Cellulose-Based Ion Exchange Membranes for Electrochemical Energy Systems: A Review
Abstract
1. Introduction
2. Overview of Strategies for the Fabrication of Cellulose-Based Membranes
2.1. Fabrication Strategies for Producing Cellulose-Based Membranes
2.2. Modification Techniques for Fabrication of Cellulose-Based Membranes
3. Overview of Cellulose-Based Membranes for Electrochemical Energy Systems
3.1. Various Cellulose Structures for the Fabrication of Cellulose-Based Membranes
3.2. Classes of Cellulose-Based Membranes
4. Electrochemical Energy Conversion Systems
5. Energy Storage Systems
6. Cellulose-Based Membranes for Fuel Cells
6.1. Proton Exchange Membranes for Proton Exchange Membrane Fuel Cells
6.2. Proton Exchange Membranes for DMFC
6.3. Anion Exchange Membranes for AEMFC
7. Batteries
7.1. Lithium-Ion Batteries
7.2. Vanadium Redox Flow Batteries
8. Supercapacitors
9. Reverse Electrodialysis
10. Challenges and Recommendations
11. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEM | Anion exchange membrane |
| AEMFC | Anion exchange membrane fuel cell |
| AEMWE | AEM water electrolyzer |
| ASC | All-nanofiber asymmetric supercapacitor |
| ATRP | Atom-transfer radical polymerization |
| BC | Bacterial cellulose |
| BC-CMC | Negatively charged carbomethyl bacterial cellulose membranes |
| BC-HACC | Positively charged chitosan quaternary ammonium bacterial cellulose membrane |
| BNC | Bacterial nanocellulose |
| BTCA CA | Butane 1,2,3,4 tetracarboxylic acid Cellulose acetate |
| CCM | Cotton cellulose membrane |
| CDCA | Cellulose-derived carbon aerogel |
| CE | Coulombic efficiency |
| CF | Carbon fiber |
| CFA | Carbon fiber aerogel |
| CMC | Carboxymethyl cellulose |
| CNCs | Cellulose nanocrystals |
| CNFs | Cellulose nanofibrils |
| CNTs | Carbon Nanotubes |
| CNW | Cellulose nano-whisker |
| CQD | Carbon quantum dot |
| CS | Chitosan |
| DMAC | Dimethylacetamide |
| DMFC | Direct methanol fuel cell |
| EDLC | Electric double-layer capacitors |
| EE | Energy efficiency |
| EES | Electrochemical energy storage system |
| EG | Ethylene glycol |
| GN | Graphene |
| GO HPMC | Graphene oxide Hydroxypropyl methyl cellulose |
| ICM | Ion conducting membrane |
| IEM | Ion exchange membrane |
| IPA | Isopropyl alcohol |
| LCNFs | Lignin-containing Cellulose Nanofibrils |
| LDH | Layered double hydroxide |
| LIBs | Lithium-ion batteries |
| LISBs | Lithium-ion sulfate batteries |
| LiTFS–LiSMC | Lithium tri-fluoromethanesulfonate-methyl cellulose |
| LS | Lignosulfonates |
| LS | Lignosulphonate |
| MBAA | N N′-methylenebisacrylamide |
| MC | Methylcellulose |
| MCC | Microcrystalline cellulose |
| MFC | Microbial fuel cell |
| MMA | Methyl methacrylate |
| MOF | Metal organic frameworks |
| MWCNT | Multi wall carbon nanotube |
| NaCMC | Sodium caboxymethylcellulose |
| NBC | Negatively charged bacterial cellulose |
| NCC | Nanocrystalline cellulose |
| NMMO | N-methylmorpholine n-oxide |
| NSHPAs | N, S dual-doped hierarchical porous carbon aerogels |
| PAA | Poly(acrylic acid) |
| PAEK | Poly(aryl ether ketone) |
| PANI | Polyaniline |
| PBC | Positively charged bacterial cellulose |
| PBI | Polybenzimidazole |
| PDA | Polydopamine |
| PDADMAC | Poly (diallyldimethylammonium chloride) |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEEK | Poly(ether ether ketone) |
| PEM | Polymer electrolyte membranes |
| PEMFC | Proton exchange membrane fuel cell |
| PEMWE | Poron exchange membrane water electrolyzer |
| PEO | Poly(ethylene oxide) |
| PET | Polyethylene terephthalate |
| PF | Phenol formaldehyde |
| PFSA | Perfluorosulfonic acid |
| pHEMA | Poly(2-Hydroxyethyl Methacrylate) |
| PMACC | Poly(methacroylcholine chloride) (pmacc) |
| PMOEP | Poly(methacryloyloxyethyl phosphate) |
| PNIMPAM | Poly(N-isopropylacrylamide) |
| Ppy | Polypyrrole |
| PRO | Pressure retarded osmosis |
| PSS | Polystyrene sulfonate |
| PSSA | 4-polystyrene sulfonic acid |
| PTA | Phosphotungstic acid |
| PVA | Polyvinyl alcohol |
| PVDF | Poly(vynilidene fluoride) |
| QPPO | Quaternized poly(phenylene oxide) |
| RDP | Bis(diphenyl phosphate) |
| RED | Reverse electrodialysis |
| RFB | Redox flow battery |
| rGO | Reduced graphene oxide |
| RH | Relative humidity |
| SA | Sulfosuccinic acid |
| SA | Sulfonic acid |
| SEC | Sulfo ethyl cellulose |
| SHP | Sodium hypophosphite |
| SiO2 | Silicone dioxide |
| SPEEK | Sulfonated poly(ether ether ketone) |
| SPEI SPSF | Sulfonated poly(ether imide) Sulfonated polysulfone |
| SPES | Sulfonated poly (ether sulfone) |
| TiO2 | Titanium dioxide |
| TOCNF | TEMPO-oxidized cellulose nanofibers |
| VE | Voltage efficiency |
| VGCF | Vapor hrown carbon fiber |
| VRFB | Vanadium redox flow battery |
| ZIF-67 | Zeolitic imidazolate framework |
References
- Li, S.; Zhu, W.; Tang, Q.; Huang, Z.; Yu, P.; Gui, X.; Lin, S.; Hu, J.; Tu, Y. Mini Review on Cellulose-Based Composite Separators for Lithium-Ion Batteries: Recent Progress and Perspectives. Energy Fuels 2021, 35, 12938–12947. [Google Scholar] [CrossRef]
- Sun, Z.; Qu, K.; You, Y.; Huang, Z.; Liu, S.; Li, J.; Hu, Q.; Guo, Z. Overview of cellulose-based flexible materials for supercapacitors. J. Mater. Chem. A 2021, 9, 7278–7300. [Google Scholar] [CrossRef]
- Thangarasu, S.; Oh, T.H. Recent Developments on Bioinspired Cellulose Containing Polymer Nanocomposite Cation and Anion Exchange Membranes for Fuel Cells (PEMFC and AFC). Polymers 2022, 14, 5248. [Google Scholar] [CrossRef] [PubMed]
- Paixão da Costa, G.; Garcia, D.M.E.; Van Nguyen, T.H.; Lacharmoise, P.; Simão, C.D. Advancements in printed components for proton exchange membrane fuel cells: A comprehensive review. Int. J. Hydrogen Energy 2024, 69, 710–728. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Calle-Gil, R.; Castillo-Martínez, E.; Carretero-González, J. Cellulose Nanocrystals in Sustainable Energy Systems. Adv. Sustain. Syst. 2022, 6, 2100395. [Google Scholar] [CrossRef]
- Bae, B.; Kim, D. Polymer Electrolyte Membranes. Membranes 2021, 11, 244. [Google Scholar] [CrossRef]
- Siekierka, A.; Smolińska-Kempisty, K.; Wolska, J. Enhanced Specific Mechanism of Separation by Polymeric Membrane Modification—A Short Review. Membranes 2021, 11, 942. [Google Scholar] [CrossRef]
- Pan, M.; Pan, C.; Li, C.; Zhao, J. A review of membranes in proton exchange membrane fuel cells: Transport phenomena, performance and durability. Renew. Sustain. Energy Rev. 2021, 141, 110771. [Google Scholar] [CrossRef]
- Junoh, H.; Jaafar, J.; Nordin, N.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Performance of Polymer Electrolyte Membrane for Direct Methanol Fuel Cell Application: Perspective on Morphological Structure. Membranes 2020, 10, 34. [Google Scholar] [CrossRef]
- Ahmad Kamaroddin, M.F.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Membrane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Iglesias van Montfort, H.-P.; Subramanian, S.; Irtem, E.; Sassenburg, M.; Li, M.; Kok, J.; Middelkoop, J.; Burdyny, T. An Advanced Guide to Assembly and Operation of CO2 Electrolyzers. ACS Energy Lett. 2023, 8, 4156–4161. [Google Scholar] [CrossRef]
- Ye, J.; Xia, L.; Li, H.; de Arquer, F.P.G.; Wang, H. The Critical Analysis of Membranes toward Sustainable and Efficient Vanadium Redox Flow Batteries. Adv. Mater. 2024, 36, e2402090. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Luk, H.M.; Tang, J.; Wu, S.; Wu, D.; Hu, Z.; Cui, D.; Zhang, J. Recent advances in non-precious metal electrocatalysts for anion exchange membrane water electrolysis: Mechanistic insights, catalyst design, and structural regulation strategies. Chem. Eng. J. 2025, 523, 168587. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Ng, W.; Wong, W.; Rosli, N.; Loh, K. Commercial Anion Exchange Membranes (AEMs) for Fuel Cell and Water Electrolyzer Applications: Performance, Durability, and Materials Advancement. Separations 2023, 10, 424. [Google Scholar] [CrossRef]
- Parnian, M.J.; Gashoul, F.; Rowshanzamir, S. Studies on the SPEEK membrane with low degree of sulfonation as a stable proton exchange membrane for fuel cell applications. Hydrog. Fuel Cell Energy Storage 2017, 3, 221–232. [Google Scholar]
- Samaniego, A.J.; Espiritu, R. Prospects on utilization of biopolymer materials for ion exchange membranes in fuel cells. Green Chem. Lett. Rev. 2022, 15, 253–275. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Liu, J.; Sun, J.; Zhang, Z.; Zhu, Q. Sustainable cellulose nanomaterials for environmental remediation—Achieving clean air, water, and energy: A review. Carbohydr. Polym. 2022, 285, 119251. [Google Scholar] [CrossRef]
- Mokhena, T.C.; John, M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2019, 27, 1149–1194. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Temane, L.T.; Ray, S.S. Nanocellulose-based conductive composites: A review of systems for electromagnetic interference shielding applications. Int. J. Biol. Macromol. 2024, 277, 133891. [Google Scholar] [CrossRef] [PubMed]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Trache, D. Nanocellulose as a promising sustainable material for biomedical applications. AIMS Mater. Sci. 2018, 5, 201–205. [Google Scholar] [CrossRef]
- Rana, R.; Ahmad, W.; Mishra, A.; Kumar, S.; Ahmad, A. Nanocellulose/nanocellulose-based membranes in wastewater treatment: A sustainable path forward for environmental protection. Food Chem. X 2025, 29, 102654. [Google Scholar] [CrossRef] [PubMed]
- Madhushree, M.; Vairavel, P.; Mahesha, G.T.; Bhat, K.S. A Comprehensive Review of Cellulose and Cellulose-Based Materials: Extraction, Modification, and Sustainable Applications. J. Nat. Fibers 2024, 21, 2418357. [Google Scholar] [CrossRef]
- Hansini, A.M.P.; Galpaya, G.; Gunasena, M.; Abeysundara, P.M.; Kirthika, V.; Bhagya, L.; Gunawardana, H.; Koswattage, K.R. From Nature to Innovation: Advances in Nanocellulose Extraction and Its Multifunctional Applications. Molecules 2025, 30, 2670. [Google Scholar] [CrossRef]
- Wang, J.; Abbas, S.C.; Li, L.; Walker, C.C.; Ni, Y.; Cai, Z. Cellulose Membranes: Synthesis and Applications for Water and Gas Separation and Purification. Membranes 2024, 14, 148. [Google Scholar] [CrossRef]
- Sharma, K.; Choudhary, P.; Majeed, A.; Guleria, S.; Kumar, M.; Rana, A.K.; Rajauria, G. Cellulose based membranes, hydrogels and aerogels for water treatment application. Ind. Crops Prod. 2025, 225, 120474. [Google Scholar] [CrossRef]
- Grzybek, P.; Dudek, G.; van der Bruggen, B. Cellulose-based films and membranes: A comprehensive review on preparation and applications. Chem. Eng. J. 2024, 495, 153500. [Google Scholar] [CrossRef]
- Padhan, B.; Ryoo, W.; Patel, M.; Dash, J.K.; Patel, R. Cutting-Edge Applications of Cellulose-Based Membranes in Drug and Organic Contaminant Removal: Recent Advances and Innovations. Polymers 2024, 16, 2938. [Google Scholar] [CrossRef]
- Qi, F.; Qi, B.; Cui, Z.; Chen, X.; Wan, Y.; Luo, J. Cellulose-based separation membranes: A sustainable evolution or fleeting trend? Adv. Membr. 2025, 5, 100153. [Google Scholar] [CrossRef]
- Duarte, P.; Pereira, S.; Cunha, I.; Pimentel, A.; Dionísio, M.; Fortunato, E.; Martins, R.; Pereira, L. Cellulose-Based Solid Electrolyte Membranes Through Microwave Assisted Regeneration and Application in Electrochromic Displays. Front. Mater. 2020, 7, 269. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Niu, S.; Wang, Q.; Wu, H.; Huang, L.; Chen, L.; Zhao, C.; Li, J. Superstable osmotic energy conversion based on strong cellulose membrane. Ind. Crops Prod. 2023, 206, 117598. [Google Scholar] [CrossRef]
- Yavuzturk Gul, B.; Pekgenc, E.; Vatanpour, V.; Koyuncu, I. A review of cellulose-based derivatives polymers in fabrication of gas separation membranes: Recent developments and challenges. Carbohydr. Polym. 2023, 321, 121296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, H.; Yi, J.; Liu, T.; Lai, H.; Zhang, S.; Huang, W.; Yin, Y.; Huang, X.; Tong, Y.; et al. Advanced cellulose-based materials for flexible energy storage systems. Resour. Chem. Mater. 2025, 4, 100120. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Feng, Y.; Cai, S.; Gao, H.; Wei, Z.; Zhao, Y. Cellulose-based separators for lithium batteries: Source, preparation and performance. Chem. Eng. J. 2023, 471, 144593. [Google Scholar] [CrossRef]
- Tafete, G.A.; Abera, M.K.; Thothadri, G. Review on nanocellulose-based materials for supercapacitors applications. J. Energy Storage 2022, 48, 103938. [Google Scholar] [CrossRef]
- Chibac-Scutaru, A.L.; Coseri, S. Advances in the use of cellulose-based proton exchange membranes in fuel cell technology: A review. Int. J. Biol. Macromol. 2023, 247, 125810. [Google Scholar] [CrossRef]
- Arif, M.B.; Ndruru, S.T.C.L.; Ghozali, M. A review on the preparation of carboxymethylcellulose-based membrane as polymer electrolyte for energy devices. Biomass Bioenergy 2025, 193, 107542. [Google Scholar] [CrossRef]
- Lizundia, E.; Costa, C.M.; Alves, R.; Lanceros-Méndez, S. Cellulose and its derivatives for lithium ion battery separators: A review on the processing methods and properties. Carbohydr. Polym. Technol. Appl. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, R.; Cheng, T.; Guo, J.; Xian, M.; Liu, H. Imidazolium-based ionic liquids for cellulose pretreatment: Recent progresses and future perspectives. Appl. Microbiol. Biotechnol. 2017, 101, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ju, Z.; Yu, M.; Luo, H.; Zhang, C.; Zhang, X.; Cheng, H.; Zheng, M.; Jin, L.; Ge, C. Cellulose Regeneration in Imidazolium-Based Ionic Liquids and Antisolvent Mixtures: A Density Functional Theory Study. ACS Omega 2022, 7, 42170–42180. [Google Scholar] [CrossRef] [PubMed]
- Barsbay, M.; Güven, O. Surface modification of cellulose via conventional and controlled radiation-induced grafting. Radiat. Phys. Chem. 2019, 160, 1–8. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gupta, B.; Shameli, K.; Verma, C.; Ali, R.R.; Ting, T.M. Engineered Bioactive Polymeric Surfaces by Radiation Induced Graft Copolymerization: Strategies and Applications. Polymers 2021, 13, 3102. [Google Scholar] [CrossRef]
- Nargatti, K.I.; Subhedar, A.R.; Ahankari, S.S.; Grace, A.N.; Dufresne, A. Nanocellulose-based aerogel electrodes for supercapacitors: A review. Carbohydr. Polym. 2022, 297, 120039. [Google Scholar] [CrossRef]
- Bolloli, M.; Antonelli, C.; Molméret, Y.; Alloin, F.; Iojoiu, C.; Sanchez, J.-Y. Nanocomposite poly(vynilidene fluoride)/nanocrystalline cellulose porous membranes as separators for lithium-ion batteries. Electrochim. Acta 2016, 214, 38–48. [Google Scholar] [CrossRef]
- Jailani, A.; Hidzer, M.H.; Firdaus, A.H.M.; Sapuan, S.M.; Zainudin, E.S.; Atiqah, A.; Wan Jaafar, W.M.; Suryanegara, L. Enhancing polyvinyl alcohol (PVA) nanocomposites: Key properties, applications and challenges in advanced engineering. Def. Technol. 2025. [Google Scholar] [CrossRef]
- Palanisamy, G.; Im, Y.M.; Muhammed, A.P.; Palanisamy, K.; Thangarasu, S.; Oh, T.H. Fabrication of Cellulose Acetate-Based Proton Exchange Membrane with Sulfonated SiO(2) and Plasticizers for Microbial Fuel Cell Applications. Membranes 2023, 13, 581. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Zhang, F.; Li, Q.; Xiao, J.; Lin, Q.; Qing, G. Robust Cellulose Nanocrystal-Based Self-Assembled Composite Membranes Doped with Polyvinyl Alcohol and Graphene Oxide for Osmotic Energy Harvesting. Small 2023, 19, e2304603. [Google Scholar] [CrossRef]
- Cheng, C.; Zhu, S.; Lu, C.; Xu, Z.J. Modification strategies for cellulose-based anion exchange membranes. Green Chem. 2025, 27, 8041–8054. [Google Scholar] [CrossRef]
- Zubair, N.A.; Moawia, R.M.; Nasef, M.M.; Hubbe, M.; Zakeri, M. A Critical Review on Natural Fibers Modifications by Graft Copolymerization for Wastewater Treatment. J. Polym. Environ. 2022, 30, 1199–1227. [Google Scholar] [CrossRef]
- Samaniego, A.J.; Arabelo, A.K.; Sarker, M.; Mojica, F.; Madrid, J.; Chuang, P.-Y.A.; Ocon, J.; Espiritu, R. Fabrication of cellulose acetate-based radiation grafted anion exchange membranes for fuel cell application. J. Appl. Polym. Sci. 2021, 138, 49947. [Google Scholar] [CrossRef]
- Jaouahar, M.; Ablouh, E.-H.; Hanani, Z.; Jaklič, B.; Spreitzer, M.; Semlali, F.-Z.; Ait Benhamou, A.; Samih, Y.; El Achaby, M.; Sehaqui, H. Preparation and characterization of sulfated nanocellulose: From hydrogels to highly transparent films. Int. J. Biol. Macromol. 2024, 260, 129464. [Google Scholar] [CrossRef] [PubMed]
- Lander, S.; Erlandsson, J.; Vagin, M.; Gueskine, V.; Korhonen, L.; Berggren, M.; Wågberg, L.; Crispin, X. Sulfonated Cellulose Membranes: Physicochemical Properties and Ionic Transport versus Degree of Sulfonation. Adv. Sustain. Syst. 2022, 6, 2200275. [Google Scholar] [CrossRef]
- Yang, H.; Gueskine, V.; Berggren, M.; Engquist, I. Cross-Linked Nanocellulose Membranes for Nanofluidic Osmotic Energy Harvesting. ACS Appl. Energy Mater. 2022, 5, 15740–15748. [Google Scholar] [CrossRef]
- Ni, C.; Wang, H.; Zhao, Q.; Liu, B.; Sun, Z.; Zhang, M.; Hu, W.; Liang, L. Crosslinking effect in nanocrystalline cellulose reinforced sulfonated poly(aryl ether ketone) proton exchange membranes. Solid State Ion. 2018, 323, 5–15. [Google Scholar] [CrossRef]
- Hambardzumyan, A.; Vayer, M.; Foulon, L.; Pernes, M.; Devers, T.; Bigarré, J.; Aguié-Béghin, V. Nafion membranes reinforced by cellulose nanocrystals for fuel cell applications: Aspect ratio and heat treatment effects on physical properties. J. Mater. Sci. 2022, 57, 4684–4703. [Google Scholar] [CrossRef]
- Raza, Z.A. Recent advances in the chitosan-based nanostructured membranes for diverse environmental applications. Next Mater. 2025, 9, 101099. [Google Scholar] [CrossRef]
- Zahid, M.; Rashid, A.; Akram, S.; Shakir, H.M.F.; Rehan, Z.A.; Javed, T.; Shabbir, R.; Hessien, M.M. Fabrication and Characterization of Sulfonated Graphene Oxide-Doped Polymeric Membranes with Improved Anti-Biofouling Behavior. Membranes 2021, 11, 563. [Google Scholar] [CrossRef]
- Islam, A.; Shahriar, M.; Islam, M.T.; Teo, S.H.; Khan, M.A.R.; Taufiq-Yap, Y.H.; Mohanta, S.C.; Rehan, A.I.; Rasee, A.I.; Kubra, K.T.; et al. Advances in filler-crosslinked membranes for hydrogen fuel cells in sustainable energy generation. Int. J. Hydrogen Energy 2025, 140, 745–776. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Habibi, Y.; Adhikari, B. Surface modifications of nanocellulose: From synthesis to high-performance nanocomposites. Prog. Polym. Sci. 2021, 119, 101418. [Google Scholar] [CrossRef]
- Yu, Z.; Tsen, W.-C.; Qu, T.; Cheng, F.; Hu, F.; Liu, H.; Wen, S.; Gong, C. Highly ion-conductive anion exchange membranes with superior mechanical properties based on polymeric ionic liquid filled functionalized bacterial cellulose for alkaline fuel cells. J. Mater. Res. Technol. 2023, 23, 6187–6199. [Google Scholar] [CrossRef]
- Brito dos Santos, F.; Whitbeck, A.; Jalaee, A.; Chalitangkoon, J.; Rojas, O.J.; Gyenge, E.; Foster, E.J. Development of a robust ion-selective membrane from sulfonated cellulose nanofibers for zinc–iodine redox flow batteries. J. Membr. Sci. 2025, 735, 124486. [Google Scholar] [CrossRef]
- Jose, J.; Thomas, V.; Vinod, V.; Abraham, R.; Abraham, S. Nanocellulose based functional materials for supercapacitor applications. J. Sci. Adv. Mater. Devices 2019, 4, 333–340. [Google Scholar] [CrossRef]
- Singh, G.; Gauba, P.; Mathur, G. Bacterial Cellulose-Based Composites: Recent Trends in Production Methods and Applications. Cellul. Chem. Technol. 2024, 58, 799–818. [Google Scholar] [CrossRef]
- Dai, Z.; Ottesen, V.; Deng, J.; Helberg, R.M.L.; Deng, L. A Brief Review of Nanocellulose Based Hybrid Membranes for CO2 Separation. Fibers 2019, 7, 40. [Google Scholar] [CrossRef]
- Ho, N.A.D.; Leo, C.P. A review on the emerging applications of cellulose, cellulose derivatives and nanocellulose in carbon capture. Environ. Res. 2021, 197, 111100. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Pinjari, D.V.; Sonawane, S.H.; Bhanvase, B.A.; Sheikh, J.; Sillanpää, M. Cellulose-based nanomaterials for water and wastewater treatments: A review. J. Environ. Chem. Eng. 2021, 9, 106626. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Oyedotun, K.O.; Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J. Cellulose derivatives and cellulose-metal-organic frameworks for CO2 adsorption and separation. J. CO2 Util. 2022, 64, 102163. [Google Scholar] [CrossRef]
- Iqbal, D.; Zhao, Y.; Zhao, R.; Russell, S.J.; Ning, X. A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment. Polymers 2022, 14, 2343. [Google Scholar] [CrossRef]
- Saud, A.; Saleem, H.; Zaidi, S.J. Progress and Prospects of Nanocellulose-Based Membranes for Desalination and Water Treatment. Membranes 2022, 12, 462. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zheng, Z.; Liu, H.; Zhu, L.; Yang, M.; Chen, Y. Nanocellulose-based membranes for highly efficient molecular separation. Chem. Eng. J. 2023, 451, 138711. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Chandel, N.; Jain, K.; Jain, A.; Raj, T.; Patel, A.K.; Yang, Y.-H.; Bhatia, S.K. The versatile world of cellulose-based materials in healthcare: From production to applications. Ind. Crops Prod. 2023, 201, 116929. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. [Google Scholar] [CrossRef] [PubMed]
- Das, P.P.; Kalyani, P.; Kumar, R.; Khandelwal, M. Cellulose-based natural nanofibers for fresh produce packaging: Current status, sustainability and future outlook. Sustain. Food Technol. 2023, 1, 528–544. [Google Scholar] [CrossRef]
- Irimia, A.; Grigoras, V.C.; Popescu, C.M. Active Cellulose-Based Food Packaging and Its Use on Foodstuff. Polymers 2024, 16, 389. [Google Scholar] [CrossRef]
- Verma, J.; Petru, M.; Goel, S. Cellulose based materials to accelerate the transition towards sustainability. Ind. Crops Prod. 2024, 210, 118078. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2021, 33, e2000619. [Google Scholar] [CrossRef]
- van den Berg, O.; Schroeter, M.; Capadona, J.R.; Weder, C. Nanocomposites based on cellulose whiskers and (semi)conducting conjugated polymers. J. Mater. Chem. 2007, 17, 2746–2753. [Google Scholar] [CrossRef]
- Aigaje, E.; Riofrio, A.; Baykara, H. Processing, Properties, Modifications, and Environmental Impact of Nanocellulose/Biopolymer Composites: A Review. Polymers 2023, 15, 1219. [Google Scholar] [CrossRef]
- Santos, F.A.d.; Iulianelli, G.C.V.; Tavares, M.I.B. The Use of Cellulose Nanofillers in Obtaining Polymer Nanocomposites: Properties, Processing, and Applications. Mater. Sci. Appl. 2016, 07, 257–294. [Google Scholar] [CrossRef]
- Kurklu-Kocaoglu, S.; Ramírez-Espinosa, D.; Casado-Coterillo, C. Enhanced OH− Transport Properties of Bio-Based Anion-Exchange Membranes for Different Applications. Membranes 2025, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, L.; Deng, Q.; Dong, W. A Sustainable and Eco-Friendly Membrane for PEM Fuel Cells Using Bacterial Cellulose. Polymers 2024, 16, 3017. [Google Scholar] [CrossRef] [PubMed]
- Chiam, C.-K.; Kamin, Z.; HueyNg, C.; AbdLahin, F.; Sarbatly, R. Cellulose and cellulose derivatives in sustainable membrane development for oil/water separation. Appl. Chem. Eng. 2024, 7, 1867. [Google Scholar] [CrossRef]
- Zuppolini, S.; Salama, A.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Cellulose Amphiphilic Materials: Chemistry, Process and Applications. Pharmaceutics 2022, 14, 386. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, J.; Yuan, Y.; Shen, J.; Lin, S. Chemical modification of cellulose membranes with sulfo ammonium zwitterionic vinyl monomer to improve hemocompatibility. Colloids Surf. B Biointerfaces 2003, 30, 249–257. [Google Scholar] [CrossRef]
- Bello, I.T.; Jolaoso, L.A.; Ahmed, R.A.; Bello, A. Electrochemical energy conversion and Storage Systems: A perspective on the challenges and opportunities for sustainable energy in Africa. Energy Rev. 2025, 4, 100109. [Google Scholar] [CrossRef]
- Nimir, W.; Al-Othman, A.; Tawalbeh, M.; Al Makky, A.; Ali, A.; Karimi-Maleh, H.; Karimi, F.; Karaman, C. Approaches towards the development of heteropolyacid-based high temperature membranes for PEM fuel cells. Int. J. Hydrogen Energy 2023, 48, 6638–6656. [Google Scholar] [CrossRef]
- Punyawudho, K.; Pugalenthi, M.R.; Shu, X.; Kaneco, S.; Wongwuttanasatian, T.; Suksri, A.; Payattikul, L.; Balasingam, S.K. Advancements and Challenges in Electrode and Electrolyte Materials for Proton Exchange Membrane Fuel Cells: A Comprehensive Review. Int. J. Energy Res. 2024, 2024, 9529659. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Lei, H.; Yang, X.; Chen, Z.; Rawach, D.; Du, L.; Liang, Z.; Li, D.S.; Zhang, G.; Tavares, A.C.; Sun, S. Multiscale Understanding of Anion Exchange Membrane Fuel Cells: Mechanisms, Electrocatalysts, Polymers, and Cell Management. Adv. Mater. 2025, 37, e2410106. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Rahman, T.U.; Tasnim, N.; Arju, J.; Rafid, M.M.; Islam, M.R.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. Microbial Fuel Cell Construction Features and Application for Sustainable Wastewater Treatment. Membranes 2023, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.E.; Hemdan, B.A.; El-Naggar, M.E.; El-Liethy, M.A.; Jadhav, D.A.; El-Hendawy, H.H.; Ali, M.; El-Taweel, G.E. Harnessing the power of microbial fuel cells as pioneering green technology: Advancing sustainable energy and wastewater treatment through innovative nanotechnology. Bioprocess Biosyst. Eng. 2025, 48, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.; Prasad Uday, U.S.; Bandyopadhyay, T.K.; Ray, R.N.; Bhunia, B. Performance improvement of microbial fuel cell (MFC) using suitable electrode and Bioengineered organisms: A review. Bioengineered 2017, 8, 471–487. [Google Scholar] [CrossRef]
- Roy, A.; Kumar, A.; Ebihara, A.; Wang, C.T.; Katiyar, V. A critical review on advancing bioinspired smart membranes for green energy recovery in microbial fuel cells: Paving the way for a sustainable future. J. Environ. Chem. Eng. 2025, 13, 118094. [Google Scholar] [CrossRef]
- Ramirez-Nava, J.; Martinez-Castrejon, M.; Garcia-Mesino, R.L.; Lopez-Diaz, J.A.; Talavera-Mendoza, O.; Sarmiento-Villagrana, A.; Rojano, F.; Hernandez-Flores, G. The Implications of Membranes Used as Separators in Microbial Fuel Cells. Membranes 2021, 11, 738. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Hassan, S.H.A.; Rahman, M.M.; Oh, S.-E. The effect of Nafion membrane fouling on the power generation of a microbial fuel cell. Int. J. Hydrogen Energy 2020, 45, 13643–13651. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Hamzat, A.K.; Whidborne, J.; Kuang, B.; Jenkins, K.W. Integration of renewable energy sources in tandem with electrolysis: A technology review for green hydrogen production. Int. J. Hydrogen Energy 2025, 107, 218–240. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Abdel-Wahab, A.; Adham, S.; Han, D.S.; Phuntsho, S.; Suwaileh, W.; Hilal, N.; Shon, H.K. Salinity gradient energy generation by pressure retarded osmosis: A review. Desalination 2021, 500, 114841. [Google Scholar] [CrossRef]
- Ju, J.; Choi, Y.; Lee, S.; Jeong, N. Comparison of fouling characteristics between reverse electrodialysis (RED) and pressure retarded osmosis (PRO). Desalination 2021, 497, 114648. [Google Scholar] [CrossRef]
- Wang, F.; Mu, D.; Lu, Y.-C. Ion-Conducting Membranes for Long-Duration Energy Storage. ACS Energy Lett. 2025, 10, 3096–3111. [Google Scholar] [CrossRef]
- Hasan, M.M.; Haque, R.; Jahirul, M.I.; Rasul, M.G.; Fattah, I.M.R.; Hassan, N.M.S.; Mofijur, M. Advancing energy storage: The future trajectory of lithium-ion battery technologies. J. Energy Storage 2025, 120, 116511. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Santos, D.M.F. Applications of Polymer Electrolytes in Lithium-Ion Batteries: A Review. Polymers 2023, 15, 3907. [Google Scholar] [CrossRef]
- Sun, Y.-K. Emerging All-Solid-State Lithium–Sulfur Batteries: Holy Grails for Future Secondary Batteries. ACS Energy Lett. 2024, 9, 5092–5095. [Google Scholar] [CrossRef]
- Swain, S.; Brahma, K.; Nayak, R.; Sonika. Recent advances in optimized polybenzimidazole-based membranes for vanadium redox flow battery applications. J. Energy Storage 2025, 129, 117372. [Google Scholar] [CrossRef]
- Wong, T.; Yang, Y.; Tan, R.; Wang, A.; Zhou, Z.; Yuan, Z.; Li, J.; Liu, D.; Alvarez-Fernandez, A.; Ye, C.; et al. Sulfonated poly(ether-ether-ketone) membranes with intrinsic microporosity enable efficient redox flow batteries for energy storage. Joule 2025, 9, 101795. [Google Scholar] [CrossRef]
- Farooq, A.; Wanyan, H.; Lu, S.; Mosisa, M.T.; Zhou, X.; Xiao, H.; Liu, K.; Huang, L.; Chen, L.; Wu, H. A review on cellulose-based derivatives and composites for sustainable rechargeable batteries. Int. J. Biol. Macromol. 2025, 308, 142528. [Google Scholar] [CrossRef]
- Wan, X.; Shen, F.; Hu, J.; Huang, M.; Zhao, L.; Zeng, Y.; Tian, D.; Yang, G.; Zhang, Y. 3-D hierarchical porous carbon from oxidized lignin by one-step activation for high-performance supercapacitor. Int. J. Biol. Macromol. 2021, 180, 51–60. [Google Scholar] [CrossRef]
- Huang, H.; Wu, H.; Xu, Y.; Xu, F. Self-discharge suppression by composite regenerated cellulose ion-selective separator for high-energy aqueous supercapacitors. Int. J. Biol. Macromol. 2024, 276, 133896. [Google Scholar] [CrossRef]
- Islam, M.A.; Ong, H.L.; Villagracia, A.R.; Halim, K.A.A.; Ganganboina, A.B.; Doong, R.-A. Biomass–derived cellulose nanofibrils membrane from rice straw as sustainable separator for high performance supercapacitor. Ind. Crops Prod. 2021, 170, 113694. [Google Scholar] [CrossRef]
- Nasef, M.M. Radiation Grafted Ion Conducting Membranes for Electrochemical Energy Systems: Status of Developmental and Upscaled Membranes. J. Appl. Membr. Sci. Technol. 2022, 26, 51–76. [Google Scholar] [CrossRef]
- Shah, M.; Hakim, N.U.D. Advances in nanocellulose proton conductivity and applications in polymer electrolyte membrane fuel cells. Next Mater. 2025, 6, 100484. [Google Scholar] [CrossRef]
- Seo, J.A.; Kim, J.C.; Koh, J.K.; Ahn, S.H.; Kim, J.H. Preparation and characterization of crosslinked cellulose/sulfosuccinic acid membranes as proton conducting electrolytes. Ionics 2009, 15, 555–560. [Google Scholar] [CrossRef]
- Phan-Huynh, T.N.; Pham, H.T.; Nguyen, P.T.; Tap, T.D.; Nguyen, T.H.; Phung, Q.; Tran, T.T.V.; Chang, Y., Jr.; Lai, Y.-T.; Hoang, D. Cellulose-Based Proton Exchange Membrane Derived from an Agricultural Byproduct. ACS Appl. Polym. Mater. 2025, 7, 2347–2358. [Google Scholar] [CrossRef]
- Brito Dos Santos, F.; Kaschuk, J.; Banvillet, G.; Jalaee, A.; Rojas, O.J.; Foster, E.J. Alternative proton exchange membrane based on a bicomponent anionic nanocellulose system. Carbohydr. Polym. 2024, 340, 122299. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef]
- Lin, C.W.; Liang, S.S.; Chen, S.W.; Lai, J.T. Sorption and transport properties of 2-acrylamido-2-methyl-1-propanesulfonic acid-grafted bacterial cellulose membranes for fuel cell application. J. Power Sources 2013, 232, 297–305. [Google Scholar] [CrossRef]
- Wen, B.; Ma, R.; Yang, G.; Li, C.; Huang, Y.; Zhong, L.; Sha, Z.; Chen, Y.; Cai, S.; Guo, D.; et al. Synergistically improve the strength and porosity of carbon paper by using a novel phenol formaldehyde resin modified with cellulose nanofiber for proton exchange membrane fuel cells. Int. J. Biol. Macromol. 2024, 278, 134205. [Google Scholar] [CrossRef]
- Yue, L.; Zheng, Y.; Xie, Y.; Liu, S.; Guo, S.; Yang, B.; Tang, T. Preparation of a carboxymethylated bacterial cellulose/polyaniline composite gel membrane and its characterization. RSC Adv. 2016, 6, 68599–68605. [Google Scholar] [CrossRef]
- Vilela, C.; Cordeiro, D.M.; Boas, J.V.; Barbosa, P.; Nolasco, M.; Vaz, P.D.; Rudić, S.; Ribeiro-Claro, P.; Silvestre, A.J.D.; Oliveira, V.B.; et al. Poly(4-styrene sulfonic acid)/bacterial cellulose membranes: Electrochemical performance in a single-chamber microbial fuel cell. Bioresour. Technol. Rep. 2020, 9, 100376. [Google Scholar] [CrossRef]
- Gadim, T.D.O.; Loureiro, F.J.A.; Vilela, C.; Rosero-Navarro, N.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Protonic conductivity and fuel cell tests of nanocomposite membranes based on bacterial cellulose. Electrochim. Acta 2017, 233, 52–61. [Google Scholar] [CrossRef]
- Gadim, T.D.O.; Vilela, C.; Loureiro, F.J.A.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nafion® and nanocellulose: A partnership for greener polymer electrolyte membranes. Ind. Crops Prod. 2016, 93, 212–218. [Google Scholar] [CrossRef]
- Kartika Sari, A.; Mohamad Yunus, R.; Majlan, E.H.; Loh, K.S.; Wong, W.Y.; Saidin, N.U.; Alva, S.; Khaerudini, D.S. Nata de Cassava Type of Bacterial Cellulose Doped with Phosphoric Acid as a Proton Exchange Membrane. Membranes 2022, 13, 43. [Google Scholar] [CrossRef]
- Vilela, C.; Gadim, T.D.O.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nanocellulose/poly(methacryloyloxyethyl phosphate) composites as proton separator materials. Cellulose 2016, 23, 3677–3689. [Google Scholar] [CrossRef]
- Vilela, C.; Martins, A.P.C.; Sousa, N.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Poly(bis [2-(methacryloyloxy)ethyl] phosphate)/Bacterial Cellulose Nanocomposites: Preparation, Characterization and Application as Polymer Electrolyte Membranes. Appl. Sci. 2018, 8, 1145. [Google Scholar] [CrossRef]
- Vilela, C.; Silva, A.C.Q.; Domingues, E.M.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.L.; Santos, S.A.O.; Freire, C.S.R. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2020, 230, 115604. [Google Scholar] [CrossRef]
- Rogalsky, S.; Bardeau, J.-F.; Makhno, S.; Babkina, N.; Tarasyuk, O.; Cherniavska, T.; Orlovska, I.; Kozyrovska, N.; Brovko, O. New proton conducting membrane based on bacterial cellulose/polyaniline nanocomposite film impregnated with guanidinium-based ionic liquid. Polymer 2018, 142, 183–195. [Google Scholar] [CrossRef]
- Vilela, C.; Morais, J.; Silva, A.; Muñoz, D.; Figueiredo, F.; Silvestre, A.; Freire, C. Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells. Nanomaterials 2020, 10, 1713. [Google Scholar] [CrossRef]
- Farzin, S.; Johnson, T.J.; Chatterjee, S.; Zamani, E.; Dishari, S.K. Ionomers From Kraft Lignin for Renewable Energy Applications. Front. Chem. 2020, 8, 690. [Google Scholar] [CrossRef]
- Rijal, M.S.; Nasir, M.; Purwasasmita, B.S.; Asri, L.A.T.W. Cellulose nanocrystals-microfibrils biocomposite with improved membrane performance. Carbohydr. Polym. Technol. Appl. 2023, 5, 100326. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Selyanchyn, R.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. High Temperature Proton Conduction in Nanocellulose Membranes: Paper Fuel Cells. Chem. Mater. 2016, 28, 4805–4814. [Google Scholar] [CrossRef]
- Guccini, V.; Carlson, A.; Yu, S.; Lindbergh, G.; Lindström, R.W.; Salazar-Alvarez, G. Highly proton conductive membranes based on carboxylated cellulose nanofibres and their performance in proton exchange membrane fuel cells. J. Mater. Chem. A 2019, 7, 25032–25039. [Google Scholar] [CrossRef]
- Smolarkiewicz, I.; Rachocki, A.; Pogorzelec-Glaser, K.; Pankiewicz, R.; Ławniczak, P.; Łapiński, A.; Jarek, M.; Tritt-Goc, J. Proton-conducting Microcrystalline Cellulose Doped with Imidazole. Thermal and Electrical Properties. Electrochim. Acta 2015, 155, 38–44. [Google Scholar] [CrossRef]
- Tritt-Goc, J.; Jankowska, I.; Pogorzelec-Glaser, K.; Pankiewicz, R.; Ławniczak, P. Imidazole-doped nanocrystalline cellulose solid proton conductor: Synthesis, thermal properties, and conductivity. Cellulose 2018, 25, 281–291. [Google Scholar] [CrossRef]
- Tritt-Goc, J.; Lindner, Ł.; Bielejewski, M.; Markiewicz, E.; Pankiewicz, R. Proton conductivity and proton dynamics in nanocrystalline cellulose functionalized with imidazole. Carbohydr. Polym. 2019, 225, 115196. [Google Scholar] [CrossRef]
- Tritt-Goc, J.; Lindner, Ł.; Bielejewski, M.; Markiewicz, E.; Pankiewicz, R. Synthesis, thermal properties, conductivity and lifetime of proton conductors based on nanocrystalline cellulose surface-functionalized with triazole and imidazole. Int. J. Hydrogen Energy 2020, 45, 13365–13375. [Google Scholar] [CrossRef]
- Etuk, S.S.; Lawan, I.; Zhou, W.; Jiang, Y.; Zhang, Q.; Wei, X.; Zhang, M.; Fernando, G.F.; Yuan, Z. Synthesis and characterization of triazole based sulfonated nanocrystalline cellulose proton conductor. Cellulose 2020, 27, 3197–3209. [Google Scholar] [CrossRef]
- Jankowska, I.; Pankiewicz, R.; Pogorzelec-Glaser, K.; Lawniczak, P.; Lapinski, A.; Tritt-Goc, J. Comparison of structural, thermal and proton conductivity properties of micro- and nanocelluloses. Carbohydr. Polym. 2018, 200, 536–542. [Google Scholar] [CrossRef]
- Cai, Z.; Li, R.; Xu, X.; Sun, G.; Zhuang, X.; Liu, Y.; Cheng, B. Embedding phosphoric acid-doped cellulose nanofibers into sulfonated poly (ether sulfone) for proton exchange membrane. Polymer 2018, 156, 179–185. [Google Scholar] [CrossRef]
- Bano, S.; Negi, Y.S.; Illathvalappil, R.; Kurungot, S.; Ramya, K. Studies on nano composites of SPEEK/ethylene glycol/cellulose nanocrystals as promising proton exchange membranes. Electrochim. Acta 2019, 293, 260–272. [Google Scholar] [CrossRef]
- Zhao, Q.; Wei, Y.; Ni, C.; Wang, L.; Liu, B.; Liu, J.; Zhang, M.; Men, Y.; Sun, Z.; Xie, H.; et al. Effect of aminated nanocrystal cellulose on proton conductivity and dimensional stability of proton exchange membranes. Appl. Surf. Sci. 2019, 466, 691–702. [Google Scholar] [CrossRef]
- Jankowska, I.; Bielejewski, M.; Ławniczak, P.; Pankiewicz, R.; Brus, J.; Tritt-Goc, J. Cellulose nanofibers vs. cellulose nanocrystals: A comparative study on their influence on the properties of imidazole-doped proton-conducting nanocomposites. Cellulose 2025, 32, 4763–4779. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, G.; Wang, H.; Li, X.; Feng, X.; Cheng, B.; Shi, L.; Kang, W.; Zhuang, X.; Yin, Y. Bio-inspired amino-acid-functionalized cellulose whiskers incorporated into sulfonated polysulfone for proton exchange membrane. J. Power Sources 2019, 409, 123–131. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Sood, T.; Chakraborty, A.; Purkait, M.K. Reduced graphene oxide incorporated polyvinylidene fluoride/cellulose acetate proton exchange membrane for energy extraction using microbial fuel cells. J. Electroanal. Chem. 2022, 907, 115890. [Google Scholar] [CrossRef]
- Jiang, G.-P.; Zhang, J.; Qiao, J.-L.; Jiang, Y.-M.; Zarrin, H.; Chen, Z.; Hong, F. Bacterial nanocellulose/Nafion composite membranes for low temperature polymer electrolyte fuel cells. J. Power Sources 2015, 273, 697–706. [Google Scholar] [CrossRef]
- Hou, X.; Liu, Z.; Wei, Y.; Zhao, Q.; Dong, J.; Liu, B.; Sun, Z.; Shi, T.; Zhang, M.; Hu, W. Proton conducting nanocomposite membranes of nanocellulose reinforced poly(arylene ether ketone)s containing sulfonic/carboxylic groups. Solid State Ion. 2017, 311, 31–40. [Google Scholar] [CrossRef]
- Xu, X.; Li, R.; Tang, C.; Wang, H.; Zhuang, X.; Liu, Y.; Kang, W.; Shi, L. Cellulose nanofiber-embedded sulfonated poly (ether sulfone) membranes for proton exchange membrane fuel cells. Carbohydr. Polym. 2018, 184, 299–306. [Google Scholar] [CrossRef]
- Esmaielzadeh, S.; Ahmadizadegan, H. Construction of proton exchange membranes under ultrasonic irradiation based on novel fluorine functionalizing sulfonated polybenzimidazole/cellulose/silica bionanocomposite. Ultrason. Sonochem. 2018, 41, 641–650. [Google Scholar] [CrossRef]
- Charradi, K.; Landolsi, Z.; Gabriel, L.; Mabrouk, W.; Koschella, A.; Ahmed, Z.; Elnaggar, A.; Heinze, T.; Keshk, S.M.A.S. Incorporating of sulfo ethyl cellulose to augment the performance of sulfonated poly (ether ether ketone) composite for proton exchange membrane fuel cells. J. Solid State Electrochem. 2023, 27, 3415–3423. [Google Scholar] [CrossRef]
- Muhmed, S.A.; Jaafar, J.; Ahmad, S.N.A.; Mohamed, M.H.; Ismail, A.F.; Ilbeygi, H.; Othman, M.H.D.; Rahman, M.A. Incorporating functionalized graphene oxide in green material-based membrane for proton exchange membrane fuel cell application. J. Environ. Chem. Eng. 2023, 11, 109547. [Google Scholar] [CrossRef]
- Muhmed, S.A.; Jaafar, J.; Ahmad, S.N.A.; Purwanto, M.; Daud, N.A.B.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Atmaja, L.; Santoso, M.; et al. Biopolymeric electrolyte-based membrane on nanocrystalline cellulose/polyvinyl alcohol for a conceivable usage in proton exchange membrane fuel cell. Chem. Eng. Res. Des. 2024, 208, 881–898. [Google Scholar] [CrossRef]
- Raut, A.; Fang, H.; Lin, Y.-C.; Farabi Rahman, M.; Fu, S.; Yin, Y.; Fang, Y.; Sprouster, D.; Isseroff, R.; Sharma, S.K.; et al. Designing a micro-cellulose membrane for hydrogen fuel cells. RSC Sustain. 2025, 3, 3025–3035. [Google Scholar] [CrossRef]
- Bhosale, A.C.; Ghosh, P.C.; Assaud, L. Preparation methods of membrane electrode assemblies for proton exchange membrane fuel cells and unitized regenerative fuel cells: A review. Renew. Sustain. Energy Rev. 2020, 133, 110286. [Google Scholar] [CrossRef]
- Sarirchi, S.; Rowshanzamir, S. An overview of organic/inorganic membranes based on sulfonated poly ether ether ketone for application in proton exchange membrane fuel cells. J. Renew. Energy Environ. 2017, 4, 46–60. [Google Scholar] [CrossRef]
- Sahu, A.K.; Pitchumani, S.; Sridhar, P.; Shukla, A.K. Nafion and modified-Nafion membranes for polymer electrolyte fuel cells: An overview. Bull. Mater. Sci. 2009, 32, 285–294. [Google Scholar] [CrossRef]
- Gadim, T.D.O.; Figueiredo, A.G.P.R.; Rosero-Navarro, N.C.; Vilela, C.; Gamelas, J.A.F.; Barros-Timmons, A.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nanostructured Bacterial Cellulose–Poly(4-styrene sulfonic acid) Composite Membranes with High Storage Modulus and Protonic Conductivity. ACS Appl. Mater. Interfaces 2014, 6, 7864–7875. [Google Scholar] [CrossRef]
- Ni, C.; Wei, Y.; Zhao, Q.; Liu, B.; Sun, Z.; Gu, Y.; Zhang, M.; Hu, W. Novel proton exchange membranes based on structure-optimized poly(ether ether ketone ketone)s and nanocrystalline cellulose. Appl. Surf. Sci. 2018, 434, 163–175. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, X.; Raut, A.; Isseroff, R.; Xue, Y.; Zhou, Y.; Sandhu, B.; Schein, T.; Zeliznyak, T.; Sharma, P.; et al. Operation of proton exchange membrane (PEM) fuel cells using natural cellulose fiber membranes. Sustain. Energy Fuels 2019, 3, 2725–2732. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Šmíd, B.; Selyanchyn, R.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. Spray deposition of sulfonated cellulose nanofibers as electrolyte membranes in fuel cells. Cellulose 2021, 28, 1355–1367. [Google Scholar] [CrossRef]
- Madih, K.; El-Shazly, A.H.; Elkady, M.F.; Aziz, A.N.; Youssef, M.E.; Khalifa, R.E. A facile synthesis of cellulose acetate reinforced graphene oxide nanosheets as proton exchange membranes for fuel cell applications. J. Saudi Chem. Soc. 2022, 26, 101435. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Nasseri, R.; Karkhaneh, A.; Majedi, F.S.; Mokarram, N.; Renaud, P.; Jacob, K.I. Cellulose nanowhiskers to regulate the microstructure of perfluorosulfonate ionomers for high-performance fuel cells. J. Mater. Chem. A 2014, 2, 11334–11340. [Google Scholar] [CrossRef]
- Gaur, S.S.; Dhar, P.; Sonowal, A.; Sharma, A.; Kumar, A.; Katiyar, V. Thermo-mechanically stable sustainable polymer based solid electrolyte membranes for direct methanol fuel cell applications. J. Membr. Sci. 2017, 526, 348–354. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Y.; Yang, J.; Shi, L.; Yang, G.; Zhuang, X.; Li, Z. UiO-66-NH2 functionalized cellulose nanofibers embedded in sulfonated polysulfone as proton exchange membrane. Int. J. Hydrogen Energy 2021, 46, 19106–19115. [Google Scholar] [CrossRef]
- Moussa, M.A.B.; Ahmed, Z.; Charradi, K.; Fraj, B.B.; Boufi, S.; Koschella, A.; Heinze, T.; Keshk, S.M.A.S.; Assaker, I.B. Performance of high sulfonated poly(ether ether ketone) improved with microcrystalline cellulose and 2,3-dialdehyde cellulose for proton exchange membranes. Mater. Renew. Sustain. Energy 2024, 13, 319–331. [Google Scholar] [CrossRef]
- Bagus Pambudi, A.; Priyangga, A.; Hartanto, D.; Atmaja, L. Fabrication and characterization of modified microcrystalline cellulose membrane as proton exchange membrane for direct methanol fuel cell. Mater. Today Proc. 2021, 46, 1855–1859. [Google Scholar] [CrossRef]
- Priyangga, A.; Atmaja, L.; Santoso, M.; Jaafar, J.; Ilbeygi, H. Utilization of mesoporous phosphotungstic acid in nanocellulose membranes for direct methanol fuel cells. RSC Adv. 2022, 12, 14411–14421. [Google Scholar] [CrossRef]
- Sriruangrungkamol, A.; Chonkaew, W. Modification of nanocellulose membrane by impregnation method with sulfosuccinic acid for direct methanol fuel cell applications. Polym. Bull. 2021, 78, 3705–3728. [Google Scholar] [CrossRef]
- Sriruangrungkamol, A.; Yongprapat, S.; Therdthianwong, A.; Chonkaew, W. Enhanced Proton Exchange Properties of Sulfonated Nanocellulose/Poly(Ether Imide) Composite Membranes for Direct Methanol Fuel Cells. J. Appl. Polym. Sci. 2025, 142, 57059. [Google Scholar] [CrossRef]
- Permana, D.; Atmaja, L.; Priyangga, A.; Kedang, Y.I.; Santoso, M. Utilization of cellulose-based carbon nanodots in sulfonated polysulfone based membrane for direct methanol fuel cell. S. Afr. J. Chem. Eng. 2024, 48, 265–275. [Google Scholar] [CrossRef]
- Palanisamy, G.; Oh, T.H.; Thangarasu, S. Modified Cellulose Proton-Exchange Membranes for Direct Methanol Fuel Cells. Polymers 2023, 15, 659. [Google Scholar] [CrossRef]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Ṋemavhola, F.; Nonjola, P.F.; Msomi, P.F. The proton conductivity and mechanical properties of Nafion®/ZrP nanocomposite membrane. Heliyon 2019, 5, e02240. [Google Scholar] [CrossRef]
- Shalaby, A.A.; Elmageed, M.H.A.; Malash, G.F.; Tamer, T.M.; Omer, A.M.; Mohy-Eldin, M.S.; Špitalský, Z.; Khalifa, R.E. Design of sulfonated polystyrene grafted cellulose acetate membrane for direct methanol fuel cells. Solid State Ion. 2024, 404, 116420. [Google Scholar] [CrossRef]
- Khalaf, M.; Saeed, A.M.; Ali, A.I.; Kamoun, E.A.; Fahmy, A. Polyelectrolyte membranes based on phosphorylated-PVA/cellulose acetate for direct methanol fuel cell applications: Synthesis, instrumental characterization, and performance testing. Sci. Rep. 2023, 13, 13011. [Google Scholar] [CrossRef]
- Feng, Z.; Gupta, G.; Mamlouk, M. A review of anion exchange membranes prepared via Friedel-Crafts reaction for fuel cell and water electrolysis. Int. J. Hydrogen Energy 2023, 48, 25830–25858. [Google Scholar] [CrossRef]
- Huang, J.; Yu, Z.; Tang, J.; Wang, P.; Tan, Q.; Wang, J.; Lei, X. A review on anion exchange membranes for fuel cells: Anion-exchange polyelectrolytes and synthesis strategies. Int. J. Hydrogen Energy 2022, 47, 27800–27820. [Google Scholar] [CrossRef]
- Hyun, J.; Kim, H.-T. Powering the hydrogen future: Current status and challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2023, 16, 5633–5662. [Google Scholar] [CrossRef]
- Zeng, G.; Han, J.; Dai, B.; Liu, X.; Li, J.; Chen, C.; Yang, J.; Sun, D. Preparation and Characterization of Alkaline Anion Exchange Membrane for Fuel Cells Application. J. Nanotechnol. 2017, 2017, 3701378. [Google Scholar] [CrossRef]
- Guo, X.; Qiao, J. Preparation of Bacterial Cellulose Based Polymer Anion Exchange Membrane and Application for Fuel Cell. ECS Meet. Abstr. 2019, MA2019-02, 1829. [Google Scholar] [CrossRef]
- Vilela, C.; Sousa, N.; Pinto, R.J.B.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Exploiting poly(ionic liquids) and nanocellulose for the development of bio-based anion-exchange membranes. Biomass Bioenergy 2017, 100, 116–125. [Google Scholar] [CrossRef]
- Nie, S.; Li, Z.; Li, X.; Lu, X.; Gong, C.; Sun, Z. Biomass-based crosslinked composite anion exchange membranes using bacterial cellulose template towards enhanced ionic conductivity and alkaline stability. Polym. Eng. Sci. 2024, 65, 620–630. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, J.; Liao, Y.; Li, C.; Wei, Z. Enhanced Conductivity of Anion-Exchange Membrane by Incorporation of Quaternized Cellulose Nanocrystal. ACS Appl. Mater. Interfaces 2018, 10, 23774–23782. [Google Scholar] [CrossRef]
- Das, G.; Park, B.J.; Kim, J.; Kang, D.; Yoon, H.H. Quaternized cellulose and graphene oxide crosslinked polyphenylene oxide based anion exchange membrane. Sci. Rep. 2019, 9, 9572. [Google Scholar] [CrossRef]
- Das, G.; Park, B.; Yoon, H. A bionanocomposite based on 1,4-diazabicyclo-[2.2.2]-octane cellulose nanofiber crosslinked-quaternary polysulfone as anion conducting membrane†. J. Mater. Chem. A 2016, 4, 15554–15564. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Wei, X.; Zhou, J.; Peng, H.; Xiao, L.; Lu, J.; Zhuang, L. Sulfonated Nanobamboo Fiber-Reinforced Quaternary Ammonia Poly(ether ether ketone) Membranes for Alkaline Polymer Electrolyte Fuel Cells. ACS Appl. Mater. Interfaces 2018, 10, 33581–33588. [Google Scholar] [CrossRef]
- Samaniego, A.J.; Sarker, M.; Najafianashrafi, Z.; Chuang, P.-Y.A.; Vasquez, M.; Ocon, J.D.; Xu, C.; Espiritu, R. Dimensionally stable cellulose acetate-chitosan semi-interpenetrating polymer network as fuel cell anion exchange membrane. ACS Appl. Polym. Mater. 2024, 6, 9047–9058. [Google Scholar] [CrossRef]
- Yunphuttha, C.; Midpanon, S.; Marr, D.W.M.; Viravathana, P. Polyvinyl alcohol/nanocellulose nanocomposites from oil palm empty fruit bunch as anion exchange membranes for direct alcohol-hydrogen peroxide fuel cells. Cellulose 2024, 31, 1569–1601. [Google Scholar] [CrossRef]
- Wang, F.; Qu, T.; Yang, H.; Yang, H.; Ou, Y.; Zhang, Q.; Cheng, F.; Hu, F.; Liu, H.; Xu, Z.; et al. Fabrication of Dual-Functional Bacterial-Cellulose-Based Composite Anion Exchange Membranes with High Dimensional Stability and Ionic Conductivity. ACS Appl. Mater. Interfaces 2024, 16, 2751–2762. [Google Scholar] [CrossRef]
- Sheng, J.; Tong, S.; He, Z.; Yang, R. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.-H.; Kim, J.-H.; Lee, S.-Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Yuan, B.; Wen, K.; Chen, D.; Liu, Y.; Dong, Y.; Feng, C.; Han, Y.; Han, J.; Zhang, Y.; Xia, C.; et al. Composite Separators for Robust High Rate Lithium Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101420. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Jia, H.; Zeng, C.; Lim, H.S.; Simmons, A.; Zhang, Y.; Weber, M.H.; Engelhard, M.H.; Gao, P.; Niu, C.; Xu, Z.; et al. Important Role of Ion Flux Regulated by Separators in Lithium Metal Batteries. Adv. Mater. 2024, 36, e2311312. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Dias, J.P.; Lanceros-Mendez, S.; Costa, C.M. Recent Advances in Poly(vinylidene fluoride) and Its Copolymers for Lithium-Ion Battery Separators. Membranes 2018, 8, 45. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Prem Kumar, T. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Song, D.; Liu, W.; Liu, C.; Li, H. Recent progress of bacterial cellulose-based separator platform for lithium-ion and lithium-sulfur batteries. Int. J. Biol. Macromol. 2024, 274, 133419. [Google Scholar] [CrossRef]
- Jiang, F.; Yin, L.; Yu, Q.; Zhong, C.; Zhang, J. Bacterial cellulose nanofibrous membrane as thermal stable separator for lithium-ion batteries. J. Power Sources 2015, 279, 21–27. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Q.-S.; Zhu, G.-R.; Wu, G.; Wang, X.-L.; Wang, Y.-Z. Cooperation of metal-organic coordination complex separator and wide-temperature-range electrolyte enables safe and high-performance lithium batteries. J. Energy Chem. 2024, 99, 692–702. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Peng, X.; Gong, W.; Ye, D.; Xu, J. Redox-active polypyrrole/bacterial cellulose bilayer separator for lithium-ion batteries. J. Energy Storage 2024, 93, 112386. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, R.; Wang, Y.; Fu, D.; Sheng, J.; Guo, X. A bacterial cellulose-based separator with tunable pore size for lithium-ion batteries. Carbohydr. Polym. 2023, 304, 120489. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Liu, J.; Yan, C. Nanofiber/ZrO2-based mixed matrix separator for high safety/high-rate lithium–ion batteries. Chem. Phys. Lett. 2017, 686, 134–139. [Google Scholar] [CrossRef]
- Ajkidkarn, P.; Manuspiya, H. Novel bacterial cellulose nanocrystals/polyether block amide microporous membranes as separators for lithium-ion batteries. Int. J. Biol. Macromol. 2020, 164, 3580–3588. [Google Scholar] [CrossRef]
- Xu, Q.; Wei, C.; Fan, L.; Peng, S.; Xu, W.; Xu, J. A bacterial cellulose/Al2O3 nanofibrous composite membrane for a lithium-ion battery separator. Cellulose 2017, 24, 1889–1899. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, J.; Du, M.; Zhang, F.; He, X. Highly porous zeolitic imidazolate framework-8@bacterial cellulose composite separator with enhanced electrolyte absorption capability for lithium-ion batteries. Cellulose 2022, 29, 5163–5176. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, C.; Gao, G.; Hu, C.; Luo, L.; Xu, J. Aramid nanofiber/bacterial cellulose composite separators for lithium-ion batteries. Carbohydr. Polym. 2020, 247, 116702. [Google Scholar] [CrossRef]
- Huang, C.; Ji, H.; Guo, B.; Luo, L.; Xu, W.; Li, J.; Xu, J. Composite nanofiber membranes of bacterial cellulose/halloysite nanotubes as lithium ion battery separators. Cellulose 2019, 26, 6669–6681. [Google Scholar] [CrossRef]
- Huang, C.; Ji, H.; Yang, Y.; Guo, B.; Luo, L.; Meng, Z.; Fan, L.; Xu, J. TEMPO-oxidized bacterial cellulose nanofiber membranes as high-performance separators for lithium-ion batteries. Carbohydr. Polym. 2020, 230, 115570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zou, S.; Lin, S.; Na, B.; Zeng, R.; Ma, Z.; Wang, L. Shish-kebab-like heterostructured cellulose nanofibers towards advanced lithium metal battery separators. Int. J. Biol. Macromol. 2025, 312, 144224. [Google Scholar] [CrossRef] [PubMed]
- Hänsel, C.; Lizundia, E.; Kundu, D. A Single Li-Ion Conductor Based on Cellulose. ACS Appl. Energy Mater. 2019, 2, 5686–5691. [Google Scholar] [CrossRef]
- Gonçalves, R.; Lizundia, E.; Silva, M.M.; Costa, C.M.; Lanceros-Méndez, S. Mesoporous Cellulose Nanocrystal Membranes as Battery Separators for Environmentally Safer Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3749–3761. [Google Scholar] [CrossRef]
- Zhou, A.; Guo, K.; Li, X.; Song, X.; Liu, X.; Ding, W.; Guo, B.; Guo, D.; Liu, G.; Wu, N.; et al. A CNC-Modified PAN Separator Improving the Cycle Stability of Lithium-Ion Batteries. Coatings 2025, 15, 351. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, L.; Li, C.; Li, X.; Xia, Z.a.; Yang, J.; Zhang, H. Preparation and Characterization of Cellulose-Based Lithium Battery Separator Materials. Polym. Adv. Technol. 2025, 36, e70299. [Google Scholar] [CrossRef]
- Chun, S.-J.; Choi, E.-S.; Lee, E.-H.; Kim, J.H.; Lee, S.-Y.; Lee, S.-Y. Eco-friendly cellulose nanofiber paper-derived separator membranes featuring tunable nanoporous network channels for lithium-ion batteries. J. Mater. Chem. 2012, 22, 16618–16626. [Google Scholar] [CrossRef]
- Sheng, J.; Chen, T.; Wang, R.; Zhang, Z.; Hua, F.; Yang, R. Ultra-light cellulose nanofibril membrane for lithium-ion batteries. J. Membr. Sci. 2020, 595, 117550. [Google Scholar] [CrossRef]
- Wang, N.; Liu, W.; Liao, H.; Li, Z.; Chen, Y.; Zeng, G. Pure cellulose nanofiber separator with high ionic conductivity and cycling stability for lithium-ion batteries. Int. J. Biol. Macromol. 2023, 250, 126078. [Google Scholar] [CrossRef]
- Li, L.; Yu, M.; Jia, C.; Liu, J.; Lv, Y.; Liu, Y.; Zhou, Y.; Liu, C.; Shao, Z. Cellulosic Biomass-Reinforced Polyvinylidene Fluoride Separators with Enhanced Dielectric Properties and Thermal Tolerance. ACS Appl. Mater. Interfaces 2017, 9, 20885–20894. [Google Scholar] [CrossRef]
- Dong, G.X.; Li, H.J.; Wang, Y.; Jiang, W.J.; Ma, Z.S. Electrospun PAN/cellulose composite separator for high performance lithium-ion battery. Ionics 2021, 27, 2955–2965. [Google Scholar] [CrossRef]
- Ko, Y.; Jung, H.Y.; Jung, H.W.; Ko, S.M.; Lee, J.T.; You, J. A new strategy for accelerating redox kinetics in lithium-ion batteries: Highly porous poly(ethylene glycol)/nanocellulose separators. Carbohydr. Polym. 2025, 356, 123372. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Xu, X.; Sun, R.; Wang, Z.; Lindh, J.; Edstrom, K.; Stromme, M.; Nyholm, L. Nanocellulose Modified Polyethylene Separators for Lithium Metal Batteries. Small 2018, 14, e1704371. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Hu, Z.; Pu, H. Lithium-ion battery separators based-on nanolayer co-extrusion prepared polypropylene nanobelts reinforced cellulose. J. Membr. Sci. 2023, 666, 121120. [Google Scholar] [CrossRef]
- Cunha, Á.; Brito, F.P.; Martins, J.; Rodrigues, N.; Monteiro, V.; Afonso, J.L.; Ferreira, P. Assessment of the use of vanadium redox flow batteries for energy storage and fast charging of electric vehicles in gas stations. Energy 2016, 115, 1478–1494. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; McCann, J.F. Vanadium redox flow batteries (VRBs) for medium- and large-scale energy storage. In Advances in Batteries for Medium and Large-Scale Energy Storage; Woodhead Publishing: Cambridge, HJ, USA, 2015; pp. 329–386. [Google Scholar]
- Wang, H.; Pourmousavi, S.A.; Soong, W.L.; Zhang, X.; Ertugrul, N. Battery and energy management system for vanadium redox flow battery: A critical review and recommendations. J. Energy Storage 2023, 58, 106384. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane development for vanadium redox flow batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef]
- Amiri, H.; Khosravi, M.; Ejeian, M.; Razmjou, A. Designing Ion-Selective Membranes for Vanadium Redox Flow Batteries. Adv. Mater. Technol. 2021, 6, 2001308. [Google Scholar] [CrossRef]
- He, S.; Chai, S.; Li, H. Nafion-Based Proton Exchange Membranes for Vanadium Redox Flow Batteries. ChemSusChem 2025, 18, e202402506. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Menictas, C. Thermal Stability of Concentrated V(V) Electrolytes in the Vanadium Redox Cell. J. Electrochem. Soc. 2019, 143, L86–L88. [Google Scholar] [CrossRef]
- Duerkop, D.; Widdecke, H.; Schilde, C.; Kunz, U.; Schmiemann, A. Polymer Membranes for All-Vanadium Redox Flow Batteries: A Review. Membranes 2021, 11, 214. [Google Scholar] [CrossRef]
- Ahn, S.M.; Jeong, H.Y.; Jang, J.-K.; Lee, J.Y.; So, S.; Kim, Y.J.; Hong, Y.T.; Kim, T.-H. Polybenzimidazole/Nafion hybrid membrane with improved chemical stability for vanadium redox flow battery application. RSC Adv. 2018, 8, 25304–25312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, D.; Zhao, L.; Wang, S.; Liu, J.; Yan, C. Excellent ion selectivity of Nafion membrane modified by PBI via acid-base pair effect for vanadium flow battery. Electrochim. Acta 2021, 394, 139144. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Cheng, Z.; Natan, A.; Ma, Y.; Yang, Y.; Cao, D.; Wang, W.; Zhu, H. Stable and Highly Ion-Selective Membrane Made from Cellulose Nanocrystals for Aqueous Redox Flow Batteries. Nano Lett. 2019, 19, 8979–8989. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Yang, Y.; Cheng, Z.; Luan, P.; Natan, A.; Zhu, H. Proton-conductive membranes with percolated transport paths for aqueous redox flow batteries. Mater. Today Nano 2021, 13, 100100. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kayal, P.; Das, S.; Raja, M.W. Sustainable paper based cellulose nano-crystal (CNC) impregnated flexible ion exchange membrane (IEM) for vanadium redox flow batteries (VRFBs). Mater. Res. Bull. 2025, 188, 113413. [Google Scholar] [CrossRef]
- Zhong, W.; Lv, L.; Wang, Z.; Wang, Z. Fabrication of Sulfonated Cellulose Nanocrystal/MXene Hybrid Proton Exchange Membrane and Its Synergistic Effect in Vanadium Redox Flow Battery. Nano Lett. 2025, 25, 4339–4346. [Google Scholar] [CrossRef]
- Kim, M.; Ha, D.; Choi, J. Nanocellulose-modified Nafion 212 Membrane for Improving Performance of Vanadium Redox Flow Batteries. Bull. Korean Chem. Soc. 2019, 40, 533–538. [Google Scholar] [CrossRef]
- Palanisamy, G.; Sadhasivam, T.; Park, W.-S.; Bae, S.T.; Roh, S.-H.; Jung, H.-Y. Tuning the Ion Selectivity and Chemical Stability of a Biocellulose Membrane by PFSA Ionomer Reinforcement for Vanadium Redox Flow Battery Applications. ACS Sustain. Chem. Eng. 2020, 8, 2040–2051. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Y.; Bian, W.; Liao, W.; Zhou, X.; Jiang, F. Robust proton exchange membrane for vanadium redox flow batteries reinforced by silica-encapsulated nanocellulose. Int. J. Hydrogen Energy 2020, 45, 9803–9810. [Google Scholar] [CrossRef]
- Yang, H.; Ding, P.; Vagin, M.; Gueskine, V.; Berggren, M.; Engquist, I. Nanocellulose-based ion-selective membranes for an aqueous organic redox flow battery. Cellulose 2024, 31, 10831–10843. [Google Scholar] [CrossRef]
- Lander, S.; Vagin, M.; Gueskine, V.; Erlandsson, J.; Boissard, Y.; Korhonen, L.; Berggren, M.; Wågberg, L.; Crispin, X. Sulfonated Cellulose Membranes Improve the Stability of Aqueous Organic Redox Flow Batteries. Adv. Energy Sustain. Res. 2022, 3, 2200016. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, W.; Zhao, H.; Li, J.; Chen, S.; Xu, F. Robust and High-Wettability Cellulose Separators with Molecule-Reassembled Nano-Cracked Structures for High-Performance Supercapacitors. Nano-Micro Lett. 2025, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Ritanjali, B.; Elanseralathan, K. A review on polyvinylidene fluoride polymer based nanocomposites for energy storage applications. J. Energy Storage 2022, 48, 103788. [Google Scholar] [CrossRef]
- Nazarpour Fard, H.; Behzadi Pour, G.; Sarvi, M.; Esmaili, P. PVA-based supercapacitors. Ionics 2019, 25, 2951–2963. [Google Scholar] [CrossRef]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Lebedeva, M.V.; Mozyleva, M.A.; Parmon, V.N. Technical Approaches for Preparation of Cellulose-Based Separators for Application in Supercapacitors. Energy Technol. 2025, 13, 2402365. [Google Scholar] [CrossRef]
- Yao, J.; Ji, P.; Sheng, N.; Guan, F.; Zhang, M.; Wang, B.; Chen, S.; Wang, H. Hierarchical core-sheath polypyrrole@carbon nanotube/bacterial cellulose macrofibers with high electrochemical performance for all-solid-state supercapacitors. Electrochim. Acta 2018, 283, 1578–1588. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, Y.; Tang, J.; Tang, W. Metal-organic frameworks governed well-aligned conducting polymer/bacterial cellulose membranes with high areal capacitance. Energy Storage Mater. 2019, 23, 594–601. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y.; Xiao, H.; Li, J.; Mei, C.; Fu, Q.; Han, J. All-solid-state wire-shaped micro-supercapacitors: A microfluidic approach to core-shell structured bacterial cellulose-GN/PPy fibers. Carbohydr. Polym. 2025, 349, 122996. [Google Scholar] [CrossRef] [PubMed]
- Rana, H.H.; Park, J.H.; Gund, G.S.; Park, H.S. Highly conducting, extremely durable, phosphorylated cellulose-based ionogels for renewable flexible supercapacitors. Energy Storage Mater. 2020, 25, 70–75. [Google Scholar] [CrossRef]
- Chen, G.; Chen, T.; Hou, K.; Ma, W.; Tebyetekerwa, M.; Cheng, Y.; Weng, W.; Zhu, M. Robust, hydrophilic graphene/cellulose nanocrystal fiber-based electrode with high capacitive performance and conductivity. Carbon 2018, 127, 218–227. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Zhu, S.; Huang, C.; Yue, Y.; Mei, C.; Xu, X.; Xia, C. Electrospun Core–Shell Nanofibrous Membranes with Nanocellulose-Stabilized Carbon Nanotubes for Use as High-Performance Flexible Supercapacitor Electrodes with Enhanced Water Resistance, Thermal Stability, and Mechanical Toughness. ACS Appl. Mater. Interfaces 2019, 11, 44624–44635. [Google Scholar] [CrossRef]
- Xu, L.H.; Wang, P.F.; Xu, Y.; Liu, J.; Peng, X.P.; Liu, J.; Shen, X.-J.; Wen, J.-L.; Li, Q.; Yuan, T.-Q. A combined genetic modification and chemical engineering strategy for designing high-performance cellulose nanofibrils separators. Chem. Eng. J. 2025, 503, 158402. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Chen, J.; Lu, Y.; Zhao, Z.; Akbar, A.R.; Yang, Q.; Shi, Z.; Xiong, C. Fabrication of porous carbon nanofibril/MnO2 composite aerogels from TEMPO-oxidized cellulose nanofibrils for high-performance supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127003. [Google Scholar] [CrossRef]
- Xia, L.; Li, X.; Wu, Y.; Hu, S.; Liao, Y.; Huang, L.; Qing, Y.; Lu, X. Electrodes derived from carbon fiber-reinforced cellulose nanofiber/multiwalled carbon nanotube hybrid aerogels for high-energy flexible asymmetric supercapacitors. Chem. Eng. J. 2020, 379, 122325. [Google Scholar] [CrossRef]
- Zhou, S.; Kong, X.; Zheng, B.; Huo, F.; Strømme, M.; Xu, C. Cellulose Nanofiber @ Conductive Metal−Organic Frameworks for High Performance Flexible Supercapacitors. ACS Nano 2019, 13, 9578–9586. [Google Scholar] [CrossRef]
- Lv, Y.; Zhou, Y.; Shao, Z.; Liu, Y.; Wei, J.; Ye, Z. Nanocellulose-derived carbon nanosphere fibers-based nanohybrid aerogel for high-performance all-solid-state flexible supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 8585–8594. [Google Scholar] [CrossRef]
- Chen, Y.; Lyu, S.; Han, S.; Chen, Z.; Wang, W.; Wang, S. Nanocellulose/polypyrrole aerogel electrodes with higher conductivity via adding vapor grown nano-carbon fibers as conducting networks for supercapacitor application. RSC Adv. 2018, 8, 39918–39928. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, Z.; Shen, M.; Chowdhury, S.; Ignaszak, A.; Sun, S.; Ni, Y. Cellulose nanofibers/reduced graphene oxide/polypyrrole aerogel electrodes for high-capacitance all-solid-state flexible supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11175–11185. [Google Scholar] [CrossRef]
- Lv, P.; Meng, Y.; Song, L.; Pang, H.; Liu, W. A self-supported electrode for supercapacitors based on nanocellulose/multi-walled carbon nanotubes/polypyrrole composite. RSC Adv. 2021, 11, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Khandelwal, S.; Rhee, K.Y.; Hui, D. Synergistic effect of reduced graphene oxide, CNT and metal oxides on cellulose matrix for supercapacitor applications. Compos. Part B Eng. 2018, 138, 45–54. [Google Scholar] [CrossRef]
- Jiran, D.; Pengfei, L.; Jinsong, Z.; Bin, W.; Wenhua, G.; Jun, X.; Kefu, C. Lignin/polypyrrole interpenetrating networks decorated Lignin-containing cellulose nanofibril composite membrane for High-performance supercapacitors. Chem. Eng. J. 2023, 470, 144180. [Google Scholar] [CrossRef]
- Kim, S.K.; Yoon, Y.; Ryu, J.H.; Kim, J.H.; Ji, S.; Song, W.; Myung, S.; Lim, J.; Jung, H.-K.; Lee, S.S.; et al. Recyclable High-Performance Polymer Electrolyte Based on a Modified Methyl Cellulose–Lithium Trifluoromethanesulfonate Salt Composite for Sustainable Energy Systems. ChemSusChem 2020, 13, 376–384. [Google Scholar] [CrossRef]
- Mona, T.A.-S.; Naglaa, N.; Gamal, T.; Qin, W.; Yajiang, Y.; Samir, K. Preparation, characterization and performance evaluation of methylcellulose/CuS@rGO/PANI membrane for energy storage application. Diam. Relat. Mater. 2025, 152, 111908. [Google Scholar] [CrossRef]
- Lee, B.-M.; Jeong, C.-U.; Hong, S.-K.; Yun, J.-M.; Choi, J.-H. Eco-friendly fabrication of porous carbon monoliths from water-soluble carboxymethyl cellulose for supercapacitor applications. J. Ind. Eng. Chem. 2020, 82, 367–373. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, J.; Zhao, T.; Hu, Q.; Xu, M.; Lei, Z.; Jin, X. Carboxymethylcellulose/poly(3,4-ethylenedioxythiophene):polystyrene sulfonate membrane after dimethyl sulfoxide treatment for flexible and high electrochemical performance asymmetric supercapacitors. Int. J. Biol. Macromol. 2023, 251, 126430. [Google Scholar] [CrossRef]
- Saborío, M.G.; Svelic, P.; Casanovas, J.; Ruano, G.; Pérez-Madrigal, M.M.; Franco, L.; Torras, J.; Estrany, F.; Alemán, C. Hydrogels for flexible and compressible free standing cellulose supercapacitors. Eur. Polym. J. 2019, 118, 347–357. [Google Scholar] [CrossRef]
- Riyadh Abdekadir, K.; Constantin, B.; Vipin, C.; Jaroslav, C.; Silvie, D.; Vladimir, S. Insight into structure-property correlations in plasticized sodium carboxymethyl cellulose/pectin blend-based polymer electrolyte for EDLC application. J. Energy Storage 2024, 101, 113769. [Google Scholar] [CrossRef]
- Zou, Z.; Xiao, W.; Zhang, Y.; Yu, H.; Zhou, W. Facile synthesis of freestanding cellulose/RGO/silver/Fe2O3 hybrid film for ultrahigh-areal-energy-density flexible solid-state supercapacitor. Appl. Surf. Sci. 2020, 500, 144244. [Google Scholar] [CrossRef]
- Salado, M.; Lanceros-Mendez, S.; Lizundia, E. Free-standing intrinsically conducting polymer membranes based on cellulose and poly(vinylidene fluoride) for energy storage applications. Eur. Polym. J. 2021, 144, 110240. [Google Scholar] [CrossRef]
- Hsu, H.; Khosrozadeh, A.; Li, B.; Luo, G.; Xing, M.; Zhong, W. An Eco-Friendly, Nanocellulose/RGO/in Situ Formed Polyaniline for Flexible and Free-Standing Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 4766–4776. [Google Scholar] [CrossRef]
- Xiao, L.; Qi, H.; Qu, K.; Shi, C.; Cheng, Y.; Sun, Z.; Yuan, B.; Huang, Z.; Pan, D.; Guo, Z. Layer-by-layer assembled free-standing and flexible nanocellulose/porous Co3O4 polyhedron hybrid film as supercapacitor electrodes. Adv. Compos. Hybrid Mater. 2021, 4, 306–316. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Xia, T.; Li, Q.; Ao, C.; Wang, Q.; Zhang, W.; Lu, C.; Deng, Y. Hollow polypyrrole/cellulose hydrogels for high-performance flexible supercapacitors. Energy Storage Mater. 2020, 31, 135–145. [Google Scholar] [CrossRef]
- Ko, J.; Kim, S.K.; Yoon, Y.; Cho, K.H.; Song, W.; Kim, T.-H.; Myung, S.; Lee, S.S.; Hwang, Y.K.; Kim, S.-W.; et al. Eco-friendly cellulose based solid electrolyte with high performance and enhanced low humidity performance by hybridizing with aluminum fumarate MOF. Mater. Today Energy 2018, 9, 11–18. [Google Scholar] [CrossRef]
- González, F.; Tiemblo, P.; Hoyos, M. In-Situ Approaches for the Preparation of Polythiophene-Derivative Cellulose Composites with High Flexibility and Conductivity. Appl. Sci. 2019, 9, 3371. [Google Scholar] [CrossRef]
- Yang, X.; Fei, B.; Ma, J.; Liu, X.; Yang, S.; Tian, G.; Jiang, Z. Porous nanoplatelets wrapped carbon aerogels by pyrolysis of regenerated bamboo cellulose aerogels as supercapacitor electrodes. Carbohydr. Polym. 2018, 180, 385–392. [Google Scholar] [CrossRef]
- Wan, C.; Jiao, Y.; Bao, W.; Gao, H.; Wu, Y.; Li, J. Self-stacked multilayer FeOCl supported on a cellulose-derived carbon aerogel: A new and high-performance anode material for supercapacitors. J. Mater. Chem. A 2019, 7, 9556–9564. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.; Chen, W.; Pastel, G.; Guo, X.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H.; et al. Nanocellulose-Enabled, All-Nanofiber, High-Performance Supercapacitor. ACS Appl. Mater. Interfaces 2019, 11, 5919–5927. [Google Scholar] [CrossRef]
- Xu, D.; Teng, G.; Heng, Y.; Chen, Z.; Hu, D. Eco-friendly and thermally stable cellulose film prepared by phase inversion as supercapacitor separator. Mater. Chem. Phys. 2020, 249, 122979. [Google Scholar] [CrossRef]
- Dang, C.; Huang, Z.; Chen, Y.; Zhou, S.; Feng, X.; Chen, G.; Dai, F.; Qi, H. Direct Dissolution of Cellulose in NaOH/Urea/α-Lipoic Acid Aqueous Solution to Fabricate All Biomass-Based Nitrogen, Sulfur Dual-Doped Hierarchical Porous Carbon Aerogels for Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 21528–21538. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, H.; Xu, Y.; Xu, F.; Zhang, X. Ultrathin separator with efficient ion transport and superior stability prepared from cotton cellulose for advanced supercapacitors. Chem. Eng. J. 2023, 470, 144089. [Google Scholar] [CrossRef]
- Kumar, S.N.; Johnsi, M.; Shalini, V.A.; Kavitha, N.; Balasubramanian, N. Cellulose nanofibril separator from Coffea arabica waste for supercapacitor applications. Ind. Crops Prod. 2024, 214, 118459. [Google Scholar] [CrossRef]
- Villafaña-López, L.; Reyes-Valadez, D.M.; González-Vargas, O.A.; Suárez-Toriello, V.A.; Jaime-Ferrer, J.S. Custom-Made Ion Exchange Membranes at Laboratory Scale for Reverse Electrodialysis. Membranes 2019, 9, 145. [Google Scholar] [CrossRef]
- Selyanchyn, O.; Selyanchyn, R.; Lyth, S. A Review of Proton Conductivity in Cellulosic Materials. Front. Energy Res. 2020, 8, 596164. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, L.; Zhang, M.; Lin, Z.; Shen, Z. Recent advances in cellulose-based polymer electrolytes. Cellulose 2022, 29, 8997–9034. [Google Scholar] [CrossRef]
- Wu, Z.; Ji, P.; Wang, B.; Sheng, N.; Zhang, M.; Chen, S.; Wang, H. Oppositely charged aligned bacterial cellulose biofilm with nanofluidic channels for osmotic energy harvesting. Nano Energy 2021, 80, 105554. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, T.; Wang, B.; Ji, P.; Sheng, N.; Zhang, M.; Liang, Q.; Chen, S.; Wang, H. Scalable bacterial cellulose biofilms with improved ion transport for high osmotic power generation. Nano Energy 2021, 88, 106275. [Google Scholar] [CrossRef]
- Sheng, N.; Zhang, M.; Song, Q.; Zhang, H.; Chen, S.; Wang, H.; Zhang, K. Enhanced salinity gradient energy harvesting with oppositely charged bacterial cellulose-based composite membranes. Nano Energy 2022, 101, 107548. [Google Scholar] [CrossRef]
- Zhouyue, L.; Ahmad, M.; Zhe, S.; Wenkai, F.; Sha, W. Advanced integrated nanochannel membrane with oppositely-charged bacterial cellulose and functionalized polymer for efficient salinity gradient energy generation. Int. J. Biol. Macromol. 2024, 277, 133975. [Google Scholar] [CrossRef]
- Sun, Z.; Ahmad, M.; Gao, Z.; Shan, Z.; Xu, L.; Wang, S.; Jin, Y. Highly ionic conductive and mechanically strong MXene/CNF membranes for osmotic energy conversion. Sustain. Energy Fuels 2022, 6, 299–308. [Google Scholar] [CrossRef]
- Wu, Y.; Xin, W.; Kong, X.-Y.; Chen, J.; Qian, Y.; Sun, Y.; Zhao, X.; Chen, W.; Jiang, L.; Wen, L. Enhanced ion transport by graphene oxide/cellulose nanofibers assembled membranes for high-performance osmotic energy harvesting. Mater. Horiz. 2020, 7, 2702–2709. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, Z.; Ahmad, M.; Liu, Y.; Wei, H.; Wang, S.; Jin, Y. Increased ion transport and high-efficient osmotic energy conversion through aqueous stable graphitic carbon nitride/cellulose nanofiber composite membrane. Carbohydr. Polym. 2022, 280, 119023. [Google Scholar] [CrossRef]
- Li, X.; Xiao, T.; Lu, B.; He, J.; Zhai, J. Double-Network Ion Channels for High-Performance Osmotic Power Generation. Adv. Mater. Interfaces 2022, 9, 2101960. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, P.; Fu, L.; Hu, Y.; Yang, L.; Wu, W.; Kong, X.-Y.; Jiang, L.; Wen, L. Engineered Cellulose Nanofiber Membranes with Ultrathin Low-Dimensional Carbon Material Layers for Photothermal-Enhanced Osmotic Energy Conversion. ACS Appl. Mater. Interfaces 2022, 14, 13223–13230. [Google Scholar] [CrossRef] [PubMed]
- Hongli, Y.; Jesper, E.; Viktor, G.; Mikhail, V.; Mehmet Girayhan, S.; Johan, E.; Lars, W.; Isak, E.; Magnus, B. The effect of crosslinking on ion transport in nanocellulose-based membranes. Carbohydr. Polym. 2022, 278, 118938. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Zhe, S.; Mehraj, A.; Wenkai, F.; Zongxia, G. Engineered cellulose nanofibers membranes with oppositely charge characteristics for high-performance salinity gradient power generation by reverse electrodialysis. Int. J. Biol. Macromol. 2023, 253, 126608. [Google Scholar] [CrossRef]
- Lin, X.; Dong, Y.; Tao, S.; Feng, X.; Wang, X.; Song, T.; Liu, J.; Zhong, Z.; Wang, Y.; Qi, H. Temperature-gated nanocellulose membrane for enhanced and controllable osmotic energy harvesting. Nano Energy 2023, 107, 108156. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Wang, C.; Wang, R.; Chen, C.; Gao, J.; Dai, J.; Liu, D.; Lin, Z.; Hu, L. Salinity-Gradient Power Generation with Ionized Wood Membranes. Adv. Energy Mater. 2020, 10, 1902590. [Google Scholar] [CrossRef]
- Xu, Y.; Song, Y.; Xu, F. TEMPO oxidized cellulose nanofibers-based heterogenous membrane employed for concentration-gradient-driven energy harvesting. Nano Energy 2021, 79, 105468. [Google Scholar] [CrossRef]
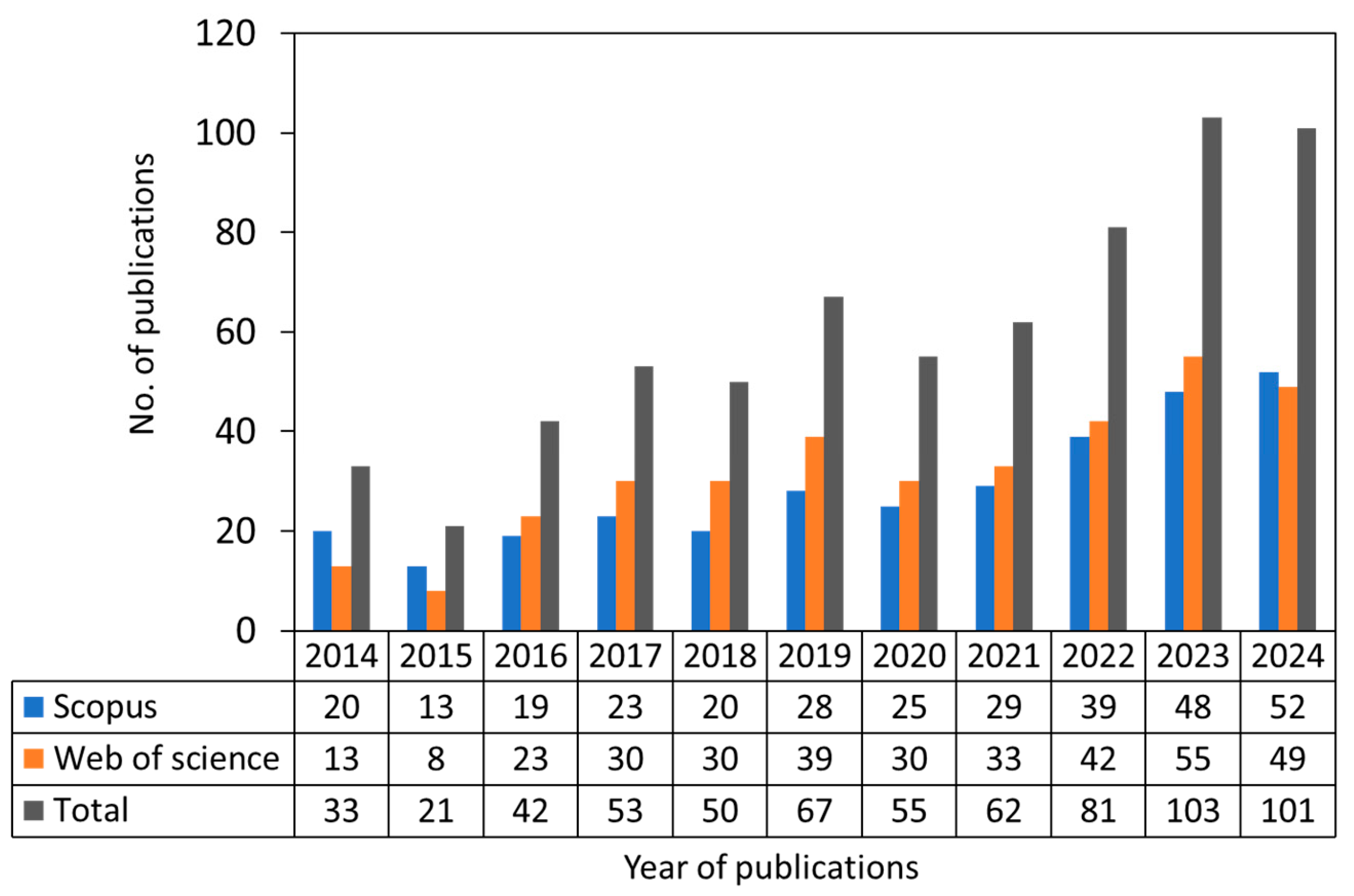
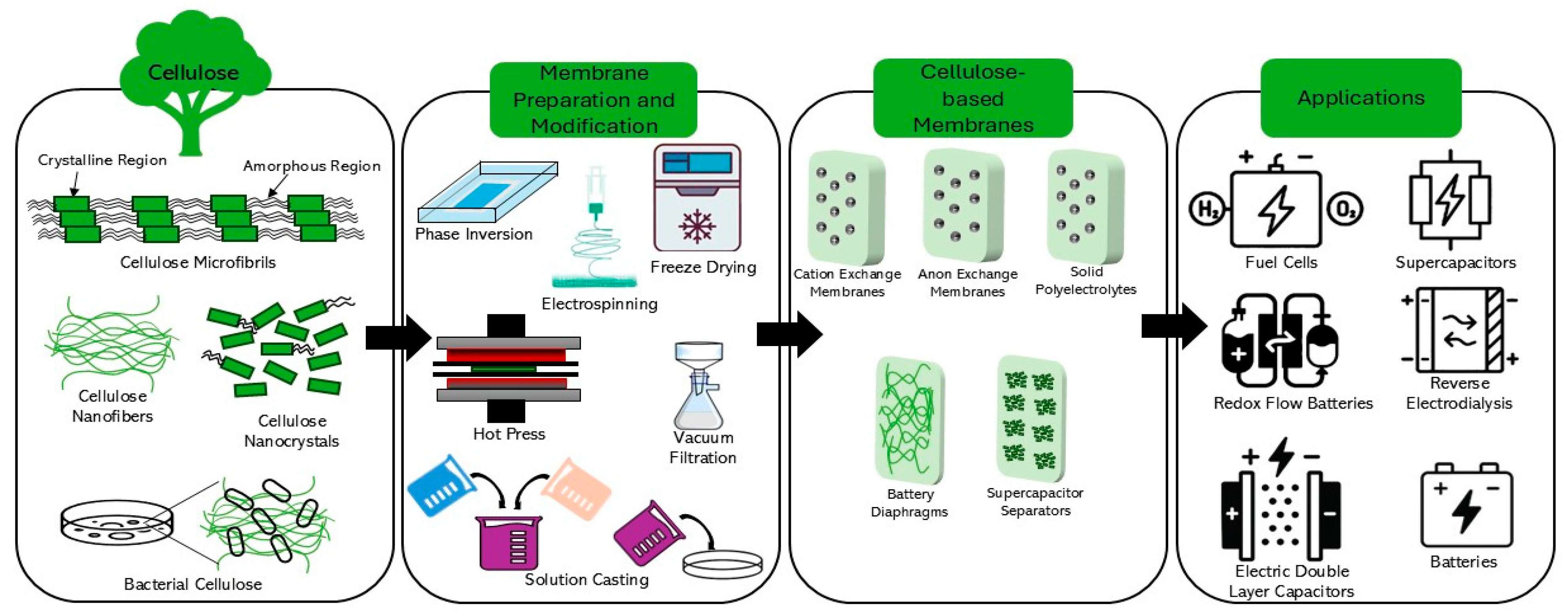
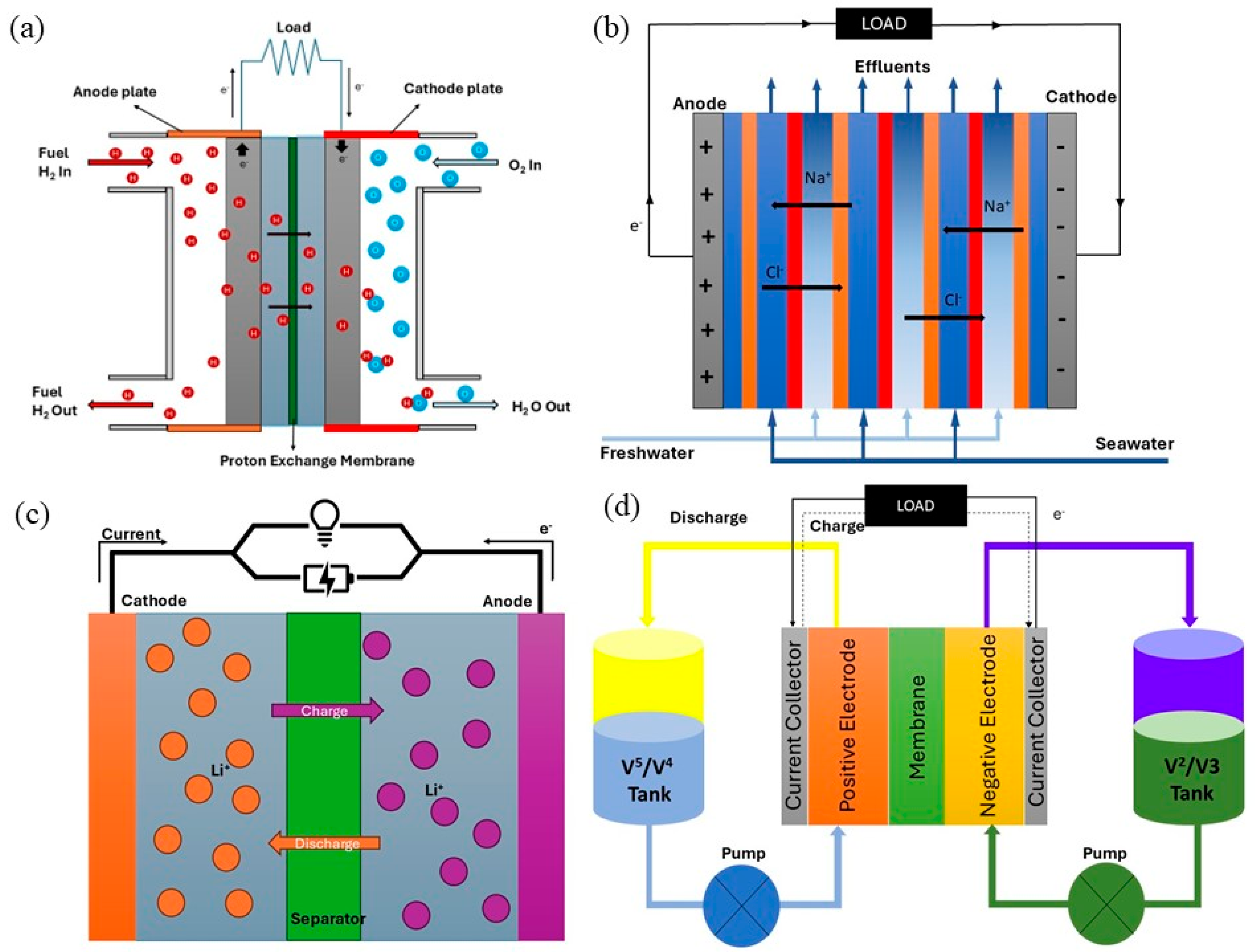
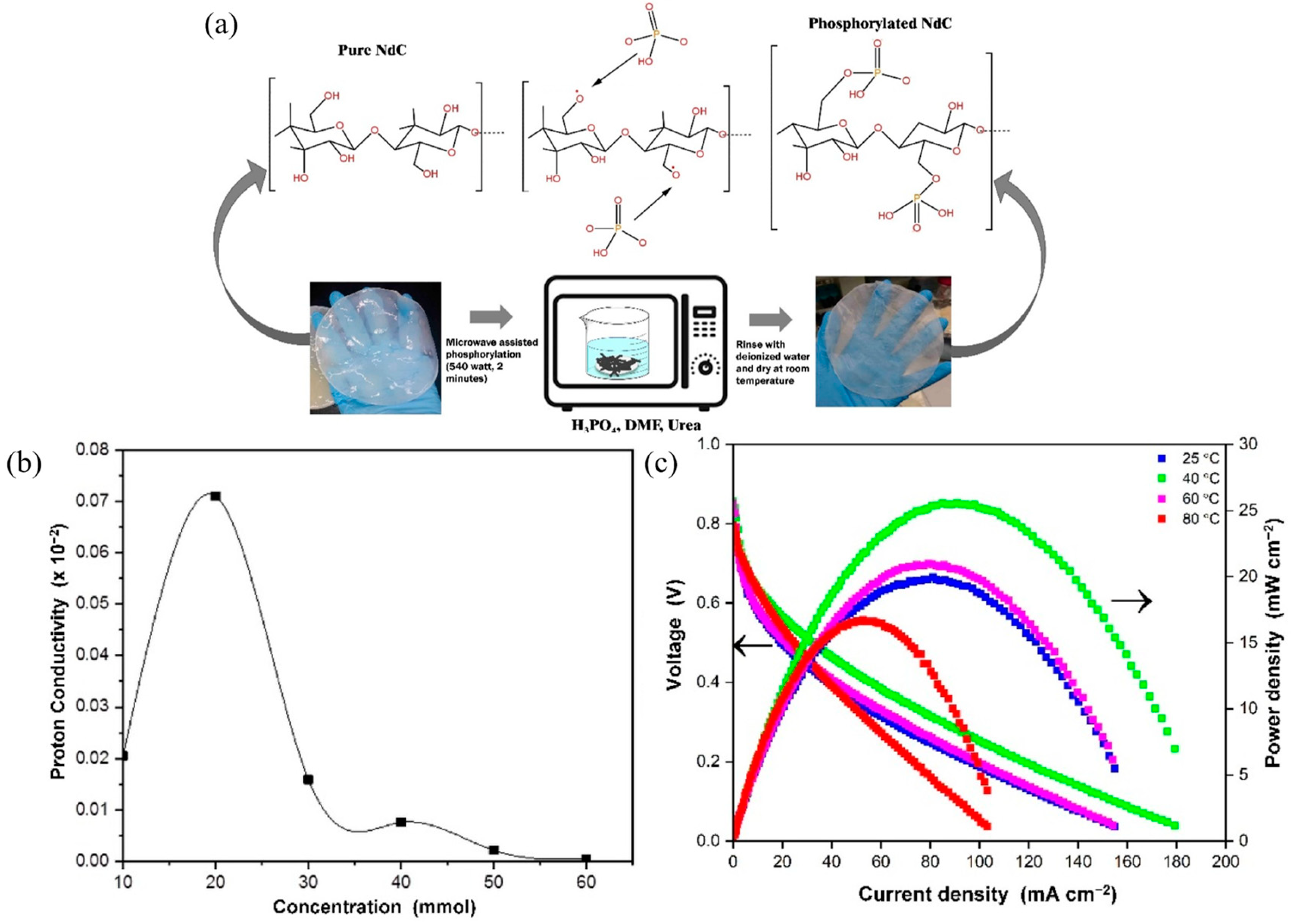
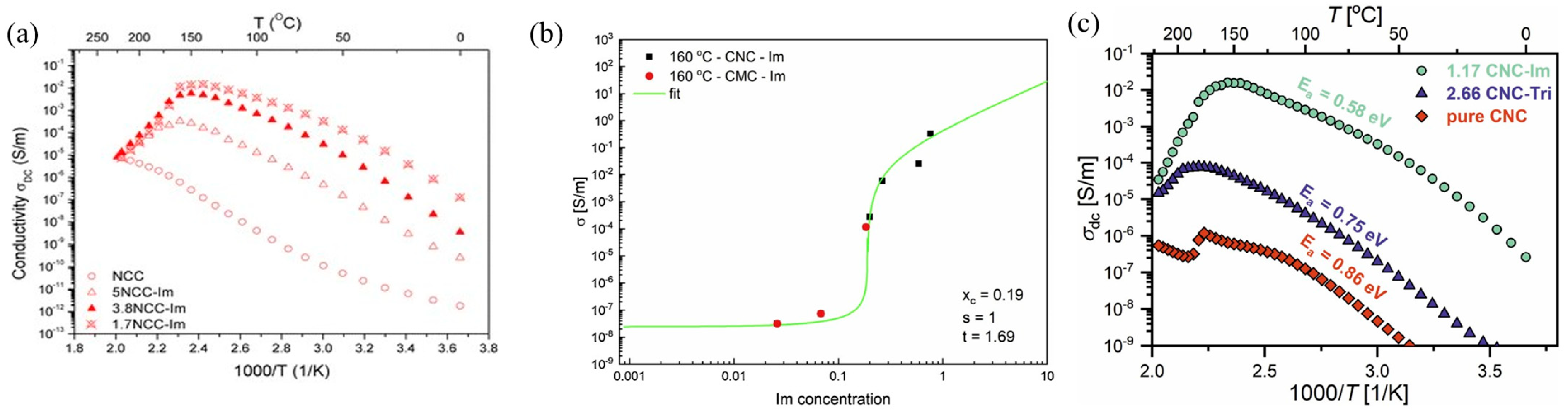
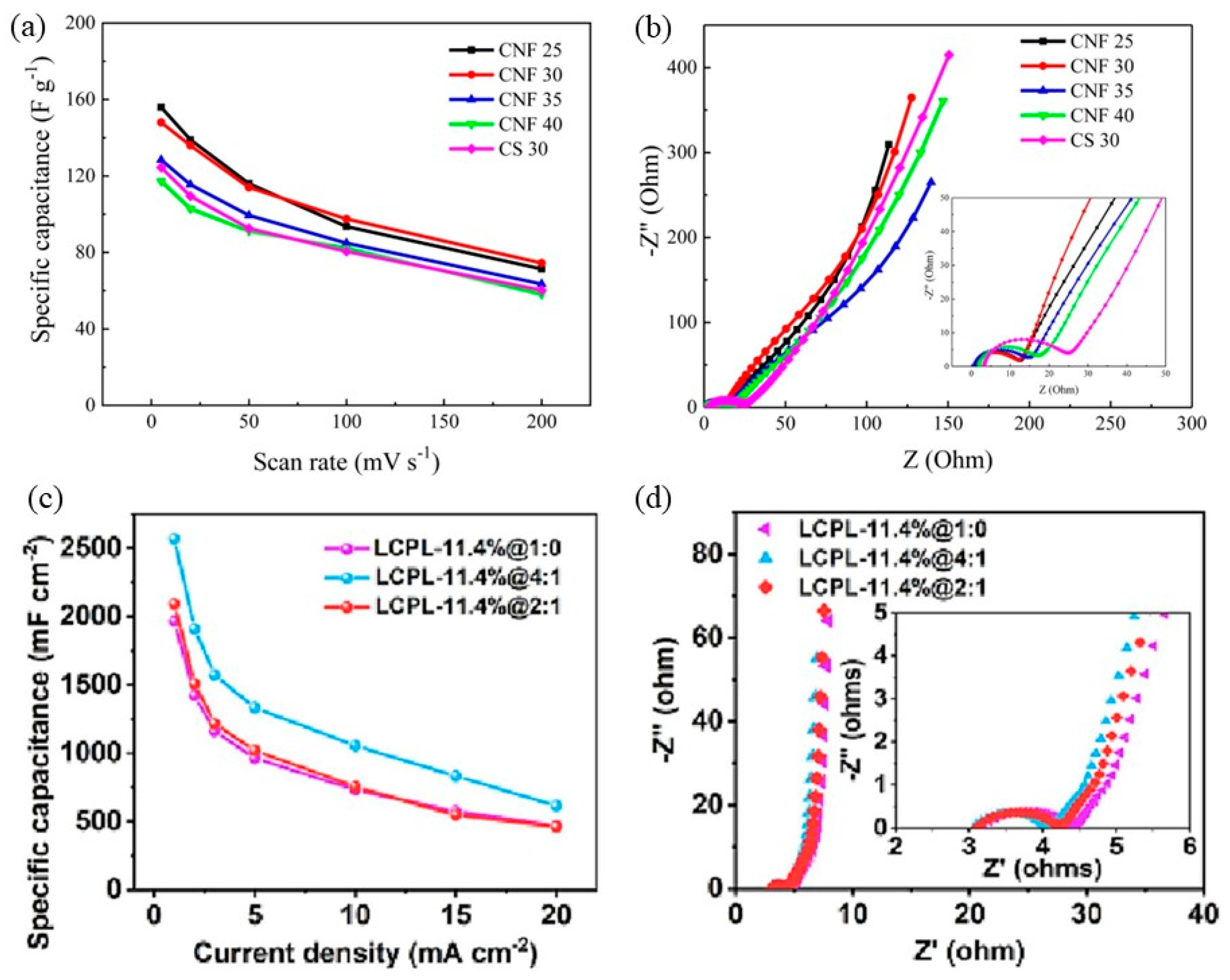
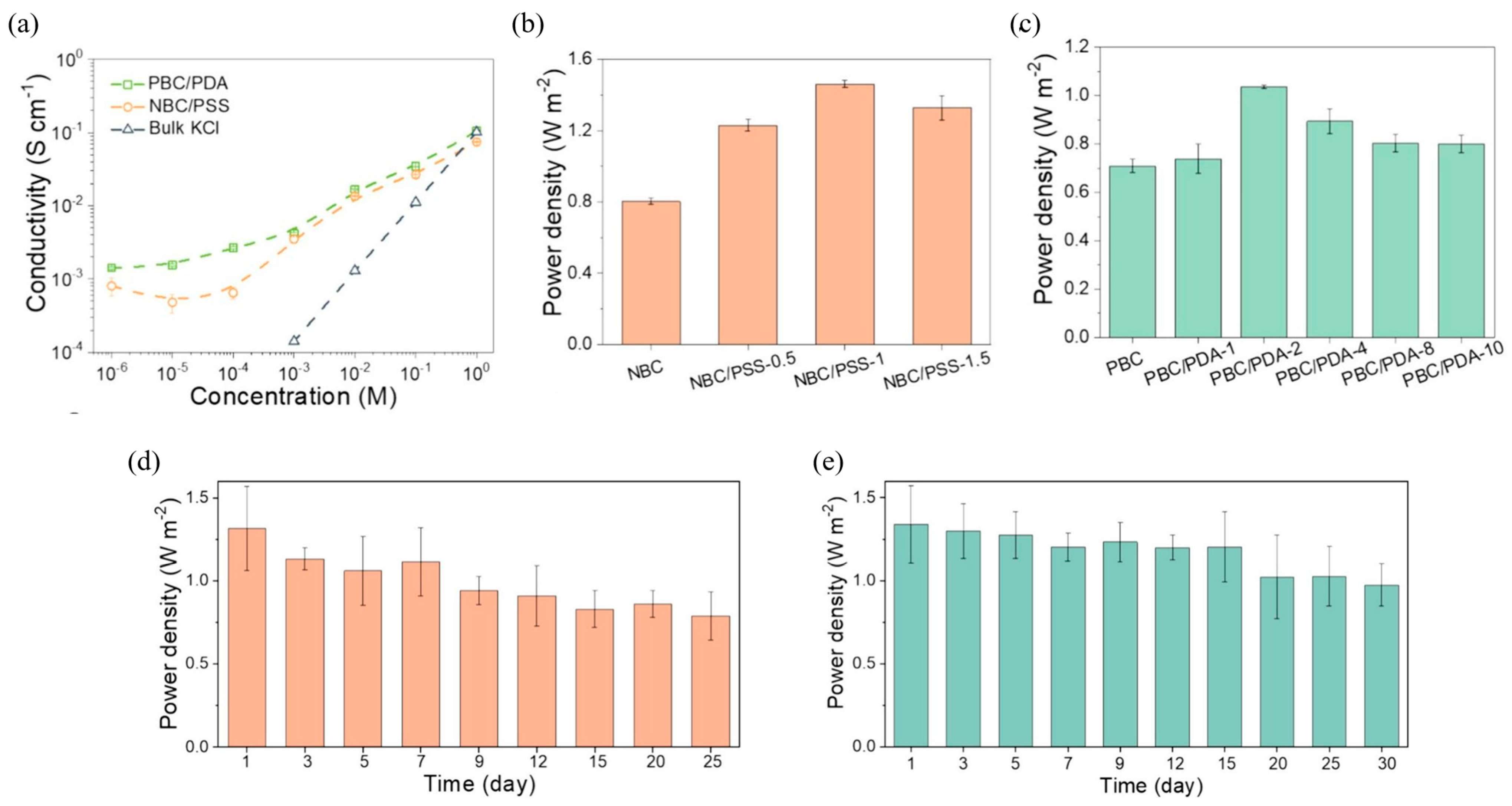
| Methods | Merits | Demerits | Applications |
|---|---|---|---|
| Solution Casting/ phase Inversion | - Simple and scalable - Good control over the thickness of membranes - Suitable for cellulose derivatives | - Use of toxic solvents - Limited porosity - Post-treatment is needed for stability | PEMs, AEMs, battery and supercapacitor separators |
| Electrospinning | - High surface area - Porous and flexible structures - Good ionic conductivity | - Requires high-voltage setup - Often involves toxic solvents - Post-regeneration is needed | Battery and supercapacitor separators, AEMs and PEMs |
| Regeneration from cellulose solutions | - Uses renewable cellulose - Environmentally friendly solvents can be used (e.g., ILs) | - Expensive or difficult-to-recycle solvents - Low mechanical strength | PEMs, AEMs, and separators |
| In-situ copolymerization/grafting | - Introduces functional groups to the cellulose backbone - Chemical bonding with cellulose backbone | - Complex process - Radiation sources or chemical initiators are required - Expensive in some cases | PEMs and AEMs for fuel cells and electrolyzers |
| Freeze-drying | - High porosity and surface area - Retains nanostructure integrity | - Energy-intensive - Poor mechanical strength unless reinforced | Supercapacitors, catalytic supports, and separators for VRFBs |
| Hot pressing/film casting of nanocellulose | - Solvent-free or low solvent use - Produces dense and robust films | - Long drying times - involve CNF/CNC fabrication, which is energy-intensive | PEMs and battery separators |
| Composite membrane formation | - Tailored properties - Combines the strengths of multiple components/fillers and substrates | - Interfacial compatibility issues - Potential phase separation - Complex fabrication | All electrochemical systems (PEMFCs, LIBs, VRFBs, etc.) |
| Cellulose Structure | Merits | Demerits | Imparted Functions |
|---|---|---|---|
| BC | - Exceptional purity - High crystallinity - Robust nanofibrillar network | - Dense microstructure restricts ion diffusion - Limited large-scale production | - Provides mechanical stability - Enhances water uptake - Facilitates ion transport |
| CNCs/NCCs | - Rigid rod-like morphology - High surface area - Abundant reactive sites for functionalization | - High loading causes brittleness - Reduces processability of composites | - Improves stiffness and dimensional stability - Enables surface chemical modification |
| CNFs | - Flexible, entangled fibrillar network - Rich in OH− - High aspect ratio | - High aqueous viscosity complicates processing - Strong tendency to aggregate | - Provides mechanical reinforcement - Offers tunable ionic conductivity - Enhances flexibility |
| CNWs | - Higher aspect ratio and crystallinity than CNCs - Strong reinforcing ability | - Aggregation and poor dispersion in polymer matrices - Processing challenges in blending | - Improves dimensional stability - Enhances proton conductivity when functionalized |
| Membrane Type | Modification | Membrane Thickness (µm) | Ion Conductivity (mS cm−1) | Power Density (mW cm−2) | Water Uptake (%) | Refs. |
|---|---|---|---|---|---|---|
| Nafion 115 | NA | 126 | 74 | 320 | NA | [156,157] |
| Nafion 117 | NA | 178 | 13.3 (30 °C/100%RH) | 30 | 35 | [158] |
| Crosslinked Cell/SA | Chemical | NA | 23 (25 °C) | NA | 53 | [116] |
| BC/AMPS | Chemical | NA | 29 | 97 | 264 | [120] |
| NCF/carboxylate & NCC/carboxylate | Chemical | 32/ 30 | 0.05 at 100 °C & 4.6 at 120 °C | 0.79 & 1.2 | NA | [134] |
| CMBC/PANI | Chemical | NA | 25.2 | NA | NA | [124] |
| BC/PSSA | Chemical | NA | 0.1 (94 °C) | NA | NA | [159] |
| BC/PSSA | Physical | 100 | NA | 40.0 | NA | [124] |
| BC/Nafion® | Physical | NA | 140 (94 °C/98%RH) | NA | NA | [125] |
| Nata de Cassava Bacterial Cellulose | Chemical | 0.4154 | 79.0 (80 °C) | 25 | 90 | [126] |
| CB/PMOEP | Chemical | 42 | 0.1 (98% RH) | NA | 206 | [127] |
| BC/P(bisMEP) | Chemical | 132 | 0.03 (80 °C/98%RH) | NA | NA | [128] |
| BC/Fuc | Physical | 50–80 | 1.6 (94 °C/98%RH) | NA | NA | [129] |
| BNC/LS | Physical | 85 | 23 (94 °C/98%RH) | NA | NA | [131] |
| BC/PANI-BG-BF4 | Physical | 20–25 | 0.052 (180 °C) | NA | NA | [130] |
| MCC-Im | Chemical | 1150 | 0.000021 (70 °C) | NA | NA | [136] |
| 1.7NCC-Im | Chemical | 100 | 0.27 (140 °C) | NA | NA | [37] |
| 1.3 NCC-Im | Chemical | 234 | 4.0 (160 °C) | NA | NA | [38] |
| 2.66 NCC-Tri | Chemical | 150 | 0.001 (175 °C) | NA | NA | [139] |
| NCC/Tri | Physical | 100–200 | 0.013 (120 °C) | NA | NA | [140] |
| CNF- COOH | Physical | 500 | 0.00001 (60 °C) | NA | NA | [143] |
| NCC-5/COOH-10 | Chemical | 0.07 | 218 (90 °C) | NA | NA | [56] |
| CNF/COOH | Physical | 15 | 1.0 (30 °C/75%RH) | NA | NA | [135] |
| P-CNF/SPES | Physical | 85–120 | 154 (80 °C, 100% RH) | NA | 45 | [142] |
| NCC/SFPEAK | Physical | 90–110 | 0.245 (90 °C) | NA | NA | [160] |
| s- PBI/cell/SiO2 | Physical | 45–55 × 103 | 9.11 (20 °C) | NA | NA | [151] |
| S-PEEKK/Am3-sNCC | Chemical | NA | 0.210 (100 °C) | NA | NA | [144] |
| CNC/SPEEK | Physical | 70 | 0.186 (95 °C and 95% RH) | NA | NA | [143] |
| Cell/Nafion & Cell/RDP | Physical | 196–206 | NA | 23 10 | NA | [161] |
| S-CNFs | Physical | 30 | 2.0 (120 °C) | 4.1 | NA | [162] |
| CA-rGO-PVDF | Physical | 120 | 0.4 | NA | NA | [147] |
| CA/GO | Physical | 25 | 15.5 | 519 | NA | [163] |
| Tempo-oxidized and sulfonated CNF | Chemical | 50 | 1.76 (25 °C, 100% RH) | NA | 63 | [118] |
| CNF-g-PMMA/PF | Chemical | 137 | NA | 542 | NA | [121] |
| NCC/PVA/SGO | Physical | NA | 11.0 (80 °C, 100% RH) | 2.9 | 147 | [153] |
| NCC/PVA | Physical | 70 | 3.0 | 10.3 | 120 | [154] |
| NCC-Im & NCF-Im | Physical | 400 & 500 | 0.0326 & 0.021 | NA | NA | [145] |
| MCC/RDP/PO3–H | Chemical | 210 | 1.12 | 16 (in air) 34.3 (in oxygen) | NA | [155] |
| SEC/SPEEK | Physical | NA | 109.2 | NA | 30 | [152] |
| Membrane Type | Modification | Membrane Thickness (µm) | Ion Conductivity (mS cm−1) | Power Density (mW cm−2) | Water Uptake (%) | Methanol Permeability (μcm2 s−1) | Selectivity (kS s cm−3) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nafion 117 | NA | 178 | 15 | 126.04 | 30 | 0.884 | NA | [175] |
| AMPS -BC | Chemical | NA | 29.0 | 61.0 | 275 | 0.564 | NA | [120] |
| PVA/CS/CNC-HNO3 | Physical | NA | 64.2 | NA | 78 | 0.031 | 18.1 | [166] |
| Nafion/5%-CW | Physical | NA | 17.0(70 °C) | 91.0 | 29 | 2,331,070 | 400 | [65] |
| CNF/SPES | Physical | 110–130 | 0.13 | NA | NA | 0.445 | 89 | [150] |
| CNF/SPES/FAA-20 | Physical | 60–100 | 0.264(80 °C) | 87.22 | NA | NA | NA | [144] |
| Cell/PTA/Im | Physical | 500 | 0.214 | NA | 38.68 | 0.0214 | 0.00004838 | [169] |
| NC/Im/m-PTA5 | Physical | 500 | 31.9 | NA | 60.3 | 0.0174 | NA | [170] |
| NC-10SSA | Physical | 20 | 3.2 | NA | 60 | NA | NA | [171] |
| SPSF/CW-Ser | Physical | NA | 0.234 (80 °C) | 73.8 | 50 | 0.76 | NA | [146] |
| CNF/UiO-66-NH2/SPS | Physical | 80–100 | 0.20 (80 °C) | NA | NA | 0.55 | NA | [167] |
| CNC/PVA | Physical | NA | 3.0 | 10.3 | NA | NA | NA | [154] |
| MCC/DAC/SPEEK | Physical | NA | 0.1 | NA | 522 | NA | NA | [168] |
| CA-g-PSSA | Chemical | 30 | 4.77 | 24.6 | 31.4 | 0.55 | 8.65 | [176] |
| CA-g-PPVA | Physical | 1200 | 0.035 | NA | 68.7 | 0.000108 | NA | [177] |
| CNF-SSASPEI | Physical | NA | 16.7(80 °C) | 4.32 | NA | 0.0822 | NA | [172] |
| SPS/CNDs | Chemical | 130 | 35.5 | NA | 45.6 | 3.5 | 0.00101 | [173] |
| Membrane Type | Modification | Membrane Thickness (µm) | Ion Conductivity (mScm−1) | Power Density (mWm−1) | Water Uptake (%) | Ref. |
|---|---|---|---|---|---|---|
| BC/TiO2/CHPTAC | Physical | NA | 93.0 (80 °C) | NA | 114 | [181] |
| BC-PDDA-OH- | Physical | NA | 51.0 | NA | NA | [182] |
| BC/PMACC | Chemical | NA | 10.0 (94 °C, 98%RH) | NA | 1549 | [183] |
| BC/LDH | Chemical | NA | 70.7 (80 °C) | 13.6 | NA | [184] |
| BC/TiO2/VBTAC | Chemical | NA | 100.5(80 °C) | 40.2 | 55 | [62] |
| Crosslinked DABCO–CNF/DABCO–PS | Chemical | NA | 74.0(25 °C) | NA | 59.5 | [187] |
| QPPO/QCNC | Chemical | 20 | 60.0 (80 °C) | 392.0 | 17 | [185] |
| QPPO/QCNF/QGO | Physical | NA | 114.0 (80 °C) | NA | 89.0 | [186] |
| CA-NF/CSSIPN | Physical | 150 | 21.0 | NA | NA | [186] |
| Crosslinked QPVA/QNC | Physical | 100 | 15.0 | 1.95 | 230 | [190] |
| PBC3/QPPO | Chemical | NA | 62.6 | 0.43 | NA | [191] |
| Membrane Type | Modification | Membrane Thickness (µm) | Ion Conductivity (mScm−1) | Thermal Shrinkage (% at °C) | Mechanical Strength Stress (MPa)/ Strain (%) | Capacity Retention (% of the Number of Cycles) | Ref. |
|---|---|---|---|---|---|---|---|
| PE | NA | 20 | 0.65 | 90 at 150 | 12.5/17 | 96 at 100 | [3] |
| PP (Celgard 2400) | 25 | 0.38 | 99 at 200 | 15.3/12 | 82 at 100 | [18] | |
| PP-PE-PP | NA | 25 | 0.18 | 99 at 200 | 90/55 | 55 at 200 | [179] |
| PP-PE-PP (Celgard 2340) | NA | 38 | 3.39 | 34 at 200 | 140/47 | 82 at 50 | [182] |
| BC | Physical | 13 | NA | 0 at 180 | 78/5.0 | NA | [177] |
| OBCS-200 | Chemical | 57 | 2.9 | 0 at 160 | 9.9/0.55 | 90 at 100 | [180] |
| PPy/BC | Chemical | 25 | 1.28 | 0 at 200 | 22.1/4.0 | 76 at 200 | [179] |
| BC-Al2O3 | Physical | 30 | 4.91 | 0 at 200 | 122/0.3 | 89 at 50 | [182] |
| BCZC | Physical | 25 | 2.14 | 3 at 200 | 18.2/10.0 | 98 at 100 | [3] |
| BCNCs/PEBAX | Physical | 35 | 9.79 | 0 at 150 | 14.9/55.2 | NA | [181] |
| BZP | Chemical | 52 | 1.15 | 0 at 180 | NA | 86 at 1600 | [178] |
| ZIF-8@BC-2 | Chemical | NA | 1.12 | 0 at 200 | 25.6/4.0 | 89 at 100 | [183] |
| ANFs/BC | Physical | 30 | 12.5 | 0 at 160 | NA | 93 at 100 | [184] |
| BC/HNTs | Physical | 30 | 5.13 | 0 at 200 | NA | 95 at 100 | [185] |
| TOBC | Chemical | 30 | 13.45 | 0 at 200 | 110/9.5 | 94 at 100 | [186] |
| PDA/BC | Chemical | NA | 1.89 | 0 at 180 | NA | NA | [187] |
| CPC | Chemical | 20 | 0.22 | 0 at 200 | NA | 98 at 65 | [197] |
| PPNBs/CS | Physical | 20 | 1.04 | 30 at 260 | 50/4.0 | 92 at 150 | [198] |
| CNCs/PAN | Physical | 30 | 2.82 | 7.3 at 200 | 22.5/11.0 | 97 at 100 | [189] |
| mCNC | Chemical | 75 | 2.00 | NA | NA | 93 at 100 | [188] |
| MCNC | Physical | 150 | 2.7 | 0 at 100 | 55/4.0 | 90 at 60 | [53] |
| PVdF/NCC | Physical | 20 | 1.45 | NA | 99/2.0 | NA | [43] |
| CNP | Physical | 19 | 0.77 | 0 at 150 | 21/NA | 87 at 100 | [191] |
| ECM | Physical | 12 | 0.26 | 0 at 160 | 60/5.0 | 78 at 100 | [192] |
| CNF | Physical | NA | 1.90 | 0 at 160 | 68/11.00 | 97.5 at 100 | [193] |
| Membrane Type | Modification | Membrane Thickness (µm) | Proton Conductivity (mS cm−1) Vanadium Permeability (×10−7 cm2 min−1) | EE (%)/Number of Cycles at Current Density (mA cm−2) | Mechanical Strength Stress (MPa)/ Strain (%) | Ref. |
|---|---|---|---|---|---|---|
| Nafion 115 | NA | 125 | 15.0/NA | NA | 20/155 | [208] |
| Nafion 115 | NA | 125 | 6.0/NA | NA | 35/238 | [209] |
| Nafion 212 | NA | 50 | NA/54 | NA | NA | [211] |
| Nafion 212 | NA | 50 | 71.6/3.2 | 82/100 at 80 | NA | [213] |
| Nafion 212 | NA | 60 | 44.0/4.4 | NA | 10/89 | [214] |
| Nafion/PBI | Physical | 25 | 111.0/0.0195 | 85/300 at 60 | NA | [234] |
| Nafion/PBI | Chemical | 51 | 32.3/13.3 | 81.3/300 at 200 | 32/81 | [235] |
| CNC/PVDF-HNF | Physical | 77 | 16.0/NA | 88.2/650 at 100 | 65/10 | [208] |
| BC/PVDF-HNF | Physical | 200 | 9.5/NA | 79.5/300 at 100 | 114/14 | [209] |
| CNC/PVDF-HNF | Physical | 20 | 6.9/NA | NA | 48/4.5 | [210] |
| SCNC/MXene/ PVDF-HFP | Physical | 40 | 15.8/660 | 90.7/120 | 52/0.8 | [11] |
| Nafion-PDDA/CNC | Physical | 51 | NA | 89/50 at 60 | NA | [212] |
| BC-PFSA | Physical | NA | 60.5/8.6 | 78.9/100 at 80 | NA | [213] |
| CNC-SPES | Physical | 70 | 29.1/3.7 | 82/200 at 100 | 2/25 | [214] |
| Membrane Type | Modification | Membrane Thickness (µm) | Ionic Conductivity (S cm−1) | Power Density (W m−2) | Ref. |
|---|---|---|---|---|---|
| Ppy/CNT/BC | Chemical | 0.12 | 21 | 228 | [250] |
| ZIF-67-Ppy/PDA/BC | Chemical | 70,000 | NA | 209.09 | [251] |
| BC/GN/Ppy | Chemical | 70 | 621 | 444 | [252] |
| C-pHEMA/C-p-cellulose | Chemical | 100 | 0.224 | 124 | [253] |
| GO/CNC | Chemical | NA | 64.7 | 123.2 | [254] |
| (PANI@CNT−CNC/PVA− PAA) | Chemical | 40 | 0.0044 | 164.6 | [255] |
| CNF | Physical | 25 | 0.00269 | 124.76 | [256] |
| CTOCN/MnO2 | Chemical | 0.025 | NA | 171.1 | [257] |
| CF-CNF/MWCNT-Has | Physical | 228.6 | NA | 174.5 | [258] |
| CNF@Ni-HITP | Physical | 350 | 103 | 103 | [259] |
| CNF/VGCF/Ppy | Chemical | NA | 11.25 | 678.66 | [261] |
| CNFs/rGO/PPy | Chemical | NA | 2.5 | 405 | [262] |
| CNF/CNT/Ppy | Chemical | NA | NA | 200.8 | [263] |
| Cellulose/MWCNT/rGO/Co3O4/SnO2 | Chemical | NA | NA | 215 | [264] |
| PDADMAC/CNF | Physical | 30 | 0.005 | 185.3 | [115] |
| (LS/PPy)/LCNFs | Physical | 225 | 160 | 197 | [265] |
| LiTFS–LiSMC | Chemical | 500 | 0.001 | 74.11 | [266] |
| MC/CuS@rGO | Physical | 70 | 1.07 × 10−7 | NA | [267] |
| CMCM | Physical | NA | NA | 210 | [268] |
| CMC/PEDOT: PSS | Physical | 98 | 45.1 | 116.4 | [269] |
| [PEDOT/Al2O3/NaCMC] PHMeDOT | Chemical | NA | NA | 0.0006 | [270] |
| Glycerol NaCMC-PC- LiClO4 | Physical | 310 | 0.000355 | 6.18 | [271] |
| Cellulose/RGO/silver/Fe2O3 | Chemical | 620 | NA | NA | [272] |
| Cell/PDA/PPy | Chemical | 134 | 0.59 | 32 | [273] |
| NC/RGO/PANI | Chemical | 40 | NA | 79.71 | [274] |
| NPC | Chemical | NA | NA | 52.74 | [275] |
| PC | Chemical | NA | 0.606 | 255 | [276] |
| A520-SC | Chemical | NA | 0.172 | 135.14 | [277] |
| Cell/PEDOT:PSS | Chemical | NA | 0.77 | NA | [278] |
| CFA | Physical | NA | NA | 409 | [279] |
| FeOCI@CDCA | Physical | NA | 0.26 | 647 | [280] |
| ASC | Chemical | NA | 0.265 | 64.83 | [281] |
| Cellulose | Chemical | 150 | 0.2986 | 130 | [282] |
| NSHPAs | Physical | 500 | NA | 329 | [283] |
| RC @PVDF | Physical | 20 | 3.87 × 10−6 | 173 | [114] |
| CCM | Physical | 15 | 0.01632 | 145.6 | [284] |
| CWCNF | Physical | 25 | 0.003 | 1768 | [285] |
| Membrane Type | Modification | Membrane Thickness (µm) | Ion Conductivity (mS cm−1) | Power Density (W m−2) | Ref. |
|---|---|---|---|---|---|
| NBC & PBC | Physical | 90 | 1.00 & 0.42 | 0.23 | [289] |
| BC-CMC & BC-HACC | Chemical | 20 | 0.78 | 2.25 (+ve) & 0.42 (−ve) | [290] |
| NBC/NGO & PBC/PLDH | Physical | 7 | 0.52–0.64 | 0.7 | [291] |
| NBC/PSS & PBC/PDA | Chemical | 160 | 1.40 & 0.8 × 10−3 | 0.99 & 1.14 | [292] |
| MXene/CNF | Physical | 46 | 4.00 | 0.15 | [293] |
| GO/CNFs | Physical | 9 | 135 | 4.19 | [294] |
| g-C3N4/CNF | Physical | 50 | 14 | 0.15 | [295] |
| CNF/CNTs | Physical | 6.76 | NA | 4.67 | [296] |
| CNF/2D-GO | Physical | 6 | 0.20 | 12.04 | [297] |
| CNF/BTCA/SHP | Physical | 20 | 8.10 | NA | [298] |
| T-CNF & E-CNF | Physical | 40 | 17 & 16 | 2.87 | [299] |
| Bamboo-CNF | Physical | 30 | 0.36 | 2.2 | [33] |
| C-CNF | Chemical | 50 | NA | 10.1 | [300] |
| P-Wood & N-Wood | Physical | 1000 | 0.20 & 0.40 | 0.00514 | [301] |
| TOCNs/PET | Physical | 21 | NA | 0.96 | [302] |
| Challenges | Recommendations | Target Applications |
|---|---|---|
| Performance trade-off between ionic conductivity, selectivity, barrier properties and mechanical strength | - Apply hybrid (composite) designs with optimum combination of CNF with polymer matrices/inorganic fillers - Adopt fabrication methods to ensure balance conductivity, selectivity, barrier property and mechanical integrity | Various fuel cell types, VRFBs, PEMWE, AEMWE and RED |
| Chemical and thermal degradation in harsh electrolytes (acidic, alkaline, oxidative/reductive) | - Employ stable cellulose derivatives (e.g., cellulose acetate, carboxymethylated cellulose, etc.) - Use CNWs or CNF matrices such as PPO- Reinforcement with oxidation-resistant fillers (Mxene, GO, SiO2, etc.). | All systems |
| Lack of sufficient in-situ durability tests in real devices | - Conduct accelerated aging and realistic cycling tests- Integrate membranes into device prototypes at early stage | All systems |
| Complex, energy-intensive, and low-throughput fabrication processes | Develop roll-to-roll or extrusion manufacturing facilities, optimize solvent recovery, use greener nanocellulose production with reduced energy demand | All systems |
| Inconsistent pore size and thickness control at scale | Apply scalable templating/phase-separation methods, integrate in-line monitoring for porosity and thickness uniformity during production | All systems |
| Lack of optimization studies on fabrication parameters | - Apply optimization techniques to obtain the best combinations allowing development of desired structures- Use DFT and AI tools to design robust membranes- Apply optimization techniques for investigating operating parameters to obtain maximum performance | All system |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shawalludin, N.S.F.; Sha’rani, S.S.; Suhot, M.A.; Sarip, S.; Nasef, M.M. Cellulose-Based Ion Exchange Membranes for Electrochemical Energy Systems: A Review. Membranes 2025, 15, 304. https://doi.org/10.3390/membranes15100304
Shawalludin NSF, Sha’rani SS, Suhot MA, Sarip S, Nasef MM. Cellulose-Based Ion Exchange Membranes for Electrochemical Energy Systems: A Review. Membranes. 2025; 15(10):304. https://doi.org/10.3390/membranes15100304
Chicago/Turabian StyleShawalludin, Nur Syahirah Faiha, Saidatul Sophia Sha’rani, Mohamed Azlan Suhot, Shamsul Sarip, and Mohamed Mahmoud Nasef. 2025. "Cellulose-Based Ion Exchange Membranes for Electrochemical Energy Systems: A Review" Membranes 15, no. 10: 304. https://doi.org/10.3390/membranes15100304
APA StyleShawalludin, N. S. F., Sha’rani, S. S., Suhot, M. A., Sarip, S., & Nasef, M. M. (2025). Cellulose-Based Ion Exchange Membranes for Electrochemical Energy Systems: A Review. Membranes, 15(10), 304. https://doi.org/10.3390/membranes15100304






