Membrane Technology for the Valorization of Wood Vinegar from Grape Pomace Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Wood Vinegar
2.2. Membrane Filtration Experiments
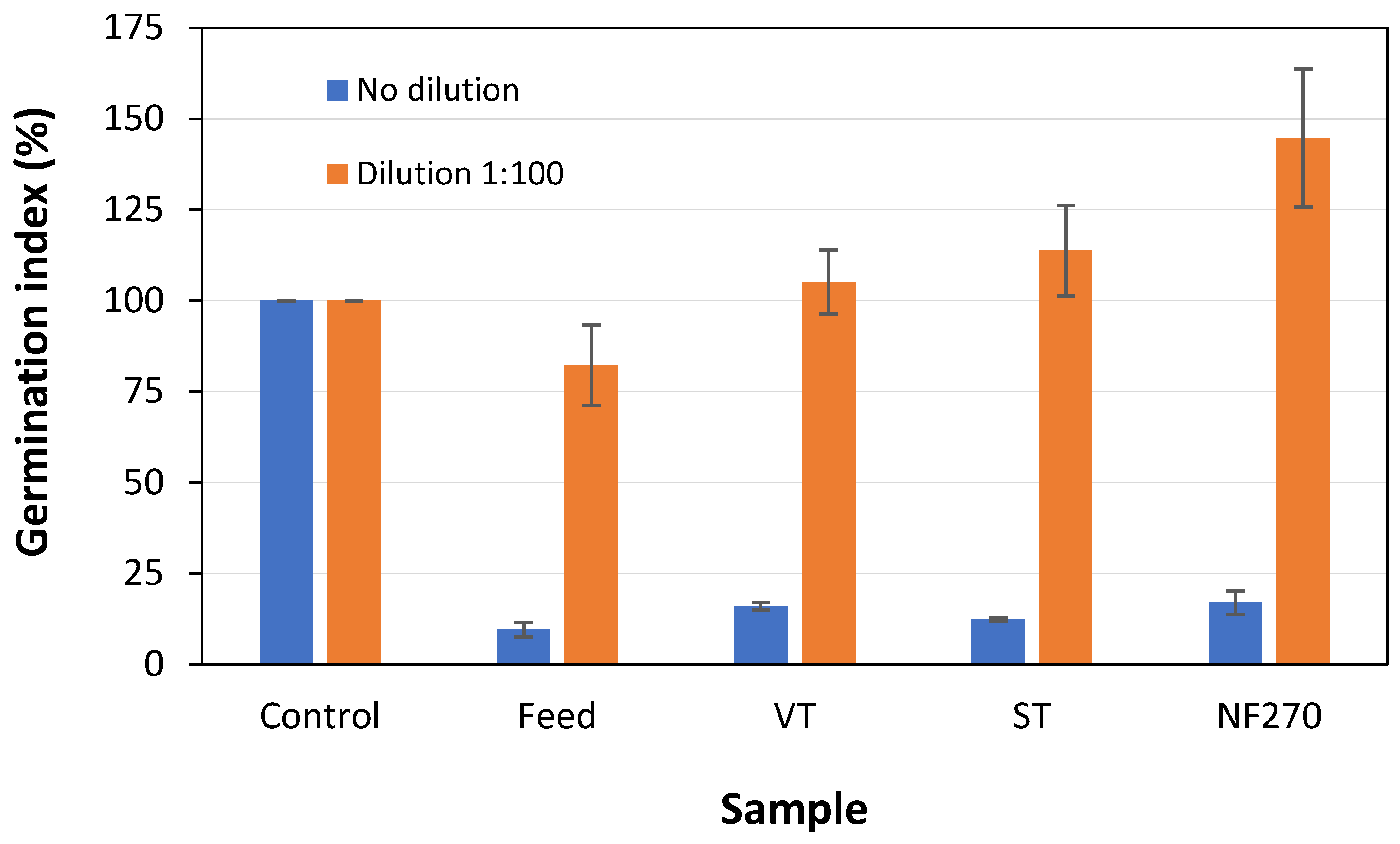
2.3. Phytotoxicity Assays
2.4. Analytical Methods
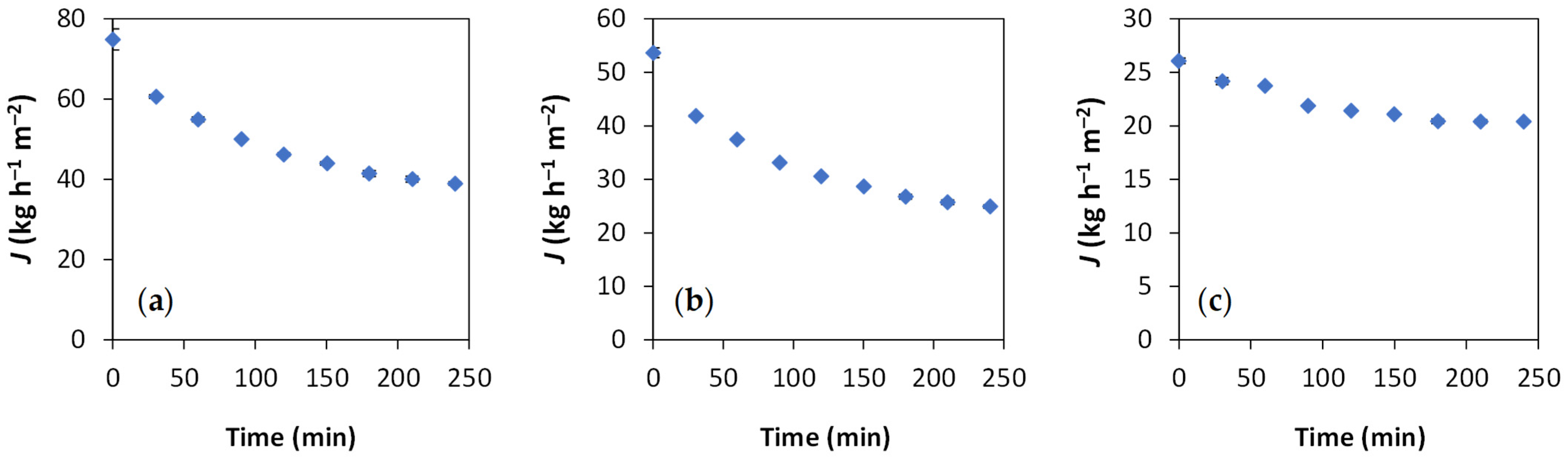
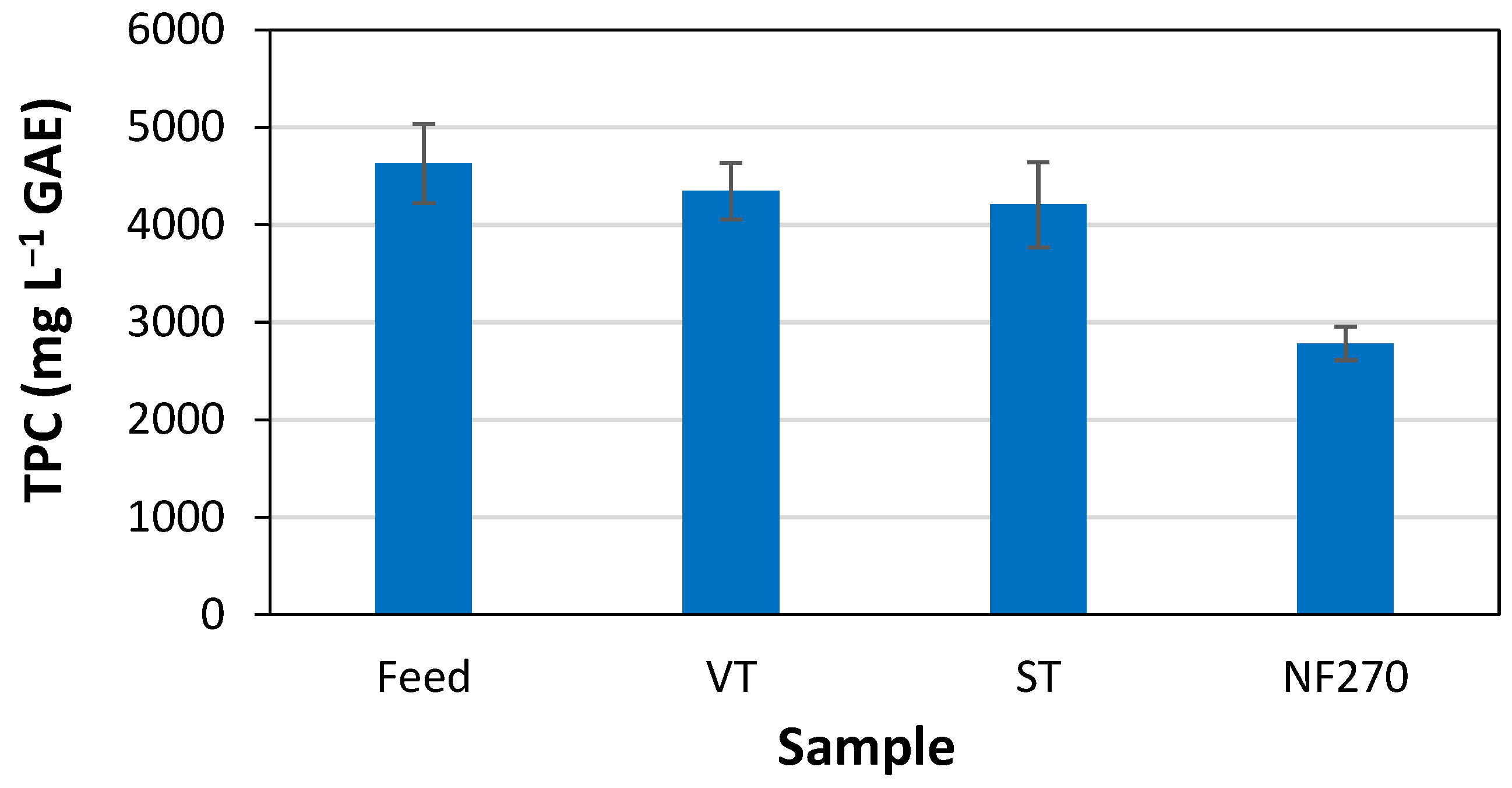
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giacobbo, A.; Cassano, A.; Conidi, C.; De Pinho, M.N. Membrane Technologies for Recovery of Bioactive Compounds. In Application of Emerging Technologies and Strategies to Extract Bioactive Compounds; Munekata, P.E.S., Ed.; Elsevier: Chennai, India, 2025; pp. 295–322. ISBN 978-0-443-18975-3. [Google Scholar]
- Nath, A.; Das, K.; Dhal, G.C. Global Status of Agricultural Waste-Based Industries, Challenges, and Future Prospects. In Agricultural Waste to Value-Added Products; Neelancherry, R., Gao, B., Wisniewski, A., Jr., Eds.; Springer Nature: Singapore, 2023; pp. 21–45. ISBN 978-981-99-4472-9. [Google Scholar]
- Prado-Acebo, I.; Cubero-Cardoso, J.; Lu-Chau, T.A.; Eibes, G. Integral Multi-Valorization of Agro-Industrial Wastes: A Review. Waste Manag. 2024, 183, 42–52. [Google Scholar] [CrossRef]
- Ezeorba, T.P.C.; Okeke, E.S.; Mayel, M.H.; Nwuche, C.O.; Ezike, T.C. Recent Advances in Biotechnological Valorization of Agro-Food Wastes (AFW): Optimizing Integrated Approaches for Sustainable Biorefinery and Circular Bioeconomy. Bioresour. Technol. Rep. 2024, 26, 101823. [Google Scholar] [CrossRef]
- Pandey, A.K.; Thakur, S.; Mehra, R.; Kaler, R.S.S.; Paul, M.; Kumar, A. Transforming Agri-Food Waste: Innovative Pathways toward a Zero-Waste Circular Economy. Food Chem. X 2025, 28, 102604. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Sial, T.A.; Ali, A.; Wahid, F. Impact of Agricultural Wastes on Environment and Possible Management Strategies. In Frontier Studies in Soil Science; Núñez-Delgado, A., Ed.; Springer: Cham, Germany, 2024; pp. 79–108. ISBN 978-3-031-50503-4. [Google Scholar]
- Giacobbo, A.; Bernardes, A.M.; de Pinho, M.N. The Role of Pressure-Driven Membrane Processes on the Recovery of Value-Added Compounds and Valorization of Lees and Wastewaters in the Wine Industry. In Improving Sustainable Viticulture and Winemaking Practices; Costa, J.M., Catarino, S., Escalona, J.M., Comuzzo, P., Eds.; Academic Press: New York, NY, USA, 2022; pp. 305–326. ISBN 978-0-323-85150-3. [Google Scholar]
- Jokinen, N.; Eronen, E.; Salami, A.; Hyttinen, M.; Jänis, J.; Vepsäläinen, J.; Lappalainen, R.; Tomppo, L. Valorization Potential of the Aqueous Products from Hydrothermal Liquefaction and Stepwise Slow Pyrolysis of Wood Bark and Hemp Hurds with Yields and Product Comparison. Bioresour. Technol. Rep. 2023, 21, 101385. [Google Scholar] [CrossRef]
- Gohar, H.; Beggio, G.; Schiavon, M.; Lavagnolo, M.C. A Systematic Review on the Potential of Agro-Industrial Waste for Pyrolysis: Feedstocks Properties and Their Impact on Biochar and Pyrolysis Gas Composition. Biomass Bioenergy 2025, 200, 108047. [Google Scholar] [CrossRef]
- Trabelsi, N.; Mhamdi, R. Wood Vinegar’s Role in Termite Control: From Mystery to Reality. Eur. J. Wood Wood Prod. 2024, 82, 1263–1272. [Google Scholar] [CrossRef]
- Calcan, S.I.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Bădulescu, L.; Dobre, T.; Egri, D.; Moț, A.; Popa, V.; Crăciun, M.E. Valorization of Vine Prunings by Slow Pyrolysis in a Fixed-Bed Reactor. Processes 2021, 10, 37. [Google Scholar] [CrossRef]
- Gama, G.S.P.; Pimenta, A.S.; Feijó, F.M.C.; de Azevedo, T.K.B.; de Melo, R.R.; de Andrade, G.S. The Potential of Wood Vinegar to Replace Antimicrobials Used in Animal Husbandry—A Review. Animals 2024, 14, 381. [Google Scholar] [CrossRef]
- Bennani, G.; Ndao, A.; Konan, D.; Brassard, P.; Le Roux, É.; Godbout, S.; Adjallé, K. Valorisation of Cranberry Residues through Pyrolysis and Membrane Filtration for the Production of Value-Added Agricultural Products. Energies 2023, 16, 7774. [Google Scholar] [CrossRef]
- Kumar, S.; Rahman, M.; Bouket, A.C.; Ahadi, R.; Meena, M.; Bhupenchandra, I.; Singh, U.B.; Arutselvan, R.; Kumar, R.; Singh, S.P.; et al. Unravelling the Multifarious Role of Wood Vinegar Made from Waste Biomass in Plant Growth Promotion, Biotic Stress Tolerance, and Sustainable Agriculture. J. Anal. Appl. Pyrolysis 2025, 185, 106851. [Google Scholar] [CrossRef]
- Ferrari, V.; Nazari, M.T.; da Silva, N.F.; Crestani, L.; Raymundo, L.M.; Dotto, G.L.; Piccin, J.S.; Oliveira, L.F.S.; Bernardes, A.M. Pyrolysis: A Promising Technology for Agricultural Waste Conversion into Value-Added Products. Environ. Dev. Sustain. 2024, 27, 17957–17991. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, Y.; Zhang, N.; Liu, Z.; Bao, L.; Xia, Y. Wood Fiber Biomass Pyrolysis Solution as a Potential Tool for Plant Disease Management: A Review. Heliyon 2024, 10, e25509. [Google Scholar] [CrossRef]
- Bikbulatova, G.M.; Grachev, A.N.; Valiullina, A.I.; Valeeva, A.R.; Zabelkin, S.A.; Dryakhlov, V.O.; Khaziakhmedova, R.M. Treatment of Wastewater Generated during the Fast Pyrolysis of Wood Waste. Biomass Convers Biorefin. 2023, 13, 13707–13714. [Google Scholar] [CrossRef]
- Justicia, J.; Baeza, J.A.; Calvo, L.; Heras, F.; Gilarranz, M.A. Valorization to Hydrogen of Bio-Oil Aqueous Fractions from Lignocellulosic Biomass Pyrolysis by Aqueous Phase Reforming over Pt/C Catalyst. Chem. Eng. J. 2023, 477, 146860. [Google Scholar] [CrossRef]
- Rahmat, B.; Pangesti, D.; Natawijaya, D.; Sufyadi, D. Generation of Wood-Waste Vinegar and Its Effectiveness as a Plant Growth Regulator and Pest Insect Repellent. Bioresources 2014, 9, 6350–6360. [Google Scholar] [CrossRef]
- Bonanomi, G.; Jesu, G.; Zotti, M.; Idbella, M.; d’Errico, G.; Laudonia, S.; Vinale, F.; Abd-ElGawad, A. Biochar-Derived Smoke-Water Exerts Biological Effects on Nematodes, Insects, and Higher Plants but Not Fungi. Sci. Total Environ. 2021, 750, 142307. [Google Scholar] [CrossRef]
- Macasait, D.R.; Roylo, B.B.; Espina, D.M. Growth Performance of Grower Pigs (Sus scrofa domesticus L.), Nutritional and Microbial Contents of Wet and Fermented Commercial Hog Ration with Different Levels of Wood Vinegar. Asian J. Dairy Food Res. 2021, 40, 220–224. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Chen, C.; Wu, H.; He, Q.; Wu, J.; Yao, J.-Y.; Chen, F. Bamboo Vinegar Powder: Unveiling Its Antioxidant and Antifungal Efficacy through Bioactive Compound Analysis and Mechanistic Insights. Food Chem. 2025, 470, 142718. [Google Scholar] [CrossRef] [PubMed]
- Desvita, H.; Faisal, M.; Mahidin; Suhendrayatna. Antimicrobial Potential of Wood Vinegar from Cocoa Pod Shells (Theobroma cacao L.) against Candida Albicans and Aspergillus Niger. Mater. Today Proc. 2022, 63, S210–S213. [Google Scholar] [CrossRef]
- Ahmadi, P.; Avesta, M.J.; Khorramdel, S.; Jonoobi, M.; Mekonnen, T.H. Production and Use of Lignocellulosic Wood Vinegar and Tar as Organic Pesticides to Fight Bacterial Canker Disease. Int. J. Biol. Macromol. 2025, 301, 140373. [Google Scholar] [CrossRef] [PubMed]
- Anokye, R.; Boadu, K.B.; Fianko, C.N.; Amegashiti, V.B. The Chemical Composition of Savannah Bamboo (Oxytenanthera abyssinica) Vinegar at Varying Pyrolysis Temperatures and Its Termiticidal Activity against Wood-Feeding Termites. Adv. Bamboo Sci. 2024, 6, 100063. [Google Scholar] [CrossRef]
- He, L.; Geng, K.; Li, B.; Li, S.; Gustave, W.; Wang, J.; Jeyakumar, P.; Zhang, X.; Wang, H. Enhancement of Nutrient Use Efficiency with Biochar and Wood Vinegar: A Promising Strategy for Improving Soil Productivity. J. Sci. Food Agric. 2025, 105, 465–472. [Google Scholar] [CrossRef]
- Yang, L.; Tang, G.; Xu, W.; Zhang, Y.; Ning, S.; Yu, P.; Zhu, J.; Wu, Q.; Yu, P. Effect of Combined Application of Wood Vinegar Solution and Biochar on Saline Soil Properties and Cotton Stress Tolerance. Plants 2024, 13, 2427. [Google Scholar] [CrossRef]
- Griebeler, A.M.; Araujo, M.M.; Turchetto, F.; Gasparin, E.; Costella, C.; Heinzmann, B.M.; Batista, B.F.; da Encarnação, F.A.; dos Santos, O.P.; Pimentel, N.; et al. Acacia Mearnsii Pyroligneous Acid as a Promoter of Rooting and Quality of Rooted Cuttings of Subtropical Eucalyptus. Trees 2024, 38, 1063–1077. [Google Scholar] [CrossRef]
- El-Fawy, M.M.; Saeed, A.S.; Abou-Shlell, M.K.; Soliman, M.A.; Ali, E.F.; Issa, A.A.; Abo-Elyousr, K.A.M.; Imran, M.; El-Nagar, A.S. Effectiveness of Biochar and Wood Vinegar from Guava Trees in Controlling Fusarium Verticillioides and Enhancing Growth and Anatomical Traits of Maize (Zea mays L.). J. Plant Dis. Prot. 2024, 131, 2029–2043. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Mostafa, L.Y.; Rihan, H.Z. Enhancing Mango Productivity with Wood Vinegar, Humic Acid, and Seaweed Extract Applications as an Environmentally Friendly Strategy. Sustainability 2024, 16, 8986. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Liu, B.; Liu, Q.; Zheng, H.; You, X.; Sun, K.; Luo, X.; Li, F. Comparative Study of Individual and Co-Application of Biochar and Wood Vinegar on Blueberry Fruit Yield and Nutritional Quality. Chemosphere 2020, 246, 125699. [Google Scholar] [CrossRef] [PubMed]
- Anggrayni, D.; Purnama, I.; Saidi, N.B.; Novianti, F.; Baharum, N.A.; Mutamima, A.; Razali, N.A.S.B.; Boukherroub, R. Antifungal and Phytotoxicity of Wood Vinegar from Biomass Waste against Fusarium oxysporum f. sp. Cubense TR4 Infecting Banana Plants. Discov. Food 2025, 5, 98. [Google Scholar] [CrossRef]
- Chai, S.; Kang, B.S.; Valizadeh, B.; Valizadeh, S.; Hong, J.; Jae, J.; Andrew Lin, K.-Y.; Khan, M.A.; Jeon, B.-H.; Park, Y.-K.; et al. Fractional Condensation of Bio-Oil Vapors from Pyrolysis of Various Sawdust Wastes in a Bench-Scale Bubbling Fluidized Bed Reactor. Chemosphere 2024, 350, 141121. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Drioli, E.; Cassano, A. Membrane-Based Agro-Food Production Processes for Polyphenol Separation, Purification and Concentration. Curr. Opin. Food Sci. 2018, 23, 149–164. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; de Pinho, M.N. Nanofiltration for the Recovery of Low Molecular Weight Polysaccharides and Polyphenols from Winery Effluents. Sep. Sci. Technol. 2013, 48, 2524–2530. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; de Pinho, M.N. Sequential Pressure-Driven Membrane Operations to Recover and Fractionate Polyphenols and Polysaccharides from Second Racking Wine Lees. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of Functional Macromolecules and Micromolecules: From Ultrafiltration to the Border of Nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Giacobbo, A.; Oliveira, M.; Duarte, E.C.N.F.; Mira, H.M.C.; Bernardes, A.M.; de Pinho, M.N. Ultrafiltration Based Process for the Recovery of Polysaccharides and Polyphenols from Winery Effluents. Sep. Sci. Technol. 2013, 48, 438–444. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Markouli, E.; Gekas, V. Recovery and Fractionation of Different Phenolic Classes from Winery Sludge Using Ultrafiltration. Sep. Purif. Technol. 2013, 107, 245–251. [Google Scholar] [CrossRef]
- Matsuura, T. Synthetic Membranes and Membrane Separation Processes, 1st ed.; CRC Press: Boca Raton, FL, USA, 1993; ISBN 9780849342028. [Google Scholar]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and Tight Ultrafiltration Membranes for the Recovery of Polyphenols from Agro-Food by-Products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fíla, V. Membrane Technologies Assisting Plant-Based and Agro-Food by-Products Processing: A Comprehensive Review. Trends Food Sci. Technol. 2020, 95, 219–232. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M. Membrane Separation Process in Wastewater and Water Purification. Membranes 2022, 12, 259. [Google Scholar] [CrossRef]
- Wen, J.; Han, Q.; Qiu, M.; Jiang, L.; Chen, X.; Fan, Y. Membrane Technologies for the Separation and Purification of Functional Oligosaccharides: A Review. Sep. Purif. Technol. 2024, 346, 127463. [Google Scholar] [CrossRef]
- DuPont—FilmTec FilmTecTM NF270 Element Data Sheet. Available online: https://www.dupont.com/content/dam/water/amer/us/en/water/public/documents/en/NF-FilmTec-NF270-PDS-45-D01529-en.pdf (accessed on 20 October 2025).
- Liu, Y.L.; Wang, X.; Yang, H.; Xie, Y.F. Quantifying the Influence of Solute-Membrane Interactions on Adsorption and Rejection of Pharmaceuticals by NF/RO Membranes. J. Memb. Sci. 2018, 551, 37–46. [Google Scholar] [CrossRef]
- Soares, E.V.; Giacobbo, A.; Rodrigues, M.A.; de Pinho, M.N.; Bernardes, A.M. The Effect of PH on Atenolol/Nanofiltration Membranes Affinity. Membranes 2021, 11, 689. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Pharmaceutical Retention Mechanisms by Nanofiltration Membranes. Environ. Sci. Technol. 2005, 39, 7698–7705. [Google Scholar] [CrossRef]
- Mänttäri, M.; Pekuri, T.; Nyström, M. NF270, a New Membrane Having Promising Characteristics and Being Suitable for Treatment of Dilute Effluents from the Paper Industry. J. Memb. Sci. 2004, 242, 107–116. [Google Scholar] [CrossRef]
- Synder Filtration VT (PES 3000Da) Sanitary UF Membrane Data Sheet. Available online: https://synderfiltration.com/2014/wp-content/uploads/2018/05/VT-PES-3kD-Sanitary-Specsheet.pdf (accessed on 20 October 2025).
- Chorhirankul, N.; Janssen, A.E.M.; Boom, R.M. Charge-Selective Separation of Amino Acids with Ultrafiltration and Nanofiltration. Sep. Purif. Technol. 2025, 362, 131874. [Google Scholar] [CrossRef]
- Synder Filtration ST (PES 10,000Da) Sanitary UF Membrane Data Sheet. Available online: https://synderfiltration.com/2014/wp-content/uploads/2018/05/ST-PES-10kD-Sanitary-Specsheet.pdf (accessed on 20 October 2025).
- Kadel, S.; Pellerin, G.; Thibodeau, J.; Perreault, V.; Lainé, C.; Bazinet, L. How Molecular Weight Cut-Offs and Physicochemical Properties of Polyether Sulfone Membranes Affect Peptide Migration and Selectivity during Electrodialysis with Filtration Membranes. Membranes 2019, 9, 153. [Google Scholar] [CrossRef]
- Du, Y.; Pramanik, B.K.; Zhang, Y.; Jegatheesan, V. Influence of Molecular Weight Cut-off (MWCO) of Commercial Ultrafiltration Substrate on the Performance of Thin Film Composite Nanofiltration Membrane. Desalination 2022, 541, 116020. [Google Scholar] [CrossRef]
- Giacobbo, A.; do Prado, J.M.; Meneguzzi, A.; Bernardes, A.M.; de Pinho, M.N. Microfiltration for the Recovery of Polyphenols from Winery Effluents. Sep. Purif. Technol. 2015, 143, 12–18. [Google Scholar] [CrossRef]
- da Trindade, C.d.M.; Giacobbo, A.; Ferreira, V.G.; Rodrigues, M.A.S.; Bernardes, A.M. Membrane Separation Processes Applied to the Treatment of Effluents from Nanoceramic Coating Operations. Desalination Water Treat. 2015, 55, 28–38. [Google Scholar] [CrossRef]
- Gerber, M.D.; Lucia, T.; Correa, L.; Neto, J.E.P.; Correa, É.K. Phytotoxicity of Effluents from Swine Slaughterhouses Using Lettuce and Cucumber Seeds as Bioindicators. Sci. Total Environ. 2017, 592, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.M.; Becker, R.; Corrêa, L.B.; Bianchi, I.; Dai Prá, M.A.; Lucia, T.; Corrêa, E.K. Phytotoxicity as an Indicator of Stability of Broiler Production Residues. J. Environ. Manag. 2016, 167, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Arsand, D.; Antunes, M.H.; Martins, G.A.; Gerber, M.D.; Corrêa, É.K. Avaliação Do Potencial Fitotóxico, Citotóxico e Genotóxico de Efluente Hemodialítico. Eng. Sanit. E Ambient. 2022, 27, 269–277. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Tam, N.F.Y. Elimination of Phytotoxicity during Co-Composting of Spent Pig-Manure Sawdust Litter and Pig Sludge. Bioresour. Technol. 1998, 65, 43–49. [Google Scholar] [CrossRef]
- Librán, C.M.; Mayor, L.; Garcia-Castello, E.M.; Vidal-Brotons, D. Polyphenol Extraction from Grape Wastes: Solvent and PH Effect. Agric. Sci. 2013, 4, 56–62. [Google Scholar] [CrossRef]
- Chungsiriporn, J.; Pongyeela, P.; Iewkittayakorn, J. Use of Wood Vinegar as Fungus and Malodor Retarding Agent for Natural Rubber Products. Songklanakarin J. Sci. Technol. 2018, 40, 87–92. [Google Scholar] [CrossRef]
- Cândido, N.R.; Pasa, V.M.D.; Vilela, A.d.O.; Campos, Â.D.; de Fátima, Â.; Modolo, L.V. Understanding the Multifunctionality of Pyroligneous Acid from Waste Biomass and the Potential Applications in Agriculture. Sci. Total Environ. 2023, 881, 163519. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Xun, H.; Yao, X.; Tang, F. Simultaneous Quantification of Twelve Compounds from Bamboo/Wood Vinegar by Gas Chromatography-Mass Spectrometry. Separations 2024, 11, 168. [Google Scholar] [CrossRef]
- Susanto, H.; Feng, Y.; Ulbricht, M. Fouling Behavior of Aqueous Solutions of Polyphenolic Compounds during Ultrafiltration. J. Food Eng. 2009, 91, 333–340. [Google Scholar] [CrossRef]
- Boussu, K.; Vandecasteele, C.; Van der Bruggen, B. Relation between Membrane Characteristics and Performance in Nanofiltration. J. Memb. Sci. 2008, 310, 51–65. [Google Scholar] [CrossRef]
- Minhalma, M.; de Pinho, M.N. Flocculation/Flotation/Ultrafiltration Integrated Process for the Treatment of Cork Processing Wastewaters. Environ. Sci. Technol. 2001, 35, 4916–4921. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-Oil Refining: A Review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Iacomino, G.; Idbella, M.; Staropoli, A.; Nanni, B.; Bertoli, T.; Vinale, F.; Bonanomi, G. Exploring the Potential of Wood Vinegar: Chemical Composition and Biological Effects on Crops and Pests. Agronomy 2024, 14, 114. [Google Scholar] [CrossRef]
- Zhang, L.; García-Pérez, P.; Arikan, B.; Elbasan, F.; Nur Alp, F.; Balci, M.; Zengin, G.; Yildiztugay, E.; Lucini, L. The Exogenous Application of Wood Vinegar Induces a Tissue- and Dose-Dependent Elicitation of Phenolics and Functional Traits in Onion (Allium cepa L.). Food Chem. 2023, 405, 134926. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Feng, X.; Yu, J. Wood Vinegar Resulting from the Pyrolysis of Apple Tree Branches for Annual Bluegrass Control. Ind. Crops Prod. 2021, 174, 114193. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Y.; Li, X.; Li, Y.; Feng, X.; Bagavathiannan, M.; Zhang, C.; Qu, M.; Yu, J. The Use of Wood Vinegar as a Non-Synthetic Herbicide for Control of Broadleaf Weeds. Ind. Crops Prod. 2021, 173, 114105. [Google Scholar] [CrossRef]
- Koltsova, T.G.; Kulagina, V.I.; Zabelkin, S.A.; Grachev, A.N.; Bikbulatova, G.M.; Khaziakhmedova, R.M.; Makarov, A.A.; Valeeva, A.R.; Valiullina, A.I.; Bashkirov, V.N. Phytotesting of Liquid Products of Wood Pyrolysis on Seeds of Higher Plants. Waste Biomass Valorization 2024, 15, 5335–5347. [Google Scholar] [CrossRef]
- Ofoe, R.; Gunupuru, L.R.; Wang-Pruski, G.; Fofana, B.; Thomas, R.H.; Abbey, L. Seed Priming with Pyroligneous Acid Mitigates Aluminum Stress, and Promotes Tomato Seed Germination and Seedling Growth. Plant Stress 2022, 4, 100083. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]


| Parameter | Membrane Type | ||
|---|---|---|---|
| NF270 | VT | ST | |
| Membrane active layer | Semi-aromatic Polyamide a,b,e | Polyethersulfone f | Polyethersulfone h |
| MWCO (Da) | 400 c | 3000 f | 10,000 h |
| pH operating range | 3–10 a | 3–9 f | 3–9 h |
| Maximum operating pressure (bar) | 41 a | 8.3 f | 8.3 h |
| Zeta potential (mV) | −22 d; −29 b | n.a. | −9.6 i |
| Contact angle (°) | 30 e | n.a. | 59 i |
| Pore radius (nm) | 0.44 b | 1.52 g | 7.84 j |
| Manufacturer | FilmTec—DuPont | Synder Filtration | Synder Filtration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacobbo, A.; Callegari, A.d.S.; Nazari, M.T.; Ferrari, V.; Basegio, T.M.; Bergmann, C.P.; Rodrigues, M.A.S.; de Pinho, M.N.; Bernardes, A.M. Membrane Technology for the Valorization of Wood Vinegar from Grape Pomace Pyrolysis. Membranes 2025, 15, 335. https://doi.org/10.3390/membranes15110335
Giacobbo A, Callegari AdS, Nazari MT, Ferrari V, Basegio TM, Bergmann CP, Rodrigues MAS, de Pinho MN, Bernardes AM. Membrane Technology for the Valorization of Wood Vinegar from Grape Pomace Pyrolysis. Membranes. 2025; 15(11):335. https://doi.org/10.3390/membranes15110335
Chicago/Turabian StyleGiacobbo, Alexandre, Amanda de Sampaio Callegari, Mateus Torres Nazari, Valdecir Ferrari, Tania Maria Basegio, Carlos Pérez Bergmann, Marco Antônio Siqueira Rodrigues, Maria Norberta de Pinho, and Andréa Moura Bernardes. 2025. "Membrane Technology for the Valorization of Wood Vinegar from Grape Pomace Pyrolysis" Membranes 15, no. 11: 335. https://doi.org/10.3390/membranes15110335
APA StyleGiacobbo, A., Callegari, A. d. S., Nazari, M. T., Ferrari, V., Basegio, T. M., Bergmann, C. P., Rodrigues, M. A. S., de Pinho, M. N., & Bernardes, A. M. (2025). Membrane Technology for the Valorization of Wood Vinegar from Grape Pomace Pyrolysis. Membranes, 15(11), 335. https://doi.org/10.3390/membranes15110335










