Cost-Effective and Durable Ceramic Membrane: Fabrication and Performance Optimization
Abstract
1. Introduction
2. Experiment
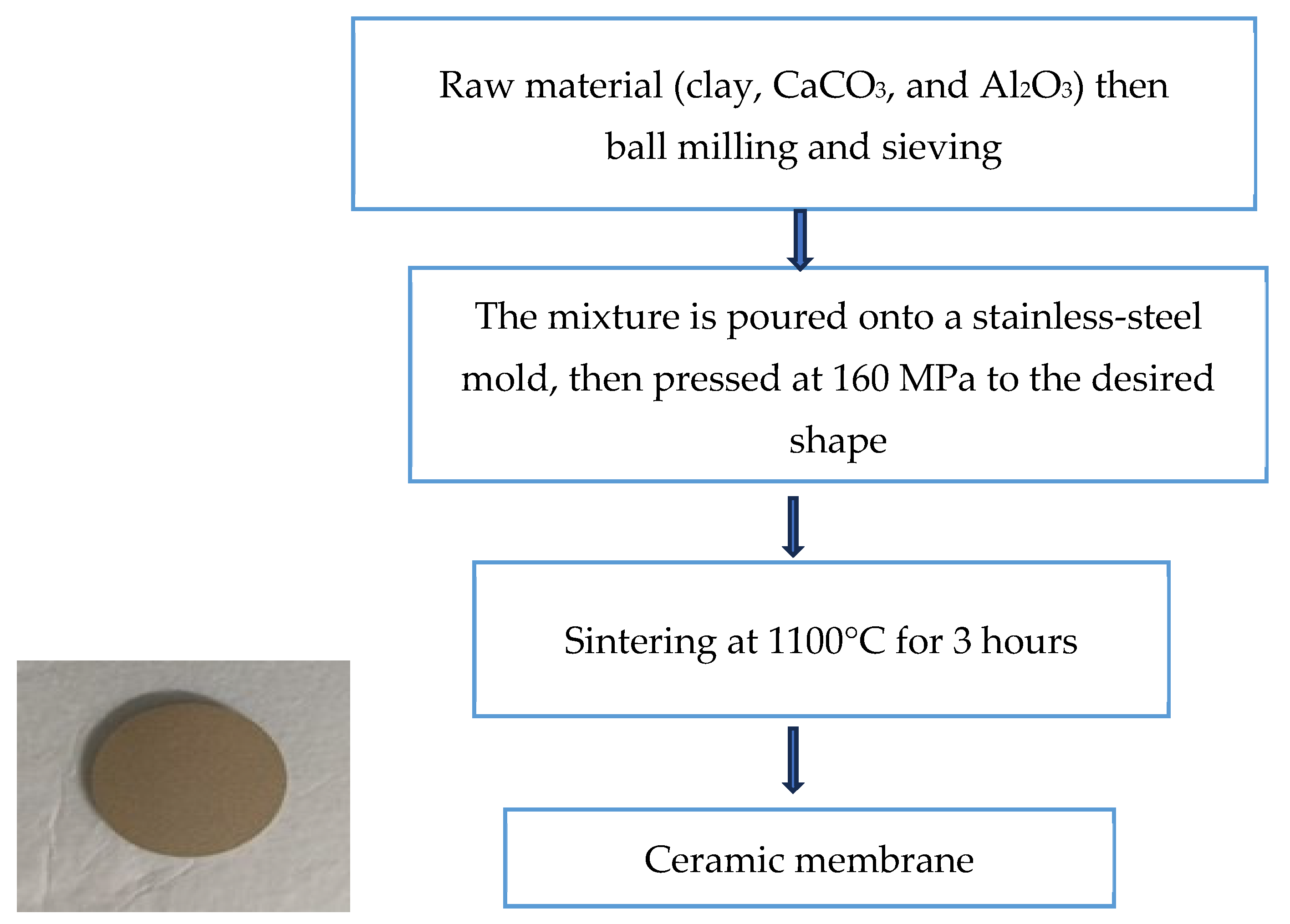
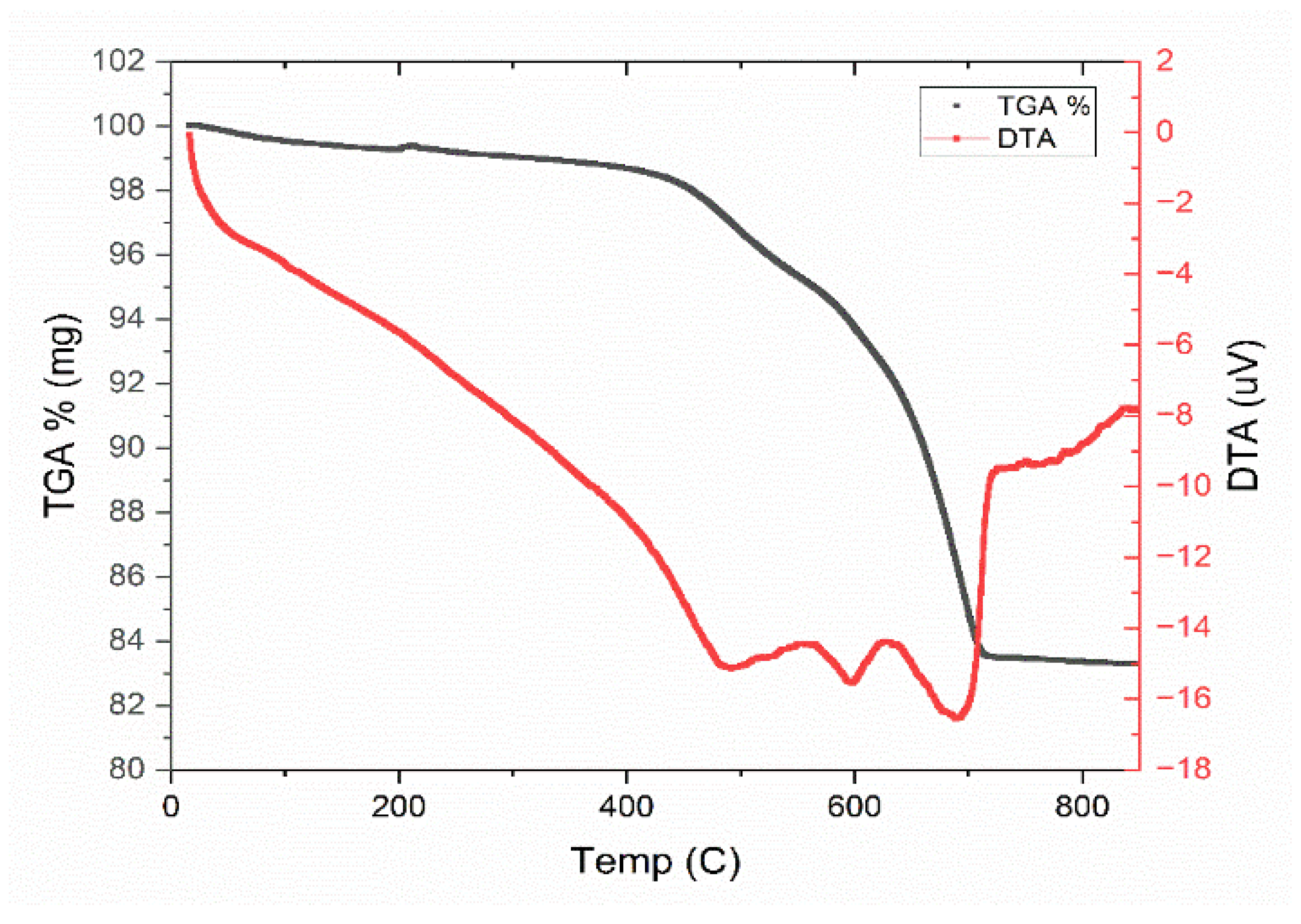
2.1. Membrane Material and Preparation of the Flat Composite
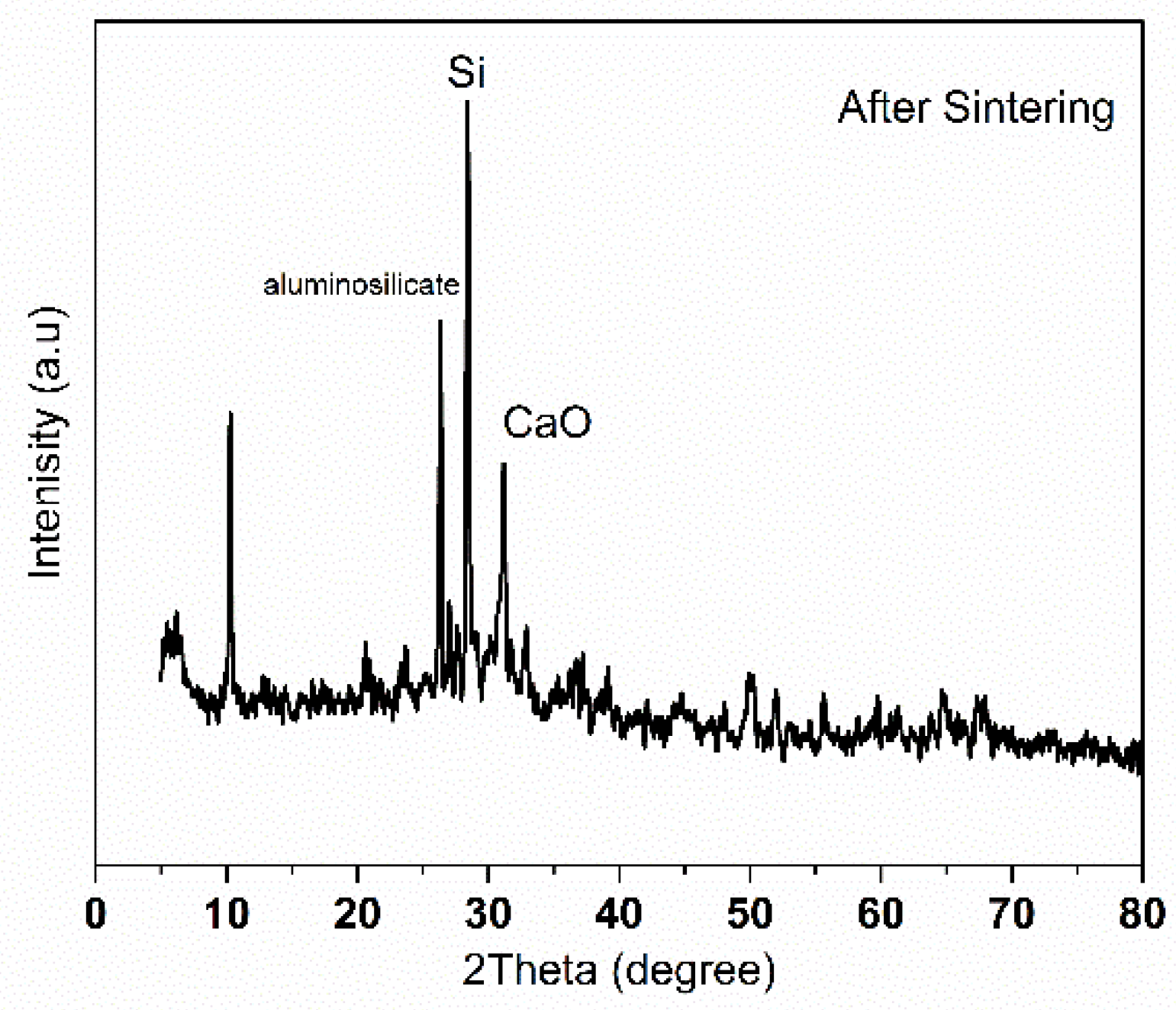
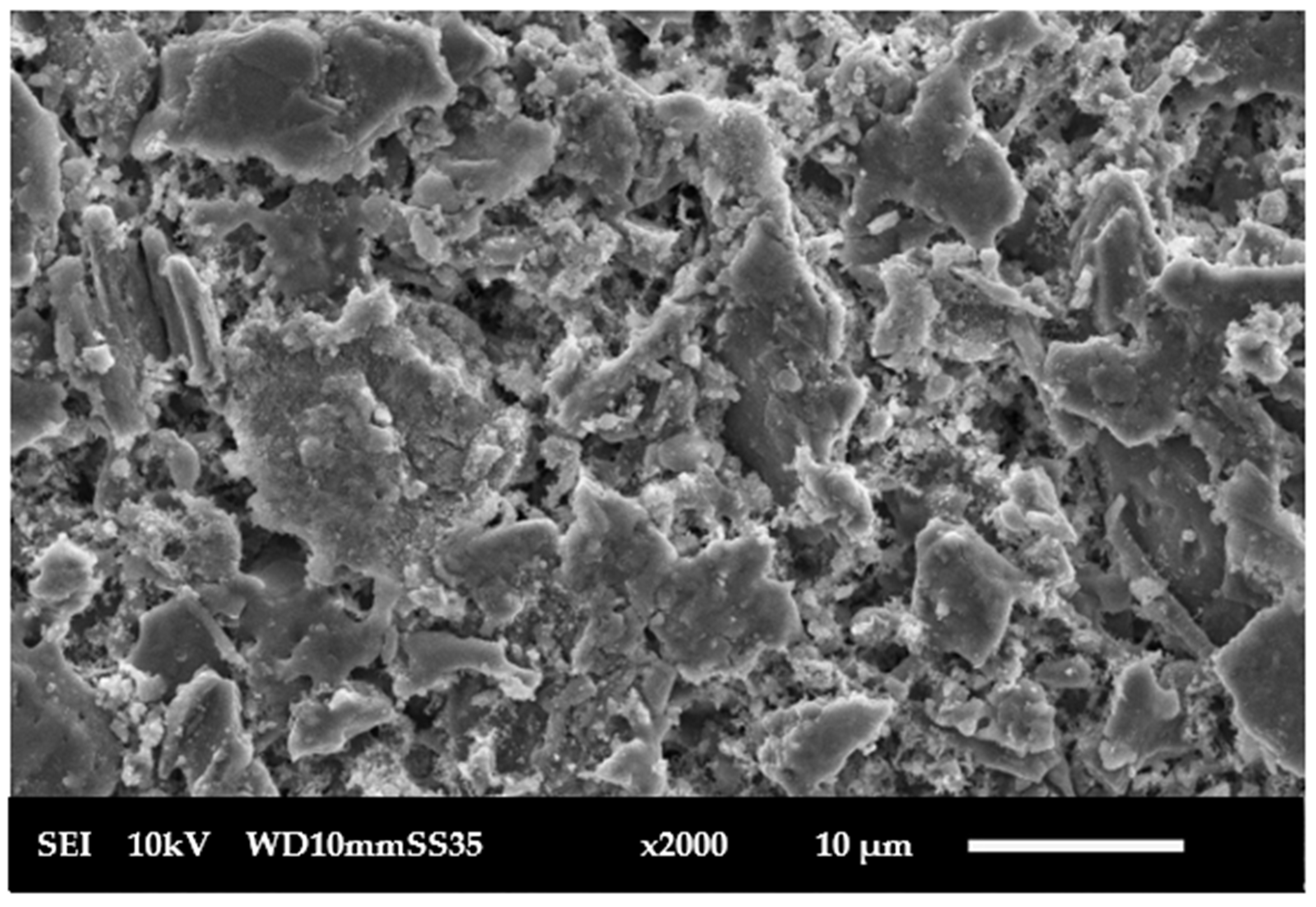
2.2. Ceramic Membrane Characterization
- Wwet: The weight of soaked membrane after 24 h;
- Wdry: The weight of the original membrane;
- : Density of water = 1000 kg/m3;
- V: Volume of membrane ().
2.3. Experimental Procedure
3. Results
3.1. Influence of Feed Concentration
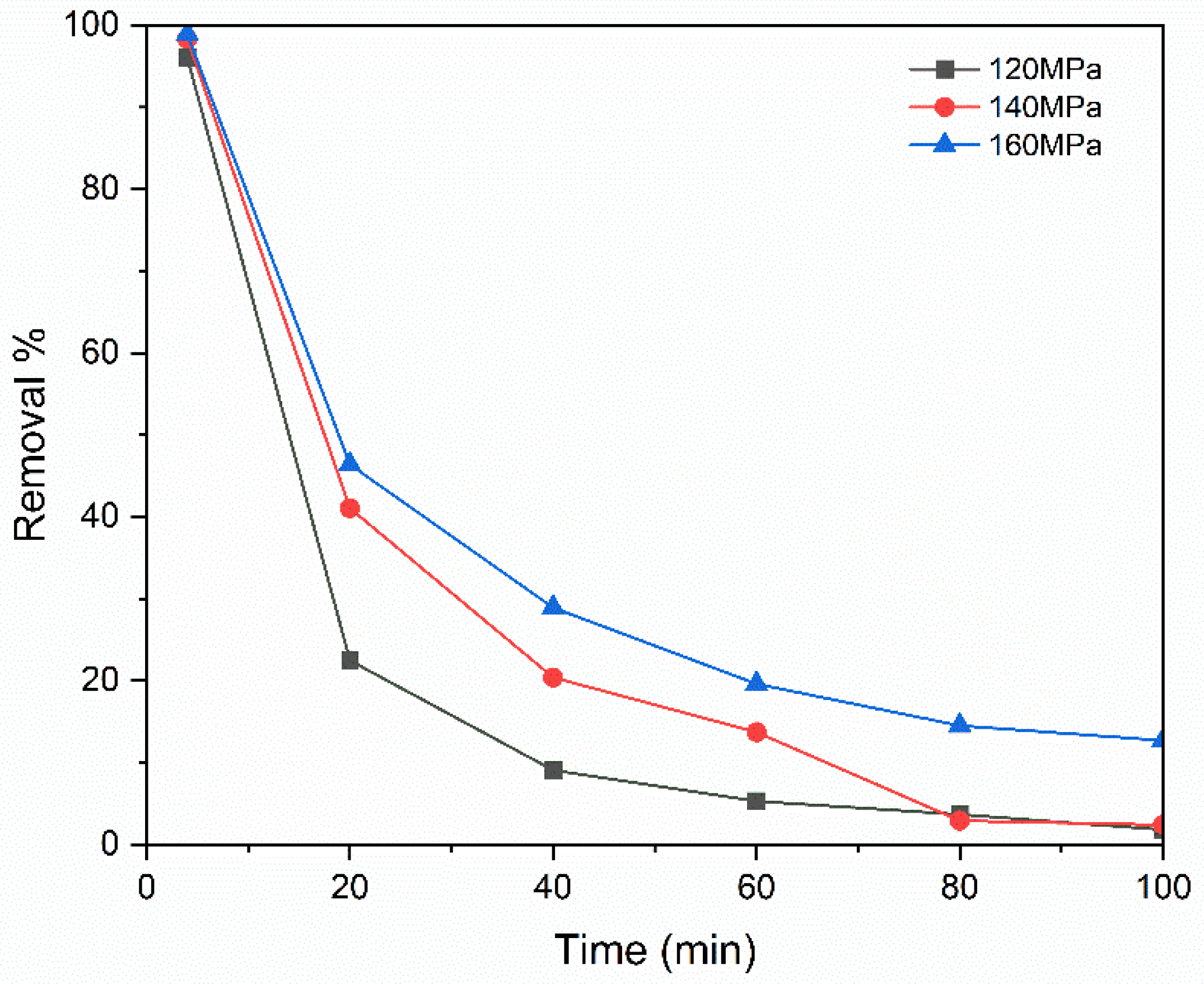
3.2. Influence of Pressing Pressure
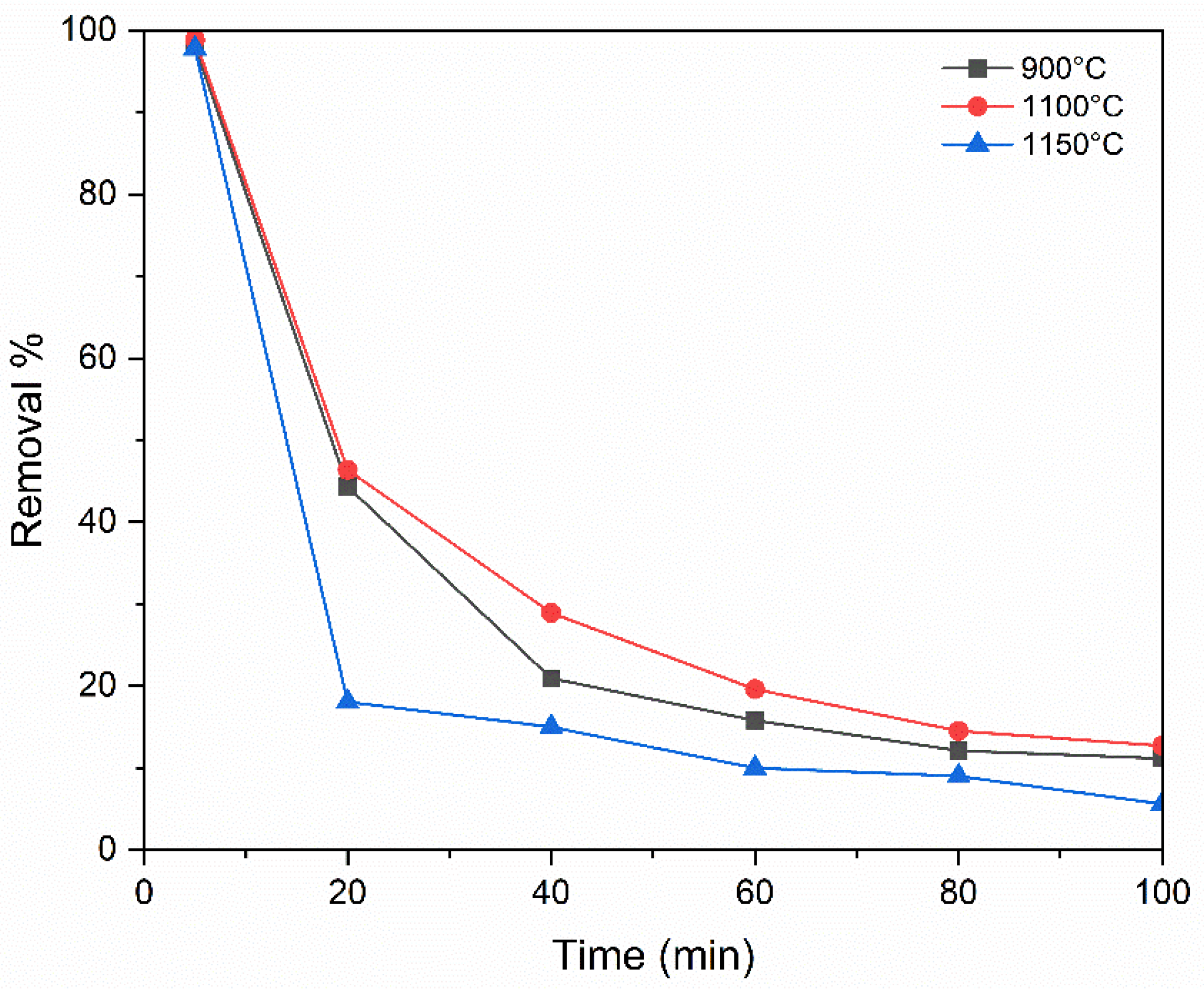
3.3. Influence of Temperature of Sintering
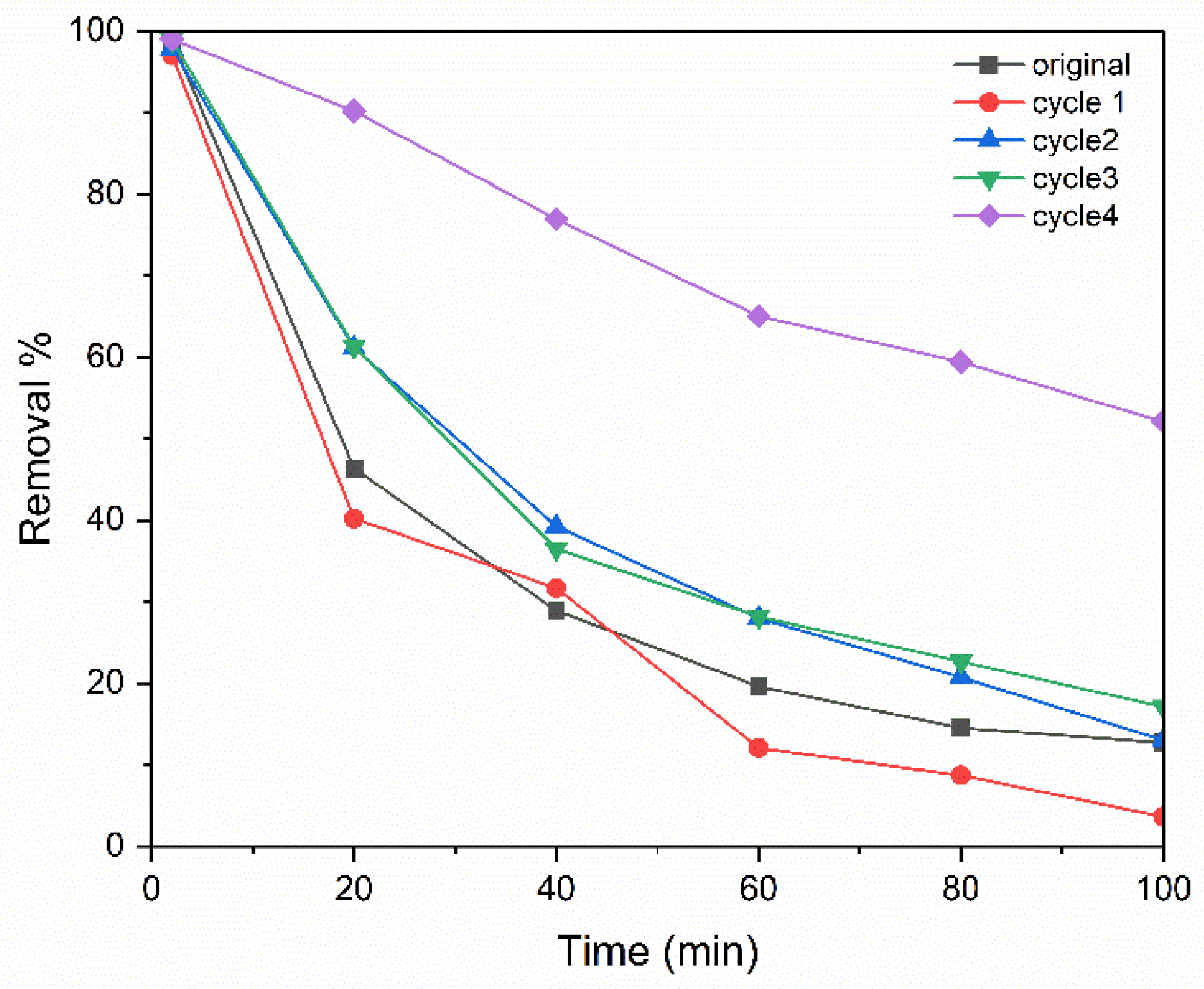
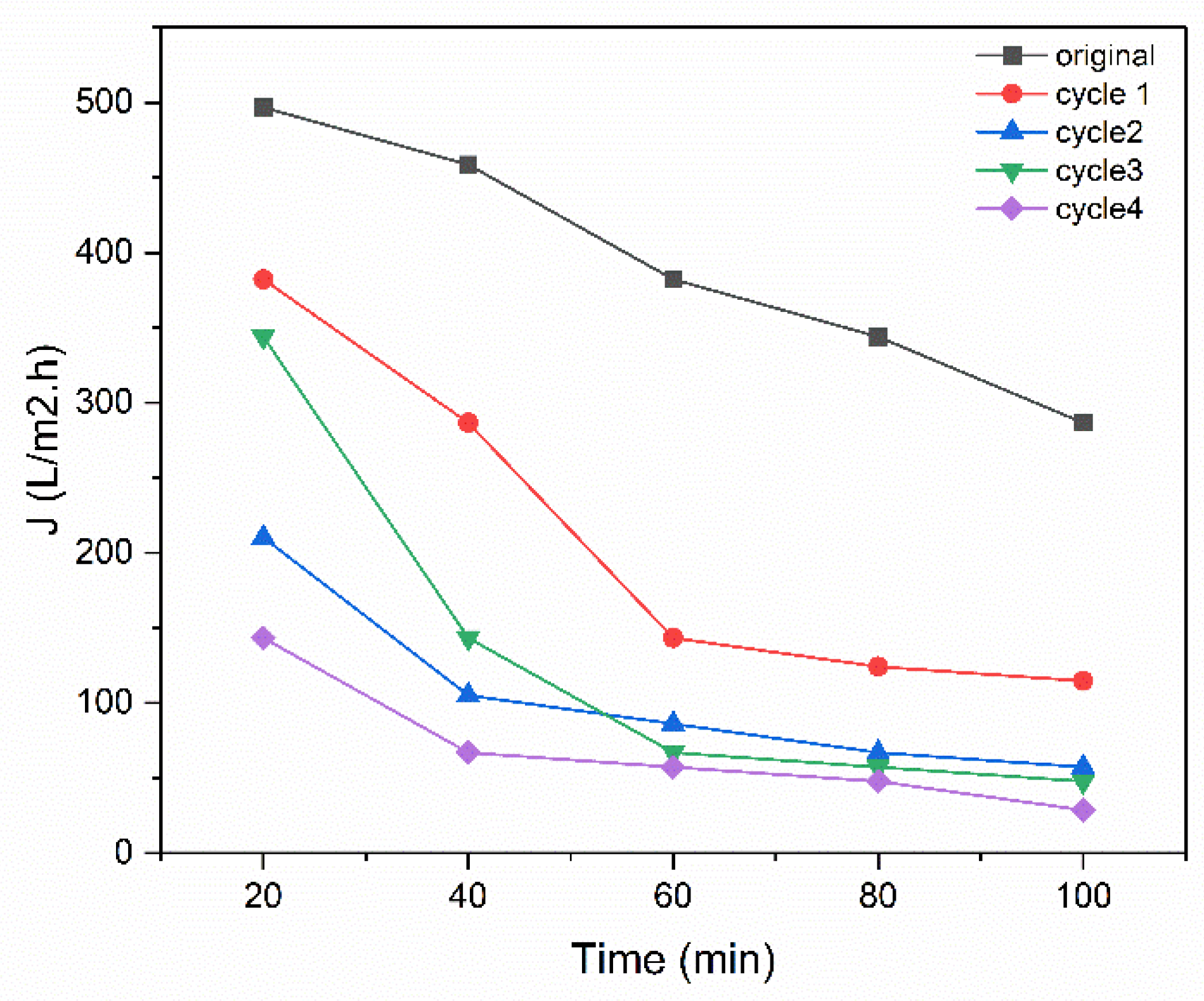
3.4. Backwash and Regeneration of the Membrane
3.5. Cost Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective Water/Wastewater Treatment Methodologies for Toxic Pollutants Removal: Processes and Applications towards Sustainable Development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, W.; Dong, X.; Wang, F.; Yang, W.; Liu, C.; Chen, D. A Review on Recent Advances of Biochar from Agricultural and Forestry Wastes: Preparation, Modification and Applications in Wastewater Treatment. J. Environ. Chem. Eng. 2024, 12, 111638. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A Review on Industrial Wastewater Treatment via Electrocoagulation Processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Ahtasham Iqbal, M.; Akram, S.; Khalid, S.; Lal, B.; Hassan, S.U.; Ashraf, R.; Kezembayeva, G.; Mushtaq, M.; Chinibayeva, N.; Hosseini-Bandegharaei, A. Advanced Photocatalysis as a Viable and Sustainable Wastewater Treatment Process: A Comprehensive Review. Environ. Res. 2024, 253, 118947. [Google Scholar] [CrossRef]
- Shehata, N.; Egirani, D.; Olabi, A.G.; Inayat, A.; Abdelkareem, M.A.; Chae, K.J.; Sayed, E.T. Membrane-Based Water and Wastewater Treatment Technologies: Issues, Current Trends, Challenges, and Role in Achieving Sustainable Development Goals, and Circular Economy. Chemosphere 2023, 320, 137993. [Google Scholar] [CrossRef] [PubMed]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Kamble, A.R.; Patel, C.M.; Murthy, Z.V.P. A Review on the Recent Advances in Mixed Matrix Membranes for Gas Separation Processes. Renew. Sustain. Energy Rev. 2021, 145, 111062. [Google Scholar] [CrossRef]
- Ma, R.; Li, J.; Zeng, P.; Duan, L.; Dong, J.; Ma, Y.; Yang, L. The Application of Membrane Separation Technology in the Pharmaceutical Industry. Membranes 2024, 14, 24. [Google Scholar] [CrossRef]
- Venzke, C.D.; Giacobbo, A.; Klauck, C.R.; Viegas, C.; Hansen, E.; De Aquim, P.M.; Rodrigues, M.A.S.; Bernardes, A.M. Integrated Membrane Processes (EDR-RO) for Water Reuse in the Petrochemical Industry. J. Membr. Sci. Res. 2018, 4, 218–226. [Google Scholar] [CrossRef]
- Sagle, A.; Freeman, B. Fundamentals of Membranes for Water Treatment; University of Texas at Austin: Austin, TX, USA, 2004. [Google Scholar]
- Abdel-Shafy, H.I.; Abdel-Shafy, S.H. Membrane Technology for Water and Wastewater Management and Application in Egypt. Egypt. J. Chem. 2017, 60, 347–360. [Google Scholar] [CrossRef]
- Pandey, L.M.; Hasan, A. Nanoscale Engineering of Biomaterials: Properties and Applications; Springer Nature: Singapore, 2022; ISBN 9789811636677. [Google Scholar]
- Souza, V.C.; Quadri, M.G.N. Organic-Inorganic Hybrid Membranes in Separation Processes: A 10-Year Review. Braz. J. Chem. Eng. 2013, 30, 683–700. [Google Scholar] [CrossRef]
- Al-Anzi, B.S.; Siang, O.C. Recent Developments of Carbon Based Nanomaterials and Membranes for Oily Wastewater Treatment. RSC Adv. 2017, 7, 20981–20994. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, R.; Chen, H.; Zhou, B.; Cui, Z.; Zhu, B. Advancements in Inorganic Membrane Filtration Coupled with Advanced Oxidation Processes for Wastewater Treatment. Molecules 2024, 29, 4267. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Nanoporous Graphene as a Reverse Osmosis Membrane: Recent Insights from Theory and Simulation. Desalination 2015, 366, 59–70. [Google Scholar] [CrossRef]
- Sandhya Rani, S.L.; Kumar, R.V. Insights on Applications of Low-Cost Ceramic Membranes in Wastewater Treatment: A Mini-Review. Case Stud. Chem. Environ. Eng. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Chen, M.; Heijman, S.G.J.; Rietveld, L.C. Ceramic Membrane Filtration for Oily Wastewater Treatment: Basics, Membrane Fouling and Fouling Control. Desalination 2024, 583, 117727. [Google Scholar] [CrossRef]
- Ezaier, Y.; Hader, A.; Latif, A.; Khan, M.E.; Ali, W.; Ali, S.K.; Bashiri, A.H.; Zakri, W.; Yusuf, M.; Rajamohan, N.; et al. Solving the Fouling Mechanisms in Composite Membranes for Water Purification: An Advance Approach. Environ. Res. 2024, 250, 118487. [Google Scholar] [CrossRef] [PubMed]
- Merouani, S.; Hamdaoui, O. Sonophotocatalytic Degradation of Refractory Textile Dyes. Photocatalytic Degrad. Dye. Curr. Trends Future Perspect. 2021, 227, 111–140. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of Membrane Filtration and Advanced Oxidation Processes for Removal of Pharmaceutical Residues: A Critical Review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Li, Q.; Kong, H.; Li, P.; Shao, J.; He, Y. Photo-Fenton Degradation of Amoxicillin via Magnetic TiO2-Graphene Oxide-Fe3O4 Composite with a Submerged Magnetic Separation Membrane Photocatalytic Reactor (SMSMPR). J. Hazard. Mater. 2019, 373, 437–446. [Google Scholar] [CrossRef]
- Liang, D.; Huang, J.; Zhang, H.; Fu, H.; Zhang, Y.; Chen, H. Influencing Factors on the Performance of Tubular Ceramic Membrane Supports Prepared by Extrusion. Ceram. Int. 2021, 47, 10464–10477. [Google Scholar] [CrossRef]
- Ma, J.; Chen, W.; Qian, J.; Shui, A.; Du, B.; He, C. Co-Pressing and Co-Sintering Preparation of Cost-Effective and High-Performance Asymmetric Ceramic Membrane for Oily Wastewater Treatment. Sep. Purif. Technol. 2023, 323, 124373. [Google Scholar] [CrossRef]
- Azaman, F.; Nor, M.A.A.M.; Abdullah, W.R.W.; Razali, M.H.; Adnen, N.A.I.; Zulkifli, R.C.; Ali, A. Fabrication of Natural Ball Clay Ceramic Membrane Using Pore Former and Additive Agents Based on Modified Slip Casting Technique. Desalination Water Treat. 2021, 223, 290–298. [Google Scholar] [CrossRef]
- Qin, H.; Guo, W.; Huang, X.; Gao, P.; Xiao, H. Preparation of Yttria-Stabilized ZrO2 Nanofiltration Membrane by Reverse Micelles-Mediated Sol-Gel Process and Its Application in Pesticide Wastewater Treatment. J. Eur. Ceram. Soc. 2020, 40, 145–154. [Google Scholar] [CrossRef]
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Park, S.; Kang, J.S.; Lee, J.J.; Vo, T.K.Q.; Kim, H.S. Application of Physical and Chemical Enhanced Backwashing to Reduce Membrane Fouling in the Water Treatment Process Using Ceramic Membranes. Membranes 2018, 8, 110. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of Ceramic and Polymeric Membrane Permeability and Fouling Using Surface Water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane Fouling in Desalination and Its Mitigation Strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Asadollahi, M.; Bastani, D.; Musavi, S.A. Enhancement of Surface Properties and Performance of Reverse Osmosis Membranes after Surface Modification: A Review. Desalination 2017, 420, 330–383. [Google Scholar] [CrossRef]
- Du, X.; Shi, Y.; Jegatheesan, V.; Ul Haq, I. A Review on the Mechanism, Impacts and Control Methods of Membrane Fouling in MBR System. Membranes 2020, 10, 24. [Google Scholar] [CrossRef]
- Li, S.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Verliefde, A.R.D.; Kemperman, A.J.B.; van Dijk, J.C.; Amy, G. Impact of Backwash Water Composition on Ultrafiltration Fouling Control. J. Memb. Sci. 2009, 344, 17–25. [Google Scholar] [CrossRef]
- Chang, H.; Liang, H.; Qu, F.; Ma, J.; Ren, N.; Li, G. Towards a Better Hydraulic Cleaning Strategy for Ultrafiltration Membrane Fouling by Humic Acid: Effect of Backwash Water Composition. J. Environ. Sci. 2016, 43, 177–186. [Google Scholar] [CrossRef]
- Heller-Kallai, L. Chapter 7.2 Thermally Modified Clay Minerals. Dev. Clay Sci. 2006, 1, 289–308. [Google Scholar] [CrossRef]
- do Carmo, E.S.; Silva, W.L.; dos Santos Barbosa, A.; Rodrigues, M.G.F. Low-Cost Ceramic Membrane Production for Dye Removal. Desalination Water Treat. 2024, 320, 100726. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Li, J.; Wang, W.; Zhao, H.; Li, Y.; Luo, J. Sintering Conversion to Porous Diopside Ceramic from Geopolymer Fabricated by Iron Tailings and Steel Slag. Ceram. Int. 2025, 51, 15687–15698. [Google Scholar] [CrossRef]
- Kumar, C.M.; Roshni, M.; Vasanth, D. Treatment of Aqueous Bacterial Solution Using Ceramic Membrane Prepared from Cheaper Clays: A Detailed Investigation of Fouling and Cleaning. J. Water Process Eng. 2019, 29, 100797. [Google Scholar] [CrossRef]
- Khebli, Z.; Bouzerara, F.; Brihi, N.; Figoli, A.; Russo, F.; Galiano, F.; Chahredine, S. Fabrication of a Zircon Microfiltration Membrane for Culture Medium Sterilization. Membranes 2023, 13, 399. [Google Scholar] [CrossRef]
- Mouiya, M.; Bouazizi, A.; Abourriche, A.; El Khessaimi, Y.; Benhammou, A.; El hafiane, Y.; Taha, Y.; Oumam, M.; Abouliatim, Y.; Smith, A.; et al. Effect of Sintering Temperature on the Microstructure and Mechanical Behavior of Porous Ceramics Made from Clay and Banana Peel Powder. Results Mater. 2019, 4, 100028. [Google Scholar] [CrossRef]
- Manni, A.; Achiou, B.; Karim, A.; Harrati, A.; Sadik, C.; Ouammou, M.; Alami Younssi, S.; El Bouari, A. New Low-Cost Ceramic Microfiltration Membrane Made from Natural Magnesite for Industrial Wastewater Treatment. J. Environ. Chem. Eng. 2020, 8, 103906. [Google Scholar] [CrossRef]
- de Oliveira Henriques, J.D.; Pedrassani, M.W.; Klitzke, W.; Mariano, A.B.; Vargas, J.V.C.; Vieira, R.B. Thermal Treatment of Clay-Based Ceramic Membranes for Microfiltration of Acutodesmus Obliquus. Appl. Clay Sci. 2017, 150, 217–224. [Google Scholar] [CrossRef]
- Zhuang, D.; Chen, Z.; Sun, B. Thermal Decomposition of Calcium Carbonate at Multiple Heating Rates in Different Atmospheres Using the Techniques of TG, DTG, and DSC. Crystals 2025, 15, 108. [Google Scholar] [CrossRef]
- Abdelhamid, A.E.; Elsayed, A.E.; Naguib, M.; Ali, E.A.B. Effective Dye Removal by Acrylic-Based Membrane Constructed from Textile Fibers Waste. Fibers Polym. 2023, 24, 2391–2399. [Google Scholar] [CrossRef]
- Zrelli, A.; Bessadok, A.J.; Alsalhy, Q. Important Parameters of Ceramic Membranes Derived from Oasis Waste and Its Application for Car Wash Wastewater Treatment. J. Membr. Sci. Res. 2022, 8, e247250. [Google Scholar] [CrossRef]
- Jalaluddin, M.L.; Azlan, U.A.A.; Abd Rashid, M.W.; Tamin, N.; Masri, M.N.; Muchlis, M. Effect of Forming Pressure on the Microstructure and Mechanical Characteristics of Dense Porous Ceramics. J. Adv. Res. Micro Nano Eng. 2025, 30, 115–129. [Google Scholar] [CrossRef]
- Simão, L.; Montedo, O.R.K.; Da Silva Paula, M.M.; Da Silva, L.; Caldato, R.F.; De Mello Innocentini, M.D. Structural and Fluid Dynamic Characterization of Calcium Carbonate-Based Porous Ceramics. Mater. Res. 2013, 16, 1439–1448. [Google Scholar] [CrossRef]
- Hu, L.F.; Wang, C.A. Effect of Sintering Temperature on Compressive Strength of Porous Yttria-Stabilized Zirconia Ceramics. Ceram. Int. 2010, 36, 1697–1701. [Google Scholar] [CrossRef]
- Rahim, F.A.M.; Noh, M.Z.; Rashid, M.W.A.; Mohamed, J.J.; Nor, M.A.A.M. Preparation and Characterization of Ceramic Membrane by Using Palm Fibers as Pore Forming Agent. AIP Conf. Proc. 2019, 2068, 020056. [Google Scholar] [CrossRef]
- Huang, C.; Yuan, N.; He, X.; Wang, C. Ceramsite Made from Drinking Water Treatment Residue for Water Treatment: A Critical Review in Association with Typical Ceramsite Making. J. Environ. Manag. 2023, 328, 117000. [Google Scholar] [CrossRef]
- Bala, J.; Patel, J. Swelling and Shrinkage Phenomena in Soils; Inderprastha Institute of Professional Studies (IIP): New Delhi, India, 2022; Volume 2, ISBN 978-93-5747-854-0. [Google Scholar]
- Barthelemy, S.; Bonan, B.; Tomas-Burguera, M.; Grandjean, G.; Bernardie, S.; Naulin, J.P.; Le Moigne, P.; Boone, A.; Calvet, J.C. Analysis of Past and Future Droughts Causing Clay Shrinkage in France. Hydrol. Earth Syst. Sci. 2025, 29, 2321–2337. [Google Scholar] [CrossRef]
- Nait-Ali, B.; Alzina, A.; Lauro, N.; Smith, D.S. Perspectives in Drying of Ceramics. Open Ceram. 2024, 17, 100554. [Google Scholar] [CrossRef]
- Li, H.; Chen, V. Membrane Fouling and Cleaning in Food and Bioprocessing. In Membrane Technology: A Practical Guide to Membrane Technology and Applications in Food and Bioprocessing; Elsevier: Amsterdam, The Netherlands, 2010; pp. 213–254. [Google Scholar] [CrossRef]
- Wang, R.; Zou, Y.; Remsing, R.C.; Ross, N.O.; Klein, M.L.; Carnevale, V.; Borguet, E. Superhydrophilicity of α-Alumina Surfaces Results from Tight Binding of Interfacial Waters to Specific Aluminols. J. Colloid. Interface Sci. 2022, 628, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, P.; Ramo, A.; Quílez, J.; Bordes Mdel, C.; Mestre, S.; Sánchez, E.; Peña, J.Á.; Menéndez, M. Low-Cost Ceramic Membrane Bioreactor: Effect of Backwashing, Relaxation and Aeration on Fouling. Protozoa and Bacteria Removal. Chemosphere 2022, 306, 135587. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Sun, Y.; Hu, Z.; Zhao, S.; Wang, X.; Li, W.; Xi, X.; Zhang, J.; Pei, Z. Influence of Various Operating Conditions on Cleaning Efficiency in Sequencing Batch Reactor (SBR) Activated Sludge Process. Part II: Backwash and Water Rinsing Introduced Membrane Filtration Process. Desalination 2011, 272, 76–84. [Google Scholar] [CrossRef]
- Issaoui, M.; Limousy, L. Low-Cost Ceramic Membranes: Synthesis, Classifications, and Applications. Comptes Rendus Chim. 2019, 22, 175–187. [Google Scholar] [CrossRef]
- Almasri, D.; Manawi, Y.; Makki, S.; Tasneem, N.; Simson, S.; Abdel-Hadi, I.; Agcaoili, J.; Lawler, J.; Kochkodan, V. A Novel, Low-Cost Clay Ceramic Membrane for the Separation of Oil-Water Emulsions. Sci. Rep. 2025, 15, 14457. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Pugazhenthi, G.; Uppaluri, R. Fly Ash Based Ceramic Microfiltration Membranes for Oil-Water Emulsion Treatment: Parametric Optimization Using Response Surface Methodology. J. Water Process Eng. 2016, 13, 27–43. [Google Scholar] [CrossRef]


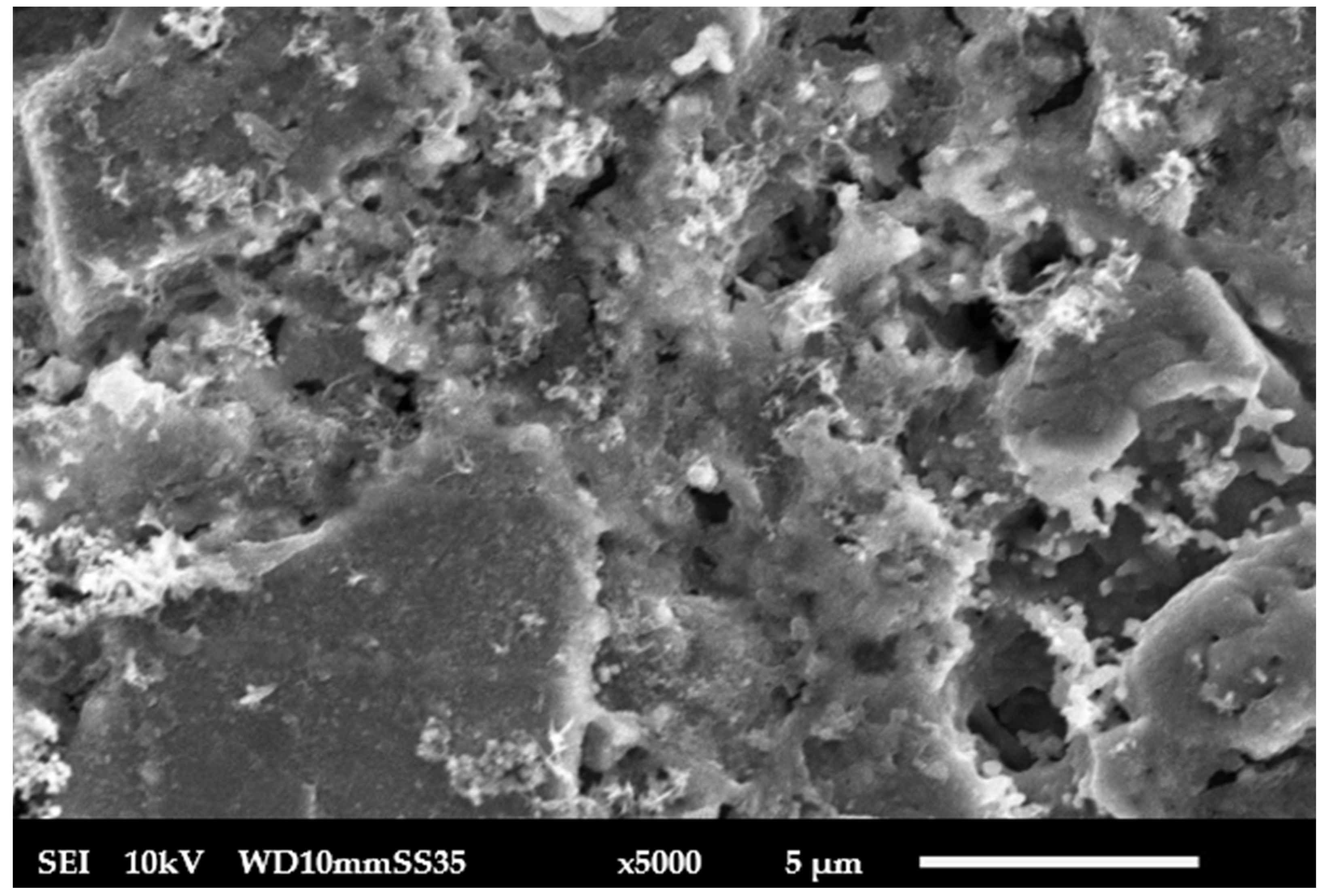
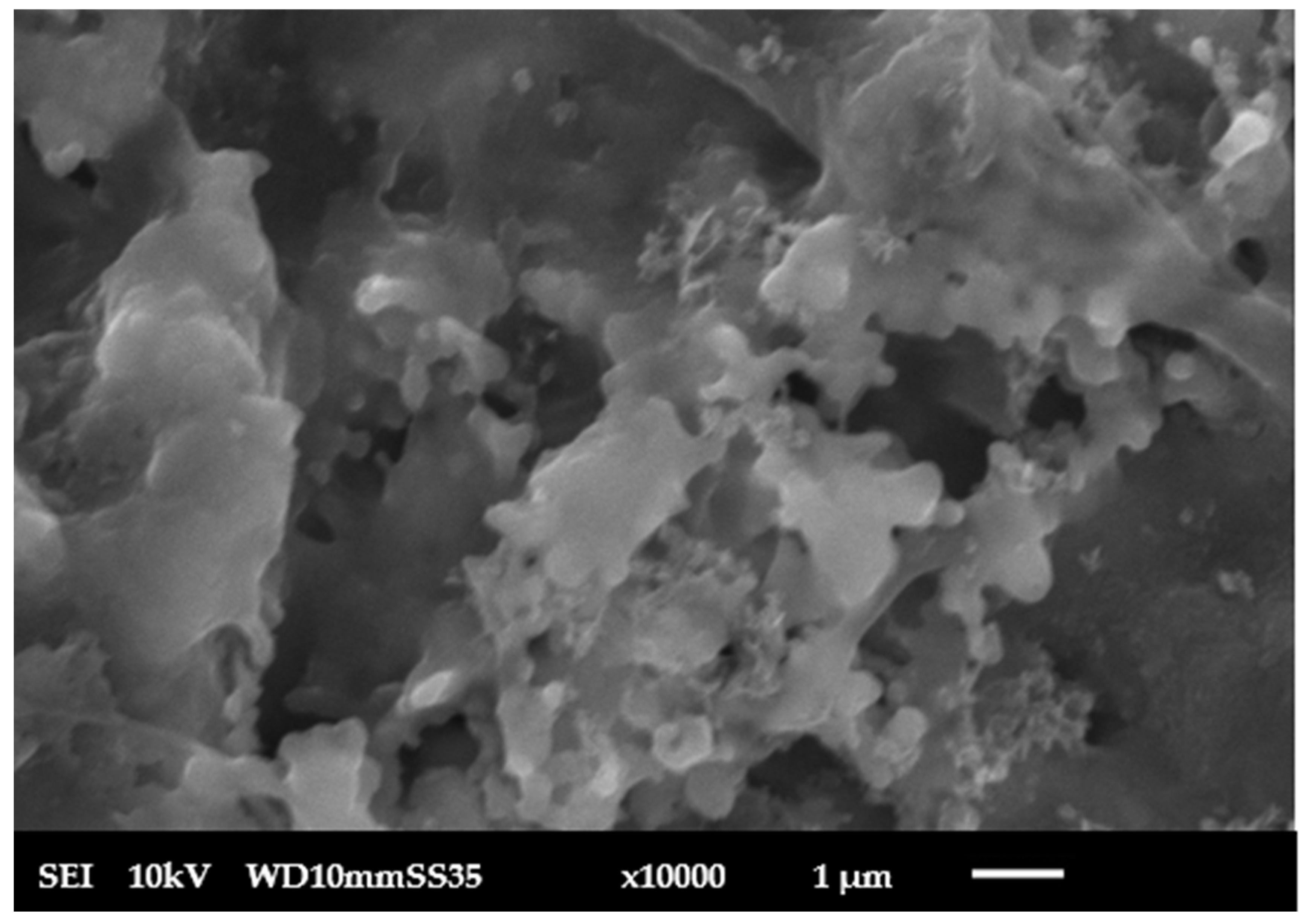




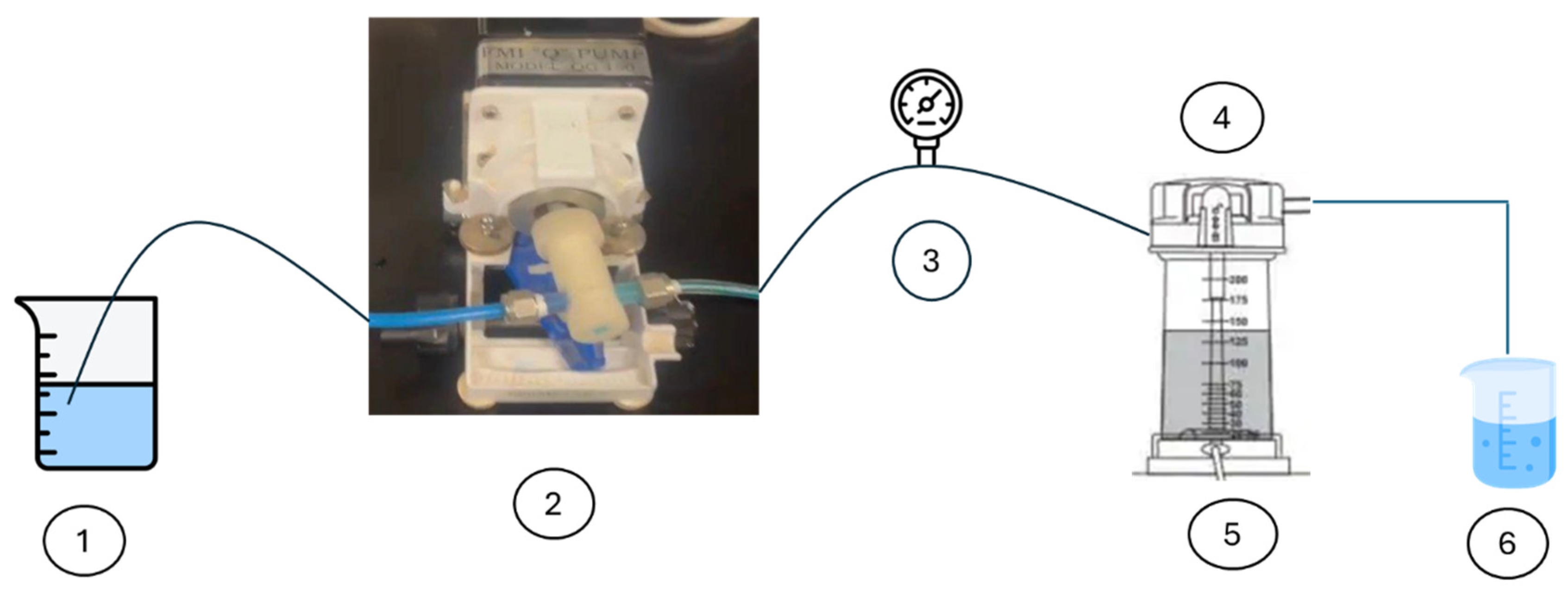
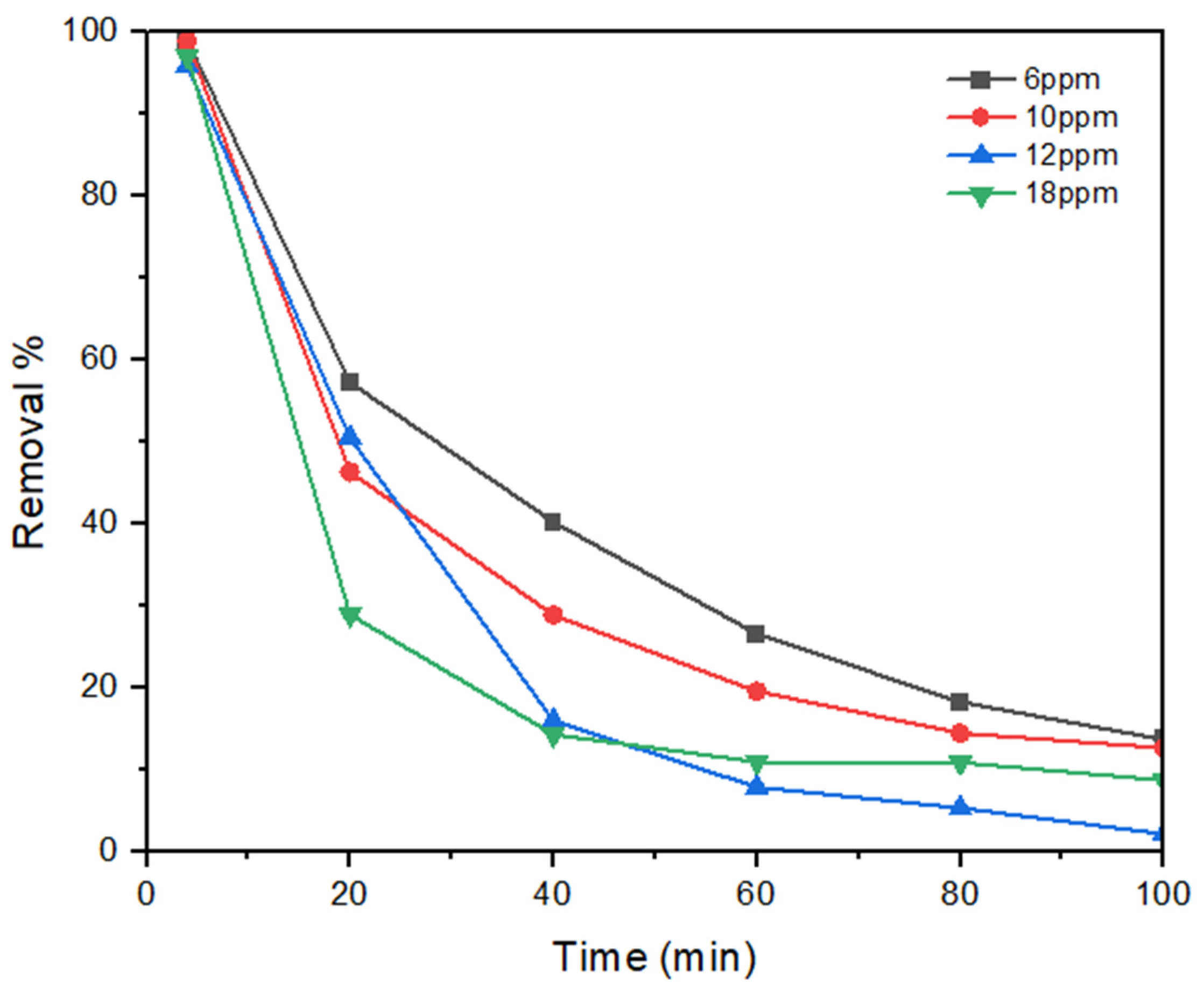
| Parameter | Value |
|---|---|
| Chemical formula | C16H18CiN3S4·H2O |
| Molecular weight (g/mol) | 319.85 |
| Absorption maxima (nm) | 664 |
| Sintering Temperature °C | Porosity% |
|---|---|
| 900 | 39.8 |
| 1100 | 38.7 |
| 1150 | 37.1 |
| Material of Fabrication | Effect of Backwash on Efficiency | Reference |
|---|---|---|
| Ceramic membrane of clay, calcium carbonate, and alumina | Increased by washing | This work |
| Ceramic of clay, chamotte, calcium carbonate, and potato starch | Remained unchanged after the first backwash and then declined from 90% to 82% after four cycles | [56] |
| Hydrophilic PES membranes | Gradual decline in efficiency with each filtration cycle | [57] |
| Raw Material of Fabrication | Total Cost of Production | Reference |
|---|---|---|
| Clay, calcium carbonate, and alumina | 170 USD/m2 | This work |
| Flay ash, quartz, and calcium carbonate | 250 USD/m2 | [60] |
| Chocobofe clay, kaolin, magnesite concentrate, and starch | 233.55 USD/m2 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shazly, A.H.; Fahmy, Y.A. Cost-Effective and Durable Ceramic Membrane: Fabrication and Performance Optimization. Membranes 2025, 15, 307. https://doi.org/10.3390/membranes15100307
El-Shazly AH, Fahmy YA. Cost-Effective and Durable Ceramic Membrane: Fabrication and Performance Optimization. Membranes. 2025; 15(10):307. https://doi.org/10.3390/membranes15100307
Chicago/Turabian StyleEl-Shazly, Ahmed H., and Yomna A. Fahmy. 2025. "Cost-Effective and Durable Ceramic Membrane: Fabrication and Performance Optimization" Membranes 15, no. 10: 307. https://doi.org/10.3390/membranes15100307
APA StyleEl-Shazly, A. H., & Fahmy, Y. A. (2025). Cost-Effective and Durable Ceramic Membrane: Fabrication and Performance Optimization. Membranes, 15(10), 307. https://doi.org/10.3390/membranes15100307





