Application of Two-Compartment Bipolar Membrane Electrodialysis for Treatment of Waste Na2SO4 Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Experimental Methods
3. Results
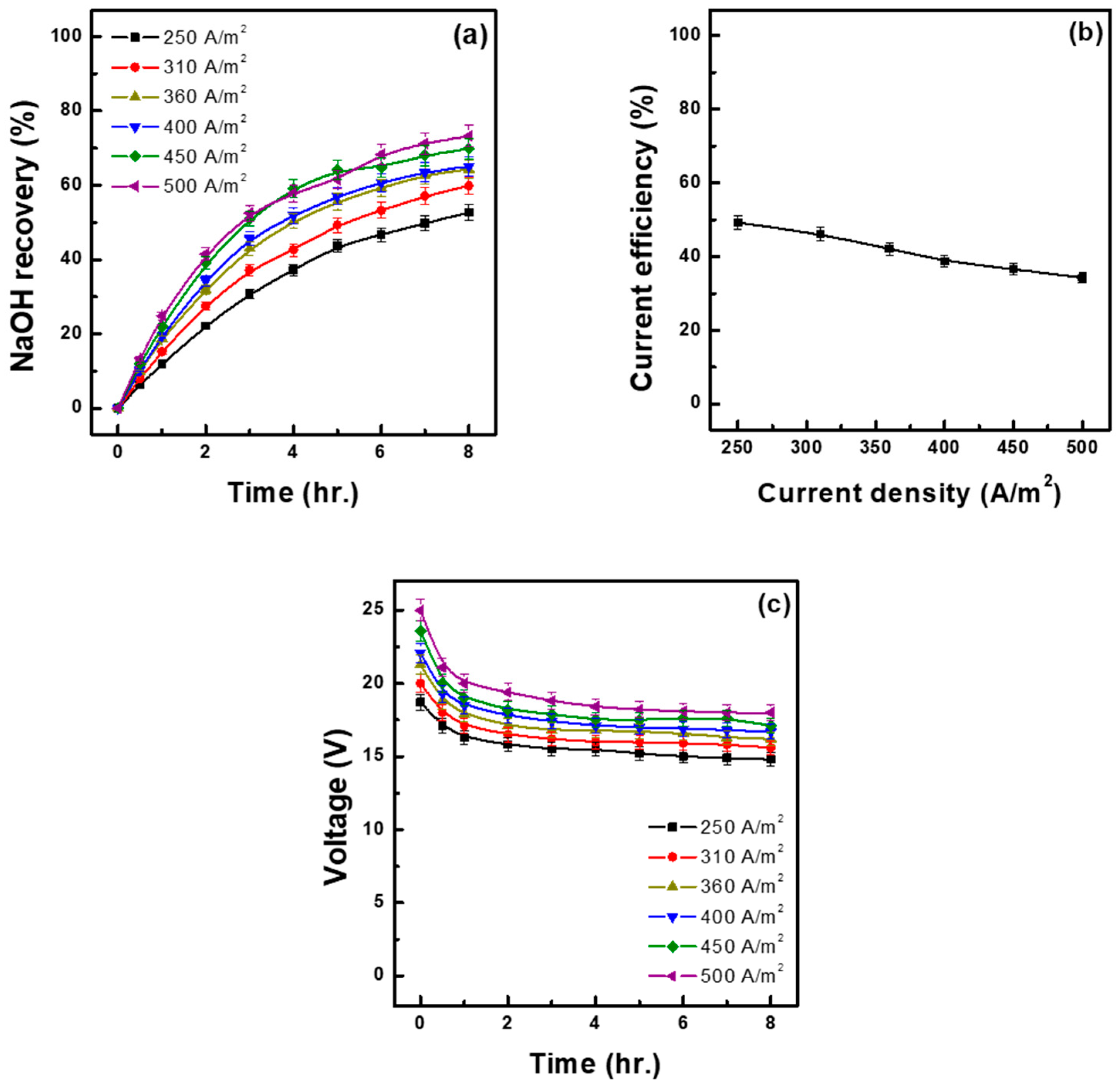
3.1. Effect of Current Density
3.2. Effect of the Initial Feed Solution Concentration
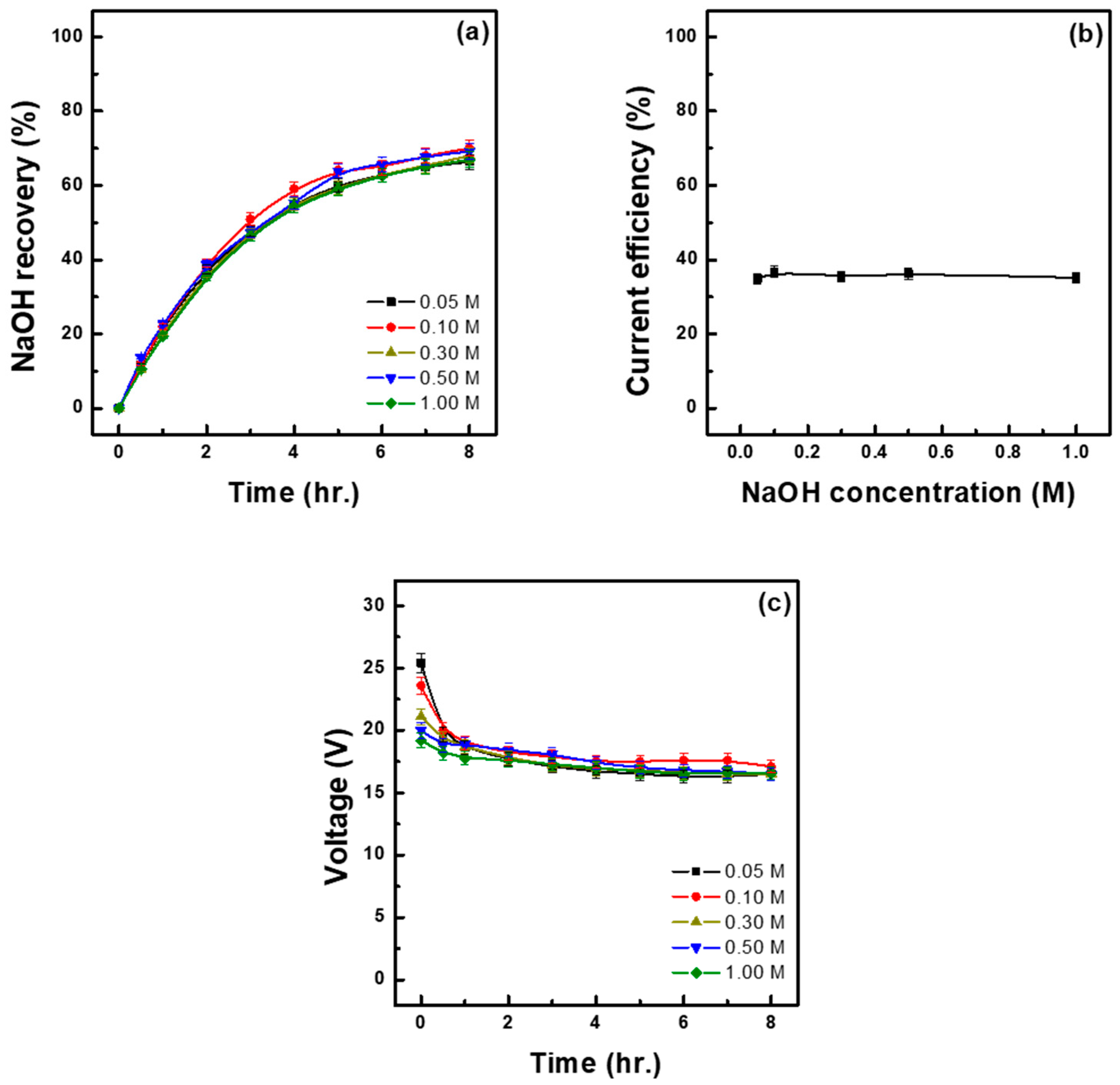
3.3. Effect of Initial Base Concentration
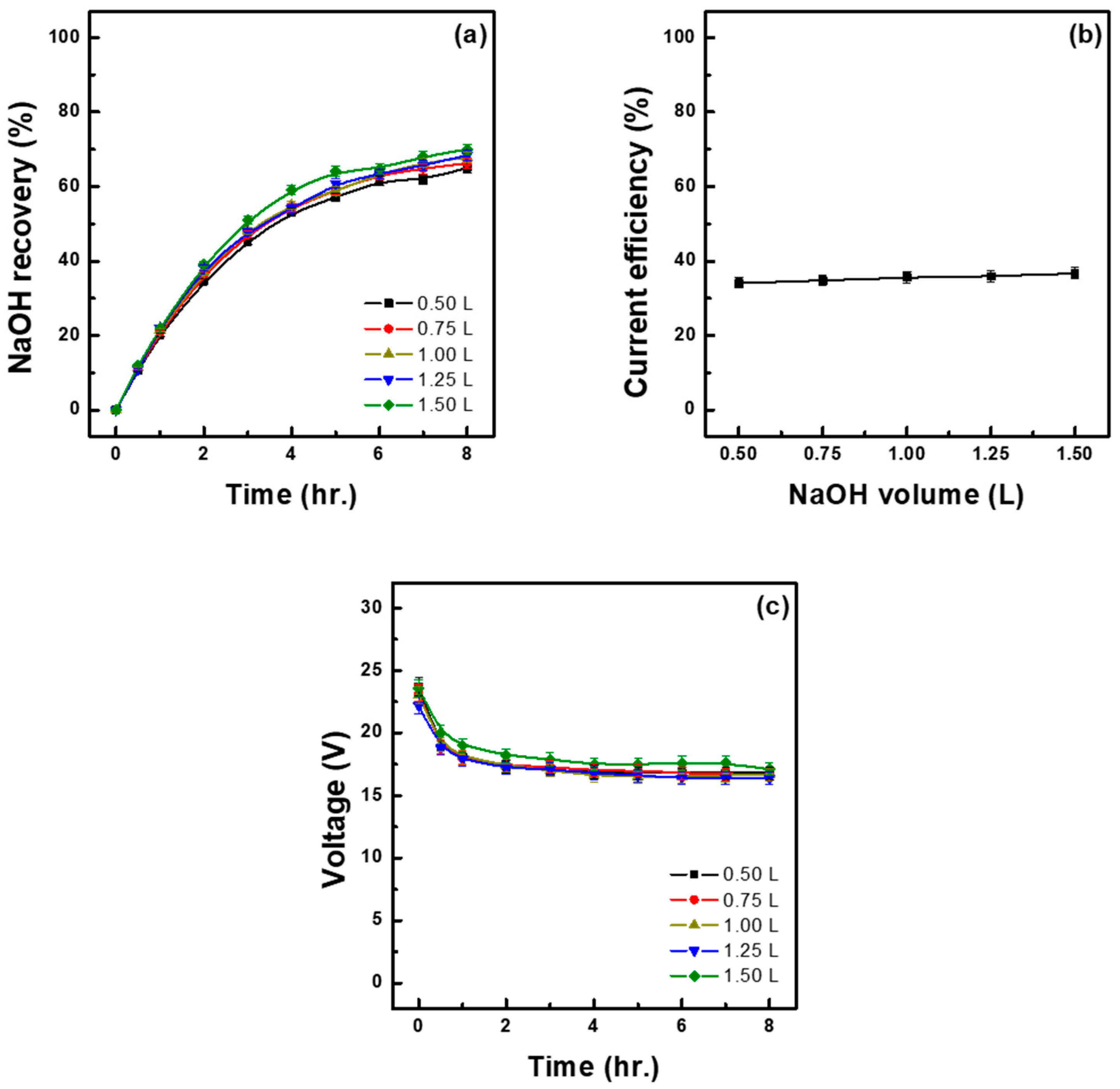
3.4. Effect of Initial Base Volume
3.5. Optimal Process Design
3.6. Treatment of Complex Salts
4. Conclusions
- As current density increased from 250 A/m2 to 500 A/m2, the base recovery rate showed an overall increasing trend, improving significantly by approximately 21%. However, excessive current density was shown to potentially lead to adverse effects such as reduced current efficiency, increased energy consumption, and increased initial applied voltage. Conversely, when current density was too low, limitations such as a reduced base recovery rate and recovery concentration were observed.
- As the concentration of the raw material solution increased, the base recovery rate decreased, whereas the recovery concentration actually increased. More specifically, 1.30 M Na2SO4 resulted in the lowest recovery rate of 70.00% but in the highest recovery concentration of 1.67 M. On the other hand, as the concentration of the raw material solution decreased, energy consumption increased, and current efficiency decreased. Therefore, from an economic perspective, it is more advantageous to use raw material solutions with higher concentrations.
- The initial concentration of the base does not directly affect the base recovery rate. However, if it is excessively low, it may lead to increased electrochemical resistance and reduced energy efficiency. Conversely, if it is excessively high, it may limit the increase in the concentration of the recovered base. Therefore, considering both energy efficiency and process stability, the optimal initial base concentration is within the range of 0.1–0.5 M.
- To increase the concentration of the recovered base, it is advisable to adjust the initial volume of the added base solution. The highest recovery concentration (2.85 M) was achieved when the initial volume was adjusted to 0.50 L, which is thus considered as the most suitable when the objective is to recover high-concentration bases. On the other hand, when the initial volume of the base solution was 1.00 L, the recovery concentration was 2.13 M (slightly lower than that achieved at 0.50 L) but resulted in the lowest energy consumption and a higher current efficiency, making this volume suitable for ensuring stability and efficiency in long-term operations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency (IEA). Global EV Outlook 2023: Catching up with Climate Ambitions; IEA: Paris, France, 2023; Available online: https://www.iea.org/reports/global-ev-outlook-2023 (accessed on 29 September 2025).
- Sai, V.; Prasanth, C. Powering the Future: Innovations in Electric Vehicle Battery Recycling. Nanotechnol. Percept. 2024, 20, 2288–2301. Available online: https://nano-ntp.com/index.php/nano/article/view/3915 (accessed on 29 September 2025).
- Zhang, J.; Azimi, G. Recycling of lithium, cobalt, nickel, and manganese from end-of-life lithium-ion battery of an electric vehicle using supercritical carbon dioxide. Resour. Conserv. Recycl. 2022, 187, 106628. [Google Scholar] [CrossRef]
- Ahn, J.-W.; Cho, Y.-C. Current Status and Prospect of Waste Lithium Ion Battery (LIB) Recycling Technology by Hydrometallurgical Process. J. Korean Inst. Resour. Recycl. 2023, 32, 3–17. [Google Scholar] [CrossRef]
- Rinne, M.; Aromaa-Stubb, R.; Elomaa, H.; Porvali, A.; Lundström, M. Evaluation of hydrometallurgical black mass recycling with simulation-based life cycle assessment. Int. J. Life Cycle Assess. 2024, 29, 1582–1597. [Google Scholar] [CrossRef]
- Verbaan, N.; Naidoo, R. A Review of Hydrometallurgical Flowsheets Considered for the Treatment of Black Mass. In Proceedings of the ALTA 2023 Nickel-Cobalt-Copper, Uranium-REE & Gold Conference, Perth, Australia, 1–5 May 2023. [Google Scholar]
- Zhou, Y.; Shi, C.; Dong, G. Analysis of a mechanical vapor recompression wastewater distillation system. Desalination 2014, 353, 91–97. [Google Scholar] [CrossRef]
- Melo, P.G.F.; Figueiredo, K.C.d.S. Review of Sulfate Removal in Low Concentration Brine Solutions. Sustain. Chem. Eng. 2024, 5, 399–410. [Google Scholar] [CrossRef]
- Melnikov, S.; Mugtamov, O.; Zabolotsky, V. Study of electrodialysis concentration process of inorganic acids and salts for the two-stage conversion of salts into acids utilizing bipolar electrodialysis. Sep. Purif. Technol. 2020, 235, 116198. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, B.; Jiang, C.; Wang, Y.; Xu, T. Improving the water dissociation efficiency in a bipolar membrane with amino-functionalized MIL-101. J. Membr. Sci. 2017, 524, 370–376. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Shen, J.; Van der Bruggen, B. Application of Bipolar Membrane Electrodialysis in Environmental Protection and Resource Recovery: A Review. Membranes 2022, 12, 829. [Google Scholar] [CrossRef]
- Zhou, R.; Dong, Z.; Ma, X.; Xue, L.; Liu, J.; He, J.; Li, W.; Sun, L.; Li, C.; Yan, H. Revisiting bipolar membrane electrodialysis for the production of monoprotic organic acids with different pKa and Mw. Chem. Eng. J. 2024, 505, 159125. [Google Scholar] [CrossRef]
- Zhu, M.; He, F.; Feng, L.; Chi, Y.; Li, Y.-Y.; Tian, B. Comparison of bipolar membrane electrodialysis, electrodialysis metathesis, and bipolar membrane electrodialysis multifunction for the conversion of waste Na2SO4: Process performance and economic analysis. J. Environ. Manag. 2024, 370, 122513. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Meng, W.; Lei, Y.; Li, C.; Cheng, J.; Qu, W.; Wang, G.; Zhang, M.; Li, S. The Novel Strategy for Increasing the Efficiency and Yield of the Bipolar Membrane Electrodialysis by the Double Conjugate Salts Stress. Polymers 2020, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhong, G.; Li, M.; Zhao, D.; Sun, Y.; Hu, X.; Sun, J.; Li, X.; Zhu, W.; Li, M.; et al. Review on electrochemical carbon dioxide capture and transformation with bipolar membranes. Chin. Chem. Lett. 2022, 34, 108075. [Google Scholar] [CrossRef]
- Tongwen, X.; Weihua, Y. Citric acid production by electrodialysis with bipolar membranes. Chem. Eng. Process.-Process. Intensif. 2002, 41, 519–524. [Google Scholar] [CrossRef]
- Paleologou, M.; Thibault, A.; Wong, P.-Y.; Thompson, R.; Berry, R. Enhancement of the current efficiency for sodium hydroxide production from sodium sulphate in a two-compartment bipolar membrane electrodialysis system. Sep. Purif. Technol. 1997, 11, 159–171. [Google Scholar] [CrossRef]
- Sun, X.; Lu, H.; Wang, J. Recovery of citric acid from fermented liquid by bipolar membrane electrodialysis. J. Clean. Prod. 2017, 143, 250–256. [Google Scholar] [CrossRef]
- Lee, Y.; Seo, M.; Ahn, J. Regeneration of NaOH from the Spent Na2SO4 Solution by Two-Compartment Bipolar Membrane Electrodialysis. Korean J. Met. Mater. 2025, 63, 281–290. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Shin, E.-K.; Na, I.-C.; Chu, C.-H.; Park, K.-P. Degradation of Electrode and Membrane in Proton Exchange Membrane Fuel Cell After Water Electrolysis. Korean Chem. Eng. Res. 2014, 52, 695–700. [Google Scholar] [CrossRef]
- Lu, H.; Wang, L.; Wycisk, R.; Pintauro, P.N.; Lin, S. Quantifying the kinetics-energetics performance tradeoff in bipolar membrane electrodialysis. J. Membr. Sci. 2020, 612, 118279. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Cao, H.; Wu, X.; Zhao, Z.; Wang, L. Bipolar membrane electrodialysis for generation of hydrochloric acid and ammonia from simulated ammonium chloride wastewater. Water Res. 2016, 89, 201–209. [Google Scholar] [CrossRef]
- Feng, X.; Cen, D.; Wu, Y. Combination of Precipitation-Adsorption-Bipolar Membrane Electrodialysis for Mine Water Treatment. Water 2024, 16, 1474. [Google Scholar] [CrossRef]
- Wei, Y.; Li, C.; Wang, Y.; Zhang, X.; Li, Q.; Xu, T. Regenerating sodium hydroxide from the spent caustic by bipolar membrane electrodialysis (BMED). Sep. Purif. Technol. 2012, 86, 49–54. [Google Scholar] [CrossRef]
- González, A.; Grágeda, M.; Quispe, A.; Ushak, S.; Sistat, P.; Cretin, M. Application and Analysis of Bipolar Membrane Electrodialysis for LiOH Production at High Electrolyte Concentrations: Current Scope and Challenges. Membranes 2021, 11, 575. [Google Scholar] [CrossRef]
- Cho, Y.C. A Study on Na2SO4 Treatment Using Bipolar Electrodialysis System. Ph.D. Thesis, Daejin University, Pocheon, Republic of Korea, January 2024. Available online: http://www.dcollection.net/handler/daejin/200000014751 (accessed on 29 September 2025).
- Cho, Y.; Kim, K.; Ahn, J.; Lee, J. Application of Multistage Concentration (MSC) Electrodialysis to Concentrate Lithium from Lithium-Containing Waste Solution. Metals 2020, 10, 851. [Google Scholar] [CrossRef]
- Huang, X.; Song, M.; Wang, H.; Lv, S.; Qi, S.; Wang, C.; Zhang, H.; Ruan, X. Waste salt separation by antisolvent crystallization process: Mechanism study of Na2SO4-NaCl-solvent ternary phase diagrams and its life cycle assessment. Desalination 2025, 613, 118985. [Google Scholar] [CrossRef]
- Ismail, A.M.; Metwalli, M.M.A.; Alamoush, A.S. Towards Safe Maritime Decarbonization: Safety Barriers of Methanol Fuel. Sustainability 2025, 17, 4896. [Google Scholar] [CrossRef]
- ITOPF (International Tanker Owners Pollution Federation). Methanol—Fate, Behaviour and Damage & Liability Report; ITOPF: London, UK, 2024; Methanol vapors are highly flammable with flash point ∼12 °C; flames are almost invisible to the eye. [Google Scholar]

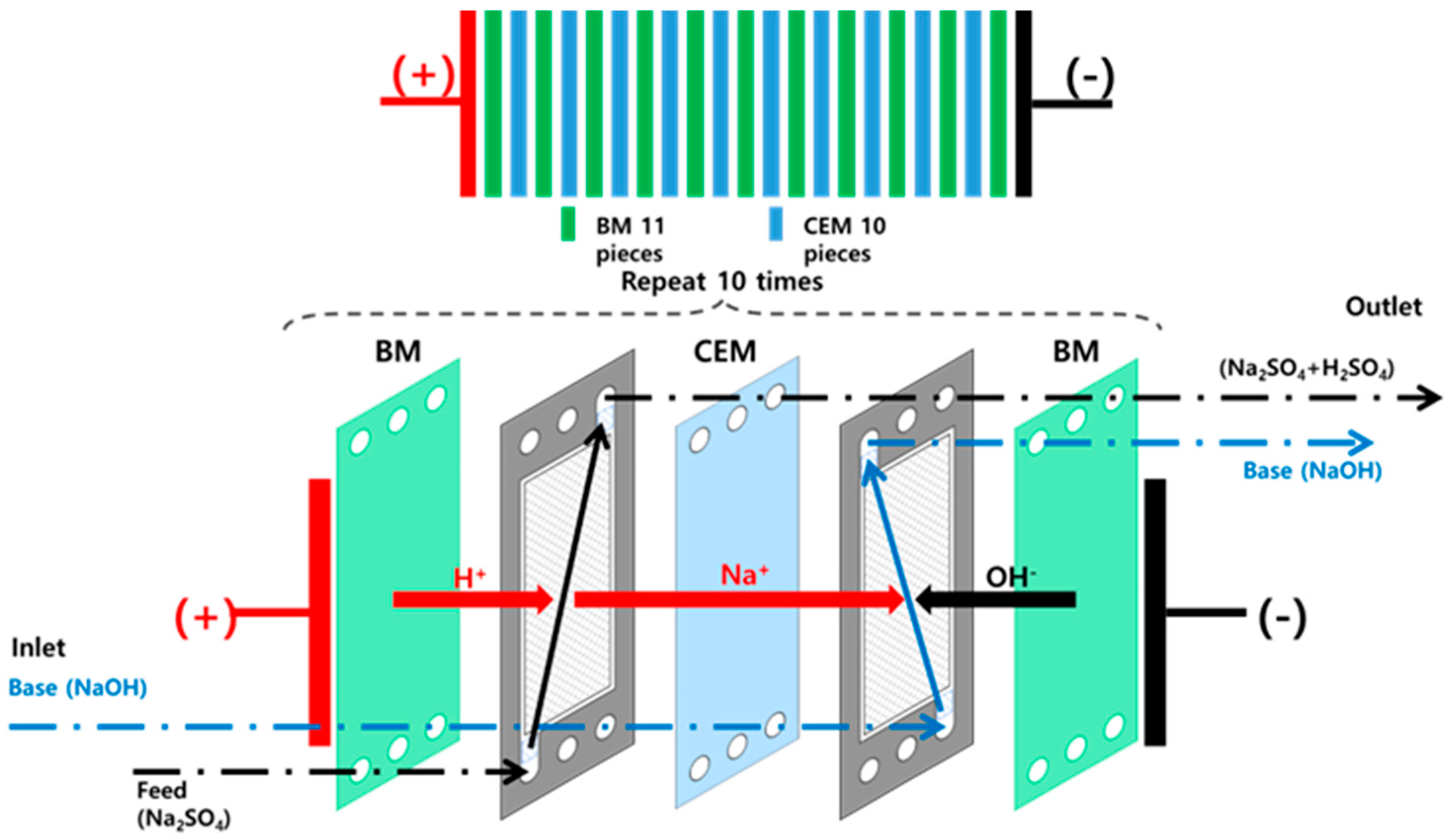

| Effective membrane area | 0.055 m2 (11 ea BM/10 ea CEM) |
| Operating mode | Constant voltage/Constant current |
| Terminating mode | Current, Conductivity, Time, pH |
| Terminating condition | Current: 0.00–6.00 A Voltage: 0~30.0 V Conductivity: 0.0~300 mS/cm pH: 0~14 Time: 0–999 min |
| Line capacity | 0.1 L |
| Upper temperature limit | 40 °C |
| Upper tank capacity limit | 2.5 L |
| Membrane | BPM | CEM |
|---|---|---|
| Type | - | Strong acid (Na type) |
| Characteristics | Water splitting -Voltage 1.0 V -Efficiency ≥ 0.98 | High mechanical strength /Alkali resistance |
| Electric resistance (Ω·cm2) | - | 4.5 |
| Burst strength | ≥0.70 | ≥0.40 |
| Thickness (mm) | 0.28 | 0.21 |
| Temperature (°C) | ≤40 | ≤60 |
| pH | - | 0~14 |
| Current Density (A/m2) | Time (h) | Water Migration to Base (%) | Recovery of NaOH (%) (M) | Acid in Mixed Sol. (M) | Ion Flux (mol/m2h) | Energy Consumption | |
|---|---|---|---|---|---|---|---|
| (kWh/kg Na2SO4) * | (kWh/kg NaOH) ** | ||||||
| 250 | 8.00 | 8.69 | 52.60 (1.35) | 0.80 | 4.68 | 1.30 | 4.39 |
| 310 | 8.00 | 10.17 | 59.84 (1.51) | 0.88 | 5.31 | 1.49 | 4.42 |
| 360 | 8.00 | 12.50 | 64.29 (1.58) | 0.97 | 5.72 | 1.68 | 4.64 |
| 400 | 8.00 | 14.24 | 65.05 (1.61) | 1.03 | 5.88 | 1.86 | 5.07 |
| 450 | 8.00 | 15.73 | 69.82 (1.67) | 1.12 | 6.21 | 2.05 | 5.21 |
| 500 | 8.00 | 17.55 | 73.19 (1.71) | 1.19 | 6.51 | 2.31 | 5.60 |
| Initial Feed Sol. (M) | Time (h) | Water Migration to Base (%) | Recovery of NaOH (%) (M) | Acid in Mixed Sol. (M) | Ion Flux (mol/m2h) | Energy Consumption | |
|---|---|---|---|---|---|---|---|
| (kWh/kg Na2SO4) * | (kWh/kg NaOH) ** | ||||||
| 0.22 | 8.00 | 16.45 | 90.64 (0.42) | 0.25 | 1.34 | 8.56 | 16.77 |
| 0.43 | 8.00 | 17.13 | 87.98 (0.73) | 0.47 | 2.61 | 4.31 | 8.70 |
| 0.65 | 8.00 | 17.25 | 82.61 (1.00) | 0.64 | 3.67 | 3.03 | 6.51 |
| 0.87 | 8.00 | 14.19 | 73.91 (1.22) | 0.78 | 4.38 | 2.51 | 6.03 |
| 1.09 | 8.00 | 15.53 | 72.73 (1.46) | 0.96 | 5.39 | 2.05 | 5.00 |
| 1.30 | 8.00 | 15.68 | 70.00 (1.67) | 1.12 | 6.17 | 1.86 | 4.72 |
| Initial Base Sol. (M) | Time (h) | Water Migration to Base (%) | Recovery of NaOH (%) (M) | Acid in Mixed Sol. (M) | Ion Flux (mol/m2h) | Energy Consumption | |
|---|---|---|---|---|---|---|---|
| (kWh/kg Na2SO4) * | (kWh/kg NaOH) ** | ||||||
| 0.05 | 8.00 | 11.76 | 66.39 (1.59) | 1.02 | 5.90 | 1.87 | 5.00 |
| 0.10 | 8.00 | 15.68 | 70.00 (1.67) | 1.12 | 6.23 | 1.86 | 4.72 |
| 0.30 | 8.00 | 14.65 | 67.99 (1.80) | 1.06 | 6.06 | 1.83 | 4.78 |
| 0.50 | 8.00 | 17.13 | 69.21 (1.90) | 1.09 | 6.15 | 1.83 | 4.70 |
| 1.00 | 8.00 | 20.30 | 67.00 (2.26) | 1.08 | 5.96 | 1.84 | 4.87 |
| Initial Base Sol. (L) | Time (h) | Water Migration (%) | Recovery of NaOH (%) (M) | Acid in Mixed Sol. (M) | Ion Flux (mol/m2h) | Energy Consumption | ||
|---|---|---|---|---|---|---|---|---|
| Base | Feed | (kWh/kg Na2SO4) * | (kWh/kg NaOH) ** | |||||
| 0.50 | 8.00 | 76.82 | −25.61 | 65.07 (2.85) | 1.15 | 5.79 | 1.91 | 5.21 |
| 0.75 | 8.00 | 44.47 | −22.23 | 66.32 (2.46) | 1.14 | 5.89 | 1.88 | 5.03 |
| 1.00 | 8.00 | 28.80 | −19.20 | 67.85 (2.13) | 1.12 | 6.03 | 1.82 | 4.76 |
| 1.25 | 8.00 | 20.30 | −16.92 | 68.41 (1.88) | 1.11 | 6.08 | 1.82 | 4.72 |
| 1.50 | 8.00 | 15.68 | −15.68 | 70.00 (1.67) | 1.12 | 6.23 | 1.86 | 4.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Seo, M.-H.; Chang, J.-H.; Kim, J.-H.; Ahn, J.-W. Application of Two-Compartment Bipolar Membrane Electrodialysis for Treatment of Waste Na2SO4 Solution. Membranes 2025, 15, 312. https://doi.org/10.3390/membranes15100312
Lee Y-J, Seo M-H, Chang J-H, Kim J-H, Ahn J-W. Application of Two-Compartment Bipolar Membrane Electrodialysis for Treatment of Waste Na2SO4 Solution. Membranes. 2025; 15(10):312. https://doi.org/10.3390/membranes15100312
Chicago/Turabian StyleLee, Young-Jae, Min-Hyuk Seo, Jae-Hyuk Chang, Jun-Hee Kim, and Jae-Woo Ahn. 2025. "Application of Two-Compartment Bipolar Membrane Electrodialysis for Treatment of Waste Na2SO4 Solution" Membranes 15, no. 10: 312. https://doi.org/10.3390/membranes15100312
APA StyleLee, Y.-J., Seo, M.-H., Chang, J.-H., Kim, J.-H., & Ahn, J.-W. (2025). Application of Two-Compartment Bipolar Membrane Electrodialysis for Treatment of Waste Na2SO4 Solution. Membranes, 15(10), 312. https://doi.org/10.3390/membranes15100312







