1. Introduction
The 21st century presents humanity with unprecedented environmental challenges, foremost among which is the urgent need to reduce greenhouse gas emissions in order to combat climate change. Among these emissions, carbon dioxide (CO
2) stands out as one of the main contributors to global warming. The continued reliance on fossil fuels for energy production, transportation, and industrial processes has led to a steady increase in CO
2 concentrations in the atmosphere and has exacerbated the climate crisis [
1].
In
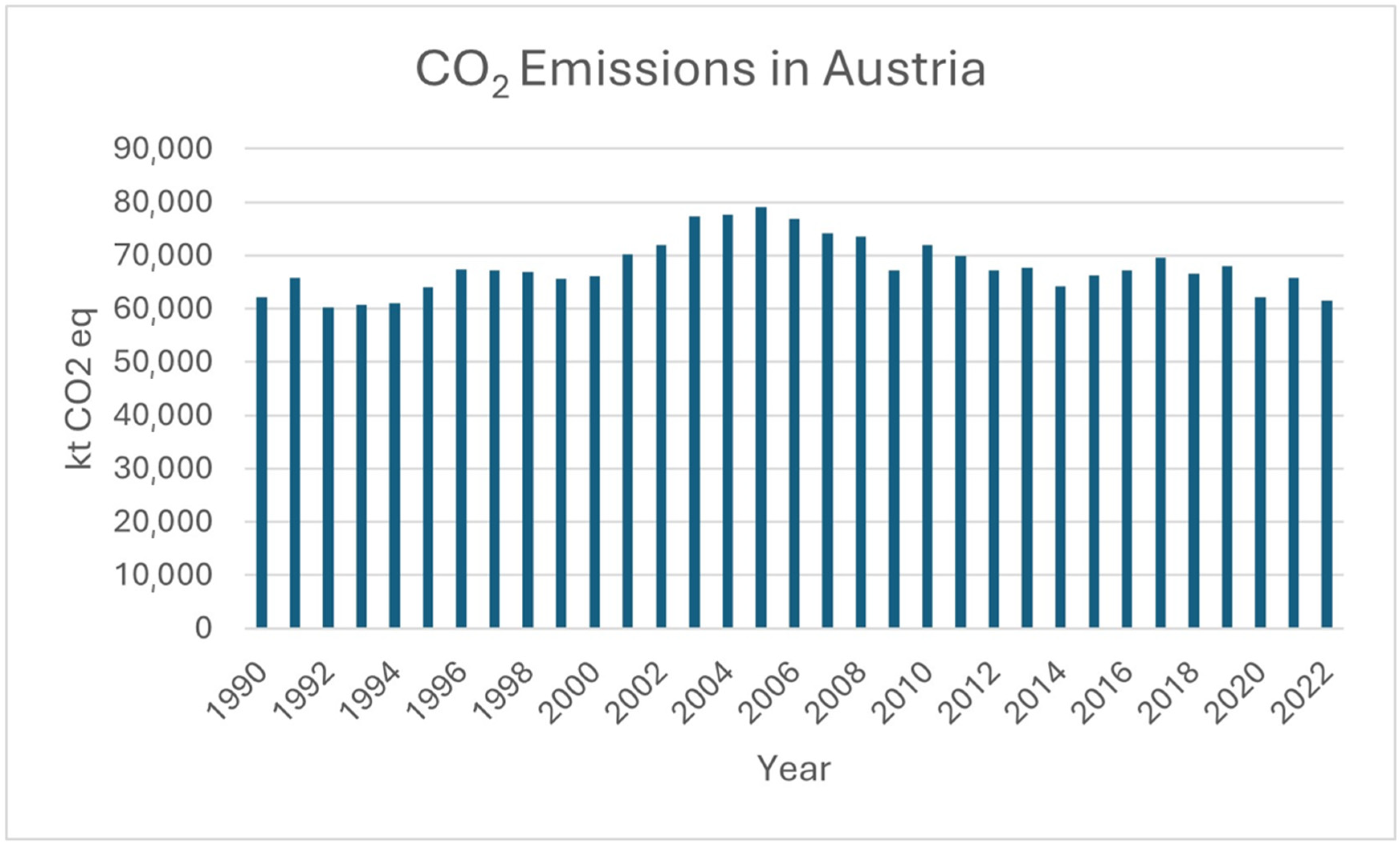
Figure 1, the CO
2 emissions in Austria from 1990 to 2022 are illustrated. To address this amount of emitted CO
2, researchers have been exploring innovative strategies to reduce CO
2 emissions while meeting energy and industrial needs. Carbon capture utilization (CCU) has proven to be a promising approach to reduce CO
2 emissions by converting captured CO
2 into valuable products. In the context of CCU, electrochemical reduction of CO
2 is particularly promising as it can convert CO
2 into useful chemicals and fuels, thus, closing the carbon cycle and contributing to a circular economy [
2].
In this review, the concept of carbon electroreduction is introduced as a key strategy within the broader CCU framework. We outline the motivations driving research in this area, including the need to decarbonize the economy, diversify energy sources, and promote sustainable development. By examining the role of CO
2 electricity reduction in achieving climate change goals, we set the stage for a comprehensive exploration of the latest advances in this area [
2].
1.1. Carbon Capture Utilization (CCU)
CCU is a promising approach to reducing CO
2 emissions while producing valuable products. CCU represents a paradigm shift in the approach to reducing CO
2 emissions by focusing on converting captured CO
2 into useful products instead of simply storing it underground. By utilizing CO
2 as a feedstock for chemical synthesis and industrial processes, CCU offers a double benefit: it reduces greenhouse gas emissions while creating economic value [
2,
3].
In the field of CCU, electrochemical CO
2 reduction has gained significant attention due to its versatility and efficiency in converting CO
2 into a range of valuable chemicals and fuels. Unlike traditional carbon capture and storage (CCS) methods, which capture and sequester CO
2 emissions, electrochemical CO
2 reduction enables the conversion of CO
2 into products such as carbon monoxide (CO), methane (CH
4), ethylene (C
2H
4) and formic acid (HCOOH) [
4].
Potential applications of CO as a feedstock for various industrial processes including metal production, hydrogen generation, and chemical synthesis. Carbon monoxide (CO) serves as a versatile building block for numerous industrial processes, ranging from metal production to chemical synthesis and hydrogen generation. As a feedstock, CO finds applications in a wide range of sectors, including petrochemicals, pharmaceuticals, and materials science. The electrochemical reduction of carbon dioxide (CO
2) offers a sustainable pathway for CO production, enabling the synthesis of CO from renewable electricity and captured CO
2 emissions [
5].
In metal production, CO serves as a reducing agent in processes such as iron smelting and steelmaking, where it reacts with metal oxides to yield pure metals. In chemical synthesis, CO is used as a precursor to produce a variety of organic compounds, including alcohols, acids, and esters. In hydrogen generation, CO can be converted into hydrogen gas via the water–gas shift reaction, providing a clean and renewable source of hydrogen for fuel cells and industrial applications [
5].
Furthermore, CO finds applications as fuel and chemical feedstock in the synthesis of methanol, ammonia, and other valuable chemicals. By harnessing CO as a platform molecule, it is possible to create a wide range of products with diverse applications in industry, transportation, and energy storage. The sustainable production of CO via electrochemical CO
2 reduction offers a promising pathway for meeting industrial demands while reducing greenhouse gas emissions and promoting a circular economy [
5].
1.2. Conventional Strategies for CO Production
The production of carbon monoxide (CO) is an essential part of various industrial processes, including the synthesis of chemicals, fuels, and materials. In the past, CO was mainly produced by conventional processes such as coal gasification, steam reforming of natural gas, and partial oxidation of hydrocarbons. These processes are based on high-temperature processes for the thermal decomposition of carbonaceous feedstocks, producing CO together with other by-products [
6].
In coal gasification, one of the oldest methods of CO production, coal is converted into a gas mixture of CO, hydrogen (H
2), and other gasses through high-temperature reactions in the presence of steam or oxygen. Similarly, steam reforming of natural gas uses steam and a catalyst to react with methane (CH
4) to produce CO and H
2. In partial oxidation, hydrocarbons are burned with a limited supply of oxygen, producing CO and H
2 [
6].
While these conventional methods must fulfill the industry’s CO production needs, they have several drawbacks, such as high energy consumption, greenhouse gas emissions, and dependence on fossil fuels. As the global transition to sustainable energy and production intensifies, there is a growing need to explore alternative ways to produce CO that are more efficient, environmentally friendly, and economically viable [
6].
2. Novel Green Conversion of CO2 into CO
With the growing urgency to mitigate climate change, researchers are increasingly turning to new technologies to convert carbon dioxide (CO2) into valuable chemicals and fuels. Among these technologies, electrochemical CO2 reduction holds promise because of its ability to selectively convert CO2 into carbon monoxide (CO) using renewable electricity as the driving force. By harnessing the power of electrons, electrochemical CO2 reduction offers a sustainable way to produce CO.
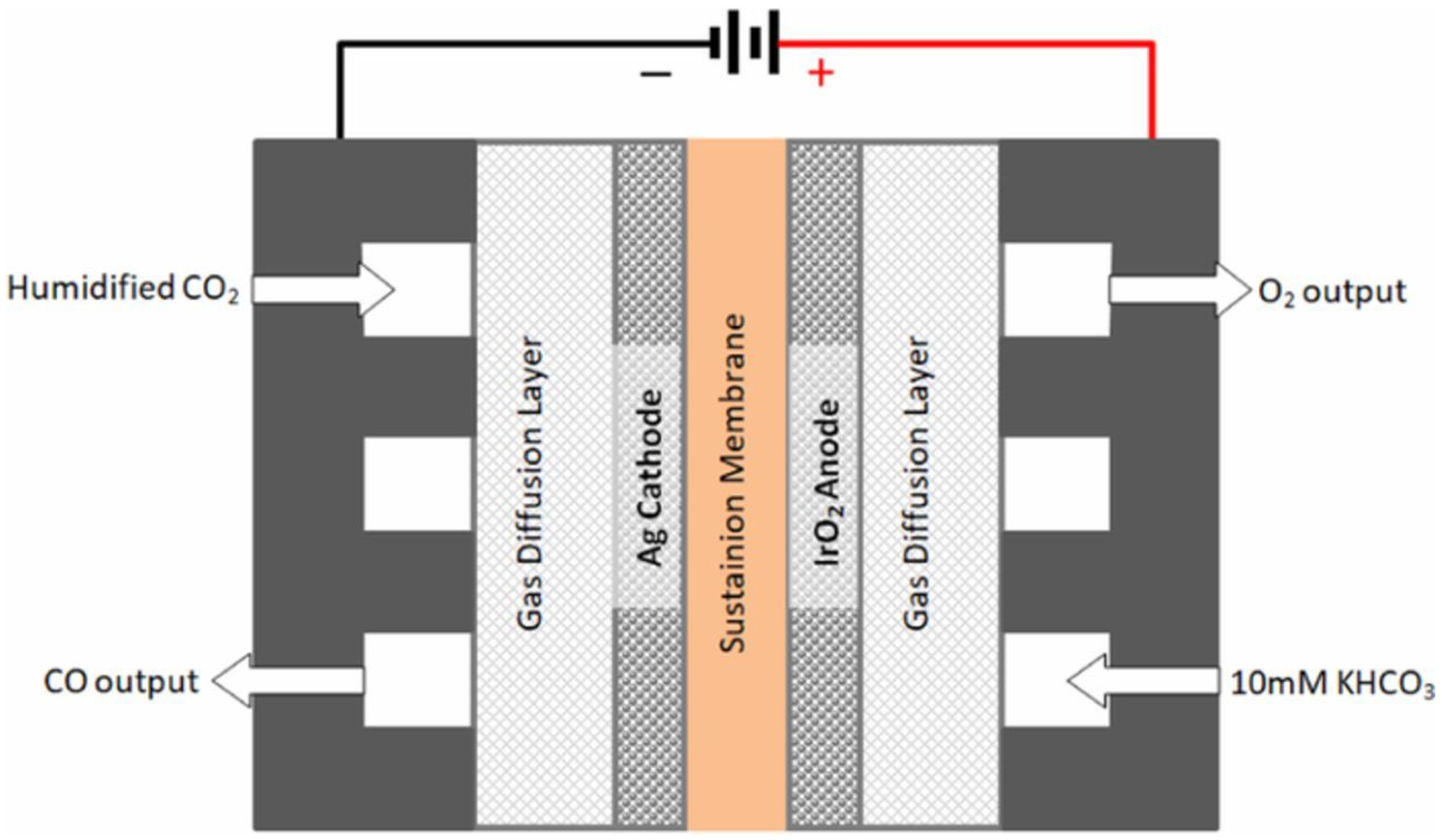
The general technical scheme of the CO
2 electroreduction cell is illustrated by
Figure 2 [
7]. The electrochemical cell is a membrane electrode assembly. The Ag-based cathode represents the catalyst, which can be varied in order to produce CO. In this setup, an aqueous solution is used as the electrolyte. In the setup, the aqueous solution is 10 mM KHCO
3.
By improving the electrochemical CO
2 reduction to CO, a syngas production route is established, which is independent on fossil fuels [
8]. In
Table 1, a comparison based on current research and literature in the field of CO
2 reduction technologies is summarized. In general, the products are generated at the cathode side via the electrochemical CO
2 reduction and the H
2O is oxidized at the anode side.
3. Electrochemical CO2 Reduction Mechanisms
In-depth analysis of electrochemical reduction pathways for the conversion of CO2 to CO is needed.
The electrochemical reduction of carbon dioxide (CO
2) offers a promising route for the conversion to carbon monoxide (CO), a valuable industrial raw material, while reducing CO
2 emissions. Central to this process is the electrochemical reactions that take place at the electrode surface and drive the conversion of CO
2 to CO via a series of intermediate steps. Understanding the mechanisms underlying these reactions is crucial for optimizing the efficiency and selectivity of CO production [
11].
The electrochemical reduction of CO
2 usually proceeds via several pathways, each comprising a sequence of elementary steps that determine the formation of specific products. These pathways are influenced by factors such as the electrode material, the reaction conditions, and the composition of the electrolyte, which determine the kinetics and thermodynamics of the electrochemical process. The main intermediates of the CO
2 reduction mechanism include adsorbed CO
2 species, CO
2 ions, and various surface-bound species formed during the reduction process (see
Figure 3) [
11].
Adsorption strength for the key intermediate (∗COOH);
proper CO adsorption energy;
inert CO reduction ability;
abundant and inexpensive raw materials;
efficient production of H2 and CO simultaneously.
4. Catalysts for Electrochemical CO2 Reduction
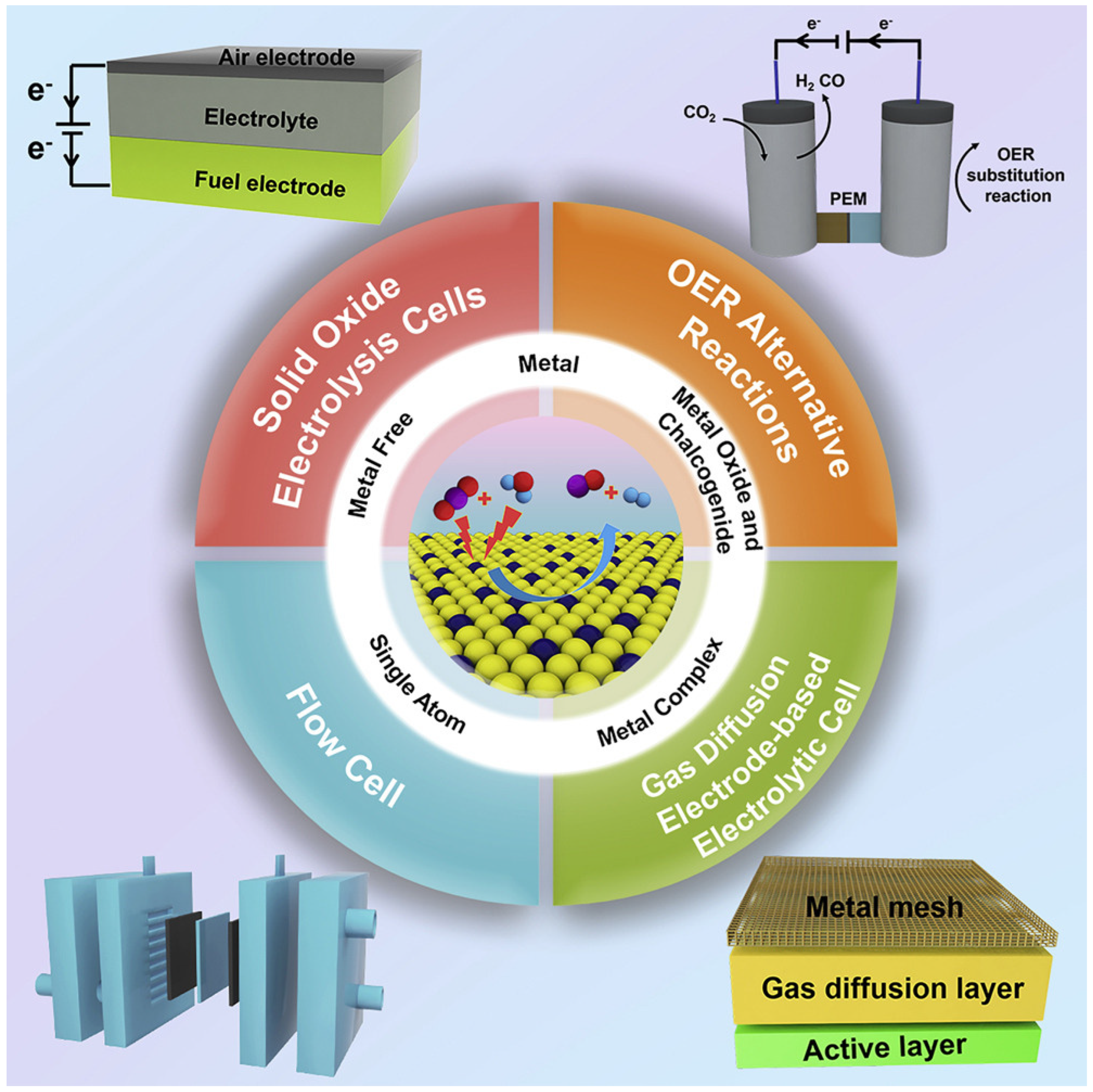
There are different types of catalysts, including metals, metal oxides, chalcogenides, metal complexes, monatomic catalysts, and metal-free catalysts, which is illustrated in
Figure 4 [
13].
Catalysts play a crucial role in the electrochemical reduction of carbon dioxide (CO
2) by facilitating the conversion of CO
2 into valuable products such as carbon monoxide (CO), methane (CH
4) and ethylene (C
2H
4). A wide range of catalysts has been explored for CO
2 electroreduction, including metals, metal oxides, chalcogenides, metal complexes, single-atom catalysts and metal-free materials. Each type of catalyst offers unique advantages and challenges in terms of activity, selectivity, stability, and cost effectiveness [
13].
In addition to inorganic catalysts, organic molecules and complexes have also been explored as catalysts for CO
2 electroreduction. Metal complexes containing transition metals such as iron (Fe), cobalt (Co) and nickel (Ni) show catalytic activity in CO
2 reduction, while single-atom catalysts provide precise control over active sites and reaction pathways. According to the literature [
14], the total reaction barrier for an Fe surface suggests a high activity on the Fe surface. The reaction barrier matches with the reaction energies as Fe < Co < Ni < Cu. Additionally, metal-free catalysts based on carbon materials, nitrogen-doped graphene, and other carbonaceous substrates are promising for CO
2 electroreduction in aqueous and non-aqueous electrolytes [
15].
As an example, copper is a useful non-noble metal catalyst, which is able to produce hydrocarbons as well as alcohols through the reduction of CO
2 [
16]. It is important to mention that by using copper a high overpotential is needed and, therefore, it influences energy efficiency negatively. The performance of Cu can be improved by changing the size and morphology design, alloying, as well as surface oxidation–reduction [
16,
17,
18,
19,
20,
21].
In order to improve the performance, nanoparticles (NP) provide a large surface area as well as active sites with a low coordination number such as surface planes, edges, and corners. For instance, by using diameters of around 7 nm, a faradaic efficiency of 76% of methane is obtained, which is higher than compared with the result of polycrystalline Cu, which is only 44% of faradaic efficiency. The trend shows that a smaller Cu-NP size enables a larger total current density. However, it generates lower selectivity in terms of hydrocarbon formation [
19].
Table 2 shows a summary of Cu-based catalysts, tested to determine the faradaic efficiency as well as the current density [
20].
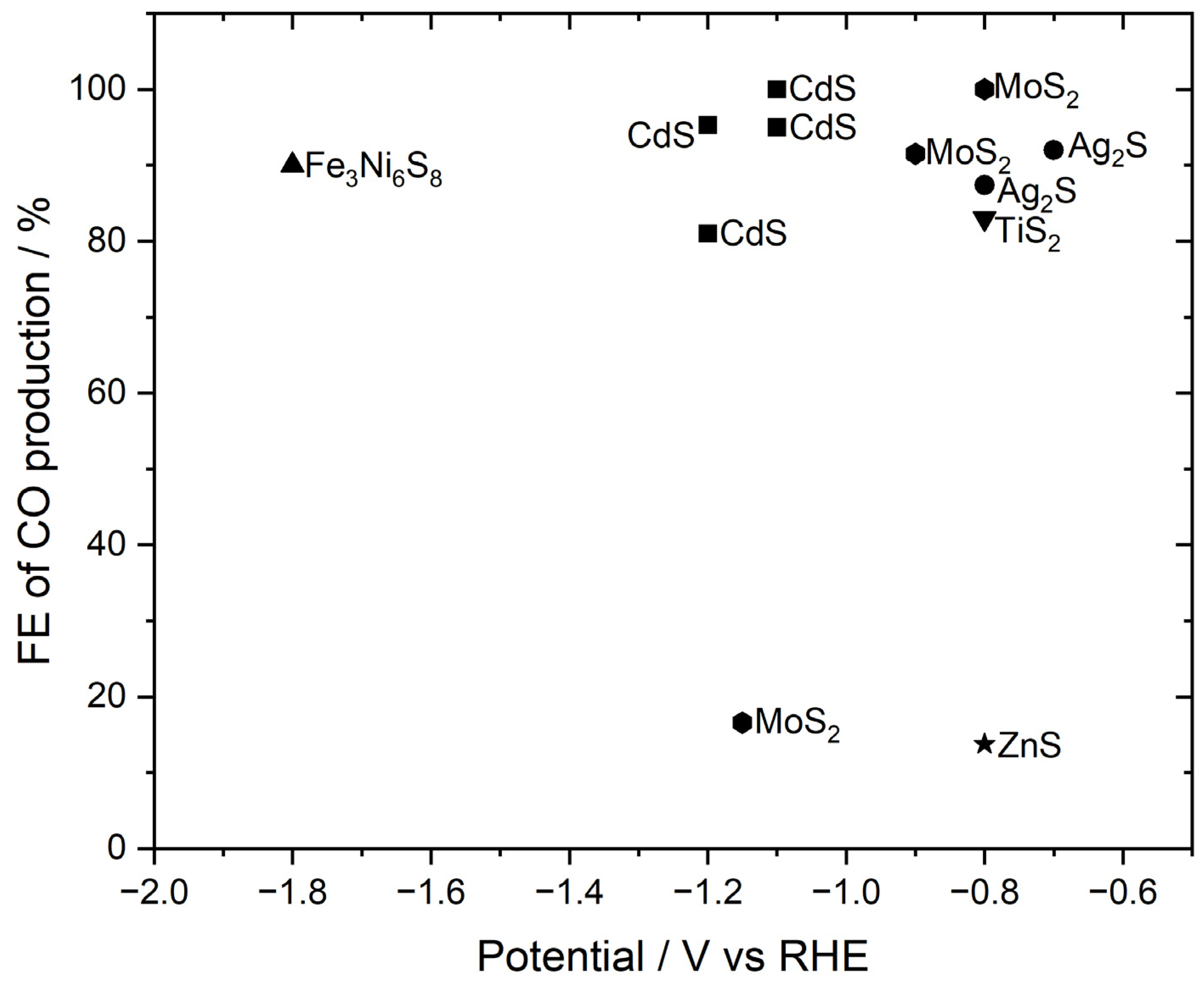
In order to produce CO, there are many inorganic-based catalysts. By changing the potential different faradaic efficiencies are reported in the literature. The conditions for the electrochemical reduction of carbon dioxide (CO2RR) are summarized in
Figure 5 [
18,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33].
According to the literature, catalysts such as CdS, MoS2, and Ag2S show a lower overpotential to award a high faradaic efficiency for producing CO. We refer this to the crystal structure of the catalyst and the conductive properties.
Strategies to Improve CO Production Efficiency
The efficiency of carbon monoxide (CO) production by electrochemical reduction of carbon dioxide (CO
2) is influenced by several factors, including catalyst activity and selectivity, electrode morphology, and electrolyte composition. To improve the efficiency of CO production, researchers have developed strategies to optimize these parameters through catalyst engineering, electrode design, and electrolyte optimization. By adjusting the properties of catalysts, electrodes, and electrolytes, it is possible to improve CO selectivity, Faradaic efficiency, and overall process performance [
15].
Catalyst engineering is about the development and synthesis of catalyst materials with tailored properties to optimize CO
2 reduction kinetics and selectivity. Strategies such as doping, alloying, and nanostructuring can increase catalyst activity and stability and, thus, improve the efficiency of CO production. Similarly, electrode design plays a crucial role in optimizing mass transfer, charge transfer, and surface area, which are essential for efficient CO
2 electroreduction. By controlling the electrode morphology, composition, and architecture, it is possible to increase CO selectivity and minimize competing side reactions [
15].
Optimizing electrolyte composition and operating conditions is another key strategy to improve CO production efficiency. Electrolytes play a critical role in facilitating CO
2 reduction reactions by providing ions for charge transfer and stabilizing reaction intermediates. By adjusting the pH, concentration and composition of the electrolyte, reaction kinetics and product selectivity can be modulated. In addition, the optimization of operating parameters such as temperature, pressure, and flow rate can further improve the efficiency of CO
2 electroreduction [
3]. The reason behind this is that temperature increases reaction rates by providing the necessary thermal energy, which accelerates CO
2 conversion. However, it must be carefully regulated to avoid overheating and damage to the system’s components. Pressure affects the CO
2 concentration at the electrode by increasing its partial pressure, which enhances the reaction rate. Higher pressure improves CO
2 availability but requires robust equipment to manage the added mechanical stresses. Flow rate ensures a steady supply of CO
2 to the electrode and efficient removal of reaction products. Proper flow management helps maintain a high reactant concentration and prevents product buildup that could inhibit the reaction.
5. Role of Membranes in CO2 Electrolyzers
In CO
2 electrolyzers, membranes are integral to the system’s ability to convert CO
2 into valuable chemicals and fuels. They serve as selective barriers that separate the anode and cathode compartments, allowing ion transport while preventing the undesired crossover of gasses like CO
2, H
2, or O
2. This selectivity is essential for high Faradaic efficiency (i.e., the efficiency of charge transfer in producing desired products) and for minimizing side reactions that can degrade the electrolyzer’s performance over time [
34,
35,
36].
Three common classes of membranes are used in a CO
2 flow reactions. Based on the ionic functional groups attached to polymer chains, ion exchange membranes (IEM) are divided into anion exchange membranes (AEM), cation exchange membranes (CEM), and bipolar membranes (BPM). The type of membrane has an influence on the pH of both sides of the membrane. Based on the pH impact, the reactant availability and reaction potential of cathodic as well as anodic reactions changes. CEM is used to transport cations from an acidic anode to the cathode. In contrast to the CEM, AEM transport anions from the basic cathode to the anode. BPM leads to dissociation of H
2O in order to enable the transportation of H
+ to the cathode and the OH
- to the anode or transportation of H
+ from the anode and OH
- from the cathode and the formation of water at the center of membrane [
37].
5.1. Proton Exchange Membranes (PEM)
Proton exchange membranes are commonly used in acidic electrolysis environments. They facilitate the movement of protons (H
+) from the anode to the cathode while preventing other species, such as CO
2, from crossing. Nafion
® is a widely used PEM due to its high ionic conductivity and chemical stability under acidic conditions. However, environments in PEM cells can limit the choice of catalysts and present challenges for CO
2 reduction due to possible catalyst degradation. PEMs also show CO
2 crossover, which can affect reaction selectivity and reduce overall efficiency [
38,
39].
A cation exchange membrane is suitable for a zero-gap CO
2 electrolyzer system. A big advantage is that carbonation can be avoided, and carbon efficiency is improved. Different cation exchange membranes are based on Nafion
® membranes (111, 112, 115, 211, and 212) with different thicknesses. Higher FECO, as well as energy efficiency, is achieved by thinner Nafion
® membranes. Non-acidic anolyte promotes the formation of carbonates and bicarbonates in the gas diffusion electrode, which would lead to clogging of the flow field channel as well as blocking of the catalytic surfaces. On the other hand, acidic anolyte remains an issue due to high hydrogen production [
40].
5.1.1. Structure and Application of CEMs in CO2RR
CEMs are designed to selectively transport cations, such as protons (H
+), while blocking anions. They are commonly employed in batch-type H-cell reactors for initial catalyst development, where CO
2-saturated aqueous electrolytes are used. CEMs enable efficient conversion of CO
2 into valuable products such as carbon monoxide (CO), formic acid (HCOOH), methane (CH
4), and alcohols under near-neutral to alkaline conditions. Nafion™, a widely used PFSA-based CEM, has shown remarkable Faradaic efficiencies (FE) for these reactions, with up to 98% for formic acid and 97% for CO. While flow reactors have begun adopting CEMs, their application is still less prevalent compared to batch-type setups [
36,
41,
42].
In flow electrolyzers, CEMs inhibit anionic product crossover, making them particularly suitable for producing formate (HCOO−). Novel cell designs leveraging the high proton conductivity of CEMs have achieved significant reductions in ohmic losses. For instance, using Nafion™ in a zero-gap configuration allows for high CO partial current densities with remarkably low cell voltages. Moreover, hybrid cell structures with buffer layers between the CEM and cathode provide opportunities to suppress undesired hydrogen evolution reactions (HER), albeit at the cost of increased energy losses due to additional resistance [
43,
44,
45].
5.1.2. Proton Conductivity
The core functionality of CEMs lies in their ability to transport protons efficiently. Proton conductivity in these membranes is governed by mechanisms such as the Grotthuss mechanism, where protons “hop” between hydrolyzed anionic sites through the formation of hydronium ions. This mechanism is faster than vehicular transport, making it ideal for CEM-based systems. Factors influencing proton conductivity include the ion exchange capacity (IEC), water uptake, and the morphology of ionic channels. For example, perfluorosulfonic acid (PFSA)-based CEMs like Nafion™ exhibit high proton conductivities (>100 mS/cm) due to their phase-separated structure, which forms a highly interconnected network of hydrated ion channels [
46].
Improving proton conductivity involves increasing the IEC, which enhances the density of ionic charges within the membrane. However, this must be balanced against potential drawbacks, such as excessive swelling and mechanical instability [
36].
5.1.3. Cation Crossover and CO2RR Selectivity
In CO2RR systems, cation crossover is a critical factor influencing product selectivity. The transport of cations such as K
+, Na
+, and Cs
+ through CEMs can affect CO
2 reduction kinetics by altering the local electric field and enhancing CO
2 adsorption. Research has demonstrated that using CEMs saturated with specific alkali cations can improve selectivity for certain products, such as CO, by suppressing proton transport and reducing competing reactions like HER [
46,
47,
48,
49].
However, cation crossover also poses challenges. For example, the leaching of substituted cations to the cathode can impact long-term stability and necessitate post-electrolysis purification. Achieving a balance between optimizing cation transport for selectivity, and minimizing undesired crossover remains an area of active research [
47,
48,
49,
50].
5.1.4. Product Crossover
Product crossover is a significant limitation in scaling CO2RR systems for industrial applications. Neutral products, such as alcohols, can diffuse through CEMs, contaminating the anolyte and increasing the complexity of downstream separation processes. Unlike anion exchange membranes (AEMs), CEMs exhibit electroosmotic drag (EOD) of water toward the cathode, mitigating anolyte contamination. However, this advantage is offset by challenges associated with high alcohol permeability and acidic product crossover [
51,
52,
53,
54,
55,
56].
Strategies to minimize product crossover include modifying PFSA membranes with inorganic materials, such as silica or zirconium phosphate, or employing nonfluorinated polymers with reduced permeability. Surface functionalization and polymer crosslinking are additional approaches to enhance selectivity and reduce crossover [
36,
57,
58,
59].
5.1.5. Stability and Durability
The long-term stability of CEMs is crucial for their viability in commercial CO2RR systems. Membrane degradation, often caused by radical-induced chain scission or excessive swelling, leads to a decline in ionic conductivity and mechanical integrity. In PEM water electrolyzers, strategies such as incorporating radical scavengers or using hydrocarbon-based membranes have shown promise in mitigating degradation. These approaches can be adapted for CEMs in CO2RR systems.
Hydrocarbon-based CEMs offer lower gas crossover rates but are more susceptible to free radical attacks than PFSA membranes. Advances in polymer chemistry, such as the development of sulfonated polyphenylenes, aim to address this trade-off by improving both chemical stability and ionic conductivity. However, achieving high stability while maintaining performance remains a key challenge [
60,
61].
5.1.6. Summary and Future Directions for CEM
CEMs have significant potential for enabling efficient and scalable CO
2 electrolysis systems. Their high proton conductivity, low anionic crossover, and compatibility with various electrolyzer designs make them an attractive choice for CO2RR applications. However, challenges such as excessive swelling, neutral product crossover, and susceptibility to degradation under acidic conditions must be addressed [
62,
63].
Future research should focus on the following:
Material Innovations: Developing novel CEM materials with tailored properties, such as improved ionic selectivity, reduced permeability, and enhanced durability;
Cell Design Optimization: Integrating buffer layers or hybrid structures to suppress HER while minimizing energy losses;
Scalability and Cost Reduction: Reducing the reliance on expensive PFSA-based membranes by exploring nonfluorinated alternatives and
scalable fabrication methods;
Stability Enhancements: Incorporating radical scavengers, surface modifications, and crosslinking to improve long-term performance.
In conclusion, while CEMs offer promising advantages for CO2RR, addressing their limitations through material science and engineering innovations will be critical for realizing their full potential in sustainable carbon utilization technologies [
64,
65].
5.2. Anion Exchange Membranes (AEM)
AEMs are typically employed in alkaline CO
2 electrolyzers, where they allow hydroxide ions (OH
−) to transport from the cathode to the anode. This alkaline environment has advantages for CO
2 reduction reactions as it can improve reaction rates and enhance product selectivity, especially for multi-carbon products. However, AEMs face issues with stability in alkaline solutions, as they can degrade over time, particularly when exposed to high pH and carbonate ions that form when CO
2 dissolves in the electrolyte. Efforts are underway to discover AEMs with improved chemical and mechanical stability by using novel polymer backbones and functional groups that resist degradation [
36,
66].
Membranes contain layers that conduct both H
+ and OH
− ions, allowing them to create a locally acidic environment at the cathode and an alkaline environment at the anode. This dual environment can help balance the pH gradients across the cell, which is beneficial for sustaining high activity in both half reactions. BPMs offer flexibility in reaction tuning, leading to higher selectivity for products like formate and ethylene, depending on catalyst choice and operating conditions. However, these membranes also face challenges related to water dissociation and require optimization to minimize energy losses associated with ion transport [
36,
67].
In
Table 3 the comparison of different membrane types are described. By comparing CEMs, AEMs, and BPMs, CEMs has high conductivity but an excessive swelling as well as low faradaic efficiency. AEMs has a high faradaic efficiency, but the main concern is the product and the carbonate ion crossover. The BPM indicates minimal crossover but has a higher resistance as well as delamination [
36].
The recent status of the IEM-based CO2RR reactors show cation exchange membranes as being mostly used in batch-type reactors. This process is used to test new developed electrocatalysts. In order to test the design and tuning of selectivity, H-cells also utilize CEM. The recent trend is that the CEM is also used in flow reactors to investigate and overcome the challenges associated with CEMs covering flux of protons toward the cathode during a continuous electrolysis producing hydrogen over the CO2RR [
36].
In order to overcome the challenges related to CEMs, AEMs are also investigated, especially when alkaline conditions are chosen. Therefore, different alkaline stable and conductive AEM materials are developed. AEMs are more popularly chosen than CEMs by usage in flow CO
2 electrolyzers [
36]. In
Table 4 the state-of-the-art performance metrics of flow CO2RR electrolyzers are summarized.
A new approach is to use an IEM as a BPM. This membrane has a laminating cation exchange layer as well as an anion exchange layer. Therefore, cationic and anionic mobile counterions are transported through their respective segments either to form water or transport from the interface where a rapid dissociation of water happens. BPMs are applied in reactors using liquid bicarbonate as feedstock [
36].
5.2.1. Anion Exchange Membranes (AEMs)
Anion Exchange Membranes (AEMs) are key components in electrochemical systems such as fuel cells, electrolyzers, and CO
2 reduction cells. Their efficiency and stability are critical in determining the overall performance of these systems, especially in applications that operate under high pH conditions. A thorough understanding of the behavior of AEMs in these environments reveals several important aspects, which include selectivity in CO
2 reduction reactions (CO2RR), rapid carbonation reactions, ionic conductivity, product crossover, and system stability [
36].
5.2.2. CO2RR Selectivity Under High-pH Conditions
One of the central concerns in using AEMs for CO2RR is maintaining high selectivity for the desired products under high pH conditions. At an elevated pH, the solubility and the reactivity of CO
2 changes significantly. AEMs, which are typically used to transport anions such as hydroxide ions (OH
−), facilitate CO2RR by enabling the transport of bicarbonate (HCO
3−) and carbonate (CO
32−) ions within the electrolyte. However, in high-pH environments, the increased presence of OH
− ions can compete with CO
2, potentially leading to undesirable side reactions. Optimizing AEMs involves adjusting their composition and structure to reduce such competition, thereby increasing the efficiency and selectivity for CO2RR products such as carbon monoxide (CO), methane (CH
4), or other carbon-based chemicals [
77,
78,
79,
80,
81].
5.2.3. Rapid Carbonation Reactions
AEMs are susceptible to carbonation reactions due to the affinity of the membrane material for CO
2. These carbonation reactions can significantly affect the membrane’s performance by altering its ionic conductivity and mechanical integrity. Under high-pH conditions, where CO
2 is readily available, the formation of bicarbonate and carbonate ions is enhanced. The reaction between CO
2 and the membrane material often leads to the formation of ionic clusters that can increase resistance and reduce the overall ionic conductivity of the AEM. Thus, controlling the rate of carbonation is critical to maintain the efficiency of the electrochemical process. Strategies to mitigate carbonation include modifying the chemical structure of the membrane or employing additives that hinder CO
2 absorption without compromising ionic conductivity [
50,
82].
5.2.4. Ionic Conductivity
Ionic conductivity is a key performance indicator for AEMs, as it directly impacts the efficiency of the electrochemical reactions. AEMs rely on the mobility of anions (typically hydroxide or carbonate ions) for ion transport within the system. High ionic conductivity is crucial for minimizing ohmic losses and ensuring efficient electron transfer across the system. However, the ionic conductivity of AEMs can be significantly impacted by high-pH conditions due to the formation of insoluble carbonate species or by changes in membrane structure resulting from carbonation. The development of AEMs with enhanced ionic conductivity under such harsh conditions is a primary goal of ongoing research. This can be achieved by modifying the polymer backbone, incorporating highly conductive ionic groups, or introducing fillers that promote ion transport without introducing detrimental effects on the membrane’s mechanical properties [
50,
83,
84,
85].
5.2.5. Product Crossover
In electrochemical systems, product crossover refers to the undesired transport of reaction products across the membrane from one side of the electrochemical cell to the other. In AEM-based systems, the main concern is the crossover of products such as CO, H
2, or other gasses, which can lead to efficiency losses and degradation of the system. High-pH conditions can exacerbate this issue by increasing the solubility of certain products or by altering the properties of the AEM. To minimize product crossover, it is essential to design membranes with appropriate ion-exchange capacities, porosities, and surface chemistries that can selectively allow the passage of ions without permitting gasses or other products to diffuse through the membrane. Enhancing the selectivity of AEMs is critical for ensuring high product yields and maintaining the overall system’s stability and efficiency [
86,
87].
5.2.6. System Stability
The long-term stability of AEMs under operating conditions is another important factor. High-pH environments, especially when combined with the presence of CO
2, can degrade the membrane materials over time. This degradation can manifest as mechanical breakdown, reduced ionic conductivity, or increased susceptibility to carbonation. In CO2RR applications, maintaining membrane integrity over long periods is crucial for ensuring stable and reliable performance. Strategies to enhance system stability include optimizing the chemical composition of the membrane, utilizing protective coatings, or incorporating stabilizing agents that prevent the formation of harmful ionic species. Additionally, improving the robustness of the membrane under high-pH conditions helps to extend the lifespan of the electrochemical system and reduce maintenance costs [
36,
88].
5.2.7. Summary and Future Directions for AEMs
In summary, AEMs play a critical role in electrochemical applications, especially in CO
2 reduction reactions, by facilitating the transport of anions like hydroxide and carbonate. Their performance is influenced by a variety of factors, including the selectivity for desired products, the occurrence of carbonation reactions, their ionic conductivity, the potential for product crossover, and their overall stability under high-pH conditions. Addressing these challenges requires continuous advancements in membrane design, material science, and process optimization to improve selectivity, minimize side reactions, and enhance long-term stability. As research progresses, it is expected that AEMs will continue to evolve, offering more efficient and sustainable solutions for CO2RR and other electrochemical applications [
36].
5.3. Bipolar Membranes (BPMs)
Bipolar membranes (BPMs) are an emerging class of ion exchange membranes (IEMs) used in electrochemical systems, particularly for CO
2 reduction reactions (CO2RR). BPMs are composed of a cation exchange layer (CEL) and an anion exchange layer (AEL), which together form a unique structure capable of supporting different pH environments on either side of the membrane, crucial for CO2RR processes. This pH difference is established through the dissociation of water at the interface between the CEL and AEL, driven by an electric field. Depending on the direction of the applied voltage, BPMs can operate with reverse or forward bias, each mode having specific advantages and challenges for CO2RR [
89,
90,
91,
92].
Water Dissociation and Overpotentials: The reverse bias mode of BPMs facilitates water dissociation at the CEL-AEL interface, generating protons and hydroxide ions that are transported across the respective layers. This electrochemical process is essential for maintaining ionic conductivity, especially under conditions where water splitting is necessary, such as in CO2RR or water electrolysis. However, the overpotentials associated with water dissociation remain significant, particularly at current densities above 20 mA cm
−2. For optimal performance, efficient water dissociation catalysts are needed at the interface to lower the overpotentials, as these can exceed 100 mV even at moderate current densities. Studies have shown that employing specific metal oxide or polymer-based catalysts at the CEL-AEL interface can improve the efficiency of water dissociation, enabling better performance under high current densities [
93,
94].
Anionic and Neutral Product Crossover: A significant advantage of BPMs in CO2RR reactors is their ability to limit the crossover of anionic species such as HCO
3− and CO
32−, which can negatively affect reactor performance. In reverse bias, the protonation of bicarbonate and carbonate species helps prevent their crossover to the anode, facilitating CO
2 capture. This selective exclusion is essential in ensuring the efficiency of CO2RR, where the presence of unwanted ions can decrease the Faradaic efficiency (FE). However, under forward bias, neutral products and CO
2 produced at the interface can lead to accumulation issues if not efficiently removed, causing potential membrane degradation [
36,
85,
95,
96].
Mechanical Degradation and Delamination: The mechanical stability of BPMs is a crucial consideration in their development, particularly under harsh electrochemical conditions. The different chemical and physical properties of the CEL and AEL can lead to mechanical degradation, such as delamination of the two layers. This can occur due to internal stressors like temperature fluctuations, pressure differentials, and water accumulation at the interface, particularly during forward bias operation. Delamination is further exacerbated by the formation of water and CO
2 at the interface in the forward bias, which can cause structural breakdown if not properly managed. Research efforts have focused on improving the adhesion between the layers, using strategies such as electrospinning to enhance the interfacial area and prevent delamination. Additionally, the differential thickness of the layers has been explored to mitigate water transport limitations and reduce mechanical degradation [
89].
Recent Developments and Applications: Recent studies have explored novel approaches to improve BPM performance. For instance, electrospinning has been used to create three-dimensional (3D) BPM structures that offer improved mechanical integrity and catalytic performance. The 3D structure increases the interfacial area, which is crucial for effective water dissociation and for preventing localized dehydration. These advanced BPMs are designed to accommodate high-current density operations, which are necessary for industrial-scale CO2RR reactors. Moreover, modifications to the membrane structure, such as roughening the interfaces between the CEL and AEL, have shown promise in reducing mechanical failures and enhancing performance under high-stress conditions [
36].
Challenges and Future Directions: Despite the potential of BPMs, several challenges remain in their application for CO2RR. The high cell voltages required in reverse bias configurations, especially under conditions of low pH at the cathode, limit their efficiency. Additionally, the low Faradaic efficiencies observed in CO2RR reactors using BPMs, due to parasitic hydrogen evolution reactions (HER) at the cathode, must be addressed. However, the use of liquid CO
2 feedstocks and strategies to improve the local pH environment at the cathode can help mitigate some of these challenges. Further development of water dissociation catalysts, membrane design improvements, and reactor configurations will be necessary to make BPMs a viable option for CO2RR at industrial scales [
36].
Summary of the Ion Exchange Membranes
BPMs represent a promising alternative to traditional anion exchange membranes (AEMs) and cation exchange membranes (CEMs) for CO2RR applications. Their ability to control pH at both the anode and cathode, minimize product crossover, and provide a mechanism for water dissociation at the CEL-AEL interface makes them particularly suitable for CO2RR systems. However, challenges related to overpotentials, mechanical degradation, and the need for efficient water dissociation catalysts remain significant hurdles to their commercial application. Future research focusing on improving BPM performance, including the development of more robust materials, better catalysts, and optimized reactor designs, will be essential to realizing their full potential in CO2 reduction and other electrochemical applications.
5.4. Performance and Challenges
The performance of electrolyzers depends heavily on membrane characteristics such as ionic conductivity, selectivity, chemical stability, and gas separation properties [
36].
Ion Conductivity: High conductivity reduces resistive losses, allowing more efficient transport of ions without needing high operating voltages, which can otherwise lead to undesirable side reactions [
36].
Chemical Stability: The acidic and alkaline environments in PEM and AEM electrolyzers, respectively, present harsh conditions that can degrade membrane materials over time. Improvements in stability are crucial for reducing maintenance costs and enhancing long-term durability [
36,
97].
Gas Crossover: Preventing the crossover of CO
2 and H
2 for maintaining product purity and minimizing side reactions. Developing membranes with low gas permeability but high ionic conductivity is an ongoing research focus [
36,
98].
Membrane degradation from high current densities and carbonate buildup (especially in alkaline environments with AEMs and BPMs) can affect system stability. For example, carbonate formation in AEMs reduces CO
2 availability for the reduction reaction, leading to lower overall efficiency [
36].
6. Challenges and Future Directions
Identifying the key challenges hindering the widespread adoption of electrochemical CO2 reduction technologies is important.
Despite the considerable progress that has been made in electrochemical carbon dioxide (CO2) reduction, there are a number of challenges that hinder the adoption of this technology for CO production on an industrial scale. These challenges include technical, economic, and regulatory barriers that need to be addressed in order to realize the full potential of electrochemical CO2 reduction as a sustainable route to CO production. Regulatory barriers that hinder the adoption of electrochemical CO2 reduction technology include the lack of specific financial incentives, inconsistent regulations across regions, and stringent environmental standards. Additionally, complex carbon emission reporting requirements, restrictions on CO2 storage, lengthy approval processes, and intellectual property issues further complicate the technology’s widespread implementation. These obstacles collectively impact the technology’s feasibility and delay its industrial adoption.
Key research challenges include the development of efficient and selective catalysts, optimization of reactor design and operation, integration with renewable energy sources, and scalability of CO2 electroreduction processes.
One of the biggest challenges in CO2 electroreduction is the development of catalysts that exhibit high activity, selectivity, and stability under realistic operating conditions. Despite advances in catalyst synthesis and characterization, precise control of reaction pathways and product distributions remain a challenging task. In addition, the integration of CO2 electroreduction with renewable energy sources such as solar and wind energy poses logistical and technical challenges related to intermittency, grid integration, and energy storage.
Economic considerations also play an important role in the commercial viability of CO2 electroreduction technologies. The high capital costs associated with electrochemical reactors, catalyst materials, and infrastructure pose financial barriers to widespread adoption. In addition, uncertainties related to market demand, regulatory frameworks, and political incentives complicate investment decisions and technology deployment strategies.
The economic assessment of electrochemical CO2 reduction methods in comparison with non-electrochemical CO production technologies is a crucial area of study. Such analyses provide valuable insights into the cost competitiveness and scalability of these emerging technologies. By evaluating the potential economic advantages of electrochemical methods, researchers can better understand their viability and the factors driving their adoption. Moving forward, it is essential to prioritize this aspect to comprehensively highlight the practical implications and transformative potential of electrochemical CO2 reduction in addressing global CO production demands.
7. Conclusions
In summary, the electrochemical reduction of carbon dioxide (CO2) represents a promising pathway for sustainable carbon monoxide (CO) production with implications for climate change mitigation, industrial decarbonization, and renewable energy integration. By utilizing renewable electricity and captured CO2 emissions, electrochemical CO2 reduction enables the synthesis of CO with high efficiency and selectivity, reducing dependence on fossil fuels and contributing to a circular carbon economy.
In this review, we have examined the mechanisms, catalysts, strategies, and applications of electrochemical CO2 reduction, highlighting its potential to address pressing environmental issues while creating economic value. A particular focus was placed on the critical role of ion exchange membranes (IEMs), including cation exchange membranes (CEMs), anion exchange membranes (AEMs), and bipolar membranes (BPMs). We discussed their advantages, such as improved ion transport and system stability, as well as their drawbacks, including material degradation and ion crossover. The current challenges associated with integrating these membranes into CO2 electroreduction systems, such as cost, scalability, and long-term performance, were also highlighted.
In the future, continued investment in research, development, and commercialization is essential to overcome the remaining challenges and realize the full potential of electrochemical CO2 reduction technologies. This includes advancing the design, functionality, and durability of ion exchange membranes, which are key to optimizing reactor performance and CO production efficiency. By fostering collaboration between academia, industry, and government, it is possible to accelerate innovation, scale up production, and create new markets for CO-derived products. Ultimately, the transition towards sustainable CO production requires concerted efforts from all stakeholders to build a resilient, resource-efficient, and low-carbon economy for future generations.
Author Contributions
Conceptualization, M.R., H.R. and A.A.; methodology, M.R. and H.R.; validation, A.A.; formal analysis, H.R. and A.A.; investigation, H.R. and A.A., data curation, H.R. and A.A.; writing—original draft preparation, H.R.; writing—review and editing, A.A. and M.R.; visualization, H.R. and A.A.; supervision, M.R.; project administration, M.R.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Österreichische Forschungsförderungsgesellschaft (FFG) (eCall number 48671894, Förderungsansuchen: Frontrunner: Electrochemical Conversion of CO2 into Valuable Basic Chemicals and Fuels FFG, Projektnummer: FO999909936, Ausschreibung: Frontrunner 2023).
Acknowledgments
We would like to acknowledge the generous funding provided by Österreichische Forschungsförderungsgesellschaft (FFG), which made this research possible (Förderungsansuchen: Frontrunner: Electrochemical Conversion of CO2 into Valuable Basic Chemicals and Fuels FFG, Projektnummer: FO999909936, eCall Antragsnummer: 48671894, Ausschreibung: Frontrunner 2023).
Conflicts of Interest
The all authors are employed by GIG Karasek GmbH.
References
- Treibhausgase. Zugegriffen: 19. December 2023. Available online: https://www.umweltbundesamt.at/klima/treibhausgase (accessed on 29 August 2024).
- Detz, R.J.; Ferchaud, C.J.; Kalkman, A.J.; Kemper, J.; Sánchez-Martínez, C.; Saric, M.; Shinde, M.V. Electrochemical CO2 conversion technologies: State-of-the-art and future perspectives. Sustain. Energy Fuels 2023, 7, 5445–5472. [Google Scholar] [CrossRef]
- Liu, Z.; Qian, J.; Zhang, G.; Zhang, B.; He, Y. Electrochemical CO2-to-CO conversion: A comprehensive review of recent developments and emerging trends. Sep. Purif. Technol. 2024, 330, 125177. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed]
- Draxler, M.; Schenk, J.; Bürgler, T.; Sormann, A. The Steel Industry in the European Union on the Crossroad to Carbon Lean Production—Status, Initiatives and Challenges. BHM Berg-Hüttenmänn. Monatshefte 2020, 165, 221–226. [Google Scholar] [CrossRef]
- Bierhals, J. Carbon Monoxide. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Kutz, R.; Masel, R.I. CO2 Electrolysis to CO and O2 at High Selectivity, Stability and Efficiency Using Sustainion Membranes. J. Electrochem. Soc. 2018, 165, J3371–J3377. [Google Scholar] [CrossRef]
- Küngas, R. Review—Electrochemical CO2 Reduction for CO Production: Comparison of Low- and High-Temperature Electrolysis Technologies. J. Electrochem. Soc. 2020, 167, 044508. [Google Scholar] [CrossRef]
- Han, G.H.; Bang, J.; Park, G.; Choe, S.; Jang, Y.J.; Jang, H.W.; Kim, S.Y.; Ahn, S.H. Recent Advances in Electrochemical, Photochemical, and Photoelectrochemical Reduction of CO2 to C2+ Products. Small 2023, 19, e2205765. [Google Scholar] [CrossRef] [PubMed]
- Wyndorps, J.; Ostovari, H.; von der Assen, N. Is electrochemical CO2 reduction the future technology for power-to-chemicals? An environmental comparison with H2-based pathways. Sustain. Energy Fuels 2021, 5, 5748–5761. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Lin, J.; Yan, S.; Zhang, C.; Hu, Q.; Cheng, Z. Electroreduction of CO2 toward High Current Density. Processes 2022, 10, 826. [Google Scholar] [CrossRef]
- Lu, S.; Shi, Y.; Meng, N.; Lu, S.; Yu, Y.; Zhang, B. Electrosynthesis of Syngas via the Co-Reduction of CO2 and H2O. Cell Rep. Phys. Sci. 2020, 1, 100237. [Google Scholar] [CrossRef]
- Liu, C.; Cundari, T.R.; Wilson, A.K. CO2 Reduction on Transition Metal (Fe, Co, Ni, and Cu) Surfaces: In Comparison with Homogeneous Catalysis. J. Phys. Chem. C 2012, 116, 5681–5688. [Google Scholar] [CrossRef]
- Sun, D.; Xu, X.; Qin, Y.; Jiang, S.P.; Shao, Z. Rational Design of Ag-Based Catalysts for the Electrochemical CO2 Reduction to CO: A Review. ChemSusChem 2020, 13, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Murata, A.; Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 2309–2326. [Google Scholar] [CrossRef]
- Manthiram, K.; Beberwyck, B.J.; Alivisatos, A.P. Enhanced Electrochemical Methanation of Carbon Dioxide with a Dispersible Nanoscale Copper Catalyst. J. Am. Chem. Soc. 2014, 136, 13319–13325. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer-Casadevall, A.; Li, C.W.; Johansson, T.P.; Scott, S.B.; McKeown, J.T.; Kumar, M.; Stephens, I.E.L.; Kanan, M.W.; Chorkendorff, I. Probing the Active Surface Sites for CO Reduction on Oxide-Derived Copper Electrocatalysts. J. Am. Chem. Soc. 2015, 137, 9808–9811. [Google Scholar] [CrossRef] [PubMed]
- Reske, R.; Mistry, H.; Behafarid, F.; Cuenya, B.R.; Strasser, P. Particle Size Effects in the Catalytic Electroreduction of CO2 on Cu Nanoparticles. J. Am. Chem. Soc. 2014, 136, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Zhong, H.; Jin, F. A brief review of electrocatalytic reduction of CO2—Materials, reaction conditions, and devices. Energy Sci. Eng. 2021, 9, 1012–1032. [Google Scholar] [CrossRef]
- Zeng, L.; Shi, J.; Luo, J.; Chen, H. Silver sulfide anchored on reduced graphene oxide as a high -performance catalyst for CO2 electroreduction. J. Power Sources 2018, 398, 83–90. [Google Scholar] [CrossRef]
- Liu, S.; Tao, H.; Liu, Q.; Xu, Z.; Liu, Q.; Luo, J.-L. Rational Design of Silver Sulfide Nanowires for Efficient CO2 Electroreduction in Ionic Liquid. ACS Catal. 2018, 8, 1469–1475. [Google Scholar] [CrossRef]
- He, R.; Zhang, A.; Ding, Y.; Kong, T.; Xiao, Q.; Li, H.; Liu, Y.; Zeng, J. Achieving the Widest Range of Syngas Proportions at High Current Density over Cadmium Sulfoselenide Nanorods in CO2 Electroreduction. Adv. Mater. 2018, 30, 170587. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Cheng, L.; Liu, P.F.; Zhang, L.; Zu, M.Y.; Wang, C.W.; Jin, Y.H.; Cao, X.M.; Yang, H.G.; Li, C. Simple Cadmium Sulfide Compound with Stable 95% Selectivity for Carbon Dioxide Electroreduction in Aqueous Medium. ChemSusChem 2018, 11, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Li, Y.; Wang, H.; Yang, G.; Cao, Y.; Yu, H.; Zhang, Q.; Liang, H.; Peng, F. Efficient electrochemical reduction of CO2 into CO promoted by sulfur vacancies. Nano Energy 2019, 60, 43–51. [Google Scholar] [CrossRef]
- Cheng, L.; Li, Y.; Chen, A.; Zhu, Y.; Li, C. Impacts on carbon dioxide electroreduction of cadmium sulfides via continuous surface sulfur vacancy engineering. Chem. Commun. 2020, 56, 563–566. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Liu, W.; Sun, Y.; Ju, Z.; Yao, T.; Wang, C.; Ju, H.; Zhu, J.; Wei, S.; et al. Carbon Dioxide Electroreduction into Syngas Boosted by a Partially Delocalized Charge in Molybdenum Sulfide Selenide Alloy Monolayers. Angew. Chem. Int. Ed. Engl. 2017, 56, 9121–9125. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, D.; Yang, H.; Liu, X.; Luo, J. Dual-Doping Promotes the Carbon Dioxide Electroreduction Activity of MoS2 Nanosheet Array. ACS Appl. Energy Mater. 2021, 4, 7492–7496. [Google Scholar] [CrossRef]
- Asadi, M.; Kumar, B.; Behranginia, A.; Rosen, B.A.; Baskin, A.; Repnin, N.; Pisasale, D.; Phillips, P.; Zhu, W.; Haasch, R.; et al. Robust carbon dioxide reduction on molybdenum disulphide edges. Nat. Commun. 2014, 5, 4470. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shen, G.; Zhang, R.; Wu, D.; Zou, C.; Ling, T.; Liu, H.; Dong, C.; Du, X.-W. Zn nanosheets coated with a ZnS subnanometer layer for effective and durable CO2 reduction. J. Mater. Chem. A 2019, 7, 1418–1423. [Google Scholar] [CrossRef]
- Tetzlaff, D.; Pellumbi, K.; Puring, K.J.; Siegmund, D.; Polet, W.S.K.; Checinski, M.P.; Apfel, U. Influence of the Fe: Ni Ratio in FexNi9-xS8 (x = 3–6) on the CO2 Electroreduction. ChemElectroChem 2021, 8, 3161–3167. [Google Scholar] [CrossRef]
- Simon, C.; Zander, J.; Kottakkat, T.; Weiss, M.; Timm, J.; Roth, C.; Marschall, R. Fast Microwave Synthesis of Phase-Pure Ni2FeS4 Thiospinel Nanosheets for Application in Electrochemical CO2 Reduction. ACS Appl. Energy Mater. 2021, 4, 8702–8708. [Google Scholar] [CrossRef]
- Piontek, S.; Puring, K.J.; Siegmund, D.; Smialkowski, M.; Sinev, I.; Tetzlaff, D.; Cuenya, B.R.; Apfel, U.-P. Bio-inspired design: Bulk iron–nickel sulfide allows for efficient solvent-dependent CO2 reduction. Chem. Sci. 2019, 10, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Gatto, I.; Patti, A.; Carbone, A. Assessment of the FAA3-50 Polymer Electrolyte for Anion Exchange Membrane Fuel Cells. ChemElectroChem 2023, 10, e202201052. [Google Scholar] [CrossRef]
- Hasa, B.; Cherniack, L.; Xia, R.; Tian, D.; Ko, B.H.; Overa, S.; Dimitrakellis, P.; Bae, C.; Jiao, F. Benchmarking anion-exchange membranes for electrocatalytic carbon monoxide reduction. Chem Catal. 2023, 3, 100450. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Mardle, P.; Zhao, N.; Riley, H.D.; Salvatore, D.A.; Berlinguette, C.P.; Holdcroft, S.; Shi, Z. Ion Exchange Membranes in Electrochemical CO2 Reduction Processes. Electrochem. Energy Rev. 2023, 6, 26. [Google Scholar] [CrossRef]
- Salvatore, D.A.; Gabardo, C.M.; Reyes, A.; O’brien, C.P.; Holdcroft, S.; Pintauro, P.; Bahar, B.; Hickner, M.; Bae, C.; Sinton, D.; et al. Designing anion exchange membranes for CO2 electrolysers. Nat. Energy 2021, 6, 339–348. [Google Scholar] [CrossRef]
- Fang, W.; Guo, W.; Lu, R.; Yan, Y.; Liu, X.; Wu, D.; Li, F.M.; Zhou, Y.; He, C.; Xia, C.; et al. Durable CO2 conversion in the proton-exchange membrane system. Nature 2024, 626, 86–91. [Google Scholar] [CrossRef] [PubMed]
- ELees, E.W.; Mowbray, B.A.W.; Parlane, F.G.L.; Berlinguette, C.P. Gas diffusion electrodes and membranes for CO2 reduction electrolysers. Nat. Rev. Mater. 2022, 7, 55–64. [Google Scholar] [CrossRef]
- Sakita, A.M.; Ticianelli, E.A. The role of cation exchange membrane characteristics in CO2 electrolysis to CO using acid anolyte. Electrochim. Acta 2025, 509, 145308. [Google Scholar] [CrossRef]
- Ma, W.; Xie, S.; Liu, T.; Fan, Q.; Ye, J.; Sun, F.; Jiang, Z.; Zhang, Q.; Cheng, J.; Wang, Y. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 2020, 3, 478–487. [Google Scholar] [CrossRef]
- Sato, M.; Ogihara, H.; Yamanaka, I. Electrocatalytic Reduction of CO2 to CO and CH4 by Co–N–C Catalyst and Ni co-catalyst with PEM Reactor. ISIJ Int. 2019, 59, 623–627. [Google Scholar] [CrossRef]
- Vennekoetter, J.-B.; Sengpiel, R.; Wessling, M. Beyond the catalyst: How electrode and reactor design determine the product spectrum during electrochemical CO2 reduction. Chem. Eng. J. 2019, 364, 89–101. [Google Scholar] [CrossRef]
- Puring, K.J.; Evers, O.; Prokein, M.; Siegmund, D.; Scholten, F.; Mölders, N.; Renner, M.; Cuenya, B.R.; Petermann, M.; Weidner, E.; et al. Assessing the Influence of Supercritical Carbon Dioxide on the Electrochemical Reduction to Formic Acid Using Carbon-Supported Copper Catalysts. ACS Catal. 2020, 10, 12783–12789. [Google Scholar] [CrossRef]
- Rasul, S.; Pugnant, A.; Xiang, H.; Fontmorin, J.-M.; Yu, E.H. Low cost and efficient alloy electrocatalysts for CO2 reduction to formate. J. CO2 Util. 2019, 32, 1–10. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Ersöz, M. Diffusion and Selective Transport of Alkali Cations on Cation-Exchange Membrane. Sep. Sci. Technol. 1995, 30, 3523–3533. [Google Scholar] [CrossRef]
- Resasco, J.; Chen, L.D.; Clark, E.; Tsai, C.; Hahn, C.; Jaramillo, T.F.; Chan, K.; Bell, A.T. Promoter Effects of Alkali Metal Cations on the Electrochemical Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2017, 139, 11277–11287. [Google Scholar] [CrossRef] [PubMed]
- Ringe, S.; Clark, E.L.; Resasco, J.; Walton, A.; Seger, B.; Bell, A.T.; Chan, K. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 2019, 12, 3001–3014. [Google Scholar] [CrossRef]
- Huang, J.E.; Li, F.; Ozden, A.; Rasouli, A.S.; de Arquer, F.P.G.; Liu, S.; Zhang, S.; Luo, M.; Wang, X.; Lum, Y.; et al. CO2 electrolysis to multicarbon products in strong acid. Science 2021, 372, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Neburchilov, V.; Martin, J.; Wang, H.; Zhang, J. A review of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2007, 169, 221–238. [Google Scholar] [CrossRef]
- Heinzel, A.; Barragán, V.M. A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells. J. Power Sources 1999, 84, 70–74. [Google Scholar] [CrossRef]
- Awang, N.; Ismail, A.; Jaafar, J.; Matsuura, T.; Junoh, H.; Othman, M.; Rahman, M. Functionalization of polymeric materials as a high performance membrane for direct methanol fuel cell: A review. React. Funct. Polym. 2015, 86, 248–258. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.; Hasran, U.; Daud, W. Overview of hybrid membranes for direct-methanol fuel-cell applications. Int. J. Hydrogen Energy 2010, 35, 2160–2175. [Google Scholar] [CrossRef]
- DeLuca, N.W.; Elabd, Y.A. Polymer electrolyte membranes for the direct methanol fuel cell: A review. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2201–2225. [Google Scholar] [CrossRef]
- Tschinder, T.; Schaffer, T.; Fraser, S.D.; Hacker, V. Electro-osmotic drag of methanol in proton exchange membranes. J. Appl. Electrochem. 2007, 37, 711–716. [Google Scholar] [CrossRef]
- Antonucci, P.L.; Aricò, A.S.; Cretì, P.; Ramunni, E.; Antonucci, V. Investigation of a direct methanol fuel cell based on a composite Nafion®-silica electrolyte for high temperature operation. Solid State Ion. 1999, 125, 431–437. [Google Scholar] [CrossRef]
- Vaivars, G.; Maxakato, N.W.; Mokrani, T.; Petrik, L.; Klavins, J.; Gericke, G.; Linkov, V. Zirconium Phosphate Based Inorganic Direct Methanol Fuel Cell. Mater. Sci. 2004, 10, 2. [Google Scholar]
- Rao, A.S.; Rashmi, K.; Manjunatha, D.; Jayarama, A.; Shastrimath, V.V.D.; Pinto, R. Methanol crossover reduction and power enhancement of methanol fuel cells with polyvinyl alcohol coated Nafion membranes. Mater. Today Proc. 2021, 35, 344–351. [Google Scholar] [CrossRef]
- Adamski, M.; Peressin, N.; Holdcroft, S. On the evolution of sulfonated polyphenylenes as proton exchange membranes for fuel cells. Mater. Adv. 2021, 2, 4966–5005. [Google Scholar] [CrossRef]
- Klose, C.; Saatkamp, T.; Münchinger, A.; Bohn, L.; Titvinidze, G.; Breitwieser, M.; Kreuer, K.; Vierrath, S. All-Hydrocarbon MEA for PEM Water Electrolysis Combining Low Hydrogen Crossover and High Efficiency. Adv. Energy Mater. 2020, 10, 1903995. [Google Scholar] [CrossRef]
- Burton, N.; Padilla, R.; Rose, A.; Habibullah, H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 2021, 135, 110255. [Google Scholar] [CrossRef]
- Masel, R.I.; Liu, Z.; Yang, H.; Kaczur, J.J.; Carrillo, D.; Ren, S.; Salvatore, D.; Berlinguette, C.P. An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat. Nanotechnol. 2021, 16, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Besha, A.T.; Tsehaye, M.T.; Aili, D.; Zhang, W.; Tufa, R.A. Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis. Membranes 2020, 10, 7. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Golubenko, D.; Shevlyakova, N.; D’yakova, M.; Tverskoi, V.; Dammak, L.; Grande, D.; Yaroslavtsev, A. New cation-exchange membranes based on cross-linked sulfonated polystyrene and polyethylene for power generation systems. J. Membr. Sci. 2016, 515, 196–203. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, A.; Garg, S.; Moss, A.B.; Chorkendorff, I.; Bligaard, T.; Seger, B. Enriching Surface-Accessible CO2 in the Zero-Gap Anion-Exchange-Membrane-Based CO2 Electrolyzer. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214383. [Google Scholar] [CrossRef]
- De Mot, B.; Hereijgers, J.; Daems, N.; Breugelmans, T. Insight in the behavior of bipolar membrane equipped carbon dioxide electrolyzers at low electrolyte flowrates. Chem. Eng. J. 2022, 428, 131170. [Google Scholar] [CrossRef]
- Duarte, M.; De Mot, B.; Hereijgers, J.; Breugelmans, T. Electrochemical Reduction of CO2: Effect of Convective CO2 Supply in Gas Diffusion Electrodes. ChemElectroChem 2019, 6, 5596–5602. [Google Scholar] [CrossRef]
- Sen, S.; Brown, S.M.; Leonard, M.; Brushett, F.R. Electroreduction of carbon dioxide to formate at high current densities using tin and tin oxide gas diffusion electrodes. J. Appl. Electrochem. 2019, 49, 917–928. [Google Scholar] [CrossRef]
- Endrődi, B.; Kecsenovity, E.; Samu, A.; Halmágyi, T.; Rojas-Carbonell, S.; Wang, L.; Yan, Y.; Janáky, C. High carbonate ion conductance of a robust PiperION membrane allows industrial current density and conversion in a zero-gap carbon dioxide electrolyzer cell. Energy Environ. Sci. 2020, 13, 4098–4105. [Google Scholar] [CrossRef]
- Gabardo, C.M.; O’Brien, C.P.; Edwards, J.P.; McCallum, C.; Xu, Y.; Dinh, C.-T.; Li, J.; Sargent, E.H.; Sinton, D. Continuous Carbon Dioxide Electroreduction to Concentrated Multi-carbon Products Using a Membrane Electrode Assembly. Joule 2019, 3, 2777–2791. [Google Scholar] [CrossRef]
- JKaczur, J.J.; Yang, H.; Liu, Z.; Sajjad, S.D.; Masel, R.I. Carbon Dioxide and Water Electrolysis Using New Alkaline Stable Anion Membranes. Front. Chem. 2018, 6, 263. [Google Scholar] [CrossRef]
- Chen, K.; Cao, M.; Lin, Y.; Fu, J.; Liao, H.; Zhou, Y.; Li, H.; Qiu, X.; Hu, J.; Zheng, X.; et al. Ligand Engineering in Nickel Phthalocyanine to Boost the Electrocatalytic Reduction of CO2. Adv. Funct. Mater. 2022, 32, 2111322. [Google Scholar] [CrossRef]
- Ye, K.; Zhang, G.; Ma, X.-Y.; Deng, C.; Huang, X.; Yuan, C.; Meng, G.; Cai, W.-B.; Jiang, K. Resolving local reaction environment toward an optimized CO2-to-CO conversion performance. Energy Environ. Sci. 2022, 15, 749–759. [Google Scholar] [CrossRef]
- Lees, E.W.; Goldman, M.; Fink, A.G.; Dvorak, D.J.; Salvatore, D.A.; Zhang, Z.; Loo, N.W.X.; Berlinguette, C.P. Electrodes Designed for Converting Bicarbonate into CO. ACS Energy Lett. 2020, 5, 2165–2173. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, R.; Zhu, X.; Han, N.; Song, B.; Liu, T.; Hu, G.; Li, Y.; Lu, J.; Li, Y. Mesoporous PdAg Nanospheres for Stable Electrochemical CO2 Reduction to Formate. Adv. Mater. 2020, 32, 2000992. [Google Scholar] [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H.A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, L.; Merinov, B.V.; Goddard, W.A. Explanation of Dramatic pH-Dependence of Hydrogen Binding on Noble Metal Electrode: Greatly Weakened Water Adsorption at High pH. J. Am. Chem. Soc. 2018, 140, 7787–7790. [Google Scholar] [CrossRef] [PubMed]
- YHori, Y.; Takahashi, R.; Yoshinami, Y.; Murata, A. Electrochemical Reduction of CO at a Copper Electrode. J. Phys. Chem. B 1997, 101, 7075–7081. [Google Scholar] [CrossRef]
- Liu, X.; Schlexer, P.; Xiao, J.; Ji, Y.; Wang, L.; Sandberg, R.B.; Tang, M.; Brown, K.S.; Peng, H.; Ringe, S.; et al. pH effects on the electrochemical reduction of CO(2) towards C2 products on stepped copper. Nat. Commun. 2019, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Bui, J.C.; Luo, X.; Cooper, J.K.; Kusoglu, A.; Weber, A.Z.; Bell, A.T. Tailored catalyst microenvironments for CO2 electroreduction to multicarbon products on copper using bilayer ionomer coatings. Nat. Energy 2021, 6, 1026–1034. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- Ma, M.; Clark, E.L.; Therkildsen, K.T.; Dalsgaard, S.; Chorkendorff, I.; Seger, B. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 2020, 13, 977–985. [Google Scholar] [CrossRef]
- Unlu, M.; Zhou, J.; Kohl, P.A. Anion Exchange Membrane Fuel Cells: Experimental Comparison of Hydroxide and Carbonate Conductive Ions. Solid-State Lett. 2009, 12, B27–B30. [Google Scholar] [CrossRef]
- Pătru, A.; Binninger, T.; Pribyl, B.; Schmidt, T.J. Design Principles of Bipolar Electrochemical Co-Electrolysis Cells for Efficient Reduction of Carbon Dioxide from Gas Phase at Low Temperature. J. Electrochem. Soc. 2019, 166, F34–F43. [Google Scholar] [CrossRef]
- Li, H.; Oloman, C. Development of a continuous reactor for the electro-reduction of carbon dioxide to formate—Part 2: Scale-up. J. Appl. Electrochem. 2007, 37, 1107–1117. [Google Scholar] [CrossRef]
- Yang, H.; Kaczur, J.J.; Sajjad, S.D.; Masel, R.I. CO2 Conversion to Formic Acid in a Three Compartment Cell with SustainionTM Membranes. ECS Trans. 2017, 77, 1425–1431. [Google Scholar] [CrossRef]
- Laconti, A.; Liu, H.; Mittelsteadt, C.; McDonald, R. Polymer Electrolyte Membrane Degradation Mechanisms in Fuel Cells—Findings Over the Past 30 Years and Comparison with Electrolyzers. ECS Trans. 2006, 1, 199–219. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Oener, S.Z.; Foster, M.J.; Boettcher, S.W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 2020, 369, 1099–1103. [Google Scholar] [CrossRef]
- Hohenadel, A.; Powers, D.; Wycisk, R.; Adamski, M.; Pintauro, P.; Holdcroft, S. Electrochemical Characterization of Hydrocarbon Bipolar Membranes with Varying Junction Morphology. ACS Appl. Energy Mater. 2019, 2, 6817–6824. [Google Scholar] [CrossRef]
- O’brien, C.P.; Miao, R.K.; Liu, S.; Xu, Y.; Lee, G.; Robb, A.; Huang, J.E.; Xie, K.; Bertens, K.; Gabardo, C.M.; et al. Single Pass CO2 Conversion Exceeding 85% in the Electrosynthesis of Multicarbon Products via Local CO2 Regeneration. ACS Energy Lett. 2021, 6, 2952–2959. [Google Scholar] [CrossRef]
- He, G.; Li, Z.; Zhao, J.; Wang, S.; Wu, H.; Guiver, M.D.; Jiang, Z. Nanostructured Ion-Exchange Membranes for Fuel Cells: Recent Advances and Perspectives. Adv. Mater. 2015, 27, 5280–5295. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, H.; Krol, J.; Rapp, H.-J.; Eigenberger, G. Limiting current density and water dissociation in bipolar membranes. J. Membr. Sci. 1997, 125, 123–142. [Google Scholar] [CrossRef]
- Li, Y.C.; Yan, Z.; Hitt, J.; Wycisk, R.; Pintauro, P.N.; Mallouk, T.E. Bipolar Membranes Inhibit Product Crossover in CO2 Electrolysis Cells. Adv. Sustain. Syst. 2018, 2, 1700187. [Google Scholar] [CrossRef]
- Pribyl-Kranewitter, B.; Beard, A.; Schuler, T.; Diklić, N.; Schmidt, T.J.; Patru, A. Investigation and Optimisation of Operating Conditions for Low-Temperature CO2 Reduction to CO in a Forward-Bias Bipolar-Membrane Electrolyser. J. Electrochem. Soc. 2021, 168, 043506. [Google Scholar] [CrossRef]
- Müller, J.; Zhegur, A.; Krewer, U.; Varcoe, J.R.; Dekel, D.R. Practical ex-Situ Technique To Measure the Chemical Stability of Anion-Exchange Membranes under Conditions Simulating the Fuel Cell Environment. ACS Mater. Lett. 2020, 2, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Kinumoto, T.; Kiriake, M.; Umebayashi, R.; Tasaka, A.; Ogumi, Z. Gas crossover and membrane degradation in polymer electrolyte fuel cells. Electrochim. Acta 2006, 51, 5746–5753. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).