Pressure-Driven Membrane Processes for Removing Microplastics
Abstract
1. Introduction
2. Methodology
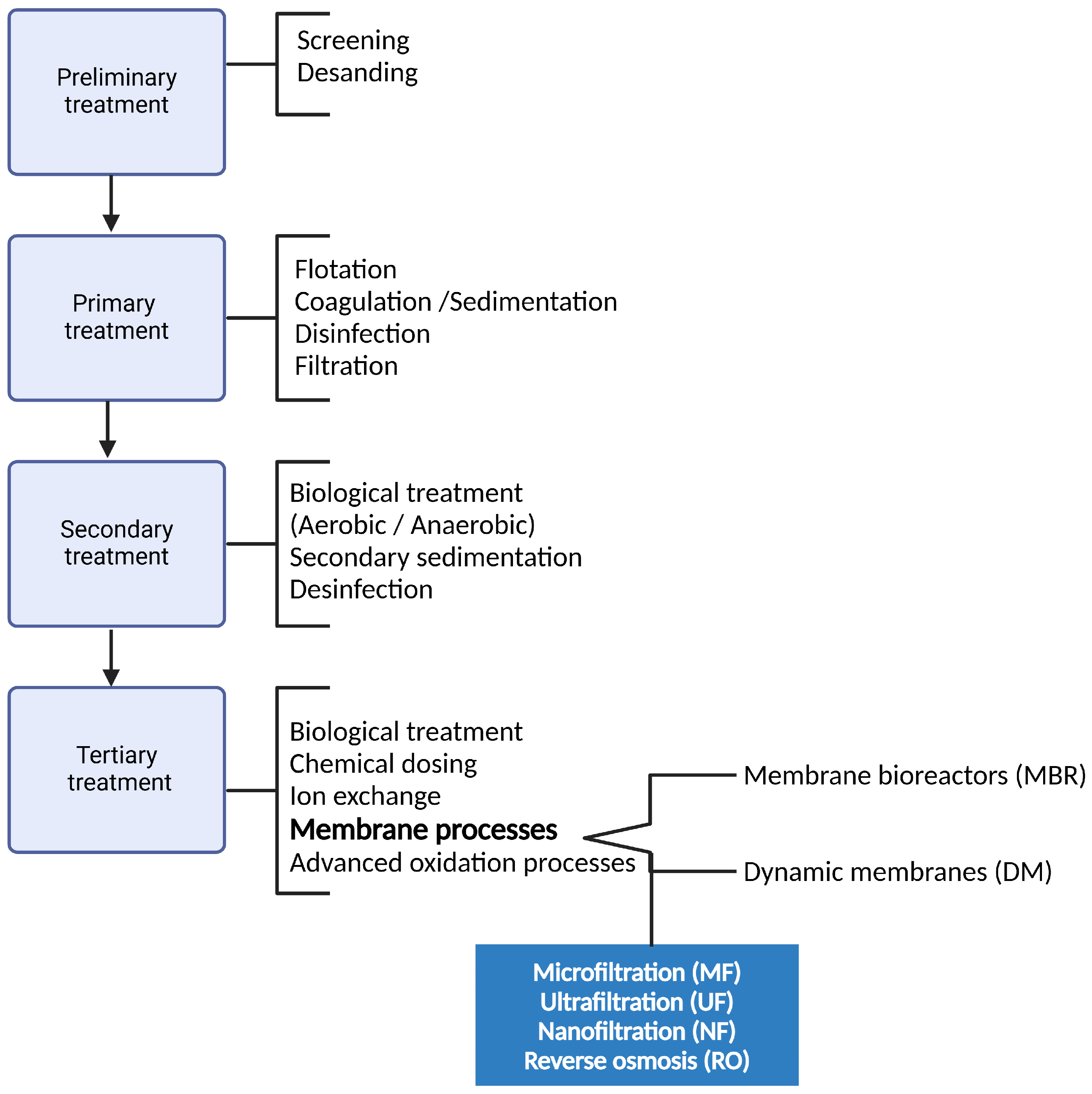
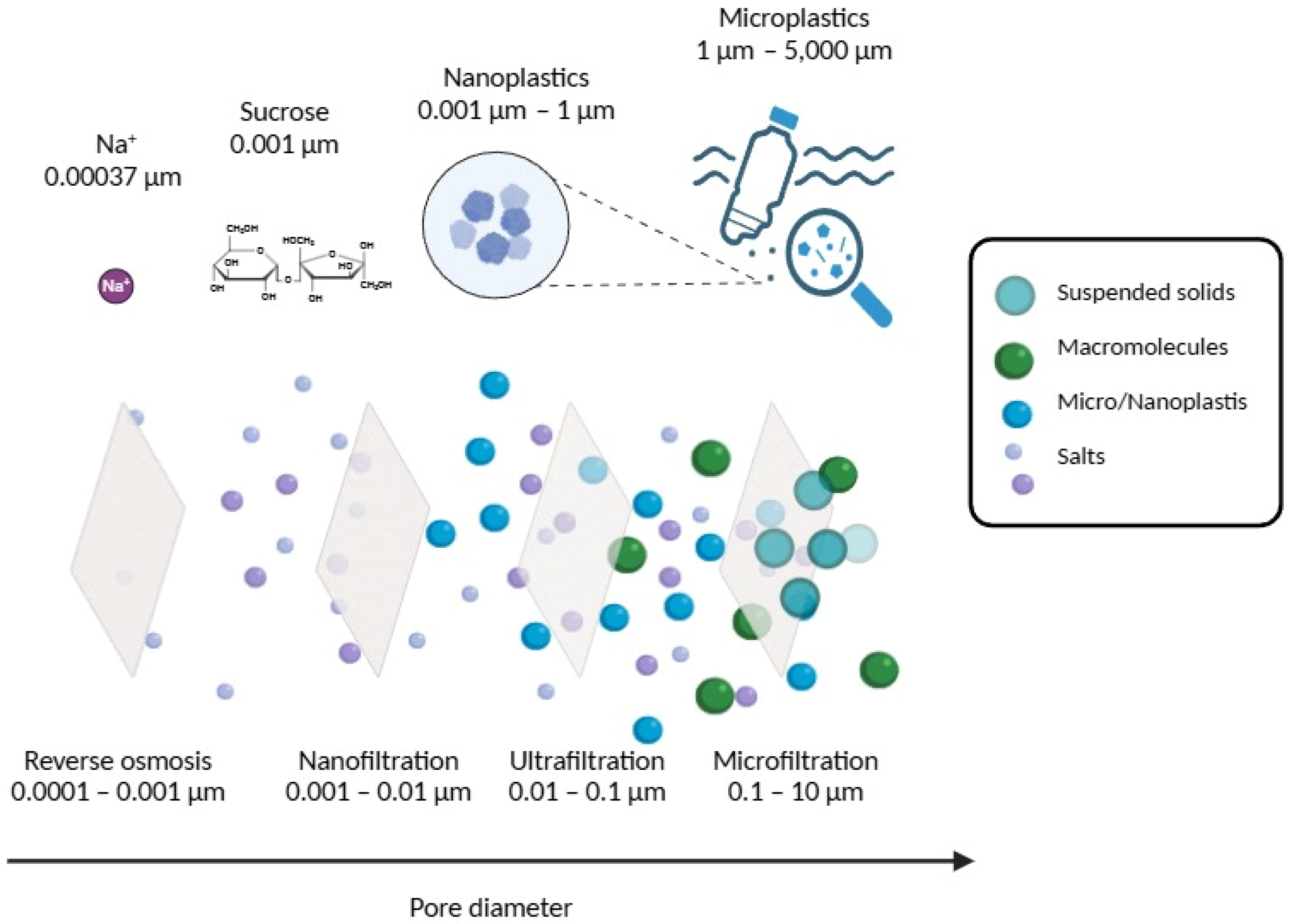
3. Removal of MPs from Aqueous Matrices by Pressure-Driven Membrane Processes
3.1. Microfiltration
| Membrane | Operating Pressure (Bar) | Aqueous Matrix | Scale | Microplastic | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Supplier | Type | Material | Pore Size (µm) | Hydrophilicity | Type | Concentration | Size (µm) | Removal (%) | ||||
| VVLP | Millipore | Flat sheet | PVDF | 0.10 | Hydrophilic | 0.7 | Milli-Q water | Lab | PE | 100 mg/L | <2 | >90 | [13] |
| PVC | 100 mg/L | ||||||||||||
| PES | 100 mg/L | ||||||||||||
| N.A. a | ≥0.2 | N.A. | N.A. | RO water | Lab | PVC | 39 ± 9 particles/L | 39–246 | 93.6 ± 2.2 | [23] | |||
| PET | 36 ± 7 particles/L | 28–121 | |||||||||||
| Nylon | 64 ± 15 fibers/L | 496–1862 | |||||||||||
| N.A. | Filter-Lab | Flat sheet | PC | 5.0 | Hydrophilic | 0.5 | Milli-Q water | Lab | PA | 100 mg/L | 20–300 | ~94 | [45] |
| CA | Hydrophilic | PS | 100 mg/L | 20–300 | |||||||||
| PTFE | Hydrophobic | 1.5 | |||||||||||
| N.A. | Cembrane | Flat sheet | Silicon carbide | 0.10 | Hydrophilic | 0.25 | Laundry wastewater | Lab | PET | 3000–45,000 fibers/L | 29–36 thickness and 220–550 length | 87.7–98.9 | [46] |
| Meiden | Meindensha | Flat sheet | Al2O3 | 0.10 | Hydrophilic | 0.25 | Laundry wastewater | Lab | PET | 3000–45,000 fibers/L | 29–36 thickness and 220–550 length | 90.3–97.7 | |
| T1-70, Membralox | Pall | Tubular | Al2O3 | 0.10 | Hydrophilic | 1.0 | Synthetic wastewater (organic UV filter + water) | Lab | PE | 100 mg/L | 75–90; 300–355; 600–710 | 80.2–97.9 | [47] |
| V0.1 | Synder Filtration | Flat sheet | PVDF | 0.10 | Hydrophilic | 1.0 | DI water + surfactant | Lab | PE; PS | 50 mg/L | 0.1–1.0 | >99 | [48] |
| SteriLUX | Meissner | Flat sheet | PVDF | 0.10 | Hydrophilic | ||||||||
| Anopore/Anodisc | Whatman | Flat sheet | Al2O3 | 0.10 | Hydrophilic | ||||||||
| N.A. | Lab-made | Flat sheet | WEPS/PI | 0.17–0.20 | Hydrophilic | 1.0 | DI water + SDS | Lab | PTFE | N.A. | 7.0 | >80 | [49] |
| N.A. | Lab-made | Hollow fiber | Sericin coated on PP | N.A. | Hydrophilic | 0.2 | 50 L DI water + 1.75 kg rock salt or sea salts | Lab | PE | 287–417 particles/kg rock salt or 1434–2284 particles/kg sea salt | ≥20–≤5000 | 99.30 | [50] |
| PET | |||||||||||||
| PP | |||||||||||||
| PVC | |||||||||||||
| Nylon | |||||||||||||
| PU | |||||||||||||
| PS | |||||||||||||
| Undefined | |||||||||||||
| N.A. | Pall b | Hollow fiber | PVDF | 0.10 | N.A. | N.A. | Wastewater | Full | HDPE, LDPE, PP | N.A. | >5 | >94 | [51] |
| Durapore | Millipore | Flat sheet | PVDF | 0.10 | Hydrophilic | 0.5 | DI water + ethanol (1:1) | Lab | PE | 50 mg/L | 10–106 | N.A. | [52] |
| 0.22 | |||||||||||||
| 0.45 | PA | 50 mg/L | 15–55 | ||||||||||
| 5.0 | |||||||||||||
| N.A. | N.A. | Flat sheet | PVDF | 0.22 | Hydrophobic | 1–3 | Secondary wastewater | Lab | PE | 200 particles/L | 150 | 100 | [53] |
| PVC | 250 | ||||||||||||
| N.A. | Mervilab | Flat sheet | CA | 5.0 | Hydrophilic | 0.1–0.7 | Milli-Q water | Lab | PA | 1–20 mg/L | 10–105 | N.A. | [54] |
| PS | 20–320 | ||||||||||||
| N.A. | N.A. | Flat sheet | PES | 0.22 | N.A. | 0.5 | DI water | Lab | PE | 10 mg/L | 40–48 | 100 | [55] |
| PET | 10 mg/L | 300 | |||||||||||
| N.A. | ADVANTEC | Flat sheet | MCE | 0.80 | Hydrophilic | 1.0 | DI water | Lab | PS | 2.5 mg/L | 1.0 | 99.9 | [56] |
| Filtanium | TAMI | Tubular | TiO2 | 0.80 | N.A. | 0.1–1.0 | Secondary wastewater | Full | N.A. | 10–100 particles/L | 17–427 | 96 | [57] |
| Filtanium | TAMI | 0.14 | |||||||||||
| TÜBITAK 1-4 | TÜBITAK | 0.40 | |||||||||||
| N.A. | Lab-made | Flat sheet | Modified PVC | 0.86–3.44 | Hydrophilic | 1.0 | DI water | Lab | PS | 2.5 mg/L | 1.0 | ~100 | [58] |
| N.A. | Lab-made | Flat sheet | Modified PVDF | 0.34–1.36 | Amphiphilic | 1.0 | DI water + 0.1% Triton X | Lab | PS | 0.1% | 0.1–0.5 | 90.41–97.2 | [59] |
| N.A. | Lab-made | Flat sheet | Glass fiber membrane + chitosan-modified geopolymer sub-microparticles | 0.058 | Hydrophilic | 0.9 | Wastewater | Lab | PS | N.A. | 0.05 | 92.04 | [60] |
| N.A. | Lab-made | Flat sheet | SA/GO/CS | 0.70 | Hydrophilic | 0.04 | Synthetic wastewater | Lab | PS | N.A. | 0.05 | 97.10 ± 1.17 | [61] |
3.2. Ultrafiltration
| Membrane | Operating Pressure (Bar) | Aqueous Matrix | Scale | Microplastic | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Supplier | Type | Material | Pore Size/MWCO | Hydrophilicity | Type | Concentration | Size (µm) | Removal (%) | ||||
| BY a | Synder Filtration | Flat sheet | PVDF | 100 kDa | Hydrophobic | 0.5 | DI water and synthetic wastewater | Lab | PET fibers | 1 mg/L | 0.142 | >99 | [10] |
| N.A. | Pureach Beijing | Flat sheet | PSU | 30 kDa | Hydrophilic | 1.0 | Milli-Q water | Lab | PE (nano/microplastics from a facial scrub) | 10 mg/L | 0.013–0.69 | N.A. | [12] |
| N.A. | Ande | Flat sheet | PVDF | 100 kDa | N.A. | 1.0 | DI water + FeCl3·6H2O + 0.1 M NaHCO3 | Lab | PE | N.A. | 500–5000 | 100 | [16] |
| N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | Landfill leachate | Full | PE, PES, PP, PA, EPM, PVAC | 1.2 ± 0.57 particles/L | 1000–5000 (41.7%); 500–1000 (16.6%); <500 (41.7%) | N.A. | [34] |
| N.A. | Yuling | Flat sheet | PES | 100 kDa | Hydrophilic | 0.8 | DI water + humic acid (10 mg/L) + AlCl3·6H2O and FeCl3·6H2O | Lab | PS | 100 mg/L | 50 | 91.2–92.7 | [66] |
| SIP-1013 | Asahi Kasei | Hollow fiber | PSU | 6 kDa | N.A. | 0.11–0.15 | DI water + alginate | Lab | PE | 10–100 ppm | 125 | N.A. | [67] |
| (SIP-1023 | |||||||||||||
| N.A. | Millipore | Flat sheet | PC | N.A. | N.A. | 1.4 | Drinking water | Lab | PET | >30 particles/L | 74 | >90 | [68] |
| PVC | 61 | ||||||||||||
| PET | 15 | ||||||||||||
| Nylon fibers | 16 | ||||||||||||
| KMS Puron | Koch | Hollow fiber | PVDF | 0.03 µm | N.A. | 0.05–0.45 | Wastewater from a PET recycling plant and urban wastewater | Pilot | HDPE | 5.8 ± 2.1 mg/L | 25–500 | 100 | [69] |
| PET | 12.3 ± 1.8 mg/L | ||||||||||||
| Other MPs | 0.17 ± 0.08 mg/L | ||||||||||||
| N.A. | Ande | Flat sheet | PVDF | 100 kDa | Hydrophilic | 0.05–0.7 | Surface water from Qinghe River | Lab | PE | 1 mg/L | 40–48 | N.A. | [70] |
| AAc | Lab-made | Flat sheet | PSU modified | N.A. | Hydrophilic | 0.25–1.2 | Milli-Q water | Lab | PE (nano/microplastics from a facial scrub) | 10 mg/L | 0.093 | N.A. | [73] |
| CPAm | Hydrophilic | ||||||||||||
| HMDSO | Hydrophobic | ||||||||||||
| N.A. | LiqTech Ceramics | Tubular | ZrO2 | 0.074 µm | Hydrophilic | 0.1–1.4 | DI water | Pilot | Nylon fibers | 180 mg/L | 80 | 100 | [74] |
| Laundry wastewater | PVC fibers | ~22.6 mg/L | N.A. | 99.2 | |||||||||
| N.A. | Lab-made | Flat sheet | rGO/PAN | 0.15 µm | Hydrophilic | 1–2 | Wastewater from a PET recycling plant | Lab | PET | N.A. | N.A. | >80 | [75] |
| N.A. | Motimo | Flat sheet | PVDF | 100 kDa | N.A. | 1.0 | DI water + AlCl3·6H2O + 0.1 M NaHCO3 | Lab | PE | N.A. | 500–5000 | 100 | [76] |
| UP150 | Microdyn-Nadir b | Flat sheet b | PES b | 150 kDa b | N.A. | 1.0 | Personal care product wastewater | Lab | N.A. | 80 particles/L | N.A. | 100 | [77] |
3.3. Nanofiltration
| Process | Membrane | Operating Pressure (Bar) | Aqueous Matrix | Scale | Microplastic | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Supplier | Type | Material | MWCO | Hydrophilicity | Type | Concentration | Size (µm) | Removal (%) | |||||
| NF and RO | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | Landfill leachate | Full | PE, PES, PP, PA, EPM, PVAC | 1.2 ± 0.57 particles/L | 1000–5000 (41.7%); 500–1000 (16.6%); <500 (41.7%) | N.A. | [34] |
| NF | NF270 | Dow—FilmTec | Flat sheet | PA b | 400 Da a | Hydrophilic a | 20 | Personal care products wastewater | Lab | N.A. | 80 particles/L | N.A. | 100 | [77] |
| NF90 | 200 Da a | |||||||||||||
| RO | SW30 | 99.4% NaCl rejection b | Hydrophilic b | |||||||||||
| BW30 | ||||||||||||||
| NF | NF90 | Dow—FilmTec | Flat sheet | PA | 200 Da a | Hydrophilic a | 4.0 | Synthetic wastewater | Lab | PET | 1 mg/L | 100 | ~100 | [81] |
| NF | NF90 | Dow—FilmTec | Flat sheet | PA | 200 Da a | Hydrophilic a | 4.0 | Synthetic wastewater | Lab | PET | 1 mg/L | 100 | 100 | [80] |
| RO | N.A. | N.A. | Spiral wound | N.A. | N.A. | N.A. | N.A. | Surface water | Full | Various types | 0.96 ± 0.46 particles/L | 20–5000 | 93 ± 5 | [85] |
3.4. Reverse Osmosis
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birley, A.W.; Heath, R.J.; Scott, M.J. Plastic Materials; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- OCDE. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD: Paris, France, 2022; ISBN 9789264654945. [Google Scholar]
- Ritchie, H.; Roser, M. Plastic Pollution—Our World in Data; Our World in Data: Oxford, UK, 2022. [Google Scholar]
- Singh, S.; Kalyanasundaram, M.; Diwan, V. Removal of Microplastics from Wastewater: Available Techniques and Way Forward. Water Sci. Technol. 2021, 84, 3689–3704. [Google Scholar] [CrossRef] [PubMed]
- Smyth, K.; Tan, S.; Van Seters, T.; Henderson, V.; Passeport, E.; Drake, J. Pavement Wear Generates Microplastics in Stormwater Runoff. J. Hazard. Mater. 2025, 481, 136495. [Google Scholar] [CrossRef] [PubMed]
- Olivatto, G.P.; Carreira, R.; Tornisielo, V.L.; Montagner, C.C. Microplastics: Contaminants of Global Concern in the Anthropocene. Rev. Virtual Quim. 2018, 10, 1968–1989. [Google Scholar] [CrossRef]
- Ho, K.T.; Bjorkland, R.; Burgess, R.M. Comparing the Definitions of Microplastics Based on Size Range: Scientific and Policy Implications. Mar. Pollut. Bull. 2024, 207, 116907. [Google Scholar] [CrossRef]
- Barbier, J.S.; Dris, R.; Lecarpentier, C.; Raymond, V.; Delabre, K.; Thibert, S.; Tassin, B.; Gasperi, J. Microplastic Occurrence after Conventional and Nanofiltration Processes at Drinking Water Treatment Plants: Preliminary Results. Front. Water 2022, 4, 886703. [Google Scholar] [CrossRef]
- Mohana, A.A.; Rahman, M.; Sarker, S.K.; Haque, N.; Gao, L.; Pramanik, B.K. Nano/Microplastics: Fragmentation, Interaction with Co-Existing Pollutants and Their Removal from Wastewater Using Membrane Processes. Chemosphere 2022, 309, 136682. [Google Scholar] [CrossRef]
- Hachemi, C.; Enfrin, M.; Rashed, A.O.; Jegatheesan, V.; Hodgson, P.D.; Callahan, D.L.; Lee, J.; Dumée, L.F. The Impact of PET Microplastic Fibres on PVDF Ultrafiltration Performance—A Short-Term Assessment of MP Fouling in Simple and Complex Matrices. Chemosphere 2023, 310, 136891. [Google Scholar] [CrossRef]
- Lee, M.; Choi, W.; Lim, G. Electrokinetic-Assisted Filtration for Fast and Highly Efficient Removal of Microplastics from Water. Chem. Eng. J. 2023, 452, 139152. [Google Scholar] [CrossRef]
- Enfrin, M.; Lee, J.; Le-Clech, P.; Dumée, L.F. Kinetic and Mechanistic Aspects of Ultrafiltration Membrane Fouling by Nano- and Microplastics. J. Memb. Sci. 2020, 601, 117890. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Pramanik, S.K.; Monira, S. Understanding the Fragmentation of Microplastics into Nano-Plastics and Removal of Nano/Microplastics from Wastewater Using Membrane, Air Flotation and Nano-Ferrofluid Processes. Chemosphere 2021, 282, 131053. [Google Scholar] [CrossRef]
- Duncan, T.V.; Khan, S.A.; Patri, A.K.; Wiggins, S. Regulatory Science Perspective on the Analysis of Microplastics and Nanoplastics in Human Food. Anal. Chem. 2024, 96, 4343–4358. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.J.; Jeong, J.; Chae, K.J. Eye-Glass Polishing Wastewater as Significant Microplastic Source: Microplastic Identification and Quantification. J. Hazard. Mater. 2021, 403, 123991. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of Microplastic Removal via Coagulation and Ultrafiltration during Drinking Water Treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- WWF. No Plastic in Nature: Assessing Plastic Ingestion from Nature to People; WWF: Gland, Switzerland, 2019. [Google Scholar]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using ΜFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino de la Serna, J. The Potential Impacts of Micro-and-Nano Plastics on Various Organ Systems in Humans. EBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Dantas, K.C.; Júnior, G.R.; Paes, V.R.; Ando, R.A.; de Oliveira Freitas, R.; da Costa, O.M.M.M.; Rabelo, R.S.; Soares Bispo, K.C.; Carvalho-Oliveira, R.; et al. Microplastics in the Olfactory Bulb of the Human Brain. JAMA Netw. Open 2024, 7, e2440018. [Google Scholar] [CrossRef]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the Mass of Microplastics Ingested—A Pivotal First Step towards Human Health Risk Assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Cherian, A.G.; Liu, Z.; McKie, M.J.; Almuhtaram, H.; Andrews, R.C. Microplastic Removal from Drinking Water Using Point-of-Use Devices. Polymers 2023, 15, 1331. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Microplastics Are Contaminants of Emerging Concern in Freshwater Environments: An Overview. In Handbook of Environmental Chemistry; Wagner, M., Lambert, S., Eds.; Springer Nature: Cham, Switzerland, 2018; Volume 58, pp. 1–23. ISBN 978-3-319-61615-5. [Google Scholar]
- Malankowska, M.; Echaide-Gorriz, C.; Coronas, J. Microplastics in Marine Environment: A Review on Sources, Classification, and Potential Remediation by Membrane Technology. Environ. Sci. 2021, 7, 243–258. [Google Scholar] [CrossRef]
- Rasheed, A.; Sharma, N.; Surampalli, R.Y.; Das, S. Evaluating Treatment Solutions: Critical Review on Technologies Employed for Microplastic Removal from Water Matrices. Curr. Opin. Environ. Sci. Health 2023, 36, 100516. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to Microplastic Pollution—Removal of Microplastics from Wastewater Effluent with Advanced Wastewater Treatment Technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Das, A.A.; Mishra, S.; Pradhan, P.; Nayak, L.; Raulo, S.; Das, K.; Sahoo, M.; Sahoo, P.K.; Naik, B.; Rout, P.R.; et al. Recent Advancements in Microplastics Treatments: Characteristics, Occurrence, and Removal Technologies. Mater. Today Proc. 2022, 67, 1211–1217. [Google Scholar] [CrossRef]
- Hidayaturrahman, H.; Lee, T.G. A Study on Characteristics of Microplastic in Wastewater of South Korea: Identification, Quantification, and Fate of Microplastics during Treatment Process. Mar. Pollut. Bull. 2019, 146, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Von Sperling, M. Comparison among the Most Frequently Used Systems for Wastewater Treatment in Developing Countries. Water Sci. Technol. 1996, 33, 59–72. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olson, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Golgoli, M.; Khiadani, M.; Shafieian, A.; Sen, T.K.; Hartanto, Y.; Johns, M.L.; Zargar, M. Microplastics Fouling and Interaction with Polymeric Membranes: A Review. Chemosphere 2021, 283, 131185. [Google Scholar] [CrossRef]
- Sanguanpak, S.; Chiemchaisri, W.; Chiemchaisri, C. Membrane Fouling and Micro-Pollutant Removal of Membrane Bioreactor Treating Landfill Leachate. Rev. Environ. Sci. Biotechnol. 2019, 18, 715–740. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, Y.; Zhu, J.; Shi, J.; Huang, H.; Xie, B. Distribution and Removal Characteristics of Microplastics in Different Processes of the Leachate Treatment System. Waste Manag. 2021, 120, 240–247. [Google Scholar] [CrossRef]
- Siddiqui, M.U.; Arif, A.F.M.; Bashmal, S. Permeability-Selectivity Analysis of Microfiltration and Ultrafiltration Membranes: Effect of Pore Size and Shape Distribution and Membrane Stretching. Membranes 2016, 6, 40. [Google Scholar] [CrossRef]
- Fijoł, N.; Aguilar-Sánchez, A.; Ruiz-Caldas, M.X.; Redlinger-Pohn, J.; Mautner, A.; Mathew, A.P. 3D Printed Polylactic Acid (PLA) Filters Reinforced with Polysaccharide Nanofibers for Metal Ions Capture and Microplastics Separation from Water. Chem. Eng. J. 2023, 457, 141153. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M. Membrane Separation Process in Wastewater and Water Purification. Membranes 2022, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Giacobbo, A.; Pasqualotto, I.F.; Filho, R.C.d.C.M.; Minhalma, M.; Bernardes, A.M.; de Pinho, M.N. Ultrafiltration and Nanofiltration for the Removal of Pharmaceutically Active Compounds from Water: The Effect of Operating Pressure on Electrostatic Solute—Membrane Interactions. Membranes 2023, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- Giacobbo, A.; do Prado, J.M.; Meneguzzi, A.; Bernardes, A.M.; de Pinho, M.N. Microfiltration for the Recovery of Polyphenols from Winery Effluents. Sep. Purif. Technol. 2015, 143, 12–18. [Google Scholar] [CrossRef]
- Bai, W.; Wang, F.; Yan, L.; Sun, H.; Zhu, Z.; Chen, L.; Li, J.; Liang, W.; Li, A. Porous Organic Polymers (POPs) Membrane via Thiol-Yne Click Chemistry for Efficient Particulate Matter Capture and Microplastics Separation. Microporous Mesoporous Mater. 2022, 329, 111509. [Google Scholar] [CrossRef]
- Yang, L.; Cao, X.; Cui, J.; Wang, Y.; Zhu, Z.; Sun, H.; Liang, W.; Li, J.; Li, A. Holey Ti3C2 Nanosheets Based Membranes for Efficient Separation and Removal of Microplastics from Water. J. Colloid Interface Sci. 2022, 617, 673–682. [Google Scholar] [CrossRef]
- Yogarathinam, L.T.; Usman, J.; Othman, M.H.D.; Ismail, A.F.; Goh, P.S.; Gangasalam, A.; Adam, M.R. Low-Cost Silica Based Ceramic Supported Thin Film Composite Hollow Fiber Membrane from Guinea Corn Husk Ash for Efficient Removal of Microplastic from Aqueous Solution. J. Hazard. Mater. 2022, 424, 127298. [Google Scholar] [CrossRef]
- Acarer, S. A Review of Microplastic Removal from Water and Wastewater by Membrane Technologies. Water Sci. Technol. 2023, 88, 199–219. [Google Scholar] [CrossRef]
- Acarer, S. Abundance and Characteristics of Microplastics in Drinking Water Treatment Plants, Distribution Systems, Water from Refill Kiosks, Tap Waters and Bottled Waters. Sci. Total Environ. 2023, 884, 163866. [Google Scholar] [CrossRef]
- Pizzichetti, A.R.P.; Pablos, C.; Álvarez-Fernández, C.; Reynolds, K.; Stanley, S.; Marugán, J. Evaluation of Membranes Performance for Microplastic Removal in a Simple and Low-Cost Filtration System. Case Stud. Chem. Environ. Eng. 2021, 3, 100075. [Google Scholar] [CrossRef]
- Hyeon, Y.; Kim, S.; Ok, E.; Park, C. A Fluid Imaging Flow Cytometry for Rapid Characterization and Realistic Evaluation of Microplastic Fiber Transport in Ceramic Membranes for Laundry Wastewater Treatment. Chem. Eng. J. 2023, 454, 140028. [Google Scholar] [CrossRef]
- Kook, H.; Cha, M.; Park, C. Transport of Emerging Organic Ultraviolet (UV) Filters in Ceramic Membranes: Role of Polyethylene (PE) Microplastics. Chemosphere 2022, 309, 136570. [Google Scholar] [CrossRef] [PubMed]
- Kook, H.; Park, C. Engineered Approaches to Facile Identification of Tiny Microplastics in Polymeric and Ceramic Membrane Filtrations for Wastewater Treatment. Membranes 2022, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Sriani, T.; Mahardika, M.; Miki, N.; Wulandari, C.P.; Prihandana, G.S. Impact of Polyimide on the Recycling of Waste Expanded Polystyrene into Flat-Sheet Filtration Membrane. J. Mater. Cycles Waste Manag. 2024, 26, 3745–3756. [Google Scholar] [CrossRef]
- Yaranal, N.A.; Subbiah, S.; Mohanty, K. Identification, Extraction of Microplastics from Edible Salts and Its Removal from Contaminated Seawater. Environ. Technol. Innov. 2021, 21, 101253. [Google Scholar] [CrossRef]
- Bitter, H.; Krause, L.; Kirchen, F.; Fundneider, T.; Lackner, S. Semi-Crystalline Microplastics in Wastewater Plant Effluents and Removal Efficiencies of Post-Treatment Filtration Systems. Water Res. X 2022, 17, 100156. [Google Scholar] [CrossRef]
- Hyeon, Y.; Kim, S.; Park, C. Exploring the Transformation of Polyethylene and Polyamide Microplastics during Membrane Filtration through FlowCam Analysis. Sep. Purif. Technol. 2024, 334, 126036. [Google Scholar] [CrossRef]
- Akarsu, C.; Kumbur, H.; Kideys, A.E. Removal of Microplastics from Wastewater through Electrocoagulation-Electroflotation and Membrane Filtration Processes. Water Sci. Technol. 2021, 84, 1648–1662. [Google Scholar] [CrossRef]
- Pizzichetti, A.R.P.; Pablos, C.; Álvarez-Fernández, C.; Reynolds, K.; Stanley, S.; Marugán, J. Kinetic and Mechanistic Analysis of Membrane Fouling in Microplastics Removal from Water by Dead-End Microfiltration. J. Environ. Chem. Eng. 2023, 11, 109338. [Google Scholar] [CrossRef]
- Lee, J.; Wang, J.; Oh, Y.; Jeong, S. Highly Efficient Microplastics Removal from Water Using In-Situ Ferrate Coagulation: Performance Evaluation by Micro-Fourier-Transformed Infrared Spectroscopy and Coagulation Mechanism. Chem. Eng. J. 2023, 451, 138556. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, U.; Lee, I.; Hong, Y.; Lee, J. Enhancement of Polystyrene Microplastic Removal by near Dissolved Organic Matter Microfiltration (NDOM MF) Coupled with Cold Plasma Treatment. J. Water Process Eng. 2023, 54, 103901. [Google Scholar] [CrossRef]
- Takeuchi, H.; Tanaka, S.; Koyuncu, C.Z.; Nakada, N. Removal of Microplastics in Wastewater by Ceramic Microfiltration. J. Water Process Eng. 2023, 54, 104010. [Google Scholar] [CrossRef]
- Minh Tran, H.; Kwak, D.; Lee, U.; Chang, S.; Thanh Tran, D.; Lee, J. A Hydrophilic near Dissolved Organic Matter Microfiltration (NDOM MF) Membrane Prepared Using Multifunctional Porogen Synthesized via Metal-Free Atom Transfer Radical Polymerization for Highly Efficient Microplastic Removal. Chem. Eng. J. 2024, 480, 147564. [Google Scholar] [CrossRef]
- Fundneider, T.; Alejo, L.; Lackner, S. Tertiary Phosphorus Removal to Extremely Low Levels by Coagulation-Flocculation and Cloth-Filtration. Water Sci. Technol. 2020, 82, 131–143. [Google Scholar] [CrossRef]
- Song, Y.; Pan, J.; Chen, M.; Wang, Y.; Li, Z.; Ge, Y. Chitosan-Modified Geopolymer Sub-Microparticles Reinforced Multifunctional Membrane for Enhanced Removal of Multiple Contaminants in Water. J. Memb. Sci. 2022, 658, 120704. [Google Scholar] [CrossRef]
- Li, Z.; Xie, W.; Zhang, Z.; Wei, S.; Chen, J.; Li, Z. Multifunctional Sodium Alginate/Chitosan-Modified Graphene Oxide Reinforced Membrane for Simultaneous Removal of Nanoplastics, Emulsified Oil, and Dyes in Water. Int. J. Biol. Macromol. 2023, 245, 125524. [Google Scholar] [CrossRef]
- Yang, J.; Monnot, M.; Sun, Y.; Asia, L.; Wong-Wah-Chung, P.; Doumenq, P.; Moulin, P. Microplastics in Different Water Samples (Seawater, Freshwater, and Wastewater): Removal Efficiency of Membrane Treatment Processes. Water Res. 2023, 232, 119673. [Google Scholar] [CrossRef]
- Cuartucci, M. Ultrafiltration, a Cost-Effective Solution for Treating Surface Water to Potable Standard. Water Pr. Technol. 2020, 15, 426–436. [Google Scholar] [CrossRef]
- Baresel, C.; Harding, M.; Fang, J. Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants. Appl. Sci. 2019, 9, 710. [Google Scholar] [CrossRef]
- Le Minh, T.; Van Ha, N.T. Surveying the Possibility of Recovering Microplastic in Waste Water from Plastic Processing and Recycling Facilities by Electrocoagulation and Ultrafiltration at Lab-Scale. Vietnam J. Sci. Technol. 2022, 60, 50–62. [Google Scholar] [CrossRef]
- Wang, W.; Yang, M.; Ma, H.; Liu, Z.; Gai, L.; Zheng, Z.; Ma, H. Removal Behaviors and Mechanism of Polystyrene Microplastics by Coagulation/Ultrafiltration Process: Co-Effects of Humic Acid. Sci. Total Environ. 2023, 881, 163408. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Yan, B.; Zargar, M.; Ling, N.N.A.; Fridjonsson, E.O.; Johns, M.L. Impact of Microplastics on Organic Fouling of Hollow Fiber Membranes. Chem. Eng. J. 2023, 467, 143320. [Google Scholar] [CrossRef]
- Yuan, C.; Almuhtaram, H.; McKie, M.J.; Andrews, R.C. Assessment of Microplastic Sampling and Extraction Methods for Drinking Waters. Chemosphere 2022, 286, 131881. [Google Scholar] [CrossRef] [PubMed]
- González-Camejo, J.; Morales, A.; Peña-Lamas, J.; Lafita, C.; Enguídanos, S.; Seco, A.; Martí, N. Feasibility of Rapid Gravity Filtration and Membrane Ultrafiltration for the Removal of Microplastics and Microlitter in Sewage and Wastewater from Plastic Industry. J. Water Process Eng. 2023, 51, 103452. [Google Scholar] [CrossRef]
- Xiong, X.; Siddique, M.S.; Graham, N.J.D.; Yu, W. Towards Microplastics Contribution for Membrane Biofouling and Disinfection By-Products Precursors: The Effect on Microbes. J. Hazard. Mater. 2022, 426, 127797. [Google Scholar] [CrossRef]
- Tadsuwan, K.; Babel, S. Microplastic Abundance and Removal via an Ultrafiltration System Coupled to a Conventional Municipal Wastewater Treatment Plant in Thailand. J. Environ. Chem. Eng. 2022, 10, 107142. [Google Scholar] [CrossRef]
- Tadsuwan, K.; Babel, S. Unraveling Microplastics Removal in Wastewater Treatment Plant: A Comparative Study of Two Wastewater Treatment Plants in Thailand. Chemosphere 2022, 307, 135733. [Google Scholar] [CrossRef]
- Enfrin, M.; Lee, J.; Fane, A.G.; Dumée, L.F. Mitigation of Membrane Particulate Fouling by Nano/Microplastics via Physical Cleaning Strategies. Sci. Total Environ. 2021, 788, 147689. [Google Scholar] [CrossRef]
- Luogo, B.D.P.; Salim, T.; Zhang, W.; Hartmann, N.B.; Malpei, F.; Candelario, V.M. Reuse of Water in Laundry Applications with Micro-and Ultrafiltration Ceramic Membrane. Membranes 2022, 12, 223. [Google Scholar] [CrossRef]
- Fryczkowska, B.; Przywara, L. Removal of Microplastics from Industrial Wastewater Utilizing an Ultrafiltration Composite Membrane Rgo/Pan Application. Desalination Water Treat. 2021, 214, 252–262. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Liu, H.; Qu, J. Removal Characteristics of Microplastics by Fe-Based Coagulants during Drinking Water Treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, C.; Isik, Z.; M’barek, I.; Bouchareb, R.; Dizge, N. Treatment of Personal Care Product Wastewater for Reuse by Integrated Electrocoagulation and Membrane Filtration Processes. J. Water Process Eng. 2022, 48, 102879. [Google Scholar] [CrossRef]
- Synder Filtration BY (PVDF 100,000Da) Sanitary UF Membrane Data Sheet. Available online: https://synderfiltration.com/2014/wp-content/uploads/2018/05/BY-PVDF-100kD-Sanitary-Specsheet.pdf (accessed on 26 January 2025).
- Microdyn-Nadir NADIR UP150 P Flat Sheet Membrane Data Sheet. Available online: https://water-membrane-solutions.mann-hummel.com/content/dam/water-membrane-solutions/download/technical-data-sheets/nadir/nadir-up150-p-flat-sheet-membrane-data-sheet.pdf/_jcr_content/renditions/original./nadir-up150-p-flat-sheet-membrane-data-sheet.pdf (accessed on 26 January 2025).
- Lin, D.; Lai, C.; Wang, X.; Wang, Z.; Kuang, K.; Wang, Z.; Du, X.; Liu, L. Enhanced Membrane Fouling by Microplastics during Nanofiltration of Secondary Effluent Considering Secretion, Interaction and Deposition of Extracellular Polymeric Substances. Sci. Total Environ. 2024, 906, 167110. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huo, L.; Yuan, Y.; Jiang, Y.; Wang, H.; Hui, K.; Li, Y.; Huang, Z.; Xi, B. Interactions between Microplastics and Heavy Metals in Leachate: Implications for Landfill Stabilization Process. J. Hazard. Mater. 2024, 480, 135830. [Google Scholar] [CrossRef]
- Kara, N.; Sari Erkan, H.; Onkal Engin, G. Characterization and Removal of Microplastics in Landfill Leachate Treatment Plants in Istanbul, Turkey. Anal. Lett. 2023, 56, 1535–1548. [Google Scholar] [CrossRef]
- Fortin, S.; Song, B.; Burbage, C. Quantifying and Identifying Microplastics in the Effluent of Advanced Wastewater Treatment Systems Using Raman Microspectroscopy. Mar. Pollut. Bull. 2019, 149, 110579. [Google Scholar] [CrossRef]
- Lin, D.; Lai, C.; Shen, X.; Wang, Z.; Xu, D.; Nie, J.; Song, W.; Du, X.; Liu, L. Electro-Coagulation Pretreatment for Improving Nanofiltration Membrane Performance during Reclamation of Microplastic-Contaminated Secondary Effluent: Unexpectedly Enhanced Membrane Fouling and Mechanism Analysis by MD-DFT Simulation. Chem. Eng. J. 2024, 498, 155779. [Google Scholar] [CrossRef]
- Dalmau-Soler, J.; Ballesteros-Cano, R.; Boleda, M.R.; Paraira, M.; Ferrer, N.; Lacorte, S. Microplastics from Headwaters to Tap Water: Occurrence and Removal in a Drinking Water Treatment Plant in Barcelona Metropolitan Area (Catalonia, NE Spain). Environ. Sci. Pollut. Res. 2021, 28, 59462–59472. [Google Scholar] [CrossRef]
- de Souza, D.I.; Dottein, E.M.; Giacobbo, A.; Rodrigues, M.A.S.; de Pinho, M.N.; Bernardes, A.M. Nanofiltration for the Removal of Norfloxacin from Pharmaceutical Effluent. J. Environ. Chem. Eng. 2018, 6, 6147–6153. [Google Scholar] [CrossRef]
- Zakmout, A.; Sadi, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Tannery Effluent Treatment by Nanofiltration, Reverse Osmosis and Chitosan Modified Membranes. Membranes 2020, 10, 378. [Google Scholar] [CrossRef]
- Venzke, C.D.; Rodrigues, M.A.S.; Giacobbo, A.; Bacher, L.E.; Lemmertz, I.S.; Viegas, C.; Striving, J.; Pozzebon, S. Application of Reverse Osmosis to Petrochemical Industry Wastewater Treatment Aimed at Water Reuse. Manag. Environ. Qual. Int. J. 2017, 28, 70–77. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater Treatment Plants as a Pathway for Microplastics: Development of a New Approach to Sample Wastewater-Based Microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Bai, X.; Ye, Z. Removal and Generation of Microplastics in Wastewater Treatment Plants: A Review. J. Clean. Prod. 2021, 291, 125982. [Google Scholar] [CrossRef]
- Giacobbo, A.; Soares, E.V.; Bernardes, A.M.; Rosa, M.J.; de Pinho, M.N. Atenolol Removal by Nanofiltration: A Case-Specific Mass Transfer Correlation. Water Sci. Technol. 2020, 81, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Farahbakhsh, J.; Najafi, M.; Golgoli, M.; Haeri, S.Z.; Khiadani, M.; Razmjou, A.; Zargar, M. Dual Modification of Reverse Osmosis Membranes with NH2-MIL-125 and Functionalised Multiwalled Carbon Nanotubes for Enhanced Nanoplastic Removal. Chemosphere 2024, 361, 142401. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, Y.; Li, D.; Wang, X.; Huang, X. Roles and Performance Enhancement of Feed Spacer in Spiral Wound Membrane Modules for Water Treatment: A 20-Year Review on Research Evolvement. Water Res. 2021, 198, 117146. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Koutsou, C.P.; Sioutopoulos, D.C. Comprehensive Performance Assessment of Spacers in Spiral-Wound Membrane Modules Accounting for Compressibility Effects. J. Memb. Sci. 2018, 549, 602–615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, P.E.; Giacobbo, A.; Almeida, G.M.d.; Rodrigues, M.A.S.; Bernardes, A.M. Pressure-Driven Membrane Processes for Removing Microplastics. Membranes 2025, 15, 81. https://doi.org/10.3390/membranes15030081
Pinto PE, Giacobbo A, Almeida GMd, Rodrigues MAS, Bernardes AM. Pressure-Driven Membrane Processes for Removing Microplastics. Membranes. 2025; 15(3):81. https://doi.org/10.3390/membranes15030081
Chicago/Turabian StylePinto, Priscila Edinger, Alexandre Giacobbo, Gabriel Maciel de Almeida, Marco Antônio Siqueira Rodrigues, and Andréa Moura Bernardes. 2025. "Pressure-Driven Membrane Processes for Removing Microplastics" Membranes 15, no. 3: 81. https://doi.org/10.3390/membranes15030081
APA StylePinto, P. E., Giacobbo, A., Almeida, G. M. d., Rodrigues, M. A. S., & Bernardes, A. M. (2025). Pressure-Driven Membrane Processes for Removing Microplastics. Membranes, 15(3), 81. https://doi.org/10.3390/membranes15030081