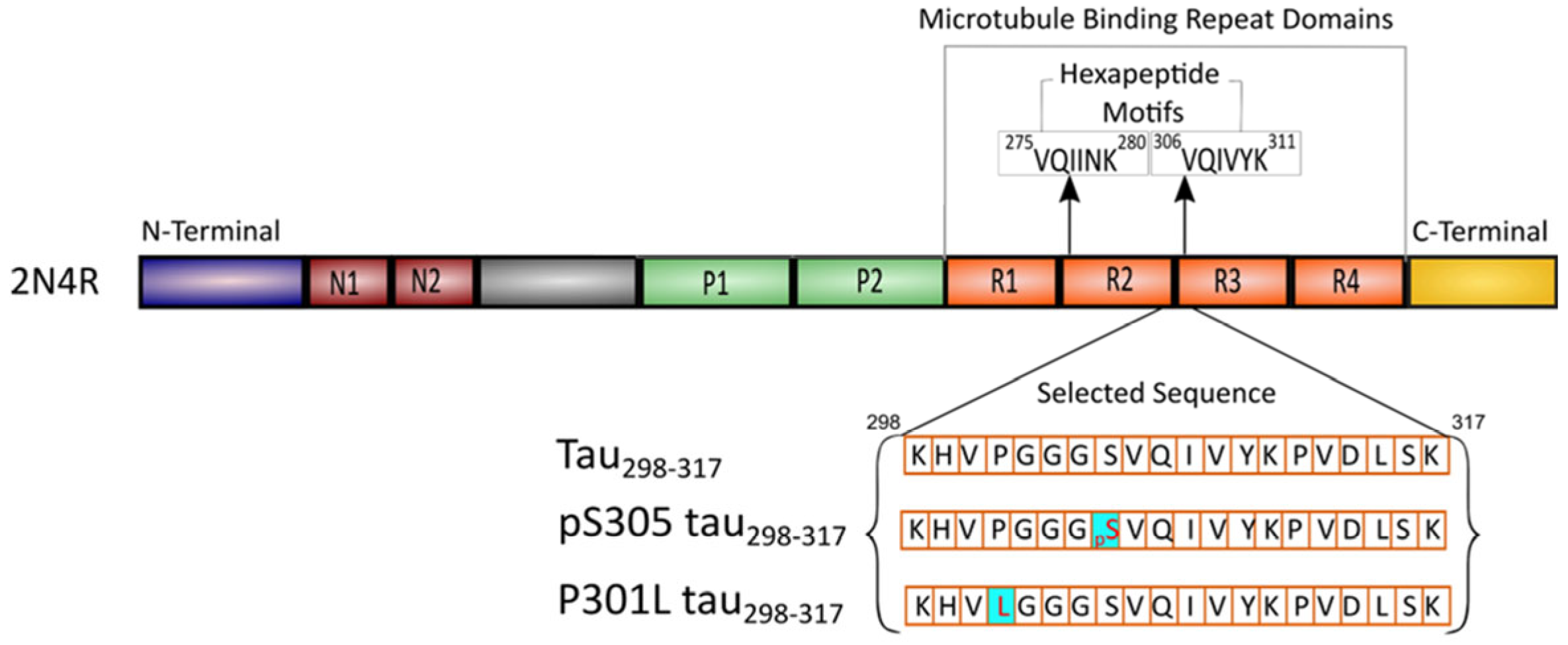
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Peptides
2.2. Lipid Vesicle Preparation
2.3. Membrane Leakage Study
2.4. Particle Size Measurement Using Dynamic Light Scattering (DLS)
2.5. Circular Dichroism (CD) Study of the Peptides
2.6. Aggregation Kinetics Study of the Peptides
2.7. Atomic Force Microscopy (AFM) Measurement
3. Results and Discussion
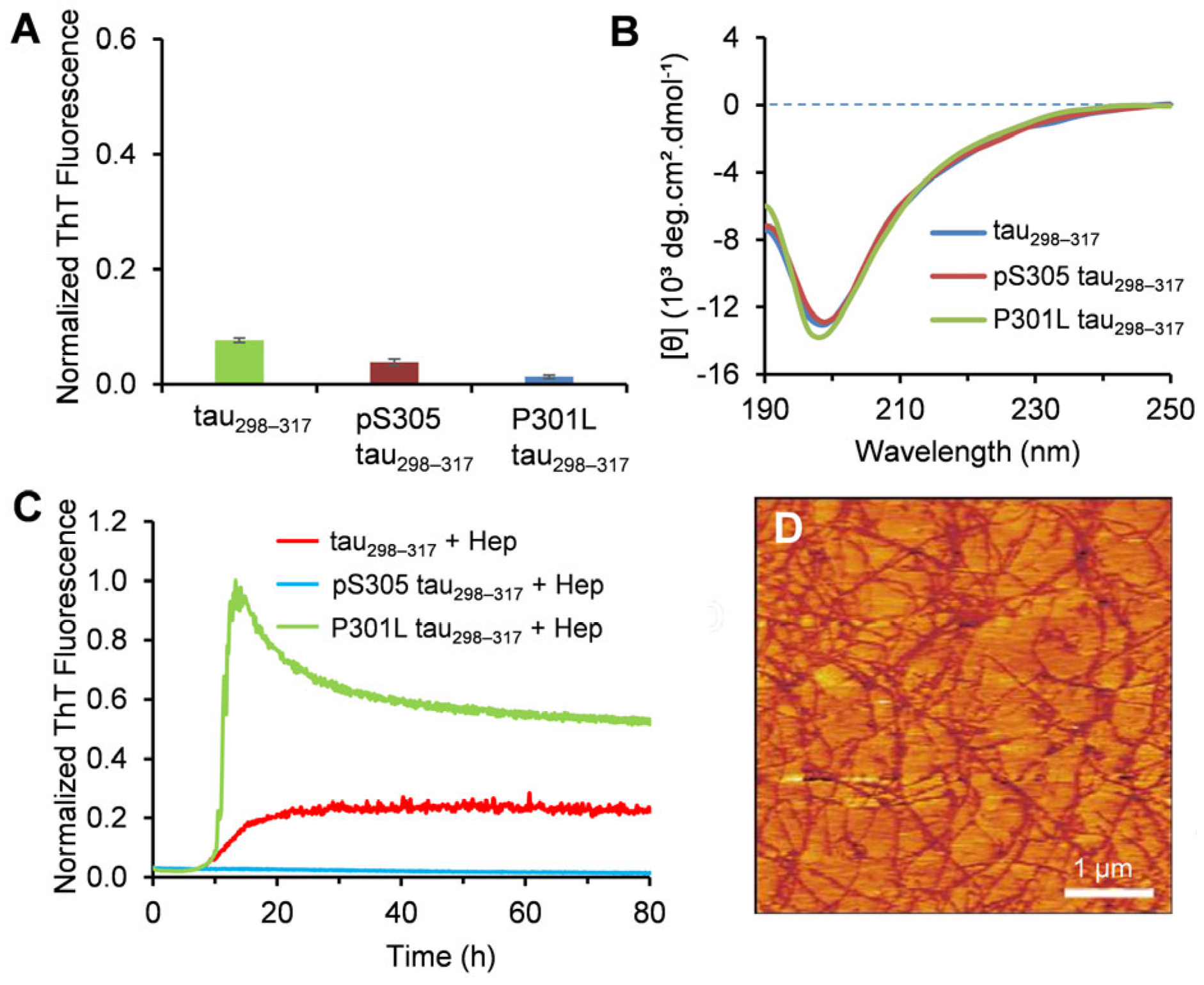
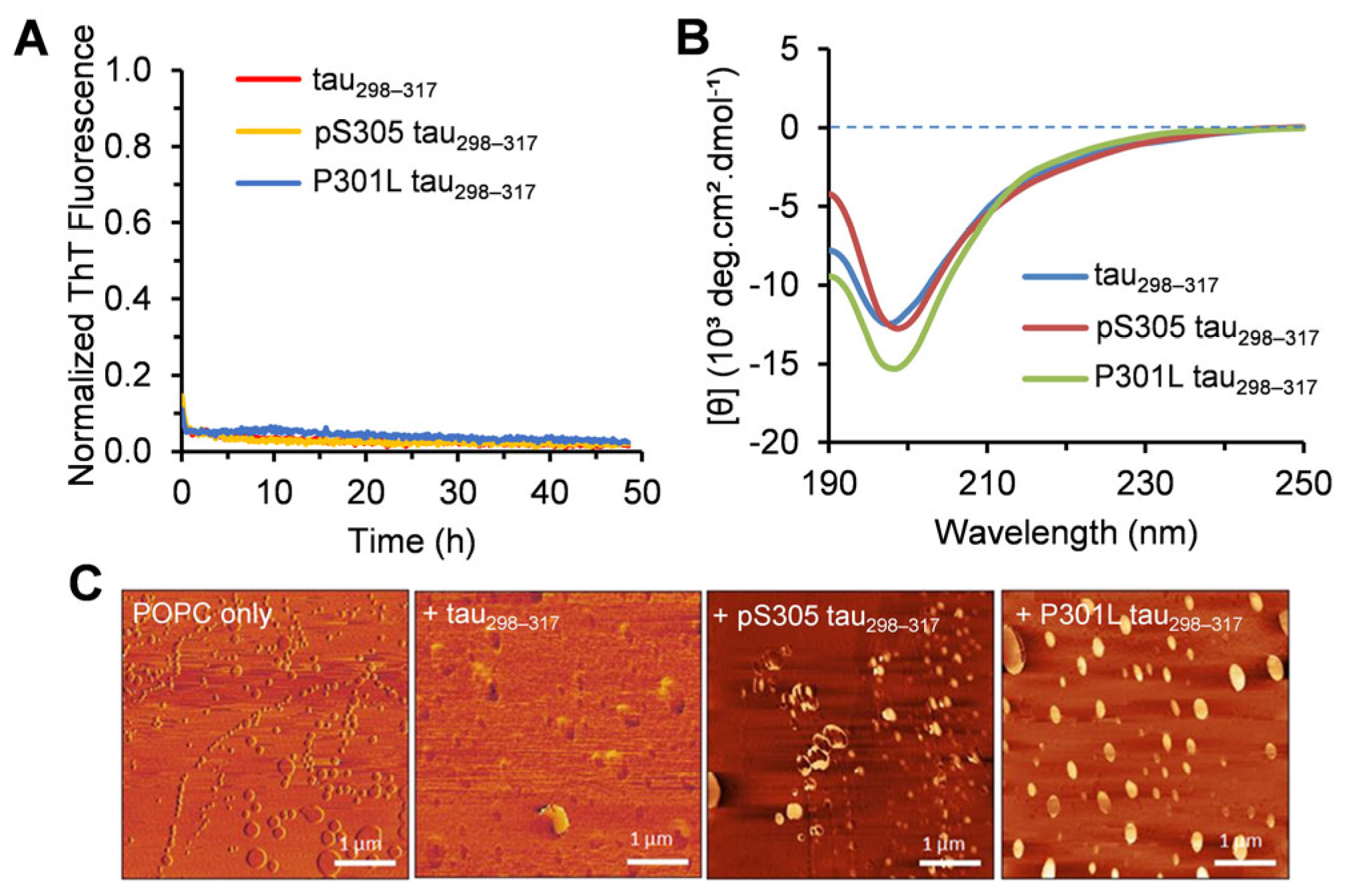
3.1. Characterization of the Aggregation Properties of Tau298–317 Mutants
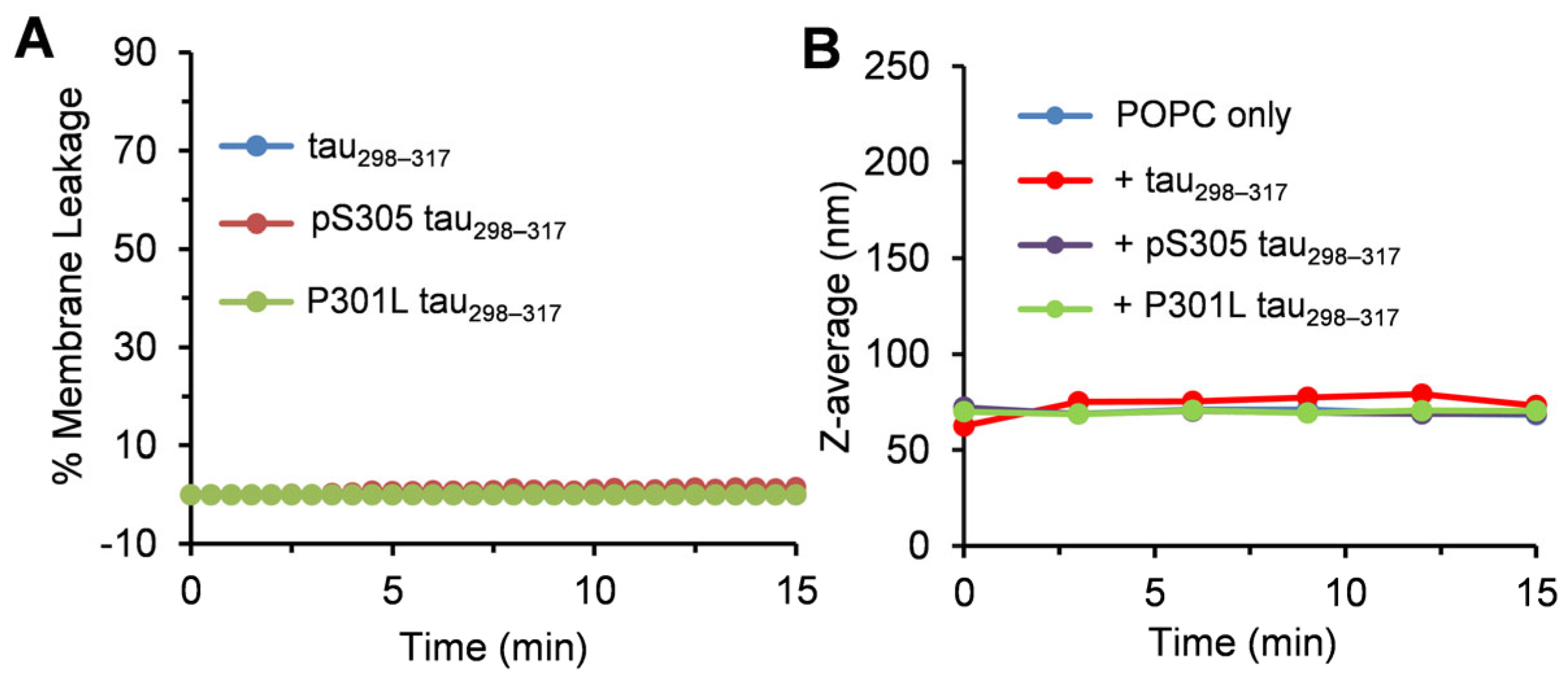
3.2. Effect of Tau298–317 and the Mutants on Leakage of Zwitterionic POPC Vesicles
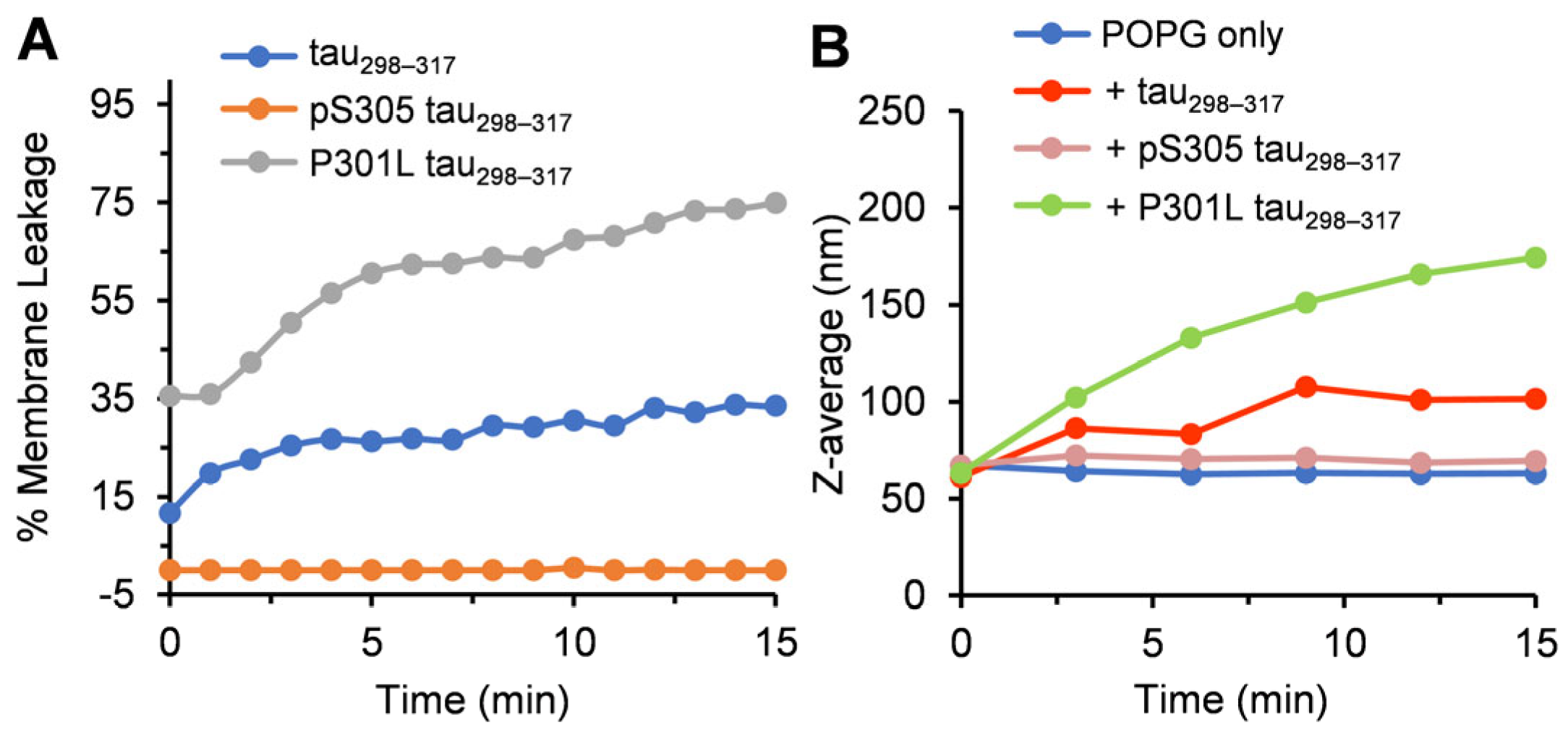
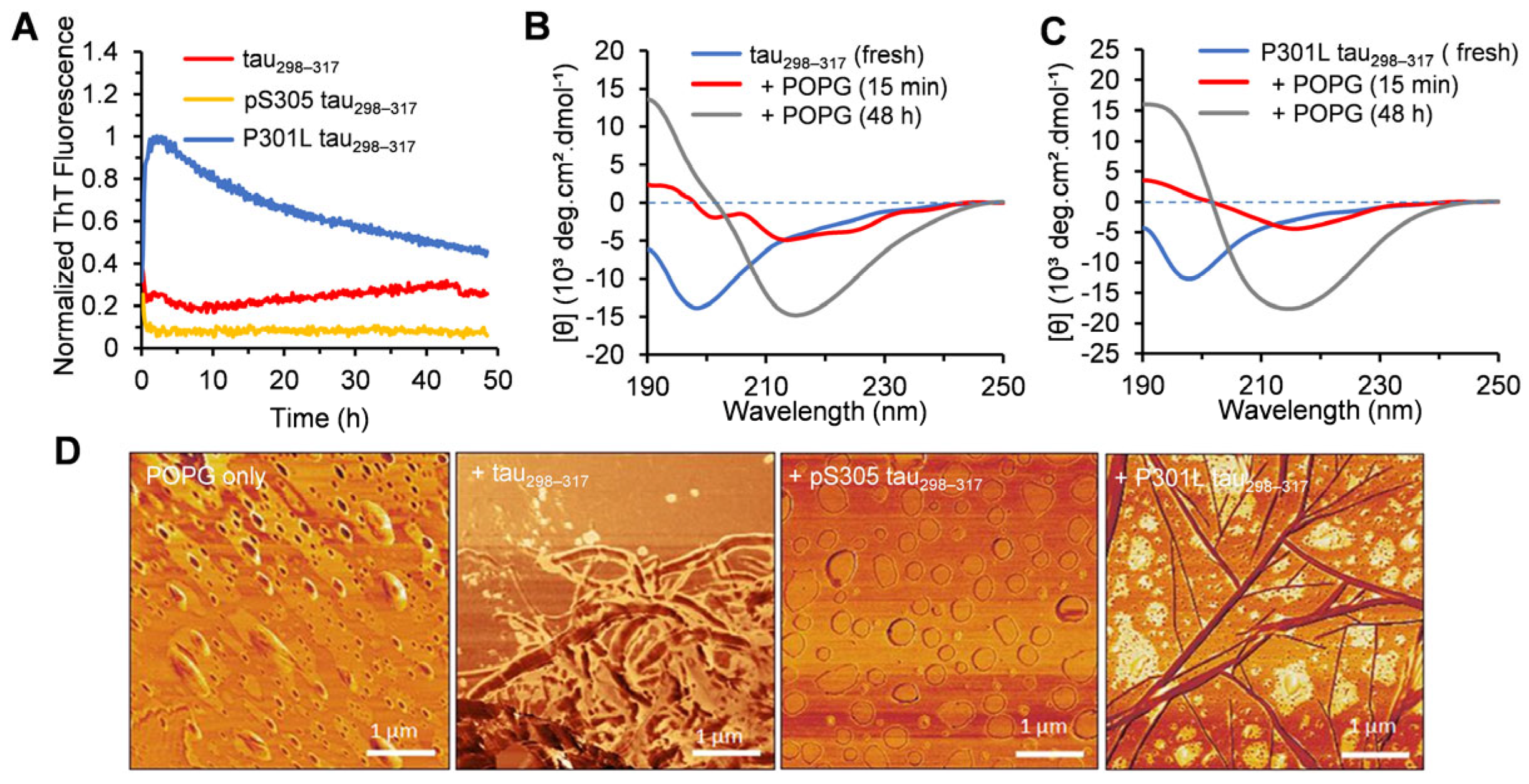
3.3. Effect of Tau298–317 and the Mutants on Leakage of Anionic POPG Vesicles
3.4. Effect of Lipid Membrane on the Aggregation of Tau Fragment
3.5. Crucial Interactions in Mediating Membrane Leakage and Peptide Aggregation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.-Y.; Kirschner, M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Cambiazo, V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol. Rev. 1995, 75, 835–864. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.-X.; Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Latypova, X.; Terro, F. Post-translational modifications of tau protein: Implications for Alzheimer’s disease. Neurochem. Int. 2011, 58, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Alquezar, C.; Arya, S.; Kao, A.W. Tau post-translational modifications: Dynamic transformers of tau function, degradation, and aggregation. Front. Neurol. 2021, 11, 595532. [Google Scholar] [CrossRef]
- Islam, M.; Shen, F.; Regmi, D.; Du, D. Therapeutic strategies for tauopathies and drug repurposing as a potential approach. Biochem. Pharmacol. 2022, 198, 114979. [Google Scholar] [CrossRef] [PubMed]
- Gellermann, G.P.; Appel, T.R.; Davies, P.; Diekmann, S. Paired helical filaments contain small amounts of cholesterol, phosphatidylcholine and sphingolipids. Biol. Chem. 2006, 387, 1267–1274. [Google Scholar] [CrossRef]
- Gray, E.; Paula-Barbosa, M.; Roher, A. Alzheimer’s disease: Paired helical filaments and cytomembranes. Neuropathol. Appl. Neurobiol. 1987, 13, 91–110. [Google Scholar] [CrossRef]
- Burke, K.A.; Yates, E.A.; Legleiter, J. Biophysical insights into how surfaces, including lipid membranes, modulate protein aggregation related to neurodegeneration. Front. Neurol. 2013, 4, 17. [Google Scholar] [CrossRef]
- Krausser, J.; Knowles, T.P.; Šarić, A. Physical mechanisms of amyloid nucleation on fluid membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 33090–33098. [Google Scholar] [CrossRef]
- Kotler, S.A.; Walsh, P.; Brender, J.R.; Ramamoorthy, A. Differences between amyloid-β aggregation in solution and on the membrane: Insights into elucidation of the mechanistic details of Alzheimer’s disease. Chem. Soc. Rev. 2014, 43, 6692–6700. [Google Scholar] [CrossRef] [PubMed]
- Ait-Bouziad, N.; Lv, G.; Mahul-Mellier, A.-L.; Xiao, S.; Zorludemir, G.; Eliezer, D.; Walz, T.; Lashuel, H.A. Discovery and characterization of stable and toxic Tau/phospholipid oligomeric complexes. Nat. Commun. 2017, 8, 1678. [Google Scholar] [CrossRef]
- Bok, E.; Leem, E.; Lee, B.-R.; Lee, J.M.; Yoo, C.J.; Lee, E.M.; Kim, J. Role of the lipid membrane and membrane proteins in tau pathology. Front. Cell Dev. Biol. 2021, 9, 653815. [Google Scholar] [CrossRef]
- De La-Rocque, S.; Moretto, E.; Butnaru, I.; Schiavo, G. Knockin’on heaven’s door: Molecular mechanisms of neuronal tau uptake. J. Neurochem. 2021, 156, 563–588. [Google Scholar] [CrossRef] [PubMed]
- Katsinelos, T.; Zeitler, M.; Dimou, E.; Karakatsani, A.; Müller, H.-M.; Nachman, E.; Steringer, J.P.; de Almodovar, C.R.; Nickel, W.; Jahn, T.R. Unconventional secretion mediates the trans-cellular spreading of tau. Cell Rep. 2018, 23, 2039–2055. [Google Scholar] [CrossRef]
- Merezhko, M.; Brunello, C.A.; Yan, X.; Vihinen, H.; Jokitalo, E.; Uronen, R.-L.; Huttunen, H.J. Secretion of tau via an unconventional non-vesicular mechanism. Cell Rep. 2018, 25, 2027–2035.e4. [Google Scholar] [CrossRef]
- Georgieva, E.R.; Xiao, S.; Borbat, P.P.; Freed, J.H.; Eliezer, D. Tau binds to lipid membrane surfaces via short amphipathic helices located in its microtubule-binding repeats. Biophys. J. 2014, 107, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Ury-Thiery, V.; Fichou, Y.; Alves, I.; Molinari, M.; Lecomte, S.; Feuillie, C. Interaction of full-length tau with negatively charged lipid membranes leads to polymorphic aggregates. Nanoscale 2024, 16, 17141–17153. [Google Scholar] [CrossRef]
- Dicke, S.S.; Tatge, L.; Engen, P.E.; Culp, M.; Masterson, L.R. Isothermal titration calorimetry and vesicle leakage assays highlight the differential behaviors of tau repeat segments upon interaction with anionic lipid membranes. Biochem. Biophys. Res. Commun. 2017, 493, 1504–1509. [Google Scholar] [CrossRef]
- Künze, G.; Barré, P.; Scheidt, H.A.; Thomas, L.; Eliezer, D.; Huster, D. Binding of the three-repeat domain of tau to phospholipid membranes induces an aggregated-like state of the protein. BBA Biomembr. 2012, 1818, 2302–2313. [Google Scholar] [CrossRef][Green Version]
- Brender, J.R.; Salamekh, S.; Ramamoorthy, A. Membrane disruption and early events in the aggregation of the diabetes related peptide IAPP from a molecular perspective. Acc. Chem. Res. 2012, 45, 454–462. [Google Scholar] [CrossRef]
- Walsh, P.; Vanderlee, G.; Yau, J.; Campeau, J.; Sim, V.L.; Yip, C.M.; Sharpe, S. The mechanism of membrane disruption by cytotoxic amyloid oligomers formed by prion protein (106–126) is dependent on bilayer composition. J. Biol. Chem. 2014, 289, 10419–10430. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, M.F.; Kotler, S.A.; Brender, J.R.; Chen, J.; Lee, D.-K. Ramamoorthy, Two-step mechanism of membrane disruption by Aβ through membrane fragmentation and pore formation. Biophys. J. 2012, 103, 702–710. [Google Scholar] [CrossRef]
- Flach, K.; Hilbrich, I.; Schiffmann, A.; Gärtner, U.; Krüger, M.; Leonhardt, M.; Waschipky, H.; Wick, L.; Arendt, T.; Holzer, M. Tau oligomers impair artificial membrane integrity and cellular viability. J. Biol. Chem. 2012, 287, 43223–43233. [Google Scholar] [CrossRef] [PubMed]
- Grabenauer, M.; Wu, C.; Soto, P.; Shea, J.-E.; Bowers, M.T. Oligomers of the prion protein fragment 106−126 are likely assembled from β-hairpins in solution, and methionine oxidation inhibits assembly without altering the peptide’s monomeric conformation. J. Am. Chem. Soc. 2009, 132, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Drajkowska, A.; Molski, A. Aggregation and partitioning of amyloid peptide fragments in the presence of a lipid bilayer: A coarse grained molecular dynamics study. Biophys. Chem. 2023, 300, 107051. [Google Scholar] [CrossRef]
- Regmi, D.; Shen, F.; Stanic, A.; Islam, M.; Du, D. Effect of phospholipid liposomes on prion fragment (106–128) amyloid formation. BBA Biomembr. 2023, 1865, 184199. [Google Scholar] [CrossRef]
- Islam, M.; Argueta, E.; Wojcikiewicz, E.P.; Du, D. Effects of Charged Polyelectrolytes on Amyloid Fibril Formation of a Tau Fragment. ACS Chem. Neurosci. 2022, 13, 3034–3043. [Google Scholar] [CrossRef]
- Goux, W.J.; Kopplin, L.; Nguyen, A.D.; Leak, K.; Rutkofsky, M.; Shanmuganandam, V.D.; Sharma, D.; Inouye, H.; Kirschner, D.A. The formation of straight and twisted filaments from short tau peptides. J. Biol. Chem. 2004, 279, 26868–26875. [Google Scholar] [CrossRef]
- Mukrasch, M.D.; Biernat, J.; von Bergen, M.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. Sites of tau important for aggregation populate beta-structure and bind to microtubules and polyanions. J. Biol. Chem. 2005, 280, 24978–24986. [Google Scholar] [CrossRef]
- Wang, Y.; Martinez-Vicente, M.; Krüger, U.; Kaushik, S.; Wong, E.; Mandelkow, E.-M.; Cuervo, A.M.; Mandelkow, E. Tau fragmentation, aggregation and clearance: The dual role of lysosomal processing. Hum. Mol. Genet. 2009, 18, 4153–4170. [Google Scholar] [CrossRef]
- Lewis, J.; McGowan, E.; Rockwood, J.; Melrose, H.; Nacharaju, P.; Van Slegtenhorst, M.; Gwinn-Hardy, K.; Murphy, M.P.; Baker, M.; Yu, X. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000, 25, 402–405. [Google Scholar] [CrossRef]
- Islam, M. Amyloidogenicity of the Peptide Fragment in Microtubule Binding Repeat Domain of Tau. Doctoral Dissertation, Florida Atlantic University, Boca Raton, FL, USA, 2023. [Google Scholar]
- Morris, C.; Kent, T.W.; Shen, F.; Wojcikiewicz, E.P.; Du, D. Effects of the hydrophilic N-terminal region on Aβ-mediated membrane disruption. J. Phys. Chem. B 2021, 125, 7671–7678. [Google Scholar] [CrossRef]
- Amselem, S.; Cohen, R.; Barenholz, Y. In vitro tests to predict in vivo performance of liposomal dosage forms. Chem. Phys. Lipids 1993, 64, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Camargo, D.C.R.; Sileikis, E.; Chia, S.; Axell, E.; Bernfur, K.; Cataldi, R.L.; Cohen, S.I.; Meisl, G.; Habchi, J.; Knowles, T.P. Proliferation of tau 304–380 fragment aggregates through autocatalytic secondary nucleation. ACS Chem. Neurosci. 2021, 12, 4406–4415. [Google Scholar] [CrossRef]
- Pretti, E.; Shell, M.S. Mapping the configurational landscape and aggregation phase behavior of the tau protein fragment PHF6. Proc. Natl. Acad. Sci. USA 2023, 120, e2309995120. [Google Scholar] [CrossRef]
- LeVine, H., 3rd. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 22–35. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R.; Spillantini, M.G.; Hasegawa, M.; Smith, M.J.; Crowther, R.A. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature 1996, 383, 550–553. [Google Scholar] [CrossRef]
- Fichou, Y.; Lin, Y.; Rauch, J.N.; Vigers, M.; Zeng, Z.; Srivastava, M.; Keller, T.J.; Freed, J.H.; Kosik, K.S.; Han, S. Cofactors are essential constituents of stable and seeding-active tau fibrils. Proc. Natl. Acad. Sci. USA 2018, 115, 13234–13239. [Google Scholar] [CrossRef] [PubMed]
- King, M.E.; Gamblin, T.C.; Kuret, J.; Binder, L.I. Differential assembly of human tau isoforms in the presence of arachidonic acid. J. Neurochem. 2000, 74, 1749–1757. [Google Scholar] [CrossRef]
- Kawasaki, R.; Tate, S.-I. Impact of the hereditary P301L mutation on the correlated conformational dynamics of human tau protein revealed by the paramagnetic relaxation enhancement NMR experiments. Int. J. Mol. Sci. 2020, 21, 3920. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Drombosky, K.W.; Hou, Z.; Sari, L.; Kashmer, O.M.; Ryder, B.D.; Perez, V.A.; Woodard, D.R.; Lin, M.M.; Diamond, M.I. Tau local structure shields an amyloid-forming motif and controls aggregation propensity. Nat. Commun. 2019, 10, 2493. [Google Scholar] [CrossRef]
- Valappil, D.K.; Mini, N.J.; Dilna, A.; Nath, S. Membrane interaction to intercellular spread of pathology in Alzheimer’s disease. Front. Neurosci. 2022, 16, 936897. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Jepson, T.; Shukla, S.; Maya-Romero, A.; Kampmann, M.; Xu, K.; Hurley, J.H. Tau fibrils induce nanoscale membrane damage and nucleate cytosolic tau at lysosomes. Proc. Natl. Acad. Sci. USA 2024, 121, e2315690121. [Google Scholar] [CrossRef]
- Reynolds, N.P.; Soragni, A.; Rabe, M.; Verdes, D.; Liverani, E.; Handschin, S.; Riek, R.; Seeger, S. Mechanism of membrane interaction and disruption by α-synuclein. J. Am. Chem. Soc. 2011, 133, 19366–19375. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Morris, C.; Cupples, S.; Kent, T.W.; Elbassal, E.A.; Wojcikiewicz, E.P.; Yi, P.; Du, D. N-terminal charged residues of amyloid-beta peptide modulate amyloidogenesis and interaction with lipid membrane. Chem. Eur. J. 2018, 24, 9494–9498. [Google Scholar] [CrossRef]
- Jones, E.M.; Dubey, M.; Camp, P.J.; Vernon, B.C.; Biernat, J.; Mandelkow, E.; Majewski, J.; Chi, E.Y. Interaction of tau protein with model lipid membranes induces tau structural compaction and membrane disruption. Biochemistry 2012, 51, 2539–2550. [Google Scholar] [CrossRef]
- Kurano, M.; Saito, Y.; Uranbileg, B.; Saigusa, D.; Kano, K.; Aoki, J.; Yatomi, Y. Modulations of bioactive lipids and their receptors in postmortem Alzheimer’s disease brains. Front. Aging Neurosci. 2022, 14, 1066578. [Google Scholar] [CrossRef] [PubMed]
- Eliezer, D.; Barré, P.; Kobaslija, M.; Chan, D.; Li, X.; Heend, L. Residual structure in the repeat domain of tau: Echoes of microtubule binding and paired helical filament formation. Biochemistry 2005, 44, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Von Bergen, M.; Friedhoff, P.; Biernat, J.; Heberle, J.; Mandelkow, E.-M.; Mandelkow, E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming beta structure. Proc. Natl. Acad. Sci. USA 2000, 97, 5129–5134. [Google Scholar] [CrossRef]
- Majewski, J.; Jones, E.M.; Zanden, C.M.V.; Biernat, J.; Mandelkow, E.; Chi, E.Y. Lipid membrane templated misfolding and self-assembly of intrinsically disordered tau protein. Sci. Rep. 2020, 10, 13324. [Google Scholar] [CrossRef]
- Tchounwou, C.; Jobanputra, A.J.; Lasher, D.; Fletcher, B.J.; Jacinto, J.; Bhaduri, A.; Best, R.L.; Fisher, W.S.; Ewert, K.K.; Li, Y. Mixtures of intrinsically disordered neuronal protein tau and anionic liposomes reveal distinct anionic liposome-tau complexes coexisting with tau liquid–liquid phase-separated coacervates. Langmuir 2024, 40, 21041–21051. [Google Scholar] [CrossRef]
- Choi, T.S.; Han, J.Y.; Heo, C.E.; Lee, S.W.; Kim, H.I. Electrostatic and hydrophobic interactions of lipid-associated α-synuclein: The role of a water-limited interfaces in amyloid fibrillation. BBA Biomembr. 2018, 1860, 1854–1862. [Google Scholar] [CrossRef]
- Rhoades, E.; Ramlall, T.F.; Webb, W.W.; Eliezer, D. Quantification of α-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys. J. 2006, 90, 4692–4700. [Google Scholar] [CrossRef] [PubMed]
- Elbaum-Garfinkle, S.; Ramlall, T.; Rhoades, E. The role of the lipid bilayer in tau aggregation. Biophys. J. 2010, 98, 2722–2730. [Google Scholar] [CrossRef]
- Huang, S.K.; Shin, K.; Sarker, M.; Rainey, J.K. Apela exhibits isoform-and headgroup-dependent modulation of micelle binding, peptide conformation and dynamics. BBA Biomembr. 2017, 1859, 767–778. [Google Scholar] [CrossRef]
- Tuerkova, A.; Kabelka, I.; Králová, T.; Sukeník, L.; Pokorná, Š.; Hof, M.; Vácha, R. Effect of helical kink in antimicrobial peptides on membrane pore formation. Elife 2020, 9, e47946. [Google Scholar] [CrossRef]
- Fanni, A.M.; Zanden, C.M.V.; Majewska, P.V.; Majewski, J.; Chi, E.Y. Membrane-mediated fibrillation and toxicity of the tau hexapeptide PHF6. J. Biol. Chem. 2019, 294, 15304–15317. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.; Karim, M.R.U.; Argueta, E.; Selim, M.N.; Wojcikiewicz, E.P.; Du, D. Effect of Tau Fragment and Membrane Interactions on Membrane Permeabilization and Peptide Aggregation. Membranes 2025, 15, 208. https://doi.org/10.3390/membranes15070208
Islam M, Karim MRU, Argueta E, Selim MN, Wojcikiewicz EP, Du D. Effect of Tau Fragment and Membrane Interactions on Membrane Permeabilization and Peptide Aggregation. Membranes. 2025; 15(7):208. https://doi.org/10.3390/membranes15070208
Chicago/Turabian StyleIslam, Majedul, Md Raza Ul Karim, Emily Argueta, Mohammed N. Selim, Ewa P. Wojcikiewicz, and Deguo Du. 2025. "Effect of Tau Fragment and Membrane Interactions on Membrane Permeabilization and Peptide Aggregation" Membranes 15, no. 7: 208. https://doi.org/10.3390/membranes15070208
APA StyleIslam, M., Karim, M. R. U., Argueta, E., Selim, M. N., Wojcikiewicz, E. P., & Du, D. (2025). Effect of Tau Fragment and Membrane Interactions on Membrane Permeabilization and Peptide Aggregation. Membranes, 15(7), 208. https://doi.org/10.3390/membranes15070208







