Uncovering the Mechanisms of Intracellular Membrane Trafficking by Reconstituted Membrane Systems
Abstract
1. Introduction
2. The Powerful Reconstituted Membrane Systems Applied in the Research of Intracellular Membrane Trafficking
2.1. The Different Types of Liposomes Employed in the Reconstituted Membrane Systems
2.1.1. Liposomes and Proteoliposomes
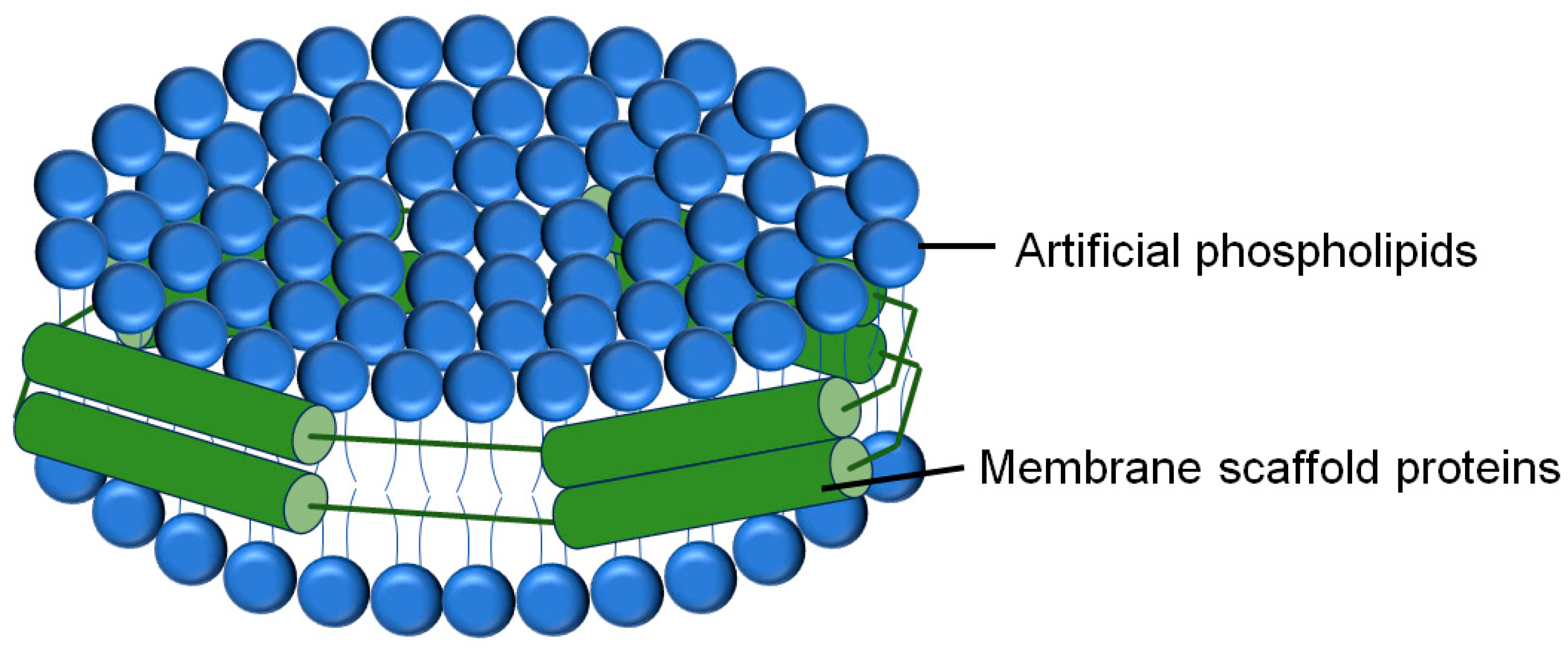
2.1.2. GUVs, LUVs and SUVs
2.2. The Liposome-Based Assays for the Measurement of Vesicle Fusion
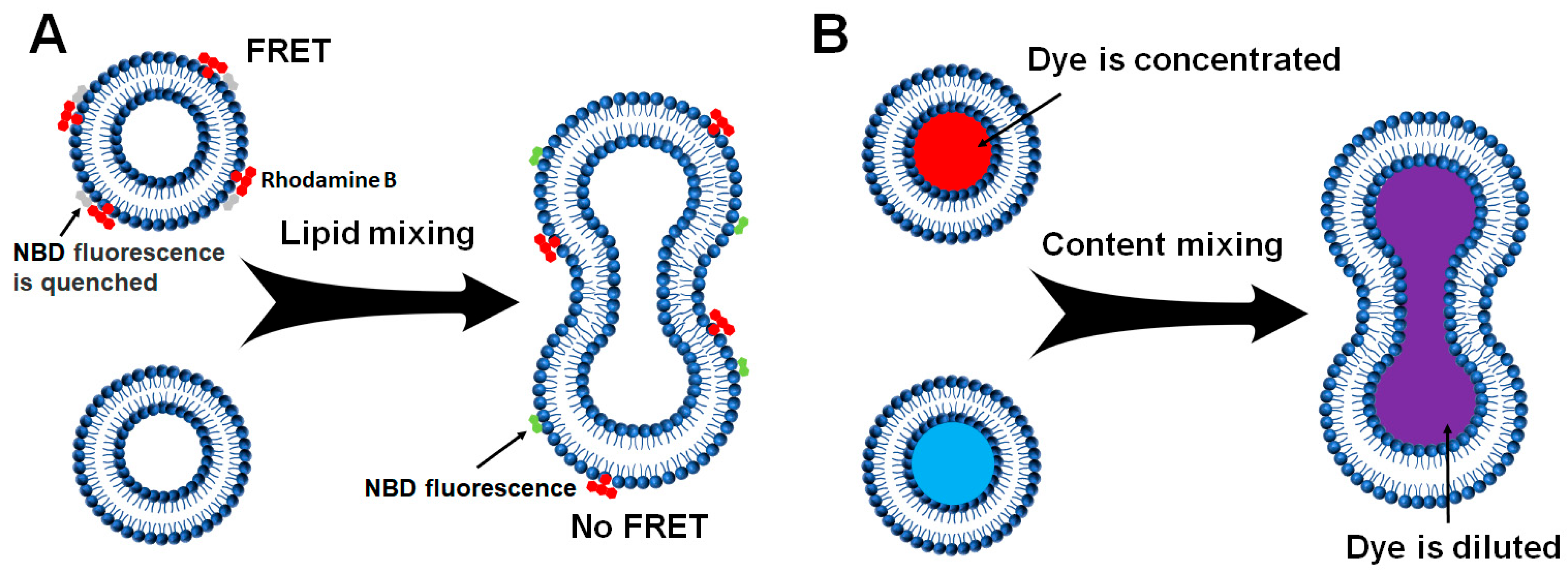
2.2.1. Liposome–Liposome Lipid Mixing
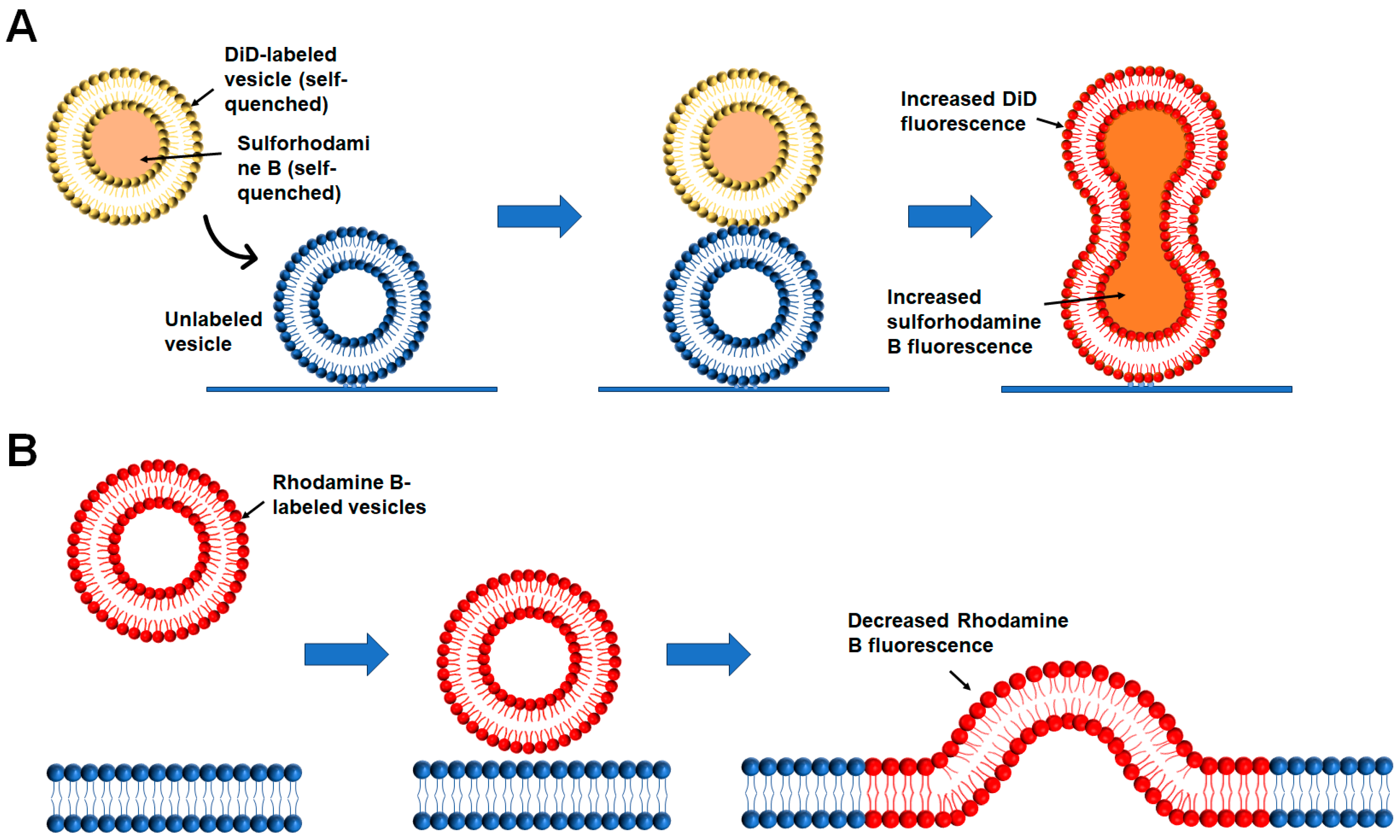
2.2.2. Liposome-Liposome Content Mixing
2.2.3. Liposome-Nanodisc Lipid Mixing and Content Mixing
2.2.4. Single-Vesicle Fusion
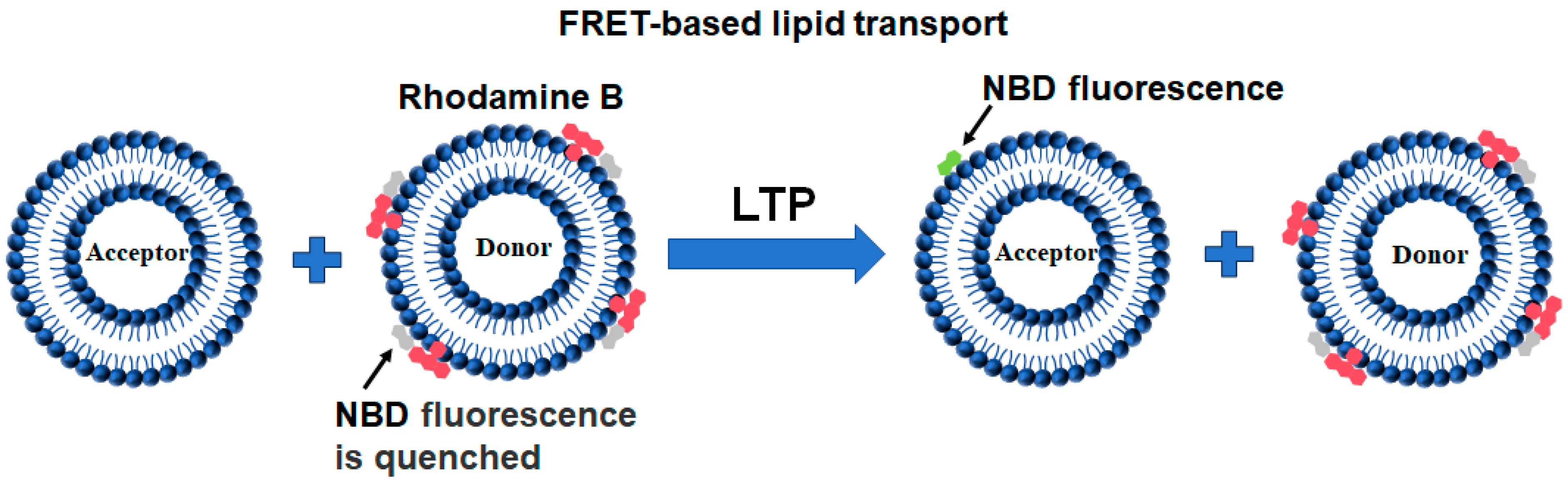
2.3. FRET-Based Lipid Transport
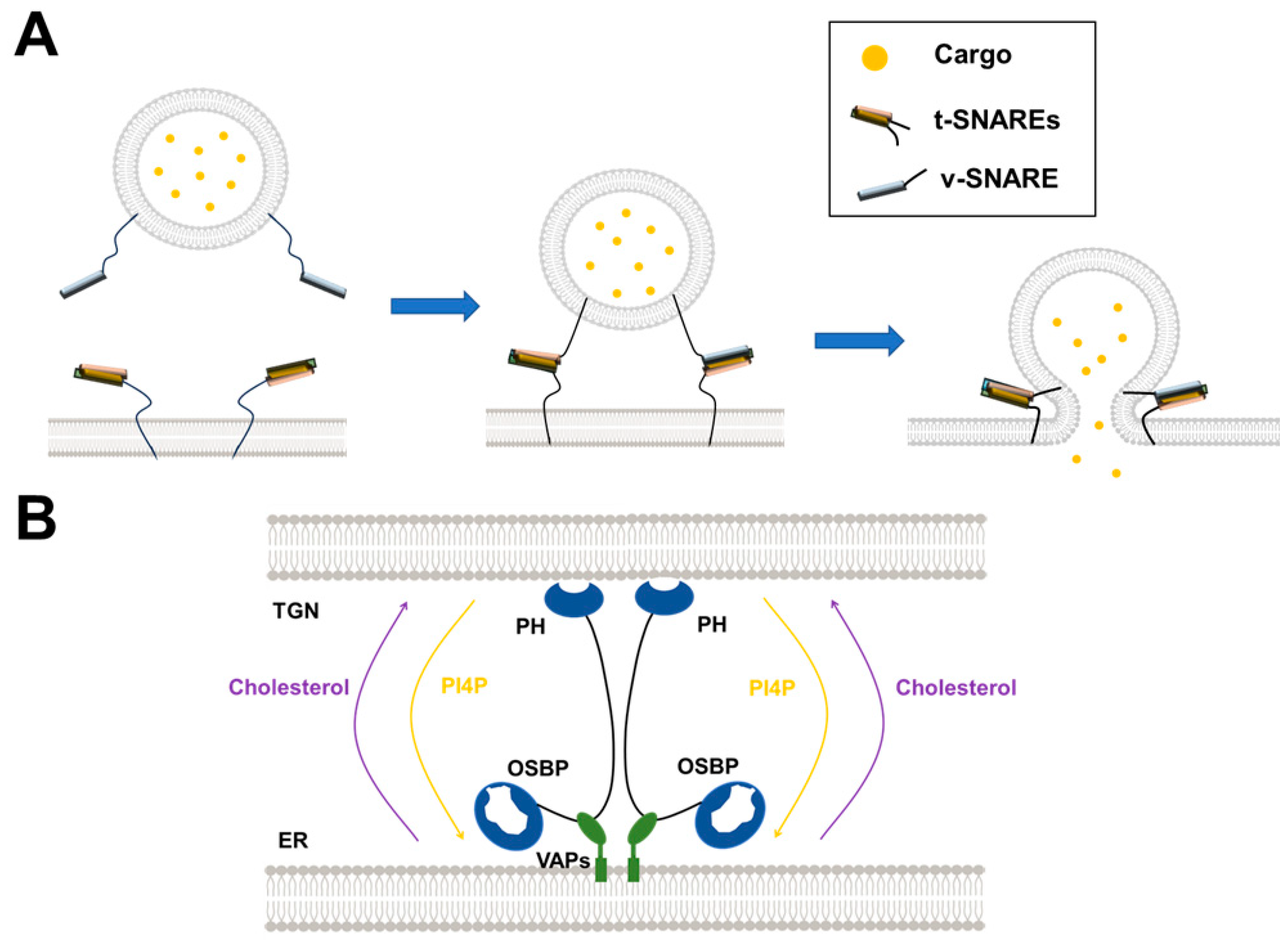
3. Uncovering the Mechanisms of SNARE-Mediated Intracellular Vesicle Fusion by Reconstituted Membrane Systems
3.1. Synaptic Vesicle Fusion
3.2. GLUT4 Vesicle Fusion
3.3. Autophagosome–Lysosome Fusion
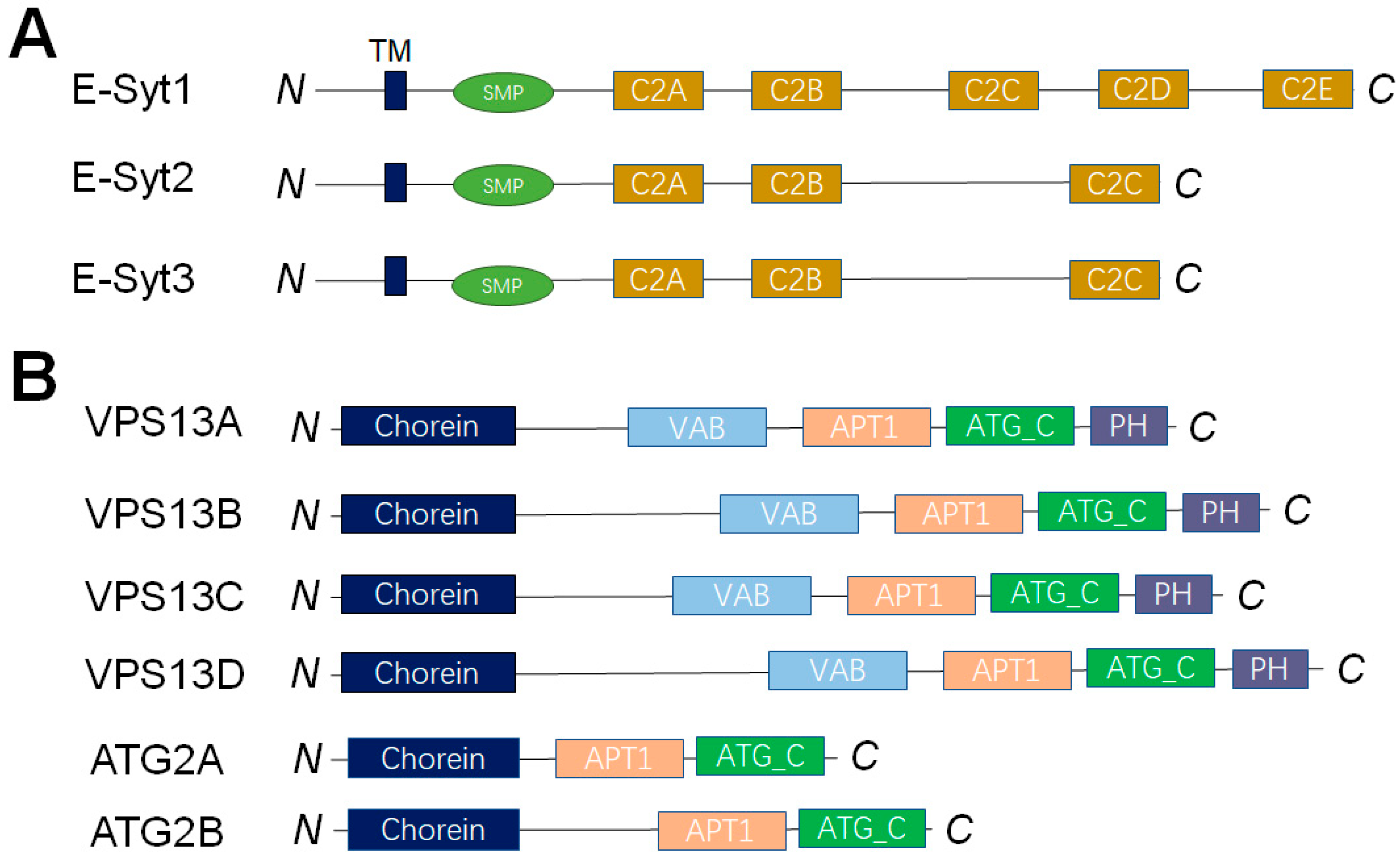
4. Dissecting the LTP-Mediated Lipid Transport at MCSs by Reconstituted Membrane Systems
4.1. LTPs Without Apparent Lipid Selectivity
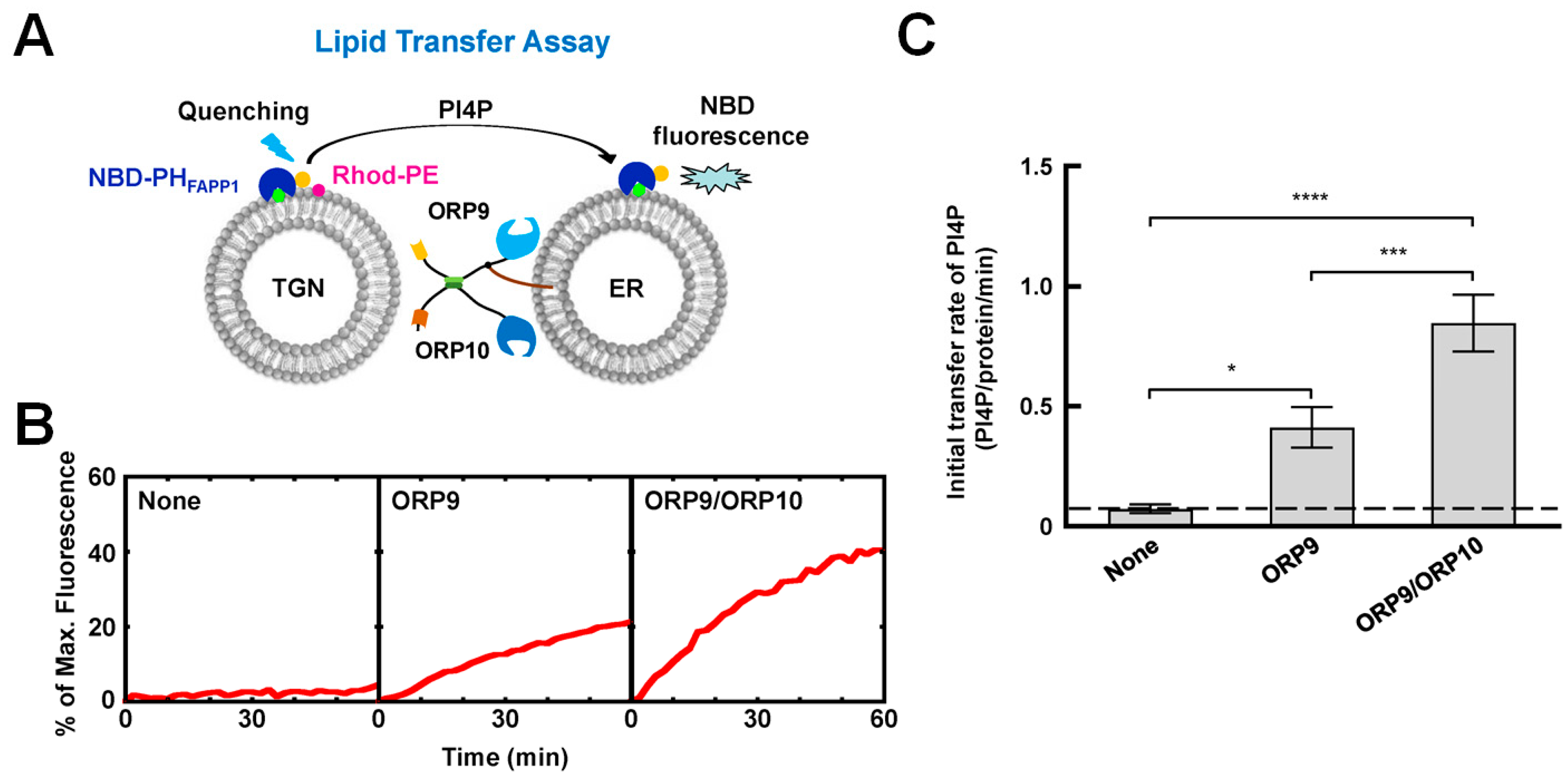
4.2. The OSBP/ORPs Family with Lipid Selectivity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Meer, G.; de Kroon, A.I. Lipid map of the mammalian cell. J. Cell Sci. 2011, 124, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Preta, G. Lipids in the cell: Organisation regulates function. Cell. Mol. Life Sci. 2018, 75, 1909–1927. [Google Scholar] [CrossRef] [PubMed]
- Acoba, M.G.; Senoo, N.; Claypool, S.M. Phospholipid ebb and flow makes mitochondria go. J. Cell Biol. 2020, 219, e202003131. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Sudhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Lev, S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 739–750. [Google Scholar] [CrossRef]
- Reinisch, K.M.; Prinz, W.A. Mechanisms of nonvesicular lipid transport. J. Cell Biol. 2021, 220, e202012058. [Google Scholar] [CrossRef]
- Rigaud, J.L.; Pitard, B.; Levy, D. Reconstitution of membrane proteins into liposomes: Application to energy-transducing membrane proteins. Biochim. Biophys. Acta 1995, 1231, 223–246. [Google Scholar] [CrossRef]
- Burgoyne, R.D.; Morgan, A. Membrane trafficking: Three steps to fusion. Curr. Biol. 2007, 17, R255–R258. [Google Scholar] [CrossRef]
- Zhang, Y.; Hughson, F.M. Chaperoning SNARE Folding and Assembly. Annu. Rev. Biochem. 2021, 90, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Pobbati, A.V.; Stein, A.; Fasshauer, D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science 2006, 313, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.B.; Wiederhold, K.; Muller, E.M.; Milosevic, I.; Nagy, G.; de Groot, B.L.; Grubmuller, H.; Fasshauer, D. Sequential N- to C-terminal SNARE complex assembly drives priming and fusion of secretory vesicles. EMBO J. 2006, 25, 955–966. [Google Scholar] [CrossRef]
- Li, F.; Pincet, F.; Perez, E.; Eng, W.S.; Melia, T.J.; Rothman, J.E.; Tareste, D. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat. Struct. Mol. Biol. 2007, 14, 890–896. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, J.; Bian, X.; Kumar, A. Quantitative Models of Lipid Transfer and Membrane Contact Formation. Contact 2022, 5, 1–21. [Google Scholar] [CrossRef]
- Olkkonen, V.M.; Ikonen, E. Getting to Grips with the Oxysterol-Binding Protein Family—A Forty Year Perspective. Contact 2024, 7, 25152564241273598. [Google Scholar] [CrossRef]
- Wong, L.H.; Gatta, A.T.; Levine, T.P. Lipid transfer proteins: The lipid commute via shuttles, bridges and tubes. Nat. Rev. Mol. Cell Biol. 2019, 20, 85–101. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Racker, E. Reconstitution of a calcium pump with phospholipids and a purified Ca++Adenosine triphosphatase from sacroplasmic reticulum. J. Biol. Chem. 1972, 247, 8198–8200. [Google Scholar] [CrossRef]
- Keller, B.U.; Hedrich, R.; Vaz, W.L.; Criado, M. Single channel recordings of reconstituted ion channel proteins: An improved technique. Pflug. Arch. Eur. J. Physiol. 1988, 411, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ciancaglini, P.; Simão, A.M.S.; Bolean, M.; Millán, J.L.; Rigos, C.F.; Yoneda, J.S.; Colhone, M.C.; Stabeli, R.G. Proteoliposomes in nanobiotechnology. Biophys. Rev. 2012, 4, 67–81. [Google Scholar] [CrossRef] [PubMed]
- de Lima Santos, H.; Lopes, M.L.; Maggio, B.; Ciancaglini, P. Na,K-ATPase reconstituted in liposomes: Effects of lipid composition on hydrolytic activity and enzyme orientation. Colloids Surf. B Biointerfaces 2005, 41, 239–248. [Google Scholar] [CrossRef]
- Witkowska, A.; Jablonski, L.; Jahn, R. A convenient protocol for generating giant unilamellar vesicles containing SNARE proteins using electroformation. Sci. Rep. 2018, 8, 9422. [Google Scholar] [CrossRef]
- Morales-Penningston, N.F.; Wu, J.; Farkas, E.R.; Goh, S.L.; Konyakhina, T.M.; Zheng, J.Y.; Webb, W.W.; Feigenson, G.W. GUV preparation and imaging: Minimizing artifacts. Biochim. Biophys. Acta 2010, 1798, 1324–1332. [Google Scholar] [CrossRef]
- Horger, K.S.; Estes, D.J.; Capone, R.; Mayer, M. Films of agarose enable rapid formation of giant liposomes in solutions of physiologic ionic strength. J. Am. Chem. Soc. 2009, 131, 1810–1819. [Google Scholar] [CrossRef]
- Girard, P.; Pécréaux, J.; Lenoir, G.; Falson, P.; Rigaud, J.L.; Bassereau, P. A new method for the reconstitution of membrane proteins into giant unilamellar vesicles. Biophys. J. 2004, 87, 419–429. [Google Scholar] [CrossRef]
- Roux, A.; Koster, G.; Lenz, M.; Sorre, B.; Manneville, J.B.; Nassoy, P.; Bassereau, P. Membrane curvature controls dynamin polymerization. Proc. Natl. Acad. Sci. USA 2010, 107, 4141–4146. [Google Scholar] [CrossRef]
- Baumgart, T.; Hammond, A.T.; Sengupta, P.; Hess, S.T.; Holowka, D.A.; Baird, B.A.; Webb, W.W. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 2007, 104, 3165–3170. [Google Scholar] [CrossRef]
- Wu, X.; Ganzella, M.; Zhou, J.; Zhu, S.; Jahn, R.; Zhang, M. Vesicle Tethering on the Surface of Phase-Separated Active Zone Condensates. Mol. Cell 2021, 81, 13–24.e17. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, H.; Jin, Y.; Kong, X.; Xu, B.; Liu, Y.; Yu, H. Synaptotagmin-1 undergoes phase separation to regulate its calcium-sensitive oligomerization. J. Cell Biol. 2024, 223, e202311191. [Google Scholar] [CrossRef] [PubMed]
- Zhemkov, V.; Ditlev, J.A.; Lee, W.R.; Wilson, M.; Liou, J.; Rosen, M.K.; Bezprozvanny, I. The role of sigma 1 receptor in organization of endoplasmic reticulum signaling microdomains. eLife 2021, 10, e65192. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.G.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of Extrusion Technique for Nanosizing Liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef]
- Liu, Y.; He, R.; Zhu, M.; Yu, H. In Vitro Reconstitution Studies of SNAREs and Their Regulators Mediating GLUT4 Vesicle Fusion. Methods Mol. Biol. 2022, 2473, 141–156. [Google Scholar]
- He, R.; Li, C.; Liu, Y.; Yu, H. Reconstitution and biochemical studies of extended synaptotagmin-mediated lipid transport. Methods Enzymol. 2022, 675, 33–62. [Google Scholar]
- Struck, D.K.; Hoekstra, D.; Pagano, R.E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry 1981, 20, 4093–4099. [Google Scholar] [CrossRef]
- Stryer, L.; Haugland, R.P. Energy transfer: A spectroscopic ruler. Proc. Natl. Acad. Sci. USA 1967, 58, 719–726. [Google Scholar] [CrossRef]
- Reese, C.; Heise, F.; Mayer, A. Trans-SNARE pairing can precede a hemifusion intermediate in intracellular membrane fusion. Nature 2005, 436, 410–414. [Google Scholar] [CrossRef]
- Yu, H.; Rathore, S.S.; Davis, E.M.; Ouyang, Y.; Shen, J. Doc2b promotes GLUT4 exocytosis by activating the SNARE-mediated fusion reaction in a calcium- and membrane bending-dependent manner. Mol. Biol. Cell 2013, 24, 1176–1184. [Google Scholar] [CrossRef]
- Yu, H.; Rathore, S.S.; Lopez, J.A.; Davis, E.M.; James, D.E.; Martin, J.L.; Shen, J. Comparative studies of Munc18c and Munc18-1 reveal conserved and divergent mechanisms of Sec1/Munc18 proteins. Proc. Natl. Acad. Sci. USA 2013, 110, E3271–E3280. [Google Scholar] [CrossRef]
- Yu, H.; Rathore, S.S.; Shen, J. Synip arrests soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent membrane fusion as a selective target membrane SNARE-binding inhibitor. J. Biol. Chem. 2013, 288, 18885–18893. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaart, G.; Holt, M.G.; Bunt, G.; Riedel, D.; Wouters, F.S.; Jahn, R. One SNARE complex is sufficient for membrane fusion. Nat. Struct. Mol. Biol. 2010, 17, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Rathore, S.S.; Gulbranson, D.R.; Shen, J. The N- and C-terminal domains of tomosyn play distinct roles in soluble N-ethylmaleimide-sensitive factor attachment protein receptor binding and fusion regulation. J. Biol. Chem. 2014, 289, 25571–25580. [Google Scholar] [CrossRef]
- Civjan, N.R.; Bayburt, T.H.; Schuler, M.A.; Sligar, S.G. Direct solubilization of heterologously expressed membrane proteins by incorporation into nanoscale lipid bilayers. Biotechniques 2003, 35, 556–560, 562–563. [Google Scholar] [CrossRef]
- Denisov, I.G.; Sligar, S.G. Nanodiscs for structural and functional studies of membrane proteins. Nat. Struct. Mol. Biol. 2016, 23, 481–486. [Google Scholar] [CrossRef]
- Bayburt, T.H.; Sligar, S.G. Membrane protein assembly into Nanodiscs. FEBS Lett. 2010, 584, 1721–1727. [Google Scholar] [CrossRef]
- Daniilidis, M.; Sperl, L.E.; Müller, B.S.; Babl, A.; Hagn, F. Efficient Segmental Isotope Labeling of Integral Membrane Proteins for High-Resolution NMR Studies. J. Am. Chem. Soc. 2024, 146, 15403–15410. [Google Scholar] [CrossRef]
- Dong, X.Q.; Lin, J.Y.; Wang, P.F.; Li, Y.; Wang, J.; Li, B.; Liao, J.; Lu, J.X. Solid-State NMR Studies of the Succinate-Acetate Permease from Citrobacter Koseri in Liposomes and Native Nanodiscs. Life 2021, 11, 908. [Google Scholar] [CrossRef]
- Lee, S.; Shin, J.; Jung, Y.; Son, H.; Shin, J.; Jeong, C.; Kweon, D.H.; Shin, Y.K. Munc18-1 induces conformational changes of syntaxin-1 in multiple intermediates for SNARE assembly. Sci. Rep. 2020, 10, 11623. [Google Scholar] [CrossRef]
- Prakash, P.; Litwin, D.; Liang, H.; Sarkar-Banerjee, S.; Dolino, D.; Zhou, Y.; Hancock, J.F.; Jayaraman, V.; Gorfe, A.A. Dynamics of Membrane-Bound G12V-KRAS from Simulations and Single-Molecule FRET in Native Nanodiscs. Biophys. J. 2019, 116, 179–183. [Google Scholar] [CrossRef]
- Roth, P.; Jeckelmann, J.M.; Fender, I.; Ucurum, Z.; Lemmin, T.; Fotiadis, D. Structure and mechanism of a phosphotransferase system glucose transporter. Nat. Commun. 2024, 15, 7992. [Google Scholar] [CrossRef]
- Hiotis, G.; Notti, R.Q.; Bao, H.; Walz, T. Nanodiscs remain indispensable for Cryo-EM studies of membrane proteins. Curr. Opin. Struct. Biol. 2025, 92, 103042. [Google Scholar] [CrossRef]
- Strickland, K.M.; Neselu, K.; Grant, A.J.; Espy, C.L.; McCarty, N.A.; Schmidt-Krey, I. Reconstitution of Detergent-Solubilized Membrane Proteins into Proteoliposomes and Nanodiscs for Functional and Structural Studies. Methods Mol. Biol. 2021, 2302, 21–35. [Google Scholar]
- Shi, L.; Howan, K.; Shen, Q.T.; Wang, Y.J.; Rothman, J.E.; Pincet, F. Preparation and characterization of SNARE-containing nanodiscs and direct study of cargo release through fusion pores. Nat. Protoc. 2013, 8, 935–948. [Google Scholar] [CrossRef]
- Shi, L.; Shen, Q.T.; Kiel, A.; Wang, J.; Wang, H.W.; Melia, T.J.; Rothman, J.E.; Pincet, F. SNARE proteins: One to fuse and three to keep the nascent fusion pore open. Science 2012, 335, 1355–1359. [Google Scholar] [CrossRef]
- Yoon, T.Y.; Okumus, B.; Zhang, F.; Shin, Y.K.; Ha, T. Multiple intermediates in SNARE-induced membrane fusion. Proc. Natl. Acad. Sci. USA 2006, 103, 19731–19736. [Google Scholar] [CrossRef]
- Mühlenbrock, P.; Sari, M.; Steinem, C. In vitro single vesicle fusion assays based on pore-spanning membranes: Merits and drawbacks. Eur. Biophys. J. 2021, 50, 239–252. [Google Scholar] [CrossRef]
- Li, M.; Oh, T.J.; Fan, H.; Diao, J.; Zhang, K. Syntaxin Clustering and Optogenetic Control for Synaptic Membrane Fusion. J. Mol. Biol. 2020, 432, 4773–4782. [Google Scholar] [CrossRef]
- Diao, J.; Grob, P.; Cipriano, D.J.; Kyoung, M.; Zhang, Y.; Shah, S.; Nguyen, A.; Padolina, M.; Srivastava, A.; Vrljic, M.; et al. Synaptic proteins promote calcium-triggered fast transition from point contact to full fusion. eLife 2012, 1, e00109. [Google Scholar] [CrossRef]
- Kyoung, M.; Zhang, Y.; Diao, J.; Chu, S.; Brunger, A.T. Studying calcium-triggered vesicle fusion in a single vesicle-vesicle content and lipid-mixing system. Nat. Protoc. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Kyoung, M.; Srivastava, A.; Zhang, Y.; Diao, J.; Vrljic, M.; Grob, P.; Nogales, E.; Chu, S.; Brunger, A.T. In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc. Natl. Acad. Sci. USA 2011, 108, E304–E313. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Diao, J.; Cipriano, D.J.; Zhang, Y.; Pfuetzner, R.A.; Padolina, M.S.; Brunger, A.T. Complexin inhibits spontaneous release and synchronizes Ca2+-triggered synaptic vesicle fusion by distinct mechanisms. eLife 2014, 3, e03756. [Google Scholar] [CrossRef] [PubMed]
- Domanska, M.K.; Kiessling, V.; Stein, A.; Fasshauer, D.; Tamm, L.K. Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J. Biol. Chem. 2009, 284, 32158–32166. [Google Scholar] [CrossRef]
- Kiessling, V.; Liang, B.; Kreutzberger, A.J.; Tamm, L.K. Planar Supported Membranes with Mobile SNARE Proteins and Quantitative Fluorescence Microscopy Assays to Study Synaptic Vesicle Fusion. Front. Mol. Neurosci. 2017, 10, 72. [Google Scholar] [CrossRef]
- Jahn, R.; Lang, T.; Südhof, T.C. Membrane fusion. Cell 2003, 112, 519–533. [Google Scholar] [CrossRef]
- Rothman, J.E. The principle of membrane fusion in the cell (Nobel lecture). Angew. Chem. Int. Ed. Engl. 2014, 53, 12676–12694. [Google Scholar] [CrossRef]
- Mesmin, B.; Bigay, J.; Moser von Filseck, J.; Lacas-Gervais, S.; Drin, G.; Antonny, B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 2013, 155, 830–843. [Google Scholar] [CrossRef]
- Schauder, C.M.; Wu, X.; Saheki, Y.; Narayanaswamy, P.; Torta, F.; Wenk, M.R.; De Camilli, P.; Reinisch, K.M. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature 2014, 510, 552–555. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Gulbranson, D.R.; Paine, A.; Rathore, S.S.; Shen, J. Extended synaptotagmins are Ca2+-dependent lipid transfer proteins at membrane contact sites. Proc. Natl. Acad. Sci. USA 2016, 113, 4362–4367. [Google Scholar] [CrossRef]
- He, R.; Liu, F.; Wang, H.; Huang, S.; Xu, K.; Zhang, C.; Liu, Y.; Yu, H. ORP9 and ORP10 form a heterocomplex to transfer phosphatidylinositol 4-phosphate at ER-TGN contact sites. Cell. Mol. Life Sci. 2023, 80, 77. [Google Scholar] [CrossRef]
- Stanton, A.E.; Hughson, F.M. The machinery of vesicle fusion. Curr. Opin. Cell Biol. 2023, 83, 102191. [Google Scholar] [CrossRef] [PubMed]
- Jahn, R.; Cafiso, D.C.; Tamm, L.K. Mechanisms of SNARE proteins in membrane fusion. Nat. Rev. Mol. Cell Biol. 2024, 25, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, L.; Bao, H. Energetics, kinetics, and pathways of SNARE assembly in membrane fusion. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 443–460. [Google Scholar] [CrossRef]
- Palfreyman, M.T.; West, S.E.; Jorgensen, E.M. SNARE Proteins in Synaptic Vesicle Fusion. Adv. Neurobiol. 2023, 33, 63–118. [Google Scholar]
- Rizo, J. Molecular Mechanisms Underlying Neurotransmitter Release. Annu. Rev. Biophys. 2022, 51, 377–408. [Google Scholar] [CrossRef]
- Halim, D.O.; Munson, M.; Gao, F.B. The exocyst complex in neurological disorders. Hum. Genet. 2023, 142, 1263–1270. [Google Scholar] [CrossRef]
- Melland, H.; Arvell, E.H.; Gordon, S.L. Disorders of synaptic vesicle fusion machinery. J. Neurochem. 2021, 157, 130–164. [Google Scholar] [CrossRef]
- Uzay, B.; Kavalali, E.T. Genetic disorders of neurotransmitter release machinery. Front. Synaptic. Neurosci. 2023, 15, 1148957. [Google Scholar] [CrossRef]
- Weber, T.; Zemelman, B.V.; McNew, J.A.; Westermann, B.; Gmachl, M.; Parlati, F.; Söllner, T.H.; Rothman, J.E. SNAREpins: Minimal machinery for membrane fusion. Cell 1998, 92, 759–772. [Google Scholar] [CrossRef]
- Brunger, A.T.; Choi, U.B.; Lai, Y.; Leitz, J.; Zhou, Q. Molecular Mechanisms of Fast Neurotransmitter Release. Annu. Rev. Biophys. 2018, 47, 469–497. [Google Scholar] [CrossRef]
- Cui, L.; Li, H.; Xi, Y.; Hu, Q.; Liu, H.; Fan, J.; Xiang, Y.; Zhang, X.; Shui, W.; Lai, Y. Vesicle trafficking and vesicle fusion: Mechanisms, biological functions, and their implications for potential disease therapy. Mol. Biomed. 2022, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, R.; Xu, X.; Zhu, M.; Yu, H.; Liu, Y. Munc18c accelerates SNARE-dependent membrane fusion in the presence of regulatory proteins α-SNAP and NSF. J. Biol. Chem. 2024, 300, 105782. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Gong, J.; Ye, S.; Yang, X.; Zhang, R.; Ma, C. Munc18 and Munc13 serve as a functional template to orchestrate neuronal SNARE complex assembly. Nat. Commun. 2019, 10, 69. [Google Scholar] [CrossRef]
- Prinslow, E.A.; Stepien, K.P.; Pan, Y.Z.; Xu, J.; Rizo, J. Multiple factors maintain assembled trans-SNARE complexes in the presence of NSF and alphaSNAP. eLife 2019, 8, e38880. [Google Scholar] [CrossRef]
- Ma, C.; Su, L.; Seven, A.B.; Xu, Y.; Rizo, J. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science 2013, 339, 421–425. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, C.; Rathore, S.S.; Stowell, M.H.B.; Yu, H.; Shen, J. SNARE Zippering Is Suppressed by a Conformational Constraint that Is Removed by v-SNARE Splitting. Cell Rep. 2021, 34, 108611. [Google Scholar] [CrossRef]
- Yu, H.; Shen, C.; Liu, Y.; Menasche, B.L.; Ouyang, Y.; Stowell, M.H.B.; Shen, J. SNARE zippering requires activation by SNARE-like peptides in Sec1/Munc18 proteins. Proc. Natl. Acad. Sci. USA 2018, 115, E8421–E8429. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Gu, Y.; Chapman, E.R. Reconstituted synaptotagmin I mediates vesicle docking, priming, and fusion. J. Cell Biol. 2011, 195, 1159–1170. [Google Scholar] [CrossRef]
- Yu, H.; Rathore, S.S.; Shen, C.; Liu, Y.; Ouyang, Y.; Stowell, M.H.; Shen, J. Reconstituting Intracellular Vesicle Fusion Reactions: The Essential Role of Macromolecular Crowding. J. Am. Chem. Soc. 2015, 137, 12873–12883. [Google Scholar] [CrossRef]
- Kiessling, V.; Ahmed, S.; Domanska, M.K.; Holt, M.G.; Jahn, R.; Tamm, L.K. Rapid fusion of synaptic vesicles with reconstituted target SNARE membranes. Biophys. J. 2013, 104, 1950–1958. [Google Scholar] [CrossRef]
- Stöckli, J.; Fazakerley, D.J.; James, D.E. GLUT4 exocytosis. J. Cell Sci. 2011, 124, 4147–4159. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, R.; Zhu, M.; Zhou, L.; Liu, Y.; Yu, H. Assembly-promoting protein Munc18c stimulates SNARE-dependent membrane fusion through its SNARE-like peptide. J. Biol. Chem. 2022, 298, 102470. [Google Scholar] [CrossRef] [PubMed]
- Reggiori, F.; Komatsu, M.; Finley, K.; Simonsen, A. Autophagy: More than a nonselective pathway. Int. J. Cell Biol. 2012, 2012, 219625. [Google Scholar] [CrossRef]
- Zaffagnini, G.; Martens, S. Mechanisms of Selective Autophagy. J. Mol. Biol. 2016, 428, 1714–1724. [Google Scholar] [CrossRef]
- Diao, J.; Liu, R.; Rong, Y.; Zhao, M.; Zhang, J.; Lai, Y.; Zhou, Q.; Wilz, L.M.; Li, J.; Vivona, S.; et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature 2015, 520, 563–566. [Google Scholar] [CrossRef]
- Huang, H.; Ouyang, Q.; Mei, K.; Liu, T.; Sun, Q.; Liu, W.; Liu, R. Acetylation of SCFD1 regulates SNARE complex formation and autophagosome-lysosome fusion. Autophagy 2023, 19, 189–203. [Google Scholar] [CrossRef]
- Huang, H.; Ouyang, Q.; Zhu, M.; Yu, H.; Mei, K.; Liu, R. mTOR-mediated phosphorylation of VAMP8 and SCFD1 regulates autophagosome maturation. Nat. Commun. 2021, 12, 6622. [Google Scholar] [CrossRef]
- Tsujishita, Y.; Hurley, J.H. Structure and lipid transport mechanism of a StAR-related domain. Nat. Struct. Biol. 2000, 7, 408–414. [Google Scholar]
- Im, Y.J.; Raychaudhuri, S.; Prinz, W.A.; Hurley, J.H. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature 2005, 437, 154–158. [Google Scholar] [CrossRef]
- Giordano, F.; Saheki, Y.; Idevall-Hagren, O.; Colombo, S.F.; Pirruccello, M.; Milosevic, I.; Gracheva, E.O.; Bagriantsev, S.N.; Borgese, N.; De Camilli, P. PI(4,5)P(2)-dependent and Ca2+-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell 2013, 153, 1494–1509. [Google Scholar] [CrossRef]
- Saheki, Y.; De Camilli, P. The Extended-Synaptotagmins. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Saheki, Y.; Bian, X.; Schauder, C.M.; Sawaki, Y.; Surma, M.A.; Klose, C.; Pincet, F.; Reinisch, K.M.; De Camilli, P. Control of plasma membrane lipid homeostasis by the extended synaptotagmins. Nat. Cell Biol. 2016, 18, 504–515. [Google Scholar] [CrossRef]
- Bian, X.; Zhang, Z.; Xiong, Q.; De Camilli, P.; Lin, C. A programmable DNA-origami platform for studying lipid transfer between bilayers. Nat. Chem. Biol. 2019, 15, 830–837. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Q.; Yang, Q.; Yang, Y.; Bian, X. DNA-Assisted Assays for Studying Lipid Transfer Between Membranes. Methods Mol. Biol. 2025, 2888, 221–236. [Google Scholar]
- Qian, T.; Li, C.; He, R.; Wan, C.; Liu, Y.; Yu, H. Calcium-dependent and -independent lipid transfer mediated by tricalbins in yeast. J. Biol. Chem. 2021, 296, 100729. [Google Scholar] [CrossRef]
- Qian, T.; Li, C.; Liu, F.; Xu, K.; Wan, C.; Liu, Y.; Yu, H. Arabidopsis synaptotagmin 1 mediates lipid transport in a lipid composition-dependent manner. Traffic 2022, 23, 346–356. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, X.; Zhao, Z.; Jiao, L.; Liu, R.; Wang, K.; Ma, R.; Yang, Y.; Chen, G.; et al. Insights into membrane association of the SMP domain of extended synaptotagmin. Nat. Commun. 2023, 14, 1504. [Google Scholar] [CrossRef]
- Rampoldi, L.; Dobson-Stone, C.; Rubio, J.P.; Danek, A.; Chalmers, R.M.; Wood, N.W.; Verellen, C.; Ferrer, X.; Malandrini, A.; Fabrizi, G.M.; et al. A conserved sorting-associated protein is mutant in chorea-acanthocytosis. Nat. Genet. 2001, 28, 119–120. [Google Scholar] [CrossRef]
- Lesage, S.; Drouet, V.; Majounie, E.; Deramecourt, V.; Jacoupy, M.; Nicolas, A.; Cormier-Dequaire, F.; Hassoun, S.M.; Pujol, C.; Ciura, S.; et al. Loss of VPS13C Function in Autosomal-Recessive Parkinsonism Causes Mitochondrial Dysfunction and Increases PINK1/Parkin-Dependent Mitophagy. Am. J. Hum. Genet. 2016, 98, 500–513. [Google Scholar] [CrossRef]
- Seong, E.; Insolera, R.; Dulovic, M.; Kamsteeg, E.J.; Trinh, J.; Brüggemann, N.; Sandford, E.; Li, S.; Ozel, A.B.; Li, J.Z.; et al. Mutations in VPS13D lead to a new recessive ataxia with spasticity and mitochondrial defects. Ann. Neurol. 2018, 83, 1075–1088. [Google Scholar] [CrossRef]
- Kumar, N.; Leonzino, M.; Hancock-Cerutti, W.; Horenkamp, F.A.; Li, P.; Lees, J.A.; Wheeler, H.; Reinisch, K.M.; De Camilli, P. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J. Cell Biol. 2018, 217, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Valverde, D.P.; Yu, S.; Boggavarapu, V.; Kumar, N.; Lees, J.A.; Walz, T.; Reinisch, K.M.; Melia, T.J. ATG2 transports lipids to promote autophagosome biogenesis. J. Cell Biol. 2019, 218, 1787–1798. [Google Scholar] [CrossRef]
- Taylor, F.R.; Kandutsch, A.A. Oxysterol binding protein. Chem. Phys. Lipids 1985, 38, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Prinz, W.A. The diverse functions of oxysterol-binding proteins. Annu. Rev. Cell Dev. Biol. 2010, 26, 157–177. [Google Scholar] [CrossRef]
- Kentala, H.; Weber-Boyvat, M.; Olkkonen, V.M. OSBP-Related Protein Family: Mediators of Lipid Transport and Signaling at Membrane Contact Sites. Int. Rev. Cell. Mol. Biol. 2016, 321, 299–340. [Google Scholar]
- Olkkonen, V.M. OSBP-related proteins: Liganding by glycerophospholipids opens new insight into their function. Molecules 2013, 18, 13666–13679. [Google Scholar] [CrossRef]
- Adams, A.; Vogl, W. ORP9 knockdown delays the maturation of junction-related endocytic structures in the testis and leads to impaired sperm release. Biol. Reprod. 2020, 103, 1314–1323. [Google Scholar] [CrossRef]
- Sohn, M.; Korzeniowski, M.; Zewe, J.P.; Wills, R.C.; Hammond, G.R.V.; Humpolickova, J.; Vrzal, L.; Chalupska, D.; Veverka, V.; Fairn, G.D.; et al. PI(4,5)P(2) controls plasma membrane PI4P and PS levels via ORP5/8 recruitment to ER-PM contact sites. J. Cell Biol. 2018, 217, 1797–1813. [Google Scholar] [CrossRef]
- Lipp, N.F.; Ikhlef, S.; Milanini, J.; Drin, G. Lipid Exchangers: Cellular Functions and Mechanistic Links with Phosphoinositide Metabolism. Front. Cell. Dev. Biol. 2020, 8, 663. [Google Scholar] [CrossRef]
- Yan, D.; Jauhiainen, M.; Hildebrand, R.B.; Willems van Dijk, K.; Van Berkel, T.J.; Ehnholm, C.; Van Eck, M.; Olkkonen, V.M. Expression of human OSBP-related protein 1L in macrophages enhances atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1618–1624. [Google Scholar] [CrossRef]
- Du, X.; Zhou, L.; Aw, Y.C.; Mak, H.Y.; Xu, Y.; Rae, J.; Wang, W.; Zadoorian, A.; Hancock, S.E.; Osborne, B.; et al. ORP5 localizes to ER-lipid droplet contacts and regulates the level of PI(4)P on lipid droplets. J. Cell Biol. 2020, 219, e201905162. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.X.; Finkel, T. A phosphoinositide signalling pathway mediates rapid lysosomal repair. Nature 2022, 609, 815–821. [Google Scholar] [CrossRef]
- Kawasaki, A.; Sakai, A.; Nakanishi, H.; Hasegawa, J.; Taguchi, T.; Sasaki, J.; Arai, H.; Sasaki, T.; Igarashi, M.; Nakatsu, F. PI4P/PS countertransport by ORP10 at ER-endosome membrane contact sites regulates endosome fission. J. Cell Biol. 2022, 221, e202103141. [Google Scholar] [CrossRef]
- Johansson, M.; Bocher, V.; Lehto, M.; Chinetti, G.; Kuismanen, E.; Ehnholm, C.; Staels, B.; Olkkonen, V.M. The two variants of oxysterol binding protein-related protein-1 display different tissue expression patterns, have different intracellular localization, and are functionally distinct. Mol. Biol. Cell 2003, 14, 903–915. [Google Scholar] [CrossRef]
- van der Kant, R.; Zondervan, I.; Janssen, L.; Neefjes, J. Cholesterol-binding molecules MLN64 and ORP1L mark distinct late endosomes with transporters ABCA3 and NPC1. J. Lipid Res. 2013, 54, 2153–2165. [Google Scholar] [CrossRef]
- Johansson, M.; Lehto, M.; Tanhuanpää, K.; Cover, T.L.; Olkkonen, V.M. The oxysterol-binding protein homologue ORP1L interacts with Rab7 and alters functional properties of late endocytic compartments. Mol. Biol. Cell 2005, 16, 5480–5492. [Google Scholar] [CrossRef]
- Dong, J.; Du, X.; Wang, H.; Wang, J.; Lu, C.; Chen, X.; Zhu, Z.; Luo, Z.; Yu, L.; Brown, A.J.; et al. Allosteric enhancement of ORP1-mediated cholesterol transport by PI(4,5)P(2)/PI(3,4)P(2). Nat. Commun. 2019, 10, 829. [Google Scholar] [CrossRef]
- Du, X.; Kumar, J.; Ferguson, C.; Schulz, T.A.; Ong, Y.S.; Hong, W.; Prinz, W.A.; Parton, R.G.; Brown, A.J.; Yang, H. A role for oxysterol-binding protein-related protein 5 in endosomal cholesterol trafficking. J. Cell Biol. 2011, 192, 121–135. [Google Scholar] [CrossRef]
- Renne, M.F.; Emerling, B.M. ORP5 regulates PI(4)P on the lipid droplet: Novel players on the monolayer. J. Cell Biol. 2020, 219, e201912010. [Google Scholar] [CrossRef]
- Chung, J.; Torta, F.; Masai, K.; Lucast, L.; Czapla, H.; Tanner, L.B.; Narayanaswamy, P.; Wenk, M.R.; Nakatsu, F.; De Camilli, P. INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 2015, 349, 428–432. [Google Scholar] [CrossRef]
- Ghai, R.; Du, X.; Wang, H.; Dong, J.; Ferguson, C.; Brown, A.J.; Parton, R.G.; Wu, J.W.; Yang, H. ORP5 and ORP8 bind phosphatidylinositol-4, 5-biphosphate (PtdIns(4,5)P2) and regulate its level at the plasma membrane. Nat. Commun. 2017, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Galmes, R.; Houcine, A.; van Vliet, A.R.; Agostinis, P.; Jackson, C.L.; Giordano, F. ORP5/ORP8 localize to endoplasmic reticulum-mitochondria contacts and are involved in mitochondrial function. EMBO Rep. 2016, 17, 800–810. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Z.; Ye, S.; Zhang, R. The crystal structure of ORP3 reveals the conservative PI4P binding pattern. Biochem. Biophys. Res. Commun. 2020, 529, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.S.; Lim, J.Y.; Turgut, A.; Servage, K.; Zhang, J.; Orth, K.; Sosale, N.G.; Lazzara, M.J.; Allegood, J.; Casanova, J.E. Calcium-stimulated disassembly of focal adhesions mediated by an ORP3/IQSec1 complex. eLife 2020, 9, e54113. [Google Scholar] [CrossRef]
- Gulyás, G.; Sohn, M.; Kim, Y.J.; Várnai, P.; Balla, T. ORP3 phosphorylation regulates phosphatidylinositol 4-phosphate and Ca(2+) dynamics at plasma membrane-ER contact sites. J. Cell Sci. 2020, 133, jcs237388. [Google Scholar] [CrossRef]
- Hynynen, R.; Laitinen, S.; Käkelä, R.; Tanhuanpää, K.; Lusa, S.; Ehnholm, C.; Somerharju, P.; Ikonen, E.; Olkkonen, V.M. Overexpression of OSBP-related protein 2 (ORP2) induces changes in cellular cholesterol metabolism and enhances endocytosis. Biochem. J. 2005, 390, 273–283. [Google Scholar] [CrossRef]
- Laitinen, S.; Lehto, M.; Lehtonen, S.; Hyvärinen, K.; Heino, S.; Lehtonen, E.; Ehnholm, C.; Ikonen, E.; Olkkonen, V.M. ORP2, a homolog of oxysterol binding protein, regulates cellular cholesterol metabolism. J. Lipid Res. 2002, 43, 245–255. [Google Scholar] [CrossRef]
- Mochizuki, S.; Miki, H.; Zhou, R.; Kido, Y.; Nishimura, W.; Kikuchi, M.; Noda, Y. Oxysterol-binding protein-related protein (ORP) 6 localizes to the ER and ER-plasma membrane contact sites and is involved in the turnover of PI4P in cerebellar granule neurons. Exp. Cell. Res. 2018, 370, 601–612. [Google Scholar] [CrossRef]
- Avalos-Padilla, Y.; Georgiev, V.N.; Dimova, R. ESCRT-III induces phase separation in model membranes prior to budding and causes invagination of the liquid-ordered phase. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183689. [Google Scholar] [CrossRef]
- Bussi, C.; Mangiarotti, A.; Vanhille-Campos, C.; Aylan, B.; Pellegrino, E.; Athanasiadi, N.; Fearns, A.; Rodgers, A.; Franzmann, T.M.; Šarić, A.; et al. Stress granules plug and stabilize damaged endolysosomal membranes. Nature 2023, 623, 1062–1069. [Google Scholar] [CrossRef]
- Qiu, H.; Wu, X.; Ma, X.; Li, S.; Cai, Q.; Ganzella, M.; Ge, L.; Zhang, H.; Zhang, M. Short-distance vesicle transport via phase separation. Cell 2024, 187, 2175–2193.e2121. [Google Scholar] [CrossRef] [PubMed]
- Bogus, S.M.; Wegeng, W.R.; Ruiz, M.; Chavez, S.R.; Cheung, S.N.; Noori, K.S.M.; Niesman, I.R.; Ernst, A.M. A hollow TFG condensate spatially compartmentalizes the early secretory pathway. Nat. Commun. 2025, 16, 3715. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ditlev, J.A.; Hui, E.; Xing, W.; Banjade, S.; Okrut, J.; King, D.S.; Taunton, J.; Rosen, M.K.; Vale, R.D. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 2016, 352, 595–599. [Google Scholar] [CrossRef]
- Case, L.B.; Zhang, X.; Ditlev, J.A.; Rosen, M.K. Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 2019, 363, 1093–1097. [Google Scholar] [CrossRef]
- Huang, W.Y.C.; Alvarez, S.; Kondo, Y.; Lee, Y.K.; Chung, J.K.; Lam, H.Y.M.; Biswas, K.H.; Kuriyan, J.; Groves, J.T. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 2019, 363, 1098–1103. [Google Scholar] [CrossRef]
- Ditlev, J.A.; Vega, A.R.; Köster, D.V.; Su, X.; Tani, T.; Lakoduk, A.M.; Vale, R.D.; Mayor, S.; Jaqaman, K.; Rosen, M.K. A composition-dependent molecular clutch between T cell signaling condensates and actin. eLife 2019, 8, e42695. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chan, S.H.; Dey, S.; Castello-Serrano, I.; Rosen, M.K.; Ditlev, J.A.; Levental, K.R.; Levental, I. Coupling of protein condensates to ordered lipid domains determines functional membrane organization. Sci. Adv. 2023, 9, eadf6205. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Liu, Y.; Yu, H. Uncovering the Mechanisms of Intracellular Membrane Trafficking by Reconstituted Membrane Systems. Membranes 2025, 15, 154. https://doi.org/10.3390/membranes15050154
Chen S, Liu Y, Yu H. Uncovering the Mechanisms of Intracellular Membrane Trafficking by Reconstituted Membrane Systems. Membranes. 2025; 15(5):154. https://doi.org/10.3390/membranes15050154
Chicago/Turabian StyleChen, Shuhan, Yinghui Liu, and Haijia Yu. 2025. "Uncovering the Mechanisms of Intracellular Membrane Trafficking by Reconstituted Membrane Systems" Membranes 15, no. 5: 154. https://doi.org/10.3390/membranes15050154
APA StyleChen, S., Liu, Y., & Yu, H. (2025). Uncovering the Mechanisms of Intracellular Membrane Trafficking by Reconstituted Membrane Systems. Membranes, 15(5), 154. https://doi.org/10.3390/membranes15050154





