Experimental Study on OC PEMFC Performance Improvement and MEA Parameter Optimization Under Water Shortage Conditions
Abstract
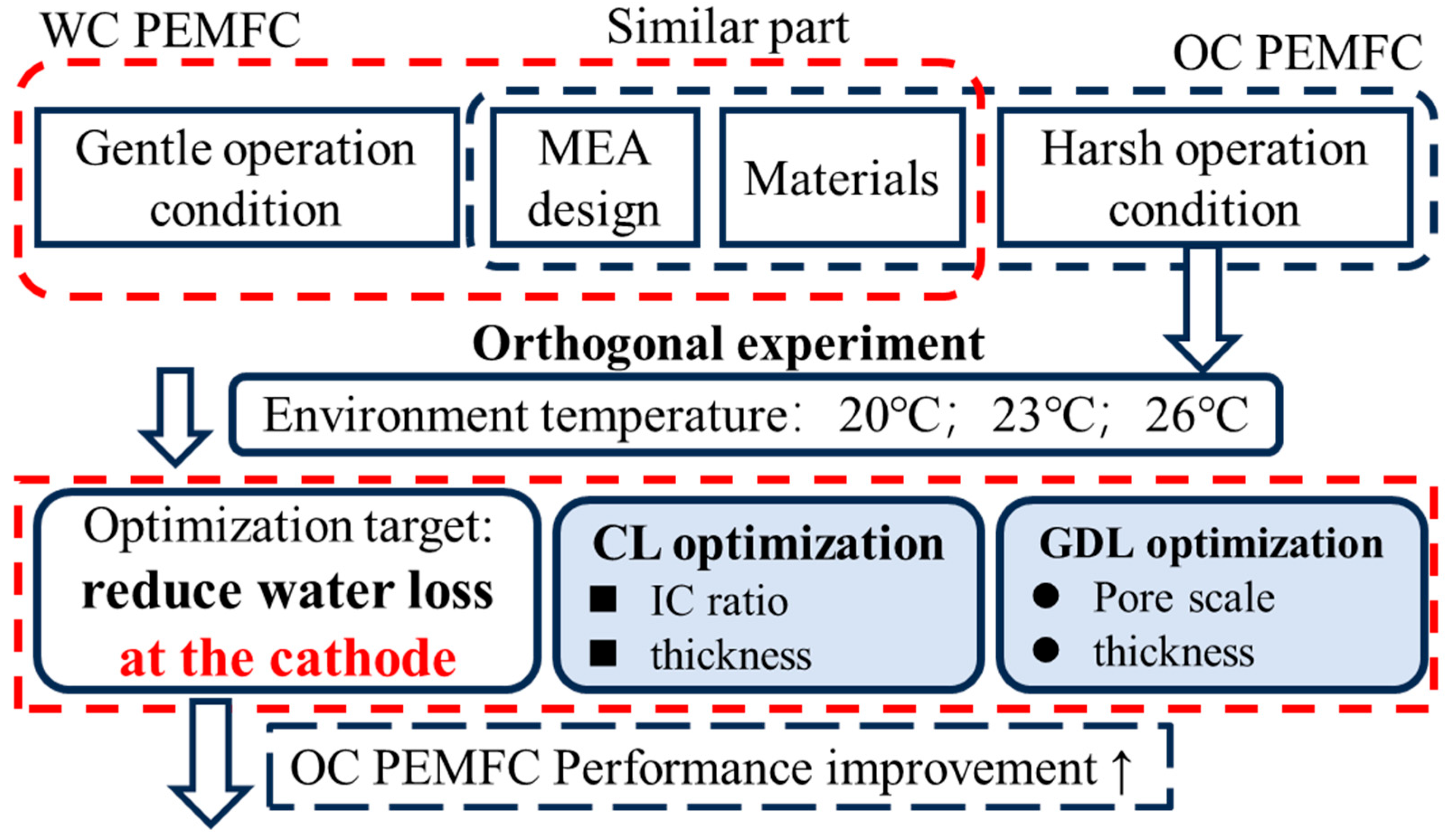
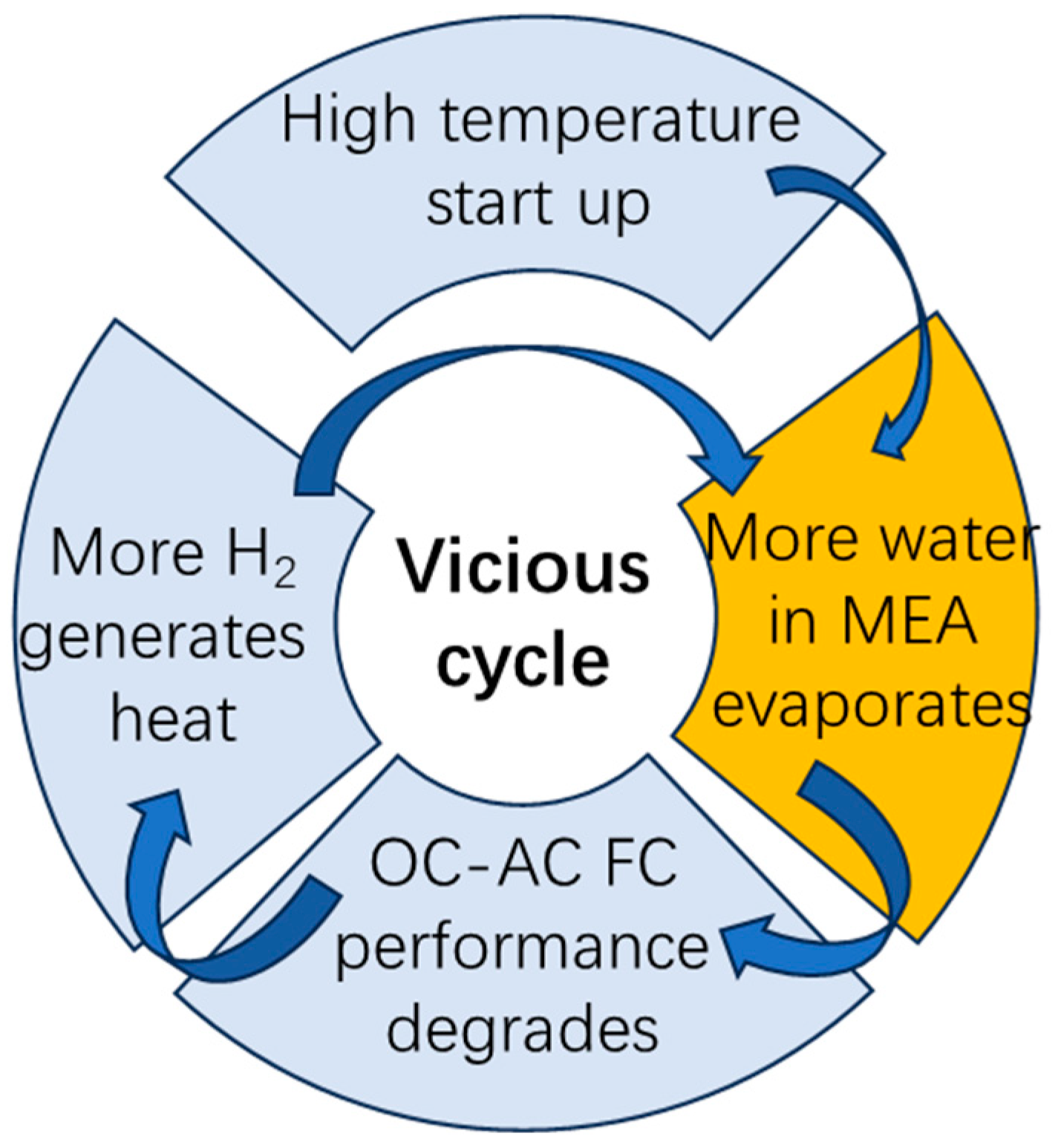
1. Introduction
2. Materials and Methods
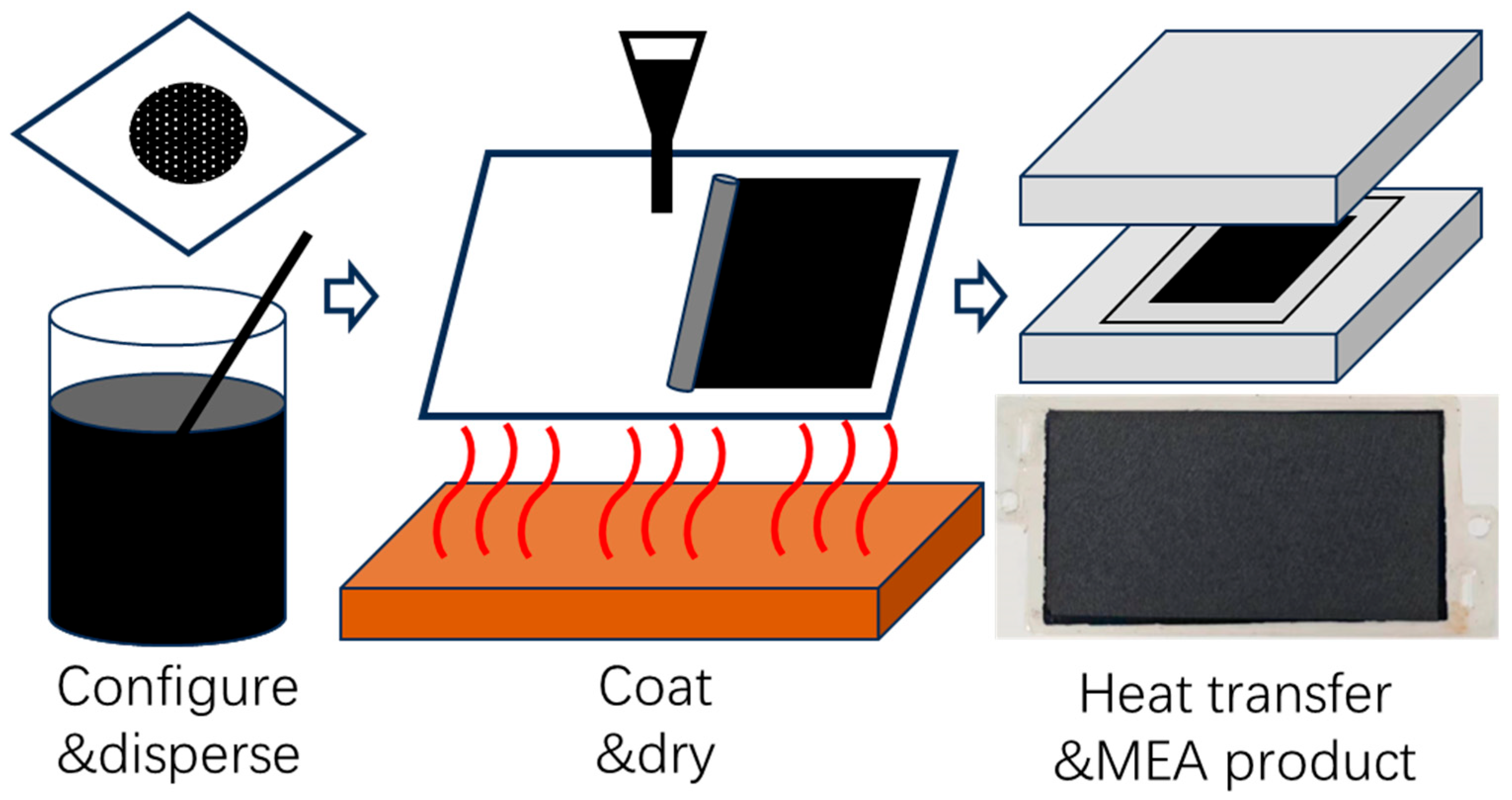
2.1. Materials and Preparation
2.2. Equipment and Procedures
3. Results
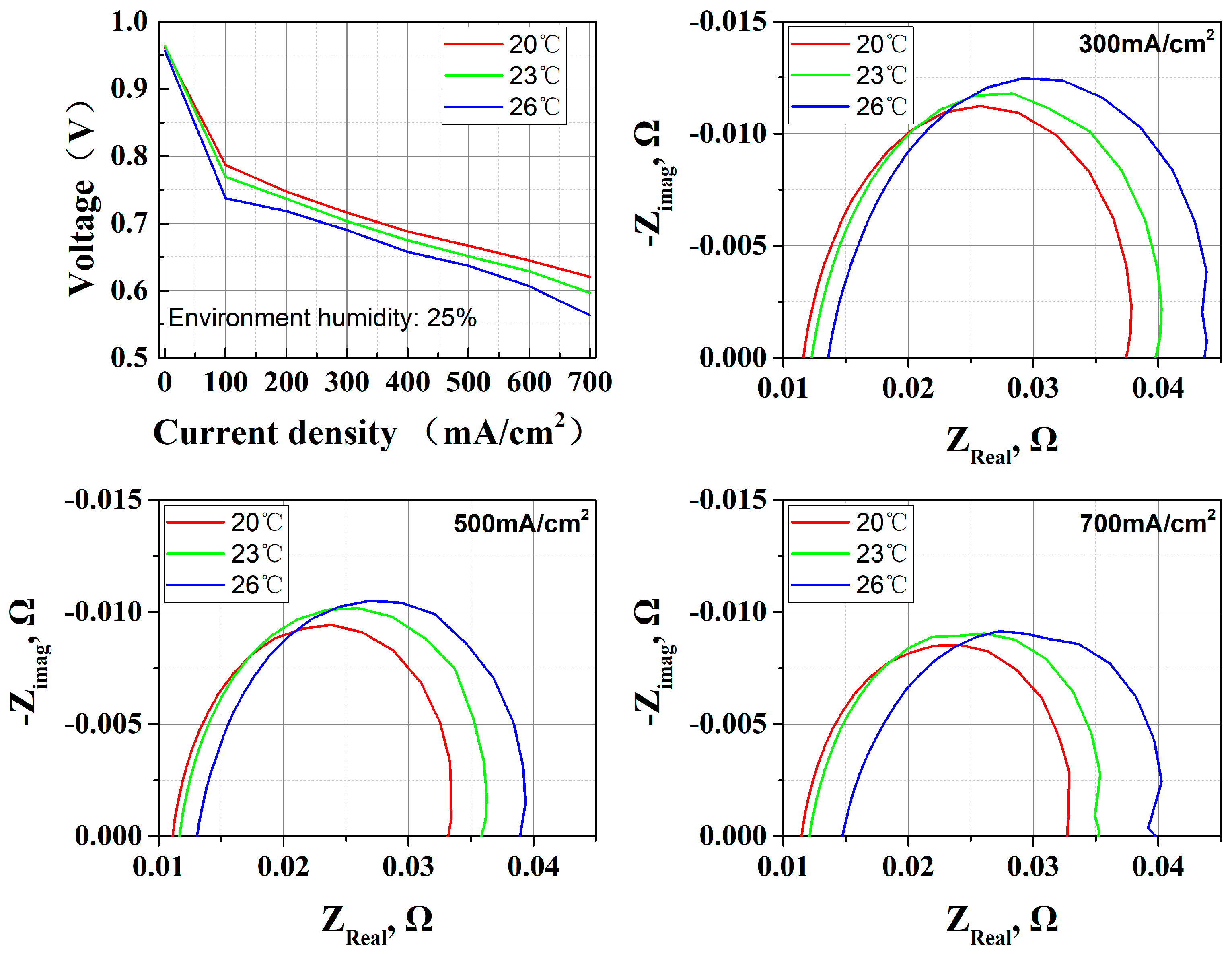
3.1. Ambient Temperature Effect
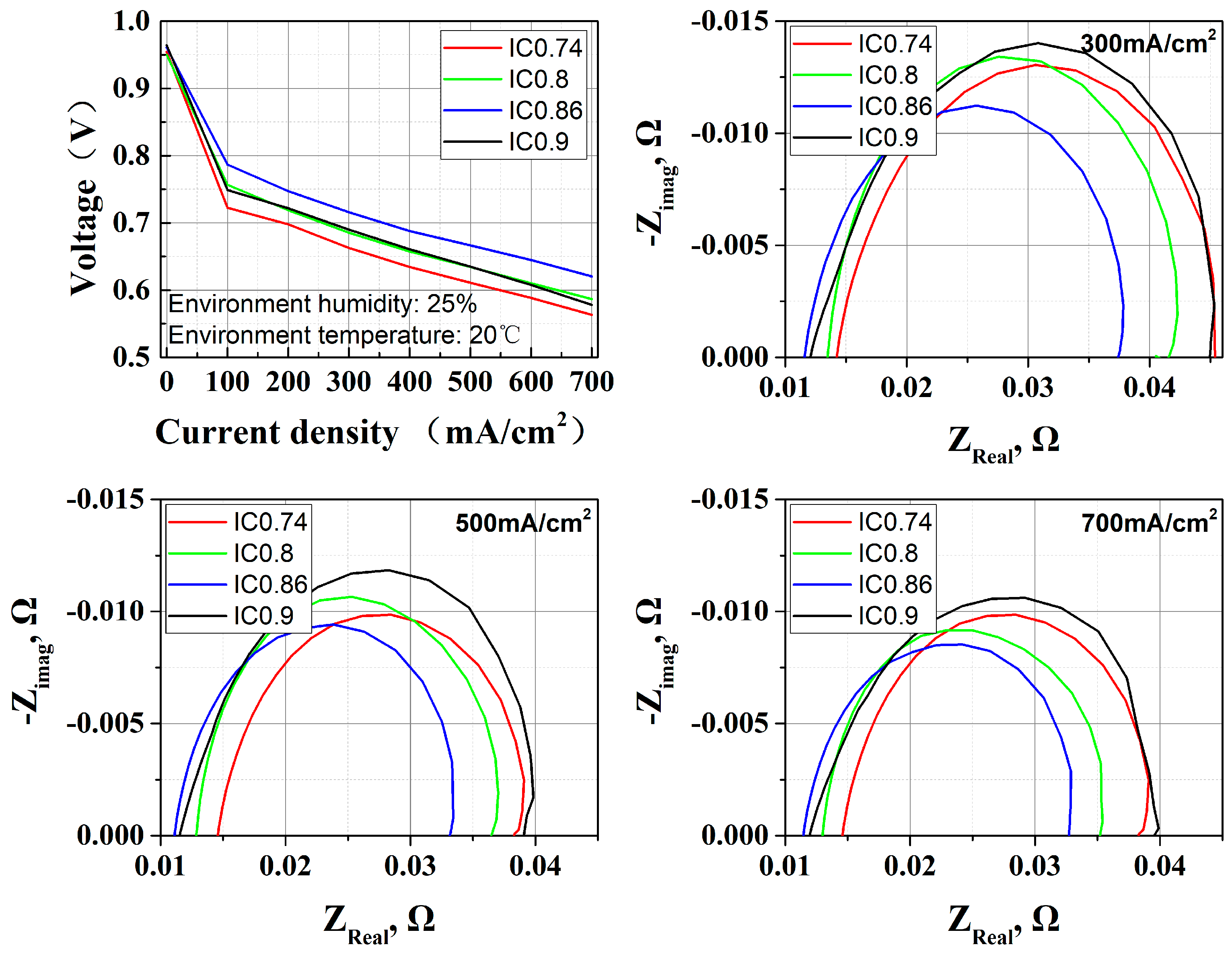
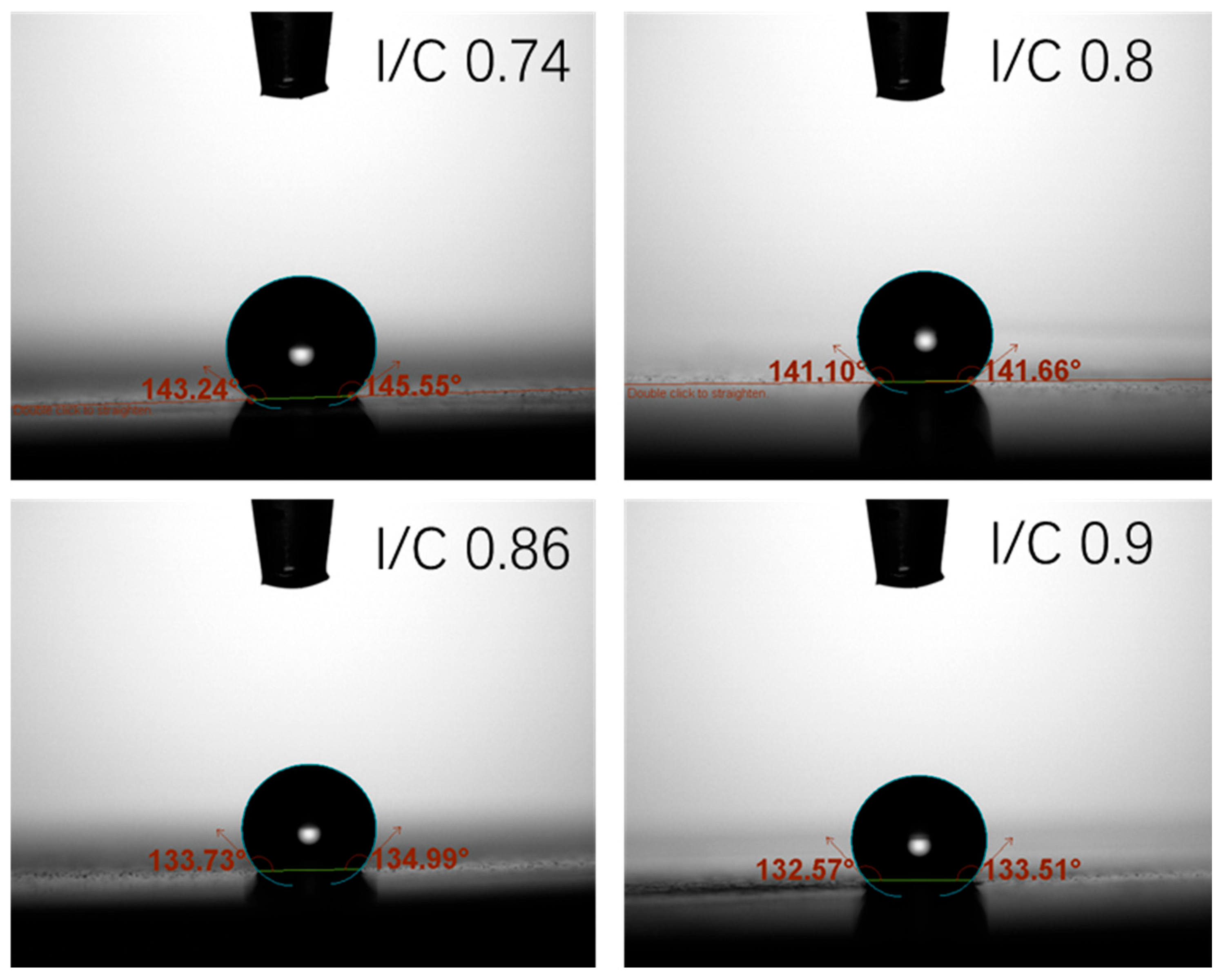
3.2. I/C Ratio Effect
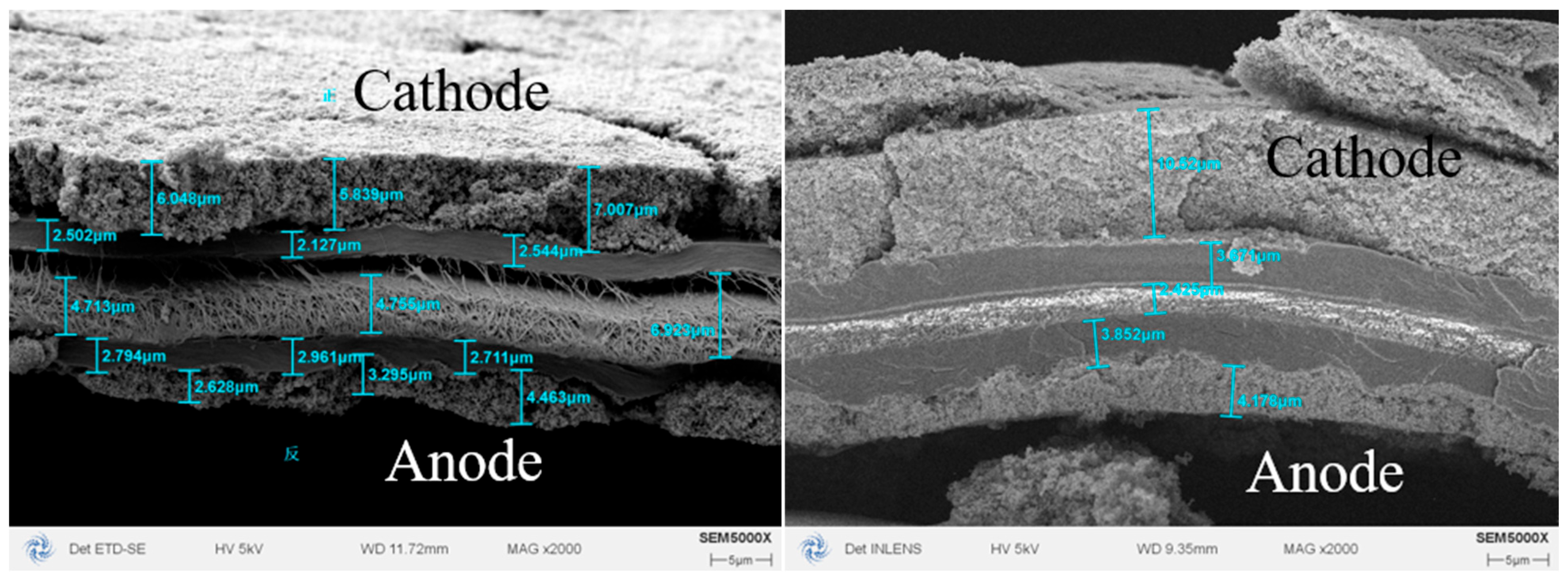
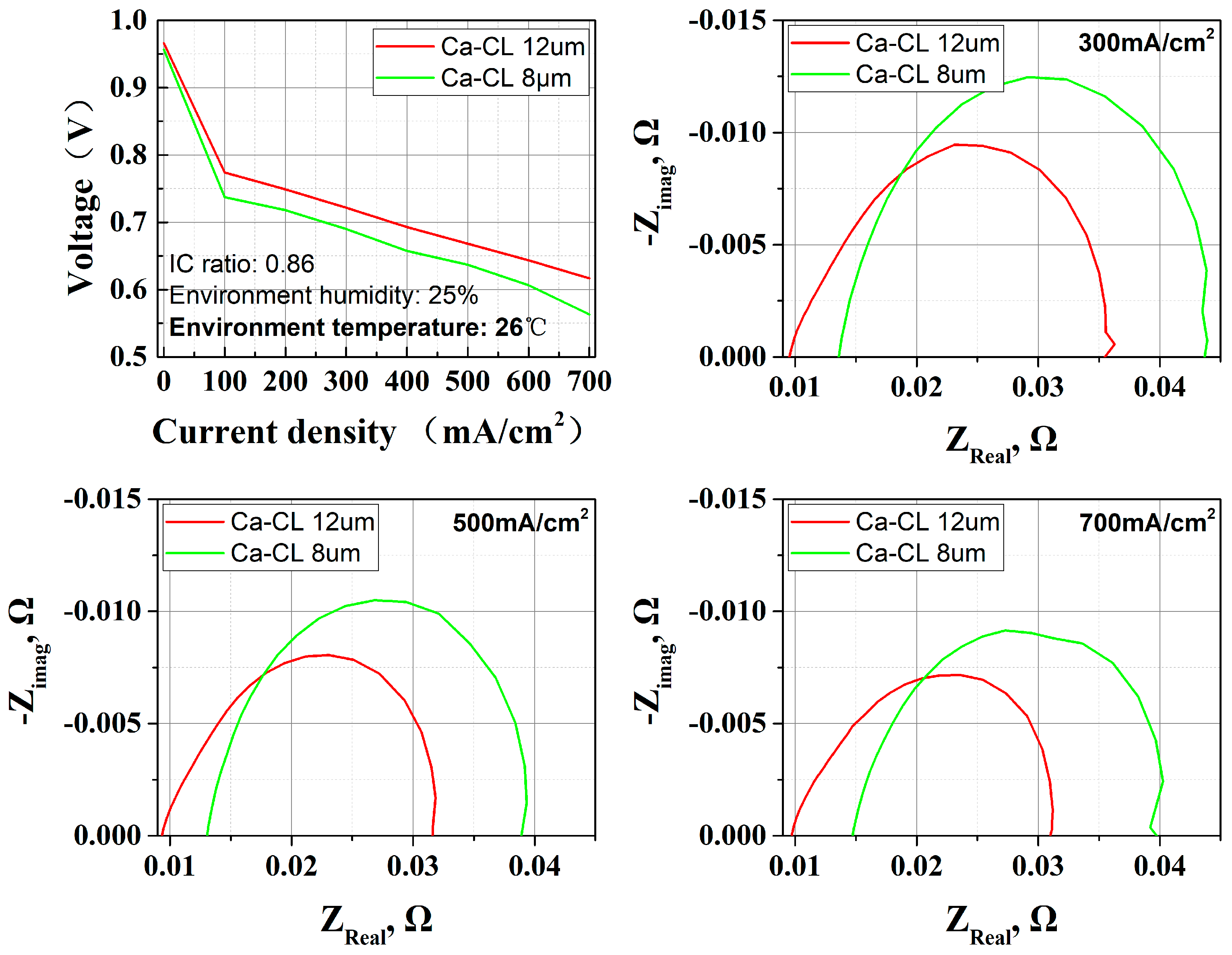
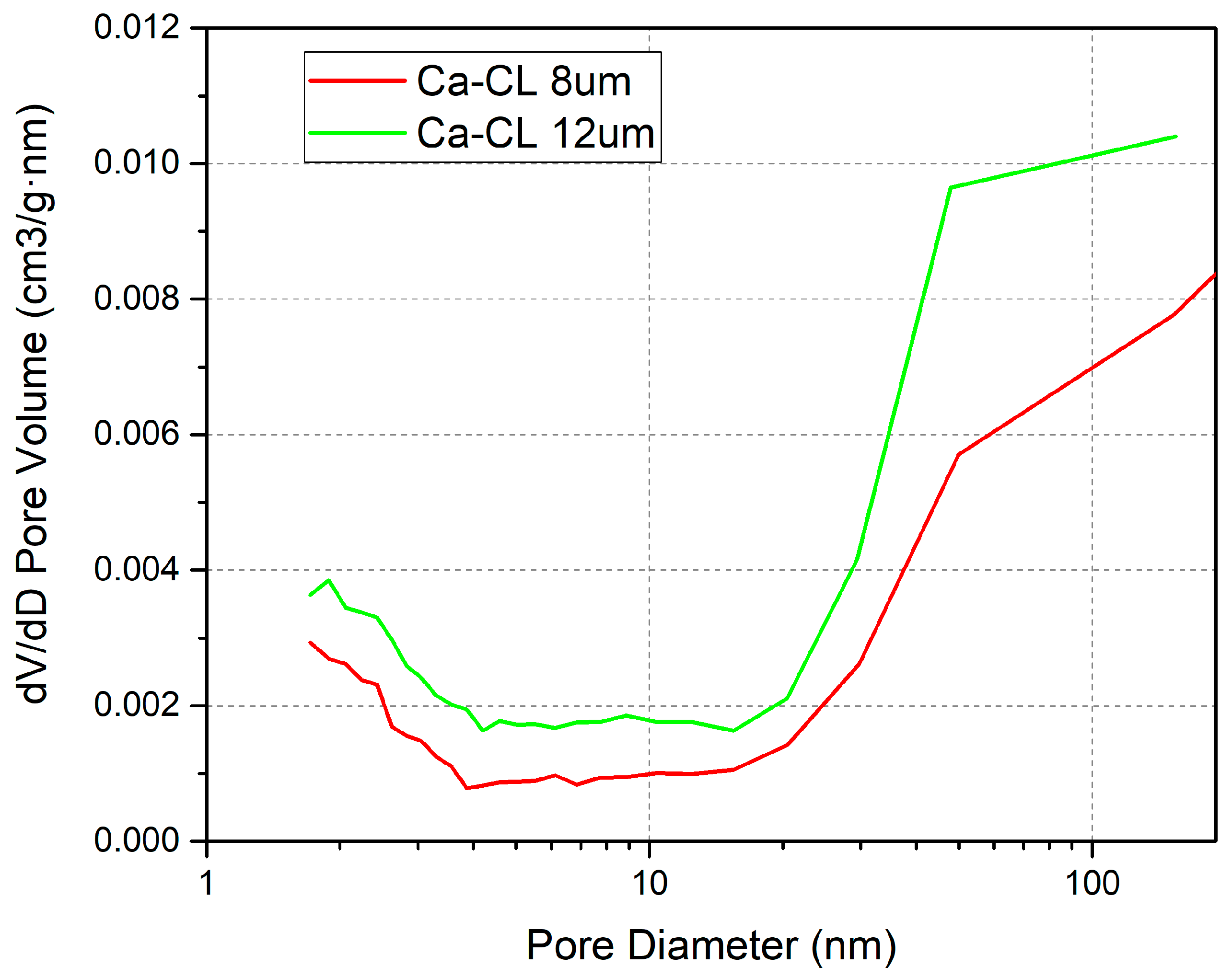
3.3. CL Pore Structure Effect
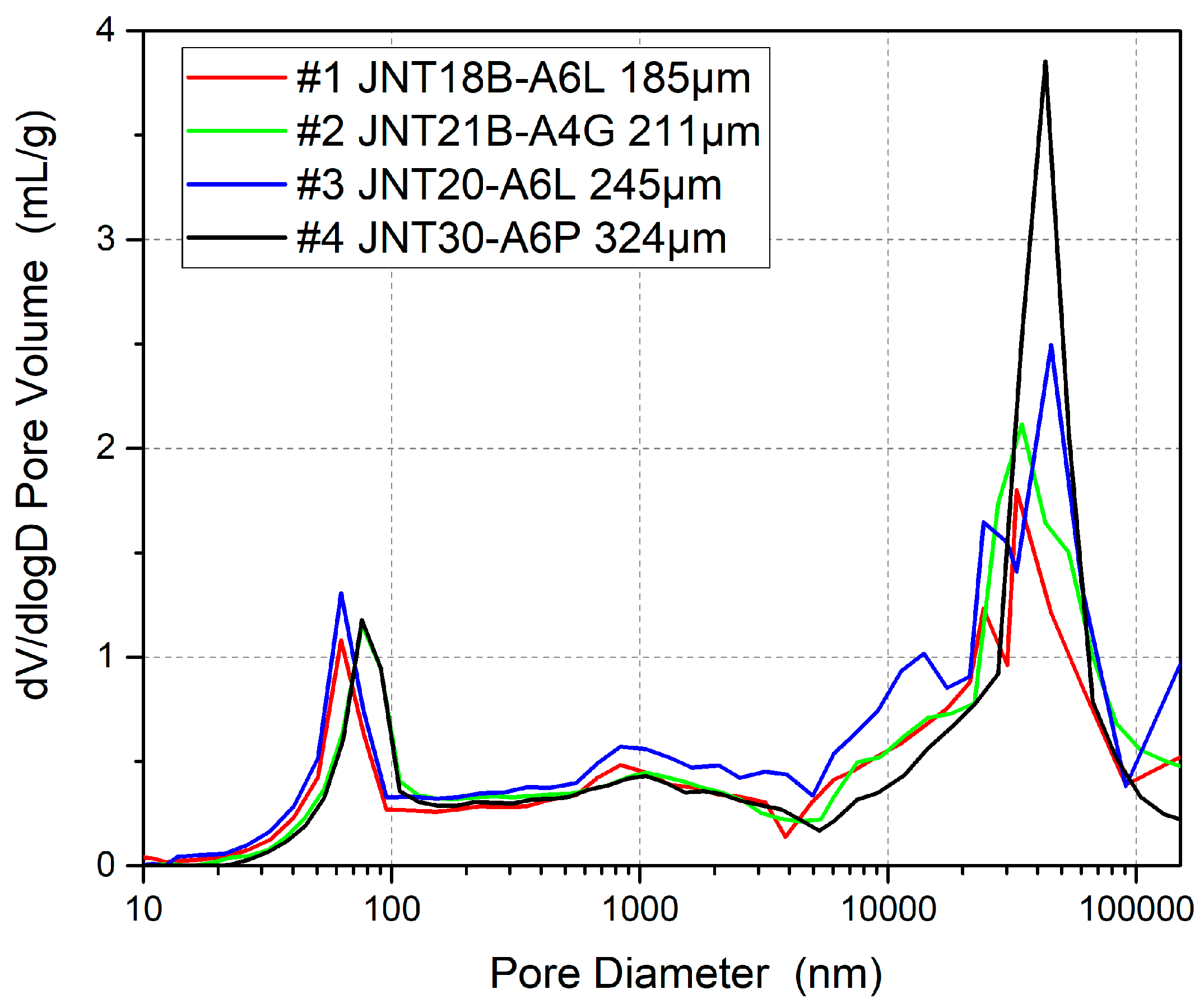
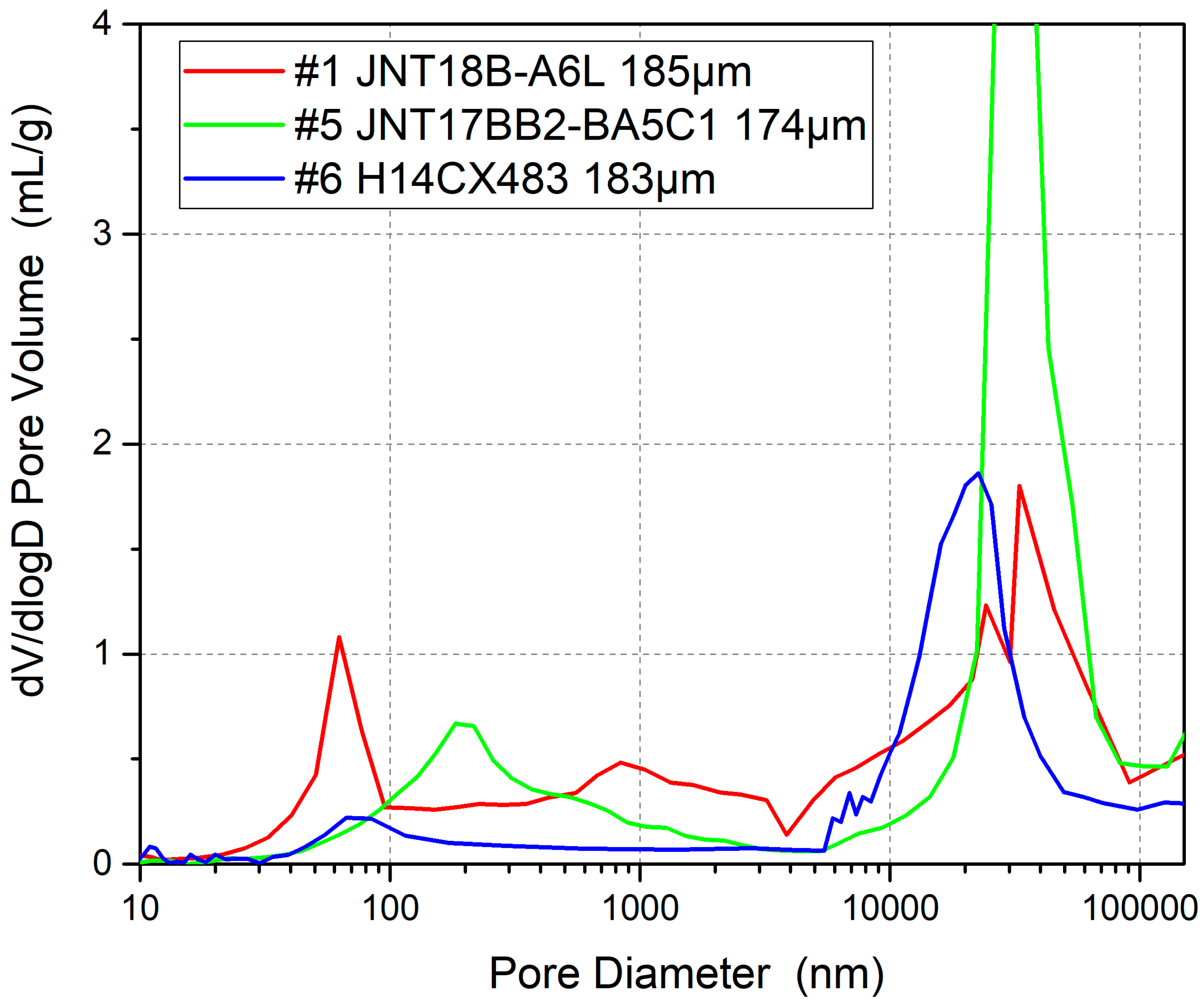
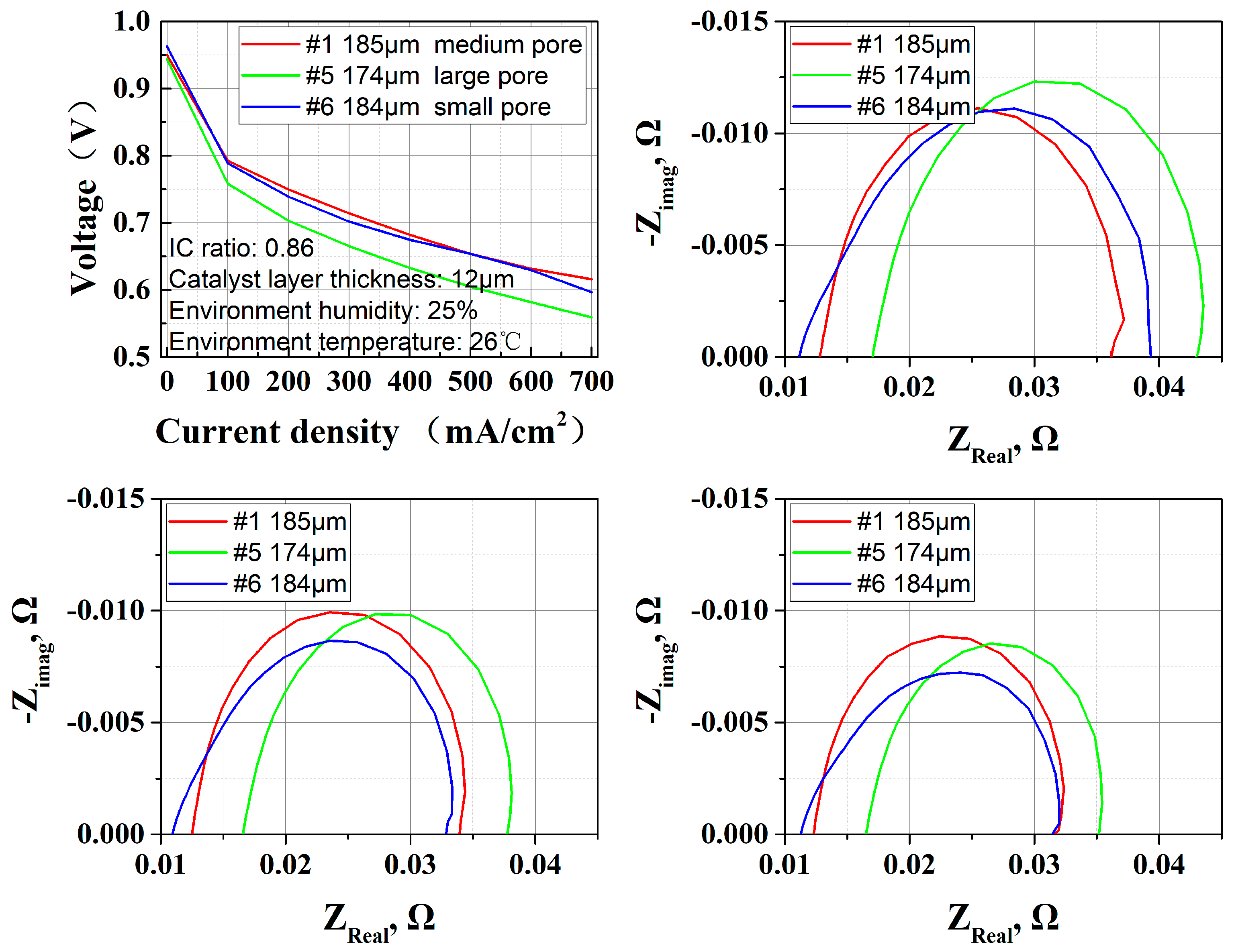
3.4. GDL Structure Effect
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| OC PEMFC | Open-cathode proton exchange membrane fuel cells |
| MEA | Membrane electrode assembly |
| CL | Catalyst layer |
| TPBs | Three-phase boundaries |
| I/C | Ionomer/carbon |
| EIS | Electrochemical impedance spectroscopy |
| GDL | Gas diffusion layer |
| BET | Brunauer–Emmett–Teller |
| MIP | Mercury intrusion porosimetry |
| MPL | Microporous layer |
References
- Deng, Z.; Li, B.; Xing, S.; Zhao, C.; Wang, H. Experimental Investigation on the Anode Flow Field Design for an Air-Cooled Open-Cathode Proton Exchange Membrane Fuel Cell. Membranes 2022, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xing, S.; Chen, M.; Liu, W.; Wang, H. Optimal design of cathode flow channel for air-cooled PEMFC with open cathode. Int. J. Hydrogen Energy 2020, 45, 17771–17781. [Google Scholar] [CrossRef]
- Yu, X.; Chang, H.; Zhao, J.; Tu, Z. Effects of anode flow channel on performance of air-cooled proton exchange membrane fuel cell. Energy Rep. 2022, 8, 4443–4452. [Google Scholar] [CrossRef]
- Zhao, C.; Xing, S.; Liu, W.; Chen, M.; Wang, H. Performance and thermal optimization of different length-width ratio for air-cooled open-cathode fuel cell. Renew. Energy 2021, 178, 1250–1260. [Google Scholar] [CrossRef]
- Xing, S.; Zhao, C.; Zou, J.; Zaman, S.; Yu, Y.; Gong, H.; Wang, Y.; Chen, M.; Wang, M.; Lin, M.; et al. Recent advances in heat and water management of forced-convection open-cathode proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2022, 165, 112558. [Google Scholar] [CrossRef]
- Zhao, C.; Xing, S.; Liu, W.; Chen, M.; Wang, Y.; Wang, H. An experimental study on pressure distribution and performance of end-plate with different optimization parameters for air-cooled open-cathode LT-PEMFC. Int. J. Hydrogen Energy 2020, 45, 17902–17915. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Tang, J.; Lai, X. Numerical analysis of air-cooled proton exchange membrane fuel cells with various cathode flow channels. Energy 2020, 198, 117334. [Google Scholar] [CrossRef]
- Omrani, R.; Seif Mohammadi, S.; Mafinejad, Y.; Paul, B.; Islam, R.; Shabani, B. PEMFC purging at low operating temperatures: An experimental approach. Int. J. Energy Res. 2019, 43, 7496–7507. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, C.; Li, M.; Chen, B.; Fan, M.; Sheng, X.; Ma, F. Experimental investigation of self-regulating capability of open-cathode PEMFC under different fan working conditions. Int. J. Hydrogen Energy 2023, 48, 26599–26608. [Google Scholar] [CrossRef]
- Liu, W.; Wan, L.; Liu, J.; Zhao, M.; Zou, Z. Performance improvement of the open-cathode proton exchange membrane fuel cell by optimizing membrane electrode assemblies. Int. J. Hydrogen Energy 2015, 40, 7159–7167. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 2011, 37, 221–291. [Google Scholar] [CrossRef]
- Tongsh, C.; Wu, S.; Jiao, K.; Huo, W.; Du, Q.; Park, J.W.; Xuan, J.; Wang, H.; Brandon, N.P.; Guiver, M.D. Fuel cell stack redesign and component integration radically increase power density. Joule 2024, 8, 175–192. [Google Scholar] [CrossRef]
- Zhao, C.; Xing, S.; Liu, W.; Chen, M.; Wang, H. Performance improvement for air-cooled open-cathode proton exchange membrane fuel cell with different design parameters of the gas diffusion layer. Prog. Nat. Sci. Mater. Int. 2020, 30, 825–831. [Google Scholar] [CrossRef]
- Sagar, A.; Chugh, S.; Kjeang, E. Experimental Design of High-Performing Open-Cathode Polymer Electrolyte Membrane Fuel Cells. ECS Adv. 2024, 3, 014504. [Google Scholar] [CrossRef]
- Yakovlev, Y.V.; Lobko, Y.V.; Vorokhta, M.; Nováková, J.; Mazur, M.; Matolínová, I.; Matolín, V. Ionomer content effect on charge and gas transport in the cathode catalyst layer of proton-exchange membrane fuel cells. J. Power Sources 2021, 490, 229531. [Google Scholar] [CrossRef]
- Ono, Y.; Ohma, A.; Shinohara, K.; Fushinobu, K. Influence of Equivalent Weight of Ionomer on Local Oxygen Transport Resistance in Cathode Catalyst Layers. J. Electrochem. Soc. 2013, 160, F779–F787. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, B.-S.; Park, J.-S. Effect of ionomer dispersions on the performance of catalyst layers in proton exchange membrane fuel cells. Electrochim. Acta 2022, 424, 140680. [Google Scholar] [CrossRef]
- Guan, S.; Zhou, F.; Tan, J.; Pan, M. Influence of pore size optimization in catalyst layer on the mechanism of oxygen transport resistance in PEMFCs. Prog. Nat. Sci. Mater. Int. 2020, 30, 839–845. [Google Scholar] [CrossRef]
- Zhu, D.; Yang, Y.; Pei, F.; Ma, T. High-precision identification of polarization processes of distribution of relaxation times by polarization curve model for proton exchange membrane fuel cell. Energy Convers. Manag. 2022, 268, 115994. [Google Scholar] [CrossRef]
- Miao, T.; Tongsh, C.; Wang, J.; Cheng, P.; Liang, J.; Wang, Z.; Chen, W.; Zhang, C.; Xi, F.; Du, Q.; et al. Current density and temperature distribution measurement and homogeneity analysis for a large-area proton exchange membrane fuel cell. Energy 2022, 239, 121922. [Google Scholar] [CrossRef]


| Materials | Type | Parameter |
|---|---|---|
| Catalyst | Umicore 550 | platinum content 50% |
| Solvent | Deionized water, isopropanol, ethanol | purity ≥ 99.7% |
| Resin | D79 | 25% resin, 75% water |
| GDL | #1-JNT18 | 185 μm |
| #2-JNT21 | 211 μm | |
| #3-JNT20 | 245 μm | |
| #4-JNT30 | 324 μm | |
| #5-JNT17 | 174 μm | |
| #6-H14CX483 | 183 μm | |
| PEM | DONGYUE | 10 μm |
| Gasket | 45 μm |
| Item | Type | Parameter |
|---|---|---|
| Reaction area | 50 cm2 | |
| Fan | FFB0412SHN | 12 V, 0.6 A |
| Number of cells | 3 |
| Factor | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|
| Environmental temperature | 20 | 23 | 26 | / |
| I/C ratio | 0.74 | 0.8 | 0.86 | 0.9 |
| Catalyst thickness | 8 | 12 | / | / |
| GDL thickness | 185 | 211 | 245 | 324 |
| GDL pore structure | Small | Medium | Large | / |
| Number | Type | GDL Thickness | Carbon Paper Thickness | MPL Thickness | Pore Structure | Manufacturer |
|---|---|---|---|---|---|---|
| #1 | JNT18B-A6L | 185 μm | 142 μm | 43 μm | Medium | JNTG, Hwaseong-si, Republic of Korea |
| #2 | JNT21B-A4G | 211 μm | 165 μm | 46 μm | ||
| #3 | JNT20-A6L | 245 μm | 202 μm | 43 μm | ||
| #4 | JNT30-A6P | 324 μm | 280 μm | 44 μm | ||
| #5 | JNT17BB2-BA5C1 | 174 μm | 143 μm | 31 μm | Large | |
| #6 | H14CX483 | 183 μm | 135 μm | 48 μm | Small | Freudenberg, Weinheim, Germany |
| Factor | Best | Factor Mechanism |
|---|---|---|
| Environmental temperature | 20 °C | Relatively low temperature reduces water gasification loss |
| I/C ratio | 0.86 | Medium I/C can improve proton conductivity while ensuring effective utilization of platinum |
| Catalyst thickness | 12 μm | Increase the pathway for water loss in the catalyst layer |
| GDL thickness | Insensitive | The thickness has little effect on the water loss under the GDL scale |
| GDL pore structure | NOT large | The relatively small pore structures help with water retention |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Tang, D.; Liao, T.; Zhang, X.; Cheng, F.; Gao, L. Experimental Study on OC PEMFC Performance Improvement and MEA Parameter Optimization Under Water Shortage Conditions. Membranes 2025, 15, 356. https://doi.org/10.3390/membranes15120356
Wang J, Tang D, Liao T, Zhang X, Cheng F, Gao L. Experimental Study on OC PEMFC Performance Improvement and MEA Parameter Optimization Under Water Shortage Conditions. Membranes. 2025; 15(12):356. https://doi.org/10.3390/membranes15120356
Chicago/Turabian StyleWang, Jianan, Di Tang, Tianshu Liao, Xiangqian Zhang, Feng Cheng, and Lingfeng Gao. 2025. "Experimental Study on OC PEMFC Performance Improvement and MEA Parameter Optimization Under Water Shortage Conditions" Membranes 15, no. 12: 356. https://doi.org/10.3390/membranes15120356
APA StyleWang, J., Tang, D., Liao, T., Zhang, X., Cheng, F., & Gao, L. (2025). Experimental Study on OC PEMFC Performance Improvement and MEA Parameter Optimization Under Water Shortage Conditions. Membranes, 15(12), 356. https://doi.org/10.3390/membranes15120356





