Nanocellulose–Graphene Derivative Composite Membranes: Recent Advances, Functional Mechanisms, and Water Purification Applications
Abstract
1. Introduction
2. Material Fundamentals
2.1. Nanocellulose

2.2. Graphene Derivatives
3. Preparation Methods for NC-GD Composite Membranes
3.1. Vacuum-Assisted Filtration
3.2. Layer-by-Layer Self-Assembly
3.3. Solution Casting
4. Functional Mechanisms of NC–GD Composite Membranes
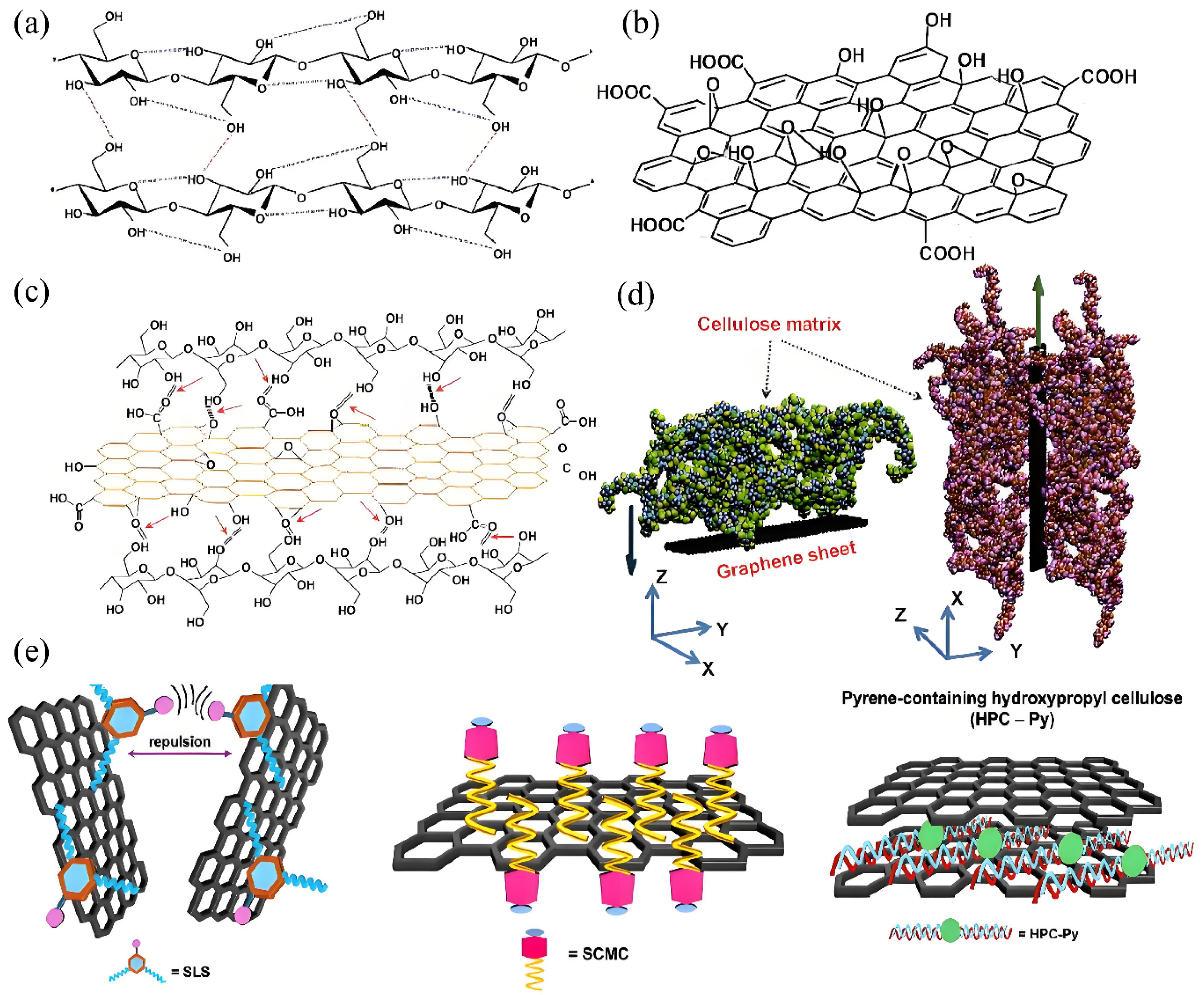
4.1. Interfacial Interactions
4.2. Mass Transfer and Separation

4.3. Anti-Fouling and Antibacterial Properties
4.4. Mechanical Stability
4.5. Synergistic Coupling of Functional Mechanisms
5. Typical Water Treatment Applications
5.1. Pollutant Adsorption
5.2. Smart Oil–Water Separation
5.3. Antibacterial and Anti-Fouling Membranes
5.4. Water Desalination
5.5. Emerging Separation Technology Applications
6. Challenges
6.1. Challenge I: Controlling GO Interlayer Swelling
6.2. Challenge II: The “Double-Edged Sword” of NC Content
6.3. Challenge III: Cost–Performance Trade-Off in Scale-Up
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NC | Nanocellulose |
| GDs | Graphene derivatives |
| CNC | Cellulose nanocrystals |
| CNF | Cellulose nanofibrils |
| BC | Bacterial cellulose |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| TEMPO | 2,2,6,6-tetramethylpiperidin-1-yl-oxyl |
| TOCNF | TEMPO-oxidized CNF |
| PVDF | Polyvinylidene fluoride |
| PES | Polyether sulfone |
| PA | Polyamide |
| RO | Reverse osmosis |
| LbL | Layer-by-layer |
References
- Unesco. The United Nations World Water Development Report 2024: Water for Prosperity and Peace; UN: New York, NY, USA, 2024. [Google Scholar]
- Canton, H. Food and agriculture organization of the United Nations—FAO. In The Europa Directory of International Organizations 2021; Routledge: London, UK, 2021; pp. 297–305. [Google Scholar]
- Li, D.; Zhang, H.; Xu, E. Spatiotemporal changes in the geographic imbalances between crop production and farmland-water resources in China. Agronomy 2022, 12, 1111. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Long-Term Performance of Ultrafiltration Membranes: Corrosion Fouling Aspect. Materials 2023, 16, 1673. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Dong, H.; Chen, Y.; Shang, B.; Wang, Y.; Wang, S.; Zhu, Z. Direct Purification of Digestate Using Ultrafiltration Membranes: Influence of Pore SIZE on Filtration Behavior and Fouling Characteristics. Membranes 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Geise, G.M. Modeling the water permeability and water/salt selectivity tradeoff in polymer membranes. J. Membr. Sci. 2016, 520, 790–800. [Google Scholar] [CrossRef]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The Critical Need for Increased Selectivity, Not Increased Water Permeability, for Desalination Membranes. Environ. Sci. Technol. Lett. 2016, 3, 112–120. [Google Scholar] [CrossRef]
- Mautner, A. Nanocellulose water treatment membranes and filters: A review. Polym. Int. 2020, 69, 741–751. [Google Scholar] [CrossRef]
- Boonyaporn, W.; Phisalaphong, M.; Painmanakul, P.; Thanyasrisung, P.; Fagkaew, P.; Hoven, V.P. Nanoporous Membranes from Bacterial Cellulose Derivatives for Water Treatment. ACS Appl. Nano Mater. 2024, 7, 24508–24521. [Google Scholar] [CrossRef]
- Wu, Z.; Ji, X.; He, Q.; Gu, H.; Zhang, W.-X.; Deng, Z. Nanocelluloses fine-tuned polyvinylidene fluoride (PVDF) membrane for enhanced separation and antifouling. Carbohydr. Polym. 2023, 323, 121383. [Google Scholar] [CrossRef]
- Zhou, K.; Guo, C.; Gan, F.; Xin, J.H.; Yu, H. Large-area ultra-thin GO nanofiltration membranes prepared by a pre-crosslinking rod coating technique. J. Colloid Interface Sci. 2023, 640, 261–269. [Google Scholar] [CrossRef]
- Ying, Y.; Ying, W.; Guo, Y.; Peng, X. Cross-Flow Assembled Ultrathin and Robust Graphene Oxide Membranes for Efficient Molecule Separation. Nanotechnology 2018, 29, 155602. [Google Scholar] [CrossRef]
- Mokhena, T.; Mochane, M.; Mtibe, A.; Sigonya, S.; Ntsendwana, B.; Masibi, E.; Sikhwivhilu, L.; Motsoeneng, T. Recent advances on nanocellulose-graphene oxide composites: A review. Cellulose 2024, 31, 7207–7249. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Suleimenova, M.; Tabynbayeva, A.; Toshtay, K.; Tauanov, Z. Composite Membranes Based on MXene and Nanocellulose for Water Purification: Structure, Efficiency, and Future Prospects. Membranes 2025, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Feng, Y.; Wang, Z.; Jia, M.; Yao, J. Fabrication of cellulose nanofibrils/UiO-66-NH2 composite membrane for CO2/N2 separation. J. Membr. Sci. 2018, 568, 10–16. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- Hobzova, R.; Hrib, J.; Sirc, J.; Karpushkin, E.; Michalek, J.; Janouskova, O.; Gatenholm, P. Embedding of Bacterial Cellulose Nanofibers within PHEMA Hydrogel Matrices: Tunable Stiffness Composites with Potential for Biomedical Applications. J. Nanomater. 2018, 2018, 5217095. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-based hydrogels as versatile materials with interesting functional properties for tissue engineering applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef]
- Valencia, L.; Handa, R.; Monti, S.; Jasso-Salcedo, A.B.; Georgouvelas, D.; Magaña, I.; Díaz de León, R.; Velikov, K.P.; Mathew, A.P.; Kumar, S. On the mineralization of nanocellulose to produce functional hybrid materials. J. Mater. Chem. A 2022, 10, 9248–9276. [Google Scholar] [CrossRef]
- Ferreira-Neto, E.P.; Ullah, S.; da Silva, T.C.A.; Domeneguetti, R.R.; Perissinotto, A.P.; de Vicente, F.S.; Rodrigues-Filho, U.P.; Ribeiro, S.J.L. Bacterial Nanocellulose/MoS2 Hybrid Aerogels as Bifunctional Adsorbent/Photocatalyst Membranes for in-Flow Water Decontamination. ACS Appl. Mater. Interfaces 2020, 12, 41627–41643. [Google Scholar] [CrossRef] [PubMed]
- Rånby, B.G. Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles. Discuss. Faraday Soc. 1951, 11, 158–164. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Morehead, F.F.; Walter, N.M. Liquid Crystal Systems from Fibrillar Polysaccharides. Nature 1959, 184, 632–633. [Google Scholar] [CrossRef]
- Revol, J.F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphology and accessibility. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 797–813. [Google Scholar]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2010, 3, 71–85. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Li, P.; Zhang, K.; Lian, H.; Liimatainen, H. Enhancement of the nanofibrillation of birch cellulose pretreated with natural deep eutectic solvent. Ind. Crops Prod. 2020, 154, 112677. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Hyypiö, K.; Asaadi, S.; Junka, K.; Liimatainen, H. High-strength cellulose nanofibers produced via swelling pretreatment based on a choline chloride–imidazole deep eutectic solvent. Green Chem. 2022, 22, 1763–1775. [Google Scholar] [CrossRef]
- Park, C.-W.; Gwon, J.; Han, S.-Y.; Park, J.-S.; Bandi, R.; Dadigala, R.; Kim, J.-K.; Kwon, G.-J.; Lee, S.-H. Effect of deep eutectic solvent pretreatment on defibrillation efficiency and characteristics of lignocellulose nanofibril. Wood Sci. Technol. 2022, 57, 197–209. [Google Scholar] [CrossRef]
- Tong, X.; He, Z.; Zheng, L.; Pande, H.; Ni, Y. Enzymatic treatment processes for the production of cellulose nanomaterials: A review. Carbohydr. Polym. 2022, 299, 120199. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, S.; Li, J.; Wang, B.; Jin, M.; Liang, Q.; Zhang, D.; Han, Z.; Deng, L.; Qu, X.; et al. Insights into Hierarchical Structure–Property–Application Relationships of Advanced Bacterial Cellulose Materials. Adv. Funct. Mater. 2023, 33, 2214327. [Google Scholar] [CrossRef]
- Jimenez-Cervantes, E.; López-Barroso, J.; Martínez-Hernández, A.L.; Velasco-Santos, C. Graphene-based materials functionalization with natural polymeric biomolecules. In Recent Advances in Graphene Research; InTech: London, UK, 2016; p. 257. [Google Scholar]
- Fatemi, S.M.; Abbasi, Z.; Rajabzadeh, H.; Hashemizadeh, S.A.; Deldar, A.N. A review of recent advances in molecular simulation of graphene-derived membranes for gas separation. Eur. Phys. J. D 2017, 71, 194. [Google Scholar] [CrossRef]
- Dai, F.; Zhou, F.; Chen, J.; Liang, S.; Chen, L.; Fang, H. Ultrahigh water permeation with a high multivalent metal ion rejection rate through graphene oxide membranes. J. Mater. Chem. A 2021, 9, 10672–10677. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Caicedo, J.P.; Joya-Cárdenas, D.R.; Corona-Rivera, M.A.; Saldaña-Robles, N.; Damian-Ascencio, C.E.; Saldaña-Robles, A. Efficiency of graphene quantum dots in water contaminant removal: Trends and future research directions. Water 2025, 17, 166. [Google Scholar] [CrossRef]
- Wu, X.; Ding, M.; Xu, H.; Yang, W.; Zhang, K.; Tian, H.; Wang, H.; Xie, Z. Scalable Ti3C2Tx MXene Interlayered Forward Osmosis Membranes for Enhanced Water Purification and Organic Solvent Recovery. ACS Nano 2020, 14, 9125–9135. [Google Scholar] [CrossRef]
- Das, M.; Aswathy, T.R.; Pal, S.; Naskar, K. Effect of ionic liquid modified graphene oxide on mechanical and self-healing application of an ionic elastomer. Eur. Polym. J. 2021, 158, 110691. [Google Scholar] [CrossRef]
- Brodie, B.C. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Berichte Der Dtsch. Chem. Ges. 2006, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Liu, W.-W.; Aziz, A. Review on the Effects of Electrochemical Exfoliation Parameters on the Yield of Graphene Oxide. ACS Omega 2022, 7, 33719–33731. [Google Scholar] [CrossRef]
- Guo, S.; Chen, J.; Zhang, Y.; Liu, J. Graphene-Based Films: Fabrication, Interfacial Modification, and Applications. Nanomaterials 2021, 11, 2539. [Google Scholar] [CrossRef]
- Wågberg, L.; Erlandsson, J. The Use of Layer-by-Layer Self-Assembly and Nanocellulose to Prepare Advanced Functional Materials. Adv. Mater. 2020, 33, 2001474. [Google Scholar] [CrossRef]
- Troncoso, O.P.; Torres, F.G. Bacterial Cellulose—Graphene Based Nanocomposites. Int. J. Mol. Sci. 2020, 21, 6532. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, P.; Sheng, C.; Zhou, L.; Duan, Y.; Zhang, J. Tunable self-assembly structure of graphene oxide/cellulose nanocrystal hybrid films fabricated by vacuum filtration technique†. RSC Adv. 2014, 4, 39301–39304. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, P.; Mathew, A.P. Self-Assembled TEMPO Cellulose Nanofibers: Graphene Oxide-Based Biohybrids for Water Purification. ACS Appl. Mater. Interfaces 2017, 9, 21048–21058. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Y.; Cui, X.; Wang, Z.; Zhang, Z.; Tosheva, L.; Guo, H. Graphene oxide membranes using MOF@ Chitosan core-shell nanoparticles as dual modulators for dye separation. Chem. Synth. 2024, 4, 27. [Google Scholar] [CrossRef]
- Kang, J.; Kwon, O.; Kim, J.P.; Kim, J.Y.; Kim, J.; Cho, Y.; Kim, D.W. Graphene Membrane for Water-Related Environmental Application: A Comprehensive Review and Perspectives. ACS Environ. Au 2024, 5, 35–60. [Google Scholar] [CrossRef]
- Abolhassani, M.; Griggs, C.S.; Gurtowski, L.A.; Mattei-Sosa, J.A.; Nevins, M.; Medina, V.F.; Morgan, T.A.; Greenlee, L.F. Scalable Chitosan-Graphene Oxide Membranes: The Effect of GO Size on Properties and Cross-Flow Filtration Performance. ACS Omega 2017, 2, 8751–8759. [Google Scholar] [CrossRef]
- Wågberg, L.; Decher, G.; Norgren, M.; Lindström, T.; Ankerfors, M.; Axnäs, K. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 2008, 24, 784–795. [Google Scholar] [CrossRef]
- Fang, Q.; Zhou, X.; Deng, W.; Zheng, Z.; Liu, Z. Freestanding bacterial cellulose-graphene oxide composite membranes with high mechanical strength for selective ion permeation. Sci. Rep. 2016, 6, 33185. [Google Scholar] [CrossRef]
- Lee, T.; Min, S.H.; Gu, M.; Jung, Y.K.; Lee, W.; Lee, J.U.; Seong, D.G.; Kim, B.-S. Layer-by-Layer Assembly for Graphene-Based Multilayer Nanocomposites: Synthesis and Applications. Chem. Mater. 2015, 27, 3785–3796. [Google Scholar] [CrossRef]
- Singh Raghuwanshi, V.; Varanasi, S.; Batchelor, W.; Garnier, G. Cellulose nanocrystals to modulate the self-assembly of graphene oxide in suspension. Mater. Des. 2022, 216, 110572. [Google Scholar] [CrossRef]
- Valencia, L.; Monti, S.; Kumar, S.; Zhu, C.; Liu, P.; Yu, S.; Mathew, A.P. Nanocellulose/graphene oxide layered membranes: Elucidating their behaviour during filtration of water and metal ions in real time†. Nanoscale 2019, 11, 22413–22422. [Google Scholar] [CrossRef]
- Sofyana; Muslim, A.; Supardan, M.D.; Ambarita, A.C.; Arahman, N. Combination of cellulose nanocrystal and graphene oxide as modifying agent for improving the performance of PVDF membranes. Case Stud. Chem. Environ. Eng. 2024, 10, 100873. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Yang, F. Graphene oxide-cellulose nanocrystal (GO-CNC) composite functionalized PVDF membrane with improved antifouling performance in MBR: Behavior and mechanism. Chem. Eng. J. 2018, 352, 765–773. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Afolabi, M.A.; Xiao, D.; Chen, Y. Incorporation of Cellulose Nanocrystals into Graphene Oxide Membranes for Efficient Antibiotic Removal at High Nutrient Recovery. ACS Appl. Mater. Interfaces 2021, 13, 14102–14111. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Aburabie, J.; Nassrullah, H.; Hashaikeh, R. Porous rGO/networked cellulose composite membranes: Towards enhanced nanofiltration performance of rGO-based membranes. Mater. Today Sustain. 2024, 25, 100682. [Google Scholar] [CrossRef]
- Mir, I.S.; Riaz, A.; Fréchette, J.; Roy, J.S.; McElhinney, J.; Pu, S.; Balakrishnan, H.K.; Greener, J.; Dumée, L.F.; Messaddeq, Y. Bacterial cellulose-graphene oxide composite membranes with enhanced fouling resistance for bio-effluents management. npj Clean Water 2024, 7, 111. [Google Scholar] [CrossRef]
- E Dawwam, G.; Abdel-Hamed, G.; Dacrory, S.; Al-Sayed, A.; Abdelmonem, M.O. Promising sheets of Bacterial cellulose/Graphene Oxide for oil-water separation. J. Basic Environ. Sci. 2025, 12, 78–88. [Google Scholar] [CrossRef]
- Phiri, J.; Johansson, L.-S.; Gane, P.; Maloney, T.C. Co-exfoliation and fabrication of graphene based microfibrillated cellulose composites—mechanical and thermal stability and functional conductive properties†. Nanoscale 2018, 10, 9569–9582. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef]
- Akatan, K.; Kuanyshbekov, T.K.; Kabdrakhmanova, S.K.; Imasheva, A.A.; Battalova, A.K.; Abylkalykova, R.B.; Nasyrova, A.K.; Ibraeva, Z.E. Synthesis of nanocomposite material through modification of graphene oxide by nanocellulose. Chem. Bull. Kazakh Natl. Univ. 2021, 102, 14–20. [Google Scholar] [CrossRef]
- Ouyang, W.; Sun, J.; Memon, J.; Wang, C.; Geng, J.; Huang, Y. Scalable preparation of three-dimensional porous structures of reduced graphene oxide/cellulose composites and their application in supercapacitors. Carbon 2013, 62, 501–509. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Strømme, M.; Nyholm, L. Nanocellulose coupled flexible polypyrrole@graphene oxide composite paper electrodes with high volumetric capacitance†. Nanoscale 2015, 7, 3418–3423. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2014, 10, 277–283. [Google Scholar] [CrossRef]
- Pan, H.; Zhu, C.; Lu, T.; Lin, J.; Ma, J.; Zhang, D.; Zhu, S. Chiral Smectic Structure Assembled by Nanosheets and Nanorods. Chem. Commun. 2017, 53, 1868–1871. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, J.; Firdaus, A.H.M.; Sapuan, S.M.; Rashid, U.; Ilyas, R.A.; Hassan, M.R.; Ansari, M.A. Nanocellulose-graphene hybrid composites: Fabrication, characterization, applications and environmental impact. Int. J. Biol. Macromol. 2024, 282, 137244. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Kim, H.S.; Zhang, L.; Korolovych, V.F.; Zhang, S.; Yingling, Y.G.; Tsukruk, V.V. Wrapping Nanocellulose Nets around Graphene Oxide Sheets. Angew. Chem. Int. Ed. 2018, 57, 8508–8513. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhai, W.; Chen, C.; Lu, D.; Wang, J.; Zheng, W. Melt Blending In situ Enhances the Interaction between Polystyrene and Graphene through π–π Stacking. ACS Appl. Mater. Interfaces 2011, 3, 3103–3109. [Google Scholar] [CrossRef]
- Wang, M.; Wei, Q.; Li, D.; Pang, C. Graphene oxide-nanocellulose-cellulose acetate mixed matrix membrane as an efficient separator for carbon dioxide from natural gas. J. Ceram. Process. Res. 2024, 25, 1060–1068. [Google Scholar] [CrossRef]
- Yifira, M.T.; Gebreslassie, G.; Mersha, A.K.; Mekonnen, K.N. Cellulose nanofiber-scaffolded rGO/WO3 membrane for photocatalytic degradation of methylene blue under visible light irradiation. RSC Adv. 2025, 15, 22649–22660. [Google Scholar] [CrossRef]
- Zou, Q.; Liu, X.; Wang, T.; Zhang, L. Enhancement of the thermal stability and mechanical properties of nanocrystalline cellulose/polyvinylidene fluoride ultrafiltration membranes by addition of graphene oxide. Desalination Water Treat. 2019, 138, 1–11. [Google Scholar] [CrossRef]
- Brakat, A.; Zhu, H. Nanocellulose-Graphene Hybrids: Advanced Functional Materials as Multifunctional Sensing Platform. Nano-Micro Lett. 2021, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.R.S.; Alcantara, J.D.S.; Alea, G.V.; Ebajo, V.D. Non-covalent strategies for the preparation of stable aqueous graphene dispersions. J. Chin. Chem. Soc. 2025, 1. [Google Scholar] [CrossRef]
- Cruz-Silva, R.; Izu, K.; Maeda, J.; Saito, S.; Morelos-Gomez, A.; Aguilar, C.; Takizawa, Y.; Yamanaka, A.; Tejiima, S.; Fujisawa, K.; et al. Nanocomposite desalination membranes made of aromatic polyamide with cellulose nanofibers: Synthesis, performance, and water diffusion study. Nanoscale 2020, 12, 19628–19637. [Google Scholar] [CrossRef] [PubMed]
- John, J.P.; Mary Nancy, T.E.; Bindu Sharmila, T.K. A comprehensive review on the environmental applications of graphene–carbon nanotube hybrids: Recent progress, challenges and prospects. Mater. Adv. 2021, 2, 6816–6838. [Google Scholar] [CrossRef]
- Nqombolo, A.; Makhetha, T.A.; Moutloali, R.M.; Nomngongo, P.N. Flux and Fouling Behavior of Graphene Oxide-Polyphenylsulfone Ultrafiltration Membranes Incorporating ZIF-67/ZIF-8 Fillers. Membranes 2025, 15, 289. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water Through Helium-Leak-Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef]
- Taleb, M.A.; Kumar, R.; Barakat, M.A. Environmental friendly fabrication of graphene oxide immobilized in chitosan coupled with carboxymethyl cellulose for removal of zinc (II) ions and oxytetracycline from aqueous solution. Earth Syst. Environ. 2025, 9, 389–402. [Google Scholar] [CrossRef]
- Nitodas, S.; Skehan, M.; Liu, H.; Shah, R. Current and Potential Applications of Green Membranes with Nanocellulose. Membranes 2023, 13, 694. [Google Scholar] [CrossRef]
- Han-jun, Y.; Chun-liang, D.; Fa-xin, D.; Yuan-yuan, L.; Kang, Y.; Run-hong, D. Preparation and properties of CTA/GO pervaporation desalination membrane. Mod. Chem. Ind. 2022, 42, 101–107. [Google Scholar] [CrossRef]
- Ren, X.; Ji, D.; Wen, X.; Bustamante, H.; Daiyan, R.; Foller, T.; Khine, Y.Y.; Joshi, R. Graphene oxide membranes for effective removal of humic acid. J. Mater. Res. 2022, 37, 3362–3371. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Li, B.; Wang, C.-G.; Surat’man, N.E.; Loh, X.J.; Li, Z. Microscopically tuning the graphene oxide framework for membrane separations: A review. Nanoscale Adv. 2021, 3, 5265–5276. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C. Biofouling and me: My Stockholm syndrome with biofilms. Water Res. 2020, 173, 115576. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, H.; Chen, J.; Mo, K.; Cui, Z. Preparation of High-Flux Composite Nanofiltration Membranes Resistant to Organic Fouling via Interfacial Polymerization of Cellulose Nanocrystals. Membr. Sci. Technol. 2025, 45, 20–29+39. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, C.; Mathew, A.P. Mechanically robust high flux graphene oxide—nanocellulose membranes for dye removal from water. J. Hazard. Mater. 2019, 371, 484–493. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Ng, I.M.J.; Shamsi, S. Graphene Oxide (GO): A Promising Nanomaterial against Infectious Diseases Caused by Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2022, 23, 9096. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Q.; Yin, Q.; Pan, G.; Tu, Z.; Liu, L. Reduced Graphene Oxide Functionalized with Gold Nanostar Nanocomposites for Synergistically Killing Bacteria through Intrinsic Antimicrobial Activity and Photothermal Ablation. ACS Appl. Bio Mater. 2019, 2, 747–756. [Google Scholar] [CrossRef]
- Xie, P.; Ge, Y.; Wang, Y.; Zhou, J.; Miao, Y.; Liu, Z. Mechanically Enhanced Nanocrystalline Cellulose/Reduced Graphene Oxide/Polyethylene Glycol Electrically Conductive Composite Film. Nanomaterials 2022, 12, 4371. [Google Scholar] [CrossRef]
- Dhamodharan, D.; Dhinakaran, V.; Ghoderao, P.N.P.; Byun, H.-S.; Wu, L. Synergistic effect of cellulose nanocrystals-graphene oxide as an effective nanofiller for enhancing properties of solventless polymer nanocomposites. Compos. Part B Eng. 2022, 238, 109918. [Google Scholar] [CrossRef]
- Trache, D.; Thakur, V.K.; Boukherroub, R. Cellulose Nanocrystals/Graphene Hybrids—A Promising New Class of Materials for Advanced Applications. Nanomaterials 2020, 10, 1523. [Google Scholar] [CrossRef]
- Ding, Z.; Tang, Y.; Zhu, P. Reduced graphene oxide/cellulose nanocrystal composite films with high specific capacitance and tensile strength. Int. J. Biol. Macromol. 2022, 200, 574–582. [Google Scholar] [CrossRef]
- Saputra, A.M.A.; Syakira, F.N.; Luthfiyah, S.A.; Azkia, S.; Hasibuan, M.I.; Marpongahtun; Andriayani; Goei, R.; Sabar, S.; Gea, S. Graphene oxide–bacterial cellulose composites for enhanced adsorption of rhodamine B from aqueous solutions. Water Sci. Eng. 2025, 18, 474–485. [Google Scholar] [CrossRef]
- Tao, J.; Yang, J.; Ma, C.; Li, J.; Du, K.; Wei, Z.; Chen, C.; Wang, Z.; Zhao, C.; Deng, X. Cellulose nanocrystals/graphene oxide composite for the adsorption and removal of levofloxacin hydrochloride antibiotic from aqueous solution. R. Soc. Open Sci. 2020, 7, 200857. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, D.T.; Akpotu, S.O.; Moodley, B. Crystalline nanocellulose anchored on reduced graphene oxide for the removal of pharmaceuticals from aqueous systems: Adsorbent characterization and adsorption performance. ChemistrySelect 2023, 8, e202202533. [Google Scholar] [CrossRef]
- Sitko, R.; Musielak, M.; Zawisza, B.; Talik, E.; Gagor, A. Graphene oxide/cellulose membranes in adsorption of divalent metal ions. RSC Adv. 2016, 6, 96595–96605. [Google Scholar] [CrossRef]
- Nan, Y.; Gomez-Maldonado, D.; Zhang, K.; Du, H.; Whitehead, D.C.; Li, M.; Zhang, X.; Peresin, M.S. Polyethylenimine functionalized graphene oxide and cellulose nanofibril composite hydrogels: Synthesis, characterization and water pollutants adsorption. Carbohydr. Polym. Technol. Appl. 2024, 8, 100585. [Google Scholar] [CrossRef]
- Liu, Y.; Coppens, M.O. Cell membrane-inspired graphene Nanomesh membrane for fast separation of oil-in-water emulsions. Adv. Funct. Mater. 2022, 32, 2200199. [Google Scholar] [CrossRef]
- Wu, J.; Su, Y.; Cui, Z.; Yu, Y.; Qu, J.; Hu, J.; Cai, Y.; Li, J.; Tian, D.; Zhang, Q. Flexible, durable, and anti-fouling nanocellulose-based membrane functionalized by block copolymer with ultra-high flux and efficiency for oil-in-water emulsions separation. Nano Res. 2023, 16, 5665–5675. [Google Scholar] [CrossRef]
- Qiao, A.; Huang, R.; Penkova, A.; Qi, W.; He, Z.; Su, R. Superhydrophobic, elastic and anisotropic cellulose nanofiber aerogels for highly effective oil/water separation. Sep. Purif. Technol. 2022, 295, 121266. [Google Scholar] [CrossRef]
- Prakash, J.; Venkataprasanna, K.; Bharath, G.; Banat, F.; Niranjan, R.; Venkatasubbu, G.D. In-vitro evaluation of electrospun cellulose acetate nanofiber containing Graphene oxide/TiO2/Curcumin for wound healing application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127166. [Google Scholar] [CrossRef]
- Luz, E.P.C.G.; da Silva, T.F.; Marques, L.S.M.; Andrade, A.; Lorevice, M.V.V.; Andrade, F.K.; Yang, L.; de Souza Filho, A.G.; Faria, A.F.; Silveira Vieira, R. Bacteria-Derived Cellulose Membranes Modified with Graphene Oxide-Silver Nanoparticles for Accelerating Wound Healing. ACS Appl. Bio Mater. 2024, 7, 5530–5540. [Google Scholar] [CrossRef]
- Bhadane, P.; Prajapati, D.G.; Rambhia, A.; Dotiyal, M.; Pandey, P.K.; Rajput, D.; Mishra, A. Antibacterial Activity of Cellulose Acetate–Graphene Oxide Composites: A Comprehensive Multimethod Assessment. ACS Omega 2025, 10, 33814–33822. [Google Scholar] [CrossRef]
- Sheng, N.; Chen, S.; Zhang, M.; Wu, Z.; Liang, Q.; Ji, P.; Wang, H. TEMPO-Oxidized Bacterial Cellulose Nanofibers/Graphene Oxide Fibers for Osmotic Energy Conversion. ACS Appl. Mater. Interfaces 2021, 13, 22416–22425. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, P.; Graham, N.; Li, G.; Yu, W. Long-term operation and biofouling of graphene oxide membrane in practical water treatment: Insights from performance and biofilm characteristics. J. Membr. Sci. 2023, 680, 121761. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Weston, J.; Valeev, R.G.; Johnson, D.J. Graphene Oxide Surface Modification of Reverse Osmosis (RO) Membrane via Langmuir–Blodgett Technique: Balancing Performance and Antifouling Properties. Membranes 2024, 14, 172. [Google Scholar] [CrossRef]
- Sui, Z.; Xue, X.; Wang, Q.; Li, M.; Zou, Y.; Zhang, W.; Lu, C. Facile fabrication of 3D Janus foams of electrospun cellulose nanofibers/rGO for high efficiency solar interface evaporation. Carbohydr. Polym. 2024, 331, 121859. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, M.; Heng, Z.; Zhang, W.; Pan, B. Soft Particles Enable Fast and Selective Water Transport through Graphene Oxide Membranes. Nano Lett. 2020, 20, 7327–7332. [Google Scholar] [CrossRef]
- Hu, J.-Q.; Liu, Z.; Chen, Z.-H.; Cai, Q.-W.; Li, X.-Y.; Xie, R.; Ju, X.-J.; Wang, W.; Chu, L.-Y. Hybrid Graphene Oxide/Laponite Layered Membranes with Stable Two-Dimensional Nanochannels for Efficient Separations in Aqueous Environments. Ind. Eng. Chem. Res. 2020, 59, 12441–12450. [Google Scholar] [CrossRef]



| Method | Suitable NC–GD Combination | Pros | Cons |
|---|---|---|---|
| Vacuum filtration [52] | CNC-GO/rGO (Rigid Scaffold) | 1. Simple operation 2. Controllable structure 3. Forms dense ordered layered structures 4. High separation selectivity | 1. Film thickness control depends on parameters; flexible materials are prone to agglomeration 2. Batch processing is challenging for continuous production |
| Layer-by-layer self-assembly [53] | CNF-GO (Surface Charge Tunable) | 1. Precise control over film thickness and morphology 2. Enables easy fabrication of ultrathin films 3. Structurally robust | 1. Time-consuming process is unsuitable for mass production 2. Requires prior design and regulation of surface charge |
| Solution casting [54] | CNF/BC and rGO/aminated GO (Well-functionalized) | 1. Intuitive and straightforward process 2. Facilitates formation of structurally uniform films | 1. Poor control over film thickness and pore structure 2. Challenges exist for large-scale production |
| NC–GD Combination | Water Flux | Separation Performance | Adsorption Capacity | Stability | Ref. |
|---|---|---|---|---|---|
| CNC–GO | Permeability increased by 2 to 4 times | Antibiotic rejection: >95%: divalent ion (Mg2+/Ca2+) rejection: 99.1% | — | — | [67] |
| CNC–rGO/PEO | 18.4 L m−2 h−1 @ 2 bar | NaCl removal rate: 98.3% | — | The flux decrease within 120 h is less than 2%. | [68] |
| CNF–GO | Five times higher than pure CNF membrane | For positive/negative dyes >90% | — | — | [64] |
| TOCNF–nanoGO | The filtration rate is 111 times that of the GO composite membrane | Suitable for dye/macromolecule separation | Cu(II): 68.1 mg/g | Stable; structure retained after three cycles. | [56] |
| BC | 0.70 ± 0.27 L m−2 h−1 kPa−1 | BSA protein: 98 ± 2% | — | Stable; flux recovery rate after three cycles: 68 ± 16%. | [9] |
| BC–rGO | 394.6 L·m−2·h−1 @ 2 bar | Organic matter and bacterial removal rate >95% | — | Antibacterial; flux recovery rate after five cycles >95%. | [69] |
| BC–GO | — | Oil separation: 90% (within 5 s) | — | — | [70] |
| Pollutant Type | Water Source | NC–GD Combination | Removal Efficiency | Ref. |
|---|---|---|---|---|
| Rhodamine B | Textile dyeing wastewater | BC–GO | 99.50% at pH 3 | [108] |
| Levofloxacin hydrochloride (antibiotic) | Antibiotic-containing wastewater | CNCs–GO | >80.1% At optimal conditions (pH 4, dosage 1.0 g L−1, 4 h) | [109] |
| Divalent metal ions (Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Pb2+) | Advanced purification of drinking water | GO–cellulose | Maximum adsorption capacities: Co2+: 15.5 mg·g−1 Ni2+: 14.3 mg·g−1 Cu2+: 26.6 mg·g−1 Zn2+: 16.7 mg·g−1 Cd2+: 26.8 mg·g−1 Pb2+: 107.9 mg·g−1 at pH 4.5 | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Lin, S.; Pan, Y.; Wang, X.; Zhang, H.; Liu, S.; Li, Z.; Wei, N. Nanocellulose–Graphene Derivative Composite Membranes: Recent Advances, Functional Mechanisms, and Water Purification Applications. Membranes 2025, 15, 347. https://doi.org/10.3390/membranes15120347
Zhang H, Lin S, Pan Y, Wang X, Zhang H, Liu S, Li Z, Wei N. Nanocellulose–Graphene Derivative Composite Membranes: Recent Advances, Functional Mechanisms, and Water Purification Applications. Membranes. 2025; 15(12):347. https://doi.org/10.3390/membranes15120347
Chicago/Turabian StyleZhang, Hui, Shuyuan Lin, Yating Pan, Xin Wang, Hanzhou Zhang, Shuhan Liu, Zhen Li, and Ning Wei. 2025. "Nanocellulose–Graphene Derivative Composite Membranes: Recent Advances, Functional Mechanisms, and Water Purification Applications" Membranes 15, no. 12: 347. https://doi.org/10.3390/membranes15120347
APA StyleZhang, H., Lin, S., Pan, Y., Wang, X., Zhang, H., Liu, S., Li, Z., & Wei, N. (2025). Nanocellulose–Graphene Derivative Composite Membranes: Recent Advances, Functional Mechanisms, and Water Purification Applications. Membranes, 15(12), 347. https://doi.org/10.3390/membranes15120347




