Improving PFAS Rejection by Ultrafiltration Membranes via Polyelectrolyte Multilayer Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Experimental Conditions
2.2. Analytical Methods and Instrumentation
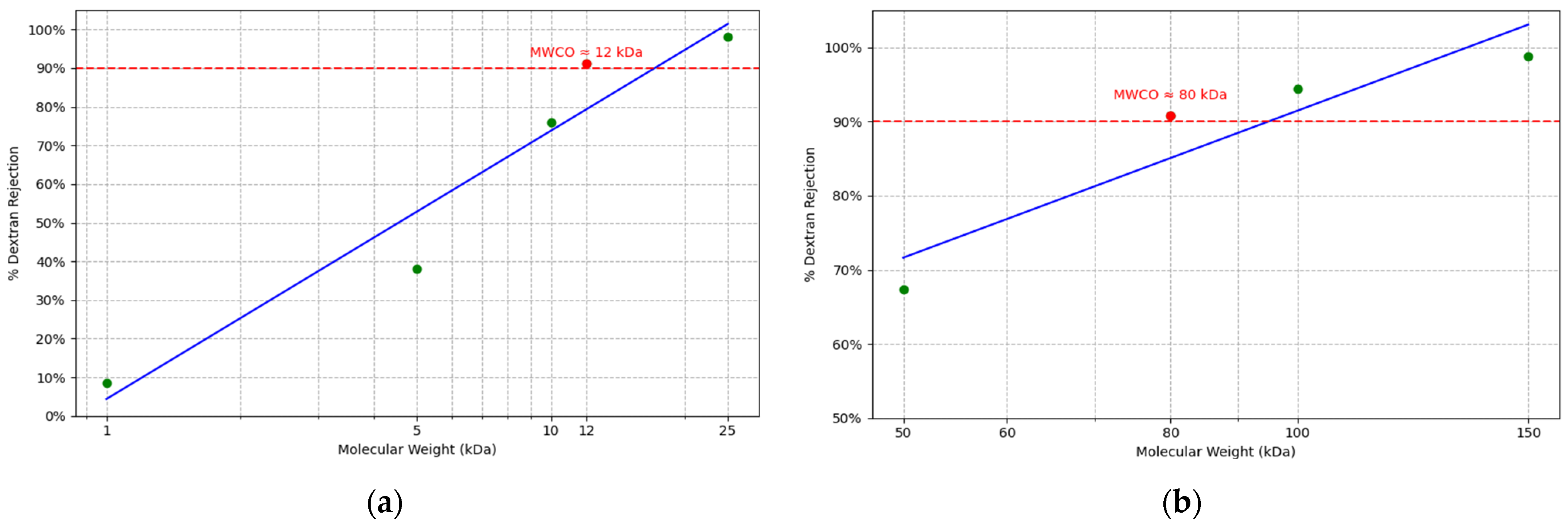
2.3. Membrane Characterization
3. Results
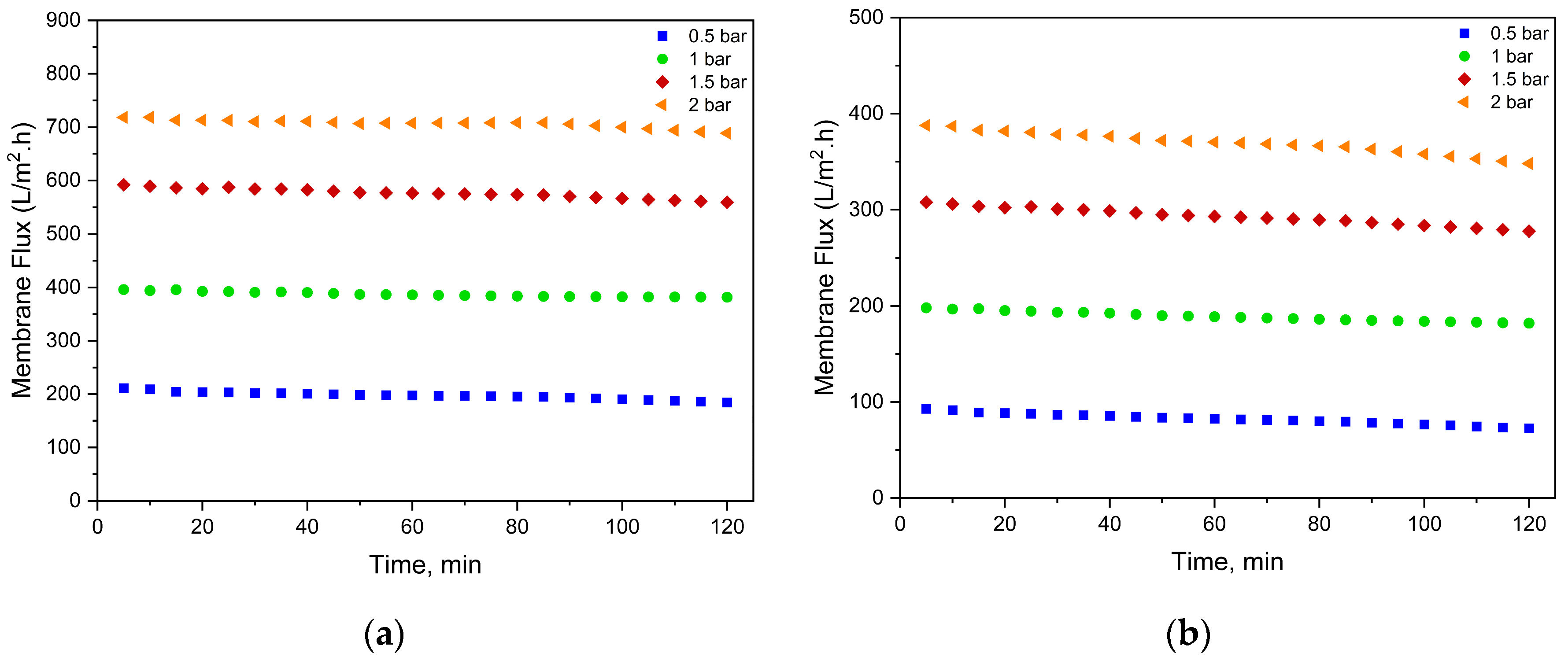
3.1. Effect of Coating on Membrane Surface Properities
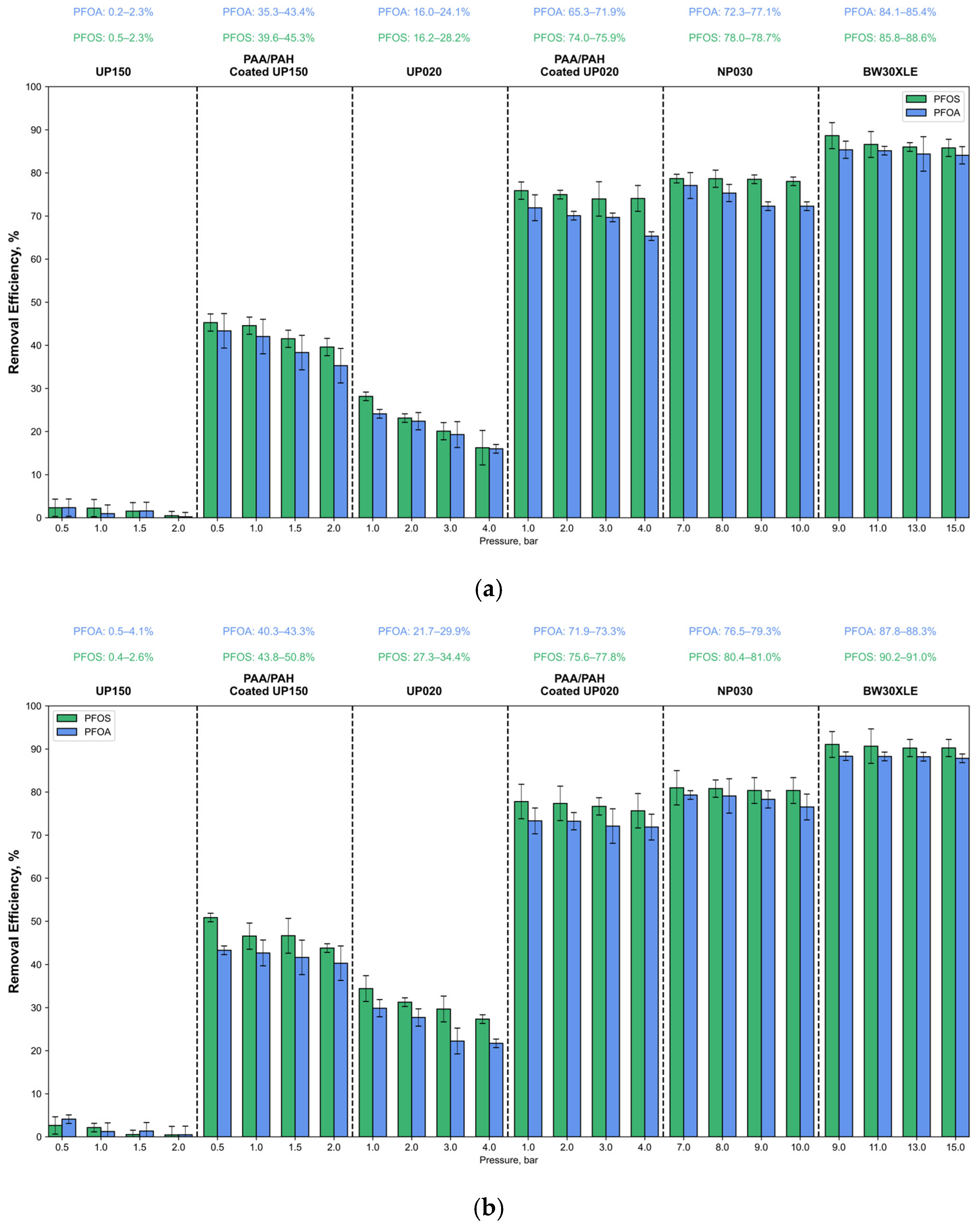
3.2. Effect of Coating on PFOA and PFOS Removal
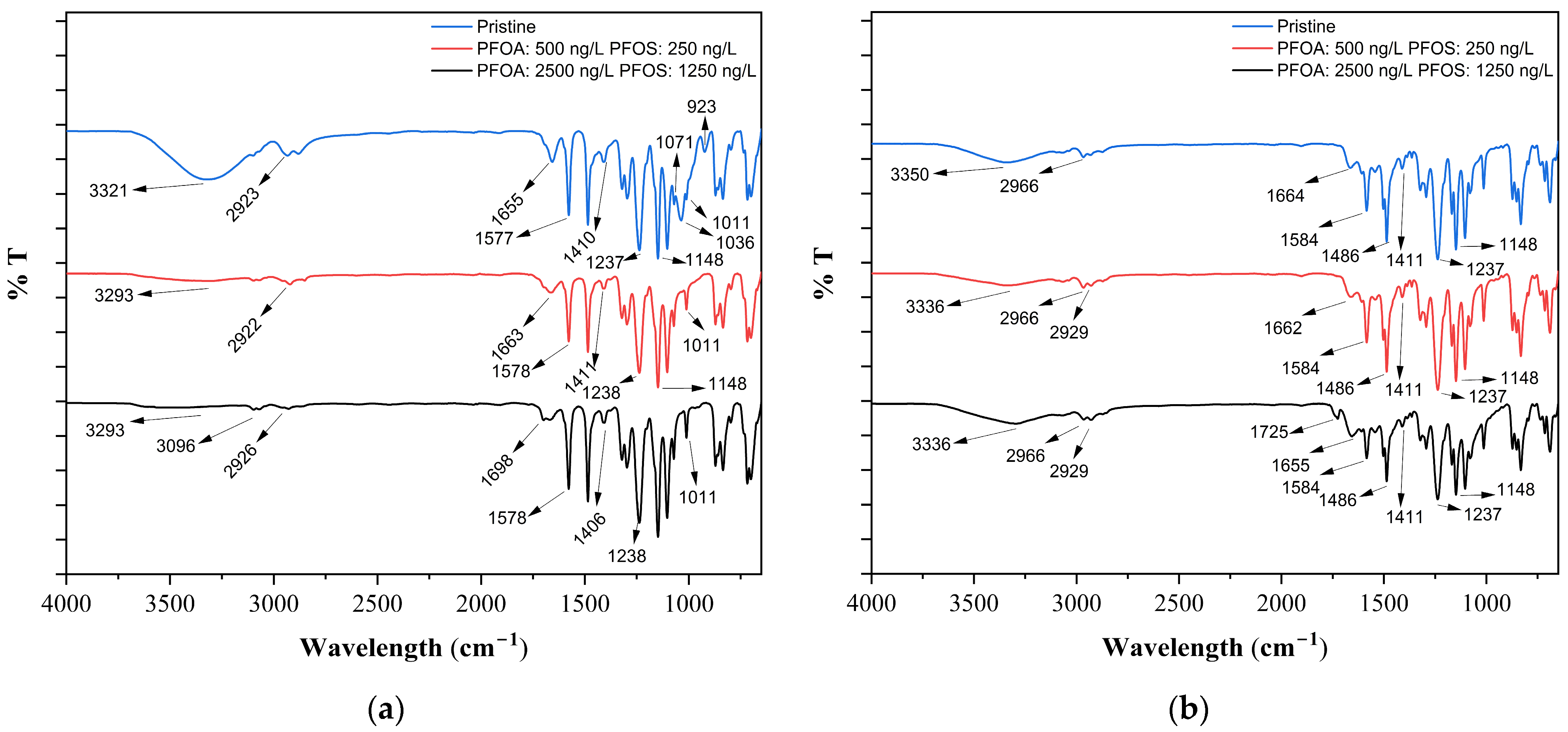
3.3. Membrane Autospy
4. Discussion
5. Conclusions
- The application of seven-layer PAH/PAA coatings resulted in a substantial increase in negative surface charge, reducing the zeta potential from −16.8 mV to −32.4 mV for UP150 and from −13.2 mV to −30.1 mV for UP020, thereby enhancing electrostatic repulsion against anionic PFAS molecules.
- Despite having larger nominal MWCO values (150 and 20 kDa), the coated membranes achieved remarkable PFAS removal, with PFOA and PFOS rejection rates increasing from ~2–34% to 43–78% depending on membrane type and pollutant concentration.
- Coating led to a measurable decrease in both MWCO (46.7% for UP150, 40% for UP020) and porosity (13.4% and 7.2%, respectively), supporting the presence of a tighter and more selective separation layer.
- Among the tested membranes, coated UP020 achieved PFAS removal efficiencies comparable to those of the NF membrane (NP030), indicating that surface charge engineering can effectively compensate for larger pore sizes in UF membranes.
- FTIR analysis revealed minor spectral changes after PFAS exposure (e.g., appearance of a 1727 cm−1 band), suggesting limited surface interaction, while EDX results showed no fluorine accumulation on the membrane surface, indicating that adsorption was not the dominant retention mechanism.
Future Challenges
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Statement on FDA’s Scientific Work to Understand per- and Polyfluoroalkyl Substances (PFAS) in Food, and Findings from Recent FDA Surveys|FDA. Available online: https://web.archive.org/web/20190614052512/https://www.fda.gov/news-events/press-announcements/statement-fdas-scientific-work-understand-and-polyfluoroalkyl-substances-pfas-food-and-findings (accessed on 21 May 2025).
- Grandjean, P.; Clapp, R. Perfluorinated Alkyl Substances: Emerging Insights into Health Risks. New Solut. 2015, 25, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Londhe, K.; Lee, C.-S.; Zhang, Y.; Grdanovska, S.; Kroc, T.; Cooper, C.A.; Venkatesan, A.K. Energy Evaluation of Electron Beam Treatment of Perfluoroalkyl Substances in Water: A Critical Review. ACS EST Eng. 2021, 1, 827–841. [Google Scholar] [CrossRef]
- Pitter, G.; Da Re, F.; Canova, C.; Barbieri, G.; Jeddi, M.Z.; Daprà, F.; Manea, F.; Zolin, R.; Bettega, A.M.; Stopazzolo, G.; et al. Serum Levels of Perfluoroalkyl Substances (PFAS) in Adolescents and Young Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy: A Cross-Sectional Study Based on a Health Surveillance Program. Environ. Health Perspect. 2020, 128, 027007. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.; Cordner, A.; Brown, P. Producing Ignorance Through Regulatory Structure: The Case of Per- and Polyfluoroalkyl Substances (PFAS). Sociol. Perspect. 2021, 64, 631–656. [Google Scholar] [CrossRef]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef]
- Sadia, M.; Nollen, I.; Helmus, R.; ter Laak, T.L.; Béen, F.; Praetorius, A.; van Wezel, A.P. Occurrence, Fate, and Related Health Risks of PFAS in Raw and Produced Drinking Water. Environ. Sci. Technol. 2023, 57, 3062–3074. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human Exposure to Per- and Polyfluoroalkyl Substances (PFAS) through Drinking Water: A Review of the Recent Scientific Literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Crone, B.C.; Speth, T.F.; Wahman, D.G.; Smith, S.J.; Abulikemu, G.; Kleiner, E.J.; Pressman, J.G. Occurrence of Per- and Polyfluoroalkyl Substances (PFAS) in Source Water and Their Treatment in Drinking Water. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2359–2396. [Google Scholar] [CrossRef]
- Horst, J.; McDonough, J.; Ross, I.; Dickson, M.; Miles, J.; Hurst, J.; Storch, P. Water Treatment Technologies for PFAS: The Next Generation. Groundw. Monit. Remediat. 2018, 38, 13–23. [Google Scholar] [CrossRef]
- Olimattel, K.; Zhai, L.; Sadmani, A.H.M.A. Enhanced Removal of Perfluorooctane Sulfonic Acid and Perfluorooctanoic Acid via Polyelectrolyte Functionalized Ultrafiltration Membrane: Effects of Membrane Modification and Water Matrix. J. Hazard. Mater. Lett. 2021, 2, 100043. [Google Scholar] [CrossRef]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Thelakkat Kochunarayanan, P.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A Review of Emerging Technologies for Remediation of PFASs. Remediation 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS Removal by Ion Exchange Resins: A Review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef] [PubMed]
- Rodowa, A.E.; Knappe, D.R.U.; Chiang, S.-Y.D.; Pohlmann, D.; Varley, C.; Bodour, A.; Field, J.A. Pilot Scale Removal of Per-and Polyfluoroalkyl Substances and Precursors from AFFF-Impacted Groundwater by Granular Activated Carbon. Environ. Sci. 2020, 6, 1083–1094. [Google Scholar] [CrossRef]
- Liang, S.; Mora, R.; Huang, Q.; Casson, R.; Wang, Y.; Woodard, S.; Anderson, H. Field Demonstration of Coupling Ion-Exchange Resin with Electrochemical Oxidation for Enhanced Treatment of per-and Polyfluoroalkyl Substances (PFAS) in Groundwater. Chem. Eng. J. Adv. 2022, 9, 100216. [Google Scholar] [CrossRef]
- Amen, R.; Ibrahim, A.; Shafqat, W.; Hassan, E.B. A Critical Review on PFAS Removal from Water: Removal Mechanism and Future Challenges. Sustainability 2023, 15, 16173. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, X.; Faria, A.F.; Quiñones, K.Y.D.; Zhang, C.; He, Q.; Ma, J.; Shen, Y.; Zhi, Y. Evaluating the Efficiency of Nanofiltration and Reverse Osmosis Membrane Processes for the Removal of Per-and Polyfluoroalkyl Substances from Water: A Critical Review. Sep. Purif. Technol. 2022, 302, 122161. [Google Scholar] [CrossRef]
- Ma, Q.; Lei, Q.; Liu, F.; Song, Z.; Khusid, B.; Zhang, W. Evaluation of Commercial Nanofiltration and Reverse Osmosis Membrane Filtration to Remove Per-and Polyfluoroalkyl Substances (PFAS): Effects of Transmembrane Pressures and Water Matrices. Water Environ. Res. 2024, 96, e10983. [Google Scholar] [CrossRef]
- Fang, F.; Chen, S.; Shi, K.; Xu, S.; Yi, Z.; Lei, L.; Zhuang, L.; Wan, H.; Xu, Z. Hydrophilic Membranes for Effective Removal of PFAS from Water: Anti-Fouling, Durability, and Reusability. Sep. Purif. Technol. 2024, 348, 127379. [Google Scholar] [CrossRef]
- Lee, T.; Speth, T.F.; Nadagouda, M.N. High-Pressure Membrane Filtration Processes for Separation of Per-and Polyfluoroalkyl Substances (PFAS). Chem. Eng. J. 2022, 431, 134023. [Google Scholar] [CrossRef]
- Das, S.; Ronen, A. A Review on Removal and Destruction of Per-and Polyfluoroalkyl Substances (PFAS) by Novel Membranes. Membranes 2022, 12, 662. [Google Scholar] [CrossRef]
- Zeng, C.; Tanaka, S.; Suzuki, Y.; Yukioka, S.; Fujii, S. Rejection of Trace Level Perfluorohexanoic Acid (PFHxA) in Pure Water by Loose Nanofiltration Membrane. J. Water Environ. Technol. 2017, 15, 120–127. [Google Scholar] [CrossRef]
- Mhlongo, S.A.; Sibali, L.L.; Ndibewu, P.P. Some Aspects of the Synthesis, Characterization and Modification of Poly (Ether) Sulfone Polymeric Membrane for Removal of Persistent Organic Pollutants in Wastewater Samples. J. Water Chem. Technol. 2023, 45, 388–401. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Kanakaraju, D.; Lim, J.W.; Lau, W.J.; Ismail, A.F. Photocatalytic Membranes: A New Perspective for Persistent Organic Pollutants Removal. Environ. Sci. Pollut. Res. 2022, 29, 12506–12530. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, G.; Yang, F. Adsorptive Removal and Oxidation of Organic Pollutants from Water Using a Novel Membrane. Chem. Eng. J. 2010, 156, 553–556. [Google Scholar] [CrossRef]
- Esfahani, A.R.; Zhai, L.; Sadmani, A.H.M.A. Removing Heavy Metals from Landfill Leachate Using Electrospun Polyelectrolyte Fiber Mat-Laminated Ultrafiltration Membrane. J. Environ. Chem. Eng. 2021, 9, 105355. [Google Scholar] [CrossRef]
- Saqib, J.; Aljundi, I.H. Membrane Fouling and Modification Using Surface Treatment and Layer-by-Layer Assembly of Polyelectrolytes: State-of-the-Art Review. J. Water Process Eng. 2016, 11, 68–87. [Google Scholar] [CrossRef]
- Baig, U.; Waheed, A.; Salih, H.A.; Matin, A.; Alshami, A.; Aljundi, I.H. Facile Modification of NF Membrane by Multi-Layer Deposition of Polyelectrolytes for Enhanced Fouling Resistance. Polymers 2021, 13, 3728. [Google Scholar] [CrossRef]
- Olimattel, K.; Church, J.; Lee, W.H.; Chumbimuni-Torres, K.Y.; Zhai, L.; Anwar Sadmani, A.H.M. Enhanced Fouling Resistance and Antimicrobial Property of Ultrafiltration Membranes via Polyelectrolyte-Assisted Silver Phosphate Nanoparticle Immobilization. Membranes 2020, 10, 293. [Google Scholar] [CrossRef]
- Bóna, Á.; Galambos, I.; Nemestóthy, N. Progress towards Stable and High-Performance Polyelectrolyte Multilayer Nanofiltration Membranes for Future Wastewater Treatment Applications. Membranes 2023, 13, 368. [Google Scholar] [CrossRef]
- Petrila, L.-M.; Bucatariu, F.; Mihai, M.; Teodosiu, C. Polyelectrolyte Multilayers: An Overview on Fabrication, Properties, and Biomedical and Environmental Applications. Materials 2021, 14, 4152. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a Novel Antifouling Mixed Matrix PES Membrane by Embedding Graphene Oxide Nanoplates. J. Memb. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- US EPA (2021) Validated Test Method 8327: Per-and Polyfluoroalkyl Substances (PFAS) Using External Standard Calibration and Multiple Reaction Monitoring (MRM) Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS). Available online: https://www.epa.gov/hw-sw846/validated-test-method-8327-and-polyfluoroalkyl-substances-pfas-using-external-standard (accessed on 20 April 2025).
- Rabiee, H.; Vatanpour, V.; Farahani, M.H.D.A.; Zarrabi, H. Improvement in Flux and Antifouling Properties of PVC Ultrafiltration Membranes by Incorporation of Zinc Oxide (ZnO) Nanoparticles. Sep. Purif. Technol. 2015, 156, 299–310. [Google Scholar] [CrossRef]
- Hamid, N.A.A.; Ismail, A.F.; Matsuura, T.; Zularisam, A.W.; Lau, W.J.; Yuliwati, E.; Abdullah, M.S. Morphological and Separation Performance Study of Polysulfone/Titanium Dioxide (PSF/TiO2) Ultrafiltration Membranes for Humic Acid Removal. Desalination 2011, 273, 85–92. [Google Scholar] [CrossRef]
- Imbrogno, A.; Calvo, J.I.; Breida, M.; Schwaiger, R.; Schäfer, A.I. Molecular Weight Cut off (MWCO) Determination in Ultra-and Nanofiltration: Review of Methods and Implications on Organic Matter Removal. Sep. Purif. Technol. 2024, 354, 128612. [Google Scholar] [CrossRef]
- Calvo, J.I.; Peinador, R.I.; Thom, V.; Schleuss, T.; ToVinh, K.; Prádanos, P.; Hernández, A. Comparison of Pore Size Distributions from Dextran Retention Tests and Liquid-Liquid Displacement Porosimetry. Microporous Mesoporous Mater. 2017, 250, 170–176. [Google Scholar] [CrossRef]
- Abraham, K.; Kunst, S.; Flöter, E. Membrane Characterisation for Fractionated Dextran Analysis in Sugar Industry. Food Anal. Methods 2019, 12, 1092–1102. [Google Scholar] [CrossRef]
- Machado, G.; Beppu, M.M.; Feil, A.F.; Figueroa, C.A.; Correia, R.R.B.; Teixeira, S.R. Silver Nanoparticles Obtained in PAH/PAA-Based Multilayers by Photochemical Reaction. J. Phys. Chem. C 2009, 113, 19005–19010. [Google Scholar] [CrossRef]
- Srivastava, S.; Kotov, N.A. Composite Layer-by-Layer (LBL) Assembly with Inorganic Nanoparticles and Nanowires. Acc. Chem. Res. 2008, 41, 1831–1841. [Google Scholar] [CrossRef]
- Xu, G.-R.; Wang, S.-H.; Zhao, H.-L.; Wu, S.-B.; Xu, J.-M.; Li, L.; Liu, X.-Y. Layer-by-Layer (LBL) Assembly Technology as Promising Strategy for Tailoring Pressure-Driven Desalination Membranes. J. Memb. Sci. 2015, 493, 428–443. [Google Scholar] [CrossRef]
- Wang, T.C.; Rubner, M.F.; Cohen, R.E. Polyelectrolyte Multilayer Nanoreactors for Preparing Silver Nanoparticle Composites: Controlling Metal Concentration and Nanoparticle Size. Langmuir 2002, 18, 3370–3375. [Google Scholar] [CrossRef]
- Joly, S.; Kane, R.; Radzilowski, L.; Wang, T.; Wu, A.; Cohen, R.E.; Thomas, E.L.; Rubner, M.F. Multilayer Nanoreactors for Metallic and Semiconducting Particles. Langmuir 2000, 16, 1354–1359. [Google Scholar] [CrossRef]
- Brant, J.A. Characterization of the Hydrophobicity of Polymeric Reverse Osmosis and Nanofiltration Membranes: Implications to Membrane Fouling; University of Nevada: Reno, Nevada, 2000; ISBN 059977276X. [Google Scholar]
- Elcik, H.; Cakmakci, M.; Ozkaya, B. The Fouling Effects of Microalgal Cells on Crossflow Membrane Filtration. J. Memb. Sci. 2016, 499, 116–125. [Google Scholar] [CrossRef]
- Polat, B.; Ozay, Y.; Bilici, Z.; Kucukkara, İ.; Dizge, N. Membrane Modification with Semiconductor Diode Laser to Reduce Membrane Biofouling for External MBR System and Modelling Study. Sep. Purif. Technol. 2020, 241, 116747. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, G.; Xiong, Y.; Zhou, M.; Zhang, S.; Tang, C.Y.; Meng, F. Unveiling the Susceptibility of Functional Groups of Poly (Ether Sulfone)/Polyvinylpyrrolidone Membranes to NaOCl: A Two-Dimensional Correlation Spectroscopic Study. Environ. Sci. Technol. 2017, 51, 14342–14351. [Google Scholar] [CrossRef]
- Saxena, N.; Prabhavathy, C.; De, S.; DasGupta, S. Flux Enhancement by Argon–Oxygen Plasma Treatment of Polyethersulfone Membranes. Sep. Purif. Technol. 2009, 70, 160–165. [Google Scholar] [CrossRef]
- Antón, E.; Álvarez, J.R.; Palacio, L.; Prádanos, P.; Hernández, A.; Pihlajamäki, A.; Luque, S. Ageing of Polyethersulfone Ultrafiltration Membranes under Long-Term Exposures to Alkaline and Acidic Cleaning Solutions. Chem. Eng. Sci. 2015, 134, 178–195. [Google Scholar] [CrossRef]
- Proner, M.C.; Ramalho Marques, I.; Ambrosi, A.; Rezzadori, K.; da Costa, C.; Zin, G.; Tres, M.V.; Di Luccio, M. Impact of MWCO and Dopamine/Polyethyleneimine Concentrations on Surface Properties and Filtration Performance of Modified Membranes. Membranes 2020, 10, 239. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Zhang, P.-B.; Sun, J.; Liu, C.-J.; Yi, Z.; Zhu, L.-P.; Xu, Y.-Y. Versatile Antifouling Polyethersulfone Filtration Membranes Modified via Surface Grafting of Zwitterionic Polymers from a Reactive Amphiphilic Copolymer Additive. J. Colloid. Interface Sci. 2015, 448, 380–388. [Google Scholar] [CrossRef]
- Long, L.; Wu, C.; Yang, Z.; Tang, C.Y. Carbon Nanotube Interlayer Enhances Water Permeance and Antifouling Performance of Nanofiltration Membranes: Mechanisms and Experimental Evidence. Environ. Sci. Technol. 2022, 56, 2656–2664. [Google Scholar] [CrossRef]
- Coates, J. Encyclopedia of Analytical Chemistry—Interpretation of Infrared Spectra, A Practical Approach; John Wiley & Sons Ltd.: Chichester, UK, 2004; pp. 1–23. [Google Scholar]
- Trzcinski, A.P.; Harada, K.H. Comparison of Perfluorooctane Sulfonate (PFOS), Perfluorooctanoic Acid (PFOA) and Perfluorobutane Sulfonate (PFBS) Removal in a Combined Adsorption and Electrochemical Oxidation Process. Sci. Total Environ. 2024, 927, 172184. [Google Scholar] [CrossRef]
- Fatima, M.; Kelso, C.; Hai, F. Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) Adsorption onto Different Adsorbents: A Critical Review of the Impact of Their Chemical Structure and Retention Mechanisms in Soil and Groundwater. Water 2025, 17, 1401. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Xu, C.; Zhi, R.; Miao, R.; Liang, T.; Yue, X.; Lv, Y.; Liu, T. Perfluorooctane Sulfonate and Perfluorobutane Sulfonate Removal from Water by Nanofiltration Membrane: The Roles of Solute Concentration, Ionic Strength, and Macromolecular Organic Foulants. Chem. Eng. J. 2018, 332, 787–797. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, T.; Hu, G.; Ma, J.; Song, R.; Li, J. Efficient Removal of Perfluorooctane Sulphonate by Nanofiltration: Insights into the Effect and Mechanism of Coexisting Inorganic Ions and Humic Acid. J. Memb. Sci. 2020, 610, 118176. [Google Scholar] [CrossRef]
- Hara-Yamamura, H.; Inoue, K.; Matsumoto, T.; Honda, R.; Ninomiya, K.; Yamamura, H. Rejection of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Severely Chlorine Damaged RO Membranes with Different Salt Rejection Ratios. Chem. Eng. J. 2022, 446, 137398. [Google Scholar] [CrossRef]
- Tang, C.Y.; Fu, Q.S.; Robertson, A.P.; Criddle, C.S.; Leckie, J.O. Use of Reverse Osmosis Membranes to Remove Perfluorooctane Sulfonate (PFOS) from Semiconductor Wastewater. Environ. Sci. Technol. 2006, 40, 7343–7349. [Google Scholar] [CrossRef]
- Wu, B.; Fane, A.G. Microbial Relevant Fouling in Membrane Bioreactors: Influencing Factors, Characterization, and Fouling Control. Membranes 2012, 2, 565–584. [Google Scholar] [CrossRef]
- Fortunato, L.; Jeong, S.; Wang, Y.; Behzad, A.R.; Leiknes, T.O. Integrated Approach to Characterize Fouling on a Flat Sheet Membrane Gravity Driven Submerged Membrane Bioreactor. Bioresour. Technol. 2016, 222, 335–343. [Google Scholar] [CrossRef]
- Dalle Vacche, S.; Geiser, V.; Leterrier, Y.; Månson, J.-A.E. Time-Intensity Superposition for Photoinitiated Polymerization of Fluorinated and Hyperbranched Acrylate Nanocomposites. Polymer 2010, 51, 334–341. [Google Scholar] [CrossRef][Green Version]






| Membrane | Type | Materials | Molecular Weight Cut Off (MWCO) (kDA) | pH Range |
|---|---|---|---|---|
| UP150 | UF | PES 1 | 150 kDA | 1–14 |
| UP020 | UF | PES | 20 kDA | 0–14 |
| NP030 | NF | PES | 0.5 kDA | 0–14 |
| BW30XLE | RO | PA-TFC 2 | 0.1 kDA | 1–13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turk, O.K.; Cakmakci, M.; Zengin, I.H.; Karadag, D.; Yuksel, E. Improving PFAS Rejection by Ultrafiltration Membranes via Polyelectrolyte Multilayer Coating. Membranes 2025, 15, 172. https://doi.org/10.3390/membranes15060172
Turk OK, Cakmakci M, Zengin IH, Karadag D, Yuksel E. Improving PFAS Rejection by Ultrafiltration Membranes via Polyelectrolyte Multilayer Coating. Membranes. 2025; 15(6):172. https://doi.org/10.3390/membranes15060172
Chicago/Turabian StyleTurk, Oruc Kaan, Mehmet Cakmakci, Ismail Hakki Zengin, Dogan Karadag, and Ebubekir Yuksel. 2025. "Improving PFAS Rejection by Ultrafiltration Membranes via Polyelectrolyte Multilayer Coating" Membranes 15, no. 6: 172. https://doi.org/10.3390/membranes15060172
APA StyleTurk, O. K., Cakmakci, M., Zengin, I. H., Karadag, D., & Yuksel, E. (2025). Improving PFAS Rejection by Ultrafiltration Membranes via Polyelectrolyte Multilayer Coating. Membranes, 15(6), 172. https://doi.org/10.3390/membranes15060172







