Enhanced CO2 Separation Performance of Mixed Matrix Membranes with Pebax and Amino-Functionalized Carbon Nitride Nanosheets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
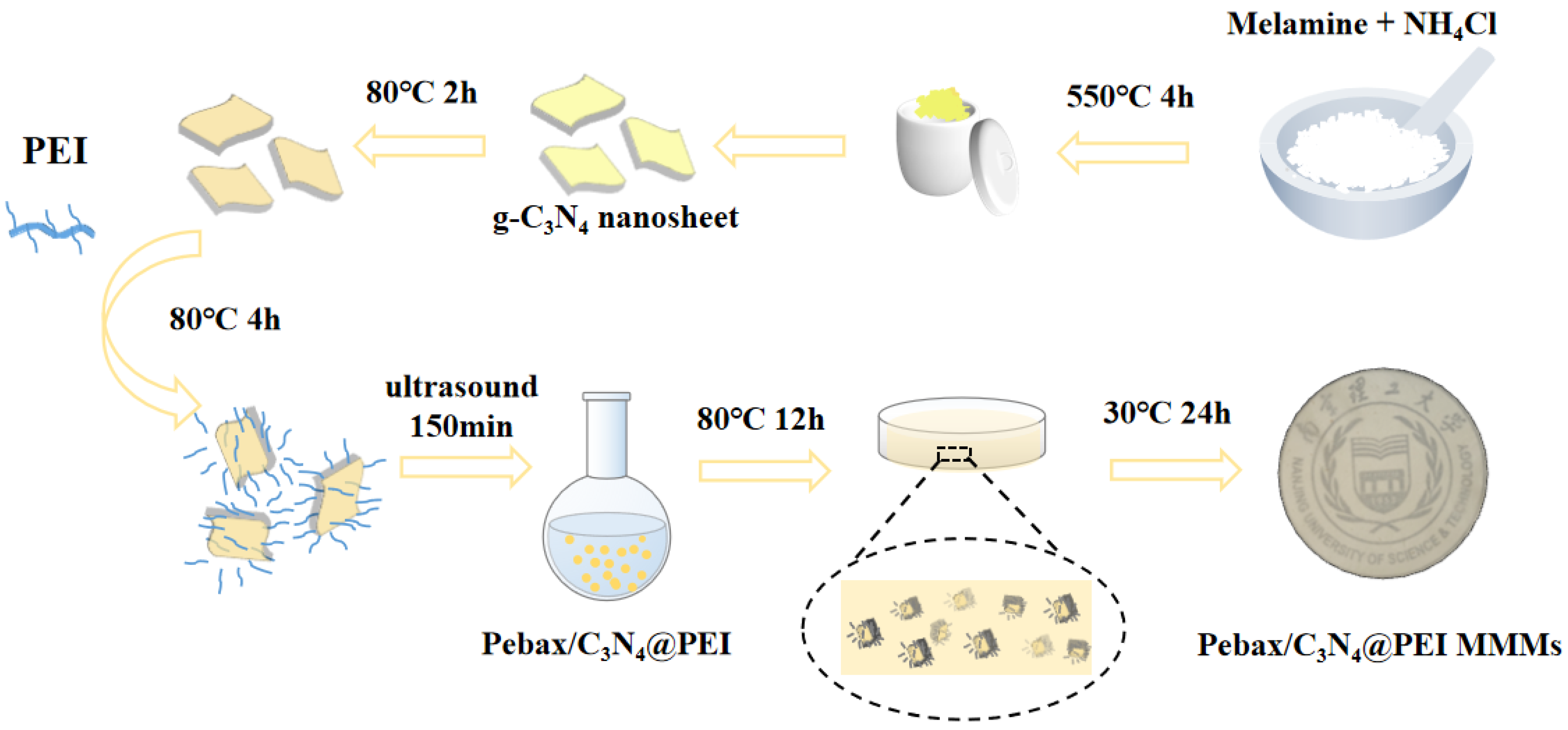
2.2. Preparation of g-C3N4 (CN) Nanosheets
2.3. Preparation of Amino-Functionalized CN Nanosheets
2.4. Preparation of the Membranes
2.5. Characterization of Nanofillers and MMMs
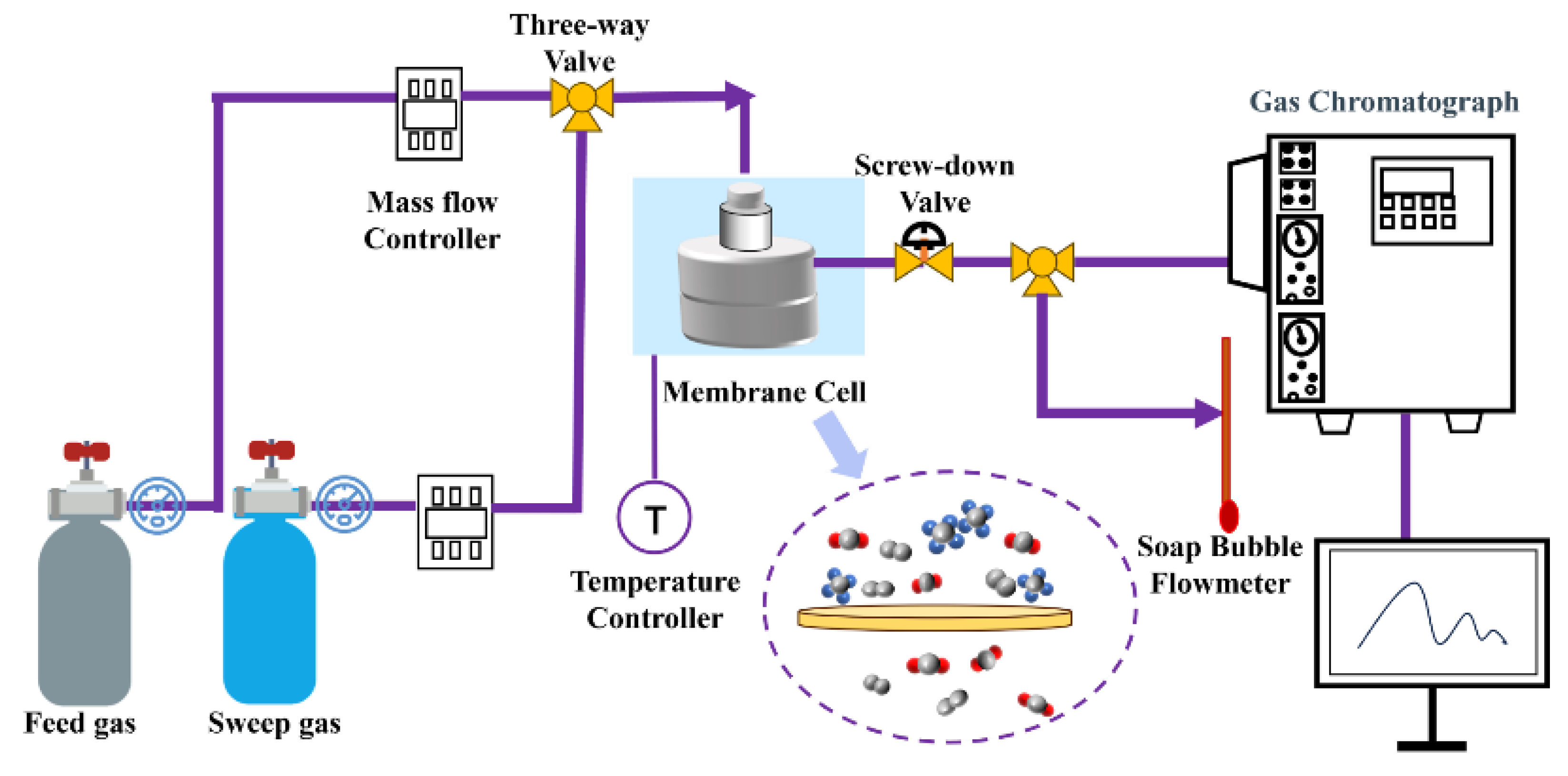
2.6. Gas Permeation Experiments
3. Results and Discussion
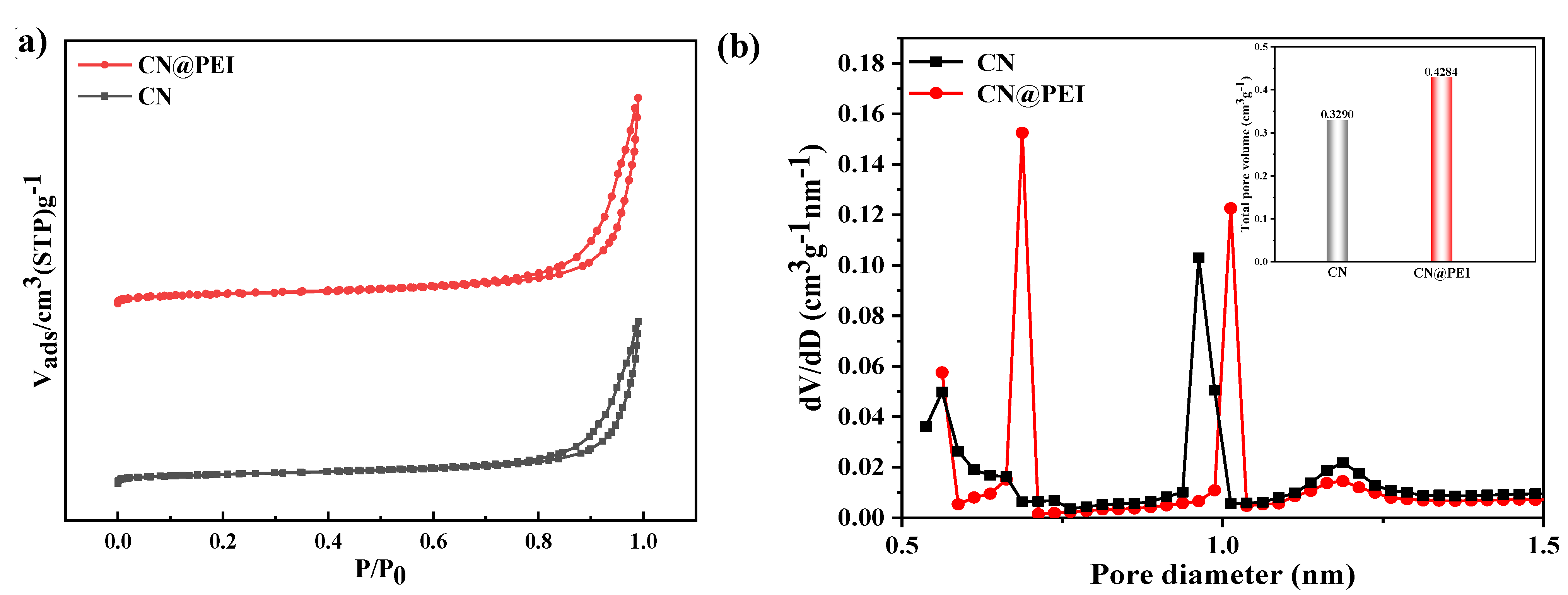
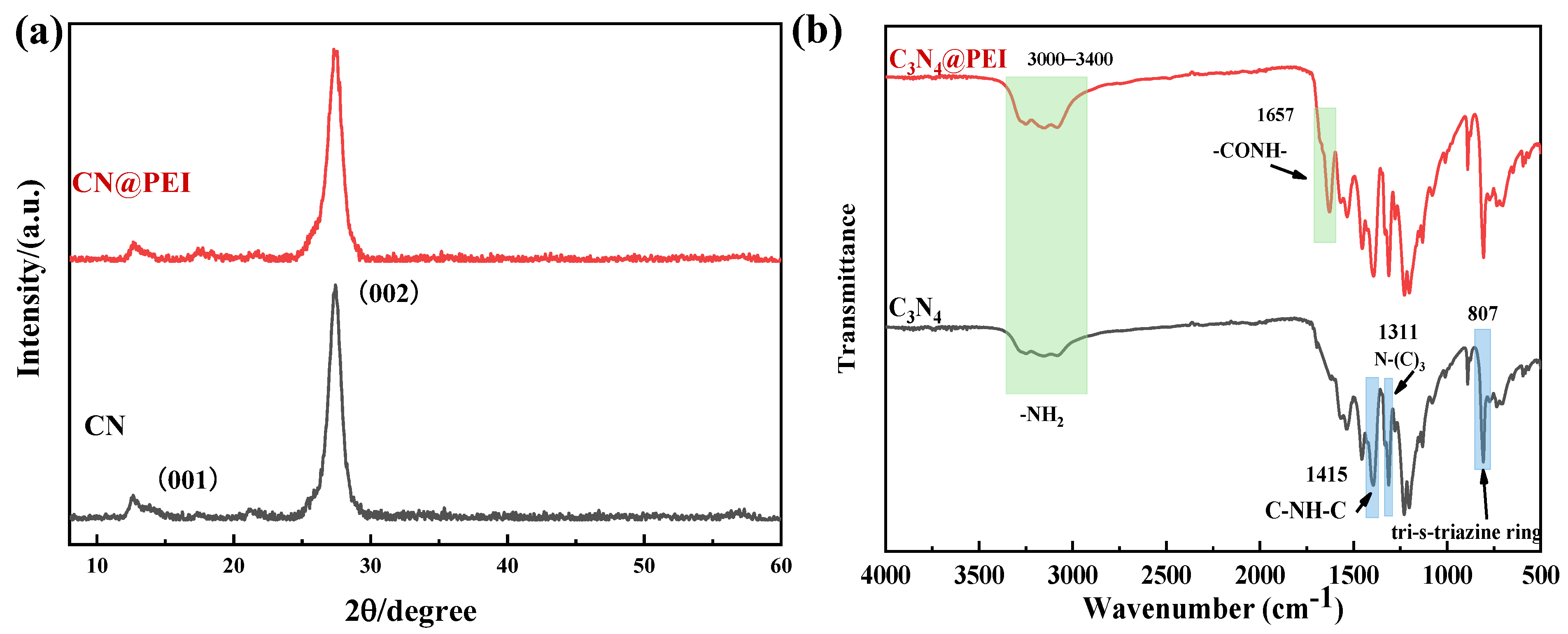
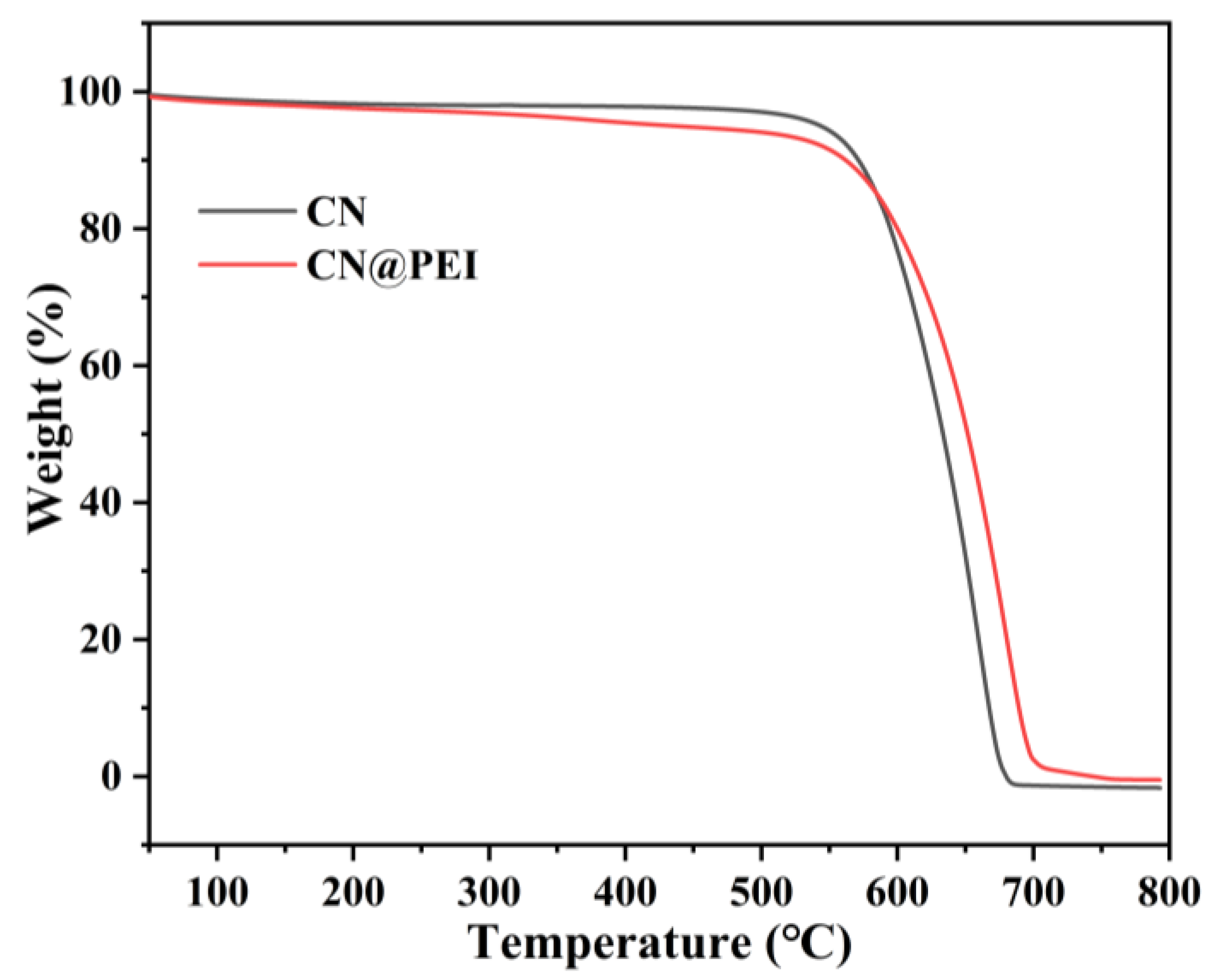
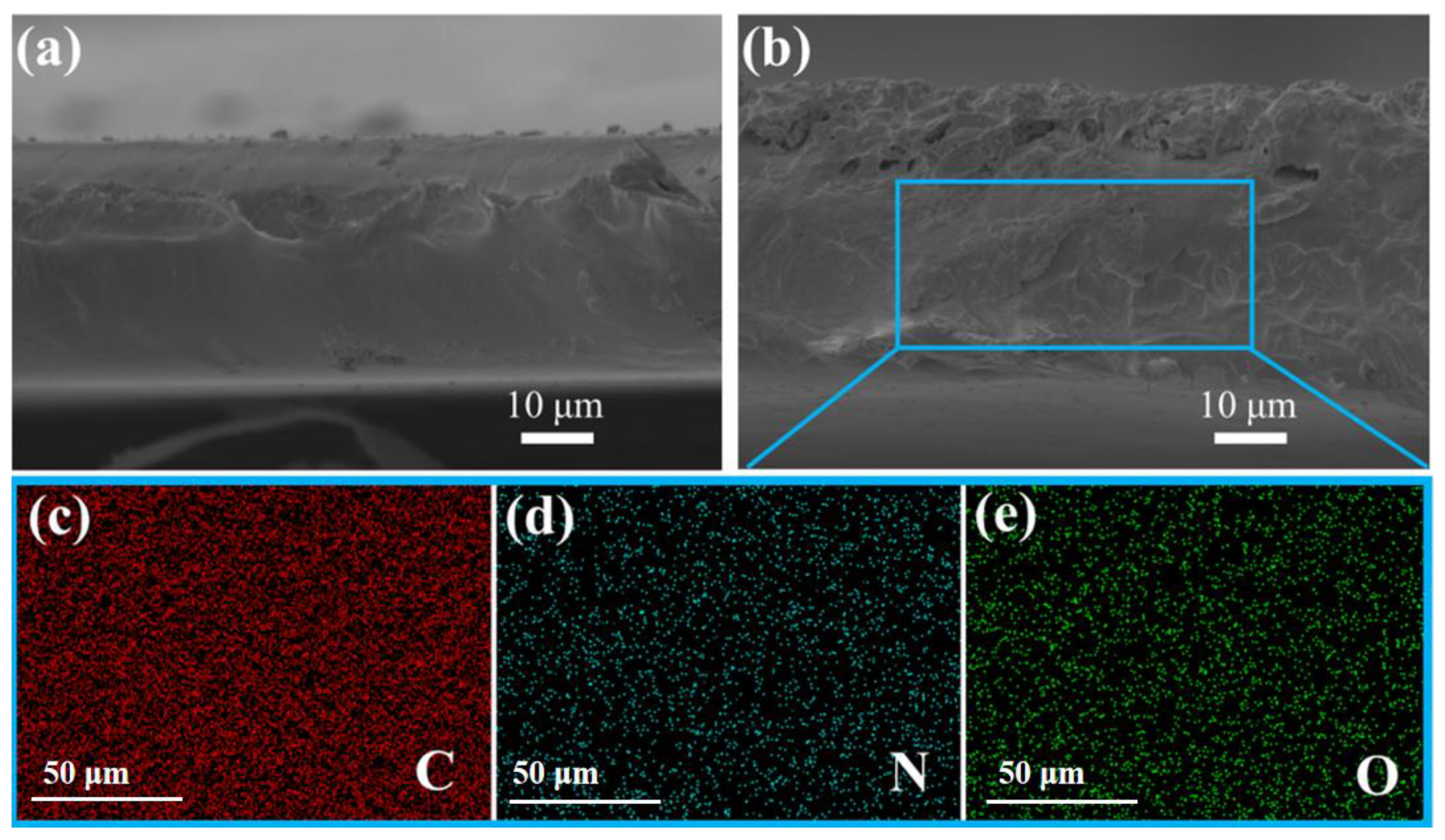
3.1. Characterization of the Nanofillers
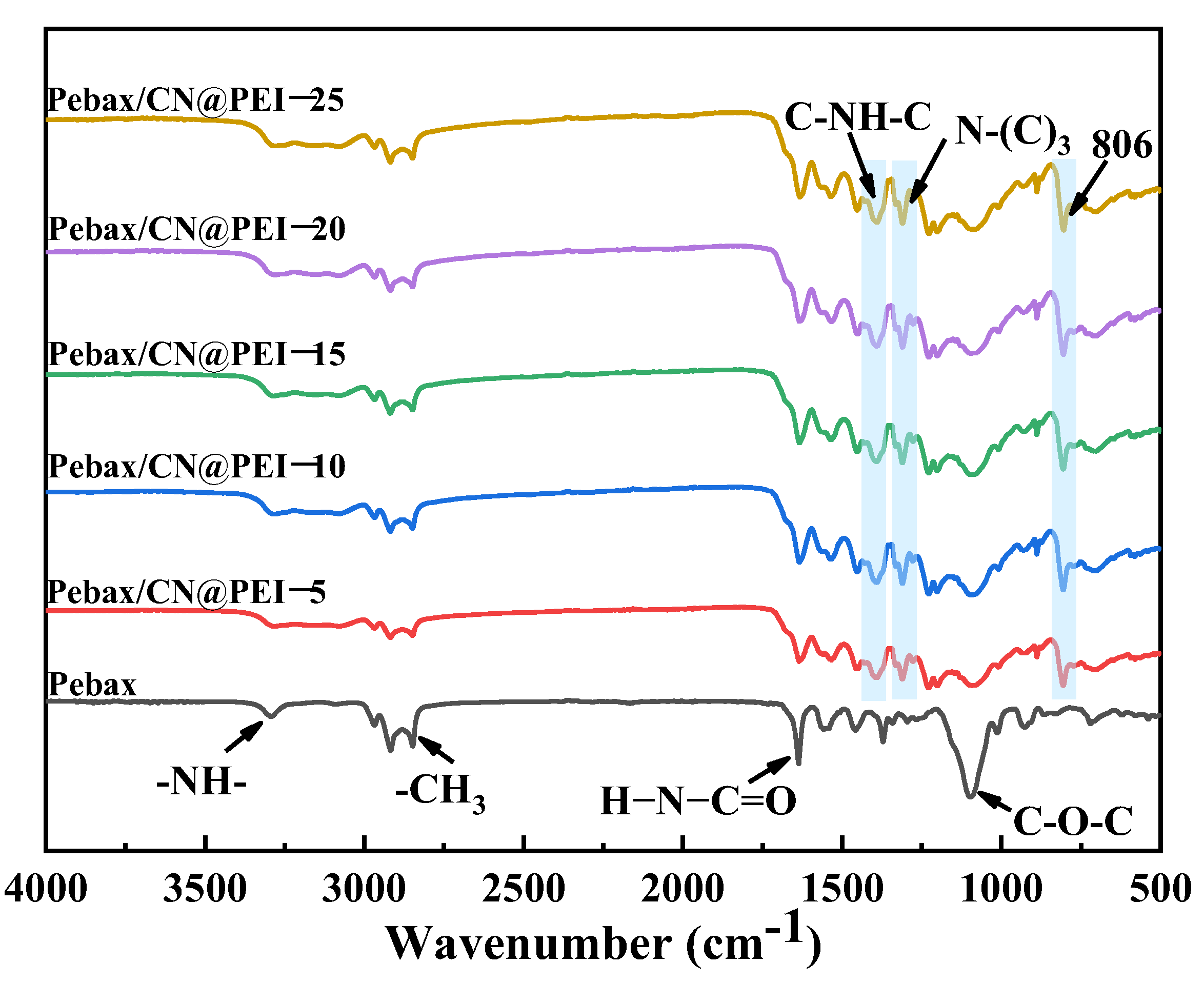
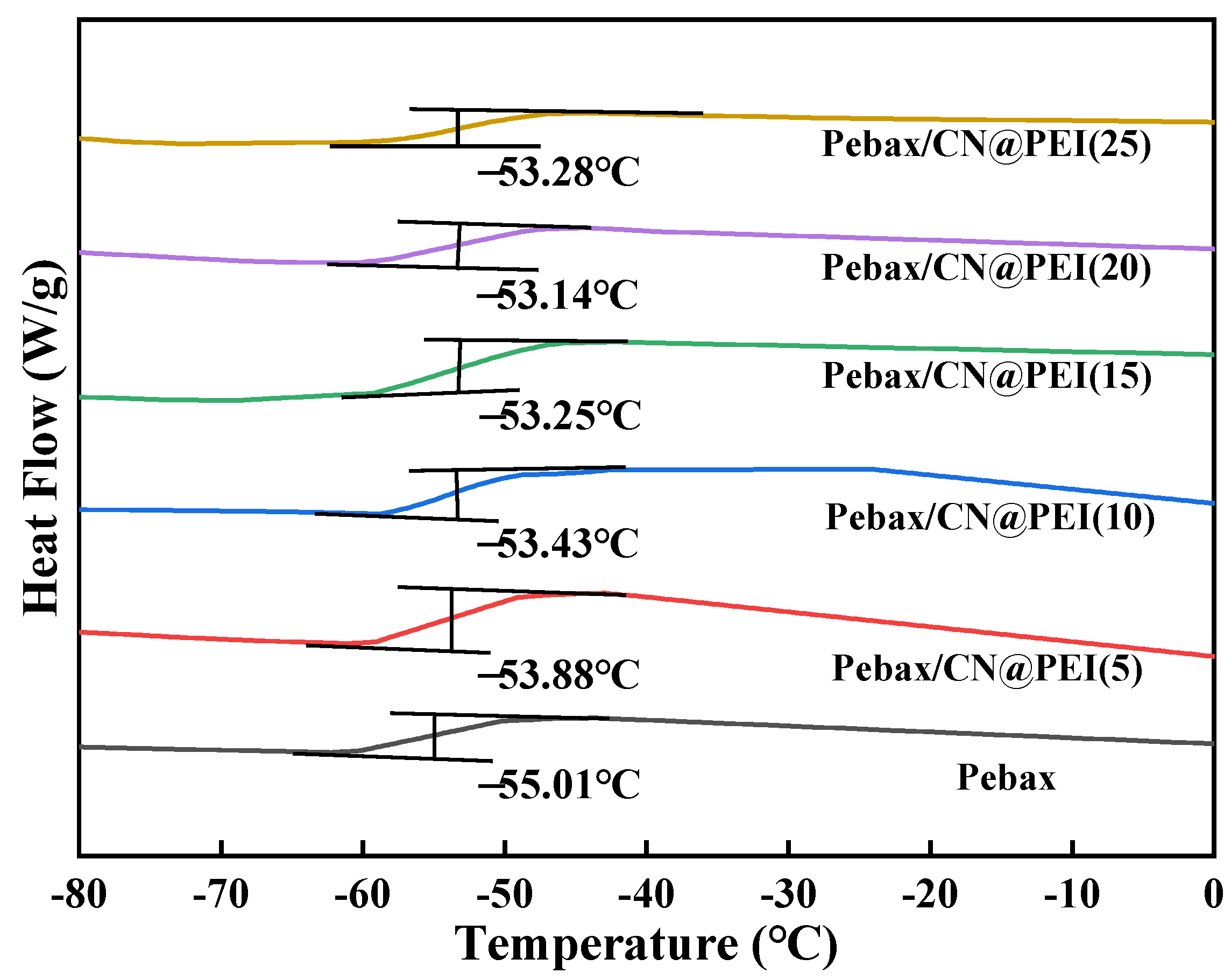
3.2. Characterization of the MMMs
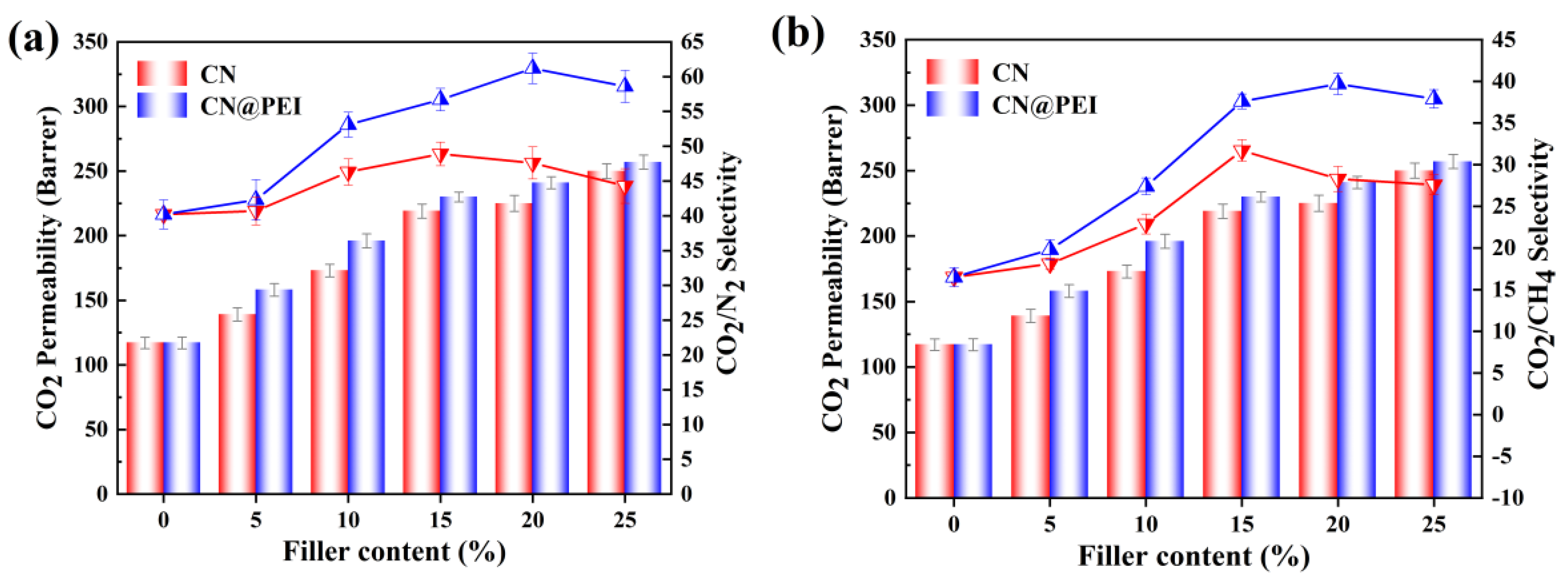
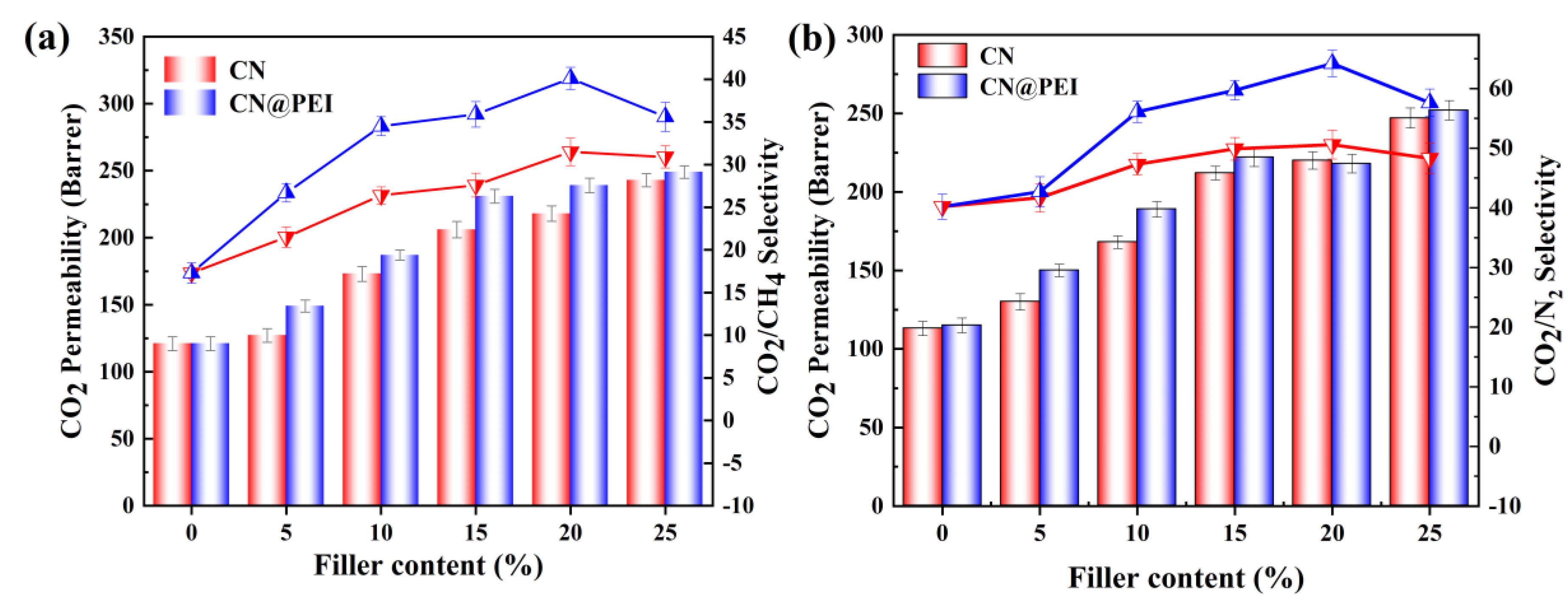
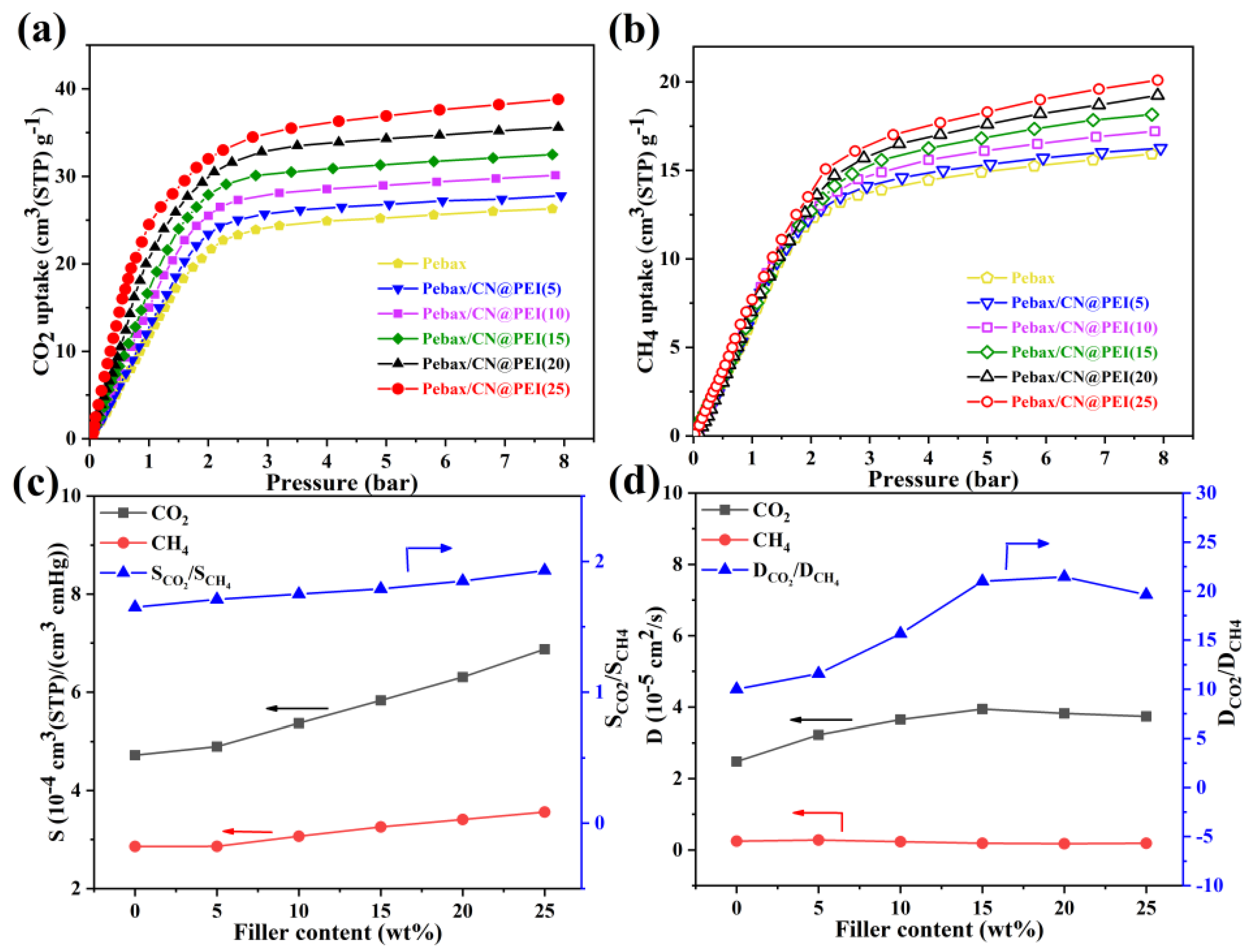
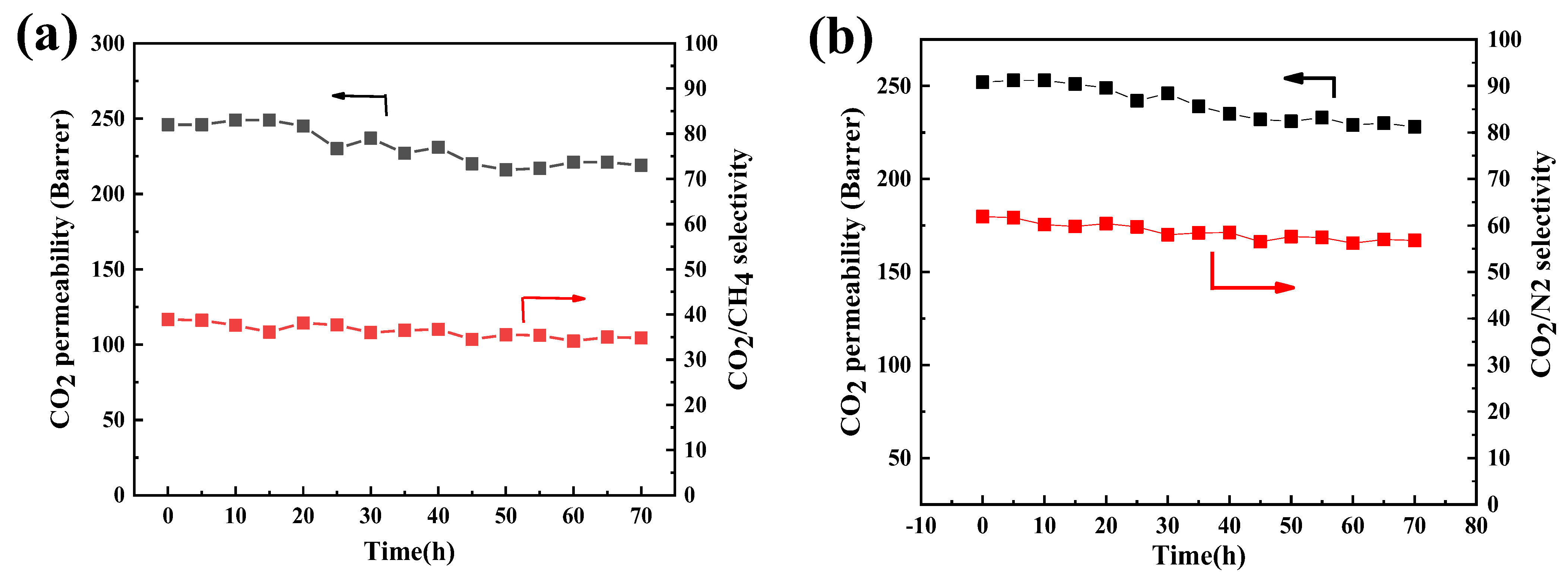
3.3. Gas Separation Performance
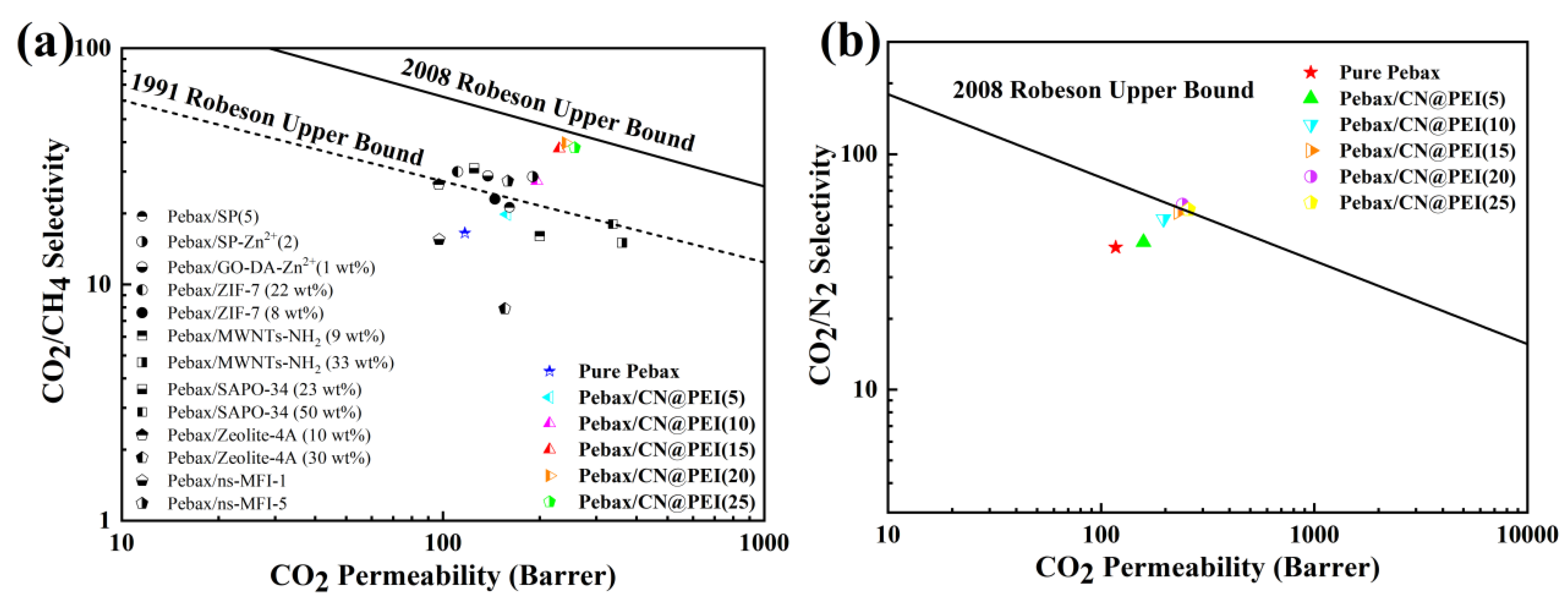
3.4. Comparison of Gas Separation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research progress on CO2 capture and utilization technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Zhao, K.; Jia, C.; Li, Z.; Du, X.; Wang, Y.; Li, J.; Yao, Z.; Yao, J. Recent advances and future perspectives in carbon capture, transportation, utilization, and storage (CCTUS) technologies: A comprehensive review. Fuel 2023, 351, 128913. [Google Scholar] [CrossRef]
- Jamil, A.; Ching, O.P.; Shariff, A.B.M. Current status and future prospect of polymer-layered silicate mixed-matrix membranes for CO2/CH4. Chem. Eng. Technol. 2016, 39, 1393–1405. [Google Scholar] [CrossRef]
- Sreedhar, I.; Vaidhiswaran, R.; Kamani, M.; Venugopal, B.A. Process and engineering trends in membrane based carbon capture. Renew. Sustain. Energy Rev. 2017, 68, 659–684. [Google Scholar] [CrossRef]
- Mannan, H.A.; Mukhtar, H.; Murugesan, T.; Nasir, R.; Mohshim, D.F.; Mushtaq, A. Recent applications of polymer blends in gas separation membranes. Chem. Eng. Technol. 2013, 36, 1838–1846. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Azmi, M.S. Membranes for gas separation current development and challenges. Appl. Mech. Mater. 2015, 773–774, 1085–1090. [Google Scholar] [CrossRef]
- Fauzan, N.A.B.; Mannan, H.A.; Nasir, R.; Mohshim, D.F.B.; Mukhtar, H. Various techniques for preparation of thin-film composite mixed-matrix membranes for CO2 separation. Chem. Eng. Technol. 2019, 42, 2608–2620. [Google Scholar] [CrossRef]
- Imtiaz, A.; Othman, M.H.D.; Jilani, A.; Khan, I.U.; Kamaludin, R.; Samuel, O. ZIF-filler incorporated mixed matrix membranes (MMMs) for efficient gas separation: A review. J. Environ. Chem. Eng. 2022, 10, 108541. [Google Scholar] [CrossRef]
- Zainuddin, M.I.F.; Ahmad, A.L. Mixed matrix membrane development progress and prospect of using 2D nanosheet filler for CO2 separation and capture. J. CO2 Util. 2022, 62, 102094. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Wang, X.; Chi, C.; Zhang, K.; Qian, Y.; Gupta, K.M.; Kang, Z.; Jiang, J.; Zhao, D. Reversed thermo-switchable molecular sieving membranes composed of two-dimensional metal-organic nanosheets for gas separation. Nat. Commun. 2017, 8, 14460. [Google Scholar] [CrossRef]
- Dakhchoune, M. Two-dimensional material membranes for gas separation. Chimia 2020, 74, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Amooghin, A.E.; Sanaeepur, H.; Luque, R.; Garcia, H.; Chen, B. Fluorinated metal-organic frameworks for gas separation. Chem. Soc. Rev. 2022, 51, 7427–7508. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Puyvelde, P.V.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, Y.; Zhang, X.; Xu, C.; Yao, J. Integrating two-dimensional MXene fillers into nanocellulose for the fabrication of CO2 separation membranes. Separ. Purif. Technol. 2023, 326, 124704. [Google Scholar] [CrossRef]
- Jia, C.; Yang, L.; Zhang, Y.; Zhang, X.; Xiao, K.; Xu, J.; Liu, J. Graphitic carbon nitride films: Emerging paradigm for versatile applications. ACS. Appl. Mater. Interfaces. 2020, 12, 53571–53591. [Google Scholar] [CrossRef]
- Chen, Z.; Lan, Y.; Hong, Y.; Lan, W. Review of 2D Graphitic carbon nitride-based membranes: Principles, syntheses, and applications. ACS Appl. Nano Mater. 2022, 5, 12343–12365. [Google Scholar] [CrossRef]
- Dai, Y.; Niu, Z.; Wang, Y.; Zhong, S.; Peng, M.; Li, J. Recent advances and prospect of emerging microporous membranes for high-performance CO2 capture. Separ. Purif. Technol. 2023, 318, 123992. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Ao, Y. Mini review on the structure and properties (photocatalysis), and preparation techniques of graphitic carbon nitride nano-based particle, and its applications. Nanoscale. Res. Lett. 2018, 13, 388. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Z.; Jin, J. Two-dimensional microporous material-based mixed matrix membranes for gas separation. Chem. Asian. J. 2020, 15, 2303–2315. [Google Scholar] [CrossRef]
- Cheng, L.; Song, Y.; Chen, H.; Liu, G.; Liu, G.; Jin, W. g-C3N4 nanosheets with tunable affinity and sieving effect endowing polymeric membranes with enhanced CO2 capture property. Separ. Purif. Technol. 2020, 250, 117200. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, S.; Wang, Y.; Ma, X.; Cao, K.; Peng, D.; Wu, X.; Wu, H.; Jiang, Z. Enhanced gas separation performance of mixed matrix membranes from graphitic carbon nitride nanosheets and polymers of intrinsic microporosity. J. Membr. Sci. 2016, 514, 15–24. [Google Scholar] [CrossRef]
- Niu, Z.; Luo, W.; Mu, P.; Li, J. Nanoconfined CO2-philic ionic liquid in laminated g-C3N4 membrane for the highly efficient separation of CO2. Separ. Purif. Technol. 2022, 297, 121513. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhou, S.; Xue, A.; Zhang, Y.; Zhao, Y.; Zhong, J. Controllable construction of polymer/inorganic interface for poly (vinyl alcohol)/graphitic carbon nitride hybrid pervaporation membranes. Chem. Eng. Sci. 2018, 181, 237–250. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53(Al) in Pebax® MH-1657 for CO2 separation. Separ. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Liu, R.; Shi, X.; Wang, C.; Gao, Y.; Xu, S.; Hao, G.; Chen, S.; Lu, A. Advances in post-combustion CO2 capture by physical adsorption: From materials innovation to separation practice. ChemSusChem. 2021, 14, 1428–1471. [Google Scholar] [CrossRef]
- Berstad, D.; Anantharaman, R.; Nekså, P. Low-temperature CO2 capture technologies-applications and potential. Int. J. Refrig. 2013, 36, 1403–1416. [Google Scholar] [CrossRef]
- Jiao, C.; Li, Z.; Li, X.; Wu, M.; Jiang, H. Improved CO2/N2 separation performance of Pebax composite membrane containing polyethyleneimine functionalized ZIF-8. Separ. Purif. Technol. 2021, 259, 118190. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Shan, Y.; Wang, X.; Yang, Y.; Zhang, F.; Chen, X. Polyethyleneimine-modified multi-walled carbon nanotubes mixed matrix membranes for CO2/N2 separation. J. Environ. Chem. Eng. 2023, 11, 109537. [Google Scholar] [CrossRef]
- Horn, N.R. A critical review of free volume and occupied volume calculation methods. J. Membr. Sci. 2016, 518, 289–294. [Google Scholar] [CrossRef]
- Park, J.Y.; Paul, D.R. Correlation and prediction of gas permeability in glassy polymer membrane materials via a modified free volume based group contribution method. J. Membr. Sci. 1997, 125, 23–39. [Google Scholar] [CrossRef]
- Kanehashi, S.; Chen, G.Q.; Scholes, C.A.; Ozcelik, B.; Hua, C.; Ciddor, L.; Southon, P.D.; Alessandr, D.M.D.; Kentish, S.E. Enhancing gas permeability in mixed matrix membranes through tuning the nanoparticle properties. J. Membr. Sci. 2015, 482, 49–55. [Google Scholar] [CrossRef]
- Kojabad, M.E.; Babaluo, A.; Tavakoli, A. A novel semi-mobile carrier facilitated transport membrane containing aniline/poly (ether-block-amide) for CO2/N2 separation: Molecular simulation and experimental study. Separ. Purif. Technol. 2021, 266, 118494. [Google Scholar] [CrossRef]
- Hou, T.; Shu, L.; Guo, K.; Zhang, X.-F.; Zhou, S.; He, M.; Yao, J. Cellulose membranes with polyethylenimine-modified graphene oxide and zinc ions for promoted gas separation. Cellulose 2020, 27, 3277–3286. [Google Scholar] [CrossRef]
- Guo, X.; Huang, H.; Liu, D.; Zhong, C. Improving particle dispersity and CO2 separation performance of amine-functionalized CAU-1 based mixed matrix membranes with polyethyleneimine-grafting modification. Chem. Eng. Sci. 2018, 189, 277–285. [Google Scholar] [CrossRef]
- Dai, Y.; Niu, Z.; Fang, T.; Wang, Y.; Zhong, S.; Mu, P.; Li, J. 1D-2D intercalated network CMC@ g-C3N4/IL membrane with high permeability and selectivity for the CO2 capture. J. Membr. Sci. 2023, 686, 122019. [Google Scholar] [CrossRef]
- Gang, M.; He, G.; Li, Z.; Cao, K.; Li, Z.; Yin, Y.; Jiang, Z. Graphitic carbon nitride nanosheets/sulfonated poly (ether ether ketone) nanocomposite membrane for direct methanol fuel cell application. J. Membr. Sci. 2016, 507, 1–11. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.; He, X.; Wang, R.; Tao, W.; Li, Z. Covalent “Bridge-crosslinking” strategy constructs facilitated transport mixed matrix membranes for highly-efficient CO2 separation. J. Membr. Sci. 2023, 680, 121755. [Google Scholar] [CrossRef]
- Li, S.; Zhang, K.; Liu, C.; Feng, X.; Wang, P.; Wang, S. Nanohybrid Pebax/PEGDA-GPTMS membrane with semi-interpenetrating network structure for enhanced CO2 separations. J. Membr. Sci. 2023, 674, 121516. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, Y.; Dai, Y.; Zhong, S.; Li, J.; Mu, P.; Li, J. Enhanced CO2 separation by functionalized g-C3N4 nanosheets as composite filler to fabricate mixed matrix membranes. Macromolecules 2023, 56, 6461–6469. [Google Scholar] [CrossRef]
- Sherugar, P.; Antony, A.M.; Nordin, N.A.H.M.; Patil, S.A.; Padaki, M. Tailoring the CH4/CO2/N2 separation performance of ultrapermeable polymeric composite membranes by altering the concentration of Pd/g-C3N4. Fuel 2024, 361, 130731. [Google Scholar] [CrossRef]
- Jomekian, A.; Bazooyar, B.; Esmaeilzadeh, J.; Behbahani, R.M. Highly CO2 selective chitosan/g-C3N4/ZIF-8 membrane on polyethersulfone microporous substrate. Separ. Purif. Technol. 2020, 236, 116307. [Google Scholar] [CrossRef]
- Kee, V.B.; Hui, S.L.; Zeng, L.C.; Fen, Y.W. Functionalized two-dimensional g-C3N4 nanosheets in PIM-1 mixed matrix membranes for gas separation. Separ. Purif. Technol. 2022, 296, 121354. [Google Scholar] [CrossRef]
- Zhang, N.; Peng, D.; Wu, H.; Ren, Y.; Yang, L.; Wu, X.; Wu, Y.; Qu, Z.; Jiang, Z.; Cao, X. Significantly enhanced CO2 capture properties by synergy of zinc ion and sulfonate in Pebax-pitch hybrid membranes. J. Membr. Sci. 2018, 549, 670–679. [Google Scholar] [CrossRef]
- Peng, D.; Wang, S.; Tian, Z.; Wu, X.; Wu, Y.; Wu, H.; Xin, Q.; Chen, J.; Cao, X.; Jiang, Z. Facilitated transport membranes by incorporating graphene nanosheets with high zinc ion loading for enhanced CO2 separation. J. Membr. Sci. 2017, 522, 351–362. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.; Peinemann, K.V.; Lai, Z. Carbon dioxide selective mixed matrix composite membrane containing ZIF-7 nano-fillers. J. Membr. Sci. 2013, 425, 235–242. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Li, X.; Deng, M. Gas separation properties of poly(amide-6-b-ethylene oxide)/amino modified multi-walled carbon nanotubes mixed matrix membranes. J. Membr. Sci. 2014, 467, 41–47. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Hua, K.; Deng, M. Poly(amide-6-b-ethylene oxide)/SAPO-34 mixed matrix membrane for CO2 separation. J. Energy Chem. 2014, 23, 227–234. [Google Scholar] [CrossRef]
- Murali, R.S.; Ismail, A.F.; Rahman, M.A.; Sridhar, S. Mixed matrix membranes of Pebax-1657 loaded with 4A zeolite for gaseous separations. Sep. Purif. Technol. 2014, 129, 1–8. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Liu, X.; Zhang, B. Pebax/two-dimensional MFI nanosheets mixed-matrix membranes for enhanced CO2 separation. J. Membr. Sci. 2021, 636, 119612. [Google Scholar] [CrossRef]
- Guo, F.; Li, D.; Ding, R.; Gao, J.; Ruan, X.; Jiang, X.; He, G.; Xiao, W. Constructing MOF-doped two-dimensional composite material ZIF-90@g-C3N4 mixed matrix membranes for CO2/N2 separation. Separ. Purif. Technol. 2022, 280, 119803. [Google Scholar] [CrossRef]


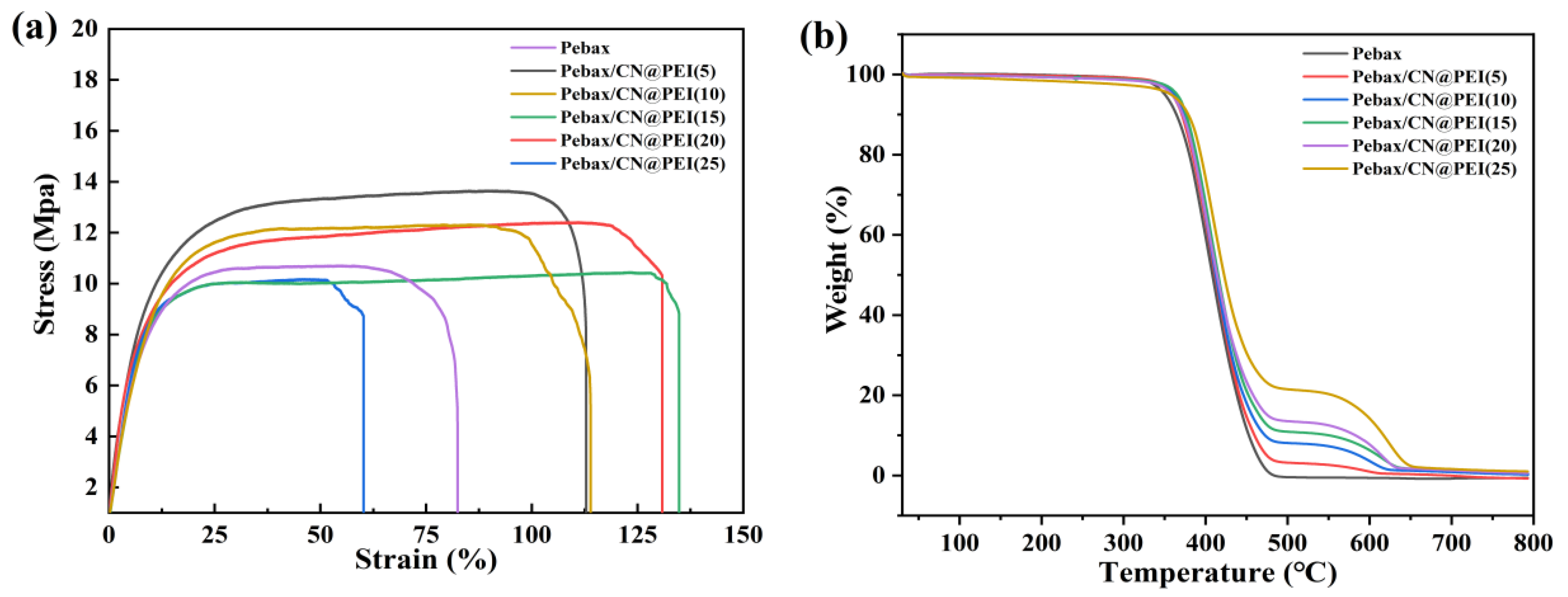
| Membranes | Breaking Elongation (%) | Young’s Module (MPa) | Tensile Strength (MPa) |
|---|---|---|---|
| Pebax | 82.5 | 11.4 | 10.9 |
| Pebax/CN@PEI (5) | 112.9 | 12.2 | 13.5 |
| Pebax/CN@PEI (10) | 113.9 | 12.8 | 12.2 |
| Pebax/CN@PEI (15) | 137.6 | 16.3 | 10.4 |
| Pebax/CN@PEI (20) | 130.9 | 15.1 | 12.6 |
| Pebax/CN@PEI (25) | 60.2 | 11.9 | 9.8 |
| Membranes | Density (g/cm3) | FFV |
|---|---|---|
| Pure Pebax | 1.042 | 0.260 |
| Pebax/CN@PEI (5) | 1.040 | 0.265 |
| Pebax/CN@PEI (10) | 1.039 | 0.269 |
| Pebax/CN@PEI (15) | 1.037 | 0.274 |
| Pebax/CN@PEI (20) | 1.035 | 0.278 |
| Pebax/CN@PEI (25) | 1.034 | 0.283 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, M.; Sun, Q.; Li, N.; Zhu, M.; Lu, Y.; Hu, Z.; Chen, S. Enhanced CO2 Separation Performance of Mixed Matrix Membranes with Pebax and Amino-Functionalized Carbon Nitride Nanosheets. Membranes 2025, 15, 306. https://doi.org/10.3390/membranes15100306
Hua M, Sun Q, Li N, Zhu M, Lu Y, Hu Z, Chen S. Enhanced CO2 Separation Performance of Mixed Matrix Membranes with Pebax and Amino-Functionalized Carbon Nitride Nanosheets. Membranes. 2025; 15(10):306. https://doi.org/10.3390/membranes15100306
Chicago/Turabian StyleHua, Mengran, Qinqin Sun, Na Li, Mingchao Zhu, Yongze Lu, Zhaoxia Hu, and Shouwen Chen. 2025. "Enhanced CO2 Separation Performance of Mixed Matrix Membranes with Pebax and Amino-Functionalized Carbon Nitride Nanosheets" Membranes 15, no. 10: 306. https://doi.org/10.3390/membranes15100306
APA StyleHua, M., Sun, Q., Li, N., Zhu, M., Lu, Y., Hu, Z., & Chen, S. (2025). Enhanced CO2 Separation Performance of Mixed Matrix Membranes with Pebax and Amino-Functionalized Carbon Nitride Nanosheets. Membranes, 15(10), 306. https://doi.org/10.3390/membranes15100306








