Recovering Ammonia as Ammonium Citrate and Ammonium Sulfate from Sludge Digestion Liquors Using Membrane Contactors in a Pilot Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Source and Pilot Plant Pretreatment
2.2. Reagents and Hydrophobic Gas-Permeable Membrane
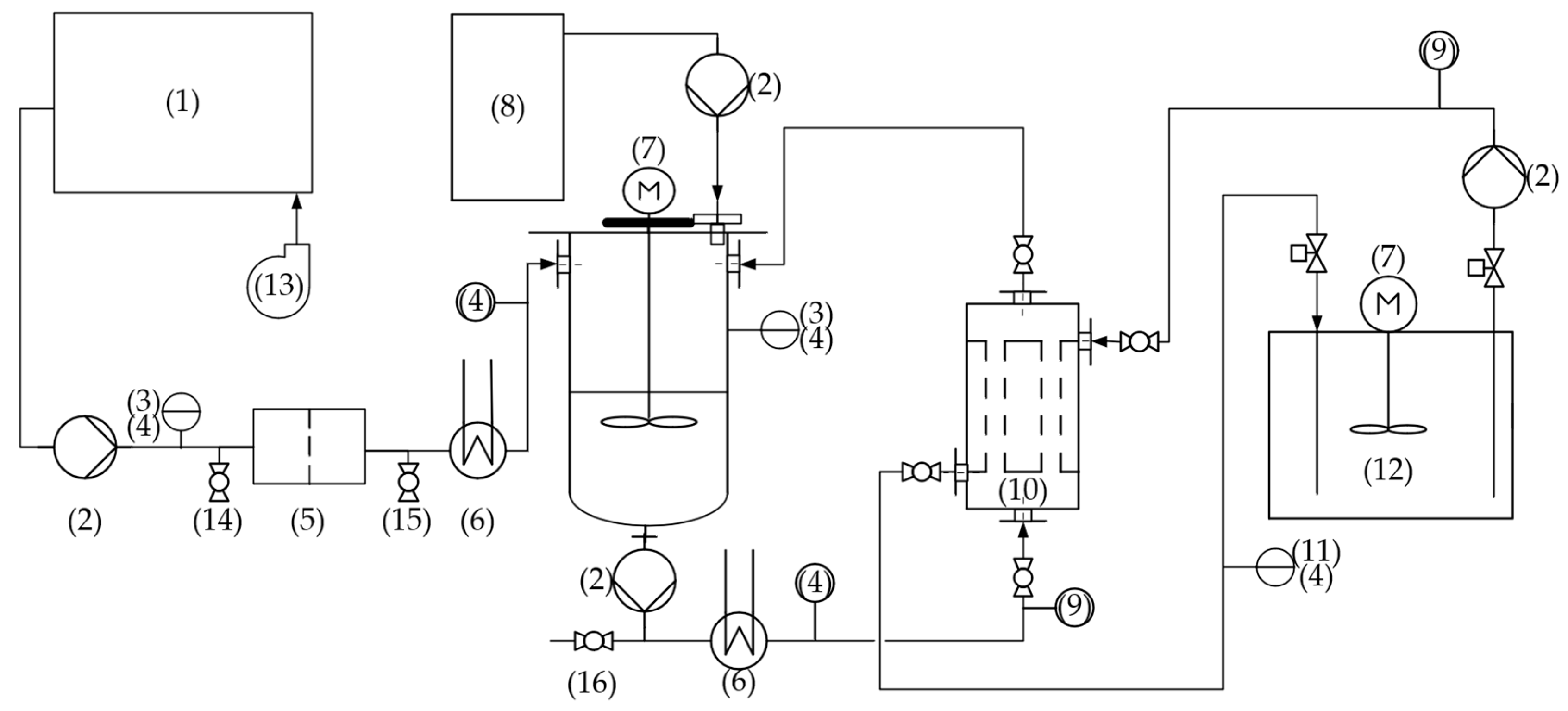
2.3. Pilot Plant and Experimental Stages
2.4. Analytical Methods
2.5. Data Analysis
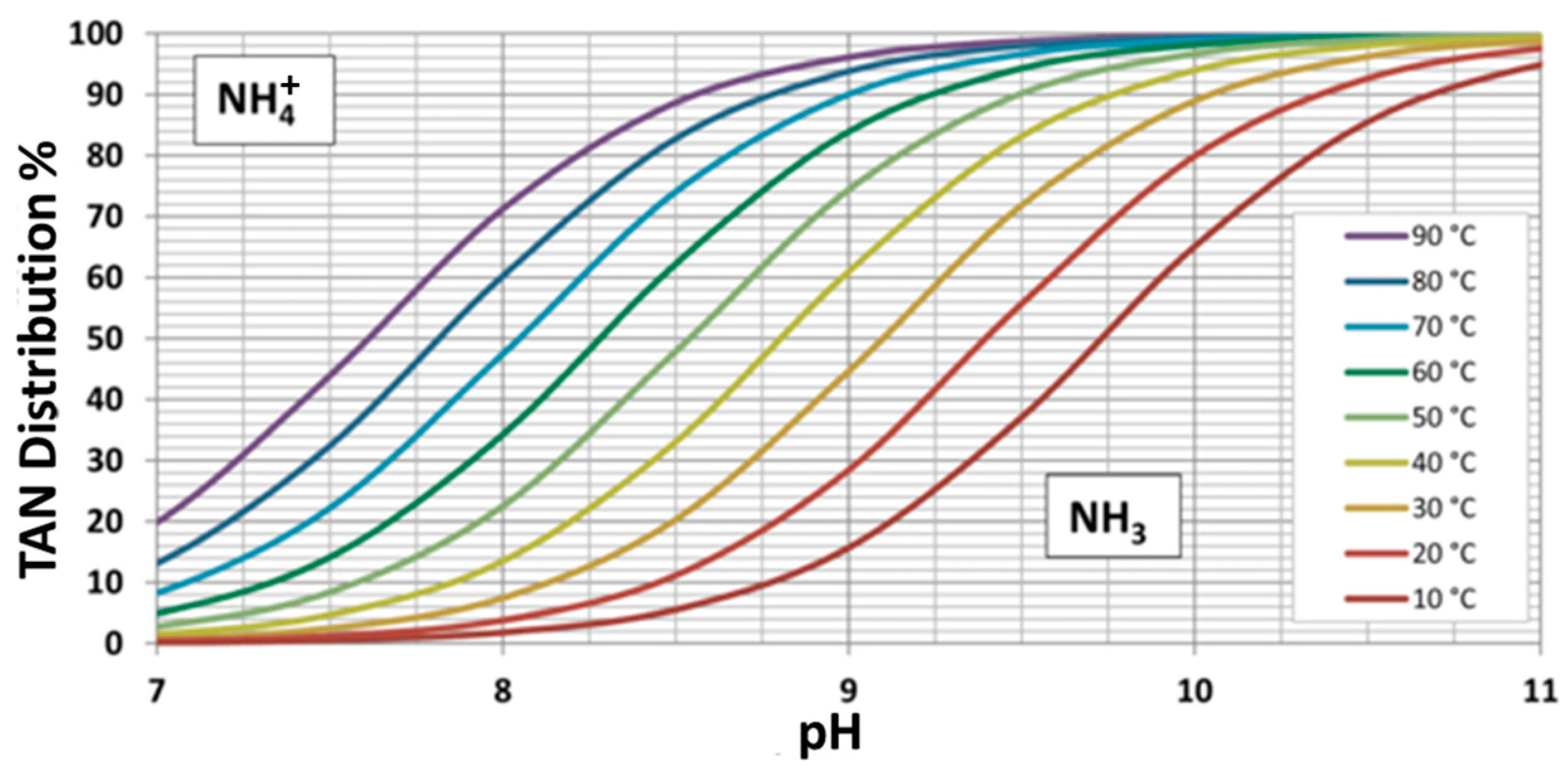
3. Results and Discussion
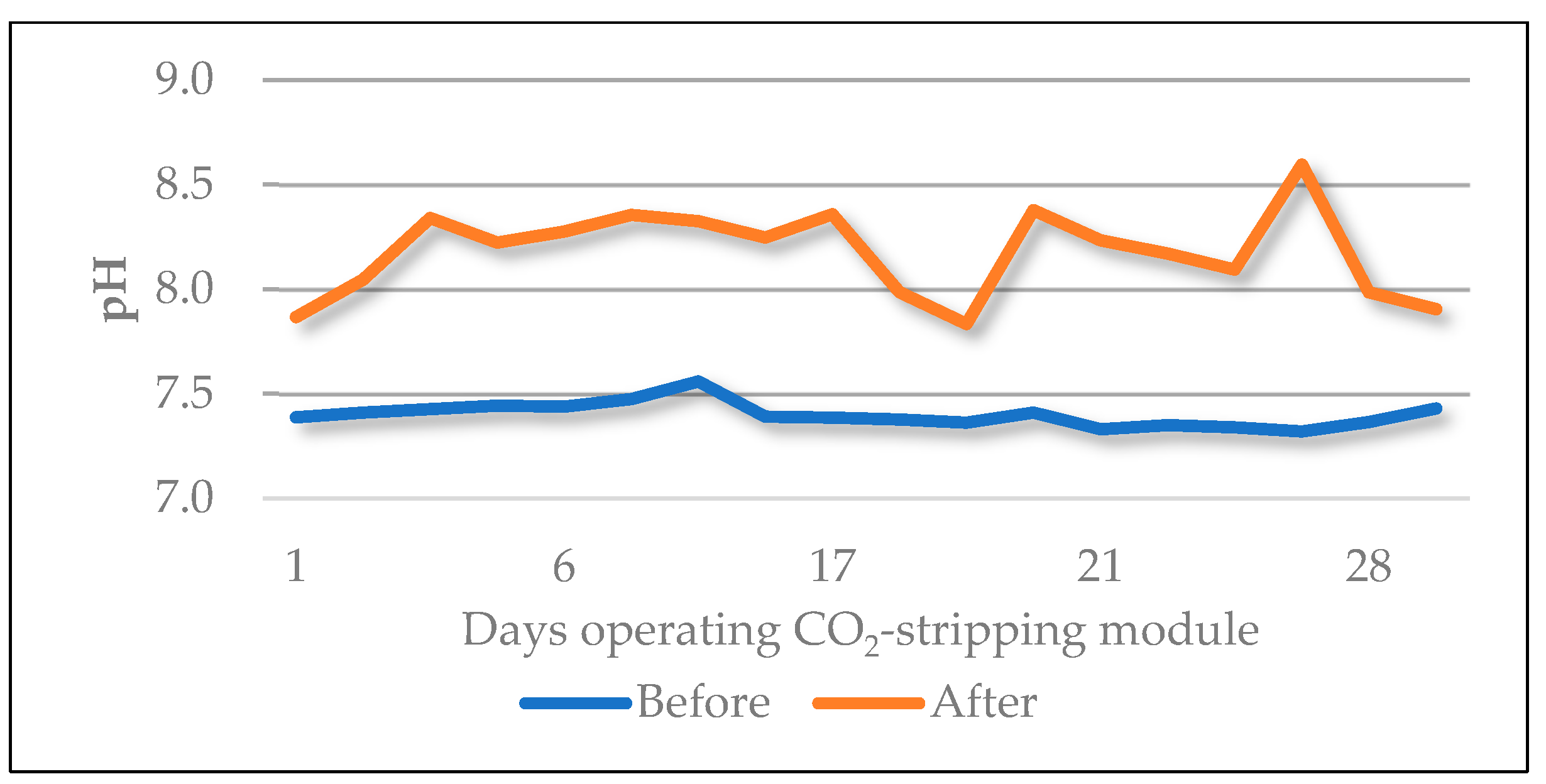
3.1. Pretreatment and Feed Water Characterization
Clean-in-Place (CIP)
3.2. TMCA and Performance of the CO2-Stripping Module
3.2.1. NH3 Mass Transfer, Energy and NaOH Consumption
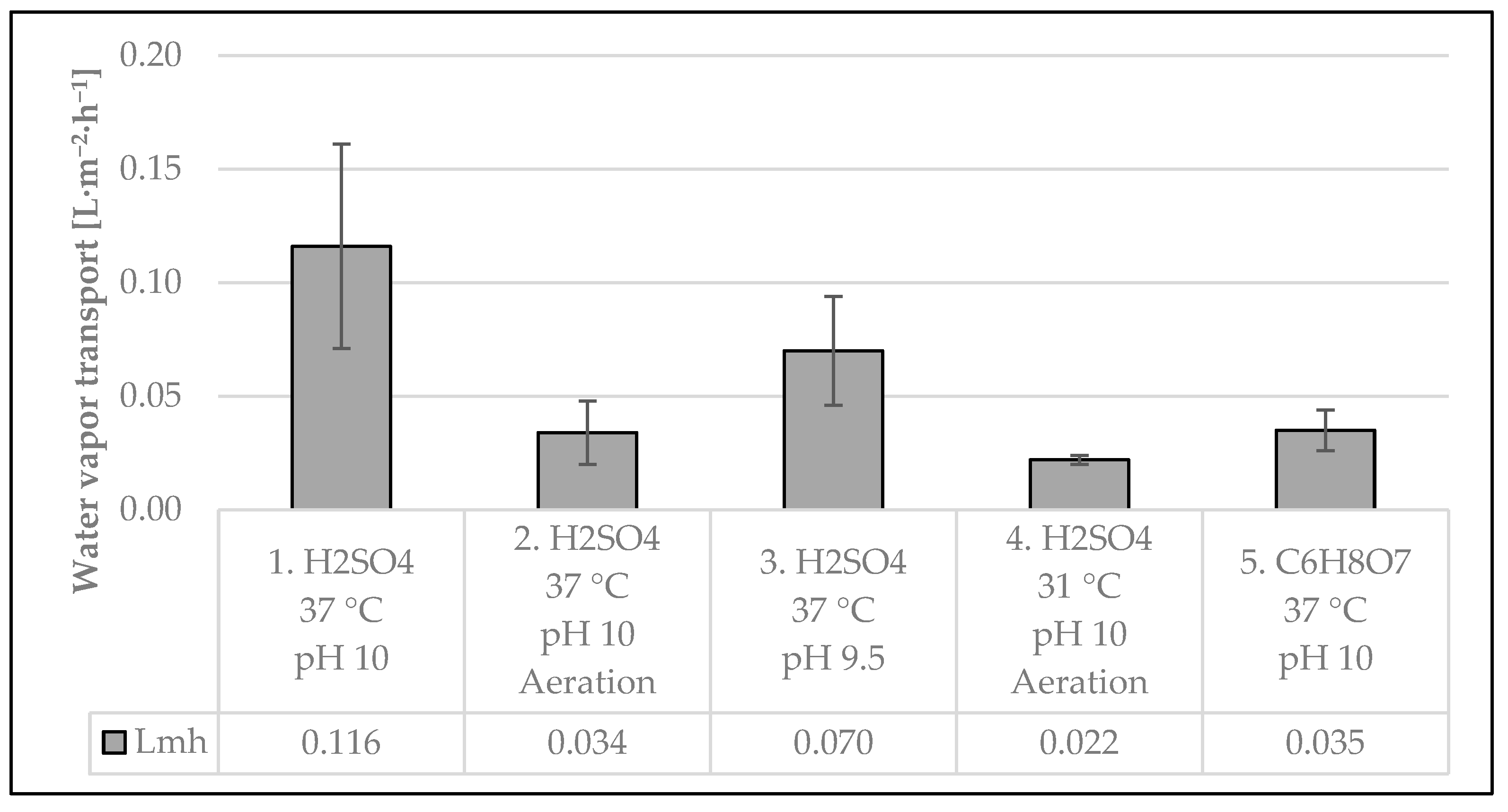
3.2.2. Water Vapor Transport
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Licon Bernal, E.E.; Maya, C.; Valderrama, C.; Cortina, J. Valorization of ammonia concentrates from treated urban wastewater using liquid–liquid membrane contactors. Chem. Eng. J. 2016, 302, 641–649. [Google Scholar] [CrossRef]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9, 580808. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Moritz, E.; Hobisch, M.; Muster-Slawitsch, B. Recovery of ammonia from centrate water in urban waste water treatment plants via direct contact membrane distillation: Process performance in long-term pilot-scale operation. J. Membr. Sci. 2023, 667, 121161. [Google Scholar] [CrossRef]
- Dow, N.; Saldin, T.F.; Duke, M.; Yang, X. Pilot demonstration of nitrogen removal from municipal wastewater by vacuum membrane distillation. J. Water Process Eng. 2022, 47, 102726. [Google Scholar] [CrossRef]
- Darestani, M.; Haigh, V.; Couperthwaite, S.J.; Millar, G.J.; Nghiem, L.D. Hollow fibre membrane contactors for ammonia recovery: Current status and future developments. J. Environ. Chem. Eng. 2017, 5, 1349–1359. [Google Scholar] [CrossRef]
- Gonzalez-Salgado, I.; Guigui, C.; Sperandio, M. Transmembrane chemical absorption technology for ammonia recovery from wastewater: A critical review. Chem. Eng. J. 2022, 444, 136491. [Google Scholar] [CrossRef]
- PONDUS Verfahrenstechnik GmbH. Stickstoffrückgewinnung aus Schlämmen und Abwässern: Das Ammonium-Ammoniak-Gleichgewicht. Available online: https://www.pondus-verfahren.de/ (accessed on 11 January 2024).
- Imai, M.; Furusaki, S.; Miyauchi, T. Separation of volatile materials by gas membranes. Ind. Eng. Chem. Proc. Des. Dev. 1982, 21, 421–426. [Google Scholar] [CrossRef]
- Ulbricht, M.; Schneider, J.; Stasiak, M.; Sengupta, A. Ammonia Recovery from Industrial Wastewater by TransMembraneChemiSorption. Chem. Ing. Tech. 2013, 85, 1259–1262. [Google Scholar] [CrossRef]
- Richter, L.; Paulsen, S.; Wichern, M.; Grömping, M.; Robecke, U.; Haberkamp, J. Hollow-fibre Membrane Contactors for Nitrogen Recovery from Municipal Wastewater. Chem. Ing. Tech. 2021, 93, 1440–1444. [Google Scholar] [CrossRef]
- Nättorp, A.; Egli, C. New Approaches and Best Practices for Closing the Materials Cycle in the Water Sector: Ammonium Sulphate Production via Membrane Stripping in Altenrhein (CH), Water Europe, 2023. Available online: https://mp.watereurope.eu/d/technology/1112/ (accessed on 28 November 2024).
- Jang, Y.; Lee, W.; Park, J.; Choi, Y. Recovery of ammonia from wastewater by liquid–liquid membrane contactor A review. Membr. Water Treat. 2022, 13, 147–166. [Google Scholar] [CrossRef]
- BUSINESS analytiQ. Ammonium Sulfate Price Index. Available online: https://businessanalytiq.com/procurementanalytics/index/ammonium-sulfate-index/ (accessed on 29 November 2024).
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Evaluation of Different Capture Solutions for Ammonia Recovery in Suspended Gas Permeable Membrane Systems. Membranes 2022, 12, 572. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Bhushan, B.; Gibert, O.; Valderrama, C.; Cortina, J.L. Liquid fertilizer production by ammonia recovery from treated ammonia-rich regenerated streams using liquid-liquid membrane contactors. Chem. Eng. J. 2019, 360, 890–899. [Google Scholar] [CrossRef]
- Reyes Alva, R.; Mohr, M.; Zibek, S. Transmembrane Chemical Absorption Process for Recovering Ammonia as an Organic Fertilizer Using Citric Acid as the Trapping Solution. Membranes 2024, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.; Wichern, M.; Grömping, M.; Robecke, U.; Haberkamp, J. Ammonium recovery from process water of digested sludge dewatering by membrane contactors. Water Pract. Technol. 2020, 15, 84–91. [Google Scholar] [CrossRef]
- Du Preez, J.; Norddahl, B.; Christensen, K. The BIOREK® concept: A hybrid membrane bioreactor concept for very strong wastewater. Desalination 2005, 183, 407–415. [Google Scholar] [CrossRef]
- Wäeger-Baumann, F.; Fuchs, W. The Application of Membrane Contactors for the Removal of Ammonium from Anaerobic Digester Effluent. Sep. Sci. Technol. 2012, 47, 1436–1442. [Google Scholar] [CrossRef]
- Stadt Erbach. Jahresbericht_PLS_2018-2021_Zusammenstellung; Excel-Datei; Wastewater Treatment Plant Erbach: Erbach, Germany, 2021. [Google Scholar]
- García-González, M.C.; Vanotti, M.B.; Szogi, A.A. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of aeration. J. Environ. Manag. 2015, 152, 19–26. [Google Scholar] [CrossRef] [PubMed]
- 3M. User Guide: 3M™ Liqui-Cel™ Membrane Contactors for TransMembrane ChemiSorption Operation. Available online: https://www.3m.com/3M/en_US/liquicel-us/resources/operating-and-technical-guides/ (accessed on 26 November 2024).
- EN 872:2005; Water Quality—Determination of Suspended Solids: Method by Filtration Through Glass Fibre Filters. European Committee for Standardization: Brussels, Belgium, 2005.
- Xu, B.; He, Z. Ammonia recovery from simulated anaerobic digestate using a two-stage direct contact membrane distillation process. Water Environ. Res. 2021, 93, 1619–1626. [Google Scholar] [CrossRef]
- Reig, M.; Vecino, X.; Gibert, O.; Valderrama, C.; Cortina, J.L. Study of the operational parameters in the hollow fibre liquid-liquid membrane contactors process for ammonia valorisation as liquid fertiliser. Sep. Purif. Technol. 2021, 255, 117768. [Google Scholar] [CrossRef]
- Alpha Analytical, Inc. Alkalinity, Titration Method; Alpha Analytical, Inc.: Westborough, MA, USA, 2012. [Google Scholar]
- Deng, Z.; van Linden, N.; Guillen, E.; Spanjers, H.; van Lier, J.B. Recovery and applications of ammoniacal nitrogen from nitrogen-loaded residual streams: A review. J. Environ. Manag. 2021, 295, 113096. [Google Scholar] [CrossRef]
- Daguerre-Martini, S.; Vanotti, M.B.; Rodriguez-Pastor, M.; Rosal, A.; Moral, R. Nitrogen recovery from wastewater using gas-permeable membranes: Impact of inorganic carbon content and natural organic matter. Water Res. 2018, 137, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Zarebska, A.; Karring, H.; Christensen, M.L.; Hjorth, M.; Christensen, K.V.; Norddahl, B. Ammonia Recovery from Pig Slurry Using a Membrane Contactor—Influence of Slurry Pretreatment. Water Air Soil Pollut. 2017, 228, 150. [Google Scholar] [CrossRef]
- Stadt Erbach. Wasserversorgung: Wasserhärte und ph-Werte. Available online: https://www.erbach-donau.de/cms/BuergerService-Ver-Entsorgung-Wasserversorgung.html (accessed on 26 December 2024).
- 3M. 3M™ Liqui-Cel™ EXF Series Membrane Contactors: Cleaning and Storage Guidelines 3M. Available online: https://www.3m.com/3M/en_US/liquicel-us/resources/operating-and-technical-guides/ (accessed on 26 November 2024).
- de la Hera, G.; Ruiz-Gutiérrez, G.; Viguri, J.R.; Galán, B. Flexible Green Ammonia Production Plants: Small-Scale Simulations Based on Energy Aspects. Environments 2024, 11, 71. [Google Scholar] [CrossRef]
- Karmann, C.; Mágrová, A.; Jeníček, P.; Bartáček, J.; Kouba, V. Advances in nitrogen removal and recovery technologies from reject water: Economic and environmental perspectives. Bioresour. Technol. 2024, 391, 129888. [Google Scholar] [CrossRef] [PubMed]
- Riaño, B.; Molinuevo-Salces, B.; Vanotti, M.B.; García-González, M.C. Ammonia Recovery from Digestate Using Gas-Permeable Membranes: A Pilot-Scale Study. Environments 2021, 8, 133. [Google Scholar] [CrossRef]
- Federal Ministry of Justice. Verordnung Über das Inverkehrbringen von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln 1 (Düngemittelverordnung—DüMV): Definition von Düngemitteltypen. Available online: https://www.gesetze-im-internet.de/d_mv_2012/anlage_1.html (accessed on 15 December 2024).
- Noriega-Hevia, G.; Serralta, J.; Seco, A.; Ferrer, J. Economic analysis of the scale-up and implantation of a hollow fibre membrane contactor plant for nitrogen recovery in a full-scale wastewater treatment plant. Sep. Purif. Technol. 2021, 275, 119128. [Google Scholar] [CrossRef]

| Feature | Unit | Value |
|---|---|---|
| Membrane type | - | Hollow fibers X-50 |
| Material | - | Polypropylene |
| Effective surface area | m2 | 53 |
| Area density | m2·m−3 | 1871 |
| Number of fibers | - | 156,000 |
| Pore size | µm | 0.04 |
| Stage | Pretreatment | CO2 Stripping | pH | T [°C] | Acid |
|---|---|---|---|---|---|
| 1 | UF Module | No | 10 | 37 | Sulfuric |
| 2 | UF Module | Yes | 10 | 37 | Sulfuric |
| 3 | UF Module | No | 9.5 | 37 | Sulfuric |
| 4 | UF Module | Yes | 10 | 31 | Sulfuric |
| 5 | UF Module | No | 10 | 37 | Citric |
| Parameter | Units | Before UF | After UF | After TMCA |
|---|---|---|---|---|
| Electrical conductivity (σ) | mS·cm−1 | 4.7 (0.6) | 4.3 (0.5) | 8.7(2.9) * |
| Total alkalinity (TA) | mg CaCO3·L−1 | 875.5 (214.9) | 768 (139.1) | 928.5 (189.5) |
| Phenolphthalein alkalinity (PA) | mg CaCO3·L−1 | 0 (0) | 0 (0) | 719.7 (194.4) |
| Total suspended solids (TSS) | mg·L−1 | 254.4 (175.8) | 25.1 (14.1) | ** |
| Total dissolved solids (TDS) | mg·L−1 | 5.3 (0.4) | 4.8 (0.5) | 10.2 (4.4) * |
| Total ammoniacal nitrogen (TAN) | mg·L−1 | 725.1 (161.8) | 647.9 (104.3) | 126.7 (135.1) * |
| Chemical oxygen demand (COD) | mg·L−1 | 206.3 (43.2) | 130.8 (28.3) | ** |
| Orthophosphate (PO4-P) | mg·L−1 | 12.4 (2.5) | 6.0 (2.7) | ** |
| Step | Parameter | Duration |
|---|---|---|
| 1 | Flushing with demin. water | 10 min |
| 2 | Cleaning with 6%v/v NaOH solution | 30–60 min |
| 3 | Drain system | |
| 4 | Cleaning with 10%v/v citric acid solution | 30–60 min |
| 5 | Drain system | |
| 6 | Flushing with demin. water | 10 min |
| 7 | Drain system |
| Experimental Stage | TANo [mg·L−1] | TANf [mg·L−1] | Removal [%] | TANTS [mg·L−1] | Vol.TS [L] | TAN Recovery Rate [g·m−2·d−1] | NaOH 30% Consumption [L·m−3] |
|---|---|---|---|---|---|---|---|
| 1 | 563.8 (96.4) | 65.3 (29.8) | 88 | 12,100 | 1624 | 15.9 | 8.3 (2.3) |
| 2 | 730.5 (90.6) | 92.7 (49.6) | 87 | 16,640 | 2581 | 13.2 | 6.4 (1.1) |
| 3 | 686.3 (67.8) | 348.5 (221.7) | 49 | * | * | * | 5.1 (1.0) |
| 4 | 659.6 (29.7) | 98.4 (23.8) | 85 | 23,150 | 1294 | 13.5 | 6.6 (0.7) |
| 5 | 583 (92.7) | 81.3 (51.6) | 86 | 14,700 | 1717 | 15.7 | 6.4 (0.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes Alva, R.; Mohr, M.; Tovar, G.E.M.; Zibek, S. Recovering Ammonia as Ammonium Citrate and Ammonium Sulfate from Sludge Digestion Liquors Using Membrane Contactors in a Pilot Plant. Membranes 2025, 15, 62. https://doi.org/10.3390/membranes15020062
Reyes Alva R, Mohr M, Tovar GEM, Zibek S. Recovering Ammonia as Ammonium Citrate and Ammonium Sulfate from Sludge Digestion Liquors Using Membrane Contactors in a Pilot Plant. Membranes. 2025; 15(2):62. https://doi.org/10.3390/membranes15020062
Chicago/Turabian StyleReyes Alva, Ricardo, Marius Mohr, Günter E. M. Tovar, and Susanne Zibek. 2025. "Recovering Ammonia as Ammonium Citrate and Ammonium Sulfate from Sludge Digestion Liquors Using Membrane Contactors in a Pilot Plant" Membranes 15, no. 2: 62. https://doi.org/10.3390/membranes15020062
APA StyleReyes Alva, R., Mohr, M., Tovar, G. E. M., & Zibek, S. (2025). Recovering Ammonia as Ammonium Citrate and Ammonium Sulfate from Sludge Digestion Liquors Using Membrane Contactors in a Pilot Plant. Membranes, 15(2), 62. https://doi.org/10.3390/membranes15020062








