Electrospun Membranes Loaded with Melanin Derived from Pecan Nutshell (Carya illinoinensis) Residues for Skin-Care Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Melanin Extraction
2.3. Melanin Purification
2.4. Fiber Preparation
2.5. Fiber Characterization
2.5.1. Morphology Analysis
2.5.2. Chemical Composition Analysis
2.5.3. Wettability Analysis
2.5.4. Mechanical Characterization
2.5.5. Liberation of Melanin
2.6. Fiber Bioactivities
2.6.1. Antioxidant Activity Against DPPH
2.6.2. Antioxidant Activity Against ABTS
2.6.3. Antimicrobial Activity
2.7. Antiaging Effect
2.7.1. Elastase Inhibition Assay
2.7.2. Tyrosinase Inhibition Assay
2.7.3. Collagenase Inhibition Assay
2.7.4. Hyaluronidase Inhibition Assay
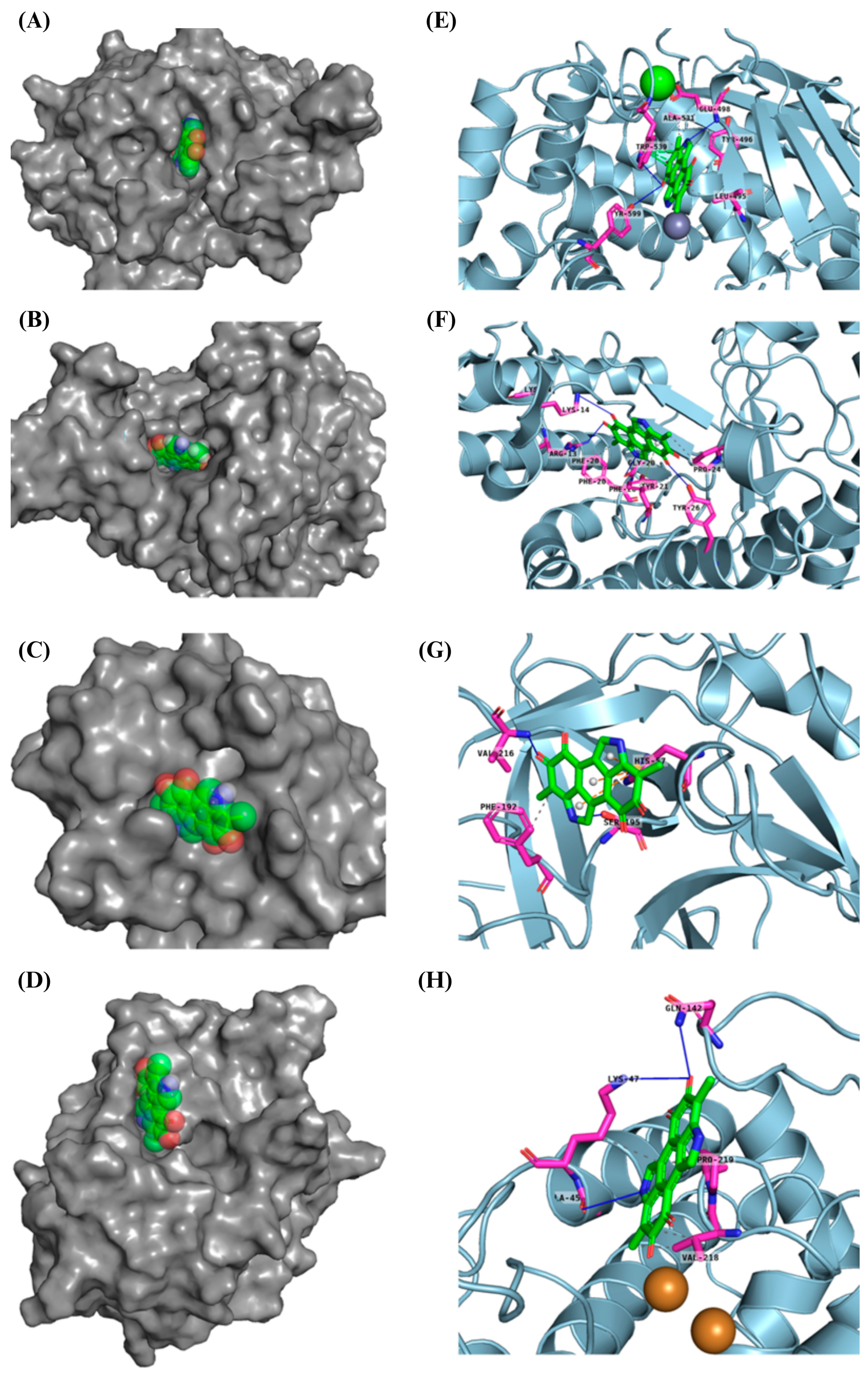
2.8. Docking In Silico of Each Tested Enzymes with Melanin Results
2.9. Statistical Analysis
3. Results and Discussions
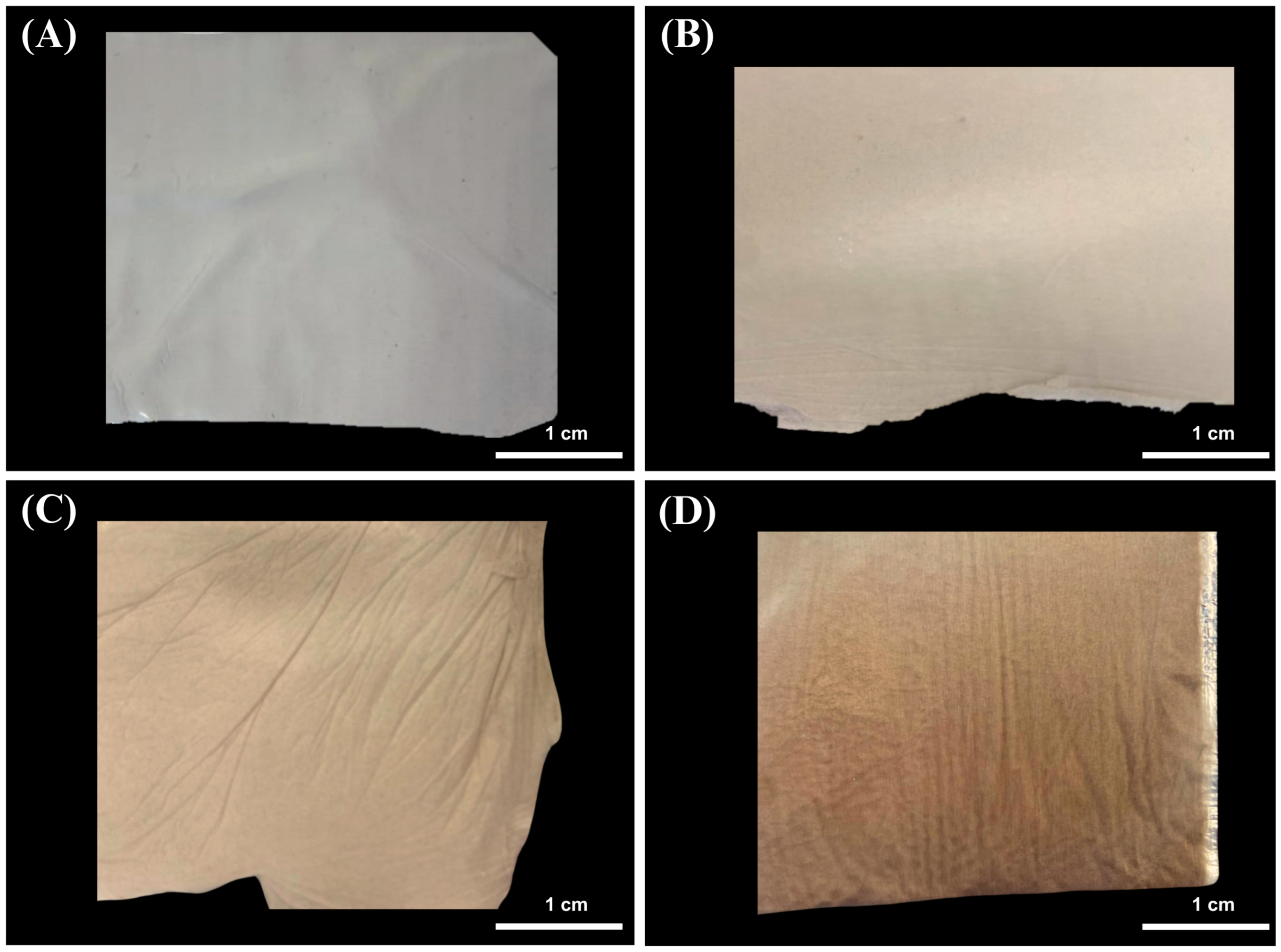
3.1. Membrane Elaboration
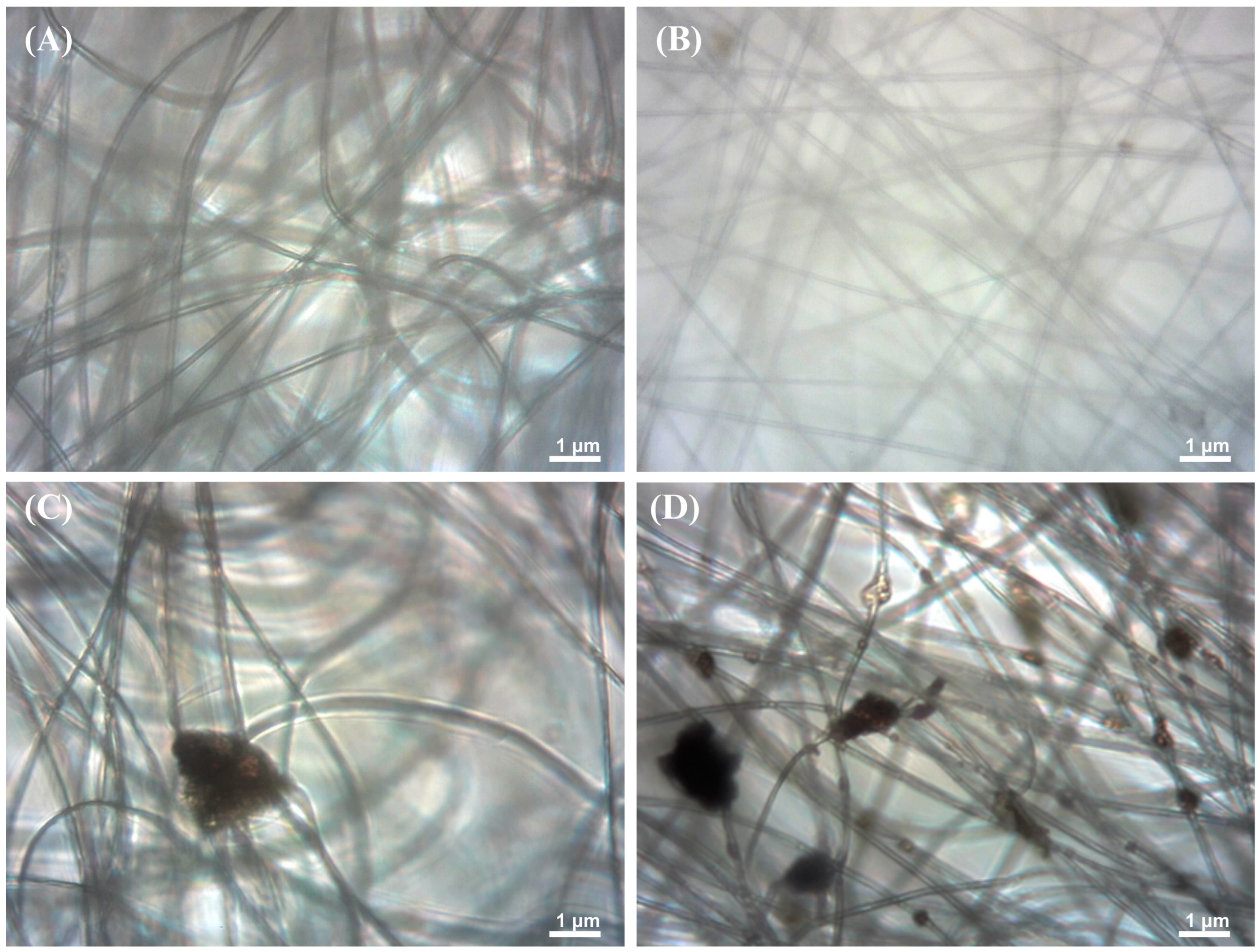
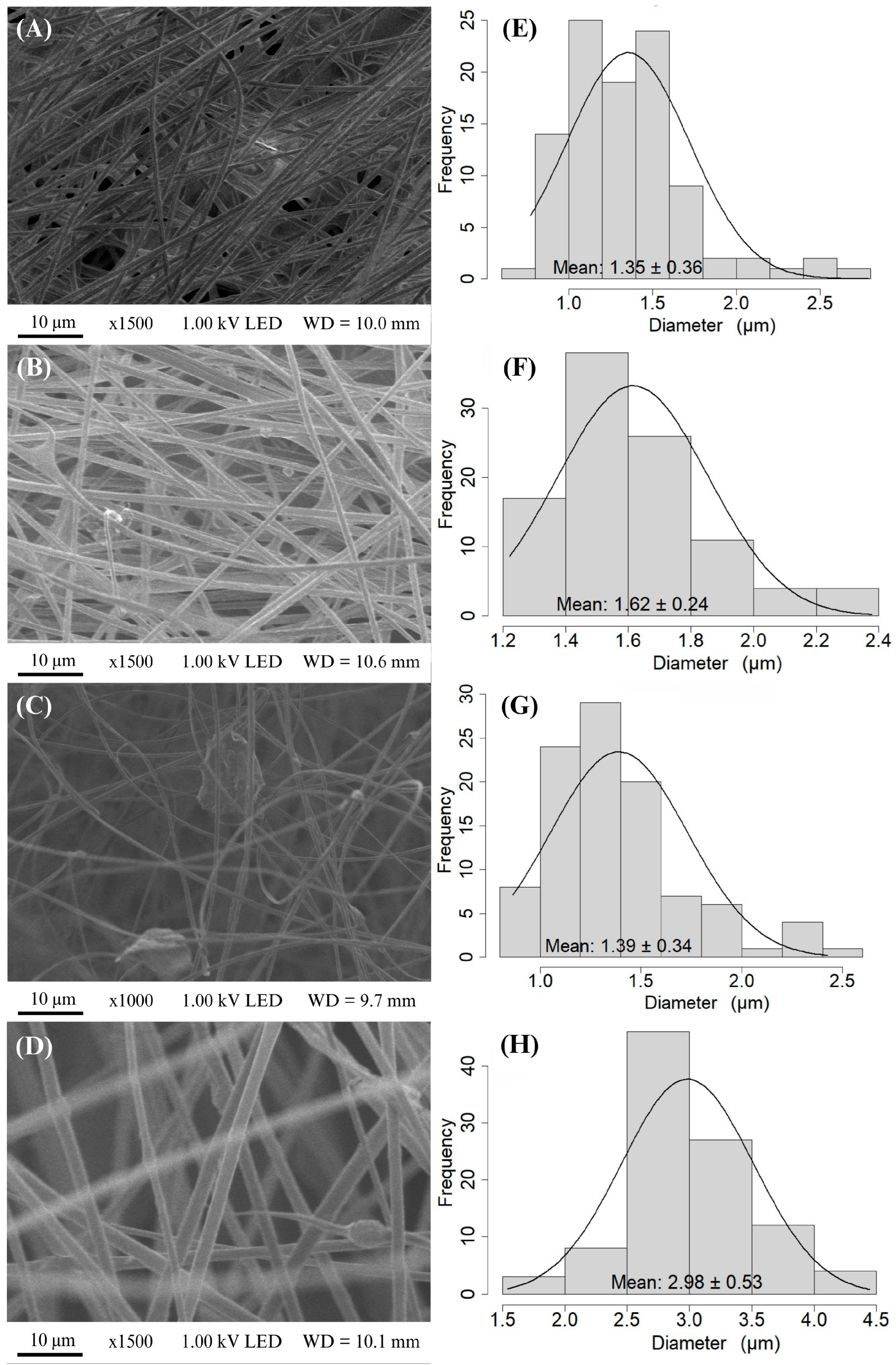
3.2. Light and Scanning Electron Microscopy
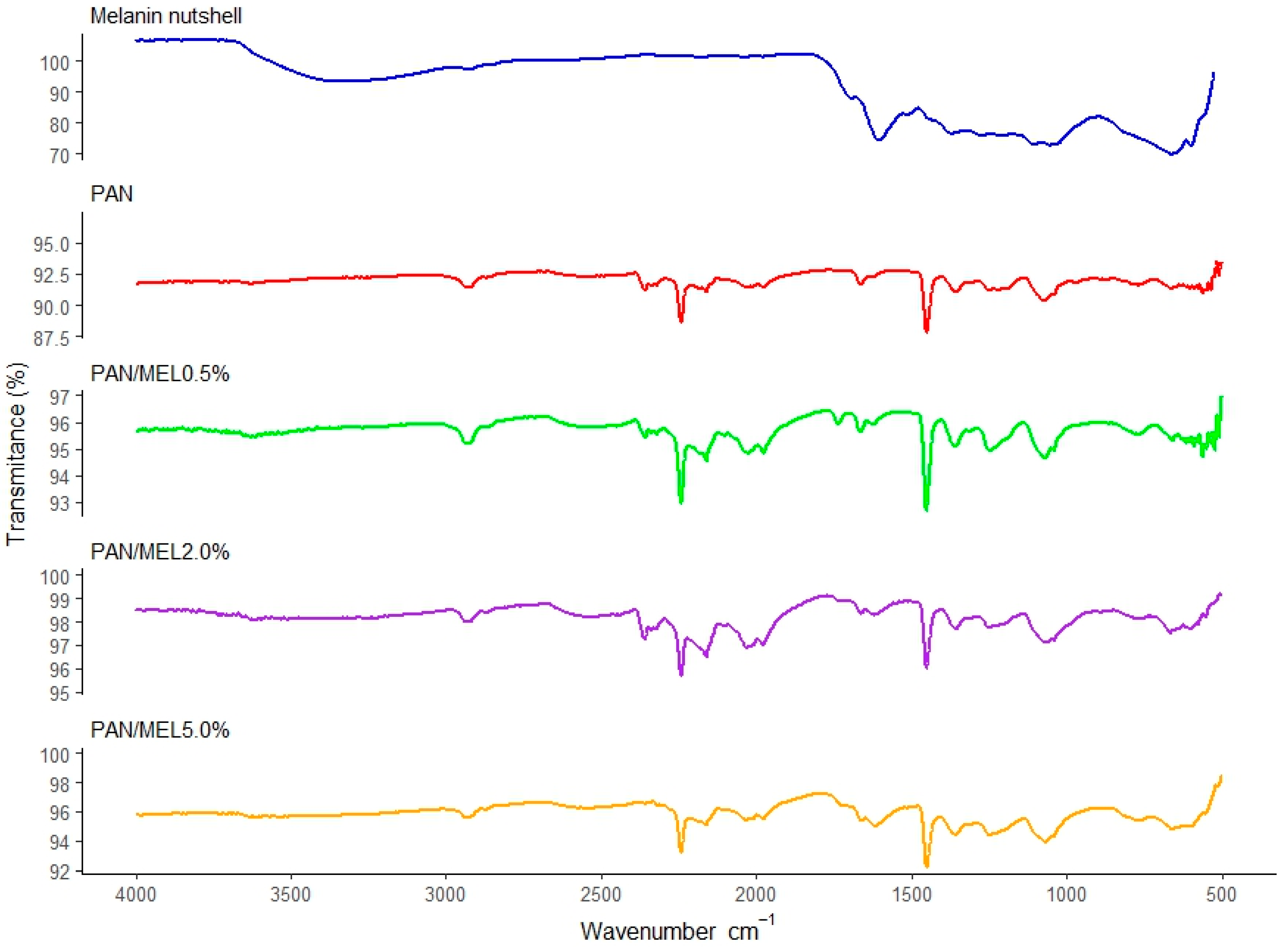
3.3. Chemical Properties of Fibers
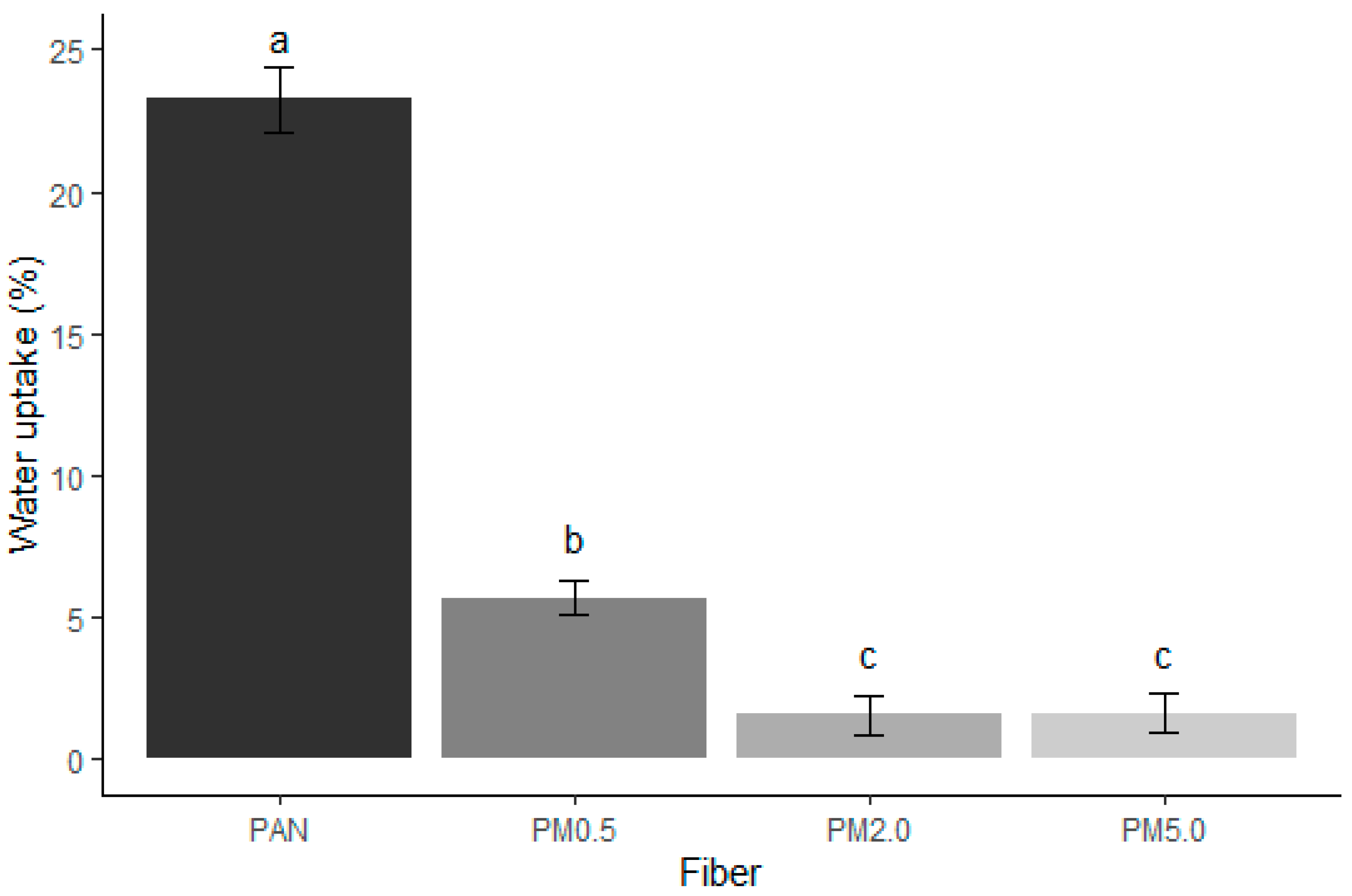
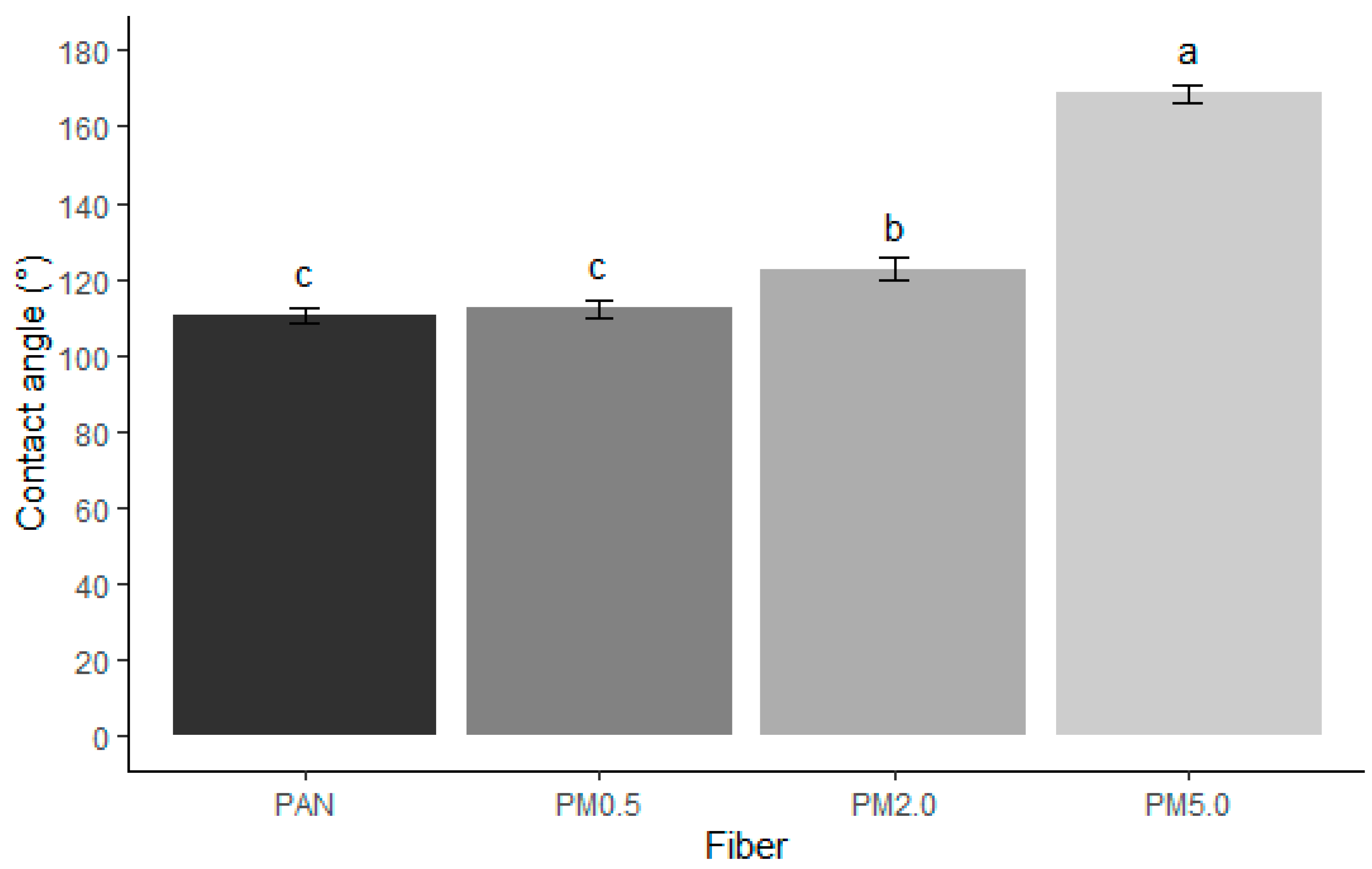
3.4. Wettability Results
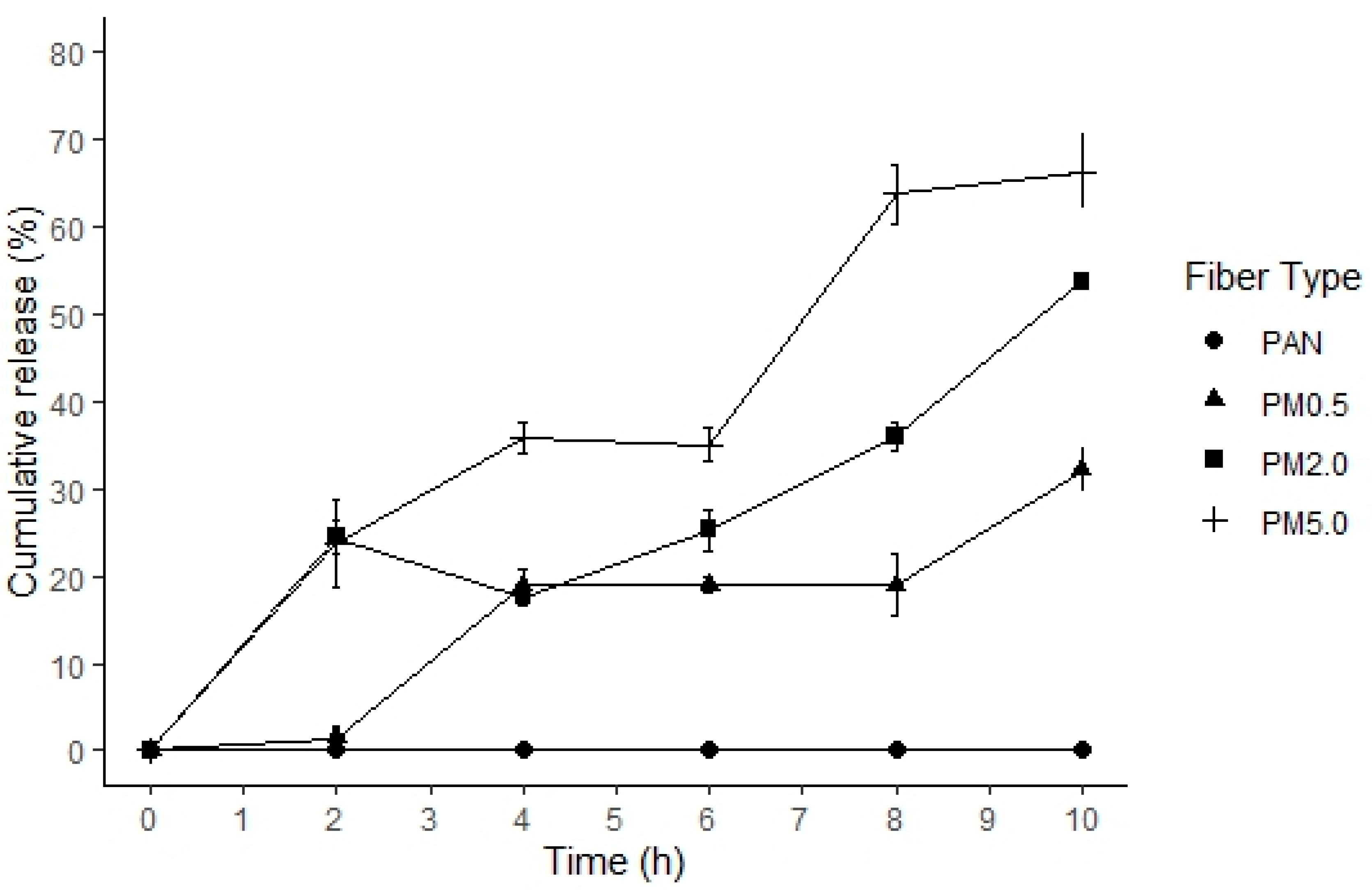
3.5. Fibers Degradation
3.6. Mechanical Properties of the Membrane
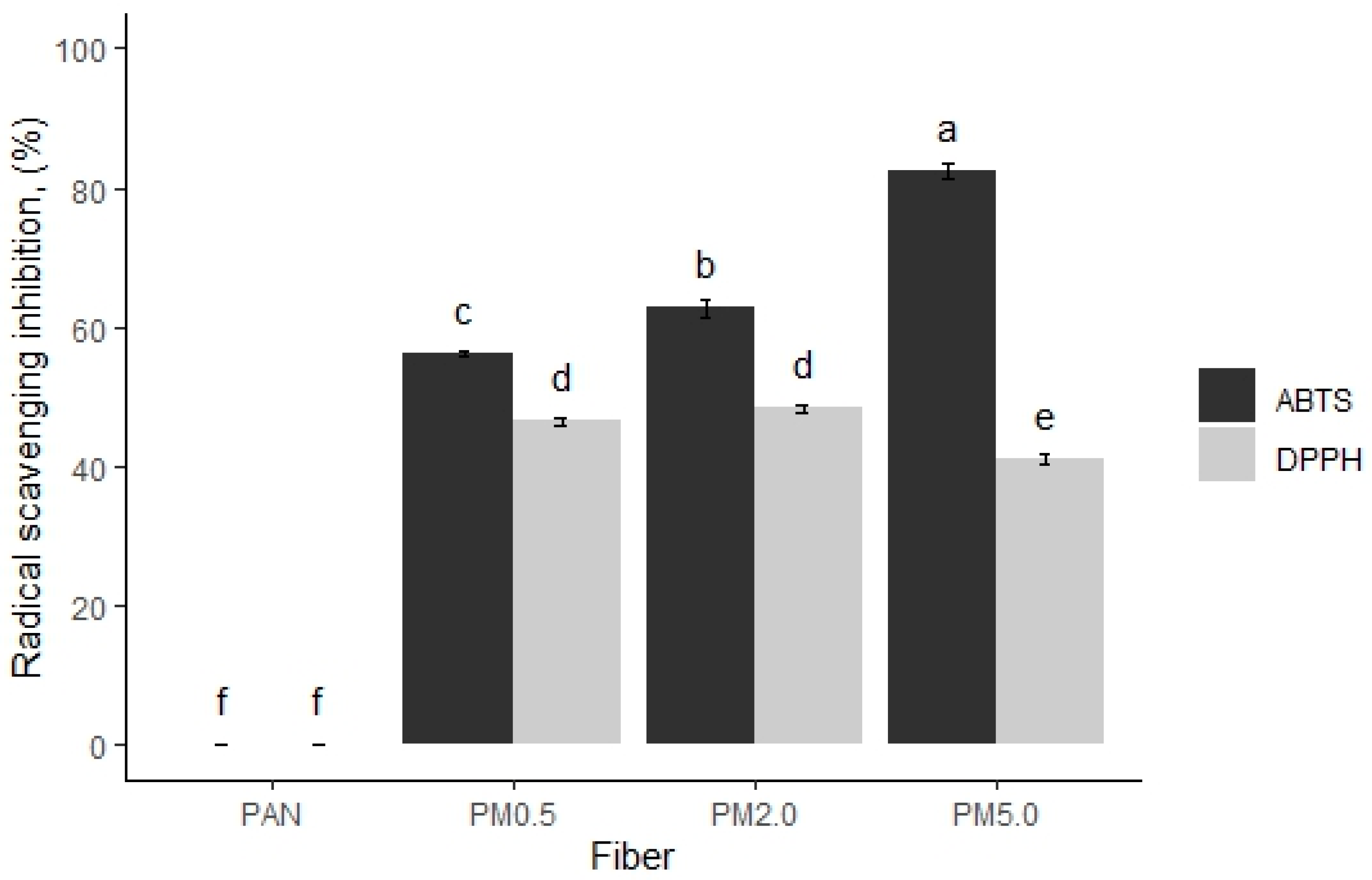
3.7. Antioxidant Activities
3.8. Antibacterial Activity
3.9. Antiaging Activity
3.10. Docking In Silico of Each Tested Enzyme with Melanin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hadgraft, J. Skin, the Final Frontier. Int. J. Pharm. 2001, 224, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Falanga, D.; Di Lorenzo, R.; Tito, A.; Carotenuto, G.; Zappelli, C.; Grumetto, L.; Sacchi, A.; Laneri, S.; Apone, F. An Extract from Ficus Carica Cell Cultures Works as an Anti-Stress Ingredient for the Skin. Antioxidants 2021, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Eidt, L.M. Cutaneous Aging and Dermatosis in Geriatric Patients. In Dermatology in Public Health Environments; Springer International Publishing: Cham, Switzerland, 2023; pp. 967–1001. [Google Scholar] [CrossRef]
- Ribeiro, A.; Estanqueiro, M.; Oliveira, M.; Sousa Lobo, J. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Kumar, V. Perspective of Natural Products in Skincare. Pharm. Pharmacol. Int. J. 2016, 4, 339–341. [Google Scholar] [CrossRef]
- Krzyżostan, M.; Wawrzyńczak, A.; Nowak, I. Use of Waste from the Food Industry and Applications of the Fermentation Process to Create Sustainable Cosmetic Products: A Review. Sustainability 2024, 16, 2757. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Assadpour, E.; Tabarestani, H.S.; Falsafi, S.R.; Jafari, S.M. Electrospinning Approach for Nanoencapsulation of Bioactive Compounds; Recent Advances and Innovations. Trends Food Sci. Technol. 2020, 100, 190–209. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.-K. Electrospinning Nanofibers for Therapeutics Delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Sethuram, L.; Thomas, J. Therapeutic Applications of Electrospun Nanofibers Impregnated with Various Biological Macromolecules for Effective Wound Healing Strategy—A Review. Biomed. Pharmacother. 2023, 157, 113996. [Google Scholar] [CrossRef]
- Serra, D.; Garroni, G.; Cruciani, S.; Coradduzza, D.; Pashchenko, A.; Amler, E.; Pintore, G.; Satta, R.; Montesu, M.A.; Kohl, Y.; et al. Electrospun Nanofibers Encapsulated with Natural Products: A Novel Strategy to Counteract Skin Aging. Int. J. Mol. Sci. 2024, 25, 1908. [Google Scholar] [CrossRef]
- Gul, A.; Gallus, I.; Tegginamath, A.; Maryska, J.; Yalcinkaya, F. Electrospun Antibacterial Nanomaterials for Wound Dressings Applications. Membranes 2021, 11, 908. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Cheng, Y.; Yang, R.; Zhao, G. Electrospun Nanofibers Promote Wound Healing: Theories, Techniques, and Perspectives. J. Mater. Chem. B 2021, 9, 3106–3130. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hu, H.; Zeng, Y.; Pan, H.; Wang, S.; Zhang, Y.; Shi, L.; Tan, G.; Pan, W.; Liu, H. Recent Advances in Electrospun Nanofibers for Wound Dressing. Eur. Polym. J. 2022, 178, 111490. [Google Scholar] [CrossRef]
- Mulholland, E.J. Electrospun Biomaterials in the Treatment and Prevention of Scars in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 481. [Google Scholar] [CrossRef]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Polymeric Wound Dressings, an Insight into Polysaccharide-Based Electrospun Membranes. Appl. Mater. Today 2021, 24, 101148. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gao, Z.; Mao, X.; Cheng, J.; Huang, L.; Tang, J. Advances in Wound Dressing Based on Electrospinning Nanofibers. J. Appl. Polym. Sci. 2024, 141, e54746. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.; Jafari, S.; Supaphol, P. A Review on Wound Dressings with an Emphasis on Electrospun Nanofibrous Polymeric Bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Ji, Y.; Song, W.; Xu, L.; Yu, D.G.; Bligh, S.W.A. A Review on Electrospun Poly(Amino Acid) Nanofibers and Their Applications of Hemostasis and Wound Healing. Biomolecules 2022, 12(6), 794. [Google Scholar] [CrossRef] [PubMed]
- Adamu, B.F.; Gao, J.; Gebeyehu, E.K.; Beyene, K.A.; Tadesse, M.G.; Liyew, E.Z. Self-Responsive Electrospun Nanofibers Wound Dressings: The Future of Wound Care. Adv. Mater. Sci. Eng. 2022, 2022, 2025170. [Google Scholar] [CrossRef]
- Abdelhakeem, E.; Monir, S.; Teaima, M.H.M.; Rashwan, K.O.; El-Nabarawi, M. State-of-the-Art Review of Advanced Electrospun Nanofiber Composites for Enhanced Wound Healing. AAPS PharmSciTech 2023, 24, 246. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun Polymeric Nanofibres as Wound Dressings: A Review. Colloids Surf. B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Ajith, G.; Tamilarasi, G.P.; Sabarees, G.; Gouthaman, S.; Manikandan, K.; Velmurugan, V.; Alagarsamy, V.; Solomon, V.R. Recent Developments in Electrospun Nanofibers as Delivery of Phytoconstituents for Wound Healing. Drugs Drug Candidates 2023, 2, 148–171. [Google Scholar] [CrossRef]
- Sharma, A.; Dheer, D.; Singh, I.; Puri, V.; Kumar, P. Phytoconstituent-Loaded Nanofibrous Meshes as Wound Dressings: A Concise Review. Pharmaceutics 2023, 15, 1058. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, P.; Song, P.; Li, N. Electrospinning of Botanicals for Skin Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 1006129. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural Composite Dressings Based on Collagen, Gelatin and Plant Bioactive Compounds for Wound Healing: A Review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun Medical Sutures for Wound Healing: A Review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in Nanofibers for Wound Dressing: A Review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Jhatial, A.K.; Kumelachew, D.M. A Review of Medicinal Plant-Based Bioactive Electrospun Nano Fibrous Wound Dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun Nanofibres Containing Antimicrobial Plant Extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhou, D.; Xia, X.; Zhou, H.; Wang, Y.; Ke, H. Preparation and Characterization of Polycaprolactone (PCL) Antimicrobial Wound Dressing Loaded with Pomegranate Peel Extract. ACS Omega 2023, 8, 20323–20331. [Google Scholar] [CrossRef]
- Al-Naymi, H.A.S.; Mahmoudi, E.; Kamil, M.M.; Almajidi, Y.Q.; Al-Musawi, M.H.; Mohammadzadeh, V.; Ghorbani, M.; Mortazavi Moghadam, F. A Novel Designed Nanofibrous Mat Based on Hydroxypropyl Methyl Cellulose Incorporating Mango Peel Extract for Potential Use in Wound Care System. Int. J. Biol. Macromol. 2024, 259, 129159. [Google Scholar] [CrossRef]
- Zdraveva, E.; Gaurina Srček, V.; Kraljić, K.; Škevin, D.; Slivac, I.; Obranović, M. Agro-Industrial Plant Proteins in Electrospun Materials for Biomedical Application. Polymers 2023, 15, 2684. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Versino, F.; López, O.V.; García, M.A. Biobased Composites from Agro-Industrial Wastes and by-Products. Emergent Mater. 2022, 5, 873–921. [Google Scholar] [CrossRef]
- Abd Elkodous, M.; El-Husseiny, H.M.; El-Sayyad, G.S.; Hashem, A.H.; Doghish, A.S.; Elfadil, D.; Radwan, Y.; El-Zeiny, H.M.; Bedair, H.; Ikhdair, O.A.; et al. Recent Advances in Waste-Recycled Nanomaterials for Biomedical Applications: Waste-to-Wealth. Nanotechnol. Rev. 2021, 10, 1662–1739. [Google Scholar] [CrossRef]
- Topić Popović, N.; Lorencin, V.; Strunjak-Perović, I.; Čož-Rakovac, R. Shell Waste Management and Utilization: Mitigating Organic Pollution and Enhancing Sustainability. Appl. Sci. 2023, 13, 623. [Google Scholar] [CrossRef]
- Ngasotter, S.; Xavier, K.A.M.; Meitei, M.M.; Waikhom, D.; Madhulika; Pathak, J.; Singh, S.K. Crustacean Shell Waste Derived Chitin and Chitin Nanomaterials for Application in Agriculture, Food, and Health—A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100349. [Google Scholar] [CrossRef]
- Villasante, J.; Pérez-Carrillo, E.; Heredia-Olea, E.; Metón, I.; Almajano, M.P. In Vitro Antioxidant Activity Optimization of Nut Shell (Carya illinoinensis) by Extrusion Using Response Surface Methods. Biomolecules 2019, 9, 883. [Google Scholar] [CrossRef]
- Ferrari, V.; Gil, G.; Heinzen, H.; Zoppolo, R.; Ibáñez, F. Influence of Cultivar on Nutritional Composition and Nutraceutical Potential of Pecan Growing in Uruguay. Front. Nutr. 2022, 9, 868054. [Google Scholar] [CrossRef] [PubMed]
- García-García, M.; Orona-Tamayo, D.; Estrada-Monje, A.; Quintana-Rodríguez, E.; Navarro-Mendoza, R.; Hernández-Perales, L.; Lozoya-Perez, N.E.; Jaime-Ferrer, J.S. Melanin from Pecan Nut Shell Waste as an Antioxidant and Antifungal Additive in Membranes for Food Packaging. J. Polym. Environ. 2025. [Google Scholar] [CrossRef]
- Fu, X.; Xie, M.; Lu, M.; Shi, L.; Shi, T.; Yu, M. Characterization of the Physicochemical Properties, Antioxidant Activity, and Antiproliferative Activity of Natural Melanin from S. Reiliana. Sci. Rep. 2022, 12, 2110. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Amorati, R.; Montalti, M. Recent Applications of Melanin-like Nanoparticles as Antioxidant Agents. Antioxidants 2023, 12, 863. [Google Scholar] [CrossRef] [PubMed]
- Khojah, H.; Ahmed, S.R.; Alharbi, S.Y.; AlSabeelah, K.K.; Alrayyes, H.Y.; Almusayyab, K.B.; Alrawiliy, S.R.; Alshammari, R.M.; Qasim, S. Skin Anti-Aging Potential of Launaea Procumbens Extract: Antioxidant and Enzyme Inhibition Activities Supported by ADMET and Molecular Docking Studies. Saudi Pharm. J. 2024, 32, 102107. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ye, M.; Song, S.; Li, L.; Shaikh, F.; Li, J. Isolation, Purification, and Anti-Aging Activity of Melanin from Lachnum Singerianum. Appl. Biochem. Biotechnol. 2014, 174, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Aguilera, J.A.; Villafaña-López, L.; Rentería-Martínez, E.C.; Anderson, S.M.; Jaime-Ferrer, J.S. Electrospinning of Polyepychlorhydrin and Polyacrylonitrile Anionic Exchange Membranes for Reverse Electrodialysis. Membranes 2021, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- ASTM E573-01(2013); Standard Practices for Internal Reflection Spectroscopy. ASTM International: West Conshohocken, PA, USA, 2013. [CrossRef]
- Liu, S.-J.; Kau, Y.-C.; Chou, C.-Y.; Chen, J.-K.; Wu, R.-C.; Yeh, W.-L. Electrospun PLGA/Collagen Nanofibrous Membrane as Early-Stage Wound Dressing. J. Memb. Sci. 2010, 355, 53–59. [Google Scholar] [CrossRef]
- Ruiz Rocha, J.E.; Moreno Tovar, K.R.; Navarro Mendoza, R.; Gutiérrez Granados, S.; Cavaliere, S.; Giaume, D.; Barboux, P.; Jaime Ferrer, J.S. Critical Electrospinning Parameters for Synthesis Control of Stabilized Polyacrylonitrile Nanofibers. Nanomaterials 2023, 13, 2648. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- Adegbola, T.A.; Agboola, O.; Fayomi, O.S.I. Review of Polyacrylonitrile Blends and Application in Manufacturing Technology: Recycling and Environmental Impact. Results Eng. 2020, 7, 100144. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, L.; Gao, A.; Tong, Y.; Shi, F.; Xu, L. Thermal Analysis and Crystal Structure of Poly(Acrylonitrile-Co-Itaconic Acid) Copolymers Synthesized in Water. Polymers 2020, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Isaac, B.; Taylor, R.M.; Reifsnider, K. Anisotropic Characterizations of Electrospun PAN Nanofiber Mats Using Design of Experiments. Nanomaterials 2020, 10, 2273. [Google Scholar] [CrossRef]
- Kalantary, S.; Golbabaei, F.; Latifi, M.; Shokrgozar, M.A.; Yaseri, M. Feasibility of Using Vitamin E-Loaded Poly(ε-Caprolactone)/Gelatin Nanofibrous Mat to Prevent Oxidative Stress in Skin. J. Nanosci. Nanotechnol. 2020, 20, 3554–3562. [Google Scholar] [CrossRef] [PubMed]
- Orona-Tamayo, D.; Valverde, M.E.; Nieto-Rendón, B.; Paredes-López, O. Inhibitory Activity of Chia (Salvia hispanica L.) Protein Fractions against Angiotensin I-Converting Enzyme and Antioxidant Capacity. LWT-Food Sci. Technol. 2015, 64, 236–242. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Liceaga, A.M. Identification of Chia Seed (Salvia hispanica L.) Peptides with Enzyme Inhibition Activity towards Skin-Aging Enzymes. Amino Acids 2020, 52, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Alhayek, A.; Abdelsamie, A.S.; Schönauer, E.; Camberlein, V.; Hutterer, E.; Posselt, G.; Serwanja, J.; Blöchl, C.; Huber, C.G.; Haupenthal, J.; et al. Discovery and Characterization of Synthesized and FDA-Approved Inhibitors of Clostridial and Bacillary Collagenases. J. Med. Chem. 2022, 65, 12933–12955. [Google Scholar] [CrossRef]
- Chao, K.L.; Muthukumar, L.; Herzberg, O. Structure of Human Hyaluronidase-1, a Hyaluronan Hydrolyzing Enzyme Involved in Tumor Growth and Angiogenesis. Biochemistry 2007, 46, 6911–6920. [Google Scholar] [CrossRef]
- Hansen, G.; Gielen-Haertwig, H.; Reinemer, P.; Schomburg, D.; Harrenga, A.; Niefind, K. Unexpected Active-Site Flexibility in the Structure of Human Neutrophil Elastase in Complex with a New Dihydropyrimidone Inhibitor. J. Mol. Biol. 2011, 409, 681–691. [Google Scholar] [CrossRef]
- Ferro, S.; Deri, B.; Germanò, M.P.; Gitto, R.; Ielo, L.; Buemi, M.R.; Certo, G.; Vittorio, S.; Rapisarda, A.; Pazy, Y.; et al. Targeting Tyrosinase: Development and Structural Insights of Novel Inhibitors Bearing Arylpiperidine and Arylpiperazine Fragments. J. Med. Chem. 2018, 61, 3908–3917. [Google Scholar] [CrossRef]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-based Graphical User Interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- PyMOL|pymol.org. Available online: https://www.pymol.org/ (accessed on 6 November 2024).
- Tran-Ly, A.N.; Ribera, J.; Schwarze, F.W.M.R.; Brunelli, M.; Fortunato, G. Fungal Melanin-Based Electrospun Membranes for Heavy Metal Detoxification of Water. Sustain. Mater. Technol. 2020, 23, e00146. [Google Scholar] [CrossRef]
- Micheal, H.S.R.; Thyagarajan, D.; Govindaraj, M.; Saravanakumar, V.K.; Mohammed, N.B.; Murugasamy Maheswari, K. Biosorption of Halophilic Fungal Melanized Membrane—PUR/Melanin Polymer for Heavy Metal Detoxification with Electrospinning Technology. Environ. Technol. 2024, 45, 5865–5877. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G. Polymer Nanofibers Electrospinning: A Review. Egypt. J. Chem. 2019, 63(4), 1279–1303. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Shen, Y.; Dong, K.; Shen, L.; Alzalab, A.A.A. Research Progress, Models and Simulation of Electrospinning Technology: A Review. J. Mater. Sci. 2022, 57, 58–104. [Google Scholar] [CrossRef]
- Rist, M.; Greiner, A. Bio-based electrospun polyamide membrane—Sustainable multipurpose filter membranes for microplastic filtration. RSC Appl. Polym. 2024, 2, 642–655. [Google Scholar] [CrossRef]
- Street, R.M.; Minagawa, M.; Vengrenyuk, A.; Schauer, C.L. Piezoelectric Electrospun Polyacrylonitrile with Various Tacticities. J. Appl. Polym. Sci. 2019, 136, 47530. [Google Scholar] [CrossRef]
- Alarifi, I.; Alharbi, A.; Khan, W.; Swindle, A.; Asmatulu, R. Thermal, Electrical and Surface Hydrophobic Properties of Electrospun Polyacrylonitrile Nanofibers for Structural Health Monitoring. Materials 2015, 8, 7017–7031. [Google Scholar] [CrossRef]
- Shekharagouda, V.; Kumar Panda, P. Electrospinning of Polyacrylonitrile Nanofiber Membrane for Bacteria Removal. J. Mater. Sci. Appl. 2018, 4, 68–74. [Google Scholar] [CrossRef]
- Yao, Y.; Liang, Y.; Navik, R.; Dong, X.; Cai, Y.; Zhang, P. Modification of Polyacrylonitrile Fibers by Coupling to Thiosemicarbazones. Materials 2019, 12, 3980. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, T.; Ma, P.; Zhang, S.; Du, M.; Dong, W.; Xie, Y.; Chen, M. Effects of Melanin on Optical Behavior of Polymer: From Natural Pigment to Materials Applications. ACS Appl. Mater. Interfaces 2018, 10, 13100–13106. [Google Scholar] [CrossRef]
- Wei, H.; Ren, J.; Han, B.; Xu, L.; Han, L.; Jia, L. Stability of Polydopamine and Poly(DOPA) Melanin-like Films on the Surface of Polymer Membranes under Strongly Acidic and Alkaline Conditions. Colloids Surf. B Biointerfaces 2013, 110, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Ito, S. Recent Advances in Characterization of Melanin Pigments in Biological Samples. Int. J. Mol. Sci. 2023, 24, 8305. [Google Scholar] [CrossRef]
- Henrici-Olivé, G.; Olivé, S. Molecular Interactions and Macroscopic Properties of Polyacrylonitrile and Model Substances. In Chemistry. Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1979; pp. 123–152. [Google Scholar] [CrossRef]
- Sanchaniya, J.V.; Kanukuntla, S. Morphology and mechanical properties of PAN nanofiber mat. J. Phys. Conf. Ser. 2023, 2423, 012018. [Google Scholar] [CrossRef]
- Sebesta, F.; John, J.; Motl, A.; Stamberg, K. Evaluation of Polyacrylonitrile (PAN) as a Binding Polymer for Absorbers Used to Treat Liquid Radioactive Wastes; Sandia National Labs.: Albuquerque, NM, USA, 1995. [Google Scholar] [CrossRef][Green Version]
- Taborda, C.P.; da Silva, M.B.; Nosanchuk, J.D.; Travassos, L.R. Melanin as a Virulence Factor of Paracoccidioides Brasiliensis and Other Dimorphic Pathogenic Fungi: A Minireview. Mycopathologia 2008, 165, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.d.C.R.; Pombeiro-Sponchiado, S.R. Antioxidant Activity of the Melanin Pigment Extracted from Aspergillus Nidulans. Biol. Pharm. Bull. 2005, 28, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Casadevall, A. Susceptibility of Melanized and Nonmelanized Cryptococcus Neoformans to Nitrogen- and Oxygen-Derived Oxidants. Infect. Immun. 1994, 62, 3004–3007. [Google Scholar] [CrossRef] [PubMed]
- Manini, P.; Lino, V.; Franchi, P.; Gentile, G.; Sibillano, T.; Giannini, C.; Picardi, E.; Napolitano, A.; Valgimigli, L.; Chiappe, C.; et al. A Robust Fungal Allomelanin Mimic: An Antioxidant and Potent Π-Electron Donor with Free-Radical Properties That Can Be Tuned by Ionic Liquids. Chempluschem 2019, 84, 1331–1337. [Google Scholar] [CrossRef]
- McGraw, K.J. The Antioxidant Function of Many Animal Pigments: Are There Consistent Health Benefits of Sexually Selected Colourants? Anim. Behav. 2005, 69, 757–764. [Google Scholar] [CrossRef]
- Peng, Z.; Luo, S.; Zhao, D.; Zhang, J. Biosynthetic Melanin with Excellent Performance Can Be Used for Heavy Metal Adsorption. J. Clean. Prod. 2023, 385, 135655. [Google Scholar] [CrossRef]
- Sunil Kumar, B.T.; Sathyendra Rao, B.V.; Swathi, S.; Vanajakshi, V.; Hebbar, H.U.; Singh, S.A. Characterization, Antioxidant, and Antimicrobial Activity of Melanin Extracted from Nigerseed Hulls. Food Biosci. 2024, 61, 104929. [Google Scholar] [CrossRef]
- Bhat, S.G.; Laxmi, M.; Kurian, N.K.; Smitha, S. Melanin and Bacteriocin from Marine Bacteria Inhibit Biofilms of Foodborne Pathogens. Indian J. Biotechnol. 2016, 15, 392–399. [Google Scholar]
- Yeroslavsky, G.; Richman, M.; Dawidowicz, L.; Rahimipour, S. Sonochemically Produced Polydopamine Nanocapsules with Selective Antimicrobial Activity. Chem. Commun. 2013, 49, 5721. [Google Scholar] [CrossRef] [PubMed]
- Eliato, T.R. Melanin Pigments as Antibacterial Agents. Doctoral Dissertations, University of New Hampshire, Durham, NH, USA, 2022. [Google Scholar]
- Mustafa, Y.F. Synthesis of 7,8-Dihydroxy-4-Phenylbenzo[g]Coumarins as Potential Multitarget Anti-Skin-Aging Candidates. J. Mol. Struct. 2025, 1321, 139806. [Google Scholar] [CrossRef]
- Widowati, W.; Ginting, C.N.; Lister, I.N.E.; Girsang, E.; Amalia, A.; Wibowo, S.H.B.; Kusuma, H.; Rizal, R. Anti-Aging Effects of Mangosteen Peel Extract and Its Phytochemical Compounds: Antioxidant Activity, Enzyme Inhibition and Molecular Docking Simulation. Trop. Life Sci. Res. 2020, 31, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential Role of Natural Bioactive Peptides for Development of Cosmeceutical Skin Products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Khelghati, N.; Alemi, F.; Bazdar, M.; Asemi, Z.; Majidinia, M.; Sadeghpoor, A.; Mahmoodpoor, A.; Jadidi-Niaragh, F.; Targhazeh, N.; et al. Stabilization of Telomere by the Antioxidant Property of Polyphenols: Anti-Aging Potential. Life Sci. 2020, 259, 118341. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Thoyajakshi, R.S.; Megha, G.T.; Ravi Kumar, H.; Mathad, S.N.; Khan, A.; Nagaraju, S.; Mahmoud, M.H.; Ansari, A.Z. Garcinol: A Novel and Potent Inhibitor of Hyaluronidase Enzyme. Int. J. Biol. Macromol. 2024, 266 Pt 2, 131145. [Google Scholar] [CrossRef] [PubMed]
- Navia, M.A.; McKeever, B.M.; Springer, J.P.; Lin, T.Y.; Williams, H.R.; Fluder, E.M.; Dorn, C.P.; Hoogsteen, K. Structure of Human Neutrophil Elastase in Complex with a Peptide Chloromethyl Ketone Inhibitor at 1.84-A Resolution. Proc. Natl. Acad. Sci. USA 1989, 86, 7–11. [Google Scholar] [CrossRef] [PubMed]


| Fiber | Antibacterial Activity | ||
|---|---|---|---|
| Mean Diameter of Inhibition Zone (mm) | |||
| E. coli | P. aeruginosa | S. aureus | |
| PAN | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| PM0.5 | 7.1 ± 0.4 b | 7.3 ± 0.1.5 b | 6.7 ± 0.5 b |
| PM2.0 | 7.4 ± 0.5 b | 7.8 ± 0.7 bc | 6.8 ± 0.5 b |
| PM5.0 | 7.6 ± 0.7 bc | 8.0 ± 0.8 bcd | 7.6 ± 0.3 bc |
| Gentamicine 10 mg | 9.8 ± 0.6 de | 9.3 ± 0.3 cde | 9.9 ± 1.4 de |
| Ampicillin 100 mg | 9.8 ± 0.1 de | 11.3 ± 2.0 e | 9.5 ± 1.8 cde |
| Enzyme Inhibition (%) | ||||
|---|---|---|---|---|
| Fiber | Tyrosinase | Hyaluronidase | Collagenase | Elastase |
| PAN | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| PM0.5 | 0.8 ± 13.3 b | 9.8 ± 0.7 b | 10.9 ± 3.8 b | 27.3 ± 9.5 b |
| PM2.0 | 35.2 ± 8.7 c | 15.3 ± 3.4 c | 25.3 ± 4.3 c | 28.2 ± 7.4 b |
| PM5.0 | 36.0 ± 5.9 c | 21.9 ± 1.4 d | 37.2 ± 3.0 d | 33.0 ± 9.6 b |
| Ligand | PubChem CID | Binding Energy (Kcal/mol) | Target Protein | Ligand Chemical Structure |
|---|---|---|---|---|
| 2HY | 11539563 | −7.6 | Elastase | 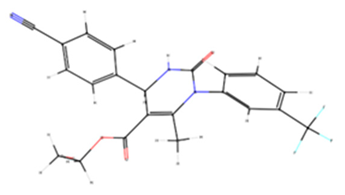 |
| B5N | 17249811 | −6.3 | Tyrosinase | 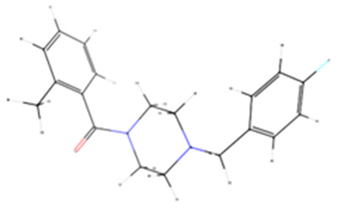 |
| IFW | 165416375 | −9.3 | Collagenase |  |
| Melanin | - | −10.4 | Collagenase | 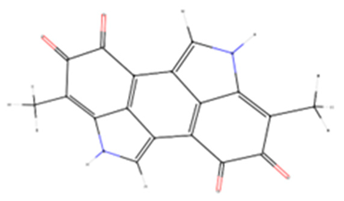 |
| −8.6 | Hyaluronidase | |||
| −7.1 | Elastase | |||
| 6.3 | Tyrosinase |
| Interaction Type | Enzyme Inhibition (%) | |||
|---|---|---|---|---|
| Collagenase | Hyaluronidase | Elastase | Tyrosinase | |
| Hydrophobic | Leu495 | Phe204 | Phe192 | Lys47 |
| Tyr496 | Pro249 | Val218 | ||
| Glu498 | Pro219 | |||
| Ala531 | ||||
| Trp539 | ||||
| Hydrogen Bonds | Glu498 | Arg134 | Ser195 | Gly46 |
| Trp539 | Lys144 | Val216 | Lys47 | |
| Tyr599 | Gly203 | Gln142 | ||
| π-π stacking π-cation | Trp539 | Tyr210 | Trp539 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, M.; Jaime-Ferrer, J.S.; Medrano-Lango, F.N.; Quintana-Rodríguez, E.; Campos-García, T.; Rodríguez-Sevilla, E.; Orona-Tamayo, D. Electrospun Membranes Loaded with Melanin Derived from Pecan Nutshell (Carya illinoinensis) Residues for Skin-Care Applications. Membranes 2025, 15, 44. https://doi.org/10.3390/membranes15020044
García-García M, Jaime-Ferrer JS, Medrano-Lango FN, Quintana-Rodríguez E, Campos-García T, Rodríguez-Sevilla E, Orona-Tamayo D. Electrospun Membranes Loaded with Melanin Derived from Pecan Nutshell (Carya illinoinensis) Residues for Skin-Care Applications. Membranes. 2025; 15(2):44. https://doi.org/10.3390/membranes15020044
Chicago/Turabian StyleGarcía-García, Michell, Jesús Salvador Jaime-Ferrer, Fernanda Nayeli Medrano-Lango, Elizabeth Quintana-Rodríguez, Tonatiu Campos-García, Erika Rodríguez-Sevilla, and Domancar Orona-Tamayo. 2025. "Electrospun Membranes Loaded with Melanin Derived from Pecan Nutshell (Carya illinoinensis) Residues for Skin-Care Applications" Membranes 15, no. 2: 44. https://doi.org/10.3390/membranes15020044
APA StyleGarcía-García, M., Jaime-Ferrer, J. S., Medrano-Lango, F. N., Quintana-Rodríguez, E., Campos-García, T., Rodríguez-Sevilla, E., & Orona-Tamayo, D. (2025). Electrospun Membranes Loaded with Melanin Derived from Pecan Nutshell (Carya illinoinensis) Residues for Skin-Care Applications. Membranes, 15(2), 44. https://doi.org/10.3390/membranes15020044






