Membrane for Pressure-Driven Separation Prepared with a Method of 3D Printing: Performance in Concentrating Orange Peel Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BSA Solution
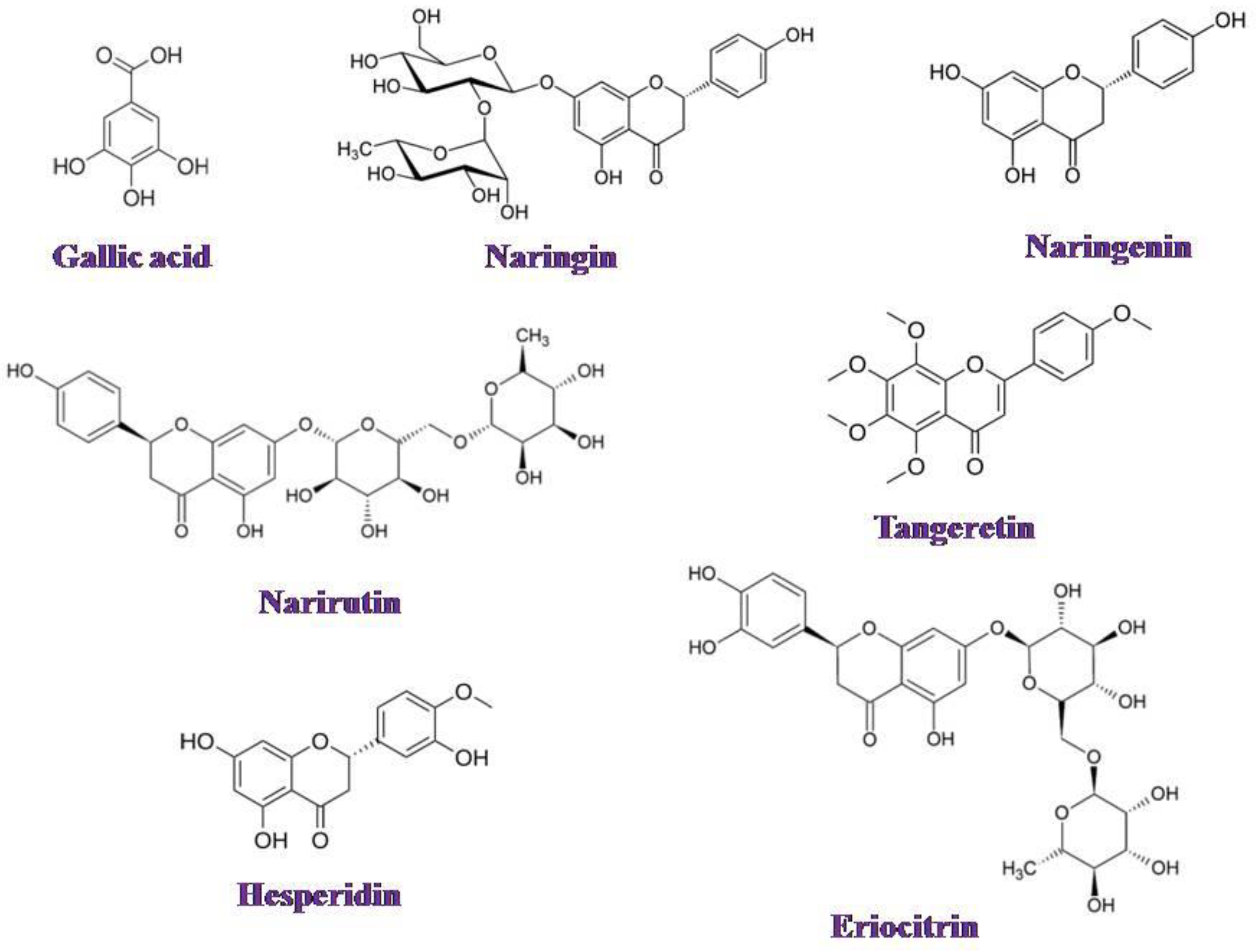
2.3. Preparation and Analysis of Orange Peel Extract
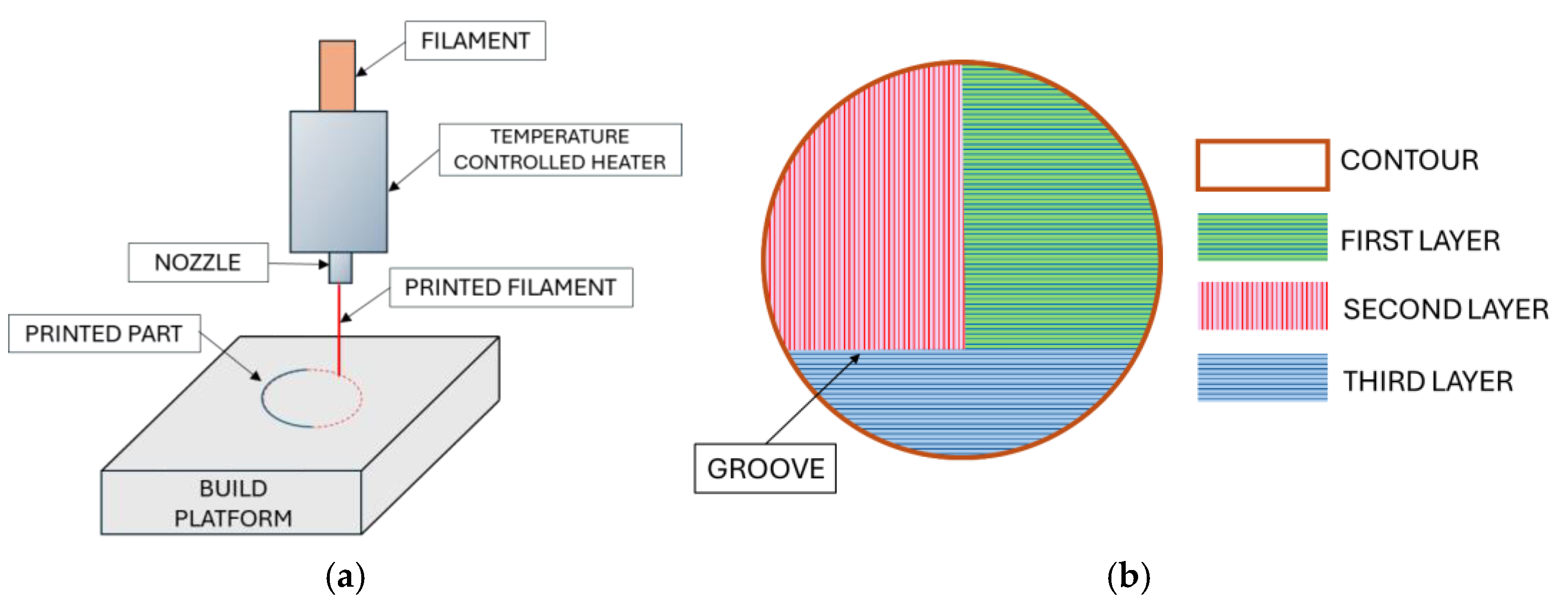
2.4. Ink for 3d Printer and Printing Membrane
2.5. Investigations of Membranes
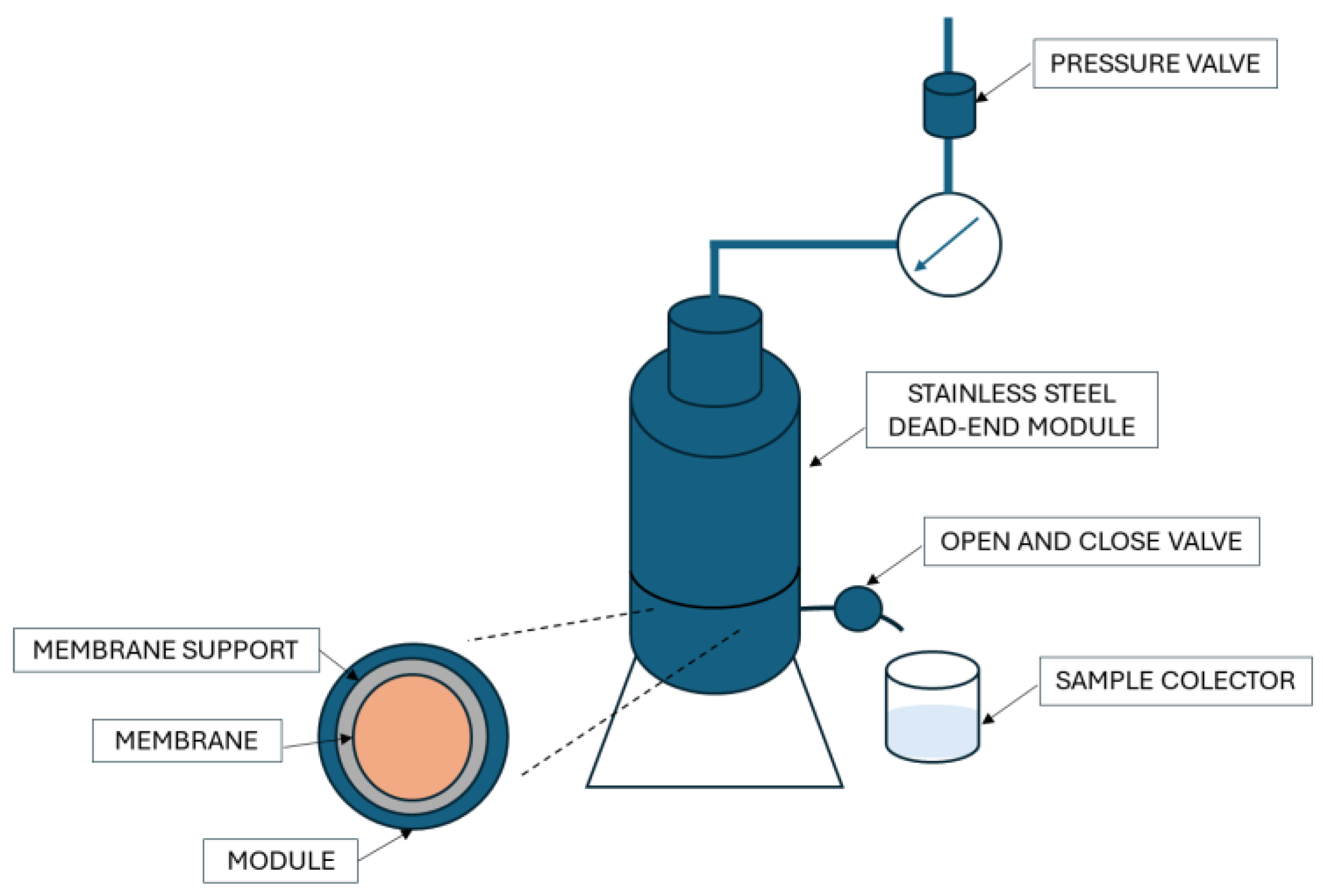
2.6. Water Filtration
2.7. Filtration of Bsa Solution and Orange Peel Extract
3. Results
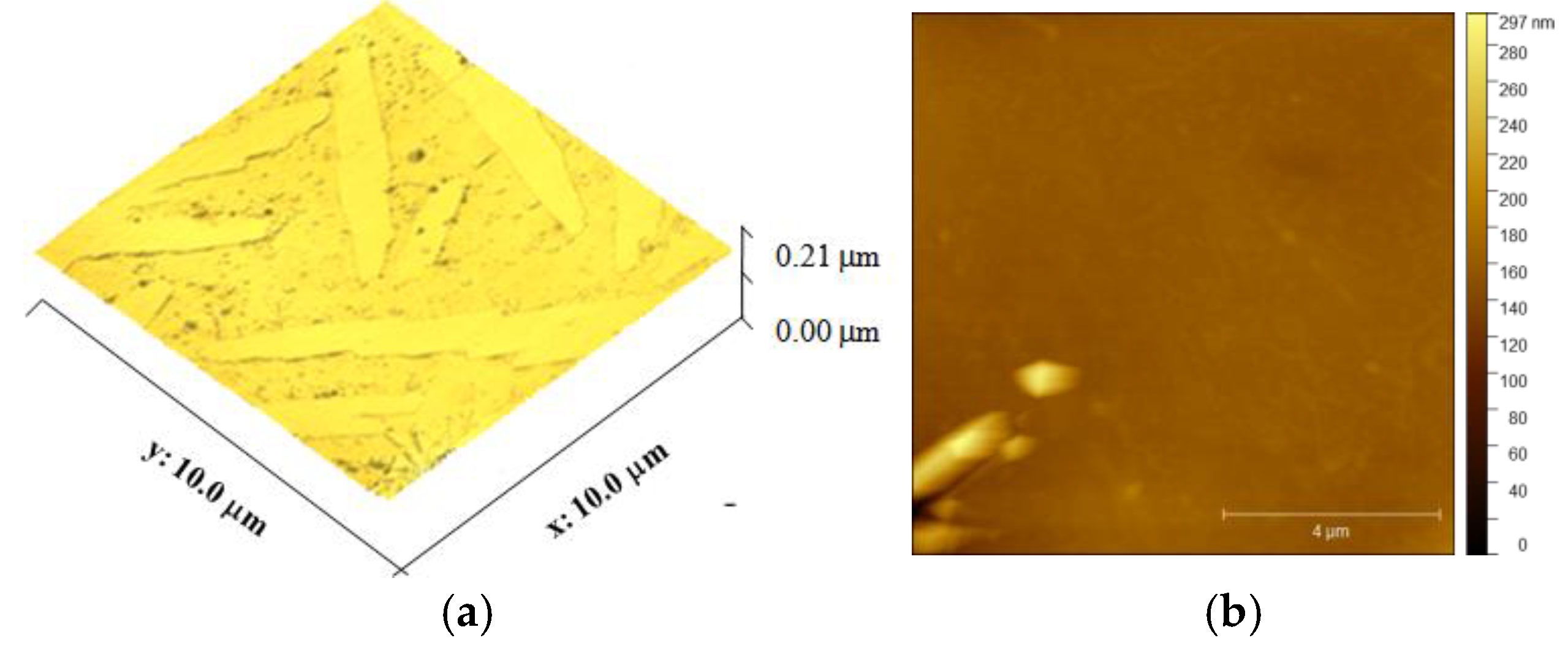
3.1. Membrane Characterization
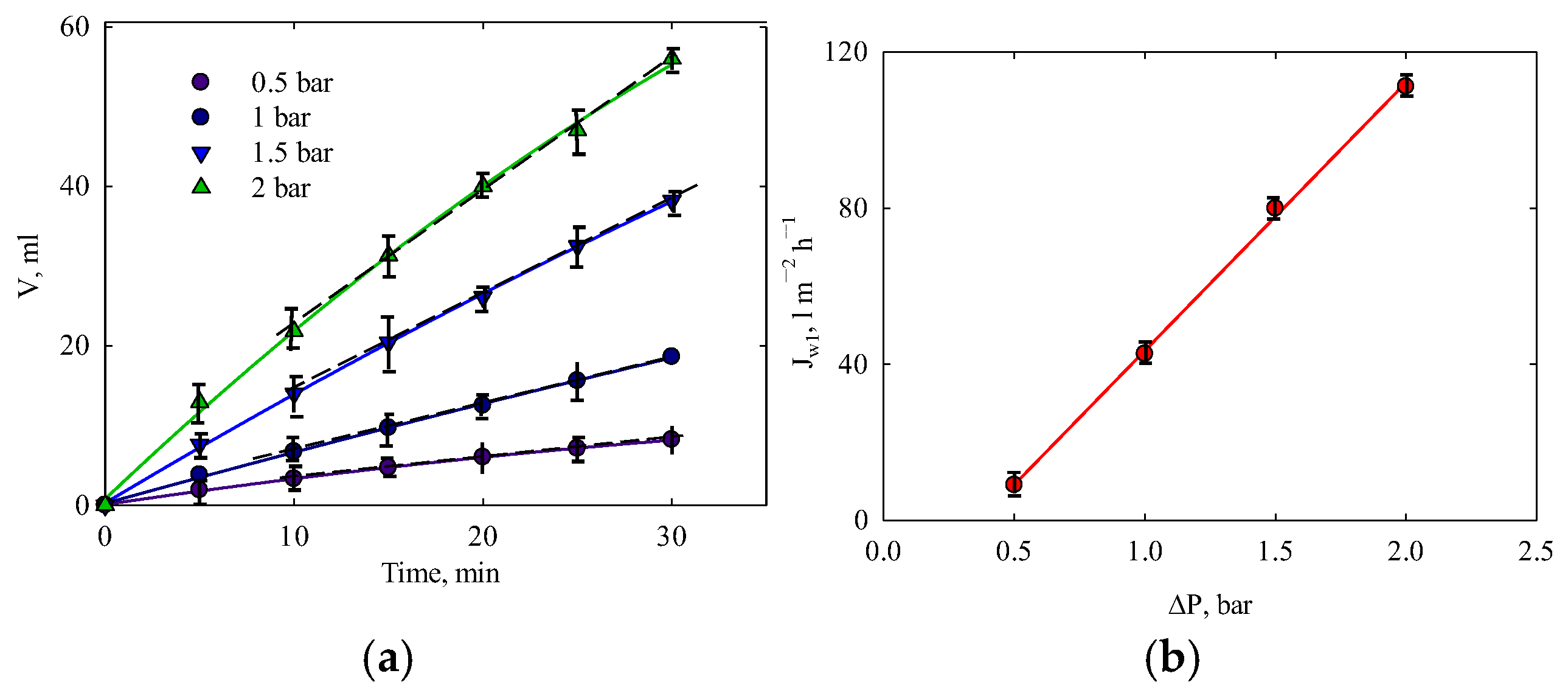
3.2. Water Test
3.3. Filtration of BSA Solution
3.4. Orange Peel Extract
3.5. Effect of Temperature on the Filtration of Orange Peel Extract
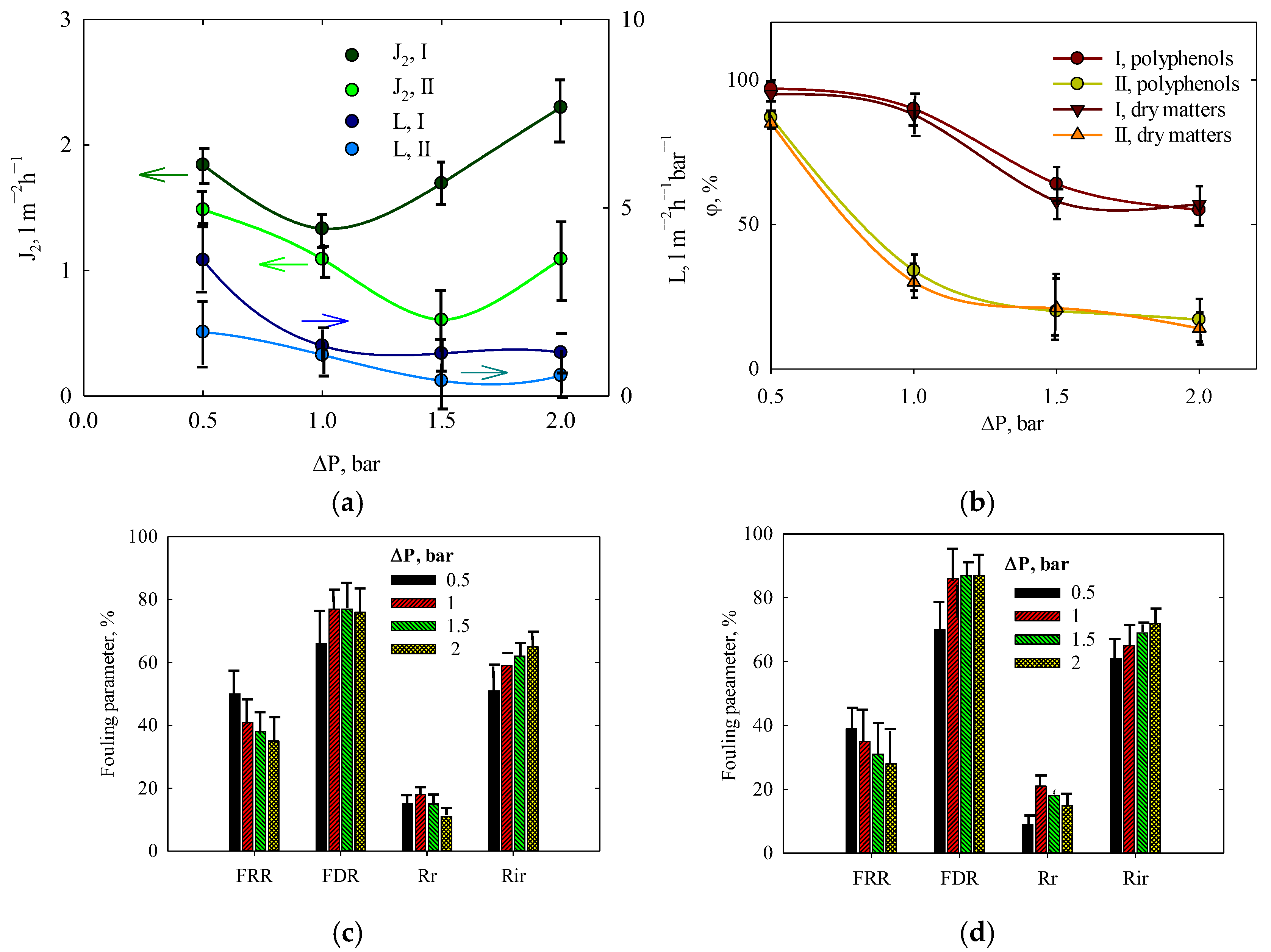
3.6. Effect of Pressure and Concentration on the Filtration of Orange Peel Extract
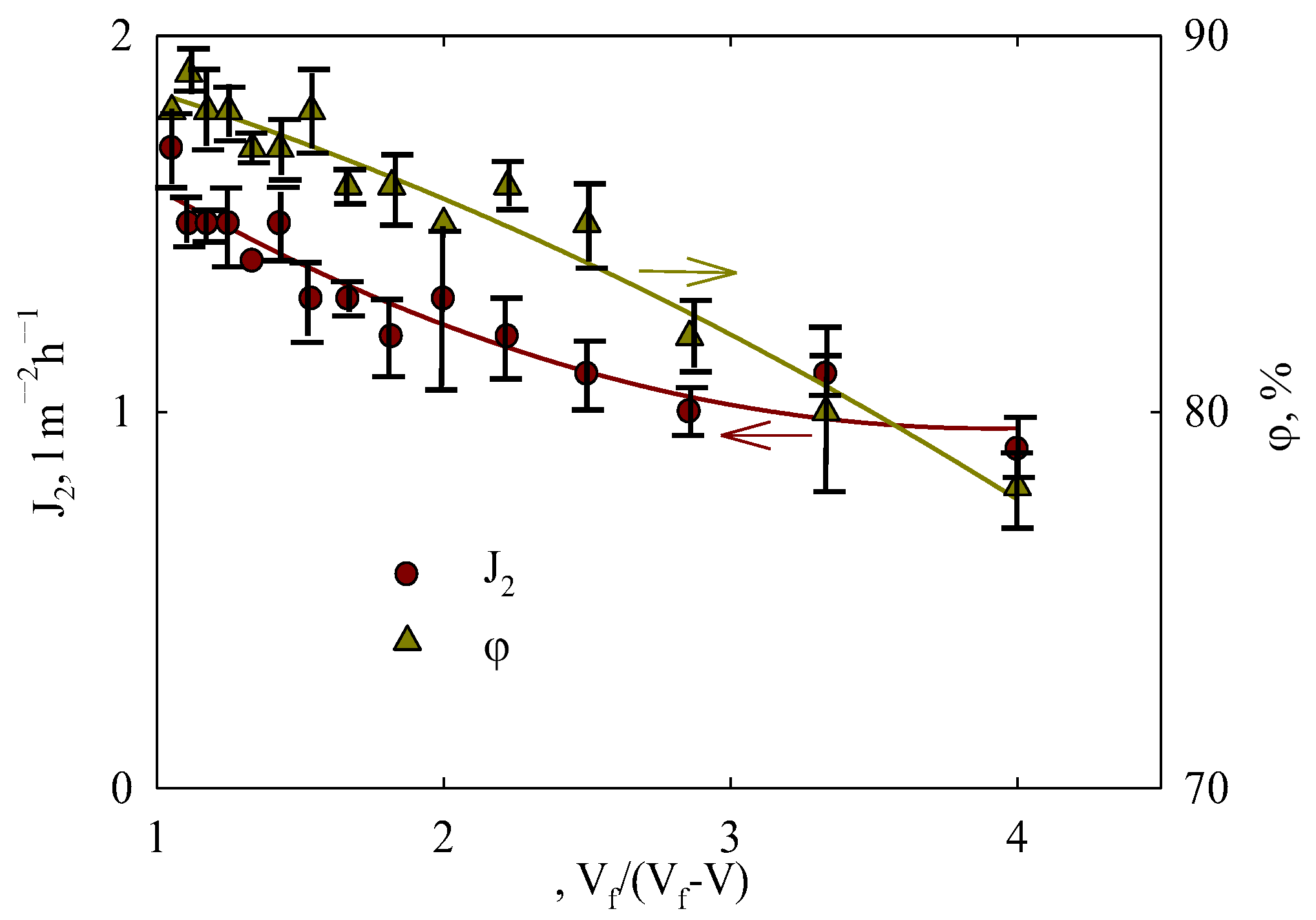
3.7. Concentrating Orange Peel Extract
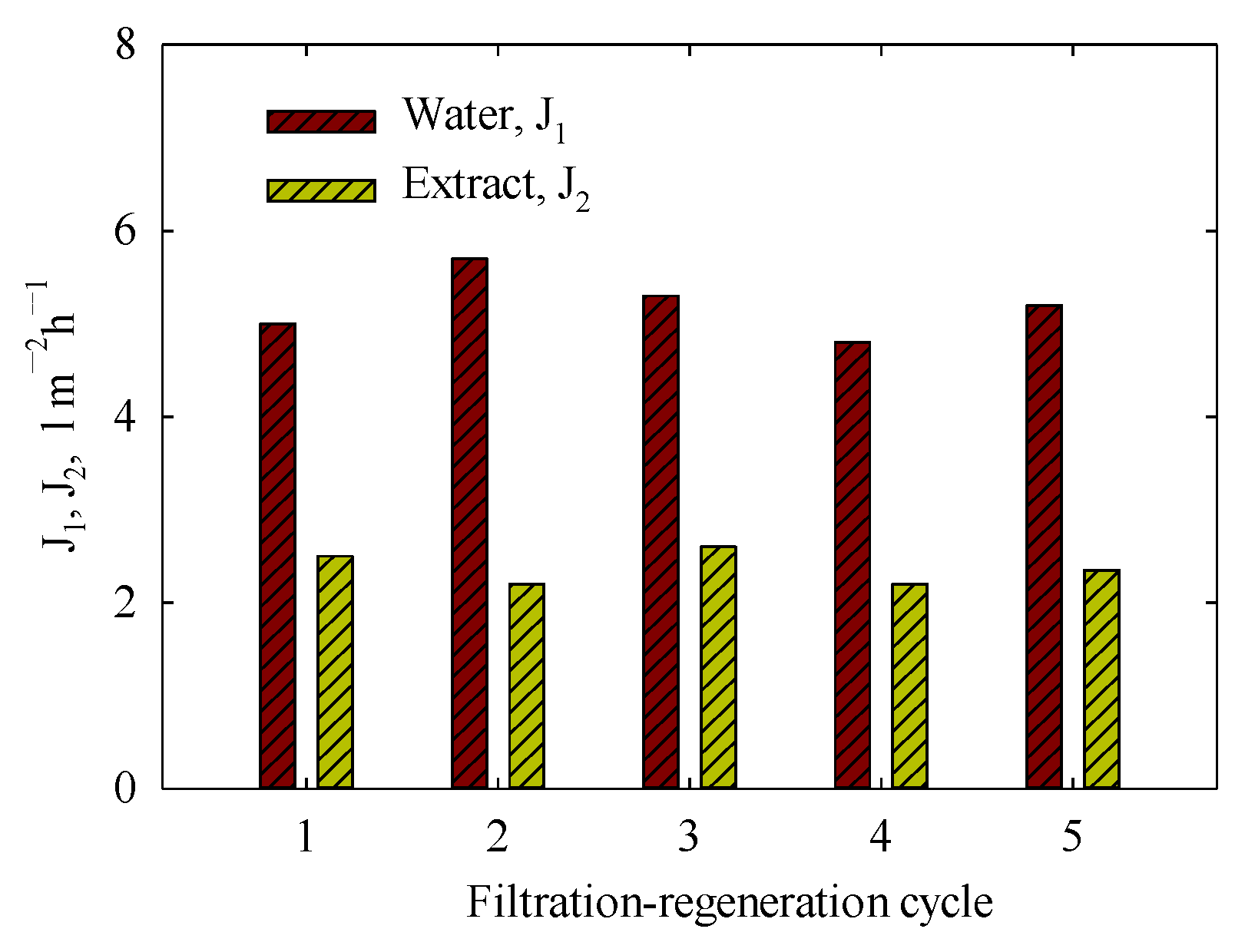
3.8. Reproducibility of Membrane Separation
3.9. Comparison of Obtained Results with Literature Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, A.; Al’Aref, S.J. History of 3D Printing. In 3D Printing Applications in Cardiovascular Medicine, 1st ed.; Al’Aref, S.J., Mosadegh, B., Dunham, S., Min, J.K., Eds.; Academic Press-Elsevier Publisher: Amsterdam, The Netherlands, 2018; Volume 1, pp. 1–10. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Haq, M.I.U. 3D printing—A review of processes, materials and applications in industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Hossain, K.R.; Shishir, M.M.A.; Rashid, S.; Ahmed, M.H.; Basher, A.; Risad, R.H.; Paul, A.C. Application of 3D printing technology in the military. J. Chem. Lett. 2023, 4, 103–116. [Google Scholar] [CrossRef]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Szabó, L.; Fodor, D. The key role of 3D printing technologies in the further development of electrical machines. Machines 2022, 10, 330. [Google Scholar] [CrossRef]
- Saharan, M.; Saharan, L. From concept to wearable: 3D printed jewellery using FDM technology. Int. J. Fash. Des. Technol. Educ. 2024, 1–12, 1–12. [Google Scholar] [CrossRef]
- Alami, A.H.; Olabi, A.G.; Khuri, S.; Aljaghoub, H.; Alasad, S.; Ramadan, M.; Abdelkareem, M.A. 3D printing in the food industry: Recent progress and role in achieving sustainable development goals. Ain. Shams. Eng. J. 2024, 15, 102386. [Google Scholar] [CrossRef]
- Barman, S.R.; Gavit, P.; Chowdhury, S.; Chatterjee, K.; Nain, A. 3D-Printed Materials for Wastewater Treatment. JACS 2023, 3, 2930–2947. [Google Scholar] [CrossRef]
- Yusoff, N.H.M.; Teo, L.R.I.; Phang, S.J.; Wong, V.L.; Cheah, K.H.; Lim, S.S. Recent advances in polymer-based 3D printing for wastewater treatment application: An overview. Chem. Eng. J. 2022, 429, 132311. [Google Scholar] [CrossRef]
- Aghaei, A.; Firouzjaei, M.D.; Karami, P.; Aktij, S.A.; Elliott, M.; Mansourpanah, Y.; Rahimpour, A.; Soares, J.B.P.; Sadrzadeh, M. The implications of 3D-printed membranes for water and wastewater treatment and resource recovery. Can. J. Chem. Eng. 2022, 100, 2309–2321. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tan, W.S.; An, J.; Chua, C.K.; Tang, C.Y.; Fane, A.G.; Chong, T.H. The potential to enhance membrane module design with 3D printing technology. J. Membr. Sci. 2016, 499, 480–490. [Google Scholar] [CrossRef]
- Li, X.; Shan, H.; Zhang, W.; Li, B. 3D printed robust superhydrophilic and underwater superoleophobic composite membrane for high efficient oil/water separation. Sep. Purif. Technol. 2020, 237, 116324. [Google Scholar] [CrossRef]
- Al-Shimmery, A.; Mazinani, S.; Ji, J.; Chew, Y.M.J.; Mattia, D. 3D printed composite membranes with enhanced anti-fouling behaviour. J. Membr. Sci. 2019, 574, 76–85. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Steffes, J.; Huey, B.D.; McCutcheon, J.R. 3D printed polyamide membranes for desalination. Science 2018, 361, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Badalov, S.; Arnusch, C.J. Ink-jet printing assisted fabrication of thin film composite membranes. J. Membr. Sci. 2016, 515, 79–85. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, L.; Tao, S. Preparation of hybrid chitosan membranes by selective laser sintering for adsorption and catalysis. Mater. Des. 2019, 173, 107780. [Google Scholar] [CrossRef]
- Kim, K.; Ratri, M.C.; Choe, G.; Nam, M.; Cho, D.; Shin, K. Three-dimensional, printed water-filtration system for economical, on-site arsenic removal. PLoS ONE 2020, 15, 0231475. [Google Scholar] [CrossRef]
- Mazinani, S.; Al-Shimmery, A.; Chew, Y.M.J.; Mattia, D. 3D printed nanofiltration composite membranes with reduced concentration polarization. J. Membr. Sci. 2022, 644, 120137. [Google Scholar] [CrossRef]
- Tian, M.; Coninck, H.D.; Zhu, J.; Zhang, Y.; Yuan, S.; Hooreweder, B.V.; Puyvelde, P.V.; Bruggen, B.V. Exploring the potential usage of 3D printed membranes combined with PVDF coating in direct contact membrane distillation. Desalination 2021, 531, 115134. [Google Scholar] [CrossRef]
- Gutierrez, D.B.; Caldona, E.B.; Yang, Z.; Suo, X.; Cheng, X.; Dai, S.; Espiritu, R.D.; Advincula, R.C. 3D-printed PDMS-based membranes for CO2 separation applications. MRS Commun. 2022, 12, 1174–1182. [Google Scholar] [CrossRef]
- Capparelli, C.; Pulido, C.R.F.; Wiencek, R.A.; Hickner, M.A. Resistance and Permselectivity of 3D-printed micropatterned anion-exchange membranes. ACS Appl. Mater. Interfaces 2019, 11, 26298–26306. [Google Scholar] [CrossRef]
- Zárybnická, L.; Stránská, E.; Janegová, K.; Vydrová, B. The effect of 3D printing parameters on electrochemical properties of heterogeneous cation exchange membrane. Rapid Prototyp. J. 2021, 27, 1538–1547. [Google Scholar] [CrossRef]
- Gao, P.; Hunter, A.; Summe, M.J.; Phillip, W.A. A method for the efficient fabrication of multifunctional mosaic membranes by inkjet printing. ACS Appl. Mater. Interfaces 2018, 8, 19772–19779. [Google Scholar] [CrossRef]
- He, J.; Yang, J.; McCutcheon, J.R.; Li, Y. Molecular insights into the structure-property relationships of 3D printed polyamide reverse-osmosis membrane for desalination. J. Membr. Sci. 2022, 658, 120731. [Google Scholar] [CrossRef]
- Tijing, L.D.; Dizon, J.R.C.; Ibrahim, I.; Nisay, A.R.N.; Shon, H.K.; Advincula, R.C. 3D printing for membrane separation, desalination and water treatment. Appl. Mater. Today 2020, 18, 100486. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Tien, H.N.; Khivantsev, K.; Chen, J.T.; Yu, M. Printing ultrathin graphene oxide nanofiltration membranes for water purification. J. Mater. Chem. A 2017, 39, 20860–20866. [Google Scholar] [CrossRef]
- Mazinani, S.; Al-Shimmery, A.; Chew, Y.M.J.; Mattia, D. 3D Printed Fouling-Resistant Composite Membranes. ACS Appl. Mater. Interfaces 2019, 11, 26373–26383. [Google Scholar] [CrossRef]
- Salentijn, G.I.; Oomen, P.E.; Grajewski, M.; Verpoorte, E. Fused Deposition Modeling 3D Printing for (Bio)analytical Device Fabrication: Procedures, Materials, and Applications. Anal. Chem. 2017, 89, 7053–7061. [Google Scholar] [CrossRef]
- Abhinay, S.; Dixit, P.; Mazumder, R. Effect of pore former sucrose on microstructure and electrical properties of porous BZT-0.5BCT ceramics. Ferroelectrics 2020, 557, 18–27. [Google Scholar] [CrossRef]
- Mohanta, K.; Kumar, A.; Parkash, O.; Kumar, D. Processing and properties of low cost macroporous alumina ceramics with tailored porosity and pore size fabricated using rice husk and sucrose. J. Eur. Ceram. Soc. 2014, 34, 2401–2412. [Google Scholar] [CrossRef]
- Gurdag, G.; Gokalp, A. Effects of Pore-Forming Agents and Polymer Composition on the Properties of Novel Poly(N,N-Dimethylaminoethyl Methacrylate Sulfate-co-N,N-dimethylacrylamide) Hydrogels. Ind. Eng. Chem. Res. 2011, 50, 8295–8303. [Google Scholar] [CrossRef]
- Fu, K.; Huang, P.; Xu, S.; Liu, L.; Huang, X.; Luo, J.; Li, Y.; Yu, H.; Fu, H.; Ou, H. Natural sucrose-assisted controllable porous PVDF-HFP films for self-powered tactile sensors with higher sensitivity. Surf. Interfaces 2024, 54, 105252. [Google Scholar] [CrossRef]
- Charcosset, C. Classical and recent applications of membrane processes in the food industry. Food Eng. Rev. 2021, 13, 322–343. [Google Scholar] [CrossRef]
- Reig, M.; Vecino, X.; Cortina, J.L. Use of membrane technologies in dairy industry: An overview. Foods 2021, 10, 2768. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, N.N.; Paraíso, C.M.; Molina, L.C.A.; Dzyazko, Y.S.; Bergamasco, R.; Vieira, A.M. Innovative trends in modified membranes: A mini review of applications and challenges in the food sector. Membranes 2024, 14, 209. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.; Barbosa, P.P.M.; Ruviaroc, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martinez, J. Recovery of phenolic compounds from citrus by-products using pressurized liquids—An application to orange peel. Food Bioprod. Proc. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Hegazy, A.E.; Ibrahium, M.I. Antioxidant Activities of Orange Peel Extracts World. Appl. Sci. J. 2012, 18, 684–688. [Google Scholar]
- Shehata, M.G.; Awad, T.S.; Asker, D.; Sohaimy, S.A.E.; El-Aziz, N.M.A.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef]
- Marranzano, M.; Rosa, L.; Malaguarnera, M.; Palmeri, R.; Tessitori, M.; Barbera, A.C. Polyphenols: Plant Sources and Food Industry Applications. Curr. Pharm. Des. 2018, 24, 4125–4130. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Axten, L.G.; Wohlers, M.W.; Waterhouse, D.S. Polyphenol-rich beverages: Insights from sensory and consumer science. J. Sci. Food Agric. 2009, 89, 2356–2363. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Liu, Y.; Benohoud, M.; Yamdeu, J.H.G.; Gong, Y.Y.; Orfila, C. Green extraction of polyphenols from citrus peel by-products and their antifungal activity against Aspergillus flavus. Food Chem. X 2021, 12, 100144. [Google Scholar] [CrossRef]
- Conidi, C.; Drioli, E.; Cassano, A. Membrane-based agro-food production processes for polyphenol separation, purification and concentration. Curr. Opin. Food Sci. 2018, 23, 149–164. [Google Scholar] [CrossRef]
- Abbassi, A.E.; Hafidi, A.; García-Payo, M.C.; Khayet, M. Concentration of olive mill wastewater by membrane distillation for polyphenols recovery. Desalination 2009, 245, 670–674. [Google Scholar] [CrossRef]
- Bazinet, L.; DeGrandpré, Y.; Porter, A. Electromigration of tobacco polyphenols. Sep. Purif. Technol. 2005, 41, 101–107. [Google Scholar] [CrossRef]
- Salehi, H.; Karimi, M.; Raofie, F. Micronization and coating of bioflavonoids extracted from Citrus sinensis L. peels to preparation of sustained release pellets using supercritical technique. J. Iran. Chem. Soc. 2021, 18, 3235–3248. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Granados, M.; Farran, A.; Cortina, J.L.; Saurina, J.; Valderrama, C. Recovery of Natural Polyphenols from Spinach and Orange By-Products by Pressure-Driven Membrane Processes. Membranes 2022, 12, 669. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Cassano, A.; Drioli, E. Recovery of phenolic compounds from orange press liquor by nanofiltration. Food Bioprod. Process 2012, 90, 867–874. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Haya, S.; Bentahar, F.; Trari, M. Optimization of polyphenols extraction from orange peel. Food Meas. 2019, 13, 614–662. [Google Scholar] [CrossRef]
- Jiang, C.; Han, Z.; Zhu, M.; Wan, X.; Zhang, L. Effects of thermal processing on transformation of polyphenols and flavor quality. Curr. Opin. Food Sci. 2023, 51, 101014. [Google Scholar] [CrossRef]
- Paraíso, C.M.; Dos Santos, S.S.; Correa, V.G.; Magon, T.; Peralta, R.M.; Visentainer, J.V.; Madrona, G.S. Ultrasound assisted extraction of hibiscus (Hibiscus sabdariffa L.) bioactive compounds for application as potential functional ingredient. J. Food Sci. Technol. 2019, 56, 4667–4677. [Google Scholar] [CrossRef]
- Zuritz, C.A.; Puntes, E.M.; Mathey, H.H.; Pérez, E.H.; Gascón, A.; Rubio, L.A.; Carullo, C.A.; Chernikoff, R.E.; Cabeza, M.S. Density, viscosity and coefficient of thermal expansion of clear grape juice at different soluble solid concentrations and temperatures. J. Food Eng. 2005, 71, 143–149. [Google Scholar] [CrossRef]
- Mahdavi, R.; Nikniaz, Z.; Rafraf, M.; Jouyban, A. Determination and comparison of the total polyphenol contents of fresh and commercial fruit juices. Br. Food J. 2011, 113, 744–752. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publisher: Dordrecht, UK, 1996. [Google Scholar]
- Gao, H.; Zhong, S.; Dangayach, R.; Chen, Y. Understanding and designing a high-performance ultrafiltration membrane using machine learning. Environ. Sci. Technol. 2023, 57, 17831–17840. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Sasanihoma, A.; Zereshki, S. Chemical cleaning of reverse osmosis membrane fouled by apple juiceJ. J. Food Process Eng. 2011, 34, 1535–1557. [Google Scholar] [CrossRef]
- Powell, L.C.; Hilal, N.; Wright, C.J. Atomic force microscopy study of the biofouling and mechanical properties of virgin and industrially fouled reverse osmosis membranes. Desalination 2017, 404, 313–321. [Google Scholar] [CrossRef]
- Paraíso, C.M.; Pizzo, J.S.; Gibin, M.S.; Volnistem, E.A.; Visentainer, J.V.; Sato, F.; Costa, S.C.; Reis, M.H.M.; Madrona, G.S. Intensified ultrafiltration process for fouling mitigation during concentration of bioactive compounds from hibiscus (Hibiscus sabdariffa L.) extract: Innovation by using ultrasound and 3D turbulence promoters. Chem. Eng. Process.—Process Intensif. 2024, 195, 109612. [Google Scholar] [CrossRef]
- Damar Huner, I.; Gulec, H.A. Fouling behavior of poly(ether)sulfone ultrafiltration membrane during concentration of whey proteins: Effect of hydrophilic modification using atmospheric pressure argon jet plasma. Colloids Surf. B Biointerfaces 2017, 160, 510–519. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Z.; Yao, Z.; Guo, H.; Xu, Z.; Tang, C.Y. Tuning roughness features of thin film composite polyamide membranes for simultaneously enhanced permeability, selectivity and anti-fouling performance. J. Colloid Interface Sci. 2019, 540, 382–388. [Google Scholar] [CrossRef]
- Kumar, S.; Nandi, B.K.; Guria, C.; Mandal, A. Oil removal from produced water by ultrafiltration using polysulfone membrane. Braz. J. Chem. Eng. 2017, 34, 583–596. [Google Scholar]
- Dzyazko, Y.; Volfkovich, Y.; Perlova, O.; Ponomaryova, L.; Perlova, N.; Kolomiets, E. Effect of Porosity on Ion Transport Through Polymers and Polymer-Based Composites Containing Inorganic Nanoparticles (Review). Springer Proc. Phys. 2019, 222, 235–253. [Google Scholar] [CrossRef]
- Cho, D.L.; Kim, S.-H.; Huh, Y.I.; Kim, D.; Cho, S.Y.; Kim, B.H. Effects of surface modification of the membrane in the ultrafiltration of waste water. Macromol. Res. 2004, 12, 553–558. [Google Scholar] [CrossRef]
- Molina, L.C.A.; Magalhães-Ghiotto, G.A.V.; Nichi, L.; Dzyazko, Y.S.; Bergamasco, R. Membranes modified with rigid polymer for processing solutions of vegetable proteins. Acta Period. Technol. 2023, 54, 313–324. [Google Scholar] [CrossRef]
- Zheng, Q.-Z.; Wang, P.; Yang, Y.-N.; Cui, D.-J. The relationship between porosity and kinetics parameter of membrane formation in PSF ultrafiltration membrane. J. Membr. Sci. 2006, 286, 7–11. [Google Scholar] [CrossRef]
- Paixão, R.M.; Silva, L.H.B.R.; Vieira, M.F.; Amorim, M.T.P.; Bergamasco, R.; Vieira, A.M.S. Enhanced filtration membranes with graphene oxide and tannic acid for textile industry wastewater dye removal. Environ. Technol. 2025, 46, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Rozhdestvenska, L.; Kudelko, K.; Ogenko, V.; Palchik, O.; Plisko, T.; Bildyukevich, A.; Zakharov, V.; Zmievskii, Y.; Vishnevskii, O. Filtration Membranes Containing Nanoparticles of Hydrated Zirconium Oxide–Graphene Oxide. Springer Proc. Phys. 2021, 246, 757–771. [Google Scholar] [CrossRef]
- Dey, S.; Bhattacharya, P.; Bandyopadhyay, S.; Roy, S.N.; Majumdar, S.; Sahoo, G.C. Single Step Preparation of Zirconia Ultrafiltration Membrane over Clay-Alumina Based Multichannel Ceramic Support for Wastewater Treatment. Membr. Sci. Res. 2018, 4, 28–33. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, J.; Peng, B.; Li, Z.; Zhang, H.; Han, C.H. A novel composite microfiltration membrane: Structure and performance. J. Membr. Sci. 2013, 439, 12–19. [Google Scholar] [CrossRef]
- Yushkin, A.; Basko, A.; Balynin, A.; Efimov, M.; Lebedeva, T.; Ilyasova, A.; Pochivalov, K.; Volkov, A. Effect of acetone as co-solvent on fabrication of polyacrylonitrile ultrafiltration membranes by non-solvent induced phase separation. Polymers 2022, 14, 4603. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Plisko, T.V.; Shustikov, A.A.; Dzyazko, Y.S.; Rozhdestvenska, L.M.; Pratsenko, S.A. Effect of the solvent nature on the structure and performance of poly(amide-imide) ultrafiltration membranes. J. Mater. Sci. 2020, 55, 9638–9654. [Google Scholar] [CrossRef]
- Lu, C.; Bao, Y.; Huang, J.-Y. Fouling in membrane filtration for juice processing. Curr. Opin. Food Sci. 2021, 42, 76–85. [Google Scholar] [CrossRef]
- Alonso-Vázquez, P.; Isola, A.; Sánchez-Arévalo, C.M.; Cuartas-Uribe, B.; Vincent-Vela, M.C.; Álvarez-Blanco, S. Concentration of phenolic compounds from an orange peel waste extract using a combination of ultrafiltration and forward osmosis. Sep. Purif. Technol. 2025, 360, 131228. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 2017, 195, 1–13. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Saurina, J.; Granados, M.; Cortina, J.L. Integration of membrane processes for the recovery and separation of polyphenols from winery and olive mill wastes using green solvent-based processing. J. Environ. Manag. 2022, 307, 114555. [Google Scholar] [CrossRef] [PubMed]
- Reig-Valor, M.J.; Rozas-Martínez, J.; López-Borrell, A.; López-Borrell, A.; Lora-García, J.; López-Pérez, M.-F. Experimental study of a sequential membrane process of ultrafiltration and nanofiltration for efficient polyphenol extraction from wine lees. Membranes 2024, 14, 82. [Google Scholar] [CrossRef]
- Filippou, P.; Mitrouli, S.T.; Vareltzis, P. Sequential membrane filtration to recover polyphenols and organic acids from red wine lees: The antioxidant properties of the spray-dried concentrate. Membranes 2022, 12, 353. [Google Scholar] [CrossRef]
- Conidi, D.; Egea-Corbacho, A.; Cassano, A. A Combination of Aqueous Extraction and Polymeric Membranes as a Sustainable Process for the Recovery of Polyphenols from Olive Mill Solid Wastes. Polymers 2019, 11, 1868. [Google Scholar] [CrossRef]
- Dushkova, M.; Vardakas, A.; Shikov, S.; Mihalev, K.; Terzyiska, M. Application of Ultrafiltration for Recovery of Polyphenols from Rose Petal Byproduct. Membranes 2023, 13, 818. [Google Scholar] [CrossRef]
- Conidi, C.; Morelli, R.; Elia, A.; Cassano, A. Towards a green process for recovering phenolic compounds from fennel wastes (Foeniculum vulgare Mill.): Identifying target molecules and assessing membrane nanofiltration for their separation. Sep. Purif. Technol. 2025, 358, 130293. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef]





| Parameter | Value | |
|---|---|---|
| Thickness, µm | 402 ± 8 | |
| Rq, nm (face side) | 63.95 ± 8.14 | |
| N2 adsorption-desorption | pore volume, cm3g−1, | 0.47 |
| specific surface area, m2g−1 | 9.2 | |
| Wetting angle, degree | 69.5 ± 1.6 | |
| Water test | ε | 48 |
| L, L m−2h−1bar−1 | 64 | |
| BSA test (0.5 bar) | L, L m−2h−1bar−1 | 7 |
| φ, % | 97 | |
| FRR, % | 70 | |
| FDR, % | 80 | |
| Rr, % | 42 | |
| Rir, % | 38 | |
| Liquid/Target Product | Membrane | ΔP, bar | L, L m−2h−1bar−1 | φ, % | Ref. |
|---|---|---|---|---|---|
| Orange peel extract | NF (aromatic polyamide, polysulfone, poly(piperazine-amide), polyethersulfone) 180–400 Da | 6 | 2–3 | 54–95 | [47] |
| Orange peel and Spinach by-products | MF (acetylcellulose) | 1.4–1.8 | ≈50 | 7–27 | [48] |
| UF (polyethersulfone), 30–50 kDa | 3–6 | ≈10–20 | 28–68 | ||
| NF (aromatic polyamide, poly(piperazine-amide), sulfonamide) | 41–82 | ≈0.4–1.8 | 70–88 | ||
| RO (aromatic polyamide) | 69 | ≈0.9–1.2 | 90–97 | ||
| Orange peel extract | UF (polysulfone), 10 kDa | 3 | ≈8 | 4 | [74] |
| Pomegranate juice | UF (polyethersulphone, fluoropolymer), 1–4 kDa | 10 | 0.1–0.3 | 30 | [75] |
| Winery lees extract, olive extract | UF (polyethersulphone), 30–50 KDa | 7 | 1–4 | 5–45 | [76] |
| Winery lees extract | UF (TiO2) | 2 | 10 | 54 | [77] |
| NF (polyamide) | 9 | 2 | 90 | ||
| Winery lees extract | UF (poly(vinylidene fluoride) | 2 | 4 | 28 | [78] |
| NF (polyamide) | 2 | 6 | 80 | ||
| Olive waste extract | UF (polyamide), 1–3.5 kDa | 25 | 2–4 | 90–95 | [79] |
| NF (polyamide) | 3- | 98–100 | |||
| Rose petal extract | UF (polyacrylonitrile), 1 and 10 kDa | 2–4 | ≈10 | 50 | [80] |
| Fennel waste extract | MF (ceramics, Al2O3), 0.8 μm | 1 | 25 | 2 | [81] |
| TS40 NF (material are not pointed) | 5–25 | 5 | 90–95 | ||
| Orange peel extract | UF (PLA) | 0.5 | 3.4 | 98 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pini Pereira, P.; Pacola Gonçalves, I.; Molina, L.C.A.; Delcolle, R.; Dzyazko, Y.S.; Moser Paraiso, C.; Batista Neto, G.L.; Diório, A.; Marquetotti Salcedo Vieira, A.; Bergamasco, R. Membrane for Pressure-Driven Separation Prepared with a Method of 3D Printing: Performance in Concentrating Orange Peel Extract. Membranes 2025, 15, 105. https://doi.org/10.3390/membranes15040105
Pini Pereira P, Pacola Gonçalves I, Molina LCA, Delcolle R, Dzyazko YS, Moser Paraiso C, Batista Neto GL, Diório A, Marquetotti Salcedo Vieira A, Bergamasco R. Membrane for Pressure-Driven Separation Prepared with a Method of 3D Printing: Performance in Concentrating Orange Peel Extract. Membranes. 2025; 15(4):105. https://doi.org/10.3390/membranes15040105
Chicago/Turabian StylePini Pereira, Priscila, Isabela Pacola Gonçalves, Luiza C. A. Molina, Roberta Delcolle, Yuliya S. Dzyazko, Carolina Moser Paraiso, Guilherme L. Batista Neto, Alexandre Diório, Angélica Marquetotti Salcedo Vieira, and Rosângela Bergamasco. 2025. "Membrane for Pressure-Driven Separation Prepared with a Method of 3D Printing: Performance in Concentrating Orange Peel Extract" Membranes 15, no. 4: 105. https://doi.org/10.3390/membranes15040105
APA StylePini Pereira, P., Pacola Gonçalves, I., Molina, L. C. A., Delcolle, R., Dzyazko, Y. S., Moser Paraiso, C., Batista Neto, G. L., Diório, A., Marquetotti Salcedo Vieira, A., & Bergamasco, R. (2025). Membrane for Pressure-Driven Separation Prepared with a Method of 3D Printing: Performance in Concentrating Orange Peel Extract. Membranes, 15(4), 105. https://doi.org/10.3390/membranes15040105







