High-Efficiency Removal of Copper and Nickel via Donnan Dialysis Using Fujifilm Cation-Exchange Membranes: Process Optimization Through Response Surface Methodology
Abstract
1. Introduction
2. Experiments
2.1. Reagents and Method
2.2. Membranes
2.3. Donnan Dialysis
2.4. Optimisation Software
3. Results and Discussion
3.1. Characterization of Membranes by FTIR
3.2. Characterization of Membranes by SEM
3.3. Preliminary Study
3.3.1. FT Influence of the Counter-Ion Nature
3.3.2. Choice of the Membrane
3.3.3. Effect of Counterion Concentration in the Receptor Compartment
3.4. Optimization by Response Surface Methodology Using Central Composite Design (CCD)
3.4.1. Pareto Chart and Contribution
3.4.2. Analysis of Variance ANOVA
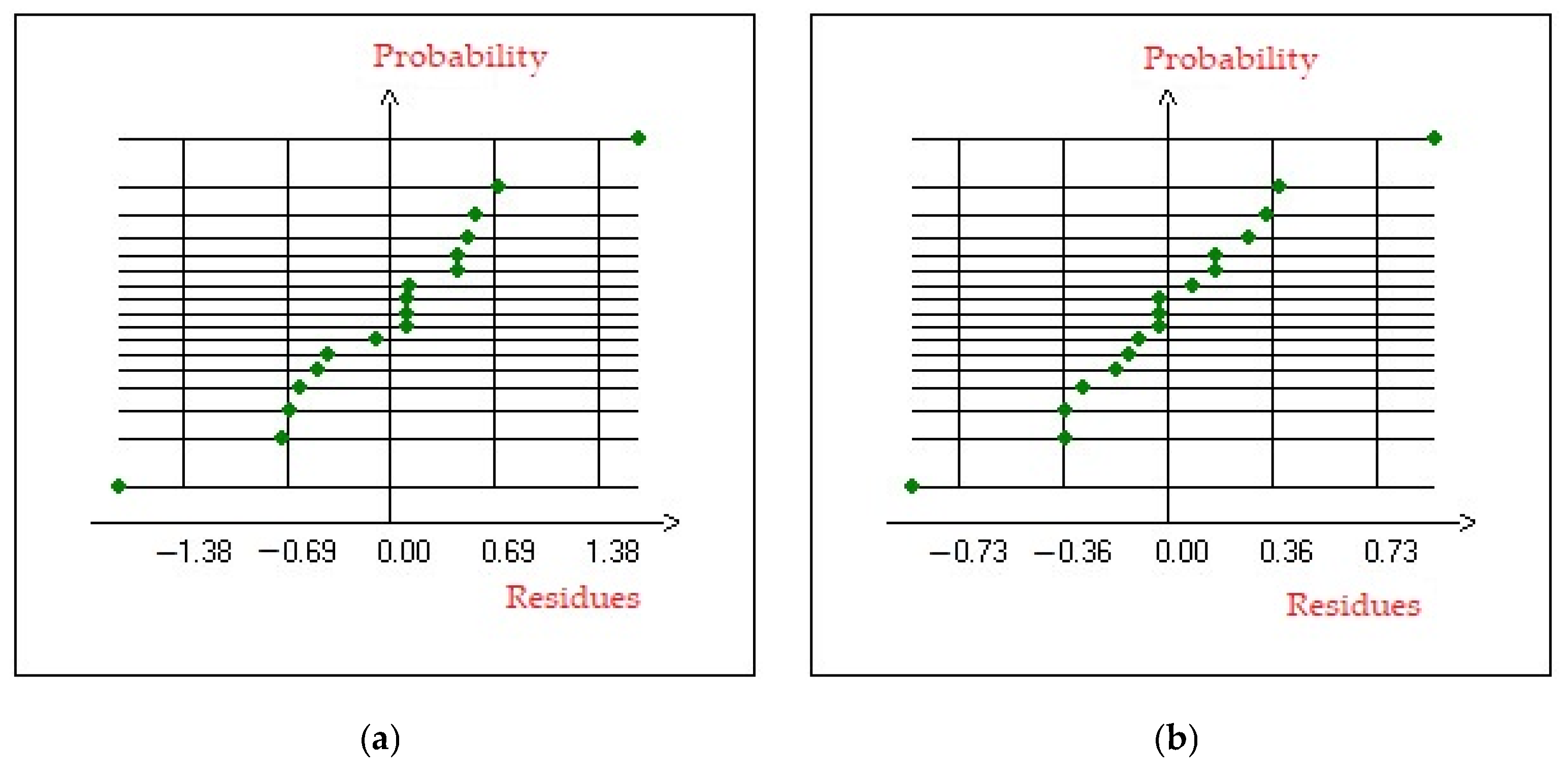
3.4.3. Residual Analysis
3.5. The 3D Surface Plots and 3D Contour Plots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, J.; Huang, J.; Wang, J.; Yu, J.; You, X.; Lin, X.; Van der Bruggen, B.; Zhao, S. High-Performance Porous Anion Exchange Membranes for Efficient Acid Recovery from Acidic Wastewater by Diffusion Dialysis. J. Membr. Sci. 2021, 624, 119116. [Google Scholar] [CrossRef]
- Golubenko, D.; Ahmed, F.E.; Hilal, N. Novel Crosslinked Anion Exchange Membranes Based on Thermally Cured Epoxy Resin: Synthesis, Structure and Mechanical and Ion Transport Properties. Membranes 2024, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Luch, A. (Ed.) Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Experientia Supplementum; Springer: Basel, Switzerland, 2012; ISBN 978-3-7643-8339-8. [Google Scholar]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Jensen, P.D.; Purnell, P.; Velenturf, A.P.M. Highlighting the Need to Embed Circular Economy in Low Carbon Infrastructure Decommissioning: The Case of Offshore Wind. Sustain. Prod. Consum. 2020, 24, 266–280. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Chapter 2—Production and Use. In Extractive Metallurgy of Copper, 5th ed.; Schlesinger, M.E., King, M.J., Sole, K.C., Davenport, W.G., Eds.; Elsevier: Oxford, UK, 2011; pp. 13–30. ISBN 978-0-08-096789-9. [Google Scholar]
- Hu, Q.; Gu, Y. Copper Economic Dynamics: Navigating Resource Scarcity, Price Volatility, and Green Growth. Resour. Policy 2024, 89, 104462. [Google Scholar] [CrossRef]
- Kartal, L. Selective Removal of Copper from Nickel–Copper Leach Solution by Electrolysis Cells with High Convection. Metals 2025, 15, 432. [Google Scholar] [CrossRef]
- Hussain, J.; Husain, I.; Arif, M.; Gupta, N. Studies on Heavy Metal Contamination in Godavari River Basin. Appl. Water Sci. 2017, 7, 4539–4548. [Google Scholar] [CrossRef]
- Kumar, A.; Jigyasu, D.K.; Kumar, A.; Subrahmanyam, G.; Mondal, R.; Shabnam, A.A.; Cabral-Pinto, M.M.S.; Malyan, S.K.; Chaturvedi, A.K.; Gupta, D.K.; et al. Nickel in Terrestrial Biota: Comprehensive Review on Contamination, Toxicity, Tolerance and Its Remediation Approaches. Chemosphere 2021, 275, 129996. [Google Scholar] [CrossRef]
- Kumar, A.; Pinto, M.C.; Candeias, C.; Dinis, P.A. Baseline Maps of Potentially Toxic Elements in the Soils of Garhwal Himalayas, India: Assessment of Their Eco-Environmental and Human Health Risks. Land Degrad. Dev. 2021, 32, 3856–3869. [Google Scholar] [CrossRef]
- Kumar, A.; Bharti; Malyan, S.K.; Kumar, S.S.; Dutt, D.; Kumar, V. An Assessment of Trace Element Contamination in Groundwater Aquifers of Saharanpur, Western Uttar Pradesh, India. Biocatal. Agric. Biotechnol. 2019, 20, 101213. [Google Scholar] [CrossRef]
- Malyan, S.K.; Singh, R.; Rawat, M.; Kumar, M.; Pugazhendhi, A.; Kumar, A.; Kumar, V.; Kumar, S.S. An Overview of Carcinogenic Pollutants in Groundwater of India. Biocatal. Agric. Biotechnol. 2019, 21, 101288. [Google Scholar] [CrossRef]
- Warlimont, H.; Martienssen, W. (Eds.) Springer Handbook of Materials Data; Springer Handbooks; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-69741-3. [Google Scholar]
- Santos, R.M.; Van Audenaerde, A.; Chiang, Y.W.; Iacobescu, R.I.; Knops, P.; Van Gerven, T. Nickel Extraction from Olivine: Effect of Carbonation Pre-Treatment. Metals 2015, 5, 1620–1644. [Google Scholar] [CrossRef]
- Panyushkina, A.; Fomchenko, N.; Babenko, V.; Muravyov, M. Effect of Temperature on Biobeneficiation of Bulk Copper-Nickel Concentrate with Thermoacidophilic Microbial Communities. Metals 2021, 11, 1969. [Google Scholar] [CrossRef]
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.M.S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Kumar, A.; et al. Bio-Remediation Approaches for Alleviation of Cadmium Contamination in Natural Resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef]
- Qu, L.; Huang, H.; Xia, F.; Liu, Y.; Dahlgren, R.A.; Zhang, M.; Mei, K. Risk Analysis of Heavy Metal Concentration in Surface Waters across the Rural-Urban Interface of the Wen-Rui Tang River, China. Environ. Pollut. 2018, 237, 639–649. [Google Scholar] [CrossRef]
- Li, Y.; Tsend, N.; Li, T.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave Assisted Hydrothermal Preparation of Rice Straw Hydrochars for Adsorption of Organics and Heavy Metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Negm, N.A.; Hefni, H.H.H.; Wahab, M.M.A. Metal Adsorption by Agricultural Biosorbents: Adsorption Isotherm, Kinetic and Biosorbents Chemical Structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef]
- Kumar, J.; Joshi, H.; Malyan, S.K. Removal of Copper, Nickel, and Zinc Ions from an Aqueous Solution through Electrochemical and Nanofiltration Membrane Processes. Appl. Sci. 2022, 12, 280. [Google Scholar] [CrossRef]
- Duan, G.; Li, X.; Ma, X.; Zhong, W.; Wang, S. High-Efficiency Adsorption Removal for Cu(II) and Ni(II) Using a Novel Acylamino Dihydroxamic Acid Chelating Resin. Sci. Total Environ. 2023, 864, 160984. [Google Scholar] [CrossRef] [PubMed]
- Kolbadinejad, S.; Ghaemi, A. Optimization of Simultaneous Adsorption of Nickel, Copper, Cadmium and Zinc from Sulfuric Solutions Using Weakly Acidic Resins. Sci. Rep. 2024, 14, 7506. [Google Scholar] [CrossRef] [PubMed]
- Abbes, M.; Barhoumi, A.; Brahmi, K.; Bouguerra, W.; Elaloui, E. Combining Electrocoagulation Process Using Aluminum Electrodes with Activated Carbon on the Removal of Humic Acid from Synthetic and Industrial Wastewater. Euro-Mediterr. J. Environ. Integr. 2024, 9, 1413–1426. [Google Scholar] [CrossRef]
- Barhoumi, A.; Ncib, S.; Bouguerra, W.; Elaloui, E. Techno-Economic Assessment of Ni Removal from Industrial Wastewater by Electrocoagulation Using Rectangular Aluminium Electrodes. Desalination Water Treat. 2022, 266, 143–153. [Google Scholar] [CrossRef]
- Barhoumi, A.; Chibani, A.; Brahmi, K.; Ncib, S.; Bouguerra, W.; Elaloui, E. Optimizing Electrochemical Parameters for Enhanced Heavy Metal and Organic Matter Removal by Electrocoagulation- Électrofloculation Reactors—A Comparative Study. Chem. Afr. 2024, 7, 2889–2898. [Google Scholar] [CrossRef]
- Ncib, S.; Chibani, A.; Barhoumi, A.; Larchet, C.; Dammak, L.; Elaloui, E.; Bouguerra, W. Separation of Copper and Nickel from Synthetic Wastewater by Polymer Inclusion Membrane Containing Di(2-Ethylhexyl)Phosphoric Acid. Polym. Bull. 2023, 80, 12177–12192. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Zhang, A.; Luo, G.; Wu, X.; Wang, S.; Feng, J.; Guo, Y. Highly Efficient and Selective Removal of Copper from Low pH Nickel Watts Solution through Hydrogen Sulfide. Chem. Pap. 2023, 77, 6707–6715. [Google Scholar] [CrossRef]
- Nascimento, M.F.; Keković, P.; Ribeiro, I.A.C.; Faria, N.T.; Ferreira, F.C. Novel Organic Solvent Nanofiltration Approaches for Microbial Biosurfactants Downstream Processing. Membranes 2023, 13, 81. [Google Scholar] [CrossRef]
- Giacobbo, A.; Pasqualotto, I.F.; Machado Filho, R.C.d.C.; Minhalma, M.; Bernardes, A.M.; de Pinho, M.N. Ultrafiltration and Nanofiltration for the Removal of Pharmaceutically Active Compounds from Water: The Effect of Operating Pressure on Electrostatic Solute—Membrane Interactions. Membranes 2023, 13, 743. [Google Scholar] [CrossRef]
- Bunani, S.; Abbt-Braun, G.; Horn, H. Heavy Metal Removal from Aqueous Solutions Using a Customized Bipolar Membrane Electrodialysis Process. Molecules 2024, 29, 1754. [Google Scholar] [CrossRef]
- Chalmers Brown, R.; Tuffou, R.; Massanet Nicolau, J.; Dinsdale, R.; Guwy, A. Overcoming Nutrient Loss during Volatile Fatty Acid Recovery from Fermentation Media by Addition of Electrodialysis to a Polytetrafluoroethylene Membrane Stack. Bioresour. Technol. 2020, 301, 122543. [Google Scholar] [CrossRef]
- Zhu, X.-Z.; Wang, L.-F.; Pan, X.-R.; Zhang, F.; Huang, M.-S.; Li, W.-W.; Liu, H.-Q. Selective Separation of Volatile Fatty Acids, Nitrogen and Phosphorus from Anaerobic Acidogenic Fermentation via Forward Osmosis Membrane Process. Chem. Eng. J. 2023, 453, 139871. [Google Scholar] [CrossRef]
- Outram, V.; Zhang, Y. Solvent-Free Membrane Extraction of Volatile Fatty Acids from Acidogenic Fermentation. Bioresour. Technol. 2018, 270, 400–408. [Google Scholar] [CrossRef]
- Tang, X.; Ju, K.; Zhao, Z. A Strategy for Deep Removal of Cu from Ni Anolyte Based on the Ion-Exchange Method. J. Environ. Chem. Eng. 2024, 12, 111786. [Google Scholar] [CrossRef]
- Chen, C.; Dong, T.; Han, M.; Yao, J.; Han, L. Ammonium Recovery from Wastewater by Donnan Dialysis: A Feasibility Study. J. Clean. Prod. 2020, 265, 121838. [Google Scholar] [CrossRef]
- Chen, H.; Rose, M.; Fleming, M.; Souizi, S.; Shashvatt, U.; Blaney, L. Recent Advances in Donnan Dialysis Processes for Water/Wastewater Treatment and Resource Recovery: A Critical Review. Chem. Eng. J. 2023, 455, 140522. [Google Scholar] [CrossRef]
- Tamersit, S.; Amrane, C.; Lalmi, A.; Akkari, I. Selective Recovery of Nickel from Electroplating Rinse Bath Wastewater by an Integrated Donnan Dialysis/Precipitation Process. Sep. Sci. Technol. 2024, 59, 494–504. [Google Scholar] [CrossRef]
- Gueccia, R.; Alhadidi, A.M.M.; Cipollina, A.; Micale, G. Donnan Dialysis for Tap-Water Softening. Desalination Water Treat. 2020, 192, 19–32. [Google Scholar] [CrossRef]
- Marzouk-Trifi, I.; Baklouti, L.; Dammak, L. Investigation of Calcium and Magnesium Removal by Donnan Dialysis According to the Doehlert Design for Softening Different Water Types. Membranes 2023, 13, 203. [Google Scholar] [CrossRef]
- Breytus, A.; Hasson, D.; Semiat, R.; Shemer, H. Ion Exchange Membrane Adsorption in Donnan Dialysis. Sep. Purif. Technol. 2019, 226, 252–258. [Google Scholar] [CrossRef]
- Trifi, I.M.; Trifi, B.; Dammak, L.; Trifi, I.M.; Trifi, B.; Dammak, L. Removal of Nitrate and Nitrite by Donnan Dialysis: Optimization According to Doehlert Design. In Recent Advances on Nitrification and Denitrification; IntechOpen: London, UK, 2023; ISBN 978-1-83768-964-4. [Google Scholar]
- Barros, K.S.; Carvalheira, M.; Marreiros, B.C.; Reis, M.A.M.; Crespo, J.G.; Pérez-Herranz, V.; Velizarov, S. Donnan Dialysis for Recovering Ammonium from Fermentation Solutions Rich in Volatile Fatty Acids. Membranes 2023, 13, 347. [Google Scholar] [CrossRef]
- Sarapulova, V.; Shkorkina, I.; Mareev, S.; Pismenskaya, N.; Kononenko, N.; Larchet, C.; Dammak, L.; Nikonenko, V. Transport Characteristics of Fujifilm Ion-Exchange Membranes as Compared to Homogeneous Membranes AMX and CMX and to Heterogeneous Membranes MK-40 and MA-41. Membranes 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Lim, Y.J.; Zhang, K. Engineering Multi-Channel Water Transport in Surface-Porous MXene Nanosheets for High-Performance Thin-Film Nanocomposite Membranes. J. Membr. Sci. 2025, 728, 124151. [Google Scholar] [CrossRef]
- Almasi, A.; Mahmoudi, M.; Mohammadi, M.; Dargahi, A.; Biglari, H. Optimizing Biological Treatment of Petroleum Industry Wastewater in a Facultative Stabilization Pond for Simultaneous Removal of Carbon and Phenol. Toxin Rev. 2021, 40, 189–197. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Dargahi, A.; Rahmani, A.; Shabanloo, A.; Ansari, A.; Nematollahi, D. Application of a Fluidized Three-Dimensional Electrochemical Reactor with Ti/SnO2–Sb/β-PbO2 Anode and Granular Activated Carbon Particles for Degradation and Mineralization of 2,4-Dichlorophenol: Process Optimization and Degradation Pathway. Chemosphere 2021, 279, 130640. [Google Scholar] [CrossRef]
- Hasani, K.; Peyghami, A.; Moharrami, A.; Vosoughi, M.; Dargahi, A. The Efficacy of Sono-Electro-Fenton Process for Removal of Cefixime Antibiotic from Aqueous Solutions by Response Surface Methodology (RSM) and Evaluation of Toxicity of Effluent by Microorganisms. Arab. J. Chem. 2020, 13, 6122–6139. [Google Scholar] [CrossRef]
- Dargahi, A.; Samarghandi, M.R.; Shabanloo, A.; Mahmoudi, M.M.; Nasab, H.Z. Statistical Modeling of Phenolic Compounds Adsorption onto Low-Cost Adsorbent Prepared from Aloe Vera Leaves Wastes Using CCD-RSM Optimization: Effect of Parameters, Isotherm, and Kinetic Studies. Biomass Convers. Biorefin. 2023, 13, 7859–7873. [Google Scholar] [CrossRef]
- Heidari, M.; Vosoughi, M.; Sadeghi, H.; Dargahi, A.; Mokhtari, S.A. Degradation of Diazinon from Aqueous Solutions by Electro-Fenton Process: Effect of Operating Parameters, Intermediate Identification, Degradation Pathway, and Optimization Using Response Surface Methodology (RSM). Sep. Sci. Technol. 2021, 56, 2287–2299. [Google Scholar] [CrossRef]
- Kazemi, A.; Ebrahimpour, E.; Esmaeilbeigi, M.; Gheitasi, F.; Einollahipeer, F.; Mohammadrezai, M. Optimizing Oxytetracycline Removal from Aqueous Solutions Using Activated Carbon from Barley Lignocellulosic Wastes with Isotherms and Thermodynamic Studies. Sci. Rep. 2024, 14, 23281. [Google Scholar] [CrossRef]
- Asante-Sackey, D.; Rathilal, S.; Pillay, L.V.; Kweinor Tetteh, E. Ion Exchange Dialysis for Aluminium Transport through a Face-Centred Central Composite Design Approach. Processes 2020, 8, 160. [Google Scholar] [CrossRef]
- Mandal, G.; Lowast, B.B. RSM-CCD Modeling and Optimization for Adsorptive Removal of Rose Bengal Dye from Aqueous Medium Using CeO2 Nanoflowers. Indian J. Sci. Technol. 2024, 17, 3199–3209. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, W.; Tufa, R.A.; Liu, C.; Aili, D.; Chanda, D.; Chang, J.; Wang, S.; Zhang, Y.; Ma, J. Studies on Anion Exchange Membrane and Interface Properties by Electrochemical Impedance Spectroscopy: The Role of pH. Membranes 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- IEM Ion Exchange Membranes for Water Purification. Brochure of Fujifilm Membrane Technology. Available online: https://asset.fujifilm.com/www/us/files/2022-09/5be284eb385e7b72906880debde5dd50/IEM_brochure_version_2.2_final_September_2022.pdf (accessed on 18 November 2024).
- Akter, M.; Park, J.-S. Fouling and Mitigation Behavior of Foulants on Ion Exchange Membranes with Surface Property in Reverse Electrodialysis. Membranes 2023, 13, 106. [Google Scholar] [CrossRef]
- Elozeiri, A.A.E.; Dykstra, J.E.; Rijnaarts, H.H.M.; Lammertink, R.G.H. Multi-Component Ion Equilibria and Transport in Ion-Exchange Membranes. J. Colloid Interface Sci. 2024, 673, 971–984. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of Monopolar and Bipolar Electrodialysis Processes for Tartaric Acid Recovery from Residues of the Winery Industry. ACS Sustain. Chem. Eng. 2020, 8, 13387–13399. [Google Scholar] [CrossRef]
- Rijnaarts, T.; Huerta, E.; Van Baak, W.; Nijmeijer, K. Effect of Divalent Cations on RED Performance and Cation Exchange Membrane Selection to Enhance Power Densities. Environ. Sci. Technol. 2017, 51, 13028–13035. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Grasman, S.; van Engelen, R.; Nijmeijer, K. Upscaling Reverse Electrodialysis. Environ. Sci. Technol. 2018, 52, 10856–10863. [Google Scholar] [CrossRef]
- Fernández de Labastida, M.; Yaroshchuk, A. Transient Membrane Potential after Concentration Step: A New Method for Advanced Characterization of Ion-Exchange Membranes. J. Membr. Sci. 2019, 585, 271–281. [Google Scholar] [CrossRef]
- Asante-Sackey, D.; Rathilal, S.; Kweinor Tetteh, E.; Ezugbe, E.O.; Pillay, L.V. Donnan Membrane Process for the Selective Recovery and Removal of Target Metal Ions—A Mini Review. Membranes 2021, 11, 358. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Ion-Exchange Membrane Systems—Electrodialysis and Other Electromembrane Processes. In Fundamental Modelling of Membrane Systems; Luis, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 251–300. ISBN 978-0-12-813483-2. [Google Scholar]
- Trifi, I.M.; Trifi, B.; Djemal, A.; Hamrouni, B. Simultaneous Removal of Nitrates and Nitrites from Water by Donnan Dialysis Using Doehlert Design. Environ. Eng. Manag. J. 2021, 20, 973–983. [Google Scholar] [CrossRef]
- Mathieu, D.; Nony, J.; Phan-Tan-Luu, R. Nemrod-W Software; LPRAI: Marseille, France, 2000. [Google Scholar]
- Huiping, L.; Guoqun, Z.; Shanting, N.; Yiguo, L. Technologic Parameter Optimization of Gas Quenching Process Using Response Surface Method. Comput. Mater. Sci. 2007, 38, 561–570. [Google Scholar] [CrossRef]
- Motri, S.; Touil, A.; Benselma, Z.; Hassini, L.; Bettaieb, E.; Zagrouba, F. Application of Factorial Design to the Study of the Effect of Drying Conditions on α-Tocopherol Content in Prickly Pear Seed Oil. J. New Sci. Agric. Biotechnol. 2015, 19, 759–765. [Google Scholar]
- Strathmann, H. Electrodialysis, a Mature Technology with a Multitude of New Applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Regmi, C.; Thamaraiselvan, C.; Zhu, Z.; Qian, X.; Wickramasinghe, S.R. Recovery of Ionic Liquid from the Model Solution Mixture Mimicking the Catalytically Hydrolyzed Cellulose Product Utilizing Amberlyst Ion-Exchange Resin. Processes 2024, 12, 55. [Google Scholar] [CrossRef]
- Zhan, W.; Xu, C.; Qian, G.; Huang, G.; Tang, X.; Lin, B. Adsorption of Cu(ii), Zn(ii), and Pb(ii) from Aqueous Single and Binary Metal Solutions by Regenerated Cellulose and Sodium Alginate Chemically Modified with Polyethyleneimine. RSC Adv. 2018, 8, 18723–18733. [Google Scholar] [CrossRef]
- Shafiqul Alam, A.M.; Ferdoushi, F.K.; Islam, A.; Rashid, H.-O.; Wahiduzzaman, M.; Habib, A. Selective Extraction of Co(II) in the Presence of Mn(II), Ni(II) and Cu(II) Using Salting-out Phase Separation Method. Pak. J. Anal. Environ. Chem. 2008, 9, 6–10. [Google Scholar]
- Zhu, C.; Liu, F.; Xu, C.; Gao, J.; Chen, D.; Li, A. Enhanced Removal of Cu(II) and Ni(II) from Saline Solution by Novel Dual-Primary-Amine Chelating Resin Based on Anion-Synergism. J. Hazard. Mater. 2015, 287, 234–242. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Lee, M.S. Separation of Co(II), Cu(II), Ni(II) and Mn(II) from Synthetic Hydrochloric Acid Leaching Solution of Spent Lithium Ion Batteries by Solvent Extraction. Physicochem. Probl. Miner. Process. 2020, 56, 599–610. [Google Scholar] [CrossRef]
- Durmaz, F.; Kara, H.; Cengeloglu, Y.; Ersoz, M. Fluoride Removal by Donnan Dialysis with Anion Exchange Membranes. Desalination 2005, 177, 51–57. [Google Scholar] [CrossRef]
- Wiśniewski, J.; Różańska, A. Donnan Dialysis for Hardness Removal from Water before Electrodialytic Desalination. Desalination 2007, 212, 251–260. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A.; Loizidou, M.D.; Grigoropoulou, H.P. The Effect of Competitive Cations and Anions on Ion Exchange of Heavy Metals. Sep. Purif. Technol. 2005, 46, 202–207. [Google Scholar] [CrossRef]
- Su, W.; Chen, C.; Xu, H.; Yang, W.; Dai, H. Filtering Whitewater with an Ultrafiltration Membrane: Effects of the Interaction between Dissolved Organics and Metal Ions on Membrane Fouling. BioResources 2015, 11, 1108–1124. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Chen, H.; Wu, Z. Removal of Cu(II) Ions from Contaminated Waters Using a Conducting Microfiltration Membrane. J. Hazard. Mater. 2017, 339, 182–190. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on Ion-Exchange Membranes: Classification, Characterization and Strategies of Prevention and Control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Dammak, L.; Fouilloux, J.; Bdiri, M.; Larchet, C.; Renard, E.; Baklouti, L.; Sarapulova, V.; Kozmai, A.; Pismenskaya, N. A Review on Ion-Exchange Membrane Fouling during the Electrodialysis Process in the Food Industry, Part 1: Types, Effects, Characterization Methods, Fouling Mechanisms and Interactions. Membranes 2021, 11, 789. [Google Scholar] [CrossRef]
- Adesina, O.A.; Abdulkareem, F.; Yusuff, A.S.; Lala, M.; Okewale, A. Response Surface Methodology Approach to Optimization of Process Parameter for Coagulation Process of Surface Water Using Moringa oleifera Seed. S. Afr. J. Chem. Eng. 2019, 28, 46–51. [Google Scholar] [CrossRef]
- Tetteh, E.; Amano, K.O.A.; Asante-Sackey, D.; Armah, E. Response Surface Optimisation of Biogas Potential in Co-Digestion of Miscanthus Fuscus and Cow Dung. Int. J. Technol. 2018, 9, 944. [Google Scholar] [CrossRef]
- John Babu, D.; King, P.; Prasanna Kumar, Y. Optimization of Cu (II) Biosorption onto Sea Urchin Test Using Response Surface Methodology and Artificial Neural Networks. Int. J. Environ. Sci. Technol. 2019, 16, 1885–1896. [Google Scholar] [CrossRef]
- Taran, M.; Aghaie, E. Designing and Optimization of Separation Process of Iron Impurities from Kaolin by Oxalic Acid in Bench-Scale Stirred-Tank Reactor. Appl. Clay Sci. 2015, 107, 109–116. [Google Scholar] [CrossRef]
- Afolabi, F.O.; Musonge, P.; Bakare, B.F. Bio-Sorption of a Bi-Solute System of Copper and Lead Ions onto Banana Peels: Characterization and Optimization. J. Environ. Health Sci. Eng. 2021, 19, 613–624. [Google Scholar] [CrossRef]
- Iqbal, M.; Iqbal, N.; Bhatti, I.A.; Ahmad, N.; Zahid, M. Response Surface Methodology Application in Optimization of Cadmium Adsorption by Shoe Waste: A Good Option of Waste Mitigation by Waste. Ecol. Eng. 2016, 88, 265–275. [Google Scholar] [CrossRef]
- Khelifi, O.; Affoune, A.M.; Nacef, M.; Chelaghmia, M.L.; Laksaci, H. Response Surface Modeling and Optimization of Ni(II) and Cu(II) Ions Competitive Adsorption Capacity by Sewage Sludge Activated Carbon. Arab. J. Sci. Eng. 2022, 47, 5797–5809. [Google Scholar] [CrossRef]
- Malenga, E.N.; Mulaba-Bafubiandi, A.F.; Nheta, W. Application of the Response Surface Method (RSM) Based on Central Composite Design (CCD) and Design Space (DS) to Optimize the Flotation and the Desliming Conditions in the Recovery of PGMs from Mine Sludge. Sep. Sci. Technol. 2022, 57, 2960–2983. [Google Scholar] [CrossRef]
- Nde Bup, D.; Abi, C.F.; Tenin, D.; Kapseu, C.; Tchiegang, C. Optimisation of the Cooking Process of Sheanut Kernels (Vitellaria paradoxa Gaertn) Using the Doehlert Experimental Design. Food Bioprocess. Technol. 2012, 5, 108–117. [Google Scholar] [CrossRef]
- Dabaibeh, R.N.; Amayrah, H.H. Competitive Adsorption of Li+, Na+, and K+ Ions on Phillip Site/Chabazite Zeolitic Tuff from Jordan. J. Med. Chem. Sci. 2023, 6, 1985–1997. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Correia, A.A.; Rasteiro, M.G. Heavy Metals Removal from Aqueous Solutions by Multiwall Carbon Nanotubes: Effect of MWCNTs Dispersion. Nanomaterials 2021, 11, 2082. [Google Scholar] [CrossRef] [PubMed]
- Ucarli, O.; Yayintas, O.T.; Engin, M.S.; Cay, S.; Saglikoglu, G.; Yilmaz, S. Investigation of Competitive and Noncompetitive Adsorption of Some Heavy Metals Ions on Leucodon sciuroides (Hedw). Schwägr. Langmuir 2020, 36, 8265–8271. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, D.T.R.; Correia, A.A.S.; Hunkeler, D.; Rasteiro, M.G.B.V. Surfactants for Dispersion of Carbon Nanotubes Applied in Soil Stabilization. Colloids Surf. Physicochem. Eng. Asp. 2015, 480, 405–412. [Google Scholar] [CrossRef]
- Radovanovic, D.; Dikić, J.; Štulović, M.; Anđić, Z.; Kamberović, Ž.; Jevtić, S. Sorption of Pb2+, Zn2+, Cu2+ and Ni2+ Ions on Na-Enriched Natural Zeolite for Wastewater Treatment Process: A Kinetic Approach. Metall. Mater. Eng. 2023, 29, 20–35. [Google Scholar] [CrossRef]
- Gao, Z.; Bandosz, T.J.; Zhao, Z.; Han, M.; Qiu, J. Investigation of Factors Affecting Adsorption of Transition Metals on Oxidized Carbon Nanotubes. J. Hazard. Mater. 2009, 167, 357–365. [Google Scholar] [CrossRef]
- Lima, I.M.; Marhsall, W.E.; Klasson, T.K. Removal of Heavy Metals from Solution by a Novel Swine Manure-Based Activated Carbon; USDA ARS Southern Regional Research Center: New Orleans, LA, USA, 2007. [Google Scholar]
- Minceva, M.; Fajgar, R.; Markovska, L.; Meshko, V. Comparative Study of Zn2+, Cd2+, and Pb2+ Removal From Water Solution Using Natural Clinoptilolitic Zeolite and Commercial Granulated Activated Carbon. Equilibrium of Adsorption. Sep. Sci. Technol. 2008, 43, 2117–2143. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Nghiem, L.D.; Hai, F.I.; Vigneswaran, S.; Nguyen, T.V. Competitive Adsorption of Metals on Cabbage Waste from Multi-Metal Solutions. Bioresour. Technol. 2014, 160, 79–88. [Google Scholar] [CrossRef]
- Gürbüz, H.; Baday, Ş. Investigating the Relationship between Chuck and Tailstock Pressure in Turning by Using Full Factorial Design. Eur. J. Tech. EJT 2023, 13, 249–255. [Google Scholar] [CrossRef]
- Hasan, S.H.; Srivastava, P.; Talat, M. Biosorption of Pb(II) from Water Using Biomass of Aeromonas hydrophila: Central Composite Design for Optimization of Process Variables. J. Hazard. Mater. 2009, 168, 1155–1162. [Google Scholar] [CrossRef]
- Box, G.E.; Hunter, W.H.; Hunter, S. Statistics for Experimenters: An Introduction to Design, Data Analysis and Model Building; John Wiley and Sons: New York, NY, USA, 1978. [Google Scholar]
- Box, G.E.P.; Draper, N.R. Empirical Model-Building and Response Surfaces; John Wiley & Sons: New York, NY, USA, 1987; ISBN 978-0-471-81033-9. [Google Scholar]
- Tiwary, S.K.; Singh, M.; Chavan, S.V.; Karim, A. Graphene Oxide-Based Membranes for Water Desalination and Purification. npj 2D Mater. Appl. 2024, 8, 27. [Google Scholar] [CrossRef]
- Arshad, F.; Al Momani, D.E.; de Vos, W.M.; Zou, L. Nanocomposite Membrane for Simultaneous Removal of Dye and Heavy Metal Ions from Wastewater. J. Environ. Manag. 2024, 371, 123242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cui, R. Separation of Copper and Nickel Metal Ions from Electroplating Wastewater by Ultrafiltration with Tartaric Acid and Sodium Citrate Reinforced Sodium Polyacrylate Complexation. Membranes 2024, 14, 240. [Google Scholar] [CrossRef] [PubMed]



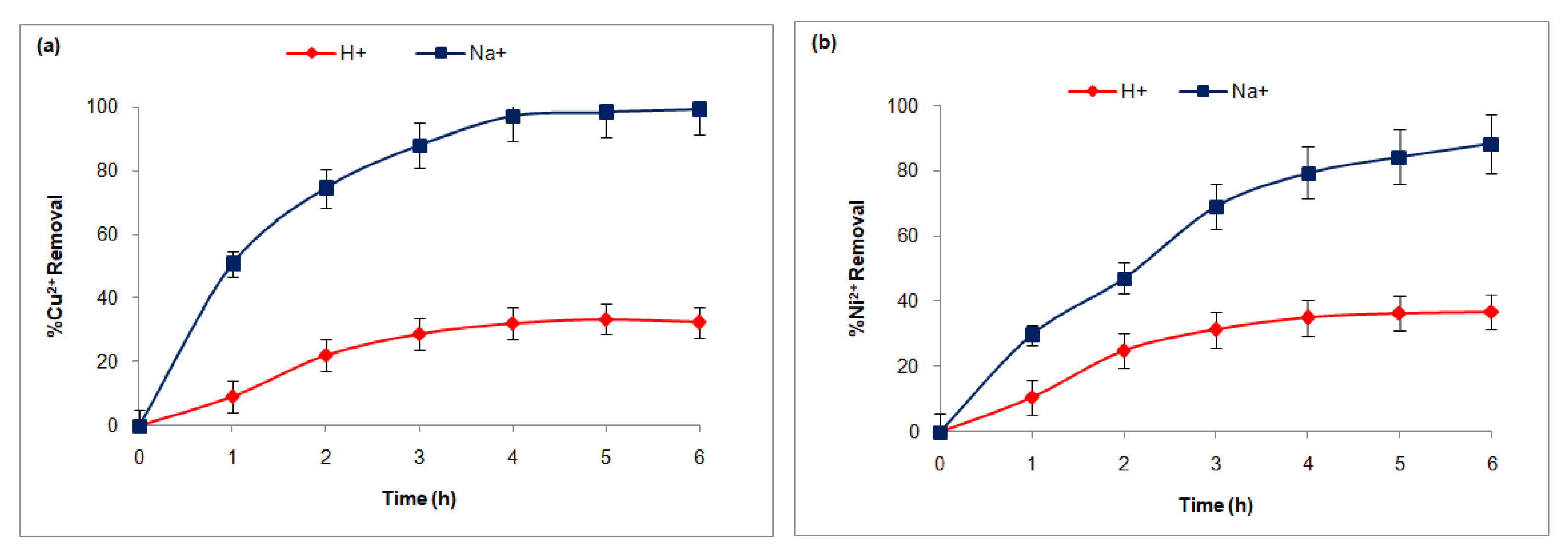
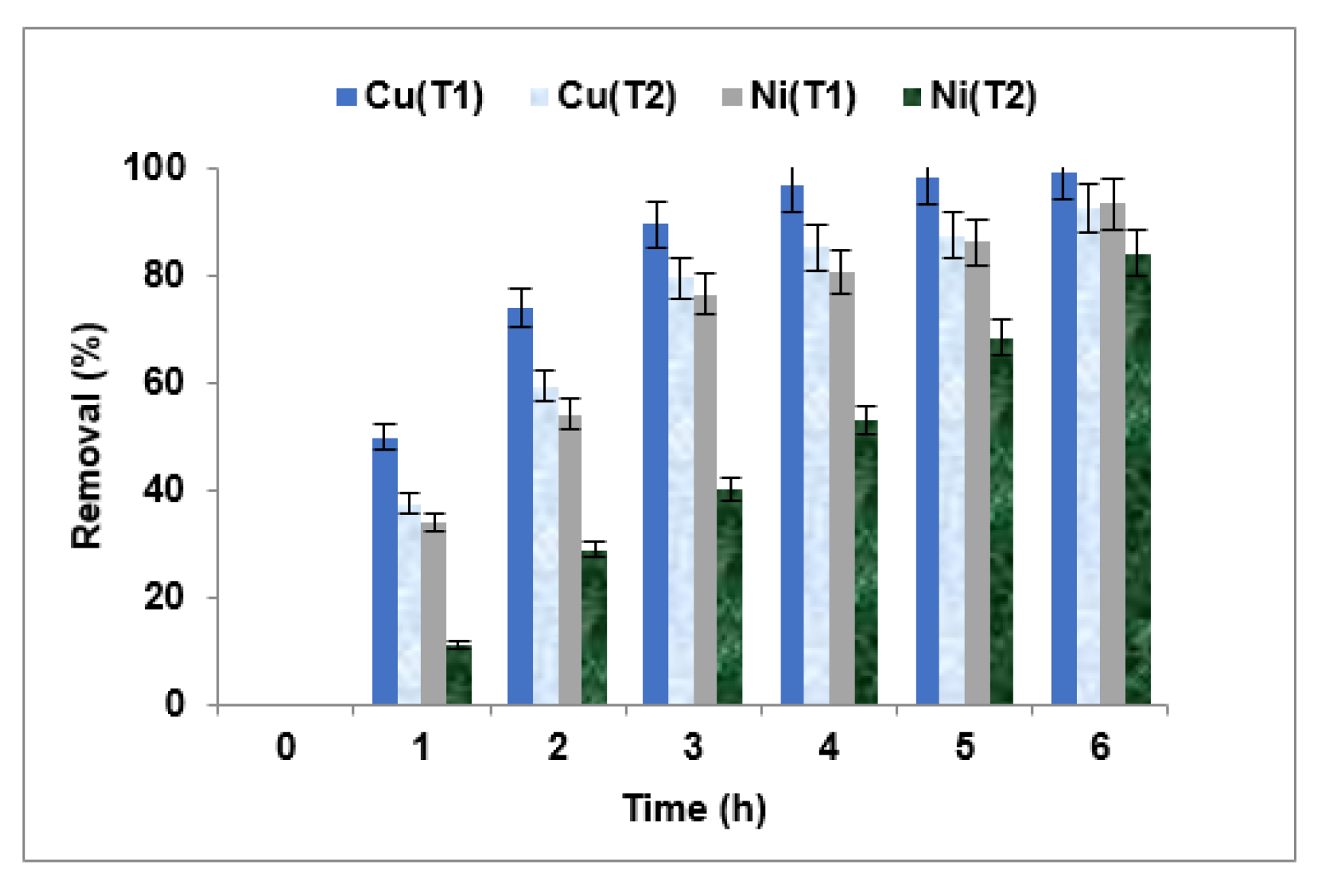

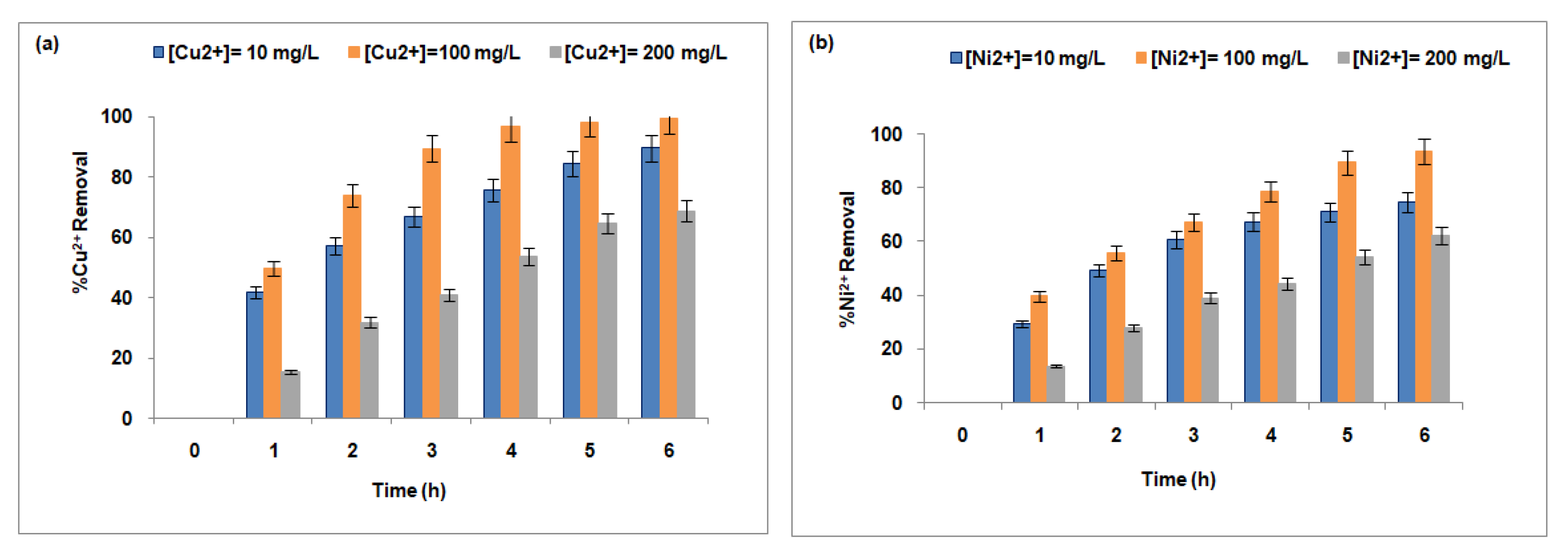

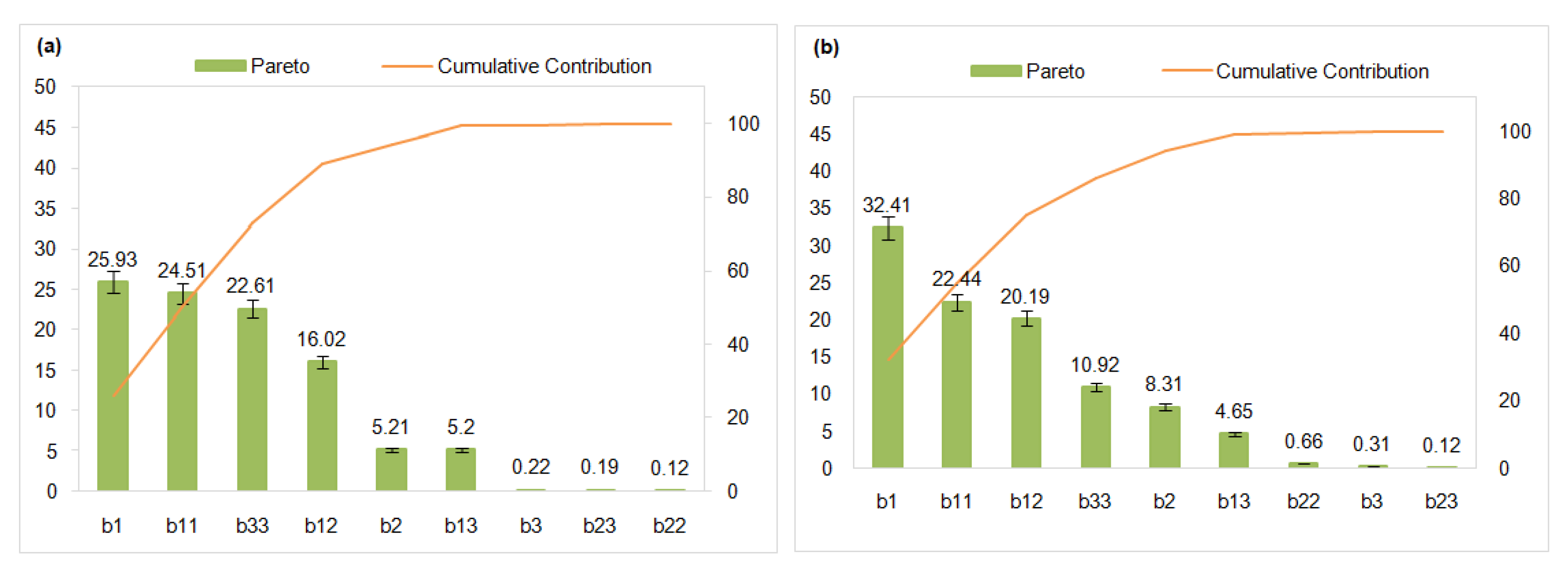



| Membranes | Fujifilm Type 1 | Fujifilm Type 2 | Reference |
|---|---|---|---|
| Type | Homogeneous | Homogeneous | [44] |
| Structure property | Aliphatic polyamide | Aliphatic polyamide | [44] |
| Fixed groups | -SO3− | -SO3− | [44] |
| Exchange capacity (mmol/g wet) | 1.43 ± 0.05 | 1.35 ± 0.05 | [44,57,58] |
| Exchange capacity (mmol/g dry) | 2.02 | 1.81 | [44] |
| Thickness of Air-Dried Membrane (µm) | 120 ± 5 | 165 ± 5 | [55,59] |
| Thickness of Wet Membrane (µm) | 140 ± 10 | 180 ± 10 | [55] |
| Conductivity (, mS/cm) | 6.1 | 2.8 | [44] |
| Density (g/cm3 wet) | 1.15 | 1.13 | [44] |
| Water Content (g H2O/g wet, %) | 29 ± 5 | 25 ± 2 | [44] |
| Permeability (mL/bar hm2) | 15 | 3.5 | [55,60] |
| Transport number | 0.985 | 0.996 | [44,61] |
| Coded | Factors | Symbol | Range and Levels | ||
|---|---|---|---|---|---|
| Low | Center | High | |||
| X1 | Concentration of Cu2+ (mg/L) | [Cu2+] | −1 10 | 055 | 1 100 |
| X2 | Concentration of Ni2+ (mg/L) | [Ni2+] | −1 10 | 055 | 1 100 |
| X3 | Concentration of Na+ (mol/L) | [Na+] | −1 0.01 | 00.055 | 1 0.1 |
| No. Exp | X1 | X2 | X3 | [Cu2+] | [Ni2+] | [Na+] | Y1exp(%) | Y1Cal(%) | Y2exp(%) | Y2Cal(%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 10 | 10 | 0.0100 | 40.23 | 40.586 | 40.00 | 40.472 |
| 2 | 1 | −1 | −1 | 100 | 10 | 0.0100 | 75.12 | 75.414 | 77.00 | 76.550 |
| 3 | −1 | 1 | −1 | 10 | 100 | 0.0100 | 75.00 | 74.915 | 74.50 | 75.172 |
| 4 | 1 | 1 | −1 | 100 | 100 | 0.0100 | 69.00 | 68.828 | 71.00 | 70.875 |
| 5 | −1 | −1 | 1 | 10 | 10 | 0.1000 | 33.33 | 33.512 | 34.75 | 34.837 |
| 6 | 1 | −1 | 1 | 100 | 10 | 0.1000 | 91.56 | 91.655 | 91.00 | 90.290 |
| 7 | −1 | 1 | 1 | 10 | 100 | 0.1000 | 63.71 | 63.426 | 66.00 | 66.412 |
| 8 | 1 | 1 | 1 | 100 | 100 | 0.1000 | 81.00 | 80.654 | 82.00 | 81.490 |
| 9 | −1 | 0 | 0 | 10 | 55 | 0.0550 | 64.53 | 64.361 | 65.11 | 63.467 |
| 10 | 1 | 0 | 0 | 100 | 55 | 0.0550 | 90.26 | 90.389 | 87.25 | 89.045 |
| 11 | 0 | −1 | 0 | 55 | 10 | 0.0550 | 86.02 | 85.093 | 78.00 | 78.601 |
| 12 | 0 | 1 | 0 | 55 | 100 | 0.0550 | 95.87 | 96.757 | 92.00 | 91.551 |
| 13 | 0 | 0 | −1 | 55 | 55 | 0.0100 | 77.08 | 76.687 | 78.80 | 78.231 |
| 14 | 0 | 0 | 1 | 55 | 55 | 0.1000 | 78.71 | 79.063 | 80.00 | 80.721 |
| 15 | 0 | 0 | 0 | 55 | 55 | 0.0550 | 90.00 | 90.026 | 87.00 | 86.898 |
| 16 | 0 | 0 | 0 | 55 | 55 | 0.0550 | 90.00 | 90.026 | 87.00 | 86.898 |
| 17 | 0 | 0 | 0 | 55 | 55 | 0.0550 | 90.00 | 90.026 | 87.00 | 86.898 |
| Y1(%) | Y2(%) | |||
|---|---|---|---|---|
| Coefficients | p-Values | Coefficients | p-Values | |
| b0 | 90.026 | 0.0000 | 86.898 | 0.0000 |
| b1 | 13.014 | 0.0000 | 12.789 | 0.0000 |
| b2 | 5.832 | 0.0000 | 6.475 | 0.0000 |
| b3 | 1.188 | 0.0004 | 1.245 | 0.0434 |
| b11 | −12.651 | 0.0000 | −10.642 | 0.0000 |
| b22 | 0.899 | 0.0422 | −1.822 | 0.0373 |
| b33 | −12.151 | 0.0000 | −7.422 | 0.0000 |
| b12 | −10.229 | 0.0000 | −10.094 | 0.0000 |
| b13 | 5.829 | 0.0000 | 4.844 | 0.0000 |
| b23 | −1.104 | 0.0012 | −0.781 | 0.0074 |
| R2 | 0.999 | 0.998 | ||
| Source Model | Degree of Freedom | Sum of Square | Mean of Square | F-Value | FtableFischer (α = 5%) | p-Value |
|---|---|---|---|---|---|---|
| Cu2+ | ||||||
| Regression | 9 | 4993.31 | 554.812 | 1575.6401 | 3.68 | 0.000< |
| Residual | 7 | 2.4649 | 0.352118 | |||
| Total | 16 | 4995.77 | ||||
| Ni2+ | ||||||
| Regression | 9 | 4258.75 | 473.194 | 360.3067 | 3.68 | 0.000< |
| Residual | 7 | 9.19317 | 1.31331 | |||
| Total | 16 | 4267.94 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaabani, N.; Ncib, S.; Dammak, L.; Larchet, C.; Bouguerra, W.; Elaloui, E. High-Efficiency Removal of Copper and Nickel via Donnan Dialysis Using Fujifilm Cation-Exchange Membranes: Process Optimization Through Response Surface Methodology. Membranes 2025, 15, 346. https://doi.org/10.3390/membranes15120346
Chaabani N, Ncib S, Dammak L, Larchet C, Bouguerra W, Elaloui E. High-Efficiency Removal of Copper and Nickel via Donnan Dialysis Using Fujifilm Cation-Exchange Membranes: Process Optimization Through Response Surface Methodology. Membranes. 2025; 15(12):346. https://doi.org/10.3390/membranes15120346
Chicago/Turabian StyleChaabani, Nabila, Sana Ncib, Lasâad Dammak, Christian Larchet, Wided Bouguerra, and Elimame Elaloui. 2025. "High-Efficiency Removal of Copper and Nickel via Donnan Dialysis Using Fujifilm Cation-Exchange Membranes: Process Optimization Through Response Surface Methodology" Membranes 15, no. 12: 346. https://doi.org/10.3390/membranes15120346
APA StyleChaabani, N., Ncib, S., Dammak, L., Larchet, C., Bouguerra, W., & Elaloui, E. (2025). High-Efficiency Removal of Copper and Nickel via Donnan Dialysis Using Fujifilm Cation-Exchange Membranes: Process Optimization Through Response Surface Methodology. Membranes, 15(12), 346. https://doi.org/10.3390/membranes15120346








