Recent Advances in Polymeric Membrane Integration for Organic Solvent Mixtures Separation: Mini-Review
Abstract
1. Introduction
2. Application of Membrane Separation in Industries
2.1. Oleochemical Industry
2.2. Pharmaceutical and Biotechnology Industry
2.3. Chemical and Petrochemical Industry
2.4. Organic Compounds Separation Using Hansen Solubility Parameters
3. Membrane Separation
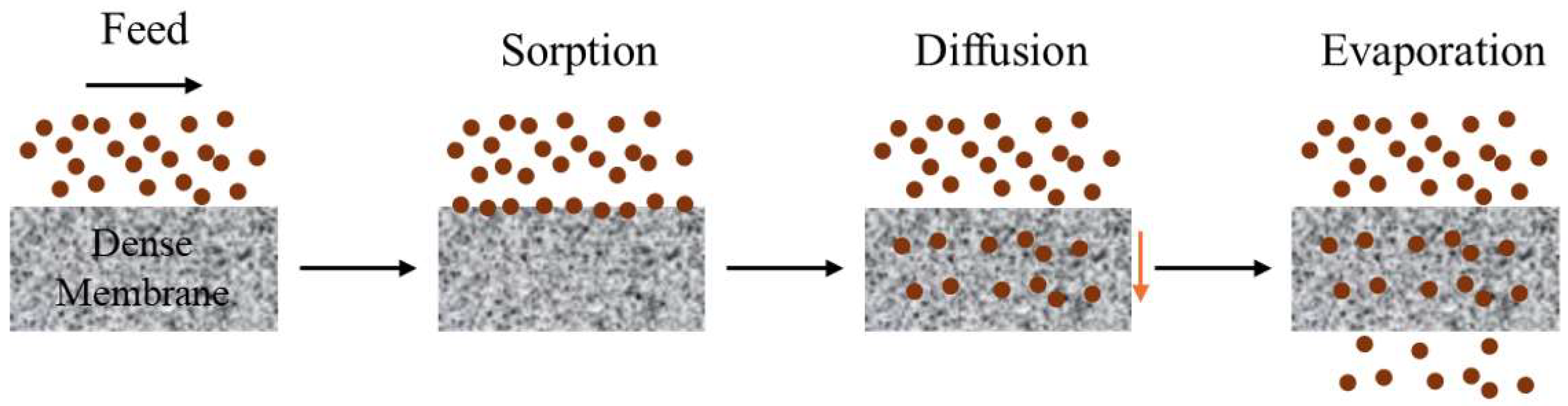
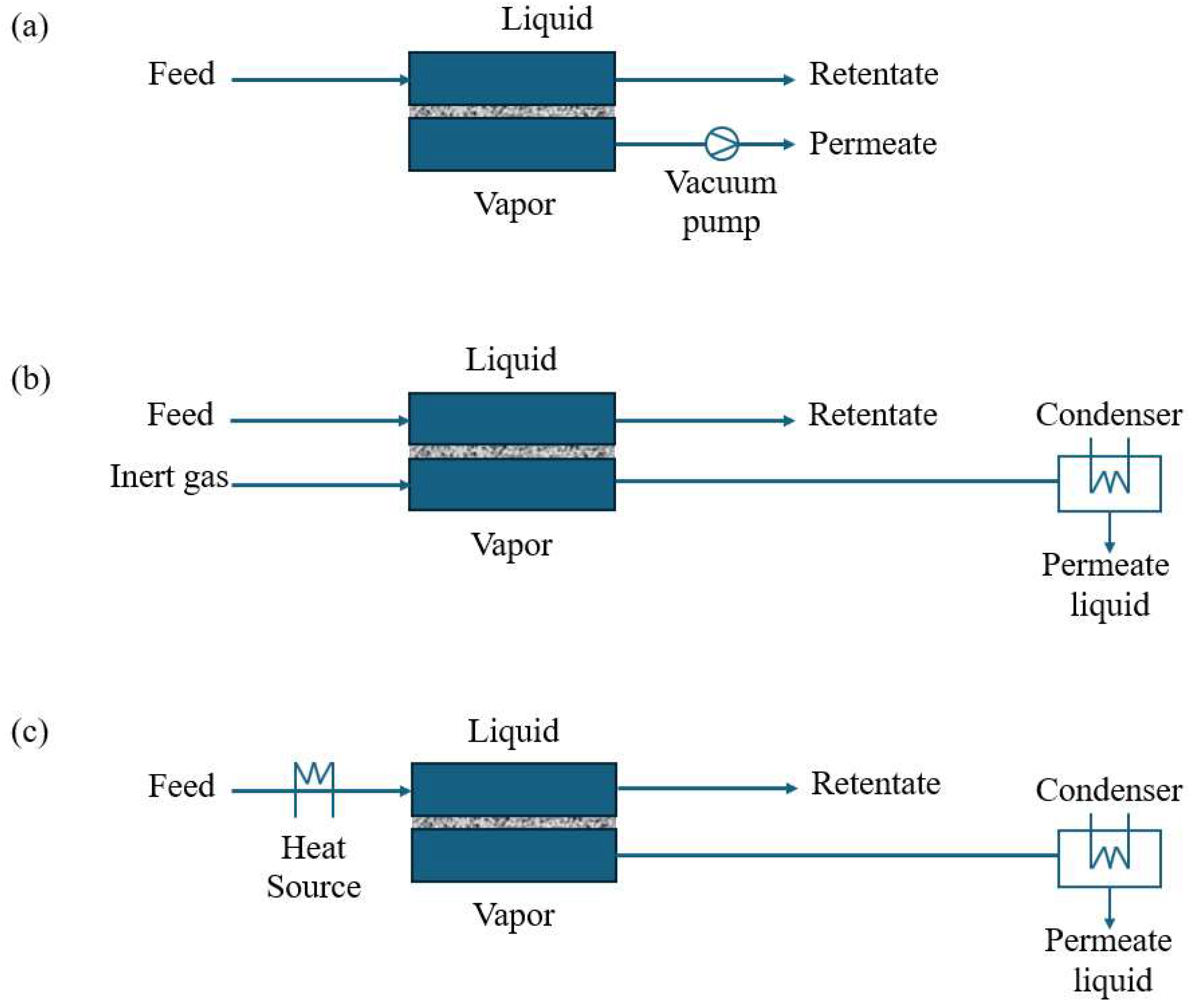
3.1. Organic Solvent Pervaporation (OSPV)
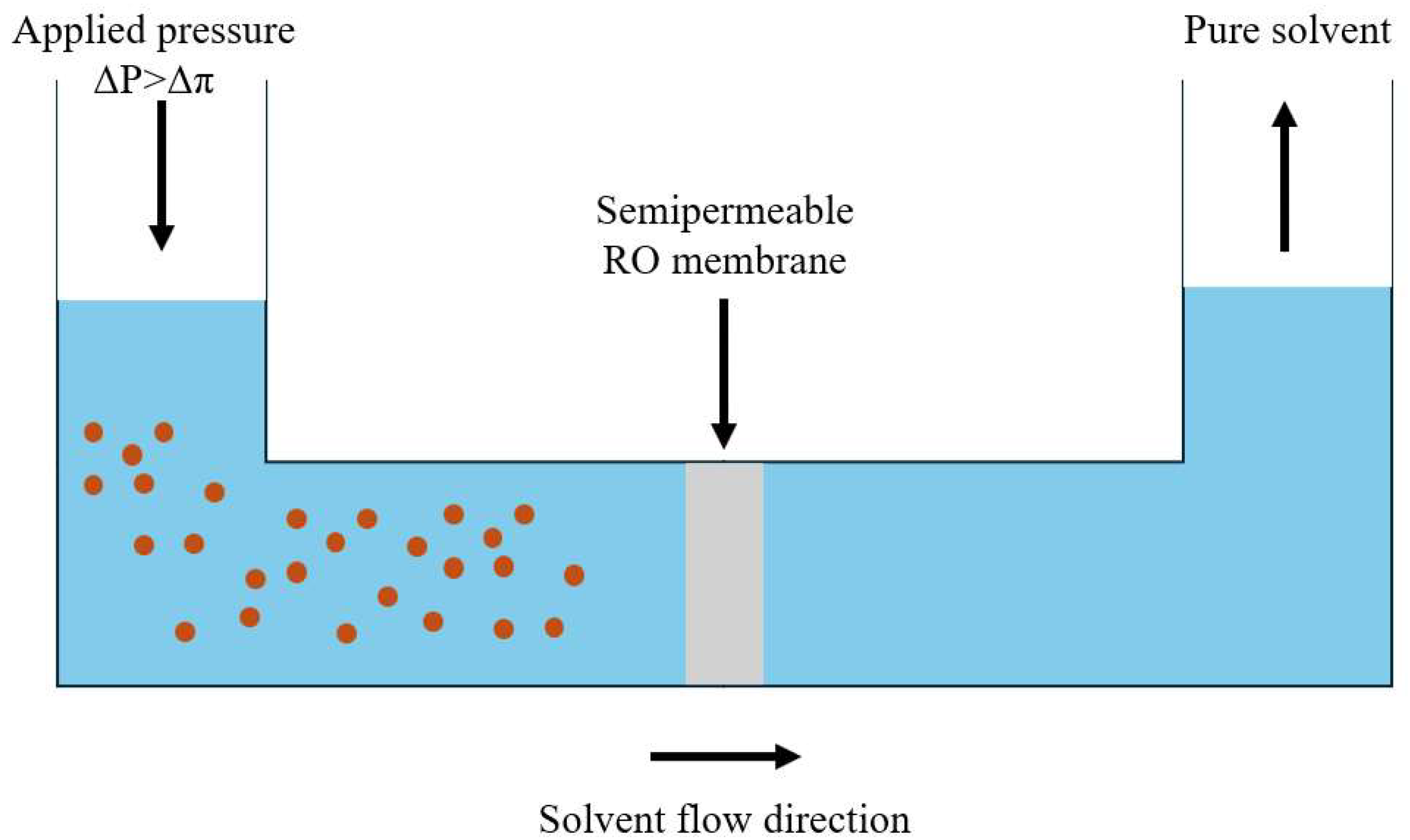
3.2. Organic Solvent Reverse Osmosis (OSRO)
3.3. Organic Solvent Nanofiltration (OSN)
3.4. Organic Solvent Ultrafiltration (OSUF)
4. Challenges and Limitations
4.1. Swelling
4.2. Fouling
4.3. Scaling
4.4. Stability
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naeem, A.; Saeed, B.; AlMohamadi, H.; Lee, M.; Gilani, M.A.; Nawaz, R.; Khan, A.L.; Yasin, M. Sustainable and Green Membranes for Chemical Separations: A Review. Sep. Purif. Technol. 2024, 336, 126271. [Google Scholar] [CrossRef]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef]
- David, S.S.; Ryan, P.L. Seven Chemical separations to Change the World. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Liang, B.; He, X.; Hou, J.; Li, L.; Tang, Z. Membrane Separation in Organic Liquid: Technologies, Achievements, and Opportunities. Adv. Mater. 2019, 31, 1806090. [Google Scholar] [CrossRef]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.A.; Priya, A.K.; Hawash, H.B.; Yap, P.S. Membrane Technology for Energy Saving: Principles, Techniques, Applications, Challenges, and Prospects. Adv. Energy Sustain. Res. 2024, 5, 2400011. [Google Scholar] [CrossRef]
- Liu, N.J.; Yu, J.Y.; Chen, X.Y.; Liu, L.F. A Novel Organic Solvent Ultrafiltration Membrane of Polyimide/Polyethyleneimine@TiO2 with High Solvent Permeability. J. Memb. Sci. 2024, 702, 122796. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, S.; Wang, J.; Zhang, Y.; Tian, M.; Van der Bruggen, B. Microporous Organic Polymer-Based Membranes for Ultrafast Molecular Separations. Prog. Polym. Sci. 2020, 110, 101308. [Google Scholar] [CrossRef]
- Wang, K.Y.; Weber, M.; Chung, T.S. Polybenzimidazoles (PBIs) and State-of-the-Art PBI Hollow Fiber Membranes for Water, Organic Solvent and Gas Separations: A Review. J. Mater. Chem. A Mater. 2022, 10, 8687. [Google Scholar] [CrossRef]
- Cao, N.; Sun, Y.; Wang, J.; Zhang, H.; Pang, J.; Jiang, Z. Strong Acid- and Solvent-Resistant Polyether Ether Ketone Separation Membranes with Adjustable Pores. Chem. Eng. J. 2020, 386, 124086. [Google Scholar] [CrossRef]
- Ravi, S.; Kang, W.S.; Lee, H.K.; Park, Y.I.; Park, H.; Kim, I.C.; Kwon, Y.N. Improvement in Acid Resistance of Polyimide Membranes: A Sustainable Cross-Linking Approach via Green-Solvent-Based Fenton Reaction. Polymers 2023, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.N.R.; Mohammad, A.W.; Mahmoudi, E.; Ang, W.L.; Leo, C.P.; Teow, Y.H. An Overview of the Modification Strategies in Developing Antifouling Nanofiltration Membranes. Membranes 2022, 12, 1276. [Google Scholar] [CrossRef]
- Aristizábal, S.L.; Lively, R.P.; Nunes, S.P. Solvent and Thermally Stable Polymeric Membranes for Liquid Molecular Separations: Recent Advances, Challenges, and Perspectives. J. Memb. Sci. 2023, 685, 121972. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of Polymer-Based Membranes Containing Ionic Liquids in Membrane Separation Processes: A Critical Review. Rev. Chem. Eng. 2018, 34, 341–363. [Google Scholar] [CrossRef]
- Sánchez-Arévalo, C.M.; Vincent-Vela, M.C.; Luján-Facundo, M.J.; Álvarez-Blanco, S. Ultrafiltration with Organic Solvents: A Review on Achieved Results, Membrane Materials and Challenges to Face. Process Saf. Environ. Prot. 2023, 177, 118–137. [Google Scholar] [CrossRef]
- Tesser, R.; Vitiello, R.; Russo, V.; Turco, R.; Di Serio, M.; Lin, L.; Li, C. Oleochemistry Products. In Industrial Oil Plant: Application Principles and Green Technologies; Di Serio, M., Xiao, Z., Li, C., Xie, X., He, L., Eds.; Springer: Singapore, 2020; pp. 201–268. ISBN 9789811549205. [Google Scholar]
- Yan, Q.; Pfleger, B.F. Revisiting Metabolic Engineering Strategies for Microbial Synthesis of Oleochemicals. Metab. Eng. 2020, 58, 35–46. [Google Scholar] [CrossRef]
- Mungwari, C.P.; King’ondu, C.K.; Sigauke, P.; Obadele, B.A. Conventional and Modern Techniques for Bioactive Compounds Recovery from Plants: Review. Sci. Afr. 2025, 27, e02509. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Robberecht, T.; Luis, P.; Degrève, J.; Van Der Bruggen, B. Performance of Nanofiltration Membranes for Solvent Purification in the Oil Industry. J. Am. Oil Chem. Soc. 2011, 88, 1255–1261. [Google Scholar] [CrossRef]
- Shi, G.M.; Davood Abadi Farahani, M.H.; Liu, J.Y.; Chung, T.S. Separation of Vegetable Oil Compounds and Solvent Recovery Using Commercial Organic Solvent Nanofiltration Membranes. J. Memb. Sci. 2019, 588, 117202. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Cai, W.; Wang, T.; Wu, Z.; Li, J. Highly Stable PDMS-PTFPMS/PVDF OSN Membranes for Hexane Recovery during Vegetable Oil Production. RSC Adv. 2017, 7, 11381–11388. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Wang, T.; Wu, Z.; Wang, J.; He, X.; Li, J. AF2400/PTFE Composite Membrane for Hexane Recovery during Vegetable Oil Production. Sep. Purif. Technol. 2017, 181, 223–229. [Google Scholar] [CrossRef]
- Ghazali, N.F.; Md Hanim, K.; Pahlawi, Q.A.; Lim, K.M. Enrichment of Carotene from Palm Oil by Organic Solvent Nanofiltration. J. Am. Oil Chem. Soc. 2022, 99, 189–202. [Google Scholar] [CrossRef]
- Ma, R.; Li, J.; Zeng, P.; Duan, L.; Dong, J.; Ma, Y.; Yang, L. The Application of Membrane Separation Technology in the Pharmaceutical Industry. Membranes 2024, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Shirke, A. Recent Developments in Membrane-Based Separations in Biotechnology Processes: Review. Prep. Biochem. Biotechnol. 2011, 41, 398–421. [Google Scholar] [CrossRef] [PubMed]
- Osuofa, J. Protein A and Multimodal Anion Exchange Membrane Adsorbers for Downstream Purification of Therapeutic Biomolecules. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2023. [Google Scholar]
- Masuda, Y.; Ogino, Y.; Yamaichi, K.; Takahashi, Y.; Nonaka, K.; Wakamatsu, K. The Prevention of an Anomalous Chromatographic Behavior and the Resulting Successful Removal of Viruses from Monoclonal Antibody with an Asymmetric Charge Distribution by Using a Membrane Adsorber in Highly Efficient, Anion-Exchange Chromatography in Flow-through Mode. Biotechnol. Prog. 2020, 36, e2955. [Google Scholar] [CrossRef]
- Xiao, H.; Feng, Y.; Goundry, W.R.F.; Karlsson, S. Organic Solvent Nanofiltration in Pharmaceutical Applications. Org. Process Res. Dev. 2024, 28, 891–923. [Google Scholar] [CrossRef]
- Thomas, S.; Thomas, S.; Kadam, S.A.; Jose, T.; Abraham, J.; George, S.C.; Thomas, S. Multiwalled Carbon Nanotubes Reinforced Flexible Blend Nanocomposites Membranes for Pervaporation Separation of Aromatic-Aliphatic Mixtures. Polym. Polym. Compos. 2022, 30, 096739112110690. [Google Scholar] [CrossRef]
- Xi, T.; Lu, Y.; Ai, X.; Tang, L.; Yao, L.; Hao, W.; Cui, P. Ionic Liquid Copolymerized Polyurethane Membranes for Pervaporation Separation of Benzene/Cyclohexane Mixtures. Polymer 2019, 185, 121948. [Google Scholar] [CrossRef]
- Zahlan, H.; Saeed, W.S.; Alqahtani, S.; Aouak, T. Separation of Benzene/Cyclohexane Mixtures by Pervaporation Using Poly (Ethylene-Co-Vinylalcohol) and Carbon Nanotube-Filled Poly (Vinyl Alcohol-Co-Ethylene) Membranes. Separations 2020, 7, 68. [Google Scholar] [CrossRef]
- Namvar-Mahboub, M.; Pakizeh, M.; Davari, S. Preparation and Characterization of UZM-5/Polyamide Thin Film Nanocomposite Membrane for Dewaxing Solvent Recovery. J. Memb. Sci. 2014, 459, 22–32. [Google Scholar] [CrossRef]
- Monjezi, S.; Soltanieh, M.; Sanford, A.C.; Park, J. Polyaniline Membranes for Nanofiltration of Solvent from Dewaxed Lube Oil. Sep. Sci. Technol. 2019, 54, 795–802. [Google Scholar] [CrossRef]
- AlQasas, N.; Eskhan, A.; Johnson, D. Hansen Solubility Parameters from Surface Measurements: A Comparison of Different Methods. Surf. Interfaces 2023, 36, 102594. [Google Scholar] [CrossRef]
- Meng, C.; Li, S.; Wu, Q.; Li, M.; Tian, S.; Tang, H.; Pan, M. Study on the Calculation Method of Hansen Solubility Parameters of Fuel Cell Ionomers. Polymers 2025, 17, 840. [Google Scholar] [CrossRef]
- Trapasso, G.; Russo, F.; Galiano, F.; McElroy, C.R.; Sherwood, J.; Figoli, A.; Aricò, F. Dialkyl Carbonates as Green Solvents for Polyvinylidene Difluoride Membrane Preparation. ACS Sustain. Chem. Eng. 2023, 11, 3390–3404. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Ferreira, A.M.; Okura, T.; Pinheiro Rolemberg, M.; Mafra, M.R.; Coutinho, J.A.P. Using COSMO-RS to Predict Hansen Solubility Parameters. Ind. Eng. Chem. Res. 2022, 61, 15631–15638. [Google Scholar] [CrossRef]
- Huang, J.; Tang, H.; Huang, X.; Feng, Z.; Su, P.; Li, W. Hansen Solubility Parameters-Guided Mixed Matrix Membranes with Linker-Exchanged Metal-Organic Framework Fillers Showing Enhanced Gas Separation Performance. J. Memb. Sci. 2023, 668, 121238. [Google Scholar] [CrossRef]
- Huang, J.H.; Shao, L.; Zhang, Y.Q.; Zhang, Y.J.; Wang, K.; Ma, J.; Drioli, E.; Cheng, X.Q. Relationship between the Hansen Solubility Parameter and Changes in Membrane Mass-Transfer Channels: A Quantitative Model. Chem. Eng. Sci. 2022, 263, 118071. [Google Scholar] [CrossRef]
- Ewah, O.I.; Zhang, Y.; Shi, J.; Escobar, I.C. Adsorptive Behavior of Poly (Vinylidene Fluoride) Membranes for the Recovery of Lignin-Derived Hydrophobic Deep Eutectic Solvents. Sci. Rep. 2025, 15, 32051. [Google Scholar] [CrossRef]
- KV, A.; Puhan, M.R.; Vasave, D.B.; Gohil, T.; Karan, S.; Sutariya, B. Are Hansen Solubility Parameters Relevant in Predicting the Post-Treatment Effect on Polyamide-Based TFC Membranes? Environ. Sci. Pollut. Res. 2024, 31, 21157–21171. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Kruber, B.; Skiborowski, M.; Gao, X. Process Intensification by Integration of Distillation and Vapor Permeation or Pervaporation—An Academic and Industrial Perspective. Results Eng. 2022, 15, 100527. [Google Scholar] [CrossRef]
- Rostovtseva, V.; Faykov, I.; Pulyalina, A. A Review of Recent Developments of Pervaporation Membranes for Ethylene Glycol Purification. Membranes 2022, 12, 312. [Google Scholar] [CrossRef]
- Chen, F.; Chen, H. Pervaporation Separation of Ethylene Glycol/Water Mixtures Using Crosslinked PVA/PES Composite Membranes. Part II. The Swelling Equilibrium Model of the Dense Active Layer in Ethylene Glycol/Water Mixtures. J. Memb. Sci. 1996, 118, 169–176. [Google Scholar] [CrossRef]
- Lipnizki, F.; Field, R.W. Integration of Vacuum and Sweep Gas Pervaporation to Recover Organic Compounds from Wastewater. Sep. Purif. Technol. 2001, 22, 347–360. [Google Scholar] [CrossRef]
- Sanchez Fernandez, E.; Geerdink, P.; Goetheer, E.L.V. Thermo Pervap: The next Step in Energy Efficient Pervaporation. Desalination 2010, 250, 1053–1055. [Google Scholar] [CrossRef]
- Knozowska, K.; Li, G.; Kujawski, W.; Kujawa, J. Novel Heterogeneous Membranes for Enhanced Separation in Organic-Organic Pervaporation. J. Memb. Sci. 2020, 599, 117814. [Google Scholar] [CrossRef]
- Knozowska, K.; Kujawa, J.; Lagzdins, R.; Figoli, A.; Kujawski, W. A New Type of Composite Membrane PVA-NaY/PA-6 for Separation of Industrially Valuable Mixture Ethanol/Ethyl Tert-Butyl Ether by Pervaporation. Materials 2020, 13, 3676. [Google Scholar] [CrossRef]
- Dong, G.; Nagasawa, H.; Yu, L.; Wang, Q.; Yamamoto, K.; Ohshita, J.; Kanezashi, M.; Tsuru, T. Pervaporation Removal of Methanol from Methanol/Organic Azeotropes Using Organosilica Membranes: Experimental and Modeling. J. Memb. Sci. 2020, 610, 118284. [Google Scholar] [CrossRef]
- Banerjee, A.; Ray, S.K. Synthesis of Novel Composite Membranes by In-Situ Intercalative Emulsion Polymerization for Separation of Aromatic-Aliphatic Mixtures by Pervaporation. J. Memb. Sci. 2020, 597, 117729. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Wang, N.; Guo, H.; Zhang, W.H.; Li, X.; Ji, S.; An, Q.F. Covalent Organic Frameworks Hybird Membrane with Optimized Mass Transport Nanochannel for Aromatic/Aliphatic Mixture Pervaporation. J. Memb. Sci. 2020, 598, 117652. [Google Scholar] [CrossRef]
- Vane, L.M. Review of Pervaporation and Vapor Permeation Process Factors Affecting the Removal of Water from Industrial Solvents. J. Chem. Technol. Biotechnol. 2020, 95, 495–512. [Google Scholar] [CrossRef]
- Wang, L.; Cao, T.; Dykstra, J.E.; Porada, S.; Biesheuvel, P.M.; Elimelech, M. Salt and Water Transport in Reverse Osmosis Membranes: Beyond the Solution-Diffusion Model. Environ. Sci. Technol. 2021, 55, 16665–16675. [Google Scholar] [CrossRef]
- Divakar, S.; Padaki, M.; Balakrishna, R.G. Review on Liquid-Liquid Separation by Membrane Filtration. ACS Omega 2022, 7, 44495–44506. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Gonzales, R.R.; Fu, W.; Xu, G.; Takagi, R.; Song, Q.; Zhou, S.; Matsuyama, H. Organic Solvent Separation Using Carbon Nanotube-Interlayered Thin Film Composite Membrane. Chem. Eng. J. 2023, 473, 145197. [Google Scholar] [CrossRef]
- Liu, C.; Dong, G.; Tsuru, T.; Matsuyama, H. Organic Solvent Reverse Osmosis Membranes for Organic Liquid Mixture Separation: A Review. J. Memb. Sci. 2021, 620, 118882. [Google Scholar] [CrossRef]
- Kushida, W.; Gonzales, R.R.; Shintani, T.; Matsuoka, A.; Nakagawa, K.; Yoshioka, T.; Matsuyama, H. Organic Solvent Mixture Separation Using Fluorine-Incorporated Thin Film Composite Reverse Osmosis Membrane. J. Mater. Chem. A Mater. 2022, 10, 4146–4156. [Google Scholar] [CrossRef]
- Guan, K.; Fang, S.; Zhou, S.; Fu, W.; Li, Z.; Gonzales, R.R.; Xu, P.; Mai, Z.; Hu, M.; Zhang, P.; et al. Thin Film Composite Membrane with Improved Permeance for Reverse Osmosis and Organic Solvent Reverse Osmosis. J. Memb. Sci. 2023, 688, 122104. [Google Scholar] [CrossRef]
- Kitamura, S.; Yoshioka, T.; Nakagawa, K.; Kitagawa, T.; Okamoto, Y.; Matsuoka, A.; Kamio, E.; Matsuyama, H. Organic Solvent Reverse Osmosis Characteristics of TiO2-ZrO2-Organic Chelating Ligand (OCL) Composite Membranes Using OCLs with Different Molecular Sizes. Sep. Purif. Technol. 2023, 315, 123576. [Google Scholar] [CrossRef]
- Chau, J.; Sirkar, K.K. Organic Solvent Mixture Separation during Reverse Osmosis and Nanofiltration by a Perfluorodioxole Copolymer Membrane. J. Memb. Sci. 2021, 618, 118663. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, L.; Shintani, T.; Matsuyama, H. AF2400/Polyketone Composite Organic Solvent Reverse Osmosis Membrane for Organic Liquid Separation. J. Memb. Sci. 2021, 628, 119270. [Google Scholar] [CrossRef]
- Liu, C.; Takagi, R.; Shintani, T.; Cheng, L.; Tung, K.L.; Matsuyama, H. Organic Liquid Mixture Separation Using an Aliphatic Polyketone-Supported Polyamide Organic Solvent Reverse Osmosis (OSRO) Membrane. ACS Appl. Mater. Interfaces 2020, 12, 7586–7594. [Google Scholar] [CrossRef] [PubMed]
- Balogun, H.A.; Lively, R.P. Independent Experimental Measurements of Diffusion, Sorption, and Permeability Support the Solution-Diffusion Model of Membrane Transport. J. Memb. Sci. 2025, in press. [CrossRef]
- Vandezande, P.; Gevers, L.E.M.; Vankelecom, I.F.J. Solvent Resistant Nanofiltration: Separating on a Molecular Level. Chem. Soc. Rev. 2008, 37, 365–405. [Google Scholar] [CrossRef]
- Tong, Y.H.; Luo, L.H.; Jia, R.; Han, R.; Xu, S.J.; Xu, Z.L. Whether Membranes Developed for Organic Solvent Nanofiltration (OSN) Tend to Be Hydrophilic or Hydrophobic?—A Review. Heliyon 2024, 10, e24330. [Google Scholar] [CrossRef]
- Shi, G.M.; Feng, Y.; Li, B.; Tham, H.M.; Lai, J.Y.; Chung, T.S. Recent Progress of Organic Solvent Nanofiltration Membranes. Prog. Polym. Sci. 2021, 123, 101470. [Google Scholar] [CrossRef]
- Ignacz, G.; Szekely, G. Deep Learning Meets Quantitative Structure–Activity Relationship (QSAR) for Leveraging Structure-Based Prediction of Solute Rejection in Organic Solvent Nanofiltration. J. Memb. Sci. 2022, 646, 120268. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Marcel, M., Ed.; Springer: Dordrecht, The Netherlands, 1996; ISBN 978-0-7923-4248-9. [Google Scholar]
- Alduraiei, F.; Kumar, S.; Liu, J.; Nunes, S.P.; Szekely, G. Rapid Fabrication of Fluorinated Covalent Organic Polymer Membranes for Organic Solvent Nanofiltration. J. Memb. Sci. 2022, 648, 120345. [Google Scholar] [CrossRef]
- Zheng, D.; Hua, D.; Yao, A.; Hong, Y.; Cha, X.; Yang, X.; Japip, S.; Zhan, G. Fabrication of Thin-Film Composite Membranes for Organic Solvent Nanofiltration by Mixed Monomeric Polymerization on Ionic Liquid/Water Interfaces. J. Memb. Sci. 2021, 636, 119551. [Google Scholar] [CrossRef]
- Su, J.; Lv, X.; Li, S.; Jiang, Y.; Liu, S.; Zhang, X.; Li, H.; Su, B. High Separation Performance Thin Film Composite and Thin Film Nanocomposite Hollow Fiber Membranes via Interfacial Polymerization for Organic Solvent Nanofiltration. Sep. Purif. Technol. 2022, 278, 119567. [Google Scholar] [CrossRef]
- Hong, Y.; Hua, D.; Pan, J.; Cheng, X.; Xu, K.; Huo, Z.; Zhan, G. Fabrication of Polyamide Membranes by Interlayer-Assisted Interfacial Polymerization Method with Enhanced Organic Solvent Nanofiltration Performance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131075. [Google Scholar] [CrossRef]
- Zhang, B.; Yi, C.; Wu, D.; Qiao, J.; Zhang, L. A High-Permeance Organic Solvent Nanofiltration Membrane via Polymerization of Ether Oxide-Based Polymeric Chains for Sustainable Dye Separation. Sustainability 2023, 15, 3446. [Google Scholar] [CrossRef]
- Li, Y.; Shahbazi, A.; Kadzere, C.T. Separation of Cells and Proteins from Fermentation Broth Using Ultrafiltration. J. Food Eng. 2006, 75, 574–580. [Google Scholar] [CrossRef]
- Elhamarnah, Y.; Hey, T.; Lipnizki, F.; Qiblawey, H. Investigating the Impact of Stormwater Fouling on Polysulfone Ultrafiltration Membranes Modified with Deep Eutectic Solvents. J. Water Process Eng. 2023, 56, 104362. [Google Scholar] [CrossRef]
- Gebru, K.A.; Das, C. Effects of Solubility Parameter Differences among PEG, PVP and CA on the Preparation of Ultrafiltration Membranes: Impacts of Solvents and Additives on Morphology, Permeability and Fouling Performances. Chin. J. Chem. Eng. 2017, 25, 911–923. [Google Scholar] [CrossRef]
- Priske, M.; Lazar, M.; Schnitzer, C.; Baumgarten, G. Recent Applications of Organic Solvent Nanofiltration. Chem. Ing. Tech. 2016, 88, 39–49. [Google Scholar] [CrossRef]
- Oxley, A.; Gaffney, P.R.J.; Kim, D.; Marchetti, P.; Livingston, A.G. Graft Modification of Polybenzimidazole Membranes for Organic Solvent Ultrafiltration with Scale up to Spiral Wound Modules. J. Memb. Sci. 2022, 647, 120199. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Scholes, C.A.; Liu, L.; Kentish, S.E. Efficient Degumming of Crude Canola Oil Using Ultrafiltration Membranes and Bio-Derived Solvents. Innov. Food Sci. Emerg. Technol. 2020, 59, 102274. [Google Scholar] [CrossRef]
- Aryanti, N.; Wardhani, D.H.; Nafiunisa, A. Ultrafiltration Membrane for Degumming of Crude Palm Oil-Isopropanol Mixture. Chem. Biochem. Eng. Q. 2018, 32, 325–334. [Google Scholar] [CrossRef]
- Goh, K.S.; Chong, J.Y.; Chen, Y.; Fang, W.; Bae, T.H.; Wang, R. Thin-Film Composite Hollow Fibre Membrane for Low Pressure Organic Solvent Nanofiltration. J. Memb. Sci. 2020, 597, 117760. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Wen, S.; Fan, L.; Chen, M.; Dong, Y.; Zhang, Q. In-Situ Formation of Polyether Membrane Selective Layer for Pervaporation Separation of Azeotropic Organic Solvent Mixtures. Sep. Purif. Technol. 2025, 359, 130522. [Google Scholar] [CrossRef]
- Esposito, R.; Abdulhamid, M.A.; Upadhyaya, L.; Volkov, A.; Nunes, S.P. Carboxyl-Functionalized Polyimide for Polar/Non-Polar Organic Solvent Separation by Pervaporation. J. Memb. Sci. 2025, 713, 123277. [Google Scholar] [CrossRef]
- Liu, C.; Takagi, R.; Saeki, D.; Cheng, L.; Shintani, T.; Yasui, T.; Matsuyama, H. Highly Improved Organic Solvent Reverse Osmosis (OSRO) Membrane for Organic Liquid Mixture Separation by Simple Heat Treatment. J. Memb. Sci. 2021, 618, 118710. [Google Scholar] [CrossRef]
- Xu, S.J.; Luo, L.H.; Tong, Y.H.; Shen, Q.; Xu, Z.L.; Wu, Y.Z.; Yang, H. Organic Solvent Nanofiltration (OSN) Membrane with Polyamantadinamide Active Layer for Reducing Separation Performance Inconformity. Sep. Purif. Technol. 2022, 278, 119582. [Google Scholar] [CrossRef]
- Yoshiwaka, Y.; Kitagawa, T.; Shintani, T.; Nakagawa, K.; Yoshioka, T.; Matsuyama, H. AF2400/Polyketone Composite OSRO Membrane for Organic Solvent Mixture Separation. Sep. Purif. Technol. 2023, 320, 124150. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Li, S.; Van der Bruggen, B. Interfacially Polymerized Thin-Film Composite Membranes for Organic Solvent Nanofiltration. Adv. Mater. Interfaces 2021, 8, 2001671. [Google Scholar] [CrossRef]
- Nie, L.; Chuah, C.Y.; Bae, T.H.; Lee, J.M. Graphene-Based Advanced Membrane Applications in Organic Solvent Nanofiltration. Adv. Funct. Mater. 2021, 31, 2006949. [Google Scholar] [CrossRef]
- Liu, J.; Fan, S.; Li, C.; Qing, H.; Xiao, Z. Sandwich Structure Membrane with Enhanced Anti-Swelling Property and Mechanical Strength for Bioethanol Separation by Pervaporation. Ind. Eng. Chem. Res. 2023, 62, 5262–5273. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, S.; Peng, G.; Sotto, A.; Ruan, H.; Shen, J.; Gao, C. Novel Crosslinked Brominated Polyphenylene Oxide Composite Nanofiltration Membranes with Organic Solvent Permeability and Swelling Property. J. Memb. Sci. 2021, 620, 118784. [Google Scholar] [CrossRef]
- Su, Q.; Wen, J.; Wang, D.; Zhang, L.; Li, M. Two-Stage Polymerization towards C–C Bonded Conjugated Microporous Polymer Membranes with Excellent Nanofiltration Performance. J. Memb. Sci. 2022, 647, 120314. [Google Scholar] [CrossRef]
- Nakoa, K.; Rahaoui, K.; Date, A.; Akbarzadeh, A. Sustainable Zero Liquid Discharge Desalination (SZLDD). Sol. Energy 2016, 135, 337–347. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling Membranes for Sustainable Water Purification: Strategies and Mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Gu, B.X.; Liu, Z.Z.; Zhang, K.; Ji, Y.L.; Zhou, Y.; Gao, C.J. Biomimetic Asymmetric Structural Polyamide OSN Membranes Fabricated via Fluorinated Polymeric Networks Regulated Interfacial Polymerization. J. Memb. Sci. 2021, 625, 119112. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, X.; Yuan, Y.; Wang, S.; Meng, X.; Huhe, T.; Wang, Q. A Facile Method to Fabricate Anti-Fouling Nanofiltration Membrane with Aminated Lignin. J. Memb. Sci. 2024, 692, 122269. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, Z.; Wang, L.; Yu, Y.; Yang, H.; Jin, H.; Lu, P.; Wang, Y.; Wu, D.; Li, Y.; et al. Facile ZIF–8 Nanocrystals Interlayered Solvent–Resistant Thin–Film Nanocomposite Membranes for Enhanced Solvent Permeance and Rejection. J. Memb. Sci. 2021, 636, 119586. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Da’na, D.A.; Qiblawey, H.; Zouari, N. Effect of Concentration of Calcium and Sulfate Ions on Gypsum Scaling of Reverse Osmosis Membrane, Mechanistic Study. J. Mater. Res. Technol. 2020, 9, 13459–13473. [Google Scholar] [CrossRef]
- Tong, T.; Wallace, A.F.; Zhao, S.; Wang, Z. Mineral Scaling in Membrane Desalination: Mechanisms, Mitigation Strategies, and Feasibility of Scaling-Resistant Membranes. J. Memb. Sci. 2019, 579, 52–69. [Google Scholar] [CrossRef]
- Shen, Y.; Van Eygen, G.; Wu, B.; Wu, C.; Yin, M.J.; Zhao, Y.; Van der Bruggen, B.; An, Q.F. In-Situ Interfacial Polymerization of Zwitterionic Nanofiltration Membranes with Anti-Scaling Performance. Adv. Membr. 2024, 4, 100095. [Google Scholar] [CrossRef]
- Peng, Y.; Song, W.; Wang, E.; Yang, Q.; Su, B. COFs Interlayer Manipulated Interfacial Polymerization for Fabricating High Performance OSN Membrane with Overall Covalently Crosslinked Structure. J. Memb. Sci. 2024, 697, 122588. [Google Scholar] [CrossRef]
- Huang, T.; Moosa, B.A.; Hoang, P.; Liu, J.; Chisca, S.; Zhang, G.; AlYami, M.; Khashab, N.M.; Nunes, S.P. Molecularly-Porous Ultrathin Membranes for Highly Selective Organic Solvent Nanofiltration. Nat. Commun. 2020, 11, 5882. [Google Scholar] [CrossRef]
- Liu, T.; Sun, H.; Wang, X.; Li, J.; Zhang, Z.; Wu, P.; Wang, N.; An, Q.-F. Hyperbranched Polymer Hollow Fiber Composite Membranes for Pervaporation Separation of Aromatic/Aliphatic Hydrocarbon Mixtures. Chin. J. Chem. Eng. 2024, 69, 13–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Chen, S.; Liu, J.; Hong, Z.; Wang, J.; Li, Z.; Gao, X.; Xu, R.; Gu, X. TiO2-Decorated NaA Zeolite Membranes with Improved Separation Stability for Pervaporation Dehydration of N, N-Dimethylacetamide. J Memb Sci 2021, 634, 119398. [Google Scholar] [CrossRef]
- Algieri, C.; Drioli, E. Zeolite Membranes: Synthesis and Applications. Sep. Purif. Technol. 2022, 278, 119295. [Google Scholar] [CrossRef]
- Wang, S.; Wei, X.; Li, Z.; Liu, Y.; Wang, H.; Zou, L.; Lu, D.; Hassan Akhtar, F.; Wang, X.; Wu, C.; et al. Recent Advances in Developing Mixed Matrix Membranes Based on Covalent Organic Frameworks. Sep. Purif. Technol. 2022, 301, 122004. [Google Scholar] [CrossRef]
| Membrane Type | Material | Fabrication Method | Application | Conditions | Performance | Limitations | Ref. |
|---|---|---|---|---|---|---|---|
| OSN | polyamide | Interfacial polymerization of Polyethyleneimine (PEI), piperazine (PIP) and trimesoyl chloride (TMC) | Rose Bengal and acid fuchsin | 0.2 MPa | 116.0 and 45.0 L m−2 h−1 MPa−1 permeability in acetone and isopropanol. 99.9% and 91.8% rejection to rose bengal (1017 Da) and acid fuchsin (585 Da). | DMF still swelling | [80] |
| OSPV | Polyether | CE (3,4-Epoxycyclohexylmethyl) and DOX (3-ethyl-3-[(3-ethyloxetan-3-yl)methoxymethyl]oxetane) cationic photopolymerization | Separation of ethanol/n-hexane and ethanol/cyclohexane | 25 °C, vacuum pump (1.3 kPa) | Separation factor of up to 162.81 for ethanol/n-hexane azeotrope and 41.89 for ethanol/cyclohexane azeotrope | Not specified | [81] |
| OSPV | carboxyl-functionalized polyimide | Solvent-casting | Separation alcohol/nonpolar solvent | 40 °C, 0–8·10−4 MPa | For methanol/toluene flux was 0.13 kg m−2 h−1 and Separation factors was 140. For methanol/methyl tert-butyl ether flux was 0.09 kg m−2 h−1 and separation factors was 3065 For ethanol/cyclohexane flux was 0.11 kg m−2 h−1 and separation factors was 406. | Not specified | [82] |
| OSRO | Polyketone supported polyamide | Interfacial polymerization | Separation alcohol/nonpolar solvent | 3–5 MPa, 25–60 °C | Separation factor: 45 MeOH from MeOH/Toluene mixture, separation factor: 210.0 MeOH form MeOH/Hexane, Flux: Around 2.1–2.4 kg m−2 h−1 | Gradual Decline in Selectivity over time due to PA chain relaxation | [83] |
| OSRO | perfluoro-2,2-dimethyl-1,3-dioxole and tetrafluoroethylene (Teflon® AF2400 from Sigma-Aldrich Co., Burlington, USA) onto a polyketone support | Spin-coating | Toluene/1,3,5-Triisopropylbenzene separation | 25 °C, 4.0 MPa | 96.2% 1,3,5-Triisopropylbenzene rejection, 0.7 L m−2 h−1 MPa−1 permeance | Limited commercial maturity | [60] |
| OSN | 3-amino-1-adamantanol (AAMO) with acyl chloride | interfacial polymerization | Fast green (FCF) in ethanol | 0.3 MPa, 25 °C | 295.0 L m−2 h−1 MPa−1 for pure MeOH and FCF/MeOH rejection of 84.4% | Not specified | [84] |
| OSUF | polyimide/polyethyleneimine@TiO2 | coupling the non-solvent-induced phase transformation (NIPs), chemical crosslinking and interfacial in situ biomineralization | Polyethylene glycol rejection from N,N-dimethylformamide (DMF) | 0.4 MPa, 25 °C | 654.0 L m−2 h−1 MPa−1 | Not specified | [6] |
| OSN | Polydopamine with Polyamide on PTFE nanofibrous substrate | Electrospinning of PTFE/FEP/PEO, PDA coating, interfacial polymerization of PIP-TMC | Rose Bengal (RB) rejection in EtOH and DMF | 0.2 MPa, 25 °C | In EtOH: 59.5 L m−2 h−1 MPa−1 flux, 96.3% RB rejection; In DMF: 23.2 L m−2 h−1 MPa−1, 92.2% RB rejection | The membrane exhibited a relatively lower permeance in DMF | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halloub, A.; Kujawski, W. Recent Advances in Polymeric Membrane Integration for Organic Solvent Mixtures Separation: Mini-Review. Membranes 2025, 15, 329. https://doi.org/10.3390/membranes15110329
Halloub A, Kujawski W. Recent Advances in Polymeric Membrane Integration for Organic Solvent Mixtures Separation: Mini-Review. Membranes. 2025; 15(11):329. https://doi.org/10.3390/membranes15110329
Chicago/Turabian StyleHalloub, Abdellah, and Wojciech Kujawski. 2025. "Recent Advances in Polymeric Membrane Integration for Organic Solvent Mixtures Separation: Mini-Review" Membranes 15, no. 11: 329. https://doi.org/10.3390/membranes15110329
APA StyleHalloub, A., & Kujawski, W. (2025). Recent Advances in Polymeric Membrane Integration for Organic Solvent Mixtures Separation: Mini-Review. Membranes, 15(11), 329. https://doi.org/10.3390/membranes15110329







