Fabrication of Polyamide Thin-Film Composite/Polyethersulfone-Coreshell-Fe3O4/ZnO Membranes for the Efficient Removal of Pb(II) from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PES/Fe3O4/ZnO Nanofiltration Membranes
2.2. Collection and Analyses of Wastewater Samples
2.3. Characterization Techniques
2.3.1. Physiochemical Analysis
2.3.2. Rejection Studies
2.3.3. Performance Studies
2.3.4. Adsorption Studies
2.3.5. Desorption Studies
3. Results and Discussion
3.1. Morphological and Topographical Studies of PES-Fe3O4/ZnO Nanofiltration Membranes
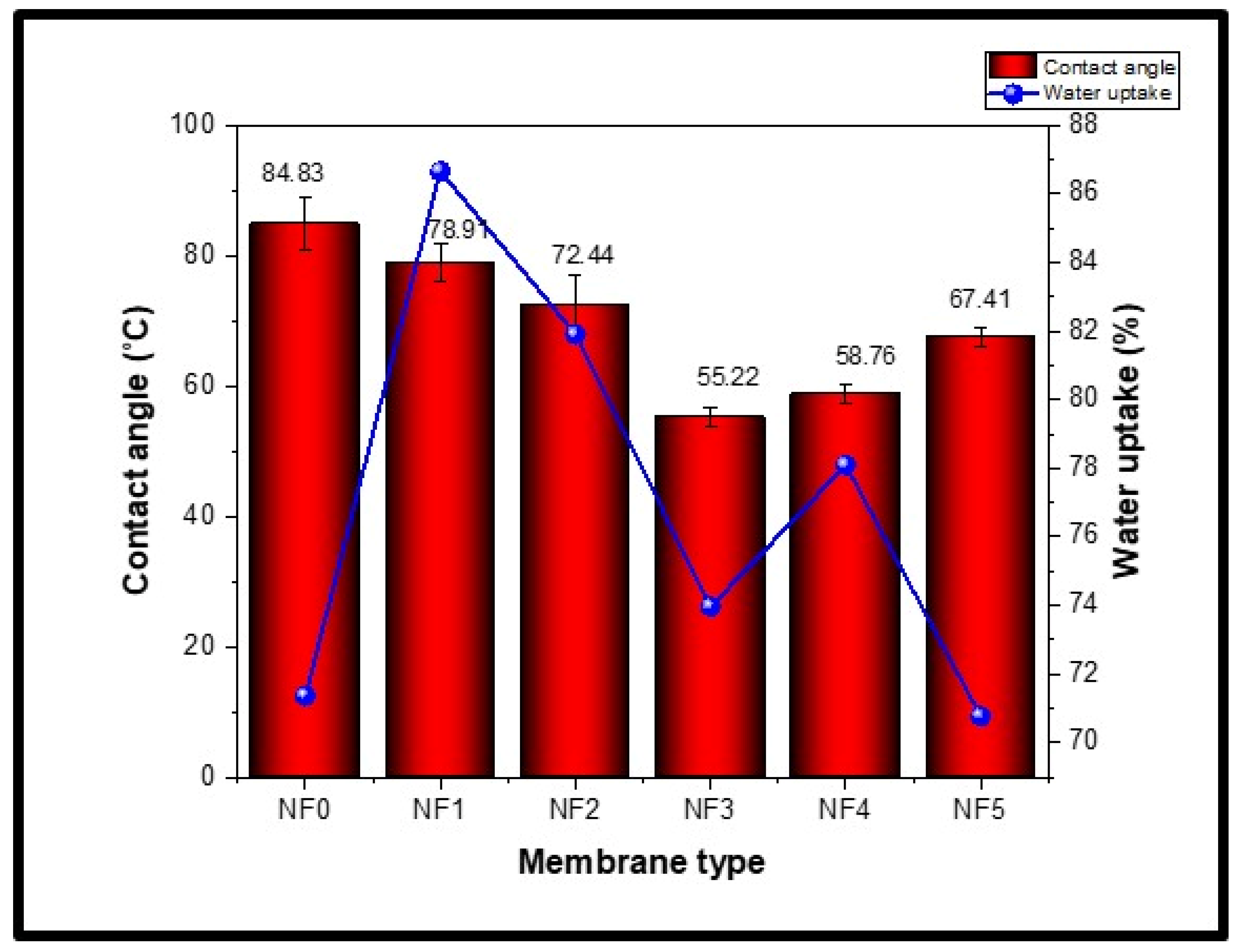
3.2. Hydrophilic Properties of PES-Fe3O4/ZnO Nanofiltration Membranes
3.3. Porosity and Pore Size
3.4. Thermal Properties of NF Membranes
3.5. Surface Chemistry of PES-Fe3O4/ZnO NF Membranes
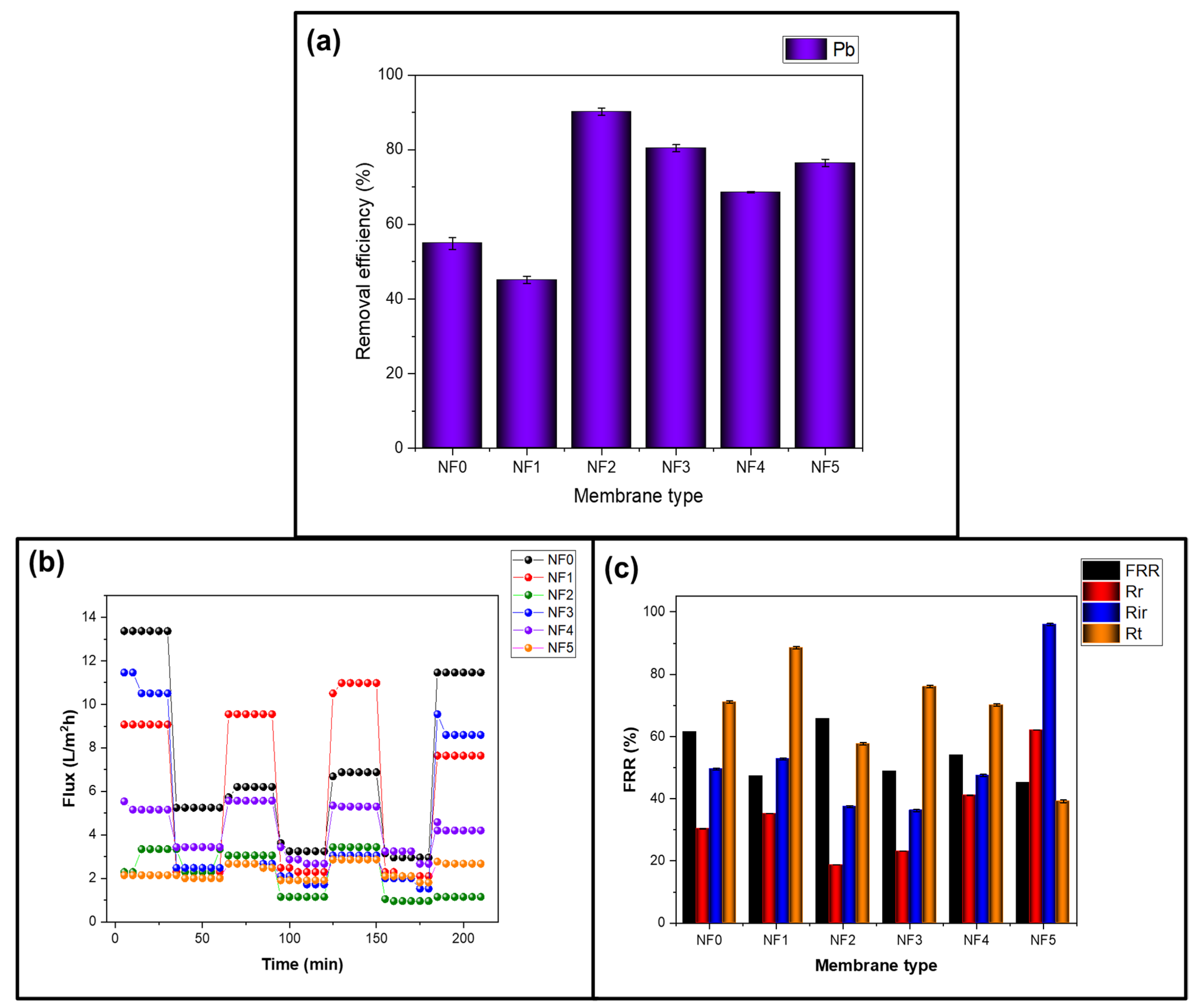
3.6. Performance Evaluation for PES-Fe3O4/ZnO Nanofiltration Membranes
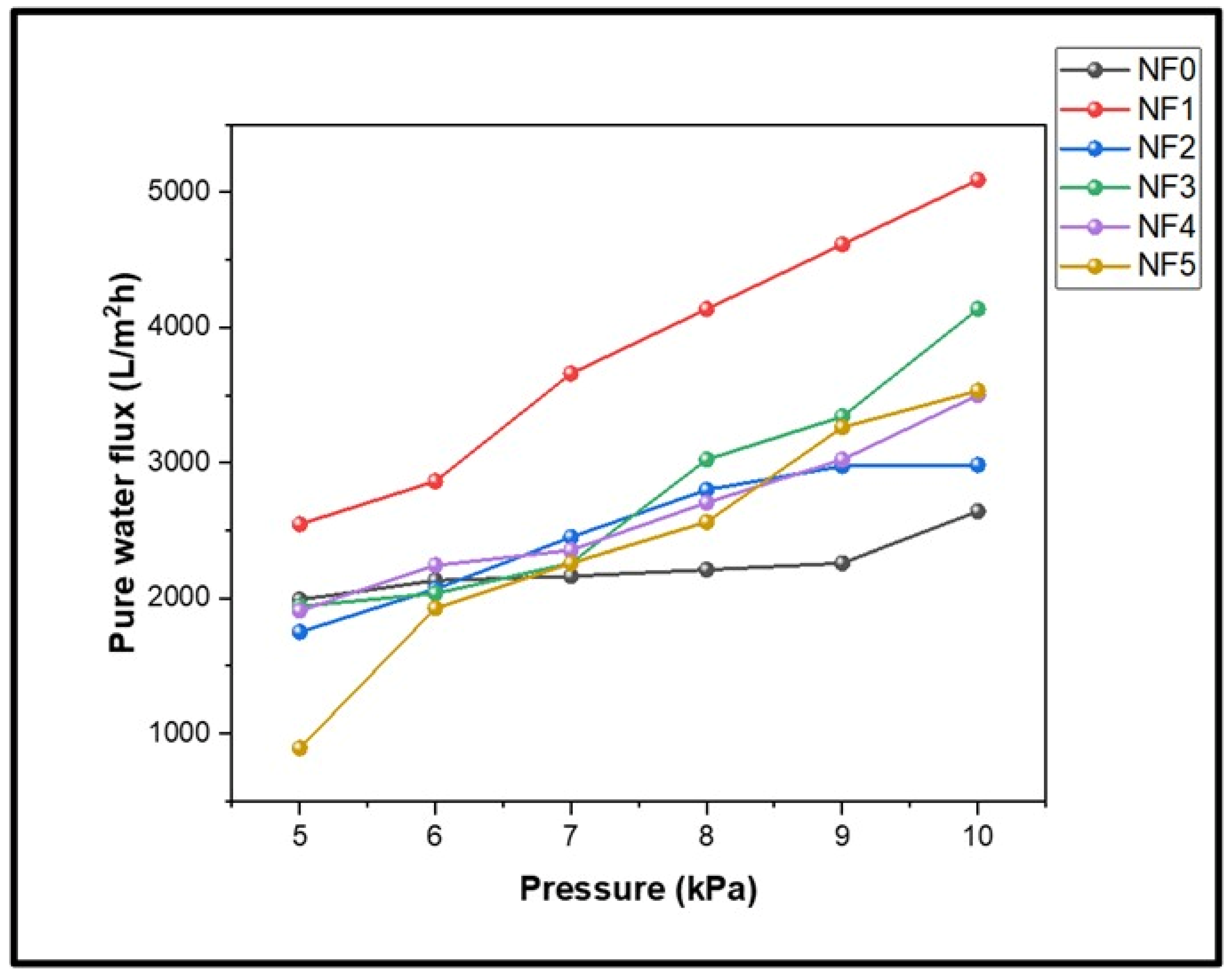
3.6.1. Permeation Performance
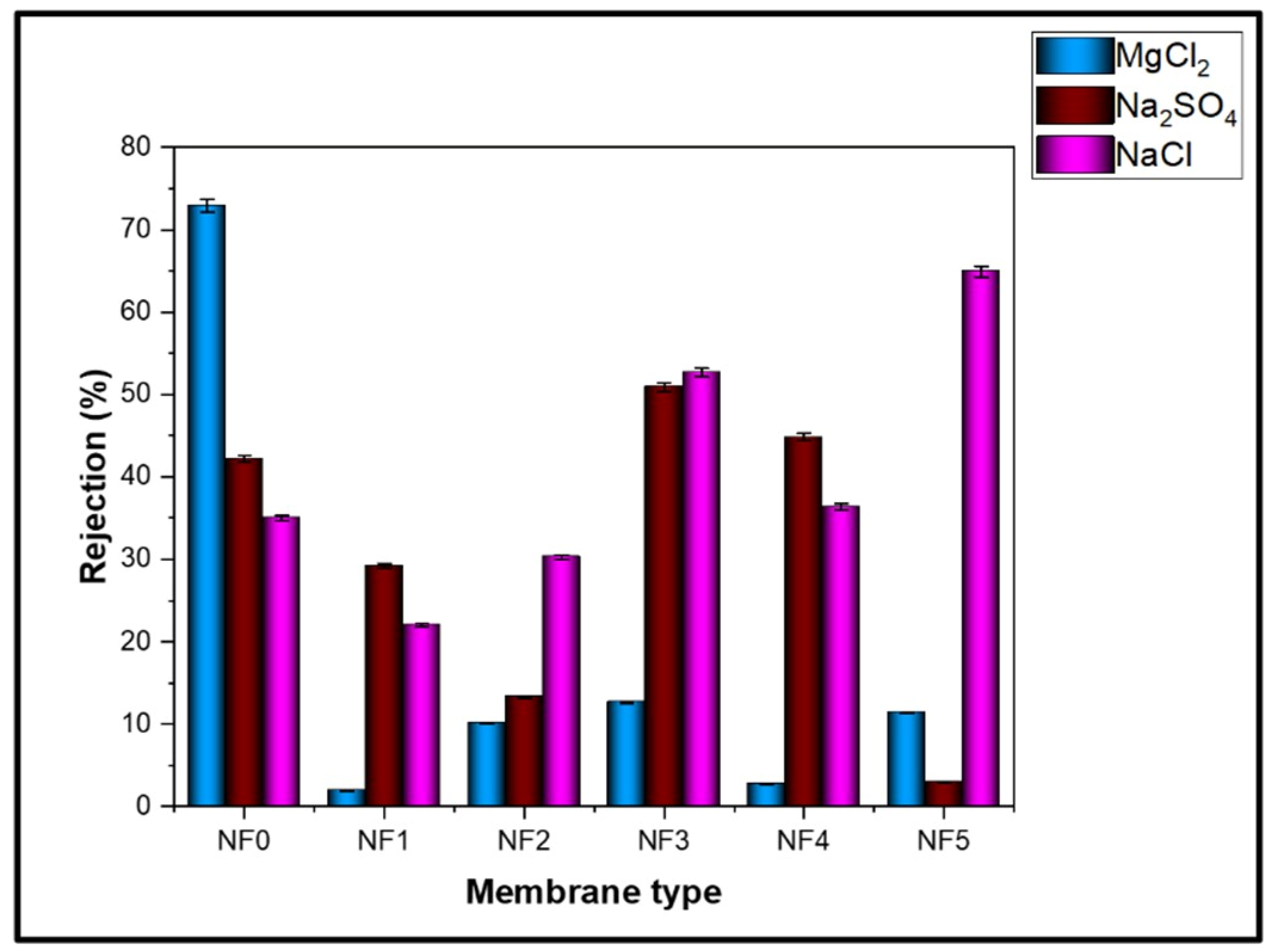
3.6.2. Rejection of Na2SO4, NaCl, and MgCl2 Salts
3.6.3. Physicochemical Characterization of Environmental Wastewater Samples
3.6.4. Heavy Metal Ions Rejection and Fouling Propensity of the Prepared Membranes
3.6.5. SEM and EDX Spectra Analysis After Performance Studies of PES-Fe3O4/ZnO Nanofiltration Membranes
| Membrane Type | Monomer Type and Concentration (w/v%) | Salt Type and Interception (%) | Heavy Metal Rejection (%) | Reference |
|---|---|---|---|---|
| NF3 | TMC (0.10) | MgCl2 (12.67) Na2SO4 (50.91) NaCl (52.64) | Pb(II) (80.39, Spectroquant) Pb(II)(87.09, ICP-MS) | This study |
| MMT-Fe3O4/PES | NaCl (39.8) Na2SO4 (31.2) MgSO4 (39.4) | Zn2+ (52.5) Cu2+ (42.8) Ni2+ (35.0) Cd2+ (37.9) | [64] | |
| Ag@ZnO-OAc/PA | TMC (0.15) | Na2SO4 (98.84) NaCl (59.94) | [65] | |
| PES with citric acid as an additive | NaCl (26.83) MgSO4 (89.36) | - | [66] | |
| Piperazine (PIP)-based polyamide (PA) | TMC (0.10) TMC (0.02) | MgCl2 (94.91) Na2SO4 (95.96) Na2SO4 (98.44) MgCl2 (92.12) NaCl (37.41) | - | [41] |
| PES/MOF | TMC (0.10) | NaCl (97.4) | [67] | |
| Dual layer polybenzimidazole/PES | Cr2+ (98) Pb2+ (93) Cd2+ (70) | [68] | ||
| Polyamide (PA) | Na2SO4 (97.7) | Cu2+ (93.9) Mn2+ (97.9) Cd2+ (87.7) | [69] | |
| NF90 NF270 | MgSO4 (≥97) NaCl (85–95) MgSO4 (≥97) NaCl (40–60) | [70] |
4. Adsorption Studies for the Removal of Lead (II) from Environmental Water Samples
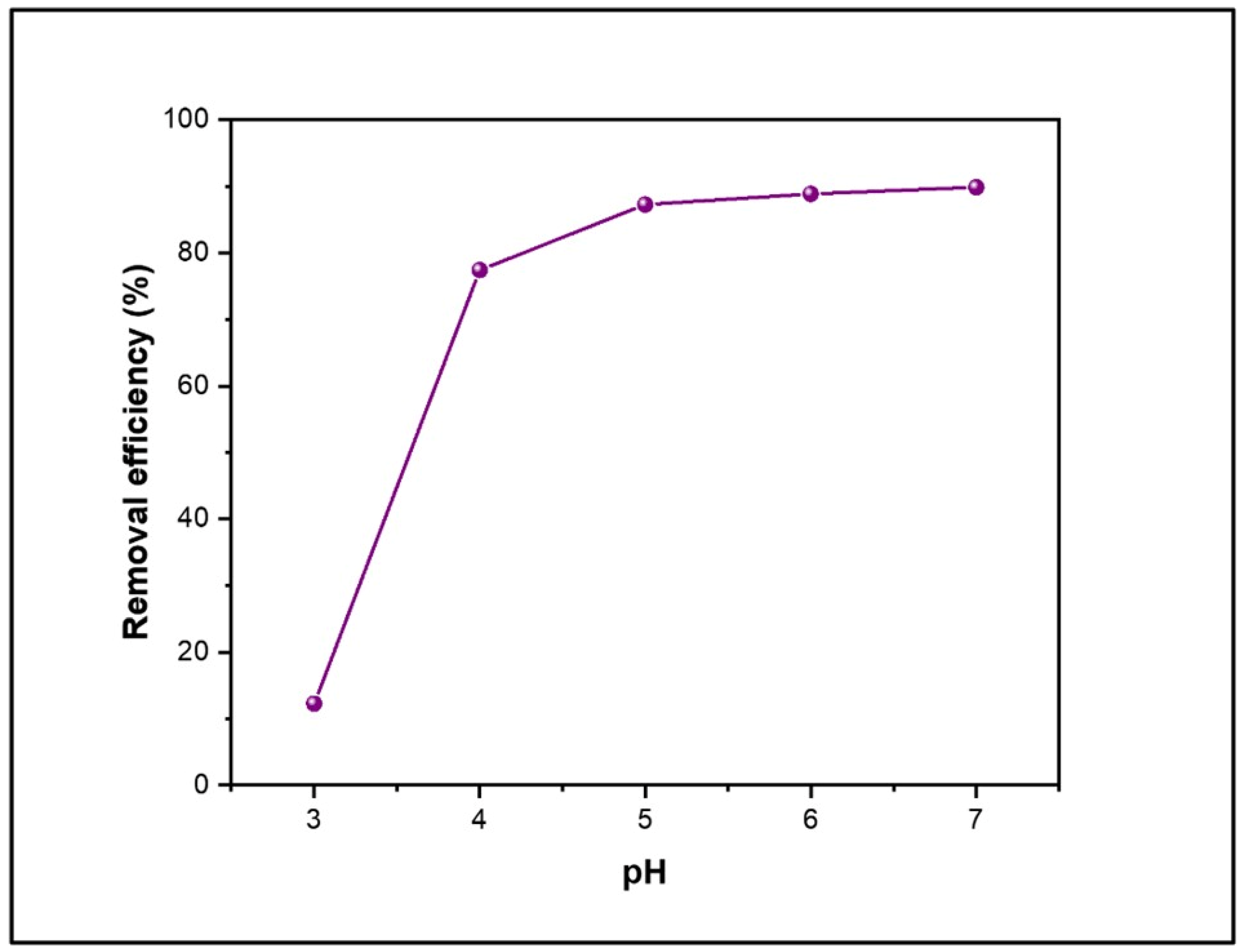
4.1. Effect of pH
4.2. Effect of Concentration
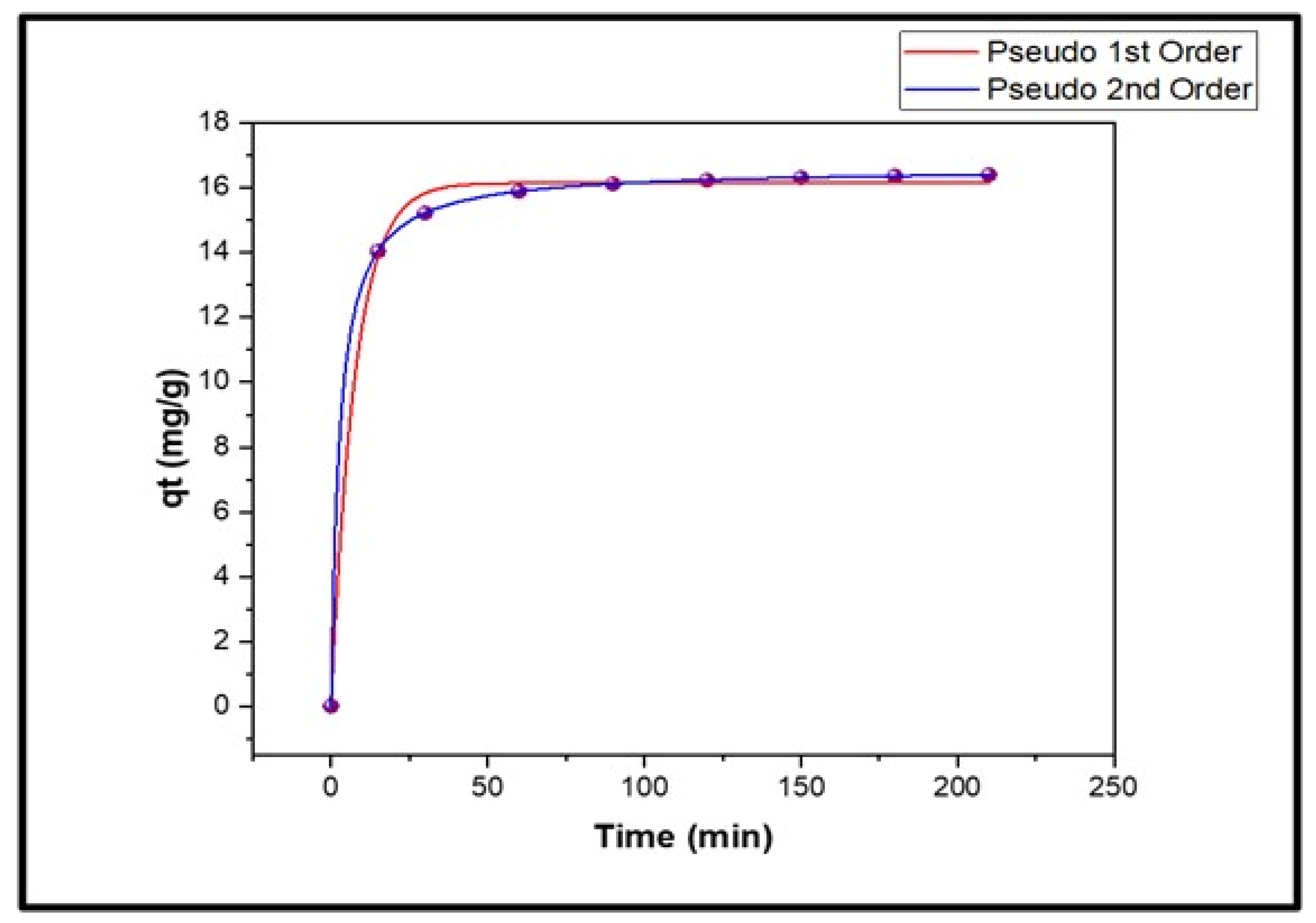
4.3. Effect of Time
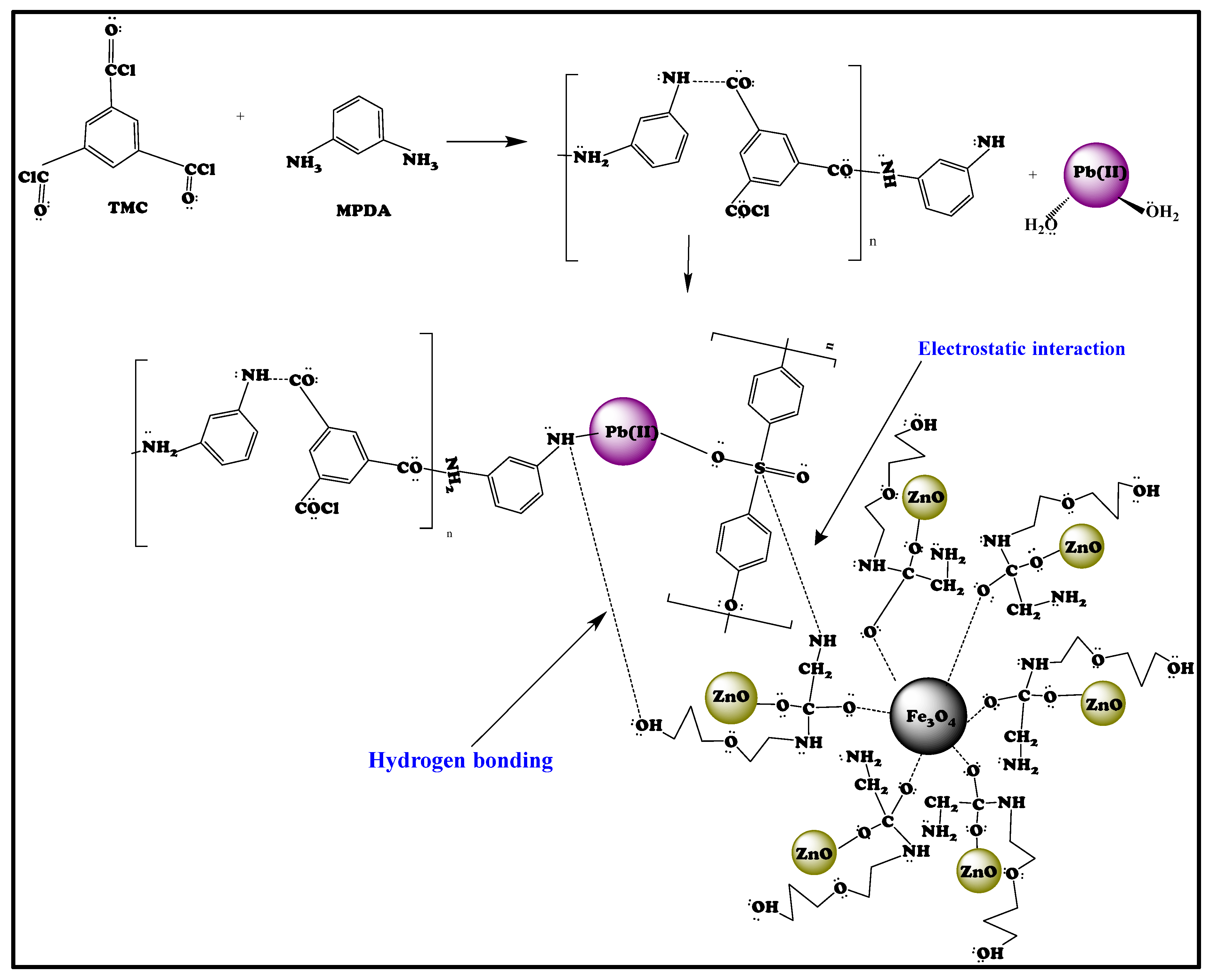
4.4. Potential Mechanism
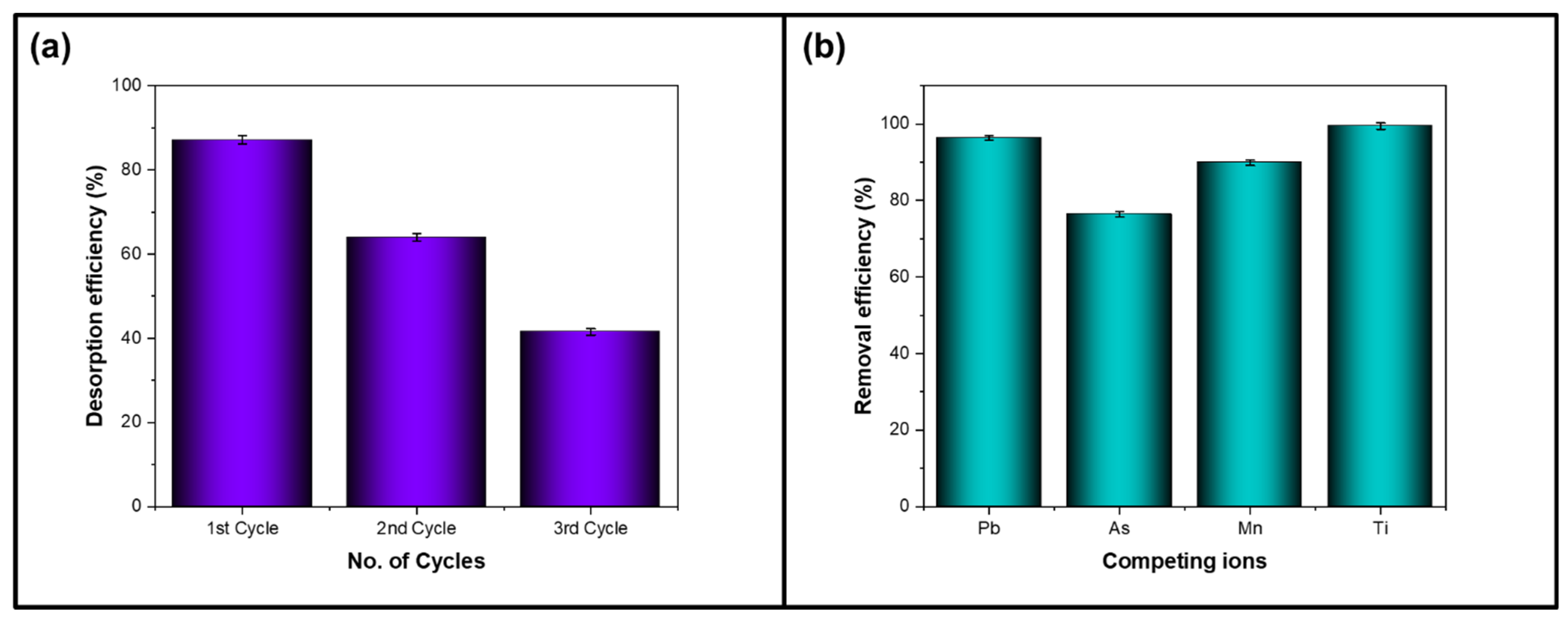
4.5. Reusability and Desorption Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boldyrev, M. Lead: Properties, History, and Applications. WikiJournal Sci. 2018, 1, 1–23. [Google Scholar] [CrossRef]
- Kumar, N.; Jha, A.K. Lead Toxicity: Challenges and Solution; Springer: New York, NY, USA, 2023. [Google Scholar]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Chapter 1. Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. In Heavy Metals in Water: Presence, Removal and Safety; RSC Publishing: Cambridge, UK, 2014; ISBN 9781782620174. [Google Scholar]
- Abadin, H.; Ashizawa, A.; Stevens, Y.-W.; Llados, F.; Diamond, G.; Sage, G.; Citra, M.; Quinones, A.; Bosch, S.J.; Swarts, S.G. Toxicological Profile for Lead; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2020; p. 582. Available online: https://stacks.cdc.gov/view/cdc/95222 (accessed on 31 July 2025).
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Praipipat, P.; Ngamsurach, P.; Joraleeprasert, T. Synthesis, Characterization, and Lead Removal Efficiency of Orange Peel Powder and Orange Peel Powder Doped Iron (III) Oxide-Hydroxide. Sci. Rep. 2023, 13, 10772. [Google Scholar] [CrossRef]
- Samra, S.E. Biosorption of Pb2+ from Natural Water using Date Pits: A Green Chemistry Approach. Mod. Chem. Appl. 2014, 2, 1000131. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, C.; Lü, T.; Zhao, H. Removal of Pb(II) Ions from Wastewater by Using Polyethyleneimine-Functionalized Fe3O4 Magnetic Nanoparticles. Appl. Sci. 2020, 10, 948. [Google Scholar] [CrossRef]
- Mirza, M.; Bodaghifard, M.A.; Darvish, F. Synthesis of a Nitrogen-Rich Dendrimer Grafted on Magnetic Nanoparticles for Efficient Removal of Pb(II) and Cd(II) Ions. RSC Adv. 2024, 14, 32559–32572. [Google Scholar] [CrossRef]
- Madlangbayan, G.A.C.; Quiton, K.G.N.; Lu, M.C. Removal of Lead and Nitrate from Simulated Lead- and Nitrate-Containing Wastewater via Hydroxide Precipitation. Processes 2024, 12, 1662. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Mostafa, E. Nanofiltration Membranes for the Removal of Heavy Metals from Aqueous Solutions: Preparations and Applications. Membranes 2023, 13, 789. [Google Scholar] [CrossRef]
- Huang, Z.-Q.; Cheng, Z.F. Recent advances in adsorptive membranes for removal of harmful cations. J. Appl. Polym. Sci. 2020, 137, 48579. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, P.; Fang, R.; Cai, J.; Zhang, L.D.; He, Q.Y.; Zhou, Z.H.; Sun, S.P.; Cao, X.L. Constructing Positively Charged Acid-Resistant Nanofiltration Membranes via Surface Postgrafting for Efficient Removal of Metal Ions from Electroplating Rinse Wastewater. Sep. Purif. Technol. 2022, 297, 121500. [Google Scholar] [CrossRef]
- Huang, B.Q.; Tang, Y.J.; Zeng, Z.X.; Xu, Z.L. Microwave Heating Assistant Preparation of High Permselectivity Polypiperazine-Amide Nanofiltration Membrane during the Interfacial Polymerization Process with Low Monomer Concentration. J. Memb. Sci. 2020, 596, 117718. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite Membranes for Water Separation and Purification: Fabrication, Modification, and Applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of Polyethersulfone Membranes—A Review of Methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Kubheka, N.S.M.; Nxumalo, E.N.; Managa, M.; Matlala, M.; Moloto, M.J. Fabrication, Modification, and Mechanism of Nanofiltration Membranes for the Removal of Heavy Metal Ions from Wastewater. ChemistrySelect 2023, 8, e202300741. [Google Scholar] [CrossRef]
- Su, W.; Liu, L.; Chen, Y.; Cui, J.; Zhao, X. Preparation of thin-film composite membrane with Turing structure by PEO-assisted interfacial polymerization combined with choline chloride modification to improve permeability. J. Taiwan Inst. Chem. Eng. 2023, 145, 104822. [Google Scholar] [CrossRef]
- Covaliu-Mierlă, C.I.; Păunescu, O.; Iovu, H. Recent Advances in Membranes Used for Nanofiltration to Remove Heavy Metals from Wastewater: A Review. Membranes 2023, 13, 643. [Google Scholar] [CrossRef]
- Kumar, S.A.; Srinivasan, G.; Govindaradjane, S. A Novel Synergistic Effect of TiO2 and ZnO Incorporation in PES-Based Thin-Film Nanocomposite Nanofiltration Membrane for Treatment of Textile Wastewater. Environ. Monit. Assess 2022, 194. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Afshari, M.; Fazlali, A.R.; Koudzari Farahani, S.; Bandehali, S.; Van der Bruggen, B.; Bagheripour, E. Mixed Matrix PES-Based Nanofiltration Membrane Decorated by (Fe3O4–Polyvinylpyrrolidone) Composite Nanoparticles with Intensified Antifouling and Separation Characteristics. Chem. Eng. Res. Des. 2019, 147, 390–398. [Google Scholar] [CrossRef]
- Richards, H.L.; Baker, P.G.L.; Iwuoha, E. Metal Nanoparticle Modified Polysulfone Membranes for Use in Wastewater Treatment: A Critical Review. Surf. Eng. Mater. Adv. Technol. 2012, 2012, 183–193. [Google Scholar] [CrossRef]
- Shahruddin, M.Z.; Binti Ishak, I.S.; Othman, N.H.; Alias, N.H.; Ramlee, N.A. The Effectiveness Study of Different Membranes in Treating Industrial Wastewater. MATEC Web Conf. 2016, 69. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Chen, X.; Feng, S.; Wan, Y.; Lu, H.; Luo, J. A Nanofiltration Membrane with Outstanding Antifouling Ability: Exploring the Structure-Property-Performance Relationship. J. Memb. Sci. 2023, 668, 121205. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Hang, X.; Wan, Y. Physicochemical Characterization of Tight Nanofiltration Membranes for Dairy Wastewater Treatment. J. Memb. Sci. 2018, 547, 51–63. [Google Scholar] [CrossRef]
- Paul, M.; Jons, S.D. Chemistry and Fabrication of Polymeric Nanofiltration Membranes: A Review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Jalanni, N.A.; Abu Seman, M.N.; Faizal, C.K.M. Synthesis and Performance of Thin Film Composite Nanofiltration Polyester Membrane for Removal of Natural Organic Matter Substances. ASEAN J. Chem. Eng. 2012, 12, 73–80. [Google Scholar] [CrossRef]
- Mohan, D.J.; Kullová, L. A Study on the Relationship between Preparation Condition and Properties/Performance of Polyamide TFC Membrane by IR, DSC, TGA, and SEM Techniques. Desalination Water Treat. 2013, 51, 586–596. [Google Scholar] [CrossRef]
- Tian, J.; Chang, H.; Gao, S.; Zhang, R. How to Fabricate a Negatively Charged NF Membrane for Heavy Metal Removal via the Interfacial Polymerization between PIP and TMC? Desalination 2020, 491, 114499. [Google Scholar] [CrossRef]
- Abedi, F.; Dubé, M.A.; Kruczek, B. Adsorption of Heavy Metals on the Surface of Nanofiltration Membranes: “A Curse or Blessing”? J. Memb. Sci. 2023, 685. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Al-Jubouri, S.M.; Zouli, N.; Mohammed, A.A.; Majdi, H.S.; Salih, I.K.; Al-Shaeli, M.; Al-Rahawi, A.M.I.; Alsalhy, Q.F.; Figoli, A. Performance Evaluation of Polyethersulfone Membranes for Competitive Removal of Cd2+, Co2+, and Pb2+Ions from Simulated Groundwater. Geofluids 2021, 2021. [Google Scholar] [CrossRef]
- Masenye, E.R.; Mabuba, N.; Phumzile, S. Nanofiltration Membrane Based on Hyperbranched Polyethyleneimine-Functionalised Multiwalled Carbon Nanotubes for Pb (II) Removal from Water. Int. J. Environ. Anal. Chem. 2020, 102, 5601–5618. [Google Scholar] [CrossRef]
- Vatanpour, V.; Soylu, S.; Osman, D.; Tuncay, G.; Mobaraki, A.; Marjani, Z.; Ziyaei Halimehjani, A.; Koyuncu, I. Improvement in Dye and Lead Removal Efficiency of PES Membranes by Blending Sulfur Containing Adsorbent Nanomaterials. J. Environ. Chem. Eng. 2024, 12, 113308. [Google Scholar] [CrossRef]
- Hanif, A.; Ali, S.; Hanif, M.A.; Rashid, U.; Bhatti, H.N.; Asghar, M.; Alsalme, A.; Giannakoudakis, D.A. A Novel Combined Treatment Process of Hybrid Biosorbent—Nanofiltration for Effective Pb (II) Removal from Wastewater. Water 2021, 13, 3316. [Google Scholar] [CrossRef]
- Kubheka, N.S.M.; Managa, M.E.; Motsa, M.M.; Nxumalo, E.N.; Moloto, M.J. Optimizing Influential Phase Separation Parameters on Polyethersulfone/Fe3O4/ZnO Membranes for Environmental Wastewater. J. Ind. Eng. Chem. 2024, 141, 228–242. [Google Scholar] [CrossRef]
- Ramezani Darabi, R.; Jahanshahi, M.; Peyravi, M. A Support Assisted by Photocatalytic Fe3O4/ZnO Nanocomposite for Thin-Film Forward Osmosis Membrane. Chem. Eng. Res. Des. 2018, 133, 11–25. [Google Scholar] [CrossRef]
- Kubheka, N.S.M.; Nxumalo, E.N.; Managa, M.; Moloto, M.J. Enhancing the Photocatalytic Activity of Co-Precipitation Synthesized Fe3O4/ZnO Nanocomposites for Rhodamine B Degradation. Asian J. Chem. 2025, 37, 641–652. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Optimization, Characterization and Nanofiltration Properties Test of MWNTs/Polyester Thin Film Nanocomposite Membrane. J. Memb. Sci. 2013, 428, 425–433. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Wang, L.; Wang, J.; Wang, X.; Hao, J. Probing the Construction Mechanism of Polyamide Membranes Regulated by Interfacial Polymerization from the Novel Micro- and Macro- Perspectives: A Review. Desalination 2024, 578. [Google Scholar] [CrossRef]
- Xu, J.; Yan, H.; Zhang, Y.; Pan, G.; Liu, Y. The Morphology of Fully-Aromatic Polyamide Separation Layer and Its Relationship with Separation Performance of TFC Membranes. J. Memb. Sci. 2017, 541, 174–188. [Google Scholar] [CrossRef]
- Bai, C.; Gu, Z.; Gao, X.; Zhang, R.; Tang, J.; Xiao, Q.; Yu, S.; Ning, R. Stepwise Increasing TMC Concentration in NF Membranes Fabrication for Controlled PA Layer Thickness, Compactness, and Charge Distribution: Maintaining High Permeance, Enhancing Desalination, and Mechanistic Insights. Sep. Purif. Technol. 2025, 359, 130404. [Google Scholar] [CrossRef]
- Behera, S.; Akkihebbal, S.K. Intrinsic Kinetics of Interfacial Polycondensation Reactions– the Reaction of MPDA with TMC. Polymer 2020, 210, 122982. [Google Scholar] [CrossRef]
- Aouled, G.; Raza, S.; Ghasali, E.; Hayat, A.; Orooji, Y. Preparation and Characterization of Polyethersulfone-Ultrafiltration Membrane Blended with Terbium-Doped Cerium Magnesium Aluminate: Analysis of Fouling Behavior. Molecules 2023, 28, 2688. [Google Scholar] [CrossRef]
- Kasim, N.; Mohammad, A.W.; Abdullah, S.R.S. Characterization of Hydrophilic Nanofiltration and Ultrafiltration Membranes for Groundwater Treatment as Potable Water Resources. Desalination Water Treat. 2016, 57, 7711–7720. [Google Scholar] [CrossRef]
- Chan, M.; Ng, S. Effect of Membrane Properties on Contact Angle. AIP Conf. Proc. 2018, 2016. [Google Scholar] [CrossRef]
- Behera, S.; Suresh, A.K. Data on of Interfacial Hydrolysis Kinetics of an Aromatic Acid Chloride. Data Brief 2019, 26, 104337. [Google Scholar] [CrossRef]
- Baig, U.; Waheed, A. Exploiting Interfacial Polymerization to Fabricate Hyper-Cross-Linked Nanofiltration Membrane with a Constituent Linear Aliphatic Amine for Freshwater Production. NPJ Clean Water 2022, 5, 46. [Google Scholar] [CrossRef]
- Ormanci-Acar, T.; Tas, C.E.; Keskin, B.; Ozbulut, E.B.S.; Turken, T.; Imer, D.; Tufekci, N.; Menceloglu, Y.Z.; Unal, S.; Koyuncu, I. Thin-Film Composite Nanofiltration Membranes with High Flux and Dye Rejection Fabricated from Disulfonated Diamine Monomer. J. Memb. Sci. 2020, 608, 118172. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Vatanpour, V.; Khoshnam, M.; Zargar, M. Recent Advancements in the Application of New Monomers and Membrane Modification Techniques for the Fabrication of Thin Film Composite Membranes: A Review. React. Funct. Polym. 2021, 166. [Google Scholar] [CrossRef]
- Lari, S.; Parsa, S.A.M.; Akbari, S.; Emadzadeh, D.; Lau, W.J. Fabrication and Evaluation of Nanofiltration Membrane Coated with Amino-Functionalized Graphene Oxide for Highly Efficient Heavy Metal Removal. Int. J. Environ. Sci. Technol. 2022, 19, 4615–4626. [Google Scholar] [CrossRef]
- Xu, C.; Shen, X.; Yang, Y.; Fu, C.; Liu, L.; Zeng, Z.; Sun, Z.; Zhu, L.; Wang, G. Turing Structured Nanofiltration Membrane Fabricated by Sulfonated Cellulose Nanocrystals Mediated Interfacial Polymerization for Enhanced Permeability Separation Performance. Sep. Purif. Technol. 2025, 357, 130112. [Google Scholar] [CrossRef]
- Suhalim, N.S.; Kasim, N.; Mahmoudi, E.; Shamsudin, I.J.; Mohammad, A.W.; Zuki, F.M.; Jamari, N.L.A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials 2022, 12, 437. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Dai, J.; Ye, Z.; Wu, L. Nanofiltration Membrane with Surface Nanostructure Induced by Interfacial Modification for Heavy Metal Ions Removal, Salt Selection and Antibacterial. J. Environ. Chem. Eng. 2024, 12, 112439. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A. Effect of Added NaX Nano-Zeolite into Polyamide as a Top Thin Layer of Membrane on Water Flux and Salt Rejection in a Reverse Osmosis Process. J. Memb. Sci. 2011, 375, 88–95. [Google Scholar] [CrossRef]
- Hu, J.; Lv, Z.; Xu, Y.; Zhang, X.; Wang, L. Fabrication of a High-Flux Sulfonated Polyamide Nanofiltration Membrane: Experimental and Dissipative Particle Dynamics Studies. J. Memb. Sci. 2016, 505, 119–129. [Google Scholar] [CrossRef]
- Roth, R.S.; Birnhack, L.; Avidar, M.; Hjelvik, E.A.; Straub, A.P.; Epsztein, R. Effect of Solution Ions on the Charge and Performance of Nanofiltration Membranes. NPJ Clean Water 2024, 7, 25. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, S.; Shi, W.; Wang, W.; Wang, X.; Zhang, Z.; Li, L.; Zhang, B.; Bao, X. A Novel Polyesteramide Thin Film Composite Nanofiltration Membrane Prepared by Interfacial Polymerization of Serinol and Trimesoyl Chloride (TMC) Catalyzed by 4-dimethylaminopyridine (DMAP). J. Memb. Sci. 2017, 542, 68–80. [Google Scholar] [CrossRef]
- Molewa, B.E. Revision of General Authorisations in Terms of Section 39 of The National Water Act, 1998 (ACT NO. 36 OF 1998) (THE ACT). Dep. Water Aff. For. 2013, 665, 3–31. [Google Scholar]
- Drop, G.; Assessment, P. Green Drop Report 2023; Department of Water and Sanitation: Pretoria, South Africa, 2023. [Google Scholar]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of Dye and Heavy Metal Ion Using a Novel Synthetic Polyethersulfone Nanofiltration Membrane Modified by Magnetic Graphene Oxide/Metformin Hybrid. J. Memb. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhu, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Construction of TiO2@graphene Oxide Incorporated Antifouling Nanofiltration Membrane with Elevated Filtration Performance. J. Memb. Sci. 2017, 533, 279–288. [Google Scholar] [CrossRef]
- Simon, A.; Price, W.E.; Nghiem, L.D. Changes in Surface Properties and Separation Efficiency of a Nanofiltration Membrane after Repeated Fouling and Chemical Cleaning Cycles. Sep. Purif. Technol. 2013, 113, 42–50. [Google Scholar] [CrossRef]
- Purushothaman, M.; Arvind, V.; Saikia, K.; Vaidyanathan, V.K. Fabrication of Highly Permeable and Anti-Fouling Performance of Poly(Ether Ether Sulfone) Nanofiltration Membranes Modified with Zinc Oxide Nanoparticles. Chemosphere 2022, 286, 131616. [Google Scholar] [CrossRef]
- Gozali Balkanloo, P.; Mahmoudian, M.; Hosseinzadeh, M.T. A Comparative Study between MMT-Fe3O4/PES, MMT-HBE/PES, and MMT-Acid Activated/PES Mixed Matrix Membranes. Chem. Eng. J. 2020, 396, 125188. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Feng, X.; Hu, X.; Zhang, Y.; Liu, L. Incorporation of Oleic Acid-Modified Ag@ZnO Core-Shell Nanoparticles into Thin Film Composite Membranes for Enhanced Antifouling and Antibacterial Properties. J. Memb. Sci. 2020, 602, 117956. [Google Scholar] [CrossRef]
- Alaei Shahmirzadi, M.A.; Hosseini, S.S.; Ruan, G.; Tan, N.R. Tailoring PES Nanofiltration Membranes through Systematic Investigations of Prominent Design, Fabrication and Operational Parameters. RSC Adv. 2015, 5, 49080–49097. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, X.; Li, X.; Wang, Z.; Tang, C.Y. Metal-Organic Framework Nanosheets for Thin-Film Composite Membranes with Enhanced Permeability and Selectivity. ACS Appl. Nano Mater. 2020, 3, 9238–9248. [Google Scholar] [CrossRef]
- Zhu, W.P.; Sun, S.P.; Gao, J.; Fu, F.J.; Chung, T.S. Dual-Layer Polybenzimidazole/Polyethersulfone (PBI/PES) Nanofiltration (NF) Hollow Fiber Membranes for Heavy Metals Removal from Wastewater. J. Memb. Sci. 2014, 456, 117–127. [Google Scholar] [CrossRef]
- Cheng, X.; Lai, C.; Li, J.; Zhou, W.; Zhu, X.; Wang, Z.; Ding, J.; Zhang, X.; Wu, D.; Liang, H.; et al. Toward Enhancing Desalination and Heavy Metal Removal of TFC Nanofiltration Membranes: A Cost-Effective Interface Temperature-Regulated Interfacial Polymerization. ACS Appl. Mater. Interfaces 2021, 13, 57998–58010. [Google Scholar] [CrossRef]
- Imbrogno, A.; Schäfer, A.I. Comparative Study of Nanofiltration Membrane Characterization Devices of Different Dimension and Configuration (Cross Flow and Dead End). J. Memb. Sci. 2019, 585, 67–80. [Google Scholar] [CrossRef]
- Hadi, S.; Mohammed, A.A.; Al-Jubouri, S.M.; Abd, M.F.; Majdi, H.S.; Alsalhy, Q.F.; Rashid, K.T.; Ibrahim, S.S.; Salih, I.K.; Figoli, A. Experimental and Theoretical Analysis of Lead Pb2+ and Cd2+ Retention from a Single Salt Using a Hollow Fiber PES Membrane. Membranes 2020, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, N. of lead ions from water using P. nanocomposite membrane incorporated with polyaniline modified G. nanoparticles: P. optimization by central composite design; Zereshki, S.; Heidari, S. Removal of Lead Ions from Water Using PES-Based Nanocomposite Membrane Incorporated with Polyaniline Modified GO Nanoparticles: Performance Optimization by Central Composite Design. Process Saf. Environ. Prot. 2017, 111, 475–490. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Rozbu, M.R.; Chowdhury, A.T.; Nuzhat, S.; Rafa, N.; Mahlia, T.M.I.; Ong, H.C.; Mofijur, M. Heavy Metal Toxicity, Sources, and Remediation Techniques for Contaminated Water and Soil. Environ. Technol. Innov. 2022, 25, 102114. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.P.; Macena, M.; Esteves, B.; Guiné, R.P.F. Ideal PH for the Adsorption of Metal Ions Cr6+, Ni2+, Pb2+in Aqueous Solution with Different Adsorbent Materials. Open Agric. 2021, 6, 115–123. [Google Scholar] [CrossRef]
- Ravishankar, H.; Wang, J.; Shu, L.; Jegatheesan, V. Removal of Pb (II) Ions Using Polymer Based Graphene Oxide Magnetic Nano-Sorbent. Process Saf. Environ. Prot. 2016, 104, 472–480. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Clean Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Rajput, S.; Pittman, C.U.; Mohan, D. Magnetic Magnetite (Fe3O4) Nanoparticle Synthesis and Applications for Lead (Pb2+) and Chromium (Cr6+) Removal from Water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef]
- Maximous, N.N.; Nakhla, G.F.; Wan, W.K. Removal of Heavy Metals from Wastewater by Adsorption and Membrane Processes: A Comparative Study. World Acad. Sci. Eng. Technol. 2010, 40, 599–604. [Google Scholar]
- Cheng, X.; Qin, Y.; Ye, Y.; Chen, X.; Wang, K.; Zhang, Y.; Figoli, A.; Drioli, E. Finely Tailored Pore Structure of Polyamide Nanofiltration Membranes for Highly-Efficient Application in Water Treatment. Chem. Eng. J. 2021, 417, 127976. [Google Scholar] [CrossRef]
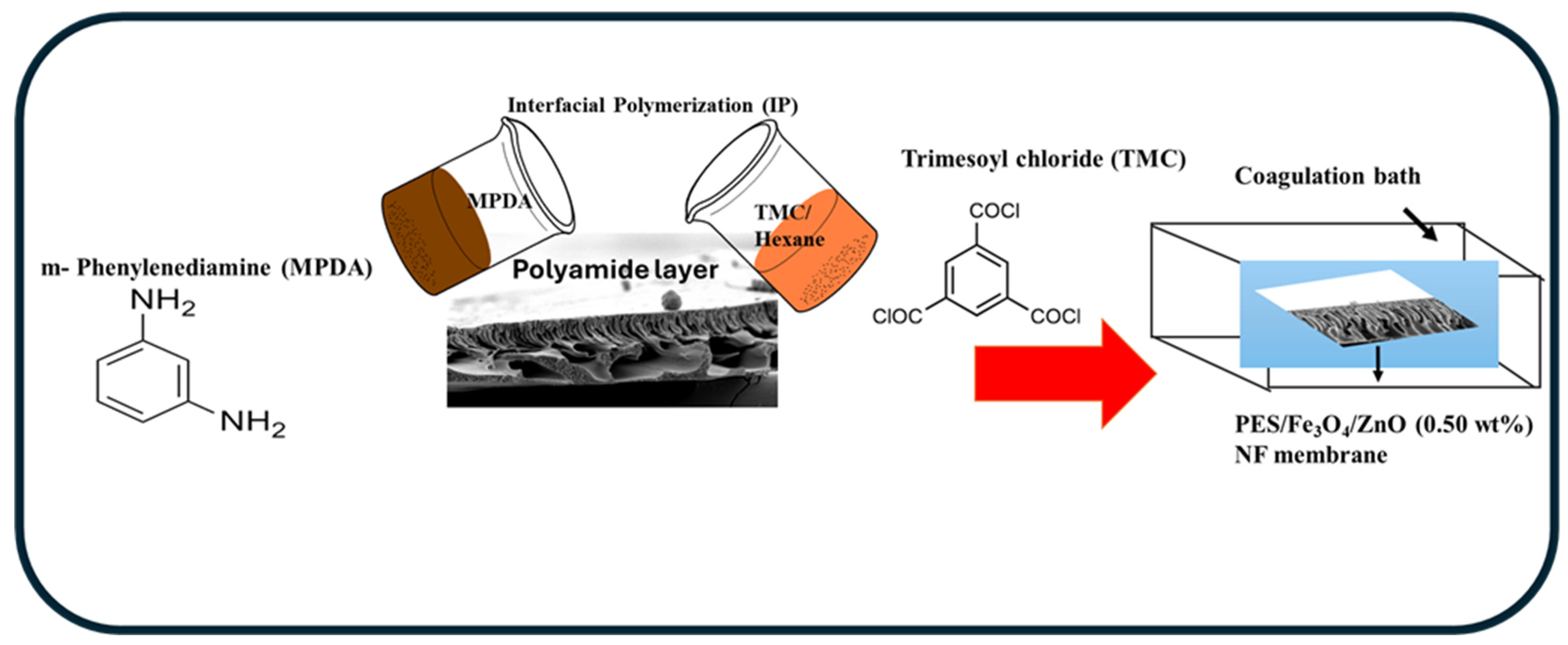







| Membrane | PES (wt.%) | PVP (wt.%) | NMP (wt.%) | Coagulation Bath Temperature (°C) | Fe3O4/ZnO (wt.%) | Solvent Evaporation Time (EVT) (s) | MPDA (wt.%) | TMC (wt.%) |
|---|---|---|---|---|---|---|---|---|
| NF0 | 18 | 0 | 82 | 25 | 0 | 0 | 2 | 0.1 |
| NF1 | 18 | 2 | 80 | 25 | 0 | 0 | 2 | 0.1 |
| NF2 | 18 | 2 | 80 | 40 | 0 | 180 | 2 | 0.1 |
| NF3 | 18 | 2 | 79.50 | 40 | 0.50 | 180 | 2 | 0.1 |
| NF4 | 18 | 2 | 79.50 | 40 | 0.50 | 180 | 2 | 0.2 |
| NF5 | 18 | 2 | 79.50 | 40 | 0.50 | 180 | 2 | 0.3 |
| Sampling Location | pH | Conductivity (µS/cm) | Dissolved Oxygen (%) | Chemical Dissolved Oxygen (mg/L) | TDS (mg/L) |
|---|---|---|---|---|---|
| Biological nutrient removal | 7.09 ± 0.05 | 15 ± 0.09 | 3.26 ± 0.37 | >4120 ± 8 | 8 ± 14 |
| Langmuir Model | Freundlich Model | ||||||
|---|---|---|---|---|---|---|---|
| Membranes | qexperimental (mg/g) | qmodel (mg/g) | KL (L/mg) | R2 | KF (mg/g) | N | R2 |
| NF0 | 5.48 | 8.32 | 13.3590 | 0.9905 | 0.00684 | 8.14551 | 0.9389 |
| NF1 | 5.72 | 8.32 | 4.505 × 10−7 | 0.9907 | 0.2771 | 3.2577 | 0.9172 |
| NF3 | 8.86 | 10.05 | 0.3296 | 0.9971 | 0.1982 | 4.7579 | 0.9076 |
| Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|
| q,exp(mg/g) | q,cal (mg/g) | K1 (min−1) | R2 | q,cal | K2 (g mg·min−1) | R2 |
| 16.1314 | 16.16 | 0.1305 | 0.9973 | 16.62 | 0.0219 | 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubheka, N.S.M.; Managa, M.; Moloto, M.J.; Nxumalo, E.N. Fabrication of Polyamide Thin-Film Composite/Polyethersulfone-Coreshell-Fe3O4/ZnO Membranes for the Efficient Removal of Pb(II) from Wastewater. Membranes 2025, 15, 341. https://doi.org/10.3390/membranes15110341
Kubheka NSM, Managa M, Moloto MJ, Nxumalo EN. Fabrication of Polyamide Thin-Film Composite/Polyethersulfone-Coreshell-Fe3O4/ZnO Membranes for the Efficient Removal of Pb(II) from Wastewater. Membranes. 2025; 15(11):341. https://doi.org/10.3390/membranes15110341
Chicago/Turabian StyleKubheka, Nompumelelo Sharol Mbali, Muthumuni Managa, Makwena Justice Moloto, and Edward Ndumiso Nxumalo. 2025. "Fabrication of Polyamide Thin-Film Composite/Polyethersulfone-Coreshell-Fe3O4/ZnO Membranes for the Efficient Removal of Pb(II) from Wastewater" Membranes 15, no. 11: 341. https://doi.org/10.3390/membranes15110341
APA StyleKubheka, N. S. M., Managa, M., Moloto, M. J., & Nxumalo, E. N. (2025). Fabrication of Polyamide Thin-Film Composite/Polyethersulfone-Coreshell-Fe3O4/ZnO Membranes for the Efficient Removal of Pb(II) from Wastewater. Membranes, 15(11), 341. https://doi.org/10.3390/membranes15110341







