Capacitive Coulometric Readout of Polyaniline Membrane-Based pH Sensors in Combination with Cyclic Voltammetry and Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Chemicals
2.2. Electrode Preparation of PANI-Based pH Sensor
2.3. Potentiometric Calibration of PANI-Based pH Sensor
2.4. Electrochemical Impedance Spectroscopy and Morphology Characterization of PANI-Based GC Electrodes
2.5. Chronoamperometric and Coulometric Response of PANI-Based GC Electrode
2.6. Flexible PANI-Based pH Sensor Preparation
2.7. Coulometric Response of Flexible PANI-Based pH Sensors in Series with Capacitor
3. Results
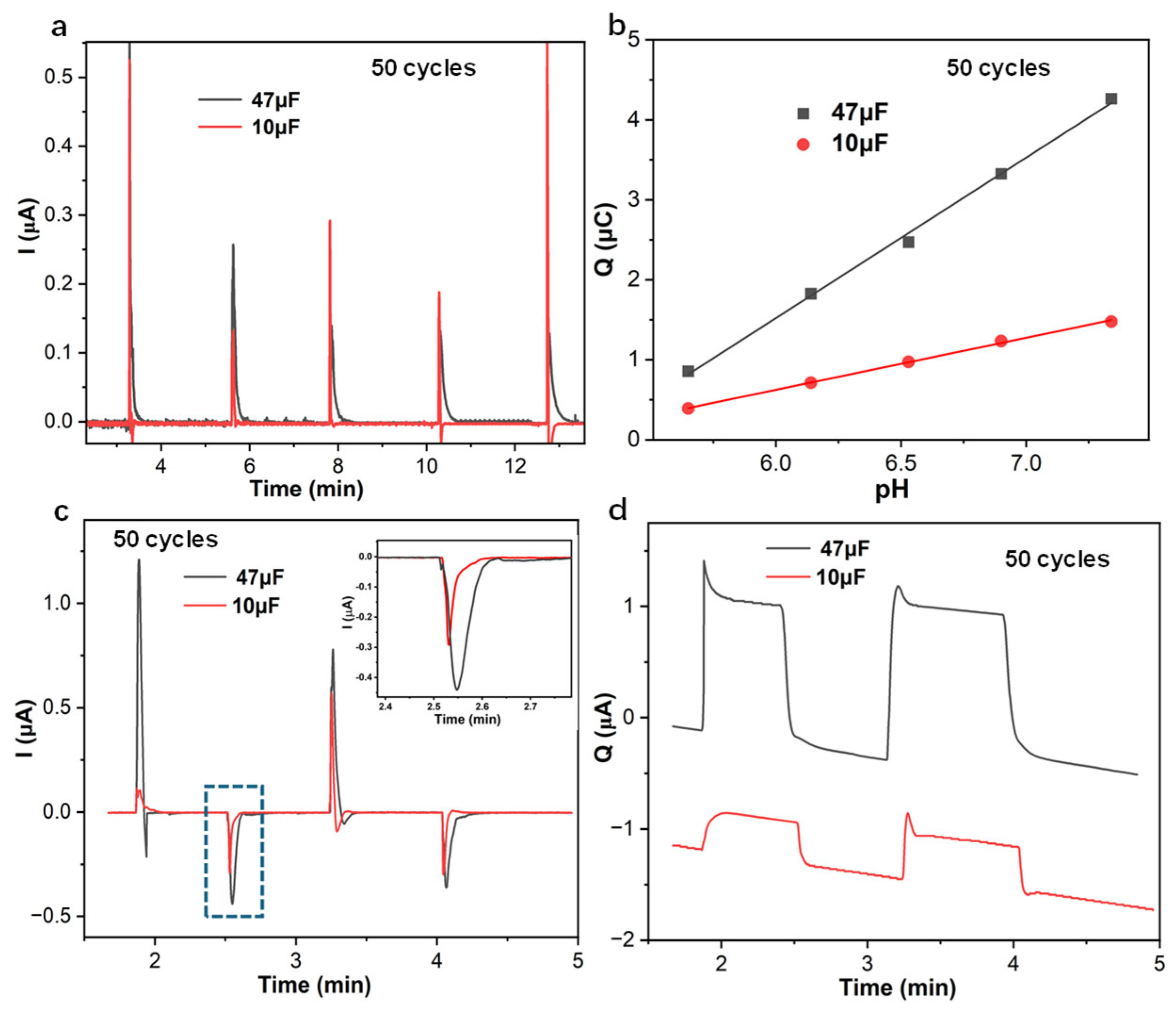
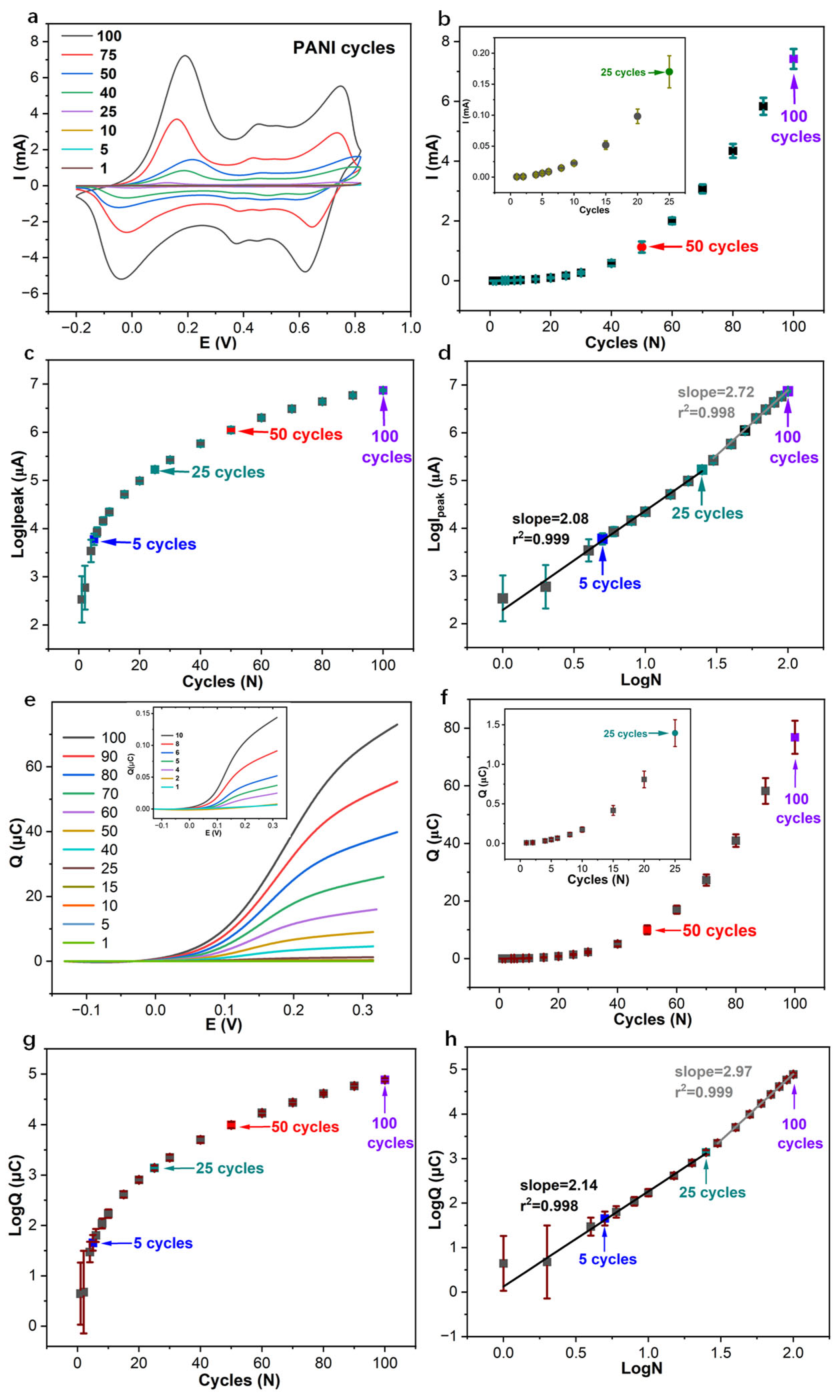
3.1. Amperometric and Coulometric Response of PANI-Based pH Sensor in Series with Capacitor
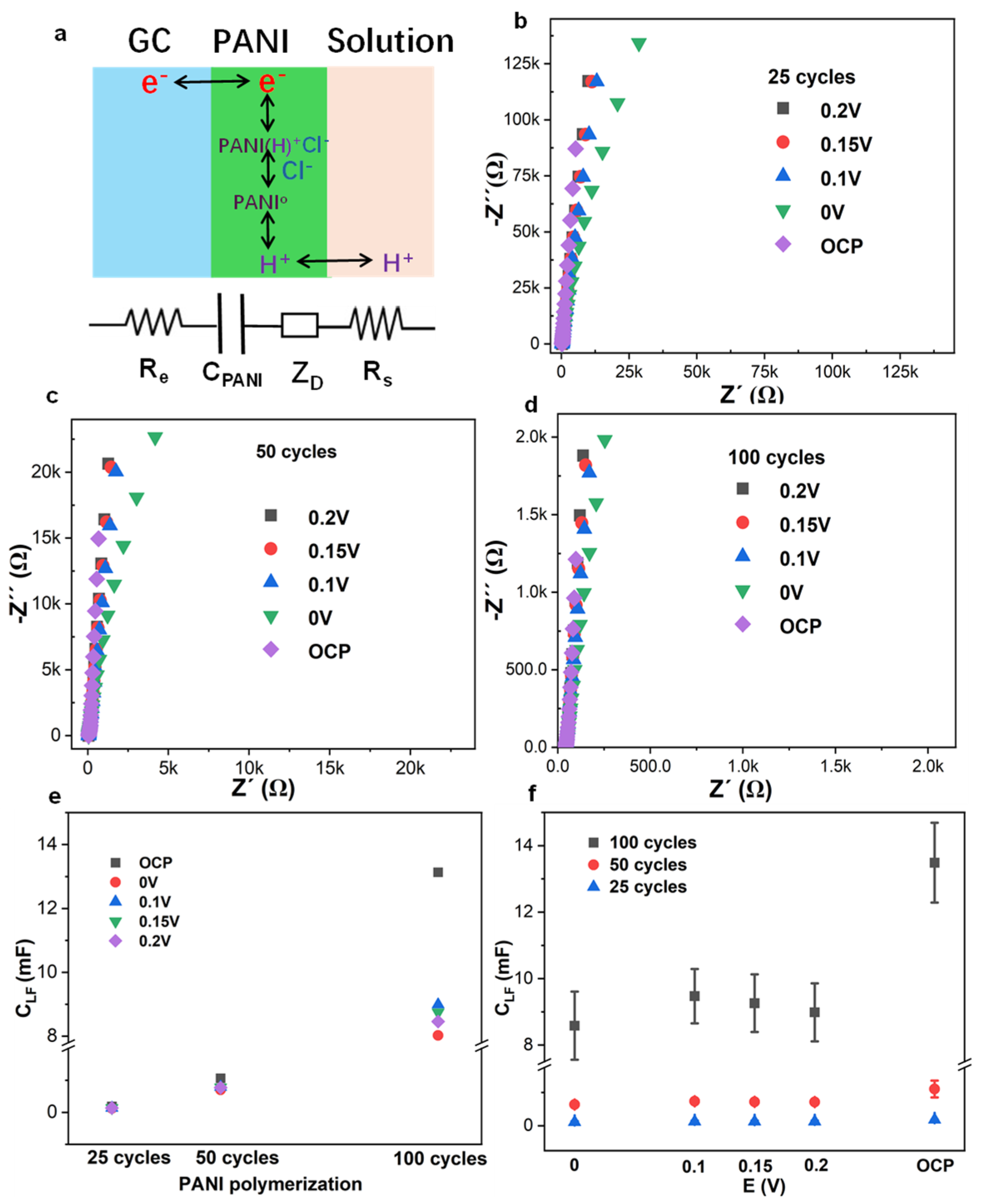
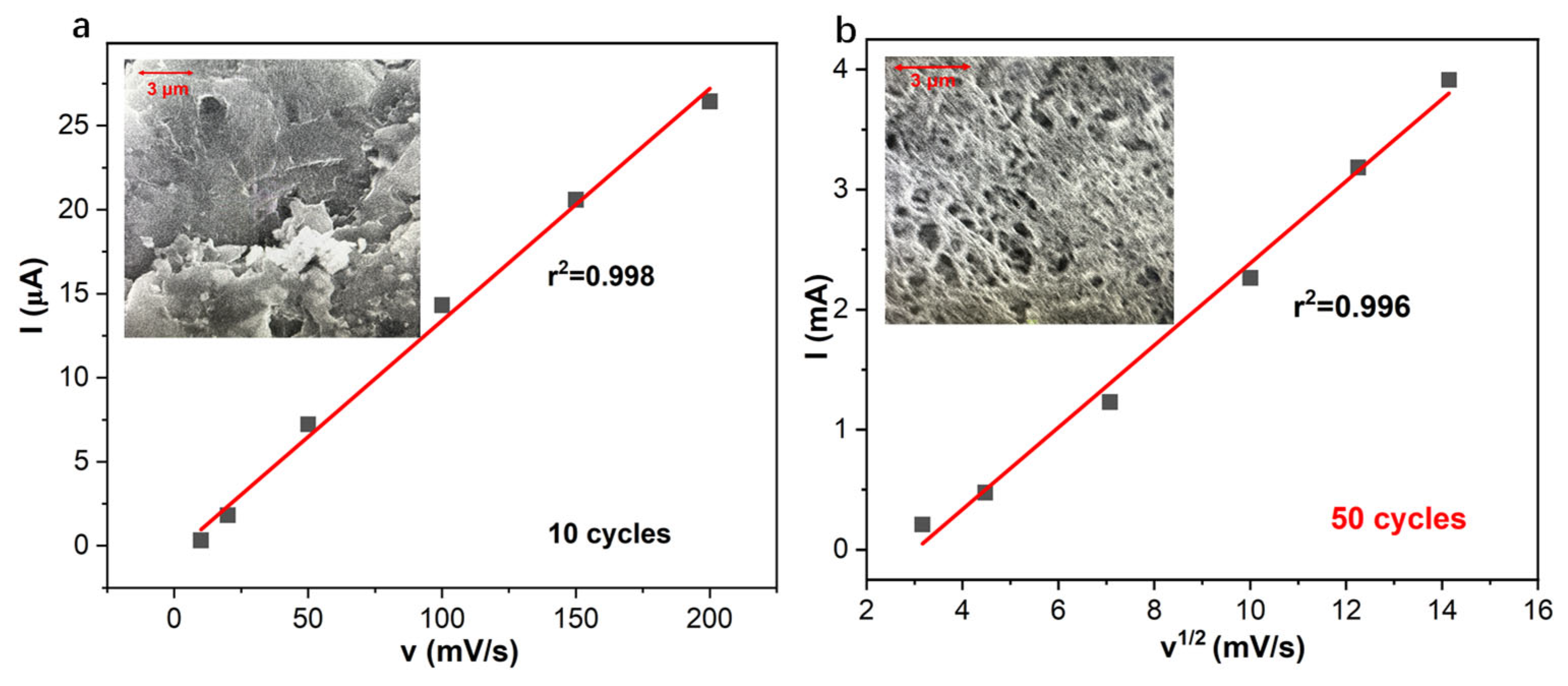
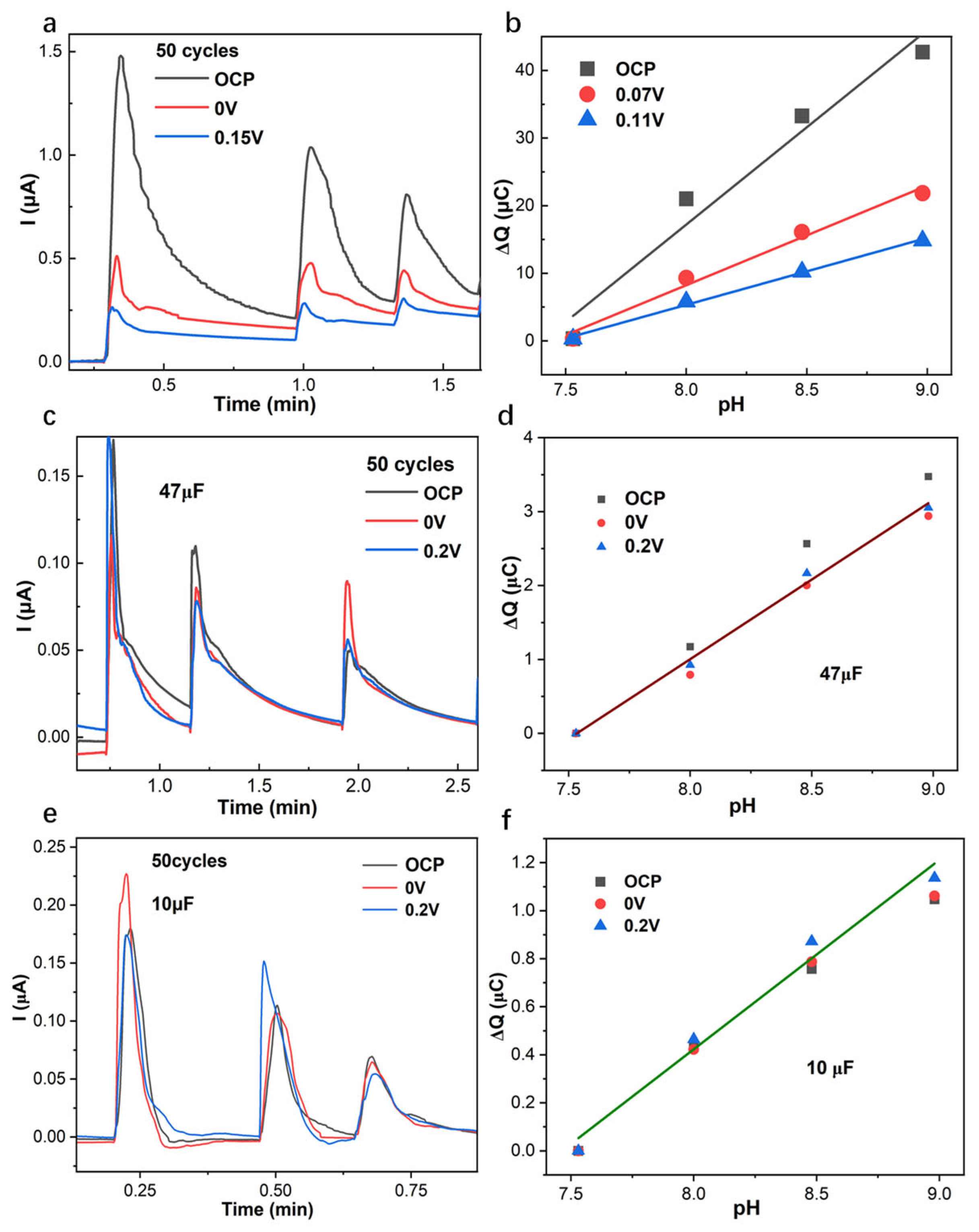
3.2. EIS Low-Frequency Capacitance, Current, and Charge of CV, and Coulometric Response of PANI-Based pH Sensor with Variable Applied Potentials
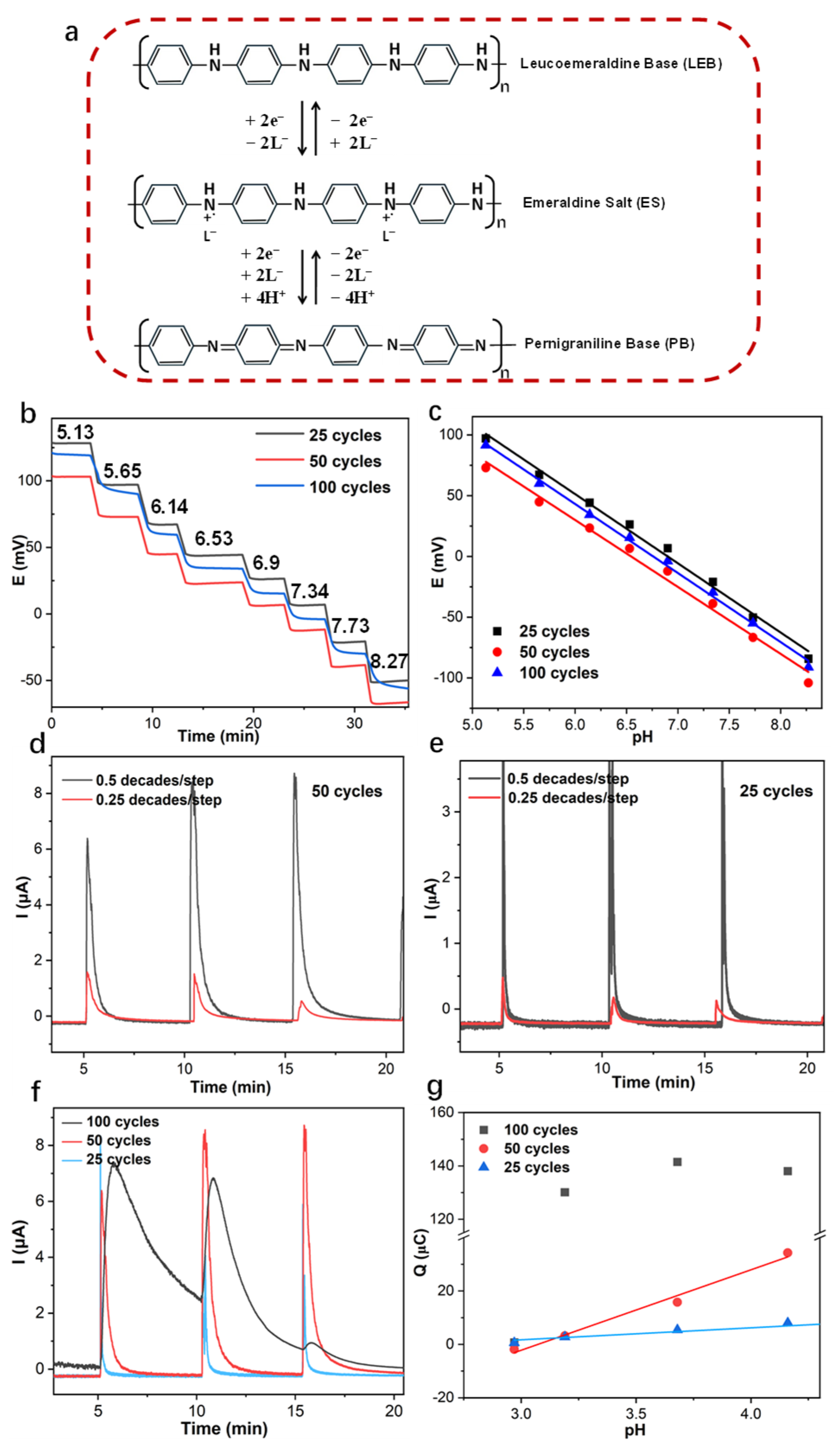
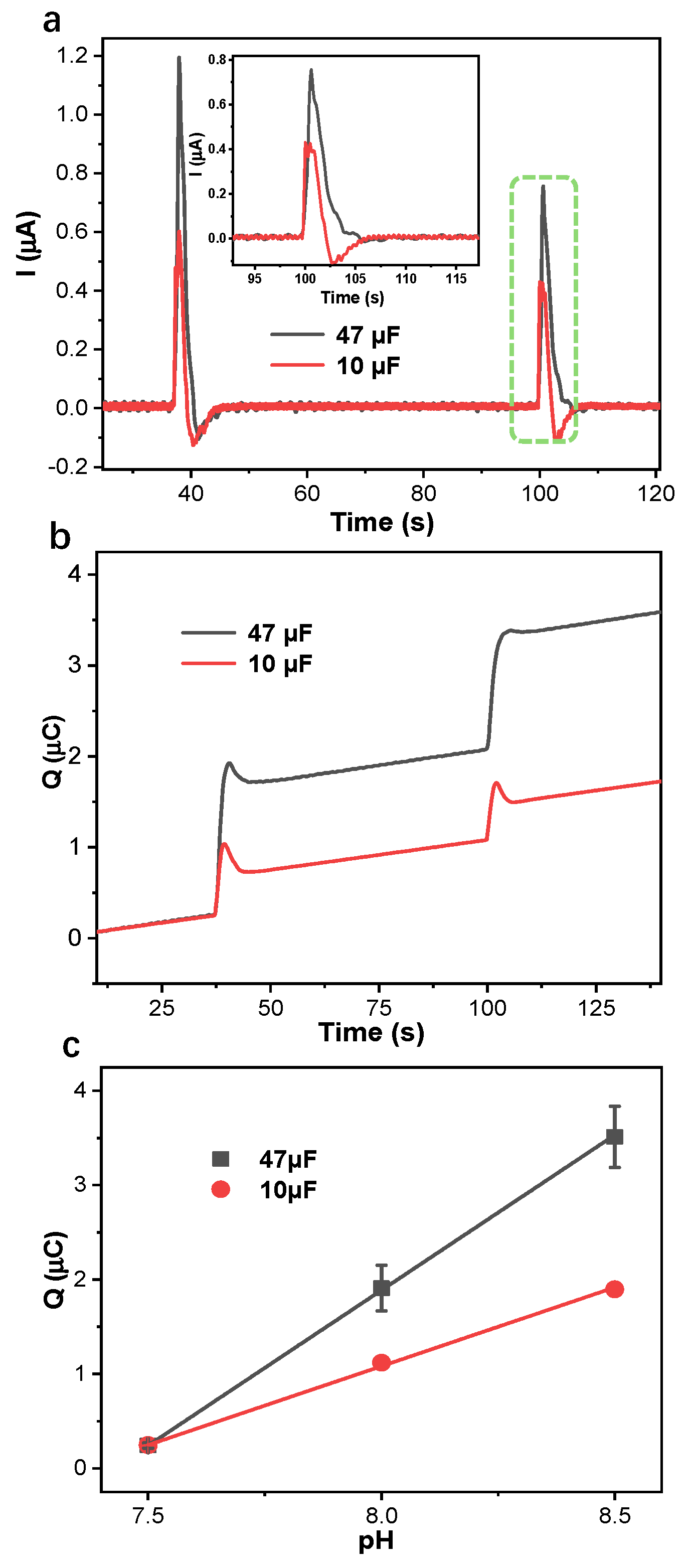
3.3. Application of Capacitive Coulometric Readout on Flexible PANI-Based pH Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewenstam, A. Routines and Challenges in Clinical Application of Electrochemical Ion-Sensors. Electroanal. 2014, 26, 1171–1181. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Bobacka, J. Perspective on the coulometric transduction principle for ion-selective electrodes. Sens. Actuators B Chem. 2024, 410, 135674. [Google Scholar] [CrossRef]
- Bakker, E. Improving robustness, sensitivity and simplicity of potentiometric sensors through symmetry and conceptual design. Chimia 2025, 79, 7–11. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Michalska, A. All-solid-state ion-selective and all-solid-state reference electrodes. Electroanalysis 2012, 24, 1253–1265. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhong, L.; Wang, W.; He, Y.; Han, T.; Xu, L.; Mo, X.; Liu, Z.; Ma, Y.; Bao, Y.; et al. Recent Advances in Wearable Potentiometric pH Sensors. Membranes 2022, 12, 504. [Google Scholar] [CrossRef]
- Kakiuchi, T.; Yoshimatsu, T.; Nishi, N. New class of Ag/AgCl Electrodes Based on Hydrophobic Ionic Liquid Saturated with AgCl. Anal. Chem. 2007, 79, 7187–7191. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Calvo, A.J.; Sierra-Padilla, A.; Bellido-Milla, D.; Cubillana-Aguilera, L.; García-Guzmán, J.J.; Palacios-Santander, J.M. Fast, Economic, and Improved Nanostructured Polymeric pH Sensor for Agrifood Analysis. Chemosensors 2025, 13, 63. [Google Scholar] [CrossRef]
- Kemer, B.; Demir, E. A novel potentiometric pH electrode based on sulfated natural Fe3O4 and analytical application in food samples. J. Food Meas. Charact. 2018, 12, 2256–2262. [Google Scholar] [CrossRef]
- Shibata, M.; Kato, M.; Iwamoto, Y.; Nomura, S.; Kakiuchi, T. Potentiometric determination of pH values of dilute sulfuric acid solutions with glass combination electrode equipped with ionic liquid salt bridge. J. Electroanal. Chem. 2013, 705, 81–85. [Google Scholar] [CrossRef]
- Liang, R.; Zhong, L.; Zhang, Y.; Tang, Y.; Lai, M.; Han, T.; Wang, W.; Bao, Y.; Ma, Y.; Gan, S.; et al. Directly Using Ti3C2Tx MXene for a Solid-Contact Potentiometric pH Sensor toward Wearable Sweat pH Monitoring. Membranes 2023, 13, 376. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Wardak, C.; Paczosa-Bator, B. Application of Metal Oxide Nanoparticles in the Field of Potentiometric Sensors: A Review. Membranes 2023, 13, 876. [Google Scholar] [CrossRef]
- Steininger, F.; Palmfeldt, J.; Koren, K.; Kalinichev, A.V. Exploting the pH-Cross Sensitivity of Ion-Selective Optodes to Broaden Their Response Range. ACS Sensors 2024, 9, 4555–4559. [Google Scholar] [CrossRef]
- Kalinichev, A.; Peshkova, M.; Pokhvishcheva, N.V.; Mikhelson, K.N. Ion-Selective Optical Sensors: A New Look at Well-Established Techniques of Signal Acquisition. Proceedings 2018, 2, 825. [Google Scholar]
- Kalisz, J.; Wegrzyn, K.; Michalska, A.; Maksymiuk, K. Resolution increase of ion selective electrodes response by using a reversed amperometric setup. Electrochim. Acta 2022, 427, 140886. [Google Scholar] [CrossRef]
- Michalak, M.; Kalisz, J.; Michalska, A.; Maksymiuk, K. High resolution electrochemical monitoring of small pH changes. Electrochim. Acta 2023, 468, 143147. [Google Scholar]
- Chen, X.; Ji, J.; Peng, Y.; Gao, Z.; Zhao, M.; Tang, B.; Liu, Y. Flexible pH sensors based on OECTs with a BTB dye-embedded ion-gel gate dielectric. J. Mater. Chem. C 2023, 11, 7722–7731. [Google Scholar] [CrossRef]
- Hupa, E.; Vanamo, U.; Bobacka, J. Novel ion-to-electron transduction principle for solid-contact ISEs. Electroanalysis 2015, 27, 591–594. [Google Scholar] [CrossRef]
- Vanamo, U.; Hupa, E.; Yrjana, V.; Bobacka, J. New signal readout principle for solid-contact ion-selective electrodes. Anal. Chem. 2016, 88, 4369–4374. [Google Scholar] [CrossRef] [PubMed]
- Jarolímova, Z.; Han, T.; Mattinen, U.; Bobacka, J.; Bakker, E. Capacitive model for coulometric readout of ion-selective electrodes. Anal. Chem. 2018, 90, 8700–8707. [Google Scholar] [CrossRef]
- Han, T.; Mattinen, U.; Bobacka, J. Improving the sensitivity of solid-contact ion-selective electrodes by using coulometric signal transduction. ACS Sens. 2019, 4, 900–906. [Google Scholar] [CrossRef]
- Bondar, A.; Mikhelson, K. Constant potential coulometric measurements with Ca2+-selective electrode: Analysis using calibration plot vs. analysis using the charge curve fitting. Sensors 2022, 22, 1145. [Google Scholar] [CrossRef] [PubMed]
- Delmo, N.; Mousavi, Z.; Sokalski, T.; Bobacka, J. Novel experimental setup for coulometric signal transduction of ion-selective electrodes. Membranes 2022, 12, 1221. [Google Scholar] [CrossRef]
- Han, T.; Song, T.; Bao, Y.; Wang, W.; He, Y.; Liu, Z.; Gan, S.; Han, D.; Bobacka, J.; Niu, L. Fast and sensitive coulometric signal transduction for ion-selective electrodes by utilizing a two-compartment cell. Talanta 2023, 262, 124623. [Google Scholar] [CrossRef]
- Kraikaew, P.; Jeanneret, S.; Soda, Y.; Cherubini, T.; Bakker, E. Ultrasensitive seawater pH measurements by capacitive readout of potentiometric sensors. ACS Sens. 2020, 5, 650–654. [Google Scholar] [CrossRef]
- Nussbaum, R.; Jeanneret, S.; Bakker, E. Increasing the sensitivity of pH electrodes with constant potential coulometry at zero current. Anal. Chem. 2024, 96, 6436–6443. [Google Scholar] [CrossRef]
- Han, T.; Song, T.; Gan, S.; Han, D.; Bobacka, J.; Niu, L.; Ivaska, A. Coulometric response of H+-selective solid-contact ion-selective electrodes and its application in flexible sensors. Chin. J. Chem. 2023, 41, 207–213. [Google Scholar] [CrossRef]
- Lindfors, T.; Ivaska, A. Stability of the inner polyaniline solid contact layer in all-solid-state K+-selective electrodes based on plasticized poly(vinyl chloride). Anal. Chem. 2004, 76, 4387–4394. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J.; Lewenstam, A.; Ivaska, A. Electrochemical impedance spectroscopy of oxidized poly(3,4-ethylenedioxythiophene) film electrodes in aqueous solutions. J. Electroanal. Chem. 2000, 489, 17–27. [Google Scholar] [CrossRef]
- Bobacka, J.; Lewenstam, A.; Ivaska, A. Equilibrium potential of potentiometric ion sensors under steady-state current by using current-reversal chronopotentiometry. J. Electroanal. Chem. 2001, 509, 27–30. [Google Scholar] [CrossRef]
- Han, T.; Chen, S.; Song, T.; Han, D.; Niu, L. Single frequency effective capacitance Cec and membrane resistance Z readout for solid-contact ion-selective electrodes. ACS Meas. Sci. Au 2025, 5, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Toth, K.; Graf, E.; Horvai, G.; Pungor, E.; Buck, R.P. Plasticized Poly(vinyl chloride) Properties and Characteristics of Valinomycin Electrodes. 2. Low-Frequency, Surface-Rate, and Warburg Impedance Characteristics. Anal. Chem. 1986, 58, 2741–2744. [Google Scholar] [CrossRef]
- Aoki, K.J.; Chen, J. Charge Transfer Rates Controlled by Frequency Dispersion of Double-Layer Capacitance. Electrochem 2025, 6, 32. [Google Scholar] [CrossRef]
- Keresten, V.; Lazarev, F.; Mikhelson, K. Transfer of Sodium Ion across Interface between Na+-Selective Electrode Membrane and Aqueous Electrolyte Solution: Can We Use Nernst Equation If Current Flows through Electrode? Membranes 2024, 14, 74. [Google Scholar] [CrossRef]
- Khripoun, G.A.; Korchak, P.A.; Mikhelson, K.N. Charge transfer resistance at the interface between valinomycin-based potassium-selective membranes and KCl aqueous solutions. Sens. Actuators B Chem. 2025, 424, 136934. [Google Scholar] [CrossRef]
- Hillman, A.R.; Mallen, E.F. A spectroelectrochemical study of the electrodeposition of polythiophene films. J. Electroanal. Chem. Interfacial Electrochem. 1988, 243, 403–417. [Google Scholar] [CrossRef]
- Hwang, B.J.; Santhanam, R.; Wu, C.R.; Tsai, Y.W. Nucleation and growth mechanism for the electropolymerization of aniline on highly oriented pyrolytic graphite at higher potentials. J. Solid State Electrochem. 2001, 5, 280–286. [Google Scholar] [CrossRef]
- Mandic, Z.; Duić, L.; Kovačiček, F. The influence of counter-ions on nucleation and growth of electrochemically synthesized polyaniline film. Electrochim. Acta 1997, 42, 1389–1402. [Google Scholar] [CrossRef]
- Glarum, S.H.; Marshall, J.H. The impedance of Poly(aniline) Electrode Films. J. Electrochem. Soc. 1987, 134, 142. [Google Scholar] [CrossRef]
- Murugappan, K.; Castell, M.R. Bridging electrode gaps with conducting polymers around the electrical percolation threshold. Electrochem. Commun. 2018, 87, 40–43. [Google Scholar] [CrossRef]
- Svoboda, V.; Liaw, B. In situ transient study of polymer film growth via simultaneous correlation of charge, mass, and ellipsometric measurements. Pure Appl. Chem. 2008, 80, 2439–2449. [Google Scholar] [CrossRef]
- Scotto, J.; Marmisolle, W.A.; Posadas, D. About the capacitive currents in conducting polymers: The case of polyaniline. J. Solid. State Electrochem. 2019, 23, 1947–1965. [Google Scholar] [CrossRef]
- Prakash, S.; Sivakumar, C.; Rajendran, V.; Vasudevan, T.; Gopalan, A.; Wen, T. Growth behavior of poly(o-toluidine-co-p-fluoroaniline) deposition by cyclic voltammetry. Mater. Chemi. Phys. 2002, 74, 74–82. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Grzeszczuk, M. Electrochemcial study of poly(3-octylthiophene) film electrodes I. Electrolyte effects on the voltammetric characteristics of the polymer. Three states of the polymer film. Synthetic met. 1991, 44, 9–19. [Google Scholar] [CrossRef]
- Bobacka, J.; Grzeszczuk, M.; Ivaska, A. Electrochemical study of poly(3-octylthiophene) film electrodes. Impedance of the polymer film semiconductor-electrolyte interface. Electrochim. Acta. 1992, 37, 1759–1765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, T.; Song, T.; Han, D.; Niu, L. Capacitive Coulometric Readout of Polyaniline Membrane-Based pH Sensors in Combination with Cyclic Voltammetry and Electrochemical Impedance Spectroscopy. Membranes 2025, 15, 320. https://doi.org/10.3390/membranes15100320
Han T, Song T, Han D, Niu L. Capacitive Coulometric Readout of Polyaniline Membrane-Based pH Sensors in Combination with Cyclic Voltammetry and Electrochemical Impedance Spectroscopy. Membranes. 2025; 15(10):320. https://doi.org/10.3390/membranes15100320
Chicago/Turabian StyleHan, Tingting, Tao Song, Dongxue Han, and Li Niu. 2025. "Capacitive Coulometric Readout of Polyaniline Membrane-Based pH Sensors in Combination with Cyclic Voltammetry and Electrochemical Impedance Spectroscopy" Membranes 15, no. 10: 320. https://doi.org/10.3390/membranes15100320
APA StyleHan, T., Song, T., Han, D., & Niu, L. (2025). Capacitive Coulometric Readout of Polyaniline Membrane-Based pH Sensors in Combination with Cyclic Voltammetry and Electrochemical Impedance Spectroscopy. Membranes, 15(10), 320. https://doi.org/10.3390/membranes15100320






