A Comparative Study of the Effects of Cholesterol and Lanosterol on Hydrated Phosphatidylethanolamine Assemblies: Focusing on Physical Parameters Related to Membrane Fusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Hydrated Samples
2.3. Volumetric Measurements
2.4. X-Ray Diffraction Measurements
2.5. Reconstruction of the Electronic Density Profiles
3. Results
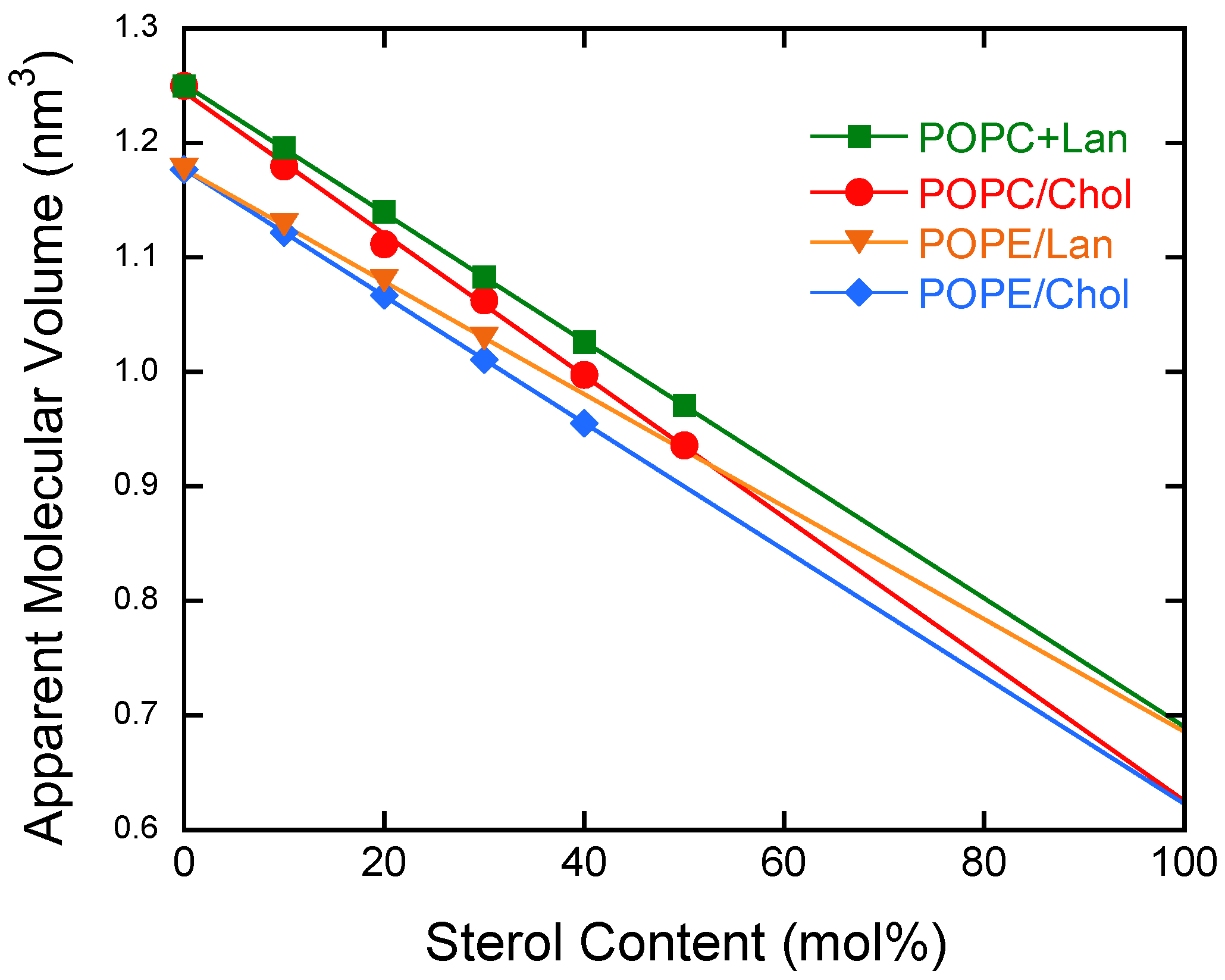
3.1. Molecular Volumes
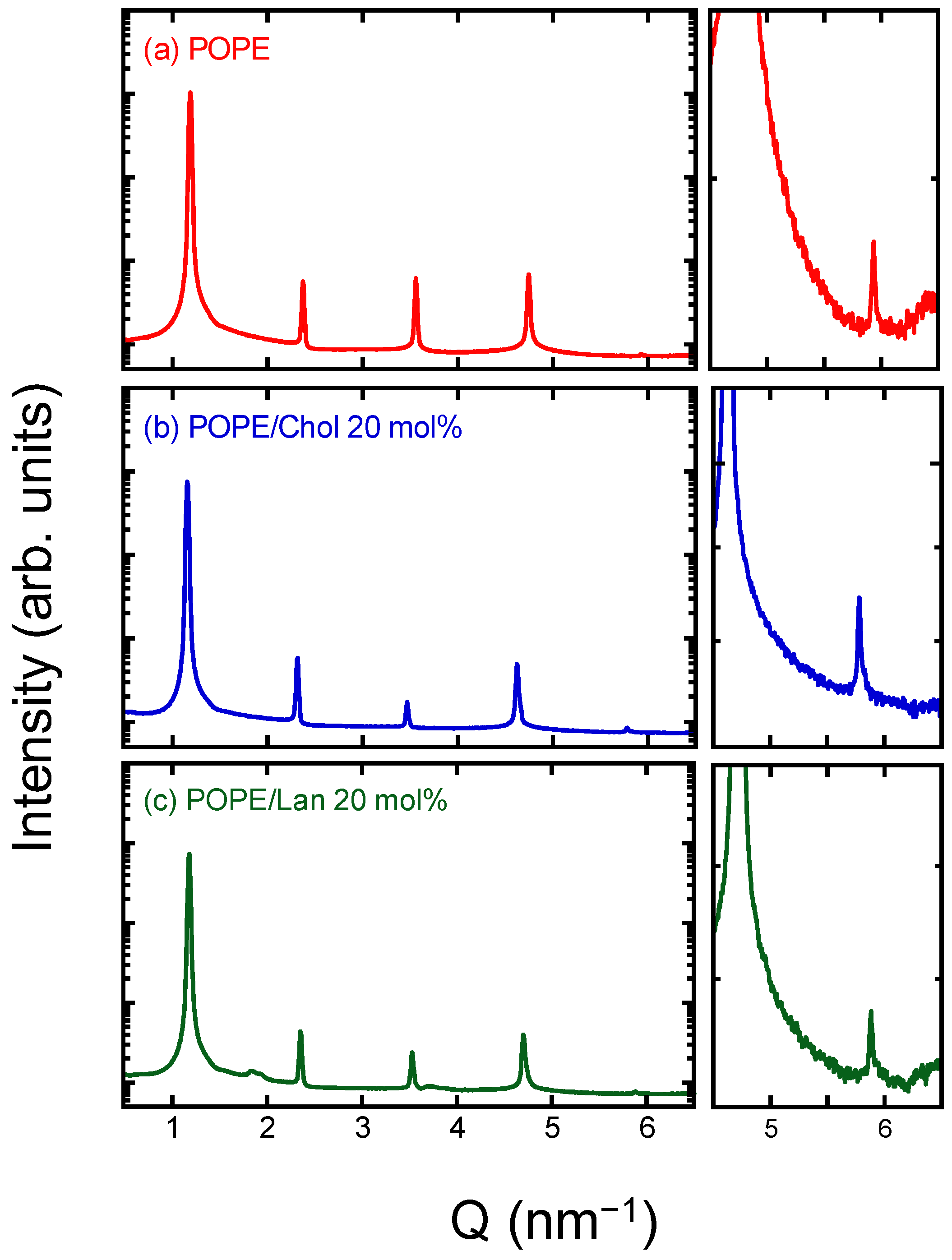
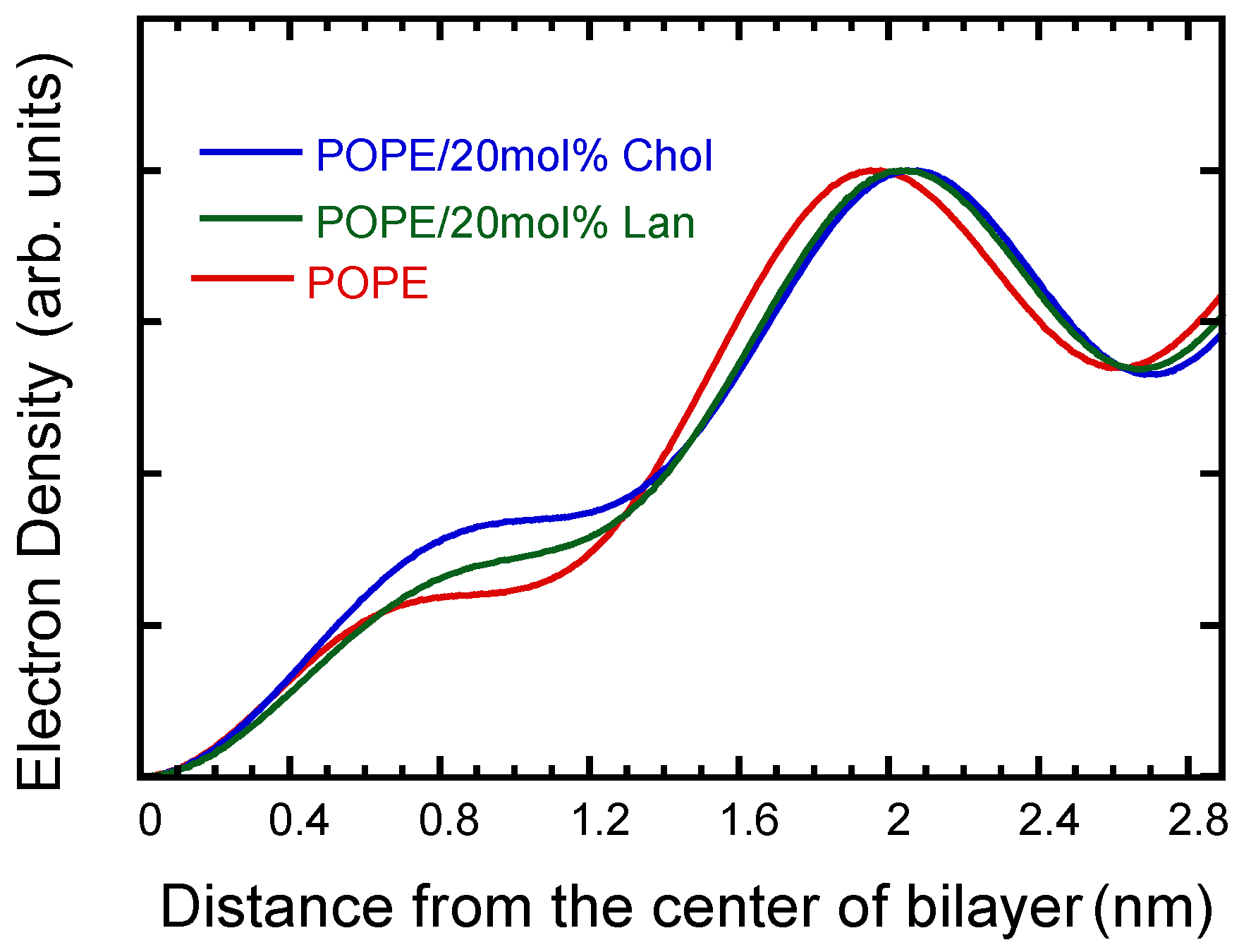
3.2. POPE/Sterol System: Lamellar Structure, Thickness, and Molecular Area
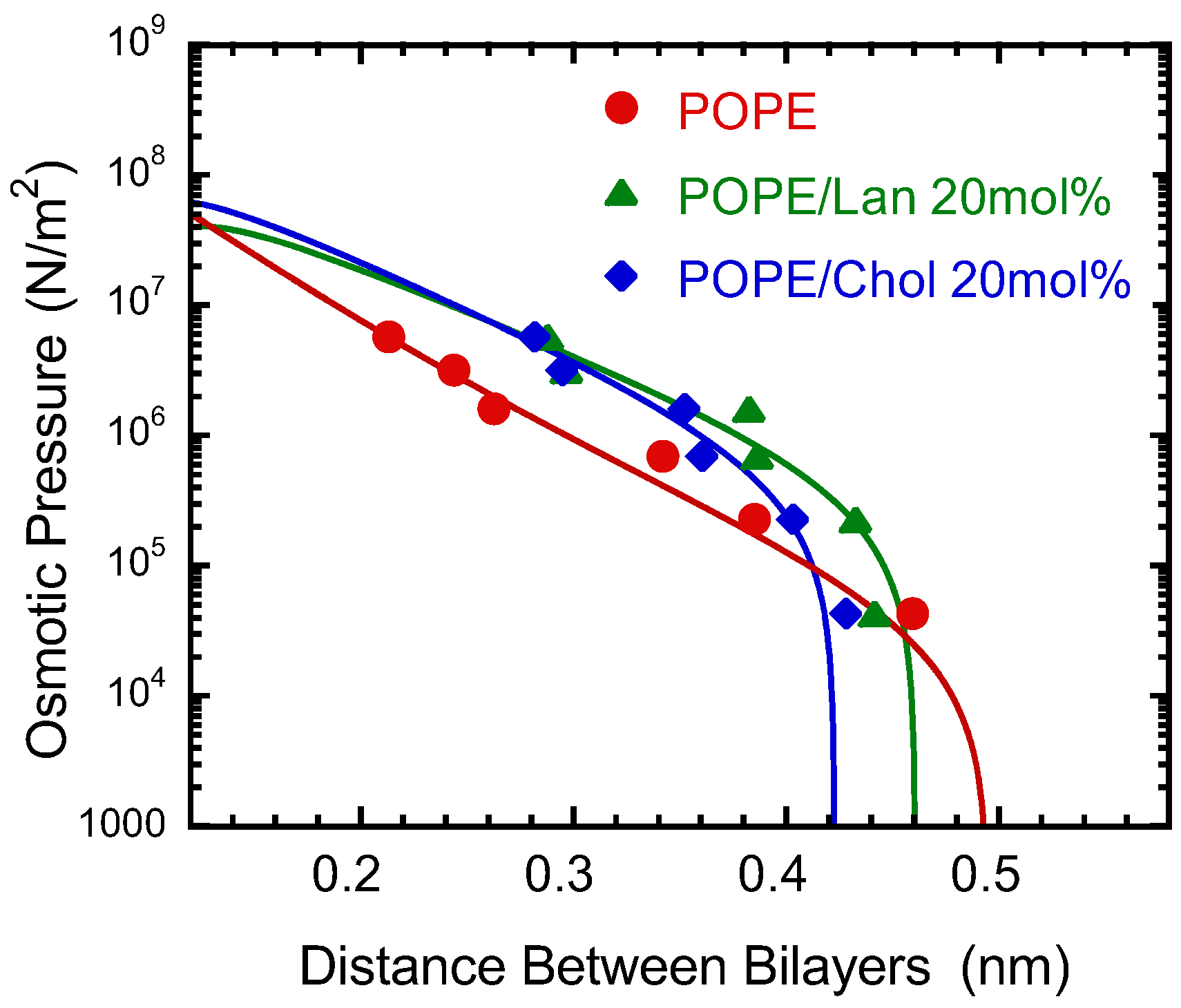
3.3. Interbilayer Interaction
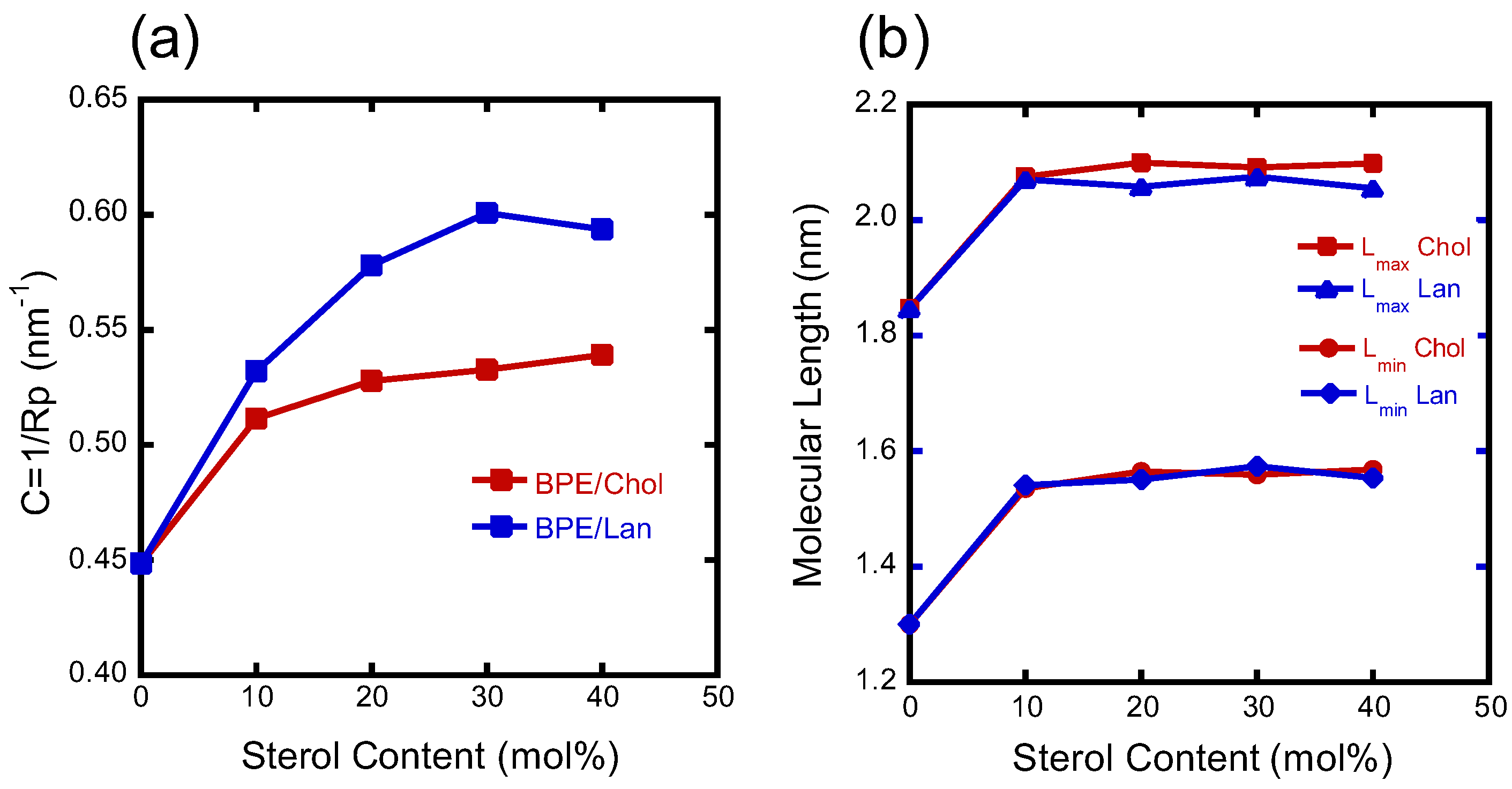
3.4. Structural Analysis of the HII Phase
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Chol | Cholesterol |
| Lan | Lanosterol |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| POPE | 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine |
| POPC | 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| BPE | L-α-Phosphatidylethanolamine from the bacterium Escherichia coli |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| HII | Inverted hexagonal |
| PVP | Polyvinylpyrrolidone |
References
- Kumar, G.A.; Chattopadhyay, A. Cholesterol: An evergreen molecule in biology. Biomed. Spectrosc. Imaging 2016, 5, S55–S66. [Google Scholar] [CrossRef]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol review: A metabolically important molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Zuckermann, M.J. What’s so special about cholesterol? Lipid 2004, 39, 1101–1113. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Róg, T.; Pasenkiewicz-Gierula, M.; Vattulainen, I.; Karttunen, M. Ordering effects of cholesterol and its analogues. Biochim. Biophys. Acta 2009, 1788, 97–121. [Google Scholar] [CrossRef]
- Doole, F.T.; Kumarage, T.; Ashkar, R.; Brown, M.F. Cholesterol stiffening of lipid membranes. J. Membr. Biol. 2022, 255, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Takegami, S.; Kitamura, K.; Kitade, T.; Hasegawa, K.; Nishihira, A. Effects of particle size and cholesterol content on the partition coefficients of chlorpromazine and triflupromazine between phosphatidylcholine-cholesterol bilayers of unilamellar vesicles and water studied by second-derivative spectrophotometry. J. Colloid. Interface Sci. 1999, 220, 81–87. [Google Scholar] [CrossRef]
- Khajeh, A.; Modarress, H. Effect of cholesterol on behavior of 5-fluorouracil (5-FU) in a DMPC lipid bilayer, a molecular dynamics study. Biophys. Chem. 2014, 187–188, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Shimizu, N.; Hikima, T.; Takata, M.; Kobayashi, T.; Takahashi, H. Effect of cholesterol on the interaction of cytochrome P450 substrate drug chlorzoxazone with the phosphatidylcholine bilayer. Biochemistry 2016, 55, 3888–3898. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Meng, F. Effects of cholesterol on chlorzoxazone translocation across POPC bilayer. J. Mol. Model. 2021, 27, 146. [Google Scholar] [CrossRef]
- Frallicciardi, J.; Melcr, J.; Siginou, P.; Marrink, S.J.; Poolman, B. Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes. Nat. Commun. 2022, 13, 1605. [Google Scholar] [CrossRef]
- Kano, S.; Takahashi, H. Cholesterol’s inhibition effect on entering of chlorzoxazone into phosphatidylethanolamine bilayer: Relevance to cytochrome P450 drug metabolism at endoplasmic reticulum membranes. Biochim. Biophys. Acta 2022, 1864, 183954. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Pike, L.J. Rafts defined: A report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid. Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Li, B.; Qin, Y.; Yu, X.; Xu, X.; Yu, W. Lipid raft involvement in signal transduction in cancer cell survival, cell death and metastasis. Cell Prolif. 2022, 55, e13167. [Google Scholar] [CrossRef]
- Rizo, J. Molecular Mechanisms Underlying Neurotransmitter Release. Annu. Rev. Biophys. 2022, 51, 377–408. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C. Viral membrane fusion. Virology 2015, 479–480, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Dai, J.; Wang, H.; Liao, Y.; Tan, L.; Sun, Y.; Song, C.; Liu, W.; Qiu, X.; Ding, C. Coronavirus infection and cholesterol metabolism. Front. Immunol. 2022, 13, 791267. [Google Scholar] [CrossRef] [PubMed]
- Jahn, R.; Cafiso, D.C.; Tamm, L.K. Mechanisms of SNARE proteins in membrane fusion. Nat. Rev. Mol. Cell Biol. 2024, 25, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Sardar, A.; Dewangan, N.; Panda, B.; Bhowmick, D.; Tarafdar, P.K. Lipid and Lipidation in Membrane Fusion. J. Membr. Biol. 2022, 255, 691–703. [Google Scholar] [CrossRef]
- Zhang, X.J.; Qin, J.J.; Cheng, X.; Shen, L.; Zhao, Y.-C.; Yuan, Y.; Lei, L.; Chen, M.-M.; Yang, F.; Bai, L.; et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020, 32, 176–187. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Fan, M.; Zhang, J.; Peng, Y.; Huang, F.; Wang, N.; He, L.; Zhang, L.; Holmdahl, R.; et al. Dependence of SARS-CoV-2 infection on cholesterol-rich lipid raft and endosomal acidification. Comput. Struct. Biotechnol. J. 2021, 19, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kreutzberger, A.J.B.; Odongo, L.; Nelson, E.A.; Nyenhuis, D.A.; Kiessling, V.; Liang, B.; Cafiso, D.S.; White, J.M.; Tamm, L.K. Ebola virus glycoprotein interacts with cholesterol to enhance membrane fusion and cell entry. Nat. Struct. Mol. Biol. 2021, 28, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Zawada, K.E.; Wrona, D.; Rawle, R.J.; Kasson, P.M. Influenza viral membrane fusion is sensitive to sterol concentration but surprisingly robust to sterol chemical identity. Sci. Rep. 2016, 6, 29842. [Google Scholar] [CrossRef]
- Cerqueira, N.M.; Oliveira, E.F.; Gesto, D.S.; Santos-Martins, D.; Moreira, C.; Moorthy, H.N.; Ramos, M.J.; Fernandes, P.A. Cholesterol biosynthesis: A mechanistic overview. Biochemistry 2016, 55, 5483–5506. [Google Scholar] [CrossRef]
- Bloch, K.E. Speculations on the evolution of sterol structure and function. CRC Crit. Rev. Biochem. 1979, 7, 1–5. [Google Scholar] [CrossRef]
- Bloch, K.E. Sterol structure and membrane function. CRC Crit. Rev. Biochem. 1983, 14, 47–92. [Google Scholar] [CrossRef]
- Bloch, K.E. Evolutionary perfection of a small molecule. In Blondes in Venetian Paintings, the Nine-Banded Armadillo, and Other Essays in Biochemistry; Bloch, K., Ed.; Yale University Press: New Haven, ST, USA, 1994; pp. 14–36. [Google Scholar]
- Dahl, C.E.; Dahl, J.S.; Bloch, K. 1980. Effect of alkyl-substituted precursors of cholesterol on artificial and natural membranes and on the viability of Mycoplasma capricolum. Biochemistry 1980, 19, 1462–1467. [Google Scholar] [CrossRef]
- Yeagle, P.L.; Martin, R.B.; Lala, A.K.; Lin, H.K.; Bloch, K. Differential effects of cholesterol and lanosterol on artificial membranes. Proc. Natl. Acad. Sci. USA 1977, 74, 4924–4926. [Google Scholar] [CrossRef]
- Urbina, J.A.; Pekerar, S.; Le, H.B.; Patterson, J.; Montez, B.; Oldfield, E. Molecular order and dynamics of phosphatidylcholine bilayer membranes in the presence of cholesterol, ergosterol and lanosterol: A comparative study using 2H-, 13C- and 31P-NMR spectroscopy. Biochim. Biophys. Acta 1995, 1238, 163–176. [Google Scholar] [CrossRef]
- Xu, X.; London, E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry 2000, 39, 843–849. [Google Scholar] [CrossRef]
- Smondyrev, A.M.; Berkowitz, M.L. Molecular dynamics simulation of the structure of dimyristoylphosphatidylcholine bilayers with cholesterol, ergosterol, and lanosterol. Biophys. J. 2001, 80, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Endress, E.; Bayerl, S.; Prechtel, K.; Maier, C.; Merkel, R.; Bayerl, T.M. The effect of cholesterol, lanosterol, and ergosterol on lecithin bilayer mechanical properties at molecular and microscopic dimensions: A solid-state NMR and micropipet study. Langmuir 2002, 18, 3293–3299. [Google Scholar] [CrossRef]
- Miao, L.; Nielsen, M.; Thewalt, J.; Ipsen, J.H.; Bloom, M.; Zuckermann, M.J.; Mouritsen, O.G. From lanosterol to cholesterol: Structural evolution and differential effects on lipid bilayers. Biophys. J. 2002, 82, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.V.; Dykstra, E.M.; Lope-Piedrafita, S.; Brown, M.F. Lanosterol and cholesterol-induced variations in bilayer elasticity probed by 2H NMR relaxation. Langmuir 2004, 20, 1043–1046. [Google Scholar] [CrossRef]
- Beattie, M.E.; Veatch, S.L.; Stottrup, B.L.; Keller, S.L. Sterol structure determines miscibility versus melting transitions in lipid vesicles. Biophys. J. 2005, 89, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, M.F.; Bayerl, T.M. Differences in the modulation of collective membrane motions by ergosterol, lanosterol, and cholesterol: A dynamic light scattering study. Biophys. J. 2005, 88, 3360–3367. [Google Scholar] [CrossRef]
- Scheidt, H.A.; Huster, D.; Gawrisch, K. Diffusion of cholesterol and its precursors in lipid membranes studied by 1H pulsed field gradient magic angle spinning NMR. Biophys. J. 2005, 89, 2504–2512. [Google Scholar] [CrossRef]
- Mannock, D.A.; Lewis, R.N.; McElhaney, R.N. Comparative calorimetric and spectroscopic studies of the effects of lanosterol and cholesterol on the thermotropic phase behavior and organization of dipalmitoylphosphatidylcholine bilayer membranes. Biophys. J. 2006, 91, 3327–3340. [Google Scholar] [CrossRef]
- Stevens, M.M.; Honerkamp-Smith, A.R.; Keller, S.L. Solubility limits of cholesterol, lanosterol, ergosterol, stigmasterol, and β-Sitosterol in electroformed lipid vesicles. Soft Matter 2010, 7, 5882–5890. [Google Scholar] [CrossRef]
- Bui, T.T.; Suga, K.; Umakoshi, H. Roles of sterol derivatives in regulating the properties of phospholipid bilayer systems. Langmuir 2016, 32, 6176–6184. [Google Scholar] [CrossRef]
- Okayama, A.; Hoshino, T.; Wada, K.; Takahashi, H. Comparison of structural effects of cholesterol, lanosterol, and oxysterol on phospholipid (POPC) bilayers. Chem. Phys. Lipids 2024, 259, 105376. [Google Scholar] [CrossRef] [PubMed]
- Verkleij, A.J.; Leunissen-Bijvelt, J.; de Kruijff, B.; Hope, M.; Cullis, P.R. Non-bilayer structures in membrane fusion. Ciba. Found Symp. 1984, 103, 45–59. [Google Scholar] [CrossRef]
- Siegel, D.P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. II. Implications for membrane-membrane interactions and membrane fusion. Biophys. J. 1986, 49, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Ellens, H.; Siegel, D.P.; Alford, D.; Yeagle, P.L.; Boni, L.; Lis, L.J.; Quinn, P.J.; Bentz, J. Membrane fusion and inverted phases. Biochemistry 1989, 28, 3692–3703. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.P.; Epand, R.M. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: Implications for membrane fusion mechanisms. Biophys. J. 1997, 73, 3089–3111. [Google Scholar] [CrossRef]
- Basanez, G.; Nieva, J.L.; Rivas, E.; Alonso, A.; Goni, F.M. Diacylglycerol and the promotion of lamellar-hexagonal and lamellar-isotropic phase transitions in lipids: Implications for membrane fusion. Biophys. J. 1996, 70, 2299–2306. [Google Scholar] [CrossRef]
- McIntosh, T.J.; Kulkarni, K.G.; Simon, S.A. Membrane fusion promoters and inhibitors have contrasting effects on lipid bilayer structure and undulations. Biophys. J. 1999, 76, 2090–2098. [Google Scholar] [CrossRef]
- Hafez, I.M.; Cullis, P.R. Roles of lipid polymorphism in intracellular delivery. Adv. Drug Deliv. Rev. 2001, 47, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Tristram-Nagle, S.; Chan, R.; Kooijman, E.; Uppamoochikkal, P.; Qiang, W.; Weliky, D.P.; Nagle, J.F. HIV fusion peptide penetrates, disorders, and softens T-cell membrane mimics. J. Mol. Biol. 2010, 402, 139–153. [Google Scholar] [CrossRef]
- Leikin, S.; Parsegian, V.A.; Rau, D.C.; Rand, R.P. Hydration forces. Annu. Rev. Phys. Chem. 1993, 44, 369–395. [Google Scholar] [CrossRef]
- McIntosh, T.J.; Simon, S.A. Hydration and steric pressures between phospholipid bilayers. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 27–51. [Google Scholar] [CrossRef]
- Shrestha, B.R.; Banquy, X. Hydration forces at solid and fluid biointerfaces. Biointerphases 2016, 11, 018907. [Google Scholar] [CrossRef]
- Helfrich, W. Steric interaction of fluid membranes in multilayer systems. Z. Naturforsch. 1978, 33, 305–315. [Google Scholar] [CrossRef]
- Sornette, D.; Ostrowsky, N. Importance of membrane fluidity on bilayer interactions. J. Chem. Phys. 1986, 84, 4062–4067. [Google Scholar] [CrossRef]
- Podgornik, R.; Parsegian, V.A. Thermal-mechanical fluctuations of fluid membranes in confined geometries: The case of soft confinement. Langmuir 1992, 8, 557–562. [Google Scholar] [CrossRef]
- Huang, C.H.; Charlton, J.P. Studies on phosphatidylcholine vesicles. Determination of partial specific volumes by sedimentation velocity method. J. Biol. Chem. 1971, 246, 2555–2560. [Google Scholar] [CrossRef]
- Greenwood, A.I.; Tristram-Nagle, S.; Nagle, J.F. Partial molecular volumes of lipids and cholesterol. Chem. Phys. Lipids 2006, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, N.; Watanabe, Y.; Shinohara, Y.; Inoko, Y.; Matsuba, G.; Okuda, H.; Mori, T.; Ito, K. Upgrade of the small angle X-ray scattering beamlines at the Photon Factory. J. Phys. Conf. Ser. 2011, 272, 012026. [Google Scholar] [CrossRef]
- Shimizu, N.; Mori, T.; Igarashi, N.; Ohta, H.; Nagatani, Y.; Kosuge, T.; Ito, K. Refurbishing of small-angle X-ray scattering beamline, BL-6A at the Photon Factory. J. Phys.: Conf. Ser. 2011, 425, 202008. [Google Scholar] [CrossRef]
- Huang, T.C.; Toraya, H.; Blanton, T.N.; Wu, Y. X-ray powder diffraction of silver behenate, a possible low-angle diffraction standard. J. Appl. Cryst. 1993, 26, 180–184. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-dimensional detector software: From real detector to idealized image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Sayre, D. Some implications of a theorem due to Shannon. Acta. Cryst. 1952, 5, 843. [Google Scholar] [CrossRef]
- Franks, N.P. Structural analysis of hydrated egg lecithin and cholesterol bilayers. I. X-ray diffraction. J. Mol. Biol. 1976, 100, 345–358. [Google Scholar] [CrossRef]
- Yu, Z.W.; Quinn, P.J. The effect of dimethyl sulphoxide on the structure and phase behaviour of palmitoleoylphosphatidylethanolamine. Biochim. Biophys. Acta 2000, 1509, 440–450. [Google Scholar] [CrossRef]
- Rappolt, M.; Hickel, A.; Bringezu, F.; Lohner, K. Lohner. Mechanism of the lamellar/inverse hexagonal phase transition examined by high resolution x-ray diffraction. Biophys. J. 2003, 84, 3111–3122. [Google Scholar] [CrossRef]
- Turner, D.C.; Gruner, S.M.; Huang, J.S. Distribution of decane within the unit cell of the inverted hexagonal (HII) phase of lipid-water-decane systems determined by neutron diffraction. Biochemistry 1992, 31, 1356–1363. [Google Scholar] [CrossRef]
- Harper, P.E.; Mannock, D.A.; Lewis, R.N.; McElhaney, R.N.; Gruner, S.M. X-ray diffraction structures of some phosphatidylethanolamine lamellar and inverted hexagonal phases. Biophys. J. 2001, 81, 2693–2706. [Google Scholar] [CrossRef]
- Ding, L.; Liu, W.; Wang, W.; Glinka, C.J.; Worcester, D.L.; Yang, L.; Huang, H.W. Diffraction techniques for nonlamellar phases of phospholipids. Langmuir 2004, 20, 9262–9269. [Google Scholar] [CrossRef]
- Pan, D.; Wang, W.; Liu, W.; Yang, L.; Huang, H.W. Chain packing in the inverted hexagonal phase of phospholipids: A study by X-ray anomalous diffraction on bromine-labeled chains. J. Am. Chem. Soc. 2006, 128, 3800–3807. [Google Scholar] [CrossRef]
- Harper, P.E.; Cavazos, A.T.; Kinnun, J.J.; Petrache, H.I.; Wassall, S.R. Vitamin E promotes the inverse hexagonal phase via a novel mechanism: Implications for antioxidant role. Langmuir 2020, 36, 4908–4916. [Google Scholar] [CrossRef]
- Vancuylenberg, G.; Sadeghpour, A.; Tyler, A.I.I.; Rappolt, M. From angular to round: In depth interfacial analysis of binary phosphatidylethanolamine mixtures in the inverse hexagonal phase. Soft Matter 2023, 19, 8519–8530. [Google Scholar] [CrossRef]
- Pan, J.; Tristram-Nagle, S.; Nagle, J.F. Effect of cholesterol on structural and mechanical properties of membranes depends on lipid chain saturation. Phys. Rev. E 2009, 80, 021931. [Google Scholar] [CrossRef]
- McIntosh, T.J.; Simon, S.A. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry 1986, 1986 25, 4948–4952. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, T.J.; Simon, S.A. Adhesion between phosphatidylethanolamine bilayers. Langmuir 1996, 12, 1622–1630. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Petrache, H.I.; Gouliaev, N.; Tristram-Nagle, S.; Zhang, R.T.; Suter, R.M.; Nagle, J.F. Interbilayer interactions from high-resolution X-ray scattering. Phys. Rev. E 1998, 57, 7014–7024. [Google Scholar] [CrossRef]
- Petrache, H.I.; Harries, D.; Parsegian, V.A. Alteration of Lipid Membrane Rigidity by Cholesterol and Its Metabolic Precursors. Macromol. Symp. 2005, 219, 39–50. [Google Scholar] [CrossRef]
- Pabst, G.; Boulgaropoulos, B.; Gander, E.; Sarangi, B.R.; Amenitsch, H.; Raghunathan, V.A.; Laggner, P. Effect of ceramide on nonraft proteins. J. Membr. Biol. 2009, 231, 125–132. [Google Scholar] [CrossRef]
- Takahashi, H.; Sinoda, K.; Hatta, I. Effects of cholesterol on the lamellar and the inverted hexagonal phases of dielaidoylphosphatidylethanolamine. Biochim. Biophys. Acta. 1996, 1289, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, K.V.; Merz, K.M., Jr. A comparison of DMPC- and DLPE-based lipid bilayers. Biophys. J. 1994, 66, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, T.J.; Magid, A.D.; Simon, S.A. Cholesterol modifies the short-range repulsive interactions between phosphatidylcholine membranes. Biochemistry 1989, 28, 17–25. [Google Scholar] [CrossRef]
- Bar, L.; Villanueva, M.E.; Neupane, S.; Cordoyiannis, G.; Losada-Pérez, P. QCM-D Study of the Formation of Solid-Supported Artificial Lipid Membranes: State-of-the-Art, Recent Advances, and Perspectives. Phys. Status Solidi A 2023, 220, 2200625. [Google Scholar] [CrossRef]
- Worthington, C.R.; Blaurock, A.E. A structural analysis of nerve myelin. Biophys. J. 1969, 9, 970–990. [Google Scholar] [CrossRef] [PubMed]
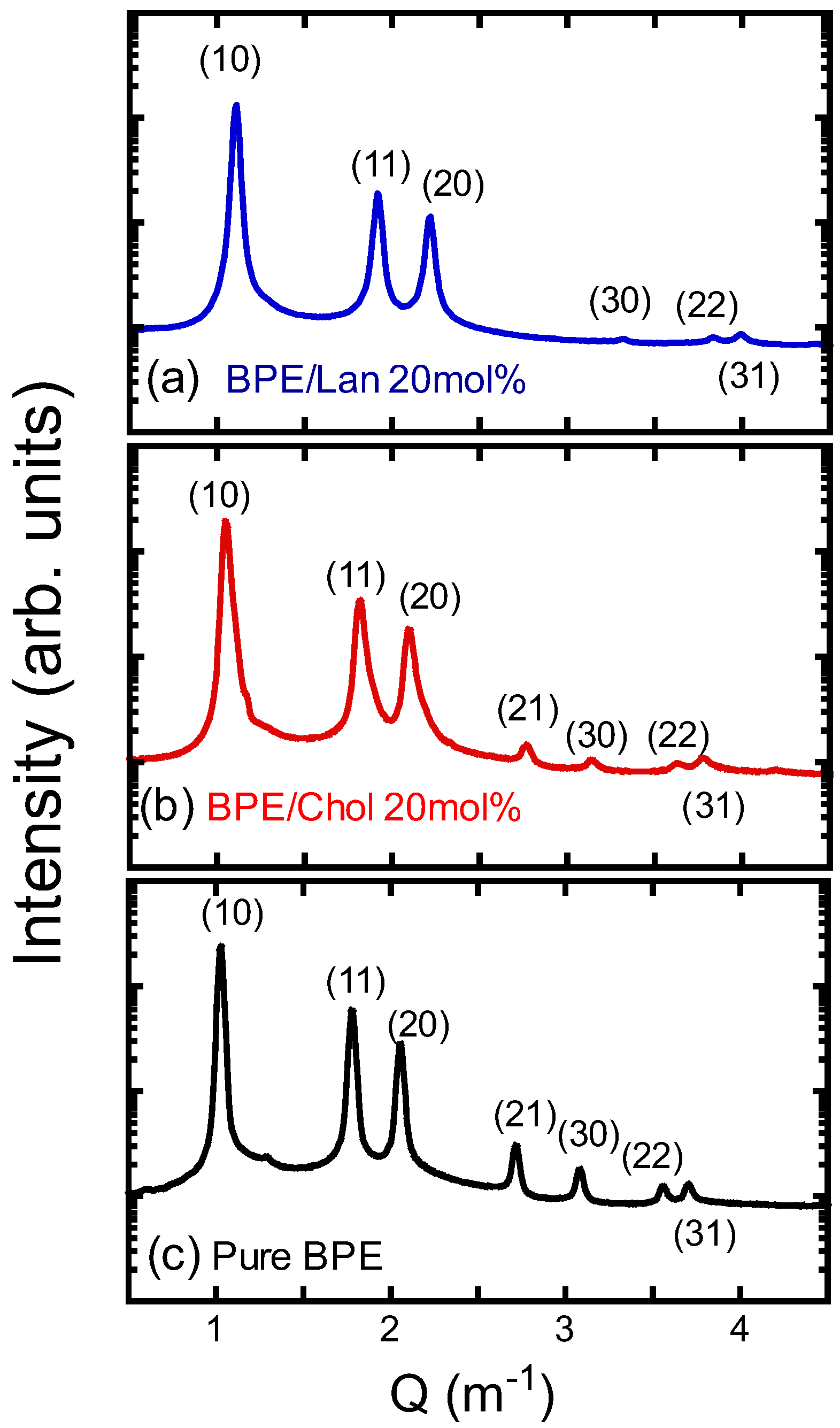
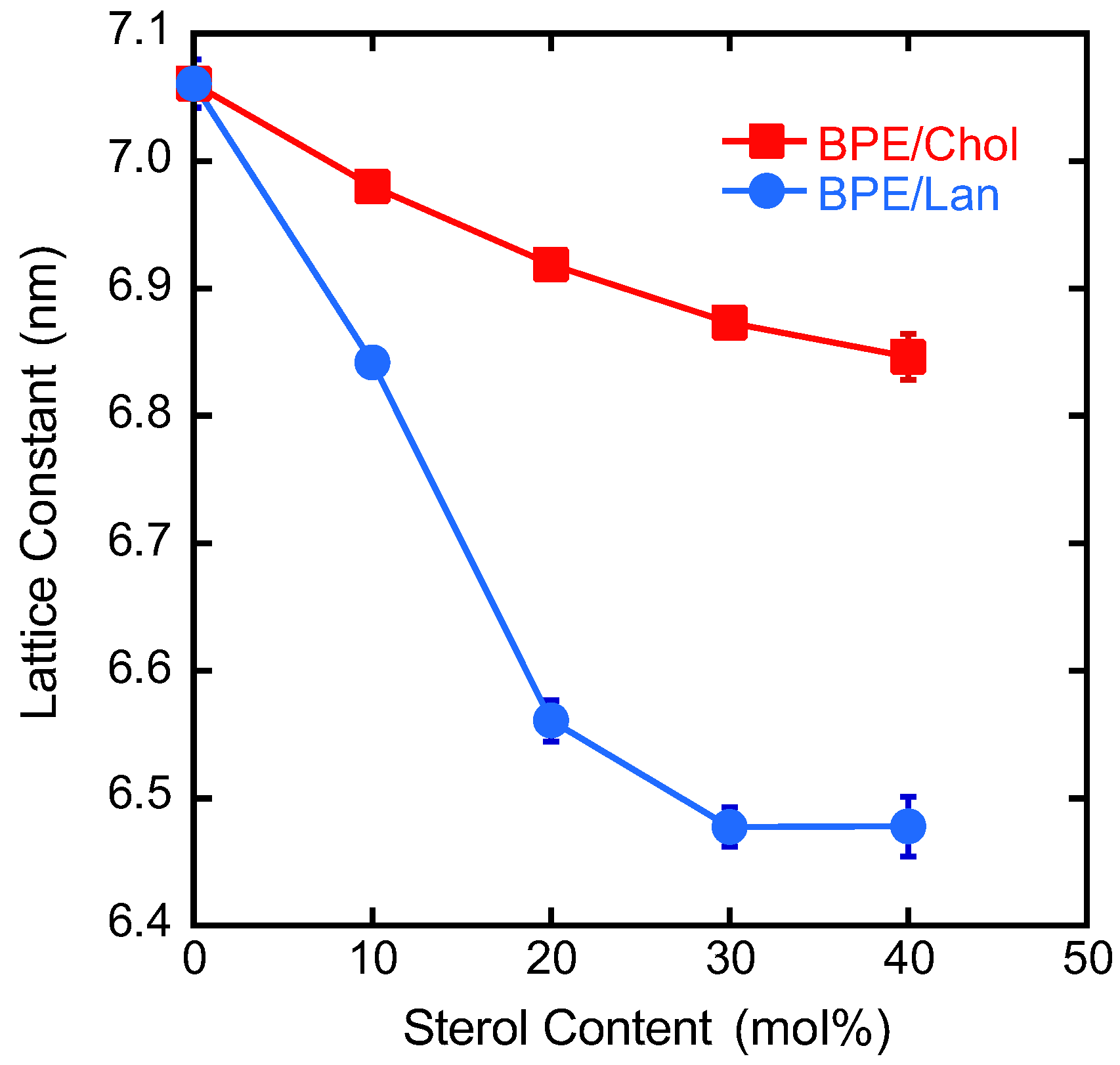
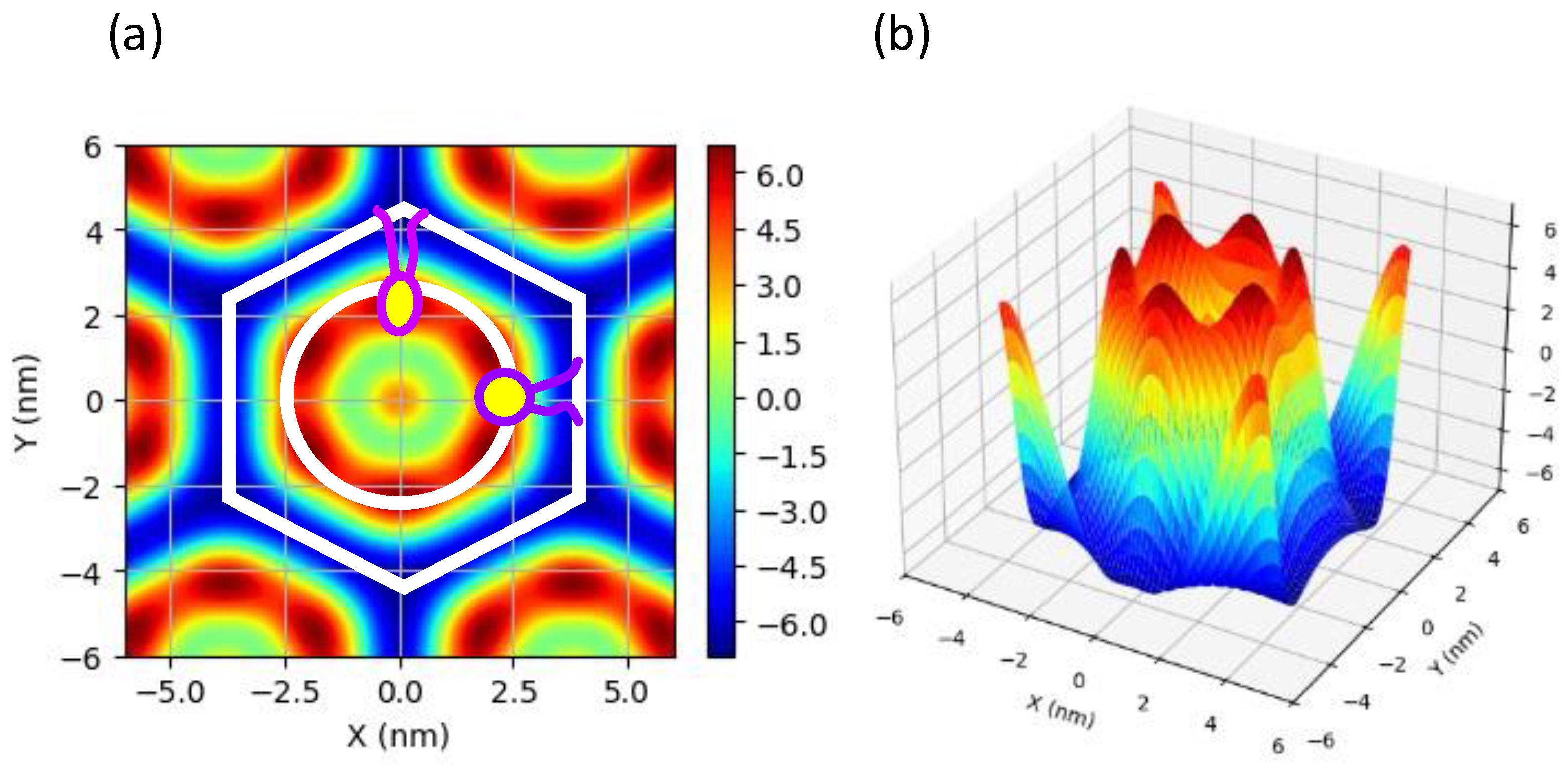
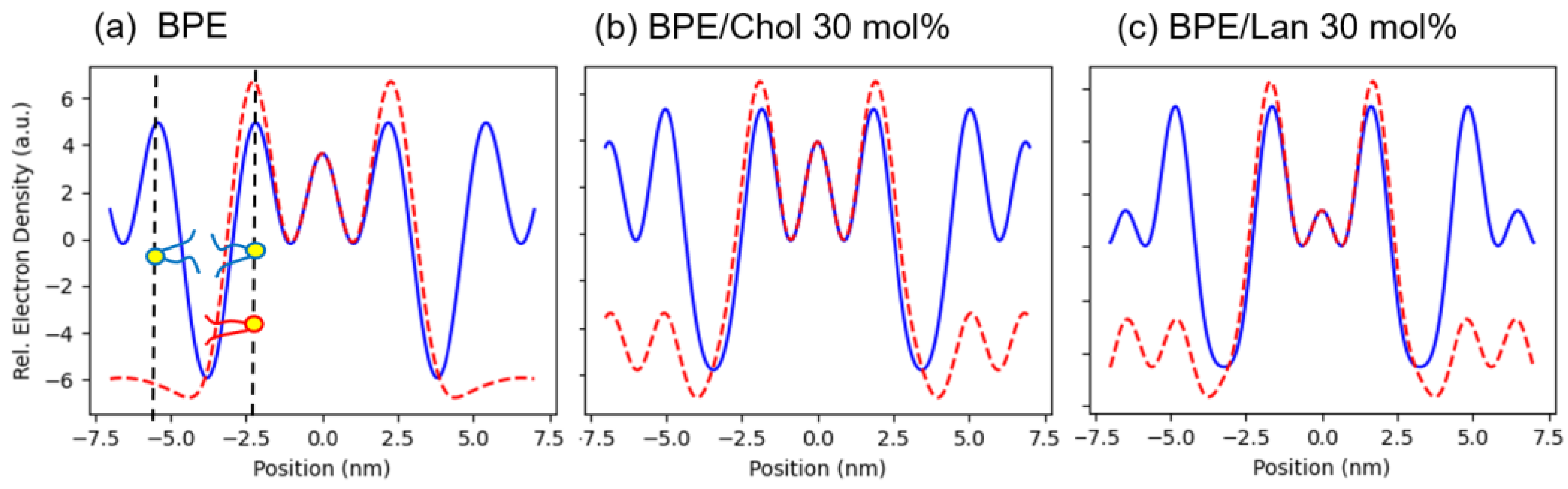
| (nm) | (nm3) | Ao (nm2) | |
|---|---|---|---|
| POPE | 4.78 | 1.177 | 0.492 |
| POPE/Chol | 4.92 | 1.067 | 0.434 |
| POPE/Lan | 4.88 | 1.080 | 0.443 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okayama, A.; Postrado, M.; Takahashi, H. A Comparative Study of the Effects of Cholesterol and Lanosterol on Hydrated Phosphatidylethanolamine Assemblies: Focusing on Physical Parameters Related to Membrane Fusion. Membranes 2025, 15, 352. https://doi.org/10.3390/membranes15120352
Okayama A, Postrado M, Takahashi H. A Comparative Study of the Effects of Cholesterol and Lanosterol on Hydrated Phosphatidylethanolamine Assemblies: Focusing on Physical Parameters Related to Membrane Fusion. Membranes. 2025; 15(12):352. https://doi.org/10.3390/membranes15120352
Chicago/Turabian StyleOkayama, Ayumi, Michael Postrado, and Hiroshi Takahashi. 2025. "A Comparative Study of the Effects of Cholesterol and Lanosterol on Hydrated Phosphatidylethanolamine Assemblies: Focusing on Physical Parameters Related to Membrane Fusion" Membranes 15, no. 12: 352. https://doi.org/10.3390/membranes15120352
APA StyleOkayama, A., Postrado, M., & Takahashi, H. (2025). A Comparative Study of the Effects of Cholesterol and Lanosterol on Hydrated Phosphatidylethanolamine Assemblies: Focusing on Physical Parameters Related to Membrane Fusion. Membranes, 15(12), 352. https://doi.org/10.3390/membranes15120352






