Abstract
L-Glutamate (L-Glu) and its salt derivatives are widely used in the food industry as flavor enhancers. Although the consumption of these compounds is generally considered safe, some studies suggest that chronically consuming L-Glu may be associated with various disorders. In this study, Wistar pregnant rats were treated daily with 1 g/L of L-Glu in their drinking water throughout the gestational period. OPA-1, DRP-1, and mitofusin 2—key proteins involved in mitochondrial fusion and fission—were analyzed by Western blot. The results showed that L-Glu exposure significantly decreased DRP-1 levels, while OPA-1 and mitofusin 2 levels were unaffected. This was accompanied by a notable decrease in mitochondrial complexes III and V. The activities of Mg2+-ATPase and Na+/K+-ATPase were also analyzed in fetal cerebellar plasma membranes. Maternal L-Glu intake significantly increased Mg2+-ATPase activity. Regarding Na+/K+-ATPase, the data showed that L-Glu exposure did not modulate the protein level or its activity. However, a positive interaction with glutamate receptors was observed in both activities, although neither AMPA nor NMDA receptors appeared to be involved. These results suggest that chronic maternal L-Glu intake during gestation modulates Mg2+-ATPase activity and protein markers of mitochondrial dynamics in the fetal cerebellum, which could affect neonatal development.
1. Introduction
Under physiological conditions, endogenous glutamate participates in multiple functions, such as energy production in enterocytes [1], which is a precursor of the powerful antioxidant glutathione [2], and as an excitatory neurotransmitter in the nervous system [3]. It is not surprising, therefore, that chronic L-Glu intake has been associated with a wide variety of disorders, including obesity, metabolic issues, alterations in the reproductive system, and neurotoxic effects [4,5]. Glutamate intake from the diet varies across different countries and cultures, but it may pose a risk when taken in excessive consumption [6]
L-Glutamate (L-Glu) and its salt derivatives, such as monosodium glutamate (MSG), are widely used by the food industry due to their ability to induce a unique taste called umami and enhance the palatability of foods [7,8]. These molecules are present in a wide variety of processed foods, including frozen meals, fast foods, soups, and canned tuna [9]. It is estimated that the average daily human intake in European countries ranges from 0.3 to 1 g [10]. The ability of exogenous glutamate to modulate brain behavior, neurochemistry, and even neuromorphology is currently being confirmed using different models [6].
Our research group has recently published that chronic maternal L-Glu intake during gestation evokes oxidative stress in the fetal cerebellum [11]. MSG-induced oxidative stress has also been reported in rat striatum and cerebellum [12,13]. The cerebellum plays a crucial role in maintaining motor coordination, sensory perception, and controlling voluntary movement. It is also involved in motor learning and is highly vulnerable to injury. Consequently, damage to this region can disrupt learning processes, leading to motor impairments or ataxia [14].
Oxidative stress is a condition characterized by an imbalance between the production and accumulation of reactive oxygen species (ROS) and the antioxidant defenses [15]. In addition to their central role in ATP production, mitochondria are also the primary source of ROS in the cell. It is estimated that during intense oxidative phosphorylation, between 1 and 2% of the oxygen consumed by the cell is converted into ROS. This generation of free radicals, such as superoxide anion, is primarily driven by the flow of electrons through the mitochondrial electron transport chain [16].
Although under physiological conditions, the production of ROS in mitochondria is necessary, as they participate in crucial processes, such as immune defense against pathogens and cell signaling [17], the presence of oxidative stress is a clear symptom of mitochondrial alteration. Mitochondria are highly dynamic organelles that undergo coordinated cycles of fusion and fission processes. During fusion, several mitochondria can merge into new mitochondria, a process in which the protein OPA-1 participates. On the other hand, mitochondrial fission allows mitochondrions to fragment into several smaller ones. The protein Drp-1 plays a key role in this process. An adequate balance between fusion and fission is necessary for many cellular processes such as the cell cycle, immunity, and apoptosis. However, it has been observed that CNS insults can promote an imbalance, favoring mitochondrial fission [18,19,20]. Currently, it remains unknown whether the chronic oral intake of L-Glu can alter normal mitochondrial dynamics.
Progressive free radical generation and reduced antioxidant levels have been linked to impaired ATPase function [21]. Evidence shows that ATPases like Ca2+-ATPase, Mg2+-ATPase, and Na+/K+-ATPase are crucial for maintaining ionic gradients and nerve cell functions, including signal transduction, neurotransmitter release, synaptic plasticity, and cognitive processes in the CNS [22,23], and even influencing glutamate signaling in neurodegenerative diseases [24].
Accordingly, the activity of Na+/K+-ATPase is crucial for maintaining proper excitability in the nervous system [17], and increased ROS levels modify its subunits, leading to the loss of activity and even the degradation of the protein [18]. Moreover, this pump can activate signaling pathways that result in the phosphorylation of proteins involved in apoptosis and cellular metabolism [25].
Several proteins and receptors can bind to and impair its activity, and even some studies have demonstrated a co-localization and physical interaction between Na+/K+ ATPase subunits and glutamate transporters [26] and receptors, [27] including ionotropic glutamate receptors such as NMDA [28] for which some findings reveal a potential cross-talk between this receptor and the pump, playing important roles in the regulation of learning and memory and AMPA, which colocalizes and associates with Na+/K+ ATPase during synaptic transmission [29]; this is critical for the formation of synaptic plasticity such as long-term potentiation and depression (LTP and LTD). The inhibition of this activity in the cerebellum may potentiate glutamate excitotoxicity, evoking cytotoxic effects such as Ca2+ influx. Previous studies have shown that glutamate can increase cerebellar Na+/K+ ATPase activity through the cyclic GMP-PKG pathway [30] via tissue preincubation with L-Glu [31]. However, to our knowledge, there are no data analyzing the consequences of the chronic oral consumption of L-Glu (not MSG) on the activity of this Na+/K+-ATPase.
Mg2+-ATPase is another essential ion transporter involved in maintaining a high intracellular concentration of magnesium ions. These ions play a key role in the synthesis and metabolism of lipids, carbohydrates, proteins, and nucleic acids. Additionally, it participates in the synthesis of the antioxidant defense system [32]. However, the impact of oxidative stress on Mg2+-ATPase activity has been less studied. Some authors have reported that oxidative stress induces an increase in the activity of this ionic pump [33,34], while others have observed a significant decrease [35,36].
Therefore, the aims of the present study were to analyze whether maternal chronic oral L-Glu treatment during gestation could alter the levels of protein markers related to mitochondrial function and dynamics and whether these changes could be associated with modifications in the activities of both Na+/K+-ATPase and Mg2+-ATPase in the cerebellum.
2. Materials and Methods
2.1. Animal Treatment
In the present work, pregnant Wistar rats were kept on a 12 h light/12 h dark cycle, with light appearing at 7:00 am and free access to food and drinking water. Pregnant rats were supplemented with a dose of 1 g/L of L-Glutamate in their drinking water from gestation day 2 onwards during the entire gestation period. At the end of this period, pregnant rats were sacrificed, and the fetuses were delivered surgically. Fetal cerebellums were then frozen in liquid nitrogen and stored at −70 °C. The mean amount of glutamate consumption for the L-Glu-treated group was 110 ± 4.6 mg/Kg·day, and daily water consumption was significantly lower in the L-glu-treated group, with no effect on food intake or body weight [37]. As the average daily human consumption of glutamate in Asian and European countries ranges from 2.2 to 12 g approximately [10,38] and the average weight is about 75 Kg, we can assume that an approximation of the human consumption of L-Glu is 30 to 160 mg/Kg·day. Therefore, the average values obtained for the consumption of this amino acid in gestational rats can represent those values usually consumed in humans. All experiments followed the European Community regulations regarding animal experimentation and those of the Animal Experimentation Committee of Castilla-La Mancha University (registration number 1103.01).
2.2. Plasma Membrane Isolation
Cerebellar plasma membranes were isolated according to the protocol previously described [39]. In brief, the fetal cerebellums were homogenized in 20 volumes of an isolation buffer (50 mM Tris-HCl, 10 mM MgCl2, pH 7.4) with protease inhibitors, using a Dounce homogenizer with pestle A (10 strokes) followed by pestle B (10 strokes). The homogenate was then centrifuged at 1000× g for 5 min in a Beckman JA 21 centrifuge (Coulter, Madrid, Spain). The resulting supernatant was further centrifuged at 27,000× g for 10 min to separate the plasma membrane (pellet) and cytosolic fractions. The pellet was resuspended in an isolation buffer, and protein concentration was determined using the Lowry method with bovine serum albumin (BSA) as the standard.
2.3. Immunoblotting Assay
Homogenates and plasma membrane samples (30 μg) were subjected to 10–15% polyacrylamide gel electrophoresis and sodium dodecyl sulfate. Proteins were transferred to nitrocellulose membranes using the iBlot™ Dry Blotting System (Invitrogen, Barcelona, Spain) and blocked for 60 min with 5% non-fat skimmed milk in phosphate-buffered saline. Then, immunodetection was performed by incubating the nitrocellulose membranes with the following subsequent antibodies: the Anti-OPA1 (1:1000, ab42364 from Abcam. Cambridge, United Kingdom), Anti-DRP1 antibody (1:1000, ab184247 from Abcam), Anti-mitofusin 2 antibody (1:1000, ab124773 from Abcam), OXPHOS antibody (1:1000, ab110413 from Abcam). β-Actin antibody (1:5000, ab8226 from Abcam), and anti-β-Tubulin, clone AA2 (1:5000, 05-661 from Millipore), were used as protein loading controls. After washing, blots were incubated with horseradish peroxidase coupled with goat anti-mouse or anti-rabbit IgG (GAMPO 170–6516 and 1:4000 and GARPO 172–1019 1:4000 from Bio-Rad, Kai Tak, Hong Kong). Bands were visualized using the ECL chemiluminescence detection kit from GE Healthcare (Madrid, Spain) in a G: Box chamber and luminescent bands were quantified by densitometry using Gene-Tools software version 4.01 (Syngene). The results are presented in arbitrary units (the ratio between the protein of interest and β-actin).
2.4. Na+/K+-ATPase and Mg2+-ATPase Activities Assay
Plasma membrane fractions (1 mg/mL) were used for this procedure. Briefly, the reaction buffer contained 5.0 mM of MgCl2, 80.0 mM of NaCl, 20.0 mM of KCl, and 40.0 mM of Tris–HCl, pH 7.4, in a final volume of 200 μL. The addition of 3 mM freshly prepared ATP initiated the reaction. The Mg2+-ATPase activity was measured after the addition of 1.0 mM of ouabain. Then, the Na+/K+-ATPase activity was measured as the difference between the total ATPase activity and the Mg2+-ATPase activity. In our experimental conditions, only 6% and 8% of this activity corresponded to ATP synthase and SERCA, respectively. The inorganic phosphate released was measured based on the spectrophotometric quantification of the phosphomolybdate–malachite green complex as described by Chan et al. 1986 [40]. The results are expressed as the nmol of Pi released/min·mg of protein. The effect of different glutamate receptor ligands such as AMPA, NMDA, and glutamate on these enzymatic activities was measured following the same procedure, but with a 6 min preincubation time with 140 μM AMPA, NMDA, and L-Glutamate each at 30 °C with stirring. The results were expressed as the percentage of the action of each ligand with respect to the basal activity of Na+/K+ and Mg2+-ATPase, respectively.
2.5. Statistical and Data Analysis
Results are expressed as the mean ± Standard Error of the Mean (SEM). Statistical comparisons were performed using unpaired two-tailed Student’s t-test and a two-way ANOVA, followed by a Bonferroni comparison post hoc test using the GraphPad Prism 8.0 program (GraphPad Software, San Diego, CA, USA). The differences between mean values were considered statistically significant at * p < 0.05.
3. Results
3.1. Maternal Chronic L-Glu Intake During Gestation Alters the Mitochondrial Oxidative Phosphorylation Complex III and V in Fetal Cerebellum
First, we aimed to determine whether chronic L-Glu intake during gestation could affect mitochondrial oxidative function by modulating mitochondrial complexes. To this end, the expression of mitochondrial complexes was analyzed in cerebellar homogenates from fetuses exposed to maternal L-Glu intake and compared with the corresponding control and L-Glu-treated groups. As shown in Figure 1, maternal L-Glu intake significantly decreased the level of mitochondrial complexes III (1.010 ± 0.166% vs. 0.526 ± 0.107 arbitrary units, p < 0.05) and V (1.370 ± 0.121% vs. 0.726 ± 0.078 arbitrary units, p < 0.01) while the other complexes (I, II and IV) were not significantly altered.
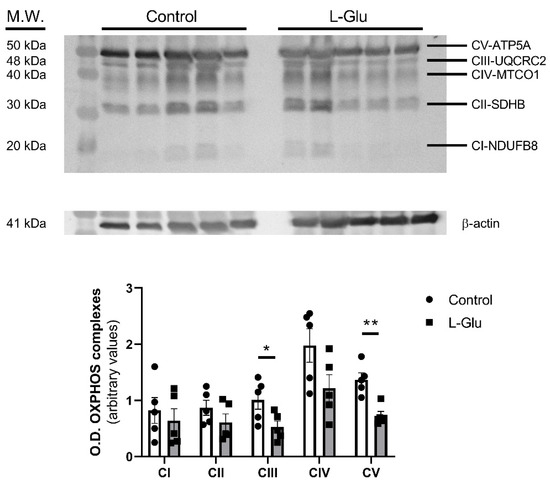
Figure 1.
Western blot of OXPHOS in fetal cerebellum exposed to L-Glutamate during gestation. Histograms show data that correspond to five different complexes, represented as mean ± SEM of 5 different experiments performed using different tissue homogenates. * p < 0.05 or ** p < 0.01, significantly different from control values according to Student’s t-test.
3.2. Maternal Chronic L-Glu Intake During Gestation Alters the Levels of the Proteins Involved in the Dynamics of the Mitochondria in Fetal Cerebellum
Next, we sought to determine whether the maternal oral consumption of L-Glu during gestation could also modulate mitochondrial dynamics in the fetal cerebellum. To investigate this, we analyzed the levels of three key proteins, OPA-1, DRP-1, and mitofusin 2, as they play crucial roles in mitochondrial fission (DRP-1) and fusion (OPA-1 and mitofusin 2). As shown in Figure 2, DRP-1 levels were significantly reduced in the cerebellum of fetuses exposed to maternal L-Glu intake (0.813 ± 0.088 vs. 0.482 ± 0.063 arbitrary units, p < 0.05). No significant variations were observed in OPA-1 and mitofusin 2 protein levels.
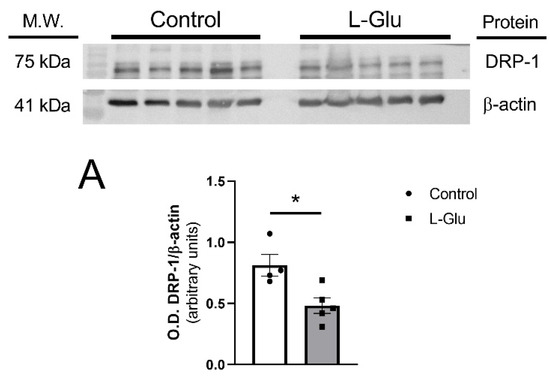
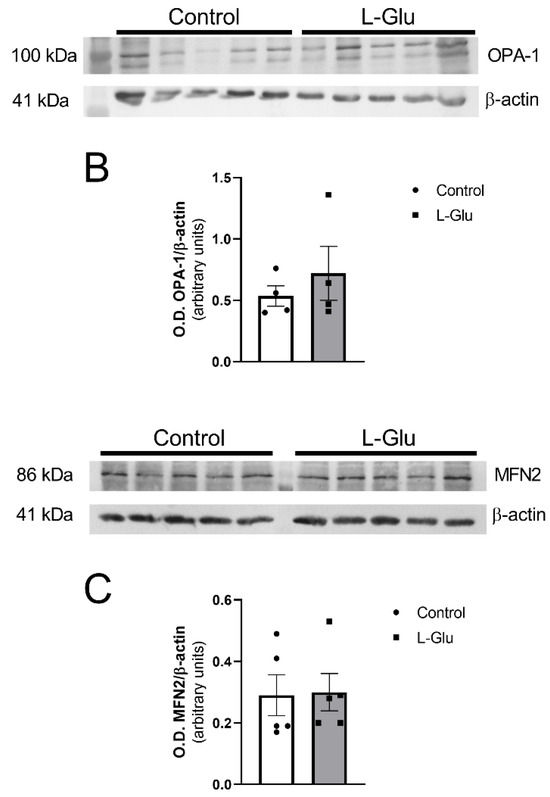
Figure 2.
Western blot and densitometric analyses of the corresponding bands of mitochondrial dynamic markers DRP-1 (A), OPA-1 (B), and mitofusin 2 (MFN2) (C) in fetal cerebellum exposed to L-Glutamate during gestation. Histograms show data that are represented as mean ± SEM of 5 different experiments performed using different tissue homogenates. * p < 0.05, significantly different from control values according to Student’s t-test.
This oxidative stress induced by chronic maternal L-Glutamate intake could also be seen as altering ATPase activity. Indeed, ATPases are the primary consumers of energy in the cell, and several studies have reported that ATP deficiency and oxidative stress may impair Na+/K+-ATPase activity, contributing to neuronal death [41]. Moreover, Mg2+-ATPase could also be affected by oxidative stress, as magnesium ions play an important role in the brain by counteracting oxidative damage, although the exact mechanisms for this remain unclear [42,43]. For this reason, we decided to investigate the effect of maternal L-Glutamate intake on ATPase activities.
3.3. Maternal Chronic L-Glu Intake During Gestation Alters the Activity of Mg2+-ATPase in Fetal Cerebellum
As indicated in the previous paragraph, the next point we investigated was the effect of maternal L-Glu consumption on Mg2+-ATPase activity. We addressed the following three questions:
- (1)
- Is Mg2+-ATPase activity affected by maternal L-Glu consumption during gestation?
- (2)
- Can the activation of glutamate receptors modulate the activity of Mg2+-ATPase?
- (3)
- If the response to question two is positive, is this effect altered by the chronic gestational intake of L-Glu?
To answer these questions, Mg2+-ATPase activity was analyzed in cerebellar plasma membranes from fetuses whose mothers consumed either water or L-Glu during gestation. Furthermore, measurements were carried out in the presence of L-Glutamate, as it is an endogenous agonist of glutamate receptors. Two-way ANOVA analyses revealed the following results: (a) gestational treatment with L-Glu significantly altered Mg2+-ATPase activity [F(1,12) = 36.47, p < 0.0001]; (b) the activity in the presence of the endogenous agonist L-Glu was also significantly altered [F(1,12) = 28.74, p = 0.0002]; and (c) no significant interaction was found between gestational L-Glu treatment and the endogenous agonist L-Glu [F(1,12) = 1.1, p = 0.31]. Bonferroni’s post-test indicated that the endogenous agonist L-Glu significantly increased Mg2+-ATPase activity in both control fetuses (74 ± 1.5 nmol Pi/mg prot/min vs. 90 ± 4 nmol Pi/mg prot/min, p < 0.05) and fetuses exposed to maternal L-Glu during gestation (88 ± 2.5 nmol Pi/mg prot/min vs. 111 ± 4 nmol Pi/mg prot/min, p < 0.01). The post hoc test further revealed that the chronic gestational consumption of L-Glu significantly increased Mg2+-ATPase activity measured in both the presence (90 ± 4 nmol Pi/mg prot/min vs. 111 ± 4 nmol Pi/mg prot/min, p < 0.01) and absence (74 ± 1.5 nmol Pi/mg prot/min vs. 88 ± 2.5 nmol Pi/mg prot/min, p < 0.05) of L-Glu (Figure 3A).
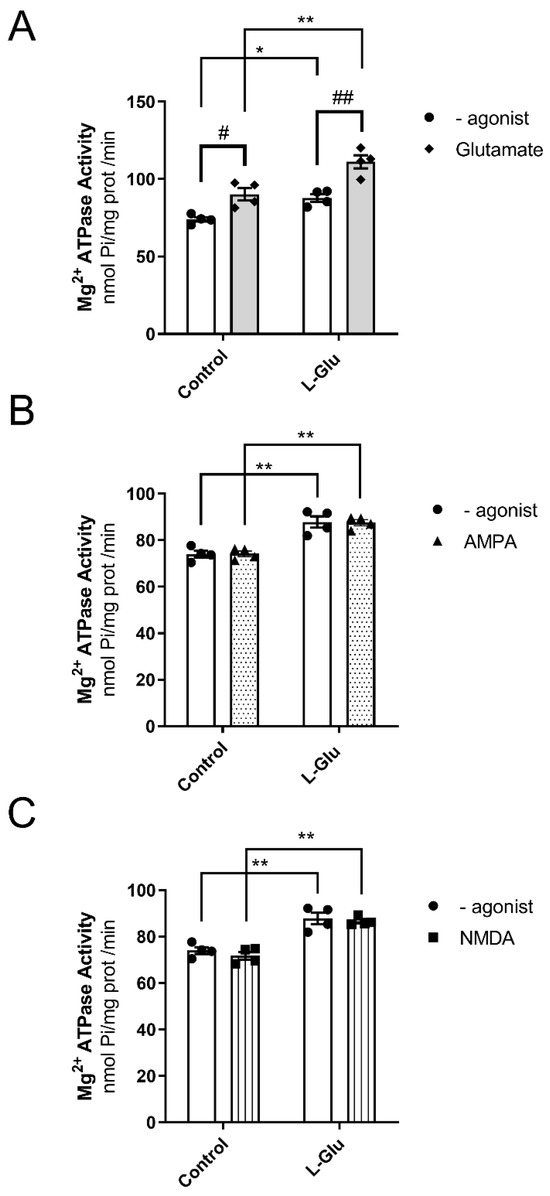
Figure 3.
Mg2+ ATPase activity in the presence of Glutamate (A), AMPA (B), and NMDA (C) in fetal cerebellum exposed to L-Glutamate during gestation. Histograms show data that are represented as mean ± SEM of 4 different experiments performed using different plasma membrane preparations. All experiments were carried out in duplicate. * p < 0.05 or ** p < 0.01, significantly different from control values, and # p < 0.05 or ## p < 0.01, significantly different from the group without agonist, according to the two-way ANOVA test.
Next, we wanted to identify the specific glutamate receptor that modulated the activity of Mg2+-ATPase. To this end, we assayed the activity of Mg2+-ATPase in the presence of two ionotropic glutamate receptor agonists, AMPA and NMDA. As shown in Figure 3B,C, maternal chronic L-Glu intake significantly increased Mg2+-ATPase activity when assayed in the absence or presence of AMPA or NMDA. However, neither AMPA nor NMDA agonists altered Mg2+-ATPase activity in either group of fetuses studied. Therefore, these results suggest the following: (a) the existence of a possible interaction between Mg2+-ATPase and the glutamate receptor other than AMPA and NMDA receptors, and (b) chronic maternal consumption of L-Glutamate throughout the gestational period increases Mg2+-ATPase activity.
3.4. Maternal Chronic L-Glu Intake Did Not Alter the Activity of Na+/K+-ATPase in Fetal Cerebellum
Next, we studied whether the level of Na+/K+-ATPase could be modulated by maternal chronic L-Glu consumption. As shown in Figure 4, the densitometric analysis of the corresponding band obtained by Western blot assays carried out with a specific antibody showed a slight, although not significant variation, in the level of Na+/K+-ATPase in cerebellar plasma membranes from fetuses exposed to maternal L-Glu.
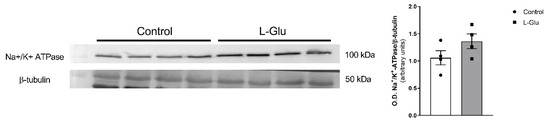
Figure 4.
Western blot of Na+/K+ ATPase in fetal cerebellum exposed to L-Glutamate during gestation. Histograms show data that are represented as mean ± SEM of 4 different experiments performed using different plasma membrane isolations.
We also wanted to investigate whether the activity of Na+/K+-ATPase or its coupling to glutamate receptors could also be modulated in response to the maternal intake of L-Glu during gestation. As shown in Figure 5A, the analysis of two-way ANOVA revealed the fact that chronic L-Glu consumption during gestation failed to alter Na+/K+-ATPase activity [F(1,12) = 1.69, p = 0.21], although the agonist L-Glu significantly affected the results [F(1,12) = 31.87, p = 0.0001]. Finally, no interaction between chronic treatment and L-Glu was observed [F(1,12) = 0.37, p = 0.55]. Bonferroni’s post-test showed that L-Glu significantly increased the activity of Na+/K+-ATPase in both control fetuses (23 ± 2.3 nmol Pi/mg prot/min vs. 59.4 ± 9.4 nmol Pi/mg prot/min, p < 0.05) and fetuses exposed to maternal L-Glu during gestation (28 ± 0.9 nmol Pi/mg prot/min vs. 73.2 ± 10.7 nmol Pi/mg prot/min, p < 0.01). Similarly to Mg2+-ATPase, the increase observed in Na+/K+-ATPase activity in response to glutamate receptor activation did not appear to involve either AMPA or NMDA receptors, as two-way ANOVA did not find any significant variation when these agonists were tested (Figure 5B,C).
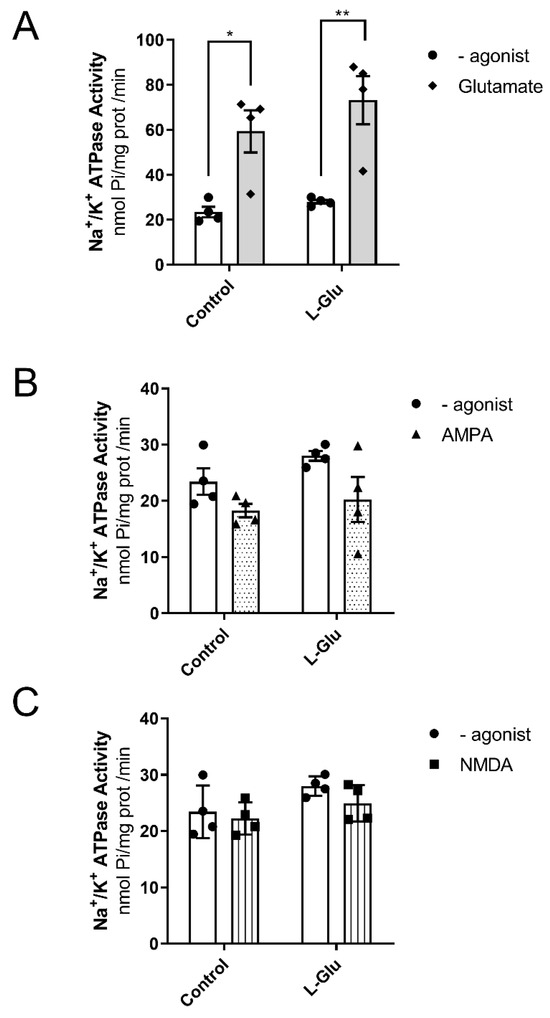
Figure 5.
Na+/K+ ATPase activity in the presence of Glutamate (A), AMPA (B), and NMDA (C) in fetal cerebellum exposed to L-Glutamate during gestation. Histograms show data that are represented as mean ± SEM of 4 different experiments performed using different plasmatic membrane preparations. All experiments were carried out per duplicate. * p < 0.05 or ** p < 0.01, significantly different from control values according to the two-way ANOVA test.
4. Discussion
The present work shows how the maternal intake of L-Glu in drinking water during pregnancy produces effects on both markers related to mitochondrial dynamics and the mitochondrial electron transport chain. These effects were accompanied by changes in Mg2+-ATPase activity, whereas Na+/K+ ATPase activity remained unaltered.
Firstly, it was observed that treatment with L-Glu significantly decreased the levels of dynamin-related protein 1 (DRP-1) in the fetal cerebellum. This protein is a GTPase that is currently considered the main regulator of mitochondrial fission [44]. It is initially located in the cytosol, and once it is activated through GTP hydrolysis, it oligomerizes around the outer mitochondrial membrane to initiate mitochondrial fragmentation and can ultimately trigger apoptosis [45]. It is not surprising, therefore, that an abnormal increase in this process is associated with the neuronal damage observed after acute or chronic damage to the Central Nervous System [46,47,48,49,50]. On the contrary, other studies have found a reduction in the levels of this protein obtained in heterozygote knock-out animals not generating mitochondrial deficiencies; however, mice showed a reduction in oxidative stress, reducing H2O2 and lipid peroxidation levels, not affecting synaptic viability [51]. Therefore, the reduction observed in the cerebellum from fetuses could indicate a possible protective mechanism to alleviate the oxidative stress in fetuses induced by mothers exposed to L-Glu, as published in previous work [11].
Regarding the fusion proteins, OPA-1 and mitofusin 2, they play a key role in mitochondrial fusion, allowing the inner and outer mitochondrial membranes of two independent mitochondria to join and form a new single functional mitochondrion [52], which is essential for cerebellar protection against neurodegeneration [53]. However, we did not observe any significant variations in these proteins.
The change in the mitochondrial fission marker was also accompanied by a significant decrease in the levels of complex V (H+-transporting two-sector ATPase) and III (ubiquinone-cytochrome c reductase) detected in the group treated with L-Glu. Complex V or ATP synthase uses the energy liberated during respiration to catalyze the condensation of ADP and inorganic phosphate into ATP, providing energy to the cell [54], so a possible downregulation of this synthase could be affecting energy balance throughout the cerebellum. Also, complex III is decreased, with this being one of the main sites of ROS production in the mitochondrial electron transport chain [55], which could also be part of the same mechanism in response to augmented lipid peroxidation observed in fetuses previously [11].
ATPases are the main consumers of energy in the cell. Some studies have stated that ATP deficiency and oxidative stress may impair Na+/K+ ATPase activity and promote mechanisms of neuronal death [41]. Changes in Na+/K+ ATPase activity may have an impact on cellular metabolism and the redox state since it controls mitochondrial ROS levels and ATP utilization rates [56,57]. As changes in the levels of the mitochondrial electron transport chain were observed, these may be affecting ROS and ATP production and, therefore, ATPase functioning.
Another interesting finding from this work is the increase in the activity of Mg2+-ATPase in the fetal cerebellum exposed to maternal L-Glu consumption. This pump is responsible for maintaining a higher intracellular concentration of magnesium ions. This cation is essential for regulating the activities of multiple Mg2+-dependent enzymes and controlling the rate of protein synthesis. Additionally, it is known that magnesium plays an important role in the brain by counteracting oxidative stress, although the exact mechanism remains unclear [42,43]. Therefore, these changes suggest the existence of a protective mechanism aimed at mitigating the oxidative stress detected in the fetal cerebellum in response to maternal L-Glu intake.
The results of this study also indicate that the pump seems to be positively coupled with glutamate receptors as the activity of the pump significantly increased in the presence of the endogenous agonist L-Glutamate. However, chronic maternal L-Glu intake did not significantly alter this coupling, as it was equally augmented in the L-Glu group.
Finally, in our work, no changes were detected either in Na+/K+ ATPase activity or in the levels of the protein itself. This absence of effects contrasts with the dependence of this pump on correct mitochondrial functioning and previous works where L-Glu affects this pump’s activity through the GMP-PKG pathway [31], activating metabotropic and ionotropic receptors [30,58] and increasing Ca2+ intracellular levels. Although some of these experiments were performed in cerebellum slices preincubated with L-Glu at a desired concentration, and our results represent a representative dose of L-Glu orally administered through gestation, this dose is potentially too small in the cerebellum of fetuses to cause change, as the cerebellum L-Glu concentrations of these fetuses were about 4 to 5 mM of L-Glu per gram of protein, while the final glutamate concentration used for the experiments in this work was significantly higher. Also, glutamate may trigger intracellular Ca2+ accumulation and apoptosis, and its extrusion via the Na+-Ca2+ exchanger may be crucial for correct cell functioning, which functionally interacts with Na+/K+ ATPase activity in neurons [59]. Moreover, this pump consumes half of the ATP molecules present in the cerebellum. It is, therefore, not strange that mitochondrial alteration and the consequent production of free radicals is accompanied by a loss in the activity of this protein. Several works have shown that this pump is sensitive to oxidative stress, as a decrease in its levels has been detected in cells that have experienced oxidative damage due to hypoxia [60,61]. It has also been shown that in raw brain synaptosomes, oxidative stress significantly reduces the activity of the pump [62]. We do not know what the reasons for this may be, but they could be related to the fact that the cerebellum does not complete its development until the postnatal period, and it has been suggested that the regulation of the Na+/K+ ATPase does not conclude until development is completed [62]. Similarly to Mg2+-ATPase activity, the results indicate that Na+/K+-ATPase is positively coupled with glutamate receptors.
Regarding how these cerebellar alterations could affect long-term cerebellum development, we have not studied the impact of maternal L-Glutamate intake during gestation on neonatal cerebellar development. However, we believe that the significant decrease in DRP-1 levels observed in this work could be relevant, as this protein is required for cerebellar development. In this sense, the brain-specific Drp1 ablation has been shown to cause developmental defects in the cerebellum, where Purkinje cells have been shown to contain fewer giant mitochondria than controls [63]. Supporting this hypothesis, a previous study also showed that the intraperitoneal injection of monosodium glutamate (3.5 mg/g bw) for 10 consecutive days caused a significant decrease in motor coordination and the number of Purkinje cells in rats [64].
In conclusion, L-Glutamate exposure during gestation affects DRP-1 and electron transport chain complexes in the fetal cerebellum, possibly as a response to oxidative stress generated by this exposure, as reported in previous studies. The activity of Mg2+-ATPase was also altered by chronic maternal L-Glutamate intake, with a possible interaction observed between this activity and glutamate receptors, excluding AMPA and NMDA receptors as mediators of this effect. Finally, while the activity of Na+/K+-ATPase, one of the primary consumers of mitochondrial energy, was not altered by chronic oral L-Glutamate intake, an interaction with glutamate receptors other than AMPA and NMDA was noted.
Limitations of the Study
This study provides indirect evidence that L-Glutamate could alter the normal functioning of mitochondria. However, more research is necessary to determine whether L-Glutamate consumption during gestation can affect mitochondrial dynamics and functioning, as well as if these possible alterations could translate into neurodevelopmental issues in offspring. In this regard, electron microscope studies could be particularly valuable, but these could not be performed in this study due to sample limitations. These will be addressed in future studies.
Author Contributions
Conceptualization, D.A.L.-N. and M.M.; methodology, D.A.L.-N. and M.M.; formal analysis, A.T.; investigation, D.A.L.-N. and M.M.; resources, M.M.; data curation, A.T.; writing—original draft preparation, D.A.L.-N.; writing—review and editing, D.A.L.-N., M.M. and A.T.; supervision, D.A.L.-N. and M.M.; project administration, M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This article is part of the research project SBPLY/23/180225/000156, funded by the EU through the FEDER and by the JCCM through INNOCAM, PID2022-140602NB-100, funded by MICIU/AEI/10-13039/501100011033 and co-funded by the FEDER, EU, Grant 2022-GRIN-34201 by UCLM cofinanced with FEDER, and the research project.
Institutional Review Board Statement
The care and use of animals were carried out in accordance with the European Directive 2010/63/EU and with Spanish laws (RD 53/2013 and Ley 32/2007) on the use of laboratory animals. All experiments were in accordance with the Experimental Animal Committee of the University of Castilla-La Mancha. Every effort was made to minimize animal suffering and to reduce the number of animals used.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Blachier, F.; Boutry, C.; Bos, C.; Tomé, D. Metabolism and Functions of L-Glutamate in the Epithelial Cells of the Small and Large Intestines. Am. J. Clin. Nutr. 2009, 90, 814S–821S. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Serritella, A.V.; Messmer, M.M.; Hayashi-Takagi, A.; Hester, L.D.; Snyder, S.H.; Sawa, A.; Sedlak, T.W. Glutathione Is a Physiologic Reservoir of Neuronal Glutamate. Biochem. Biophys. Res. Commun. 2011, 409, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed]
- Niaz, K.; Zaplatic, E.; Spoor, J. Extensive Use of Monosodium Glutamate: A Threat to Public Health? EXCLI J. 2018, 17, 273–278. [Google Scholar] [CrossRef]
- Kayode, O.T.; Bello, J.A.; Oguntola, J.A.; Kayode, A.A.A.; Olukoya, D.K. The Interplay between Monosodium Glutamate (MSG) Consumption and Metabolic Disorders. Heliyon 2023, 9, e19675. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Onaolapo, O.J. Dietary Glutamate and the Brain: In the Footprints of a Jekyll and Hyde Molecule. Neurotoxicology 2020, 80, 93–104. [Google Scholar] [CrossRef]
- Yamamoto, T.; Inui-Yamamoto, C. The Flavor-Enhancing Action of Glutamate and Its Mechanism Involving the Notion of Kokumi. npj Sci. Food 2023, 7, 3. [Google Scholar] [CrossRef]
- Jinap, S.; Hajeb, P. Glutamate. Its Applications in Food and Contribution to Health. Appetite 2010, 55, 1–10. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nițulescu, G.M.; Kouretas, D.; Veskoukis, A.; Tsoukalas, D.; Engin, A.B.; Aschner, M.; Margină, D. A Review of the Alleged Health Hazards of Monosodium Glutamate. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef]
- Beyreuther, K.; Biesalski, H.K.; Fernstrom, J.D.; Grimm, P.; Hammes, W.P.; Heinemann, U.; Kempski, O.; Stehle, P.; Steinhart, H.; Walker, R. Consensus Meeting: Monosodium Glutamate—An Update. Eur. J. Clin. Nutr. 2007, 61, 304–313. [Google Scholar] [CrossRef]
- Tejero, A.; León-Navarro, D.A.; Martín, M. Effect of Chronic Maternal L-Glu Intake during Gestation and/or Lactation on Oxidative Stress Markers, AMPA Glu1 Receptor and Adenosine A1 Signalling Pathway from Foetal and Neonatal Cerebellum. Purinergic Signal 2024, 20, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Gbadamosi, I.; Yawson, E.O.; Akesinro, J.; Adeleke, O.; Tokunbo, O.; Bamisi, O.; Ibrahim-Abdulkareem, R.; Awoniran, P.; Gbadamosi, R.; Lambe, E.; et al. Vitamin D Attenuates Monosodium Glutamate-Induced Behavioural Anomalies, Metabolic Dysregulation, Cholinergic Impairment, Oxidative Stress, and Astrogliosis in Rats. Neurotoxicology 2024, 103, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.L.; Agu, V.A.; Idowu, G.A.; Ezejiaku, B.C.; Atunnise, A.K. The Role of Vitamin C on ATPases Activities in Monosodium Glutamate-Induced Oxidative Stress in Rat Striatum and Cerebellum. Neurotox. Res. 2024, 42, 40. [Google Scholar] [CrossRef] [PubMed]
- Reeber, S.L.; Otis, T.S.; Sillitoe, R. V New Roles for the Cerebellum in Health and Disease. Front. Syst. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free Radicals, Antioxidant Defense Systems, and Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Balog, J.; Mehta, S.L.; Vemuganti, R. Mitochondrial Fission and Fusion in Secondary Brain Damage after CNS Insults. J. Cereb. Blood Flow. Metab. 2016, 36, 2022–2033. [Google Scholar] [CrossRef]
- Zhou, H.; Toan, S. Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury. Biomolecules 2020, 10, 85. [Google Scholar] [CrossRef]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial Dynamics: Overview of Molecular Mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Bulygina, E.R. Na/K-ATPase and Oxidative Stress. Ann. N. Y Acad. Sci. 1997, 834, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Komali, E.; Venkataramaiah, C.; Rajendra, W. Antiepileptic Potential of Bacopa Monnieri in the Rat Brain during PTZ-Induced Epilepsy with Reference to Cholinergic System and ATPases. J. Tradit. Complement. Med. 2021, 11, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A. Plasma Membrane Ca-ATPases: Targets of Oxidative Stress in Brain Aging and Neurodegeneration. World J. Biol. Chem. 2010, 1, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, P.F.; Leite, J.A.; Orellana, A.M.M.; Vasconcelos, A.R.; Quintas, L.E.M.; Kawamoto, E.M.; Scavone, C. The Influence of Na+, K+-ATPase on Glutamate Signaling in Neurodegenerative Diseases and Senescence. Front. Physiol. 2016, 7, 195. [Google Scholar] [CrossRef]
- Pierre, S.V.; Blanco, G. Na/K-ATPase Ion Transport and Receptor-Mediated Signaling Pathways. J. Membr. Biol. 2021, 254, 443–446. [Google Scholar] [CrossRef]
- Rose, E.M.; Koo, J.C.P.; Antflick, J.E.; Ahmed, S.M.; Angers, S.; Hampson, D.R. Glutamate Transporter Coupling to Na,K-ATPase. J. Neurosci. 2009, 29, 8143–8155. [Google Scholar] [CrossRef]
- Cholet, N.; Pellerin, L.; Magistretti, P.J.; Hamel, E. Similar Perisynaptic Glial Localization for the Na+,K+-ATPase Alpha 2 Subunit and the Glutamate Transporters GLAST and GLT-1 in the Rat Somatosensory Cortex. Cereb. Cortex 2002, 12, 515–525. [Google Scholar] [CrossRef]
- De Lores Arnaiz, G.R.; Bersier, M.G. Relationship between Na+, K+-ATPase and NMDA Receptor at Central Synapses. Curr. Protein Pept. Sci. 2014, 15, 761–777. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, Q.; Wang, M.; Lin, A.; Jarzylo, L.; Navis, A.; Raissi, A.; Liu, F.; Man, H.-Y. Na,K-ATPase Activity Regulates AMPA Receptor Turnover through Proteasome-Mediated Proteolysis. J. Neurosci. 2009, 29, 4498–4511. [Google Scholar] [CrossRef]
- Nathanson, J.A.; Scavone, C.; Scanlon, C.; McKee, M. The Cellular Na+ Pump as a Site of Action for Carbon Monoxide and Glutamate: A Mechanism for Long-Term Modulation of Cellular Activity. Neuron 1995, 14, 781–794. [Google Scholar] [CrossRef]
- Scavone, C.; Munhoz, C.D.; Kawamoto, E.M.; Glezer, I.; de Lima, L.S.; Marcourakis, T.; Markus, R.P. Age-Related Changes in Cyclic GMP and PKG-Stimulated Cerebellar Na,K-ATPase Activity. Neurobiol. Aging 2005, 26, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Szentmihályi, K.; Szilágyi, M.; Balla, J.; Ujhelyi, L.; Blázovics, A. In Vitro Antioxidant Activities of Magnesium Compounds Used in Food Industry. Acta Aliment. 2014, 43, 419–425. [Google Scholar] [CrossRef]
- De Freitas, R.M.; Feng, D.; Jordán, J. Neuropharmacological Effects of Lipoic Acid and Ubiquinone on δ-Aminolevulinic Dehydratase, Na+, K+-ATPase, and Mg2+-ATPase Activities in Rat Hippocampus after Pilocarpine-Induced Seizures. Fundam. Clin. Pharmacol. 2011, 25, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Na+/K+- and Mg2+-ATPases and Their Interaction with AMPA, NMDA and D2 Dopamine Receptors in an Animal Model of Febrile Seizures. Int. J. Mol. Sci. 2022, 23, 14638. [Google Scholar] [CrossRef]
- Shagirtha, K.; Bashir, N.; MiltonPrabu, S. Neuroprotective Efficacy of Hesperetin against Cadmium Induced Oxidative Stress in the Brain of Rats. Toxicol. Ind. Health 2017, 33, 454–468. [Google Scholar] [CrossRef]
- Garoui, E.; Ben Amara, I.; Driss, D.; Elwej, A.; Chaabouni, S.E.; Boudawara, T.; Zeghal, N. Effects of Cobalt on Membrane ATPases, Oxidant, and Antioxidant Values in the Cerebrum and Cerebellum of Suckling Rats. Biol. Trace Elem. Res. 2013, 154, 387–395. [Google Scholar] [CrossRef]
- León Navarro, D.; Albasanz, J.L.; Iglesias, I.; Ruiz, M.A.; Martín, M. Effect of Chronic Glutamate Administration to Pregnant Rats during Gestation on Metabotropic Glutamate Receptors from Mothers and Full-Term Fetuses Brain. Amino Acids 2005, 28, 127–137. [Google Scholar] [CrossRef]
- He, K.; Du, S.; Xun, P.; Sharma, S.; Wang, H.; Zhai, F.; Popkin, B. Consumption of Monosodium Glutamate in Relation to Incidence of Overweight in Chinese Adults: China Health and Nutrition Survey (CHNS). Am. J. Clin. Nutr. 2011, 93, 1328–1336. [Google Scholar] [CrossRef]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Cerebellar Oxidative Stress and Fine Motor Impairment in Adolescent Rats Exposed to Hyperthermia-Induced Seizures Is Prevented by Maternal Caffeine Intake during Gestation and Lactation. Eur. J. Pharmacol. 2018, 822, 186–198. [Google Scholar] [CrossRef]
- Chan, K.M.; Delfert, D.; Junger, K.D. A Direct Colorimetric Assay for Ca2+-Stimulated ATPase Activity. Anal. Biochem. 1986, 157, 375–380. [Google Scholar] [CrossRef]
- Wang, X.Q.; Xiao, A.Y.; Sheline, C.; Hyrc, K.; Yang, A.; Goldberg, M.P.; Choi, D.W.; Yu, S.P. Apoptotic Insults Impair Na+, K+-ATPase Activity as a Mechanism of Neuronal Death Mediated by Concurrent ATP Deficiency and Oxidant Stress. J. Cell Sci. 2003, 116, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Shamshirian, A.; Eslami, S.; Shamshirian, D.; Ebrahimzadeh, M.A. Magnesium Sulfate Attenuates Lethality and Oxidative Damage Induced by Different Models of Hypoxia in Mice. Biomed. Res. Int. 2020, 2020, 2624734. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M.; Locatelli, L.; Fedele, G.; Cazzaniga, A.; Mazur, A. Magnesium and the Brain: A Focus on Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2022, 24, 223. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Huang, Y.; Li, L. Drp1-Dependent Mitochondrial Fission Plays Critical Roles in Physiological and Pathological Progresses in Mammals. Int. J. Mol. Sci. 2017, 18, 144. [Google Scholar] [CrossRef]
- Wang, S.; Tan, J.; Miao, Y.; Zhang, Q. Mitochondrial Dynamics, Mitophagy, and Mitochondria-Endoplasmic Reticulum Contact Sites Crosstalk Under Hypoxia. Front. Cell Dev. Biol. 2022, 10, 848214. [Google Scholar] [CrossRef]
- Brooks, C.; Cho, S.-G.; Wang, C.-Y.; Yang, T.; Dong, Z. Fragmented Mitochondria Are Sensitized to Bax Insertion and Activation during Apoptosis. Am. J. Physiol. Cell Physiol. 2011, 300, C447–C455. [Google Scholar] [CrossRef]
- Reddy, P.H.; Reddy, T.P.; Manczak, M.; Calkins, M.J.; Shirendeb, U.; Mao, P. Dynamin-Related Protein 1 and Mitochondrial Fragmentation in Neurodegenerative Diseases. Brain Res. Rev. 2011, 67, 103–118. [Google Scholar] [CrossRef]
- Grohm, J.; Kim, S.-W.; Mamrak, U.; Tobaben, S.; Cassidy-Stone, A.; Nunnari, J.; Plesnila, N.; Culmsee, C. Inhibition of Drp1 Provides Neuroprotection in Vitro and in Vivo. Cell Death Differ. 2012, 19, 1446–1458. [Google Scholar] [CrossRef]
- Kumari, S.; Mehta, S.L.; Li, P.A. Glutamate Induces Mitochondrial Dynamic Imbalance and Autophagy Activation: Preventive Effects of Selenium. PLoS ONE 2012, 7, e39382. [Google Scholar] [CrossRef]
- Knott, A.B.; Perkins, G.; Schwarzenbacher, R.; Bossy-Wetzel, E. Mitochondrial Fragmentation in Neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 505–518. [Google Scholar] [CrossRef]
- Manczak, M.; Sesaki, H.; Kageyama, Y.; Reddy, P.H. Dynamin-Related Protein 1 Heterozygote Knockout Mice Do Not Have Synaptic and Mitochondrial Deficiencies. Biochim. Biophys. Acta 2012, 1822, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Zappaterra, M.D.; Hasegawa, A.; Wright, A.P.; Newman-Smith, E.D.; Buttle, K.F.; McDonald, K.; Mannella, C.A.; van der Bliek, A.M. The C. Elegans Opa1 Homologue EAT-3 Is Essential for Resistance to Free Radicals. PLoS Genet. 2008, 4, e1000022. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; McCaffery, J.M.; Chan, D.C. Mitochondrial Fusion Protects against Neurodegeneration in the Cerebellum. Cell 2007, 130, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Vizarra, E.; Zeviani, M. Mitochondrial Disorders of the OXPHOS System. FEBS Lett. 2021, 595, 1062–1106. [Google Scholar] [CrossRef]
- Lenaz, G. Role of Mitochondria in Oxidative Stress and Ageing. Biochim. Biophys. Acta 1998, 1366, 53–67. [Google Scholar] [CrossRef]
- Petrushanko, I.; Bogdanov, N.; Bulygina, E.; Grenacher, B.; Leinsoo, T.; Boldyrev, A.; Gassmann, M.; Bogdanova, A. Na-K-ATPase in Rat Cerebellar Granule Cells Is Redox Sensitive. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R916–R925. [Google Scholar] [CrossRef]
- Bogdanova, A.; Petrushanko, I.; Boldyrev, A.; Gassmann, M. Oxygen- and Redox-Induced Regulation of the Na/K ATPase. Curr. Enzym. Inhib. 2006, 2, 37–59. [Google Scholar] [CrossRef]
- Garthwaite, J.; Balázs, R. Supersensitivity to the Cyclic GMP Response to Glutamate during Cerebellar Maturation. Nature 1978, 275, 328–329. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Bolshakov, A.E.; Abushik, P.A.; Krivoi, I.I.; Antonov, S.M. Na+,K+-ATPase Functionally Interacts with the Plasma Membrane Na+,Ca2+ Exchanger to Prevent Ca2+ Overload and Neuronal Apoptosis in Excitotoxic Stress. J. Pharmacol. Exp. Ther. 2012, 343, 596–607. [Google Scholar] [CrossRef]
- Gusarova, G.A.; Dada, L.A.; Kelly, A.M.; Brodie, C.; Witters, L.A.; Chandel, N.S.; Sznajder, J.I. Alpha1-AMP-Activated Protein Kinase Regulates Hypoxia-Induced Na,K-ATPase Endocytosis via Direct Phosphorylation of Protein Kinase C Zeta. Mol. Cell Biol. 2009, 29, 3455–3464. [Google Scholar] [CrossRef]
- Dada, L.A.; Chandel, N.S.; Ridge, K.M.; Pedemonte, C.; Bertorello, A.M.; Sznajder, J.I. Hypoxia-Induced Endocytosis of Na,K-ATPase in Alveolar Epithelial Cells Is Mediated by Mitochondrial Reactive Oxygen Species and PKC-Zeta. J. Clin. Investig. 2003, 111, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- De Lores Arnaiz, G.R.; Ordieres, M.G.L. Brain Na+, K+-ATPase Activity In Aging and Disease. Int. J. Biomed. Sci. 2014, 10, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, J.; Zhang, Z.; Wakabayashi, N.; Tamura, Y.; Fukaya, M.; Kensler, T.W.; Iijima, M.; Sesaki, H. The Dynamin-Related GTPase Drp1 Is Required for Embryonic and Brain Development in Mice. J. Cell Biol. 2009, 186, 805–816. [Google Scholar] [CrossRef]
- Prastiwi, D.; Djunaidi, A.; Partadiredja, G. High Dosage of Monosodium Glutamate Causes Deficits of the Motor Coordination and the Number of Cerebellar Purkinje Cells of Rats. Hum. Exp. Toxicol. 2015, 34, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).