Engineering Thermal Cross-Linking in Nanofiltration Membranes for Efficient Nicotine Extraction from Tobacco Extract
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Preparation of Membranes
2.3. Characterizations
2.4. Measurements of Separation Properties
3. Results and Discussion
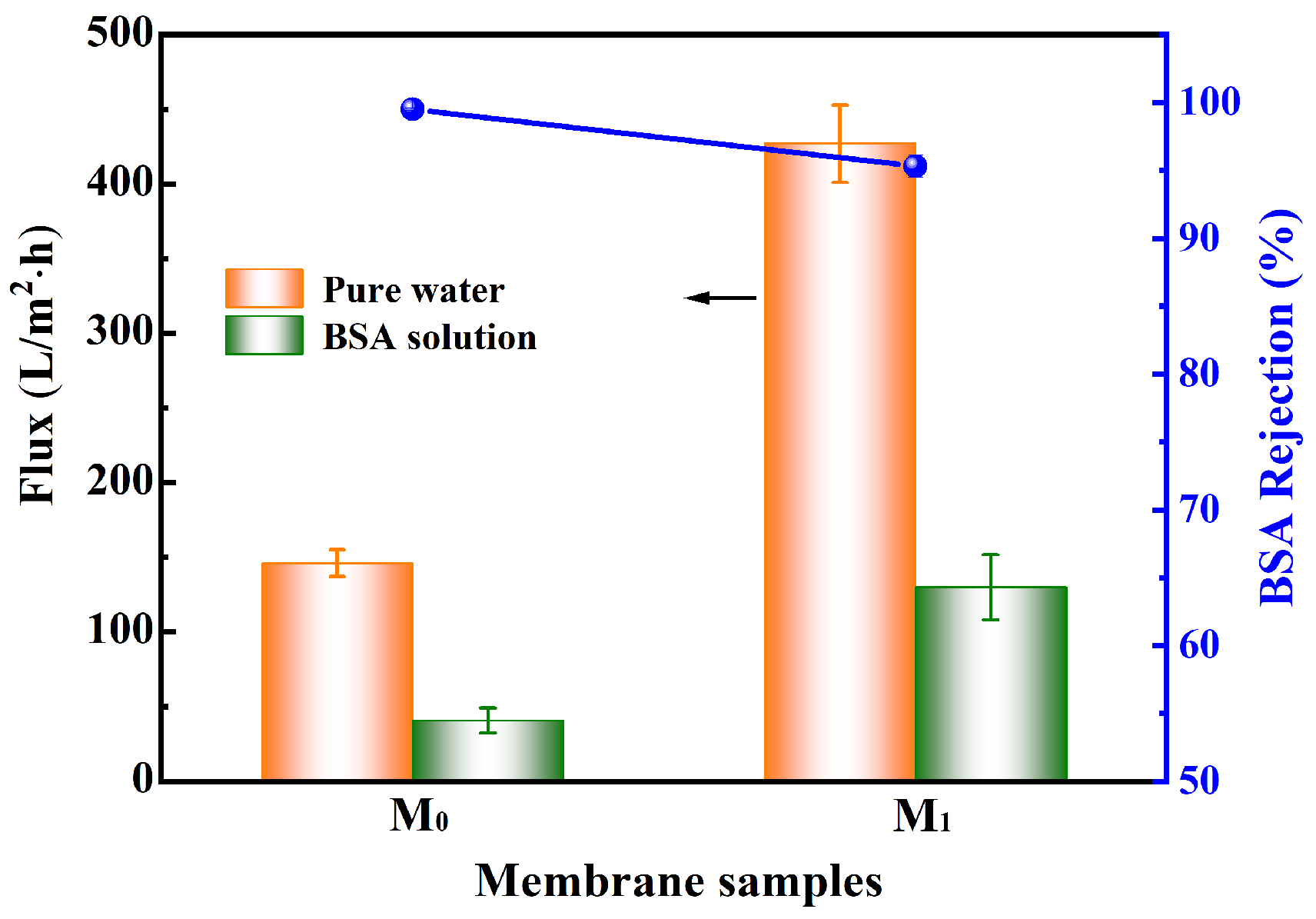
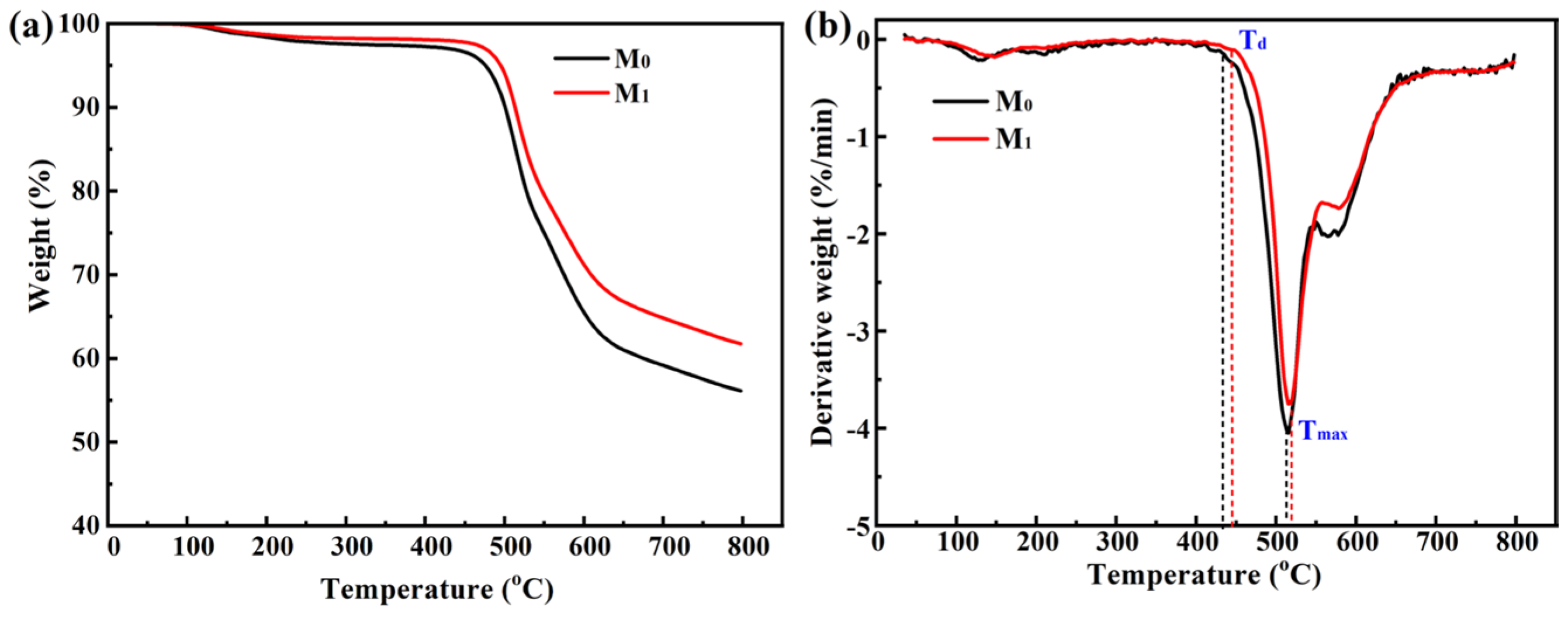
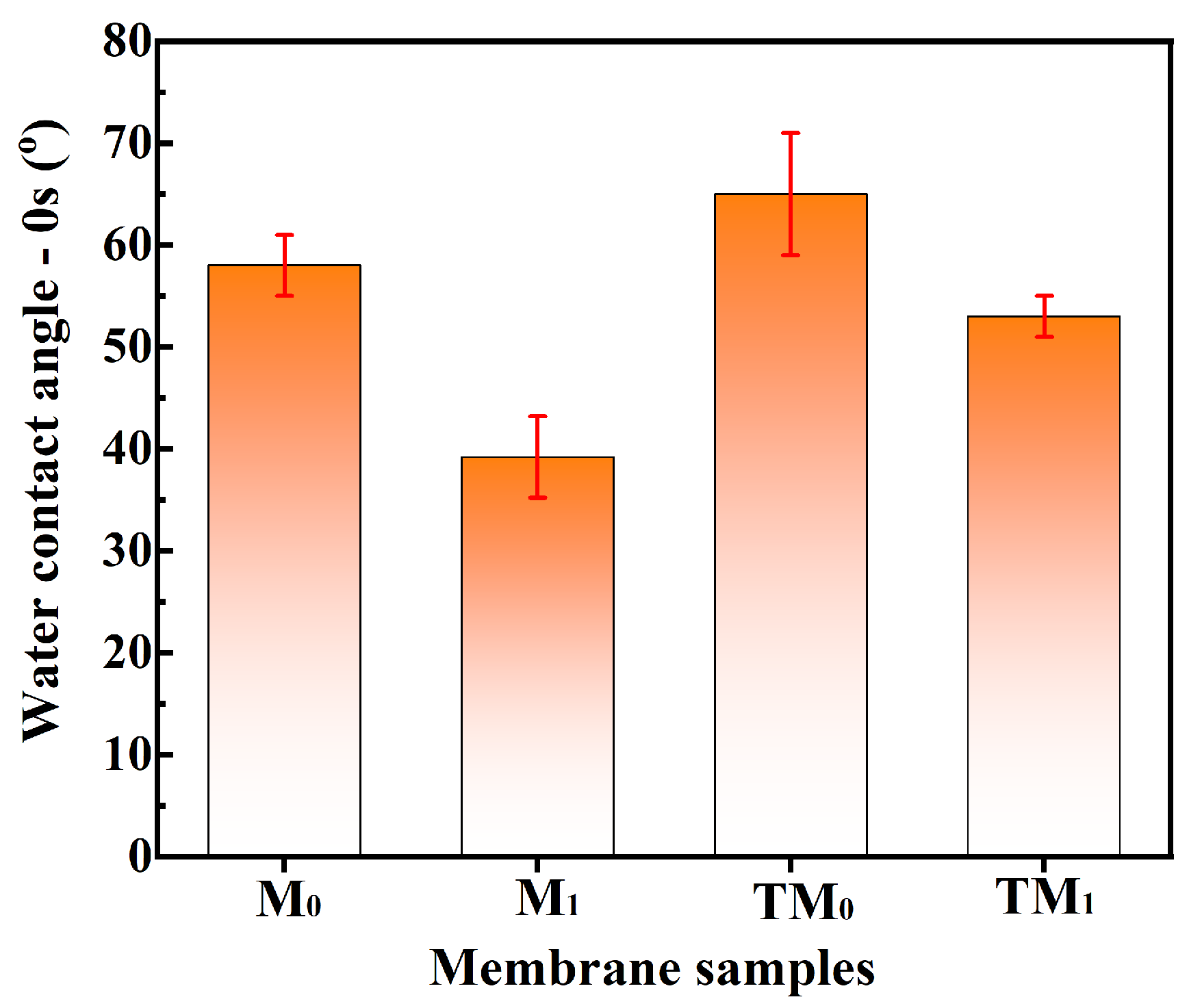
3.1. Properties of UF Membranes
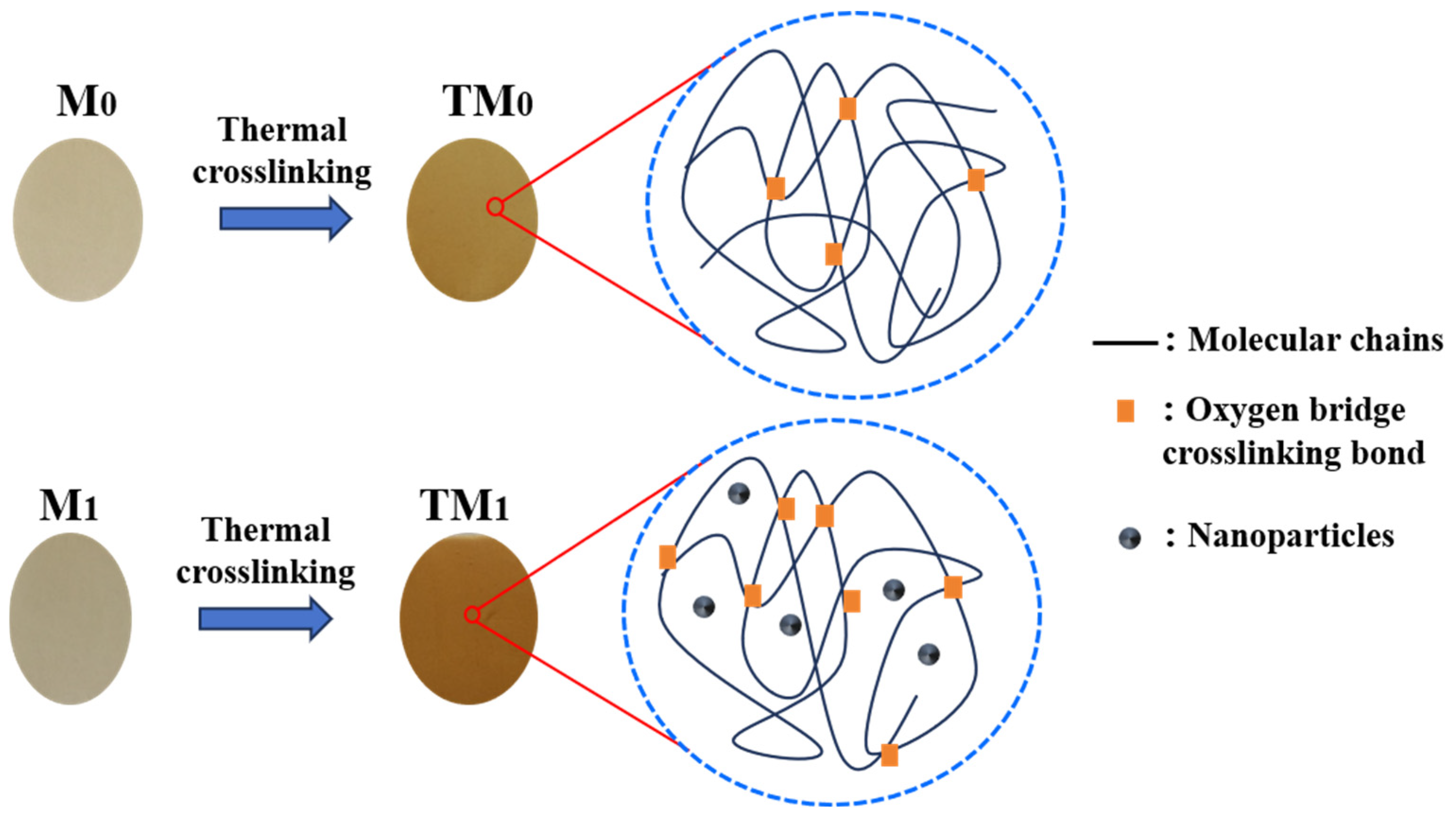
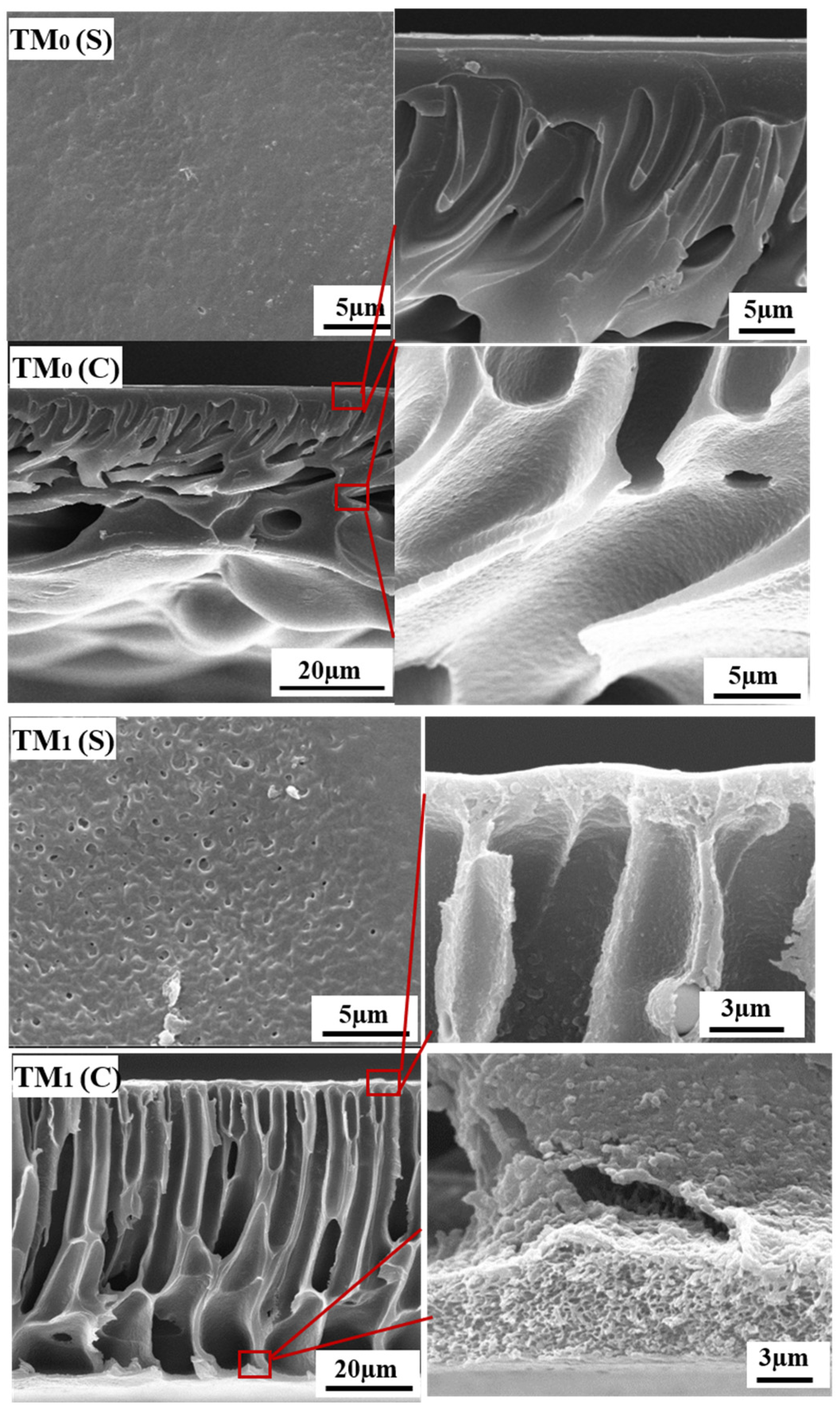
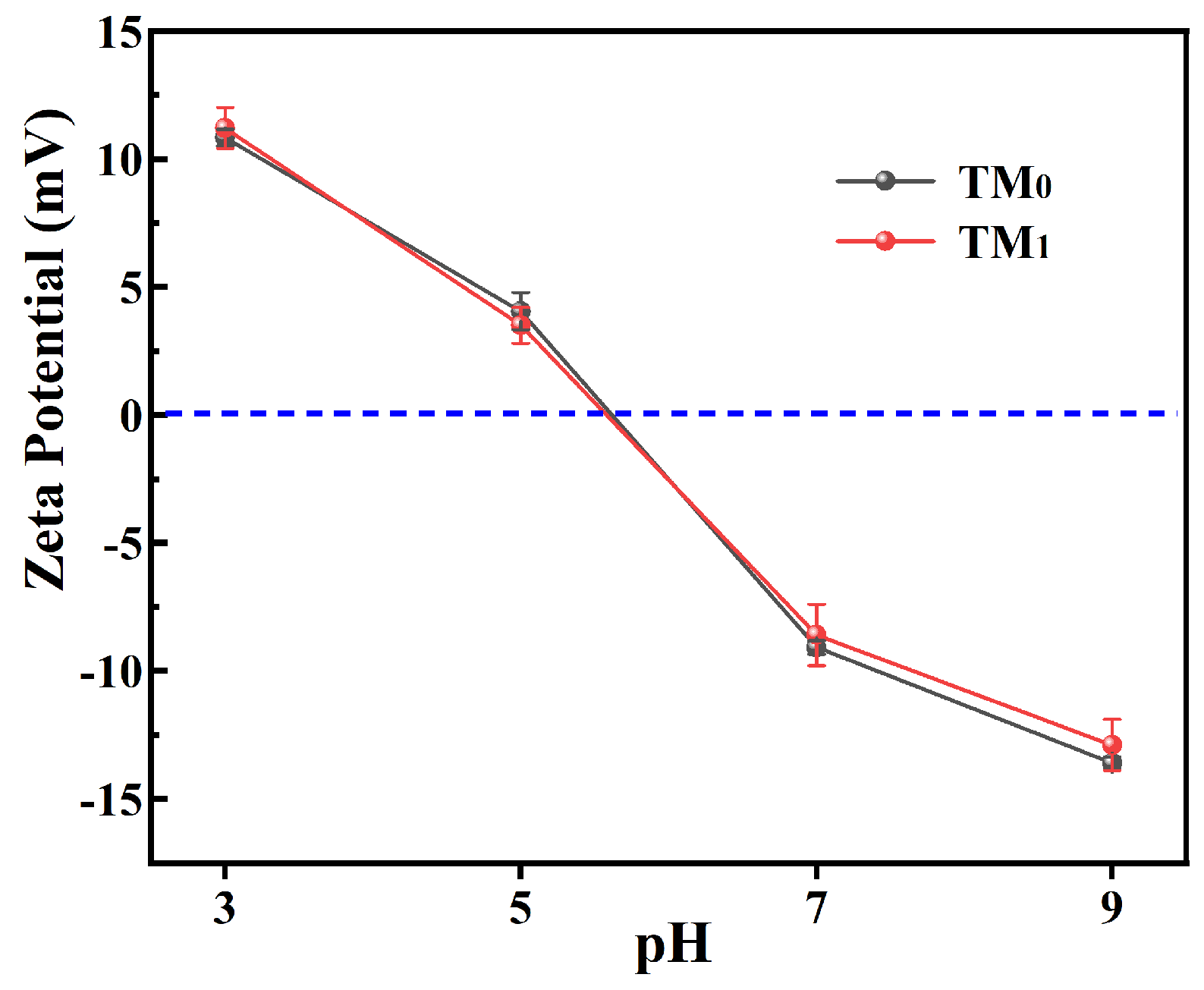
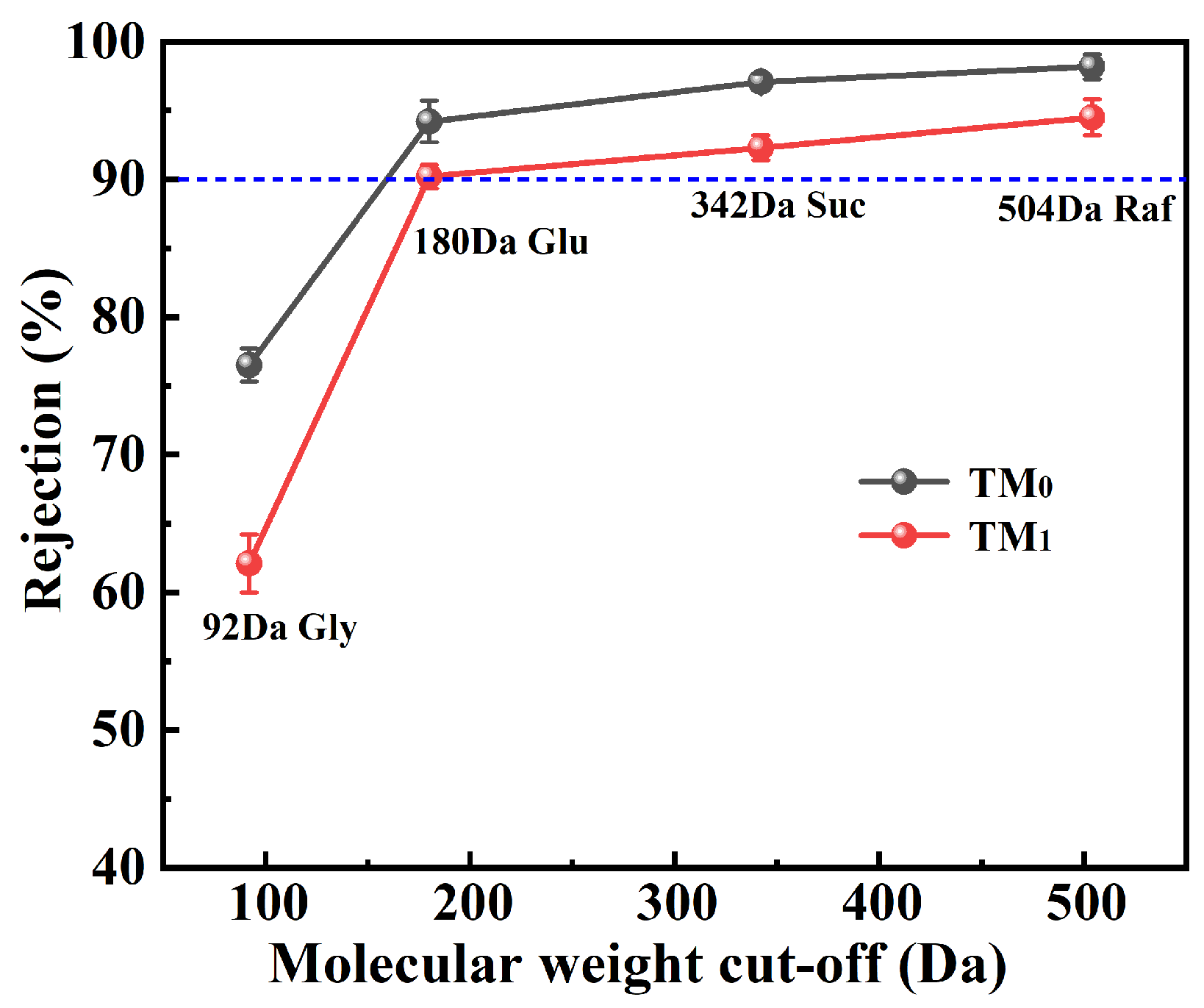
3.2. Properties of Thermal Crosslinking Membranes
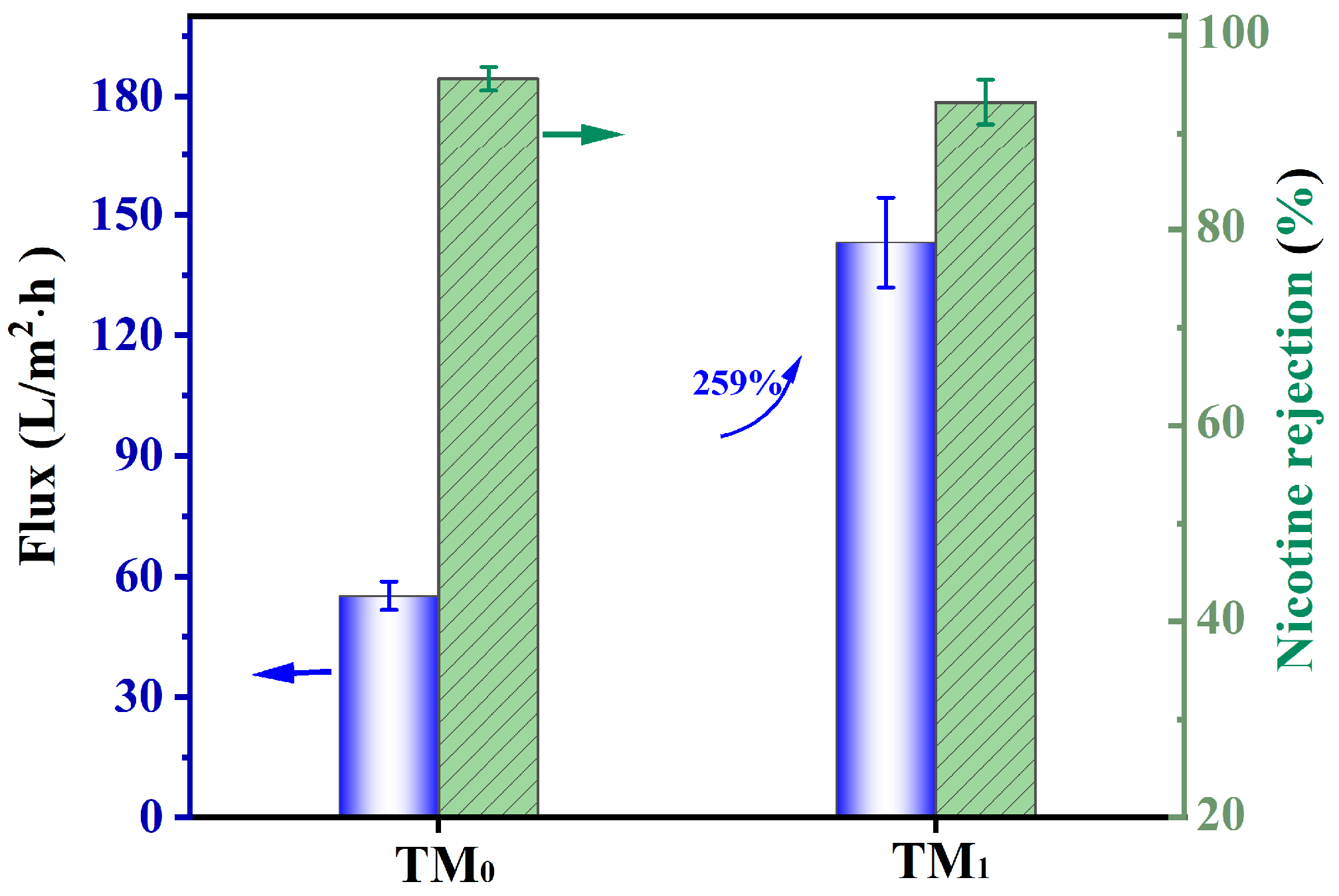
3.3. Separation Performance Toward Nicotine
3.4. Stability of Thermal Crosslinking Membrane
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, Q.; Yang, B.; Guan, Y.; Bi, P.; Huang, H.; Zeng, W.; Xiang, H.; Liu, X.; Mi, Q.; Li, X. Study on nicotine-induced autophagy in human intestinal epithelial cells. Acta Tabacaria Sin. 2019, 25, 117–122. [Google Scholar] [CrossRef]
- Yang, L.; Shen, J.; Liu, C.; Kuang, Z.; Tang, Y.; Qian, Z.; Guan, M.; Yang, Y.; Zhan, Y.; Li, N.; et al. Nicotine rebalances NAD+ homeostasis and improves aging-related symptoms in male mice by enhancing NAMPT activity. Nat. Commun. 2023, 14, 900. [Google Scholar] [CrossRef]
- Zou, X.; Amrit, B.K.; Tareq, A.; Aziz, A.; Devnath, P.; Raul, A.; Mitra, S.; Emran, T.; Mujawah, A.; Lorenzo, J.; et al. Current advances of functional phytochemicals in Nicotiana plant and related potential value of tobacco processing waste: A review. Biomed. Pharmacother. 2021, 143, 112191. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R.; Zare, S.; Asadollahi, M.; Schuman, M.C. Ecological Roles and Biological Activities of Specialized Metabolites from the Genus Nicotiana. Chem. Rev. 2017, 117, 12227–12280. [Google Scholar] [CrossRef]
- Banožić, M.; Babić, J.; Jokić, S. Recent advances in extraction of bioactive compounds from tobacco industrial waste—A review. Ind. Crop. Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Guan, M.; Zhang, J.; Zhou, S.; Zhang, X.; Wang, X.; Cao, Y.; Tian, H.; Ding, N.; Li, Y.; Chen, W.; et al. Three-dimensional structure-property relationships for filter membranes used to refine tobacco extracts based on high-resolution CT scanning and deep machine learning. Tob. Sci. Technol. 2025, 58, 11–19. [Google Scholar]
- Liu, C.; Xu, R.; Jiang, W.; Li, Y.; Si, X.; Yang, J.; Zhu, R.; Zhang, F.; Tang, S.; Yang, M.; et al. Preparation and properties of composite nanofiltration membrane for fine separation of aromatic components from tobacco. Membr. Sci. Technol. 2024, 44, 115–121, 131. [Google Scholar]
- Liu, C.; Zhang, H.; Xu, R.; Hou, M.; Jiang, W.; Tang, S.; Si, X.; Zhang, F.; Tang, J.; Zhang, S.; et al. Precise separation of flavor constituents from tobacco extracts via tailored nanofiltration membranes. J. Membr. Sci. 2025, 734, 124432. [Google Scholar] [CrossRef]
- Lu, P.; Wu, R.; Xu, G.; Zhang, B. Effective separation of nicotine from tobacco extract by silica gel. Chem. Eng. Res. Des. 2025, 217, 502–513. [Google Scholar] [CrossRef]
- Sha, Y.; Yu, H.; Xiong, J.; Wang, J.; Fei, T.; Wu, D.; Yang, K.; Zhang, L. Separation and purification of active ingredients in tobacco by free-flow electrophoresis. Anal. Methods 2023, 15, 5885–5890. [Google Scholar] [CrossRef]
- Guo, S.; Zheng, L.; Luo, J.; Yuan, C. Application progress of nanofiltration membrane in treatment of high salinity wastewater. Membr. Sci. Technol. 2022, 42, 175–182. [Google Scholar]
- Zhang, Z.; Fan, K.; Liu, Y.; Xia, S. A review on polyester and polyester-amide thin film composite nanofiltration membranes: Synthesis, characteristics and applications. Sci. Total. Environ. 2022, 858, 159922. [Google Scholar] [CrossRef]
- de Almeida, F.R.; Ferreira, I.L.d.M.; dos Reis, R.A. Recent Progress on the Development and Application of Polymeric Nanofiltration Membranes: A Mini-Review. Mini-Rev. Org. Chem. 2024, 21, 3–21. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, C.; Ren, X.; Li, G.; Tang, S.; Feng, X.; Lu, D.; Yao, Z.; Wang, T.; Zhang, S.; et al. Manipulating the ionization of polyamide nanofiltration membrane for cascade separation of low-molecular-weight tobacco extracts. J. Membr. Sci. 2025, 728, 124149. [Google Scholar] [CrossRef]
- Tsibranska, I.; Karabojikova, V.; Jeliazkov, J. Concentration of flavonoids in ethanolic extracts from tobacco leaves through nanofiltration. Bulg. Chem. Commun. 2016, 48, 232–237. [Google Scholar]
- Mohammad, A.; Teow, Y.; Ang, W.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Gan, Q.; Hu, Y.; Wu, C.; Yang, Z.; Peng, L.E.; Tang, C.Y. Nanofoamed Polyamide Membranes: Mechanisms, Developments, and Environmental Implications. Environ. Sci. Technol. 2024, 58, 20812–20829. [Google Scholar] [CrossRef]
- Peng, L.E.; Yang, Z.; Long, L.; Zhou, S.; Guo, H.; Tang, C.Y. A critical review on porous substrates of TFC polyamide membranes: Mechanisms, membrane performances, and future perspectives. J. Membr. Sci. 2022, 641, 119871. [Google Scholar] [CrossRef]
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A.F. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Sahu, L.R.; Yadav, D.; Ingole, P.G. Recent developments and innovations in thin-film nanocomposite nanofiltration: The next-generation selective membrane for heavy metal ion removal from water. Chem. Eng. J. 2025, 513, 162579. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Z.; Hu, Y.; Chen, Y. Thin-Film Composite Membrane Compaction: Exploring the Interplay among Support Compressive Modulus, Structural Characteristics, and Overall Transport Efficiency. Environ. Sci. Technol. 2024, 58, 8587–8596. [Google Scholar] [CrossRef]
- Saleh, E.A.M.; Kumar, A.; Alghazali, T.; Ganesan, S.; Shankhyan, A.; Sharma, G.C.; Naidu, K.S.; Rahbari-Sisakht, M. Recent advances in thin film composite (TFC) membrane development: Materials and modification methods. Environ. Sci. Water Res. Technol. 2025, 11, 1059–1085. [Google Scholar] [CrossRef]
- Rahbari-Sisakht, M.; Mousavian, S.; Ariana, M.A.; Ismail, A.F. Thin-film nanocomposite membranes for water treatment: Evolution, applications, challenges, and advancement strategies. J. Environ. Chem. Eng. 2025, 13, 116392. [Google Scholar] [CrossRef]
- Pang, H.Z.; Jin, X.-G.; Wang, J.; Ma, X.-H.; Xu, Z.-L. High temperature resistant polyamide thin film composite nanofiltration membrane based on polyethylene substrate. J. Membr. Sci. 2025, 721, 123811. [Google Scholar] [CrossRef]
- Jia, Y.; Huo, X.; Gao, L.; Shao, W.; Chang, N. Controllable Design of Polyamide Composite Membrane Separation Layer Structures via Metal–Organic Frameworks: A Review. Membranes 2024, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, W.; Wang, H.; Luo, Y.; Liu, Q.; Wang, X.; Wang, L.; Zhang, T. Reverse osmosis and nanofiltration processes in industrial wastewater treatment: The recent progress, challenge, and future opportunity. Sep. Purif. Technol. 2025, 362, 131687. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, Y.; Xu, X.; Peng, X.; Niu, Y.; Xu, P.; Li, T. Research on the factors influencing nanofiltration membrane fouling and the prediction of membrane fouling. J. Water Process. Eng. 2024, 59, 104876. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, X.; Zhao, D.; Li, M. Organic solvent nanofiltration polymeric membranes: Recent progress, applications, challenges, and perspectives. Chin. J. Chem. Eng. 2025, 82, 196–208. [Google Scholar] [CrossRef]
- Xu, R.; Li, L.; Jin, X.; Hou, M.; He, L.; Lu, Y.; Song, C.; Wang, T. Thermal crosslinking of a novel membrane derived from phenolphthalein-based cardo poly(arylene ether ketone) to enhance CO2/CH4 separation performance and plasticization resistance. J. Membr. Sci. 2019, 586, 306–317. [Google Scholar] [CrossRef]
- Xu, R.; Li, L.; Hou, M.; Xue, J.; Liu, Y.; Pan, Z.; Song, C.; Wang, T. Enhanced CO2 permeability of thermal crosslinking membrane via sulfonation/desulfonation of phenolphthalein-based cardo poly(arylene ether ketone). J. Membr. Sci. 2020, 598, 117824. [Google Scholar] [CrossRef]
- Xu, R.; Li, L.; Wang, Y.; Hou, M.; Pan, Z.; Song, C.; Wang, T. Thermal crosslinking membrane with enhanced CO2 separation performance derived from nitrile-containing phenolphthalein-based poly(arylene ether ketone). J. Membr. Sci. 2021, 637, 119634. [Google Scholar] [CrossRef]
- Bhuwania, N.; Labreche, Y.; Achoundong, C.S.; Baltazar, J.; Burgess, S.K.; Karwa, S.; Xu, L.; Henderson, C.L.; Williams, P.J.; Koros, W.J. Engineering substructure morphology of asymmetric carbon molecular sieve hollow fiber membranes. Carbon 2014, 76, 417–434. [Google Scholar] [CrossRef]
- Chung, Y.; Park, D.; Kim, H.; Nam, S.-E.; Kang, S. Novel method for the facile control of molecular weight cut-off (MWCO) of ceramic membranes. Water Res. 2022, 215, 118268. [Google Scholar] [CrossRef] [PubMed]

| Fabricating Conditions | M0 | M1 |
|---|---|---|
| Polymer type and concentration | 16 wt% PEK | 16 wt% PEK |
| Amount of nanoparticle additive | without | 25 wt% TBT |
| Coagulation bath composition and temperature | deionized water, 25 °C | hydrochloric acid bath (pH = 2), 60 °C |
| Thickness | 95~105 μm | 120~130 μm |
| Thermal cross-linking conditions | 280 °C for 5 h | 280 °C for 5 h |
| Thermally cross-linked membranes and thickness | TM0 30~40 μm | TM1 70~85 μm |
| Types of Solvent | Flux Change Rate (%) | Rejection Change Rate (%) |
|---|---|---|
| n-hexane | 4.30 | −1.2 |
| isopropanol | 1.20 | −0.86 |
| acetone | −1.21 | 1.78 |
| ethyl acetate | −2.24 | 1.53 |
| toluene | 1.56 | −2.22 |
| 1 mol/L HCl | 1.45 | −0.83 |
| 1 mol/L NaOH | −2.14 | 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Wang, X.; Na, B.; Ye, Y.; Qiao, Y.; Li, L.; Tian, Y.; Ning, X.; Wang, Z.; Zhao, X.; et al. Engineering Thermal Cross-Linking in Nanofiltration Membranes for Efficient Nicotine Extraction from Tobacco Extract. Membranes 2025, 15, 327. https://doi.org/10.3390/membranes15110327
Du H, Wang X, Na B, Ye Y, Qiao Y, Li L, Tian Y, Ning X, Wang Z, Zhao X, et al. Engineering Thermal Cross-Linking in Nanofiltration Membranes for Efficient Nicotine Extraction from Tobacco Extract. Membranes. 2025; 15(11):327. https://doi.org/10.3390/membranes15110327
Chicago/Turabian StyleDu, He, Xinyuan Wang, Baodan Na, Yajun Ye, Yuemei Qiao, Linda Li, Ye Tian, Xiaoping Ning, Zhigang Wang, Xingquan Zhao, and et al. 2025. "Engineering Thermal Cross-Linking in Nanofiltration Membranes for Efficient Nicotine Extraction from Tobacco Extract" Membranes 15, no. 11: 327. https://doi.org/10.3390/membranes15110327
APA StyleDu, H., Wang, X., Na, B., Ye, Y., Qiao, Y., Li, L., Tian, Y., Ning, X., Wang, Z., Zhao, X., & Chen, C. (2025). Engineering Thermal Cross-Linking in Nanofiltration Membranes for Efficient Nicotine Extraction from Tobacco Extract. Membranes, 15(11), 327. https://doi.org/10.3390/membranes15110327





