Preparation of Polysilsesquioxane-Based RO Membranes with Urea Units for Water Desalination
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
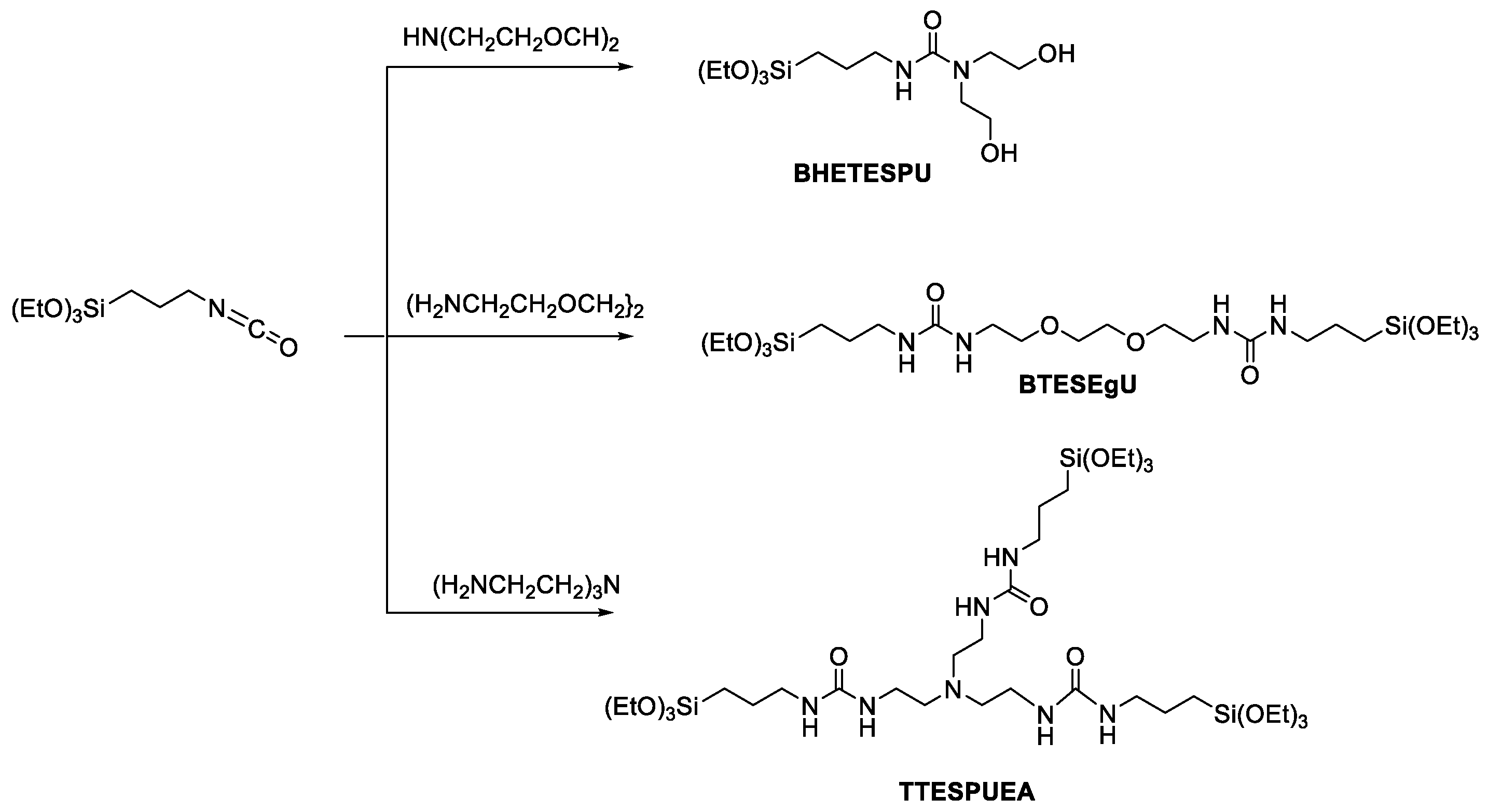
2.2. Preparation of BHETESPU
2.3. Preparation of BTESEgU
2.4. Preparation of TTESPUEA
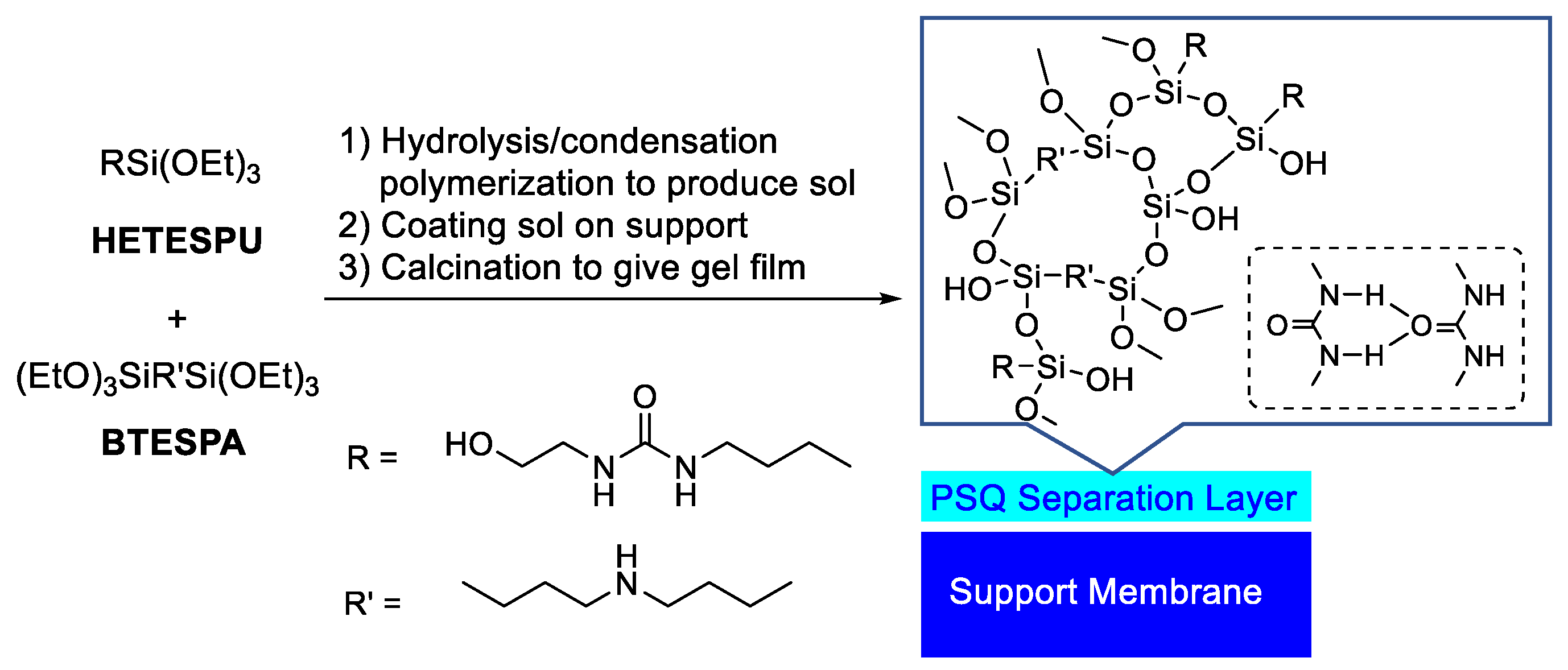
2.5. Sol–Gel Process
2.6. Membrane Preparation
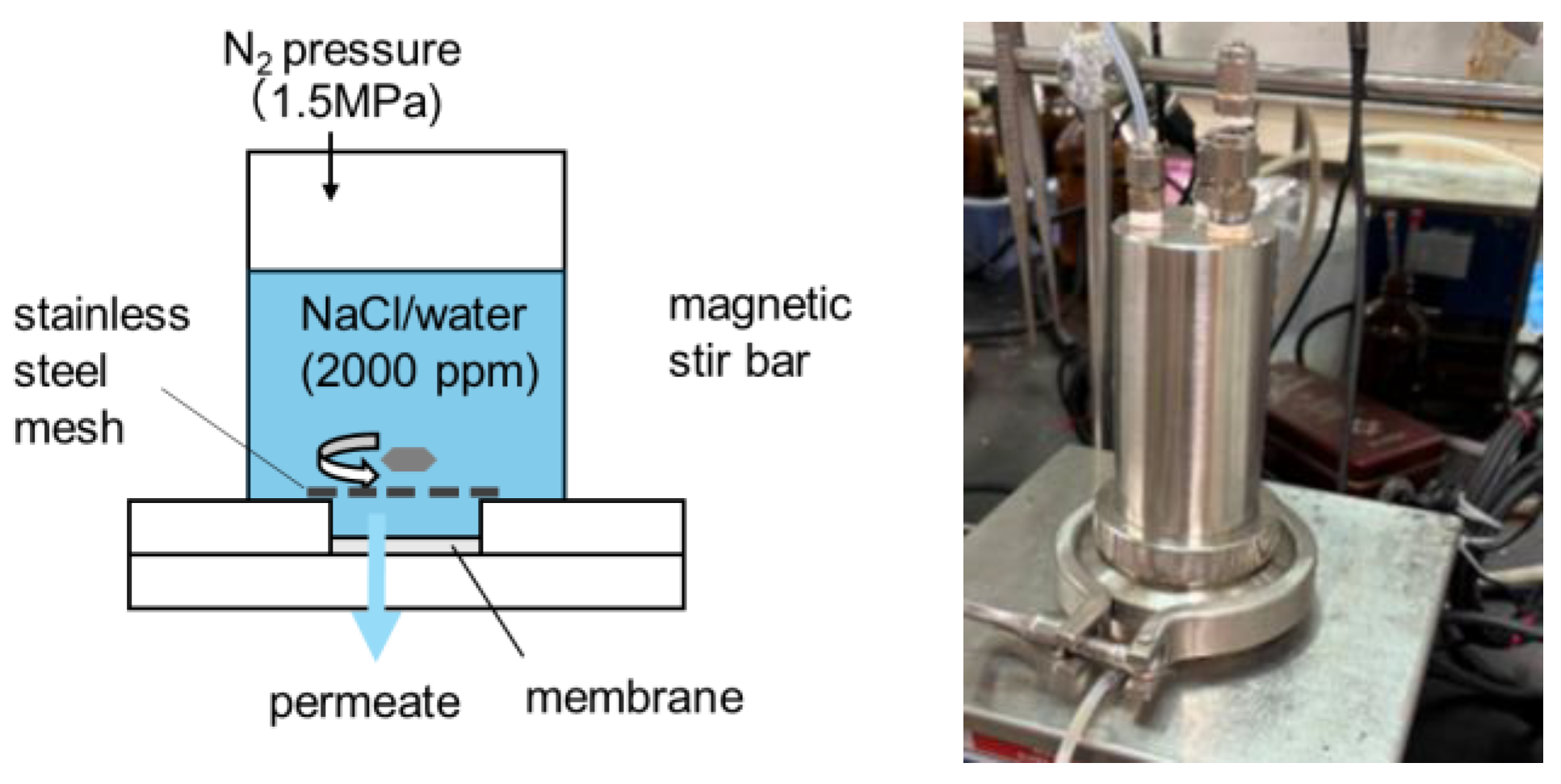
2.7. Evaluation of RO Performance
3. Results and Discussion
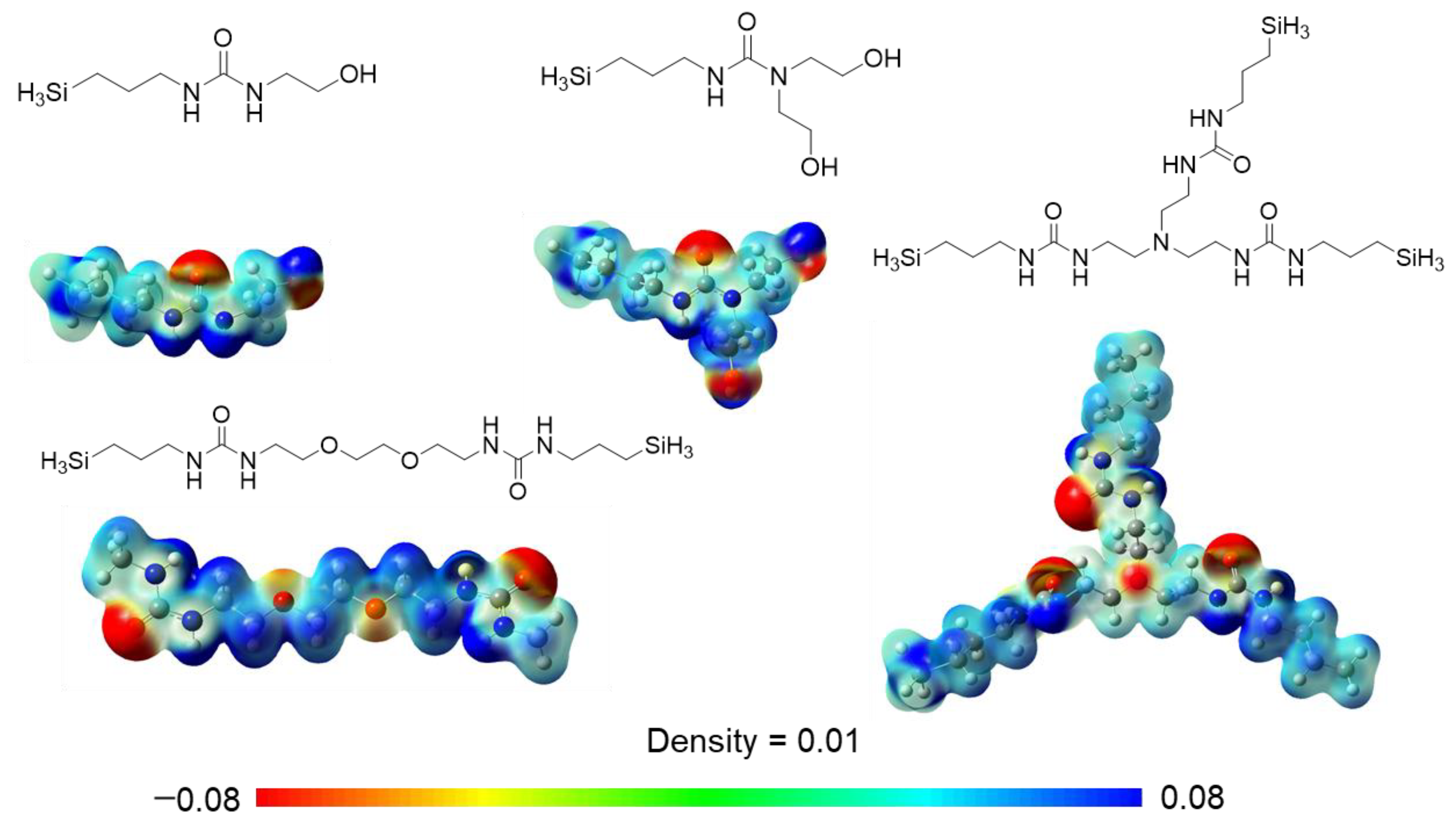
3.1. Membrane Preparation
3.2. RO Performance
3.3. Chlorine and Heat Resistance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, H. Recent developments in reverse osmosis desalination membranes. J. Mater. Chem. 2010, 20, 4551–4566. [Google Scholar] [CrossRef]
- Li, D.; Yan, Y.; Wang, H. Recent advances in polymer and polymer composite membranes for reverse and forward osmosis processes. Prog. Polym. Sci. 2016, 61, 104–155. [Google Scholar] [CrossRef]
- Hailemariam, R.H.; Woo, Y.C.; Damtie, M.M.; Kim, B.C.; Park, K.-D.; Choi, J.-S. Reverse osmosis membrane fabrication and modification technologies and future trends: A review. Adv. Colloid Interface Sci. 2020, 276, 102100. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. Chemically functionalized polyamide thin film composite membranes: The art of chemistry. Desalination 2020, 495, 114655. [Google Scholar] [CrossRef]
- Zuo, H.-R.; Shi, P.; Duan, M. A review on thermally stable membranes for water treatment: Material, fabrication, and application. Serp. Purif. Technol. 2020, 236, 116223. [Google Scholar] [CrossRef]
- Kang, G.-D.; Gao, C.-J.; Chen, W.-D.; Jie, X.-M.; Cao, Y.-M.; Yuan, Q. Study on hypochlorite degradation of aromatic polyamide reverse osmosis membrane. J. Membr. Sci. 2007, 300, 165–171. [Google Scholar] [CrossRef]
- Liu, M.; Wu, D.; Yu, S.; Gao, C. Influence of the polyacyl chloride structure on the reverse osmosis performance, surface properties and chlorine stability of the thin-film composite polyamide membranes. J. Membr. Sci. 2009, 26, 205–214. [Google Scholar] [CrossRef]
- Xu, G.-R.; Wang, J.-N.; Li, C.-J. Strategies for improving the performance of the polyamide thin film composite (PA-TFC) reverse osmosis (RO) membranes: Surface modifications and nanoparticles incorporations. Desalination 2013, 328, 83–100. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, Z.; Wang, M.; Wang, X.; Zhang, H.; Wang, Z. Optimizing separation layer structure of polyamide composite membrane for high permselectivity based on post-treatment: A review. Desalination 2024, 580, 117585. [Google Scholar] [CrossRef]
- Shao, F.; Dong, L.; Dong, H.; Zhang, Q.; Zhao, M.; Yu, L.; Pang, B.; Chen, Y. Graphene oxide modified polyamide reverse osmosis membranes with enhanced chlorine resistance. J. Membr. Sci. 2017, 525, 9–17. [Google Scholar] [CrossRef]
- Gohil, J.M.; Suresh, A.K. Chlorine attack on reverse osmosis membranes: Mechanisms and mitigation strategies. J. Membr. Sci. 2017, 541, 108–126. [Google Scholar] [CrossRef]
- Khorshidi, B.; Biswas, I.; Ghosh, T.; Thundat, T.; Sadrzadeh, M. Robust fabrication of thin film polyamide-TiO2 nanocomposite membranes with enhanced thermal stability and anti-biofouling propensity. Sci. Rep. 2018, 8, 784. [Google Scholar] [CrossRef]
- He, M.; Wang, L.; Zhang, Z.; Zhang, Y.; Zhu, J.; Wang, X.; Lv, Y.; Miao, R. Stable Forward Osmosis Nanocomposite Membrane Doped with Sulfonated Graphene Oxide@Metal–Organic Frameworks for Heavy Metal Removal. ACS Appl. Mater. Interfaces 2020, 12, 57102–57116. [Google Scholar] [CrossRef]
- Matshetshe, K.; Sikhwivhilu, K.; Ndlovu, G.; Tetyana, P.; Moloto, N.; Tetana, Z. Antifouling and antibacterial β-cyclodextrin decorated graphene oxide/polyamide thin-film nanocomposite reverse osmosis membranes for desalination applications. Sep. Purif. Technol. 2022, 278, 119594. [Google Scholar] [CrossRef]
- Chang, C.-M.; Zhao, Q.; Chen, S.B. Solvent-assisted insertion of molecular supports for enhanced separation performance and stability of thin film composite reverse osmosis membranes. J. Membr. Sci. 2025, 725, 124005. [Google Scholar] [CrossRef]
- Abe, Y.; Gunji, T. Oligo- and polysiloxanes. Prog. Polym. Sci. 2004, 29, 149–182. [Google Scholar] [CrossRef]
- Gon, M.; Tanaka, K.; Chujo, Y. Recent progress in the development of advanced element-block materials. Polym. J. 2018, 50, 109–126. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.; Kwon, M.S. Recent progress in ladder-like polysilsesquioxane: Synthesis and applications. Mater. Chem. Front. 2024, 8, 2689–2726. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ohshita, J. Bridged polysilsesquioxane membranes for water desalination. Polym. J. 2019, 51, 1103–1116. [Google Scholar] [CrossRef]
- Zhang, D.; Kanezashi, M.; Tsuru, T.; Yamamoto, K.; Gunji, T.; Adachi, Y.; Ohshita, J. Development of PSQ-RO membranes with high water permeability by copolymerization of bis [3-(triethoxysilyl)propyl]amine and triethoxy(3-glycidyloxypropyl)silane. J. Membr. Sci. 2022, 644, 120162. [Google Scholar] [CrossRef]
- Zhang, D.; Kanezashi, M.; Tsuru, T.; Yamamoto, K.; Gunji, T.; Adachi, Y.; Ohshita, J. Preparation of thermally stable 3-glycidyloxypropyl-POSS-derived polysilsesquioxane RO membranes for water desalination. J. Membr. Sci. 2023, 668, 121213. [Google Scholar] [CrossRef]
- Zhang, D.; Kanezashi, M.; Tsuru, T.; Yamamoto, K.; Gunji, T.; Adachi, Y.; Ohshita, J. Development of Highly Water-Permeable Robust PSQ-Based RO Membranes by Introducing Hydroxyethylurea-Based Hydrophilic Water Channels. ACS Appl. Mater. Interfaces 2022, 14, 21426–21435. [Google Scholar] [CrossRef]
- Yamamoto, K.; Amaike, Y.; Tani, M.; Saito, I.; Kozuma, T.; Kaneko, Y.; Gunji, T. Bridged organosilica membranes incorporating, carboxyl-functionalized cage silsesquioxanes for water desalination. J. Sol-Gel Sci. Technol. 2022, 101, 315–322. [Google Scholar] [CrossRef]
- Yamamoto, K. Development of reverse osmosis membranes by incorporating polyhedral oligomeric silsesquioxanes (POSSs). Polym. J. 2022, 54, 1153–1160. [Google Scholar] [CrossRef]
- Xu, R.; Cheng, S.; Cheng, X.; Qi, L.; Zhong, J.; Liu, G.P.; Huang, M.; Wasnik, P.; Jiang, Q. In situ carboxyl functionalization of hybrid organosilica reverse osmosis membranes for water desalination. Adv. Compos. Hybrid Mater. 2023, 6, 153. [Google Scholar] [CrossRef]
- Kamitani, T.; Ishida, A.; Imoto, H.; Naka, K. Supramolecular organogel of polyureas containing POSS units in the main chain: Dependence on POSS and co-monomer structures. Polym. J. 2022, 54, 161–167. [Google Scholar] [CrossRef]
- Takeuchi, J.; Tokuami, I.; Sakurai, S.; Imoto, H.; Naka, K. Formation of supramolecular gels by self-assembly of dumbbell-shaped polyhedral oligomeric silsesquioxane derivatives linked with bisurea groups. Bull. Soc. Chem. Jpn. 2025, 98, uoaf023. [Google Scholar] [CrossRef]
- Hatakeyama, E.S.; Gabriel, C.J.; Wiesenauer, B.R.; Lohr, J.L.; Zhou, M.; Noble, R.D.; Gin, D.L. Water filtration performance of a lyotropic liquid crystal polymer membrane with uniform, sub-1-nm pores. J. Membr. Sci. 2011, 366, 62–72. [Google Scholar] [CrossRef]
- Niimi, T.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Ito, K.; Tsuru, T. Preparation of BTESE-derived organosilica membranes for catalytic membrane reactors of methylcyclohexane dehydrogenation. J. Membr. Sci. 2014, 455, 375. [Google Scholar] [CrossRef]
- Blatchley, E.R., III; Chen, M. Reaction Mechanism for Chlorination of Urea. Environ. Sci. Technol. 2010, 44, 8529–8534. [Google Scholar] [CrossRef] [PubMed]
- Bergsman, D.S.; Closser, R.G.; Tassone, C.J.; Clemens, B.M.; Nordlund, D.; Bent, S.F. Effect of Backbone Chemistry on the Structure of Polyurea Films Deposited by Molecular Layer Deposition. Chem. Mater. 2017, 29, 1192–1203. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, T.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal Decomposition (Pyrolysis) of Urea in an Open Reaction Vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Horata, K.; Yoshio, T.; Miyazaki, R.; Adachi, Y.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Preparation of Polysilsesquioxane-based CO2 Separation Membranes with Thermally Degradable Succinic Anhydride and Urea Units. Separations 2024, 11, 110. [Google Scholar] [CrossRef]
- Yamamoto, K.; Koge, S.; Sasahara, K.; Mizumo, T.; Kaneko, Y.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Preparation of bridged polysilsesquioxane membranes from bis [3-(triethoxysilyl)propyl]amine for water desalination. Bull. Chem. Soc. Jpn. 2017, 90, 1035–1040. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Nadzri, N.; Wang, R. Thin-film composite (TFC) membranes for sustainable desalination and water reuse: A perspective. Desalination 2025, 599, 118451. [Google Scholar] [CrossRef]
- Zhang, D.; Kanezashi, M.; Tsuru, T.; Yamamoto, K.; Gunji, T.; Adachi, Y.; Ohshita, J. Development of robust and high-performance polysilsesquioxane reverse osmosis membranes modified by SiO2 nanoparticles for water desalination. Sep. Purif. Technol. 2022, 296, 121421. [Google Scholar] [CrossRef]
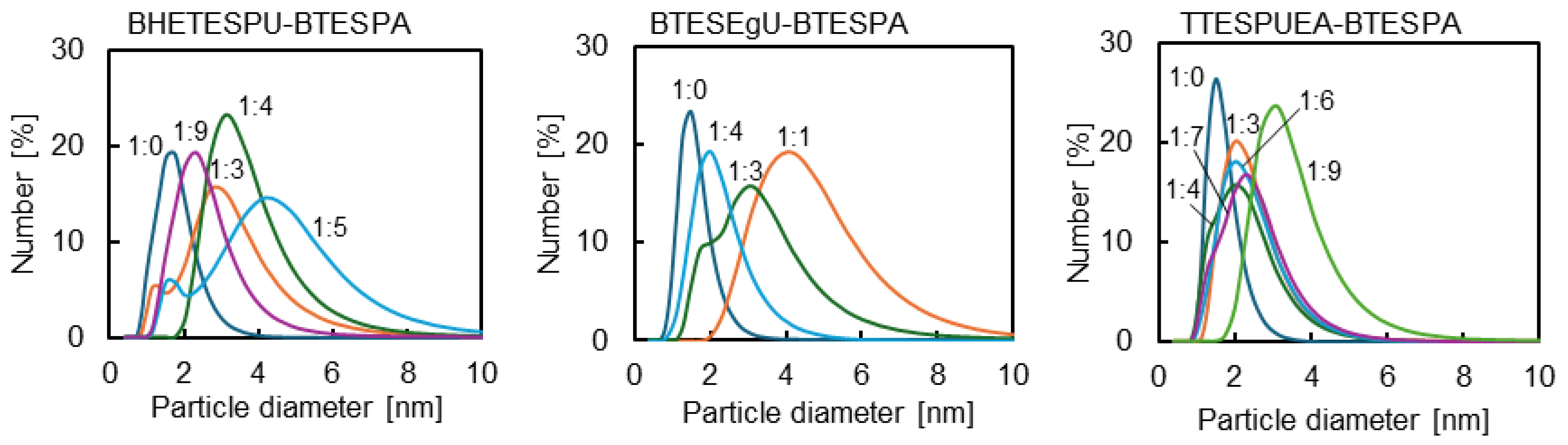

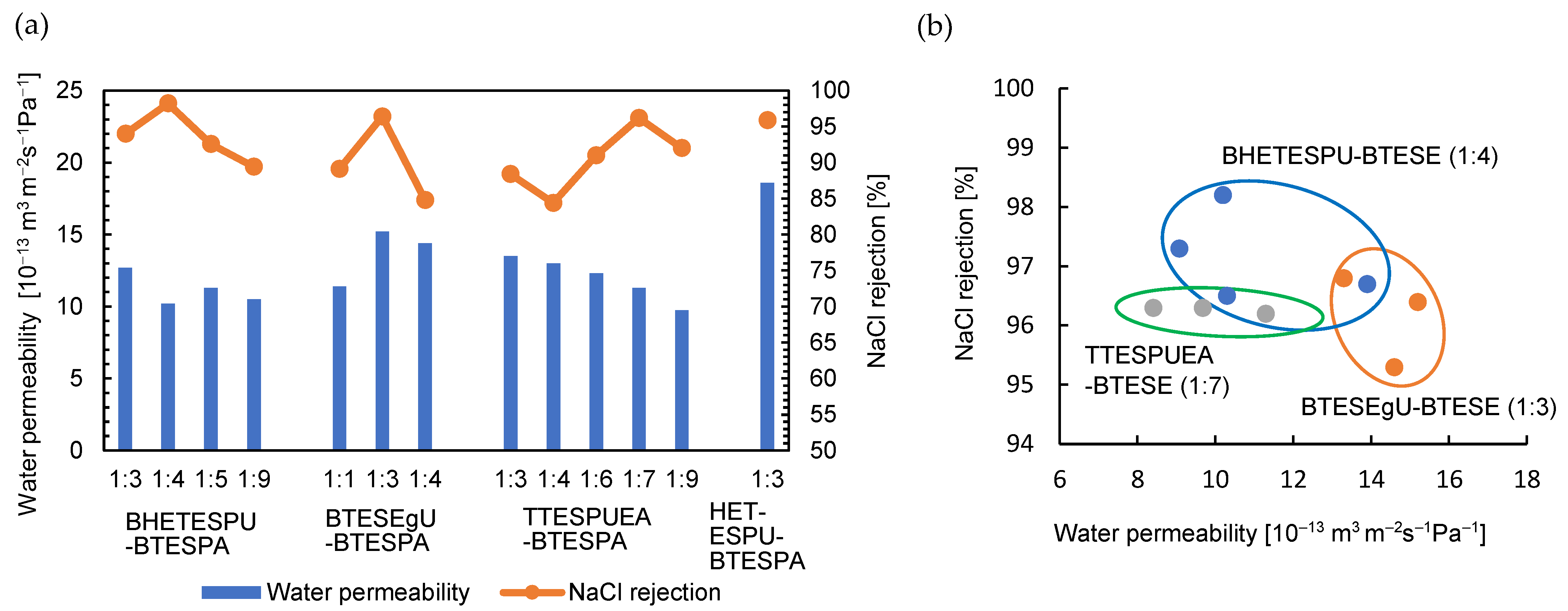

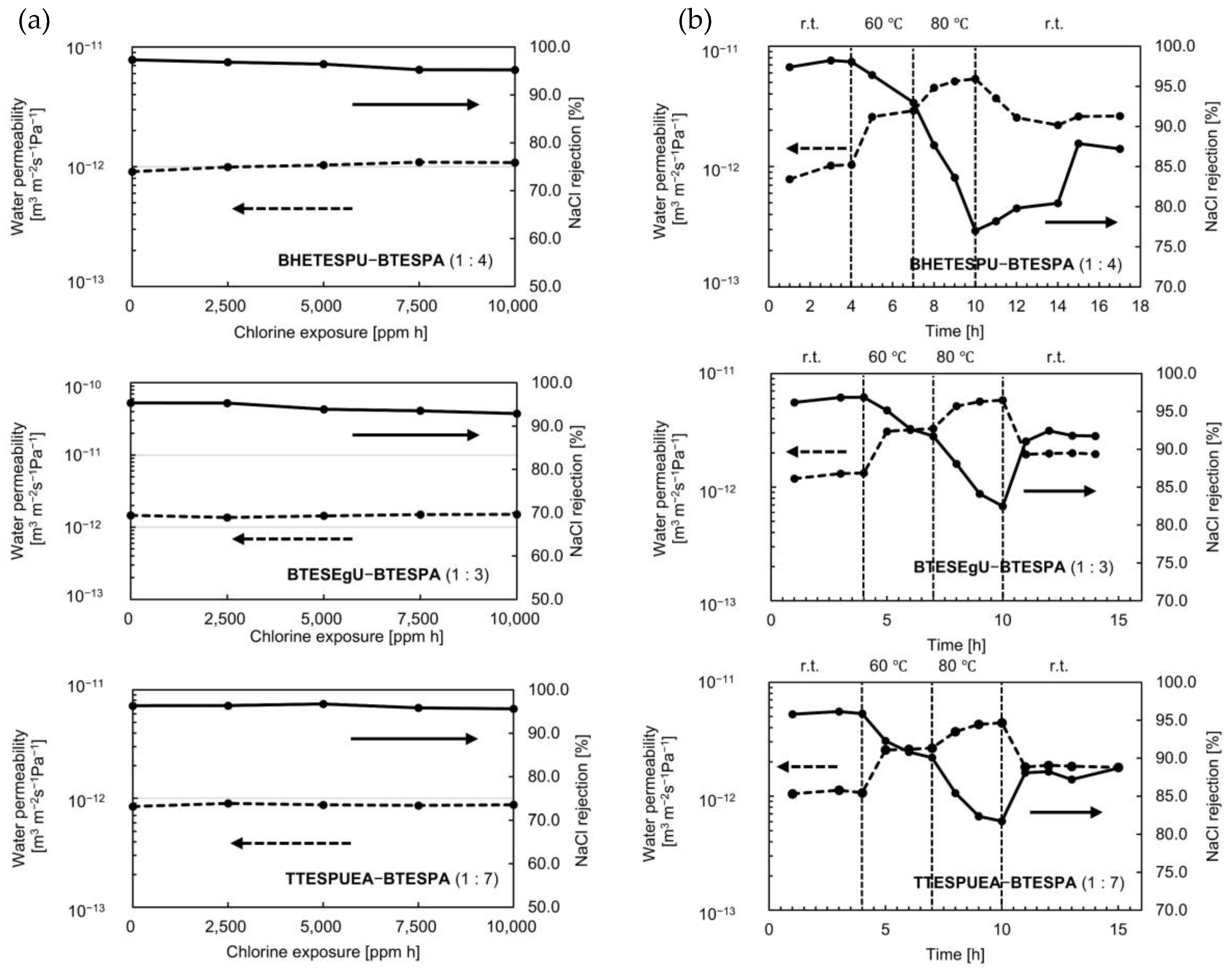
| Monomer /g (/mmol) | BTESPA /g (/mmol) | Ethanol /g | Water /g (/mmol) | Time /h |
|---|---|---|---|---|
| BHETESPU | ||||
| 0.100 (0.287) | 0 | 1.440 | 0.460 (25.52) | 2 |
| 0.075 (0.213) | 0.225 (0.529) | 4.899 | 0.801 (44.45) | 3 |
| 0.050 (0.142) | 0.200 (0.470) | 4.089 | 0.661 (36.68) | 2 |
| 0.025 (0.071) | 0.125 (0.294) | 2.456 | 0.394 (21.86) | 3 |
| 0.025 (0.071) | 0.225 (0.528) | 4.102 | 0.648 (36.00) | 2 |
| BTESEgU | ||||
| 0.150 (0.233) | 0 | 2.598 | 0.252 (14.00) | 8 |
| 0.100 (0.156) | 0.100(0.235) | 3.378 | 0.422 (23.42) | 3 |
| 0.050 (0.078) | 0.150 (0.352) | 3.335 | 0.465 (25.80) | 3 |
| 0.050 (0.078) | 0.200 (0.470) | 4.158 | 0.592 (32.85) | 2 |
| TTESPUEA | ||||
| 0.100 (0.113) | 0 | 1.717 | 0.183 (10.16) | 8 |
| 0.050 (0.056) | 0.150 (0.352) | 3.358 | 0.442 (24.53) | 2 |
| 0.050 (0.056) | 0.200 (0.470) | 4.181 | 0.569 (31.58) | 2 |
| 0.025 (0.028) | 0.150 (0.352) | 2.914 | 0.411 (22.81) | 2 |
| 0.025 (0.028) | 0.175 (0.411) | 3.325 | 0.475 (26.36) | 2 |
| 0.025 (0.028) | 0.225 (0.528) | 4.148 | 0.602 (33.41) | 2 |
| Monomer | BTESPA | sol Conc | Water Permeability | NaCl |
| /Monomer | /wt% | /10−13 m3 m−2s−1Pa−1 | Rejection/% | |
| BHETESPU | 0 | 0.5 | 56.6 | 63.5 |
| 3 | 0.5 | 12.7 | 94.0 | |
| 4 | 0.5 | 10.2 | 98.2 | |
| 5 | 0.5 | 11.3 | 92.6 | |
| 9 | 0.5 | 10.5 | 89.4 | |
| BTESEgU | 0 | 0.5 | 18.8 | 48.9 |
| 0 | 2.5 | 5.69 | 78.5 | |
| 1 | 0.5 | 11.4 | 89.1 | |
| 1 | 1.0 | 3.70 | 90.7 | |
| 1 | 2.5 | 6.68 | 76.5 | |
| 3 | 0.5 | 15.2 | 96.4 | |
| 3 | 1.0 | 2.89 | 92.0 | |
| 4 | 0.5 | 14.4 | 84.8 | |
| TTESPUEA | 0 | 0.5 | 28.5 | 73.6 |
| 3 | 0.5 | 13.5 | 88.4 | |
| 4 | 0.5 | 13.0 | 84.4 | |
| 6 | 0.5 | 12.3 | 91.0 | |
| 7 | 0.5 | 11.3 | 96.2 | |
| 9 | 0.5 | 9.74 | 92.0 | |
| HETESPU | 3 | 0.5 | 18.6 | 95.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohshita, J.; Horata, K.; Kaneko, T.; Adachi, Y.; Kanezashi, M. Preparation of Polysilsesquioxane-Based RO Membranes with Urea Units for Water Desalination. Membranes 2025, 15, 322. https://doi.org/10.3390/membranes15100322
Ohshita J, Horata K, Kaneko T, Adachi Y, Kanezashi M. Preparation of Polysilsesquioxane-Based RO Membranes with Urea Units for Water Desalination. Membranes. 2025; 15(10):322. https://doi.org/10.3390/membranes15100322
Chicago/Turabian StyleOhshita, Joji, Katsuhiro Horata, Toshiki Kaneko, Yohei Adachi, and Masakoto Kanezashi. 2025. "Preparation of Polysilsesquioxane-Based RO Membranes with Urea Units for Water Desalination" Membranes 15, no. 10: 322. https://doi.org/10.3390/membranes15100322
APA StyleOhshita, J., Horata, K., Kaneko, T., Adachi, Y., & Kanezashi, M. (2025). Preparation of Polysilsesquioxane-Based RO Membranes with Urea Units for Water Desalination. Membranes, 15(10), 322. https://doi.org/10.3390/membranes15100322






